Abstract
Exaggerated levels of the leukotriene B4 (LTB4) frequently coexist at sites of inflammation and tissue remodeling. Therefore, we hypothesize that the LTB4 pathway plays an important role in the pathogenesis of neutrophilic inflammation that contributes to pulmonary emphysema. In this study, significant levels of LTB4 were detected in human lung tissues with emphysema compared with lungs without emphysema (9,497 ± 2,839 vs. 4,142 ± 1,173 pg/ml, n = 9 vs. 10, P = 0.04). To further determine the biological role of LTB4 in the pathogenesis of emphysema, we compared the lungs of wild-type (WT) and LTA4 hydrolase−/− mice (LTB4 deficient, LTA4H−/−) exposed to intranasal elastase or vehicle control. We found that intranasal elastase induced accumulation of LTB4 in the lungs and caused progressively worsening emphysema between 14 and 28 days after elastase exposure in WT mice but not in LTA4H−/− mice. Premortem physiology documented increased lung compliance in elastase-exposed WT mice compared with elastase-exposed LTA4H−/− mice as measured by Flexivent (0.058 ± 0.005 vs. 0.041 ± 0.002 ml/cmH2O pressure). Postmortem morphometry documented increased total lung volume and alveolar sizes in elastase-exposed WT mice compared with elastase-exposed LTA4H−/− mice as measured by volume displacement and alveolar chord length assessment. Furthermore, elastase-exposed LTA4H−/− mice were found to have significantly delayed influx of the CD45highCD11bhighLy6Ghigh leukocytes compatible with neutrophils compared with elastase-exposed WT mice. Mechanistic insights to these phenotypes were provided by demonstrating protection from elastase-induced murine emphysema with neutrophil depletion in the elastase-exposed WT mice and by demonstrating time-dependent modulation of cysteinyl leukotriene biosynthesis in the elastase-exposed LTA4H−/− mice compared with elastase-exposed WT mice. Together, these findings demonstrated that LTB4 played an important role in promoting the pathogenesis of pulmonary emphysema associated with neutrophilic pulmonary inflammation.
Keywords: chronic obstructive pulmonary disease, leukotriene B4, neutrophil
inflammation and tissue remodeling are prominent features of many pulmonary disorders. This is readily apparent in chronic obstructive pulmonary disease (COPD), which is characterized by lymphocyte-, macrophage-, eosinophil-, and granulocyte-rich inflammation, leading to associated pathological alterations in the airways and alveoli (16, 18, 33, 38). Previous observations also demonstrated significant variability of the disease phenotypes and inflammatory responses among COPD patients (36). Population-based epidemiological studies have reported that a considerable number of cigarette smokers with a history of significant smoking do not develop COPD, and 25% of smokers with COPD develop an emphysematous variant of COPD (36). The importance of genetics in the pathogenesis of emphysematous COPD is further reflected by the discovery of α1-antitrypsin deficiency, which manifests as an aggressive form of emphysema at younger ages (31). These observations suggest that unique host characteristics may either protect or predispose patients, respectively, from or to developing cigarette smoke-induced pulmonary emphysema. Unfortunately, to date, specific mechanisms that are responsible for the initiation, generation, and proliferation of emphysema have not been adequately defined.
Leukotrienes (LTs), one subpopulation of the lipid metabolites, have been suggested as possible molecules involved in the pathogenesis of several pulmonary disorders. LTs are lipid mediators of inflammation derived from the 5-lipoxygenase (5-LO) pathways of the arachidonic acid metabolism. They fall into two classes, the cysteinyl LTs (cLT) (LTC4, LTD4, LTE4) and LTB4. Exaggerated levels of the cLTs have been documented in asthma, where they contribute to asthmatic smooth muscle contraction, bronchospasm, microvascular permeability, mucous hypersecretion, airway remodeling, and eosinophilic inflammation (5, 7, 11, 43, 46). Exaggerated levels of the LTB4 have been documented in sepsis, bacterial infection, cystic fibrosis, nonsteroid-dependent asthma, and COPD (3, 13, 14, 19, 29, 43). However, few studies have specifically investigated the roles of the LTB4 pathways in the pathogenesis of pulmonary emphysema.
Previous studies from our laboratory utilized a transgenic knock-in model of IL-13, and it demonstrated that the transgenic IL-13 potently stimulated the 5-LO metabolite pathways during the process of emphysematous pulmonary tissue destruction and inflammation (40). These studies also demonstrated that in mouse lungs the tissue effects of the 5-LO metabolites in IL-13-induced pulmonary emphysema were possibly modulated by the LTB4 biosynthetic pathways (40). Several studies have also highlighted the importance of LTB4 and the significant induction of genes within the LT metabolism that contribute to neutrophilic inflammation and tissue remodeling (4, 17, 21, 25, 47). Interestingly, cellular and molecular mechanisms by which LTB4 induces emphysematous alveolar remodeling have not been specifically investigated. Therefore, we hypothesized that the rate-limiting LTB4 biosynthetic enzyme, leukotriene A4 hydrolase (LTA4H), may play an important role in the pathogenesis of neutrophilic pulmonary inflammation and emphysema. To test this hypothesis, we first assessed the amount of LTB4 in human lungs with emphysema. These studies demonstrated that emphysematous human lungs contained higher levels of LTB4 compared with non-emphysematous human lungs. To further investigate the possible biological role of LTB4 in the pathogenesis of pulmonary emphysema, we characterized the development of emphysema in the lungs of the LTA4H wild-type (WT) and LTA4H knockout (LTA4H−/−) mice exposed to intranasal elastase. Our findings demonstrated that LTA4H−/− mice were protected from pulmonary emphysema induced by elastase, in part, as a result of reduced neutrophilic inflammation.
MATERIALS AND METHODS
Human lung specimens.
After University of Virginia Institutional Review Board approval, 19 patients who underwent lung resection for the purposes of lung cancer were enrolled. All subjects signed informed consent. Once the lung tissues were resected, lung tissues from non-cancerous surrounding areas were excised and snap-frozen at −80°C until biochemical analyses. To determine the presence or absence of emphysema, clinical data including basic demographic information, official report of preoperative chest computer tomography (CT) scan, and original result of the preoperative pulmonary function test (PFT) was collected. Only those who had significant cigarette smoking history defined as minimally 20 pack-year cigarette smoking (number of packs of cigarette smoked per day multiplied by the total number of years smoking) were enrolled. PFT findings with less than 0.80 ratio of the forced expiratory volume in 1 s (FEV1) to functional vital capacity (FVC) and less than 80% of the predicted carbon monoxide diffusion capacity (DLCO) by European Community for Coal and Steel 1993 reference were necessary to be classified as emphysematous COPD subjects. These emphysematous PFT findings were also correlated with the presence of CT report describing emphysematous parenchymal lung findings. All other subjects with greater than 80% of the predicted DLCO were considered non-emphysematous smokers even if the FEV1 to FVC ratio was less than 0.80. These non-emphysematous PFT findings were also correlated with the CT report to confirm the absence of any description suggestive of pulmonary emphysema. For biochemical analysis, lungs were suspended in ice-cold homogenization buffer (Complete Protease Inhibitor Cocktail Tablets, Roche Diagnostics), homogenized by a tissue homogenizer (Fisher Scientific), and sonicated in ice. Fifty microliters of each lung homogenate was used to measure the protein concentration by Coomassie dry protein assay plates (Pierce Lab). HPLC grade methanol (JT Baker) with 0.1% 1 N acetic acid was added to a portion of lung homogenate to precipitate protein and then centrifuged at 2,000 rpm for 15 min. Lipid from the supernatant was extracted by C18 Sep-pak cartridges (Waters) after preconditioning it with 100% HPLC methanol (JT Baker) three times and 100% HPLC H2O (JT Baker) three times. After initially passing the lung homogenate samples through the cartridges, C18 cartridges were washed three times with 60% methanol-40% H2O solution, and then the lipid was eluted with 100% methanol-0.1% 1 N acetic acid (30). Eluted methanol was dried completely under vacuum in a Speedvac. Pellet was resuspended in enzyme immunoassay (EIA) buffer, and the LTB4 was assessed by a commercially available LTB4 EIA kit (Cayman Chemical). LTB4 levels were normalized to total protein in each specimen.
LTA4H−/− mice.
In these studies, we used knockout mice in which LTA4 hydrolase loci were targeted with null mutation. These mice were provided as a kind gift from Dr. Beverly Koller's laboratory (Univ. of North Carolina, Chapel Hill, NC). The mice were on a 129J genetic background and were characterized as previously described (6). The mice used in these studies were 8–12 wk of age. The null mutation in these mice caused deficiency in the production of LTB4, which was associated with blunted neutrophilic inflammatory responses (6). The genotype of the mice was confirmed as previously described (6). 129J WT mice were purchased from the National Cancer Institute (NCI Animal Production Program). In all experiments, we compared the phenotype of the WT and LTA4H−/− mice. Use of these mice in our studies was approved by the University of Virginia School of Medicine Institutional Animal Care and Use Committee.
Elastase-induced pulmonary emphysema.
WT and LTA4H−/− mice, 8 to 12 wk of age, were sedated by 0.06 ml of 60/5 mg ketamine-5 mg xylazine mixture per kg mouse weight. Once sedated, 100 μl of porcine elastase in PBS, 0.75 μg of elastase per gram mouse weight (Calbiochem), or PBS alone (vehicle control, Quality Biological) were intranasally instilled using a method previously described (20, 22, 27). Once exposed to elastase or vehicle control, mice were kept upright in 50-ml conical tubes for 10 min to prevent any reflux of the instilled agents. Once recovered, mice were killed on days 3, 7, 14, and 28 postexposure.
Premortem pulmonary physiological assessment.
Total lung compliance was assessed using a Flexivent (SCIREQ, Montreal, Canada) as previously described (24, 28). In brief, animals were deeply anesthetized with a ketamine and xylazine mixture (60 mg/5 mg), the trachea was cannulated using p10 tubing, the sternum was completely opened, the diaphragm was cleared by opening the abdominal cavity, and animals were ventilated at a respiratory rate of 120 breaths/min with PEEP 3 cmH2O per a recommended protocol from the manufacturer. This technique ensured that the live animals' voluntary effort could not influence the physiological values detected by the Flexivent. Once the animals were acclimated to the Flexivent ventilator, prescribed Flexivent algorithm was performed as previously described (24, 28), and lung compliance was calculated using the software supplied with the ventilator. Animals were killed following physiological measurements, and tissues were harvested for postmortem physiological and morphometric assessment. For the purposes of biochemical analysis and flow cytometry, tissues from animals were harvested without Flexivent assessment.
Postmortem lung volume assessment.
Lung volume was assessed as previously described (40, 50). In brief, animals were anesthetized, the trachea was cannulated, and the lungs were removed en bloc and inflated at a constant 25 cmH2O pressure of 1% melted low-melting point agarose gel (Promega) in PBS. The sizes of the lungs were evaluated by volume displacement technique as previously described (40, 50).
Histological morphometric assessment.
Once the volume displacement of the lungs was determined, the trachea was tied to keep the lungs inflated, then fixed in 10 ml of paraformaldehyde for 18 h. Fixed lungs were stained with H&E by the Research Histology Laboratory of the Department of Pathology at the University of Virginia School of Medicine. Alveolar size was determined from the mean chord length of the air space as previously described (40, 50, 51). Sequential digital pictures of the entire lungs were captured by an Axiostar microscope (Carl Zeiss Microimaging) and then processed by NIH Image 1.63 in an iMac (Apple computer) with a macro downloaded from the NIH server user macro directory (chord length macro by Dr. Robert Homer, public web link: http://rsb.info.nih.gov/nih-image/download/contrib/ChordLength.SurfaceArea). Chord length measurement is similar to the mean linear intercept, a standard measure of air space size, but has the advantage that it is independent of alveolar septal thickness (40, 49).
Bronchalveolar lavage fluid analysis for LTB4 and cLTs.
Animals were anesthetized as described above at the specified time points, and the trachea was cannulated with Insyte Autoguard Winged 22 Ga 1.00 inch 0.9 × 25 mm angiocatheter (BD). Two aliquots of 0.6 ml PBS were used to collect whole lung BALF. The BALF was immediately frozen at −80°C. Lipid from the BALF was extracted by C18 Sep-pak cartridges in the same manner described above for the human lung tissues. Extracted lipid was then resuspended in EIA buffer, and LTB4 and cLTs levels were assessed by a commercially available LTB4 or cLT EIA kit (Cayman Chemical).
mRNA analysis.
Total lung mRNA was isolated by TRIzol (Invitrogen) per the manufacturer's recommended protocol. The PCR primers are described in Table 1. GAPDH was used as an internal standard. Real-time RT-PCR was performed with Bio-Rad iCycler Real-Time RT-PCR machine using Sybergreen RT-PCR kits (Bio-Rad). The levels of the detected target gene products were first quantified by an already constructed standard curve, normalized with GAPDH, and then expressed as % ratio of the target gene transcript compared with negative control animals (intranasal vehicle-exposed WT mice). Levels of the target gene transcription were first analyzed by one sample t-test with 100% as a hypothetical mean.
Table 1.
Primer sequence used for Sybergreen real time RT-PCR
| Target Gene | Left | Right | AT |
|---|---|---|---|
| cPLA2 | GTTTGTTCATGCCCAGACCT | ATCCCCGACTCATACAGTGC | 59 |
| LTA4H | AACCAGAGGGTTCCCATACC | GCAGATTTCTCCACCTGCTC | 59 |
| GAPDH | TGCCTGCTTCACCACCTTC | GCCTTCCGTGTTCCTACCC | 60 |
cPLA2, cytosolic phospholipase A2; LTA4H, leukotriene A4 hydrolase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; AT, annealing temperature in °C.
FACS analysis of whole lung leukocyte infiltration.
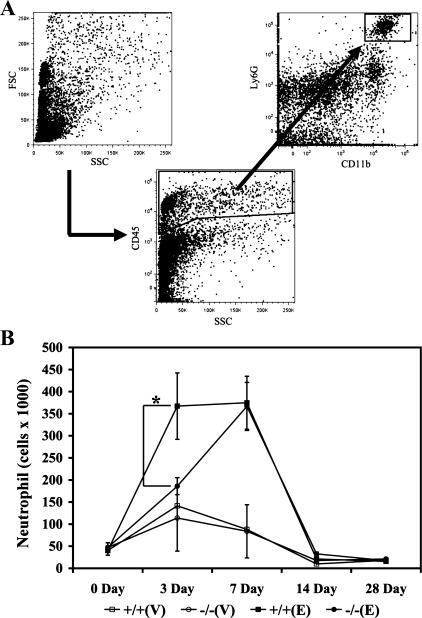
Time course of the lung inflammatory cell trafficking into the lungs was assessed from whole lung single cell suspension by FACS analysis. Briefly, mouse lungs were harvested at 3, 7, 14, and 28 days postexposure to intranasal elastase or vehicle control. The lungs were isolated after flushing the right ventricle with PBS to clear leukocytes and erythrocytes from the pulmonary circulation. Lungs were then digested in lung digestion media with 1.0 mg/ml collagenase A (Roche Diagnostics) in RPMI (Quality Biological), and erythrocytes were lysed with ACK buffer (Quality Biological). Cells isolated from the lungs were stained with PerCP-labeled CD45 (BD), APC-Cy7-labeled CD11b (BD), and PE-labeled Ly6G (BD). Infiltrating leukocytes were gated from other lung cells by the expression of CD45. Next, all CD45high cells were gated into Ly6Ghigh and CD11bhigh cells, neutrophils. Multicolor analysis of the stained cells was performed on a FACScan flow cytometer (BD Biosciences). FlowJo (version 8.8.6.) was used to analyze the data.
Neutrophil depletion in the murine model of intranasal elastase-induced emphysema.
Starting 1 day before intranasal exposure, 129J WT mice were treated with intraperitoneal (ip) 200 μg anti-Ly6G antibody (BioXcell) or 200 μg Isotype antibody (BioXcell) per mouse every other day for 28 days. Twenty-four hours after the first ip antibody treatment, mice were exposed to intranasal elastase as described above. Twenty-eight days postexposure, lung compliance, total lung volume, and chord length were assessed as described above. Peripheral blood leukocytes were assessed to confirm successful depletion of neutrophils by performing flow cytometry with PerCP-labeled CD45, APC-Cy7-labeled CD11b, and PE-labeled Ly6G.
Statistics.
All data was expressed as means ± SE and assessed for significance by one sample t-test, Student's t-test or one-way ANOVA with subgroup comparison as appropriate. All statistics were performed using Prism software (GraphPad Software). In all analyses, P value < 0.05 was considered significant.
RESULTS
LTB4 levels are found to be elevated in human emphysema.
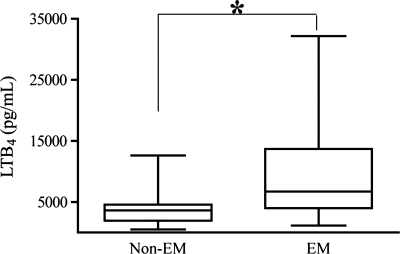
To begin to address the role(s) of LTB4 in the pathogenesis of pulmonary emphysema, studies were first undertaken to assess the levels of the LTB4 in the whole lung tissues from human subjects with and without pulmonary emphysema. All subjects had a diagnosis of non-small cell lung cancer and underwent curative lung resection. Non-cancerous tissues surrounding the cancer were collected as far away as possible from the primary tumor, and absence of cancer was confirmed by clinical pathologists. Nine subjects had no evidence of emphysematous COPD (Non-EM) by CT scan and PFT, whereas 10 had clear evidence of emphysematous COPD (EM). Basic demographic information showed a slightly older and more female-predominant EM group compared with non-EM group with expected patterns in the PFT assessments (Table 2). Measured levels of lung tissue LTB4 were first normalized to total protein concentration of tissue from each subject. When the levels of LTB4 were compared, EM group had significantly higher levels of LTB4 in its lung tissue compared with non-EM group, supporting the notion that LTB4 levels were higher in emphysema (Fig. 1). These findings demonstrated that emphysematous lungs of cigarette smoke-exposed humans contained higher levels of LTB4 compared with non-emphysematous lungs of cigarette smoke-exposed humans.
Table 2.
Basic demographics of non-EM control and EM groups
| Non-EM | EM | P Value | |
|---|---|---|---|
| n | 9 | 10 | |
| Age | 63.8 ± 13.0 | 71.0 ± 7.8 | 0.17 |
| Gender (Male:Female) | 6:3 | 4:6 | |
| FEV1/FVC | 0.76 ± 0.044 | 0.53 ± 0.152 | 0.0009 |
| %FEV1 | 86.2 ± 19.6 | 49.7 ± 19.1 | 0.0008 |
| %FVC | 87 ± 13.7 | 72.3 ± 21.8 | 0.096 |
| %DLCO | 97.7 ± 7.9 | 53.4 ± 9.3 | <0.0001 |
Values are means ± SD; n = number of subjects. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; FEV1/FVC, ratio of FEV1 to FVC; %FEV1, percent predicted of FEV1 according to National Health and Nutrition Examination Survey III (NHANES III) reference. %FVC, percent predicted of FVC according to NHANES III; %DLCO, percent predicted of carbon monoxide diffusion capacity according to the European Community for Coal and Steel 1993 reference.
Fig. 1.
Assessment of leukotriene B4 (LTB4) in human whole lung tissues by an enzyme immunoassay. Amounts of LTB4 are standardized by protein concentrations of each human lung tissue block. Non-EM, without emphysema (n = 9); EM, with emphysema (n = 10). *P < 0.05.
LTB4 biosynthetic pathways in elastase-induced murine emphysema.
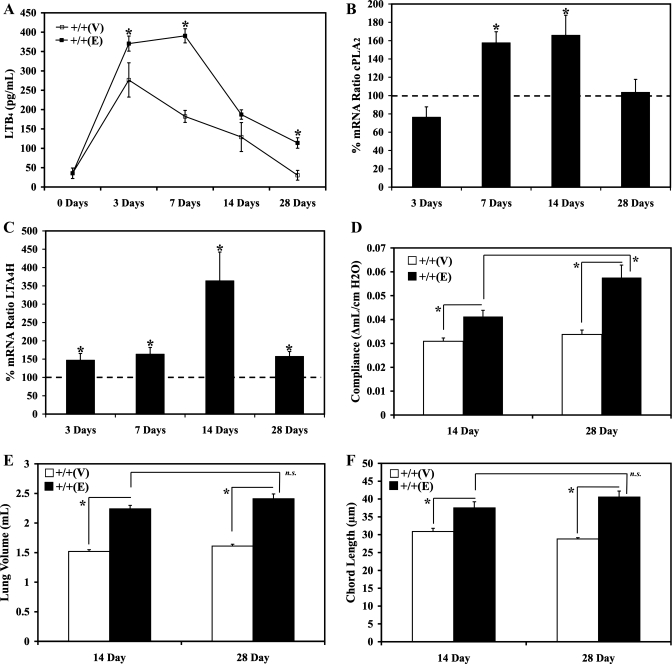
On the basis of the findings in emphysematous human lungs, a cohort of WT mice was exposed to either intranasal vehicle control or elastase at 0.75 μg of elastase per kilogram of mouse weight to determine whether the LTB4 pathway contributed to the pathogenesis of the elastase-induced murine pulmonary inflammation and remodeling. The mice were killed 0, 3, 7, 14, and 28 days post either intranasal elastase or vehicle exposure and assessed for LTB4 biosynthesis and for the severity of pulmonary emphysema. Total lung BALF was collected and examined for LTB4 levels. LTB4 biosynthesis was significantly upregulated in the BALF of the WT mice exposed to intranasal elastase (up to 2.5-fold higher by day 7) compared with mice exposed to vehicle alone (Fig. 2A). To investigate the mechanisms associated with the upregulated LTB4 biosynthesis, levels of genes transcribing cytosolic phospholipase 2 (cPLA2) and LTA4H were assessed with real-time RT-PCR in whole lung mRNA of the WT mice exposed to either elastase or vehicle (Fig. 2, B and C). Statistically significant upregulation was observed with the levels of the gene transcribing cPLA2 and LTA4H after elastase exposure, and transcriptional upregulation of LTA4H was greater in magnitude and persisted longer than that of cPLA2. This demonstrated that the increased levels of the LTB4 may have been caused by simultaneously increased bioavailability of the precursor arachidonic acid and increased biosynthesis of the LTB4. To assess the progression in the severity of pulmonary emphysema, mouse lungs were assessed at 14 and 28 days after intranasal elastase or vehicle exposure pre- and postmortem. The severity of emphysema was assessed by measuring premortem lung compliance by Flexivent method. Lung compliance measured by this method was expected to be directly proportional to the severity of the emphysematous alveolar destruction. As expected, significantly increased lung compliance was noted in WT mice exposed to elastase compared with WT mice exposed to vehicle control at 14 and 28 days postexposure (Fig. 2D). Moreover, significantly higher compliance was noted in the mouse lungs assessed at 28 days postelastase exposure compared with the mouse lungs assessed at 14 days postelastase exposure (Fig. 2D). The severity of emphysema was also assessed by measuring postmortem whole lung volume and chord length. It was expected that the lung volume would increase with worsening emphysema (40, 49). To correlate the gross anatomical lung volume assessment with the microscopic alterations in the alveolar sizes, histological assessment was conducted. Lungs were inflated with 25-cm pressure of melted 1% low-melting point agarose gel and fixed for 18 h in paraformaldehyde. Larger chord length values indicate larger alveolar sizes, which suggest more severe emphysema (40, 49). Again as expected, significantly increased whole lung volume and chord length were noted in WT mice exposed to elastase compared with WT mice exposed to vehicle control at 14 and 28 days postelastase intranasal exposure (Fig. 2, E and F). Although larger lung volumes and chord lengths were appreciated in the mouse lungs assessed at 28 days postelastase exposure, these values were not statistically different from the values of the lungs assessed at 14 days postexposure (Fig. 2, E and F). These results demonstrated that the emphysematous remodeling was progressive from days 14 to 28 postelastase exposure in our mouse model, and, as a result, the day 28 time point was chosen for all subsequent phenotypic assessment.
Fig. 2.
All wild-type 129J mice were exposed to either PBS vehicle or 0.75 μg elastase per kg mouse weight via an intranasal route. A: whole lung bronchoalveolar lavage fluid LTB4 0, 3, 7, 14, and 28 days after elastase exposure was assessed by an enzyme immunoassay. B: gene transcribing for cPLA2 was assessed by Sybergreen real-time RT-PCR 3, 7, 14, and 28 days after elastase exposure, normalized by GAPDH, expressed as % of target gene expression compared with the matched wild-type controls, and assessed for significance from the expected baseline (100%). C: genes transcribing for LTA4H were assessed by Sybergreen real-time RT-PCR 3, 7, 14, and 28 days after elastase exposure, normalized by GAPDH, expressed as % of target gene expression compared with the matched wild-type controls, and assessed for significance from the expected baseline (100%). D: premortem lung compliance was assessed with Flexivent 14 and 28 days after elastase exposure. E: postmortem whole lung volume was assessed by volume displacement technique after inflating the lungs at 25 cmH2O pressure melted 1% low-melting point agarose gel pressure. F: postmortem chord length was assessed in H&E-stained lungs after inflating the lungs with techniques described above. +/+, Wild type; V, vehicle; E, elastase. *P < 0.05; n.s., P value not significant.
Absence of LTB4 ameliorated emphysematous pulmonary remodeling as assessed by lung compliance measurement.
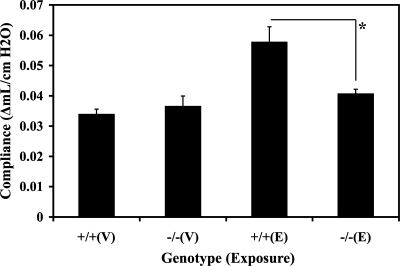
To define the role of LTB4 in the pathogenesis of elastase-induced emphysematous alveolar remodeling, we compared the alterations in the premortem lung compliance invasively measured by Flexivent in WT and LTA4H−/− mice after exposure to either elastase or vehicle. Twenty-eight days after the intranasal vehicle exposure, there were no changes in the lung compliance between WT and LTA4H−/− mice postintranasal vehicle exposure (Fig. 3). As expected, the average lung compliance of the WT mice at 28 days postintranasal elastase exposure was significantly increased compared with the average lung compliances of the WT and LTA4H−/− mice at 28 days postintranasal vehicle exposure. However, the average lung compliance of the LTA4H−/− mice at 28 days postintranasal elastase exposure was significantly reduced compared with the average lung compliance of the WT mice at 28 days postintranasal elastase exposure (P < 0.05). These findings demonstrated that the absence of LTB4 protected mouse lungs from elastase-induced pulmonary emphysema.
Fig. 3.
Premortem lung compliance measurement by Flexivent 28 days after intranasal elastase or vehicle exposure. +/+, WT; −/−, LTA4H−/−; V, vehicle; E, elastase. *P < 0.05.
Absence of LTB4 ameliorated emphysematous pulmonary remodeling as assessed by gross inspection and whole lung volume and chord length measurement.
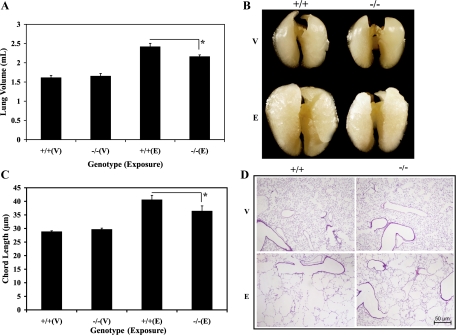
On the basis of these pulmonary physiological findings, we next assessed the postmortem alterations in the whole lung volumes to define the role of LTB4 in the pathogenesis of elastase-induced emphysematous alveolar remodeling. Similar lung volumes were noted between the vehicle-exposed WT and LTA4H−/− mice (Fig. 4A). As expected, intranasal elastase caused a marked increase in lung volumes of the WT mice compared with the vehicle-exposed WT and LTA4H−/− mice (Fig. 4A). However, when the elastase-exposed WT mice were compared with the elastase-exposed LTA4H−/− mice, LTA4H−/− mice were found to have significantly reduced lung volume (Fig. 4A, P < 0.05). After being fixed for 18 h in paraformaldehyde, gross inspection demonstrated significant reduction in lung volumes among the elastase-exposed LTA4H−/− mice compared with the elastase-exposed WT mice (Fig. 4B). We also assessed the postmortem alterations in the alveolar structure by chord length measurement. Similar chord lengths were noted between the vehicle-exposed WT and LTA4H−/− mice (Fig. 4C). As expected, intranasal elastase caused a marked increase in the alveolar sizes of the WT mice compared with the vehicle-exposed WT and LTA4H−/− mice (Fig. 4C). Significantly reduced alveolar sizes were noted in the elastase-exposed LTA4H−/− mice compared with the elastase-exposed WT mice (Fig. 4C, P < 0.05). However, these protective effects noted in the elastase-exposed LTA4H−/− mice were partial as demonstrated by modest but persistent emphysema noted in these animals. Representative pictures were taken to appreciate the differences found in the chord length assessment (Fig. 4D). These findings demonstrated that the absence of LTB4 protected mouse lungs from the elastase-induced pulmonary emphysema.
Fig. 4.
Lungs were inflated at 25 cmH2O pressure of melted 1% low-melting point agarose gel 28 days after intranasal elastase or vehicle exposure. A: whole lung volumes assessed by PBS volume displacement; n = 7–10/group. B: a representative picture of inflated lungs after being fixed in paraformaldehyde for 18 h. C: serial adjacent images were captured, and then the chord length was assessed by a computerized macro script; n = 6–8/group. D: a representative picture of H&E slides at 5× power after being fixed with paraformaldehyde for 18 h. +/+, WT; −/−, LTA4H−/−; V, vehicle; E, elastase. *P < 0.05.
Absence of LTB4 reduces neutrophilic inflammatory responses in the murine model of elastase-induced pulmonary emphysema.
Previous studies have reported that LTB4 plays a major role in the biology of neutrophils (4, 6, 13, 29, 48). Therefore, we set out to investigate the roles that LTB4 plays on the influx of neutrophils into the lungs in our murine model. We isolated cells from the lungs 0, 3, 7, 14, and 28 days postintranasal elastase or vehicle exposure, triple-stained for CD45, Ly6G, and CD11b, and examined by flow cytometry. Cells positively stained with CD45, Ly6G, and CD11b were considered neutrophils, and a previously published gating strategy by Park et al. (35) was applied to ensure clear separation of CD45highCD11bhighLy6Ghigh neutrophils from CD45highCD11bhighLy6Gintermediate monocytes (Fig. 5A). The number of neutrophils was similar between the unchallenged WT and LTA4H−/− mice (Fig. 5B). After intranasal exposure, our results revealed that a significantly increased number of neutrophils infiltrated the lungs of the elastase-exposed WT mice compared with the vehicle-exposed WT and LTA4H−/− mice. The infiltration of neutrophils appeared by day 3, was maintained until day 7, and then tapered off by days 14 and 28 (Fig. 5B). Compared with the elastase-exposed WT mice, infiltration of the neutrophils into the lungs was significantly reduced in the elastase-exposed LTA4H−/− mice 3 days after elastase exposure (Fig. 5B). However, between days 3 and 7 postelastase exposure, significant infiltration of the neutrophils into the lungs continued in greater intensity with the elastase-exposed LTA4H−/− mice compared with the elastase-exposed WT mice. As a result, there was no significant difference in the number of infiltrating neutrophils between the elastase-exposed WT and elastase-exposed LTA4H−/− mice by day 7 (Fig. 5B). These findings suggested that the LTB4 played a major role in the recruitment of neutrophils into the lungs in a kinetic-dependent manner in our murine model.
Fig. 5.
WT and LTA4H−/− mice were exposed to intranasal vehicle or elastase, lungs were harvested 0, 3, 7, 14, and 28 days after exposure, cells were triple-stained with CD45, CD11b, and Ly6G, and cells were counted by flow cytometry. A: gating strategy to count CD45highCD11bhighLy6Ghigh (neutrophils). B: neutrophils were counted, and significant reduction was noted in the elastase-exposed LTA4H−/− mice compared with elastase-exposed WT mice 3 days postelastase exposure. No difference was noted between the elastase-exposed WT and LTA4H−/− mice 7 days postelastase exposure. +/+, WT; −/−, LTA4H−/−; V, vehicle; E, elastase. *P < 0.05.
Role of neutrophils in elastase-induced murine emphysema.
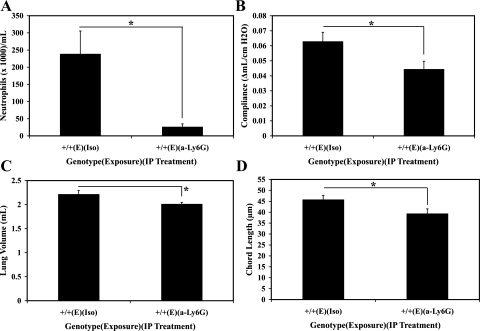
Based on the results of the flow cytometry, we hypothesized that the LTB4 deficiency may have protected murine lungs from emphysematous destruction by alleviating the acute phase of the neutrophilic inflammation. To establish the causal relationship between the neutrophilic inflammation and pulmonary emphysema in our murine model, we investigated the effects of neutrophil depletion on the elastase-induced pulmonary emphysema. Starting on day −1, WT mice were treated every other day with either neutrophil-depleting anti-Ly6G antibody or isotype control antibody (8, 35), and 28 days after elastase exposure, lung compliance, whole lung volume, and chord length were measured to assess emphysema. Effectiveness of neutrophil depletion strategy was confirmed by demonstrating neutropenia in the peripheral blood with flow cytometry analysis (Fig. 6A). As shown in Fig. 6, B–D, anti-Ly6G antibody-treated murine lungs were significantly protected from emphysema compared with the isotype control antibody-treated murine lungs in all measures of the severity of emphysema. This demonstrated that neutrophils contributed significantly to the pathogenesis of elastase-induced murine pulmonary emphysema.
Fig. 6.
Effects of neutrophil depletion on pulmonary emphysema in elastase-exposed WT mice 28 days after elastase or vehicle exposure. A: flow cytometry of the peripheral blood leukocytes stained with CD45, CD11b, and Ly6G to identify neutrophils. B: premortem lung compliance measurement by Flexivent. C: postmortem whole lung volumes assessed by PBS volume displacement; n = 6/group. D: serial adjacent images were captured, and then the chord length was assessed by a computerized macro script; n = 6/group. +/+, WT; E, elastase; Iso, isotype IgG; a-Ly6G, anti-Ly6G monoclonal antibody. *P < 0.05.
Absence of LTA4H altered cLT metabolite pathway.
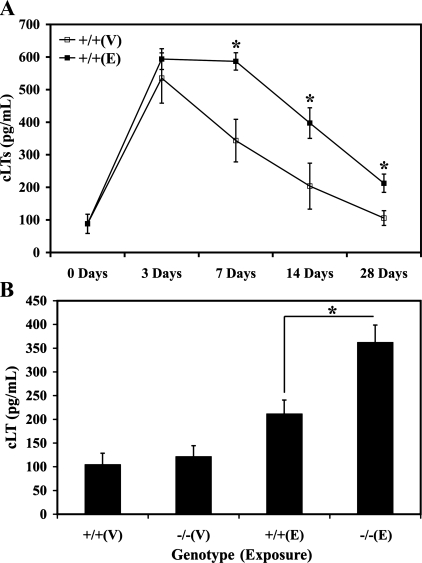
In the absence of LTA4H activity and presence of enhanced cPLA2 activity, one can envision a scenario in which an increased amount of precursor substrate is available for production of cLTs. To assess the effects of cLT biosynthesis in the LTA4H−/− mice, we first measured the levels of BALF cLTs in WT mice 0, 3, 7, 14, and 28 days after vehicle or elastase exposure. The levels of cLTs in the BALF were significantly increased in elastase-exposed WT mice at all time points compared with the vehicle-exposed WT mice (Fig. 7A). Subsequently, the levels of cLTs were measured in the BALF of the WT and LTA4H−/− mice 28 days after vehicle or elastase exposure, and they was found to be significantly higher in the elastase-exposed LTA4H−/− mice compared with the elastase-exposed WT mice (Fig. 7B). These results suggested that alteration in the cLT metabolic pathways may have possibly contributed to the protection against pulmonary emphysema observed in the elastase-exposed LTA4H−/− mice.
Fig. 7.
A: WT mice were exposed to intranasal vehicle or elastase, and whole lung BALF were harvested 0, 3, 7, 14, and 28 days after exposure. Levels of cLTs in the BALF were measured by EIA, and two groups of mice at each time point were compared by t-test. *P < 0.05. B: levels of cLTs in the BALF were measured from WT and LTA4−/− mice 28 days postintranasal vehicle or elastase exposure. *P < 0.05. +/+, WT; −/−, LTA4H−/−; V, vehicle; E, elastase.
DISCUSSION
Exaggerated levels of LTB4 frequently coexist with and are felt to contribute to the pathogenesis of a variety of diseases including sepsis, bacterial infection, cystic fibrosis, non-steroid-dependent asthma, and COPD (3, 13, 14, 19, 29, 43). Several studies have highlighted the importance of LTB4 and significant induction of genes within the LT metabolism that contribute to neutrophilic inflammation and tissue remodeling (4, 17, 21, 25, 47). In accordance with these observations, our previous study demonstrated that the LTB4 biosynthesis was significantly induced and may play an important role in the emphysematous form of COPD (40). In keeping with the appreciation that the LTB4 biosynthesis may be dysregulated at sites of neutrophilic inflammation and tissue damages in emphysematous COPD, we investigated the biology of LTB4 in the pathogenesis of elastase-induced pulmonary emphysema.
Our studies demonstrated that the LTB4 made an important contribution to the pulmonary inflammation and emphysematous destruction. Initially, we demonstrated that levels of LTB4 were significantly elevated in the emphysematous human lungs compared with the non-emphysematous human lungs in smokers. This suggested that the LTB4 may play a significant biological role in the pathogenesis of human pulmonary emphysema. To provide a mechanistic understanding, we employed a murine model of elastase-induced pulmonary emphysema. In this mouse model, LTB4 biosynthesis was significantly upregulated, and time-dependent progression of emphysema was found by premortem physiological assessment. Pulmonary emphysema became progressively more severe 28 days after elastase exposure compared with 14 days. LTA4H−/− mice are unable to produce LTB4, and when these mice were exposed to intranasal elastase, absence of LTB4 protected murine lungs from emphysematous destruction. Trafficking of the neutrophils into the lungs was significantly delayed in the absence of LTB4, and neutrophils were demonstrated to be important, deleterious inflammatory cells in our model by applying a strategy to deplete neutrophils with anti-Ly6G antibody. Finally, the LTA4H null mutation caused significant alterations in the cLT biosynthetic pathways.
These results demonstrated several notable observations related to the biology of LTB4 and the murine model of the elastase-induced pulmonary emphysema. First, we reported that LTB4 biosynthesis was upregulated after intranasal elastase exposure, and this confirmed that the murine model of the elastase-induced pulmonary emphysema was an appropriate murine model to investigate the biology of LTB4. Although LTB4 biosynthesis was significantly upregulated at all time points, absolute amounts of LTB4 from BALF were 2.5- to-3-fold greater 3 and 7 days postelastase exposure compared with 14 and 28 days. Unlike the IL-13-induced pulmonary emphysema model, intranasal elastase significantly upregulated cLT biosynthesis throughout all time points (40). Amounts of cLTs were even greater in the elastase-exposed LTA4H−/− mice compared with the elastase-exposed WT mice 28 days postexposure. These results demonstrated that the elastase-induced murine model of emphysema was biologically different from IL-13-induced pulmonary emphysema and that the alteration in the cLT biosynthesis may have contributed to the protection of the murine lungs from emphysema.
Second, absence of LTB4 delayed infiltration of the neutrophils into the lungs. Several studies reported that LTB4 played important roles in the recruitment of neutrophils (12, 15, 26, 34). Our results are consistent with these previous studies. However, further examination of neutrophil trafficking demonstrates that LTB4 may be involved only in the trafficking of these cells into the lungs during the acute phases of our model. Significantly fewer neutrophils infiltrated the lungs of the elastase-exposed LTA4H−/− mice compared with the elastase-exposed WT mice 3 days postexposure. However, from 3 to 7 days postelastase exposure, significantly more neutrophils were found in the elastase-exposed LTA4H−/− mice, and the numbers of neutrophils were virtually equivalent between the elastase-exposed WT and LTA4H−/− mice by day 7. This strongly suggests that LTB4-independent mechanism(s) may take over 3 days after elastase exposure and increase the number of neutrophils in lungs of these animals. Several possible mechanisms may influence influx, efflux, and or apoptosis of the neutrophils. In the past, neutrophils have been mostly described as a causative leukocyte in the pathogenesis of pulmonary emphysema (16, 19, 31, 41, 42). We confirmed this hypothesis by depleting neutrophils with anti-Ly6G antibody and by demonstrating protection against emphysema in the elastase-exposed WT mice treated with anti-Ly6G antibody. Absence of LTB4 ameliorated emphysematous lung destruction in the setting of delayed but still increased number of neutrophils infiltrating the lungs. This suggests the possibility of LTB4- and neutrophil-independent or LTB4-dependent but neutrophil-independent mechanism(s) working to protect the elastase-exposed LTA4H−/− mice from progressive emphysematous alveolar destruction. Future studies are necessary to investigate this intriguing question of why lungs were still protected from progressive emphysematous destruction in the absence of LTB4 but in the presence of significantly delayed infiltration of neutrophils into the lungs.
Third, we described the phenotypes of the elastase-induced mouse pulmonary emphysema by means of both premortem and postmortem assessments. Although alveolar morphometry has been frequently performed to characterize murine pulmonary emphysema (40, 49), reliability and accuracy of the postmortem anatomic assessments have been also questioned (44, 45). Several postmortem factors such as activation of coagulation, rigor mortem, and techniques to inflate whole lungs may influence and skew experimental endpoints in postmortem anatomic assessment. On the other hand, premortem assessment with Flexivent has been applied with increasing frequency in recent years to provide more in-depth understanding of pulmonary pathology in murine models (9, 10, 23, 24, 37). However, to date, only a few studies have utilized both premortem and postmortem assessments to characterize pulmonary emphysema in murine models (28). We believe that our results correlating premortem lung compliance and postmortem anatomic/histologic measurements have considerable significance in this aspect. We also believe that assessment by Flexivent can provide more direct, accurate, sensitive, and reliable measurement of alveolar pathology. This was demonstrated by the fact that premortem assessment by Flexivent was able to detect progressively worsened pulmonary emphysema in the elastase-exposed WT mice 28 days postexposure compared with 14 days. On the other hand, the postmortem assessment by lung volume displacement and chord length measurement was not able to reliably detect such changes.
Fourth, absence of LTB4 protected murine lungs from pulmonary emphysema, but these effects were not complete. Direct instillation of elastase by the intranasal route causes immediate, acute damages to the lungs with significant bleeding, plasma extravasation, and destruction of lung architecture (data not shown), and these direct insults to the lungs may have been too great to reverse completely. This methodological aspect of our murine model can at least partially explain incomplete protection in the absence of LTB4.
We also acknowledge that our study has some limitations. First, the presence of non-small cell lung cancer in our human lung tissues may have altered the amounts of LTB4. Several previous studies have demonstrated association between lung cancer and leukotrienes (1, 2, 32, 39). However, we believe that our experimental design was well controlled by the presence of lung cancer diagnosis in all human subjects, by the presence of cigarette smoking history in all human subjects, and by the absence of cancer in all tissues analyzed for LTB4. Second, although intranasal elastase causes significant emphysema, it is not representative of typical pathogenesis of human emphysema. Therefore, we set our hypothesis narrowly and conducted investigation to define mechanistic relationships among LTB4, neutrophil, and pulmonary emphysema with clearly stated limitation of our model. Third, all our analyses of the whole lung inflammatory cells were observational and limited only to neutrophils. Therefore, future studies are required to establish causal relationship among LTB4 biosynthetic pathway, pulmonary emphysema, and other types of inflammatory responses in the pulmonary tissues of this murine model. Last, our previous study demonstrated that the timely activation of 5-LO may be necessary to protect murine lungs from IL-13-induced pulmonary emphysema (40). However, our current study suggests that one of the 5-LO metabolites, LTB4, may be a deleterious metabolite in the pathogenesis of pulmonary emphysema. We can speculate three possible reasons for the differences between these studies. First, our previous study was conducted in mice with C57B/6 background instead of 129J background, and the difference in genetic backgrounds may explain the different results. Second, our previous study investigated the emphysematous remodeling by applying transgenic IL-13 knockin strategy compared with direct instillation of elastase. The intranasal elastase did not induce IL-13 production (data not shown), and the absence of IL-13 induction may be the reason for the observed differences. Third, the transgenic IL-13 selectively induced LTB4 biosynthesis (40), but the intranasal elastase significantly induced cLT and LTB4 biosynthesis. These biological differences suggest that the phenotypes observed in the current study may have been composite results of the exaggerated presence of the cLTs in the absence of LTB4. When viewed in combination, one can envision complex biological effects with the null mutation at the LTA4H loci channeling unmetabolized precursors to LTC4 or other lipid metabolite biosynthetic pathways. This, in turn, may have contributed to protection from emphysematous tissue destruction. It is tempting to speculate that the long-observed heterogeneity of COPD phenotypes may be explained by this sort of complex modulation in the lipid metabolic pathways based on genetic variations. However, additional experiments are required to draw such conclusion.
In summary, our studies demonstrated that emphysematous human lungs of cigarette smokers had higher levels of LTB4 compared with the non-emphysematous human lungs of cigarette smokers. This finding led us to a murine study demonstrating that intranasal elastase stimulated production of the LTA4 hydrolase metabolite, LTB4. Our study demonstrated that the absence of LTB4 significantly ameliorated elastase-induced pulmonary emphysematous remodeling and tissue destruction. Last, our study provided mechanistic insights by demonstrating that the absence of the LTB4 altered neutrophil infiltration into the lungs and cLT biosynthesis in the lungs in a kinetic manner. Our present study suggested that the severity of the emphysematous destruction in lungs may be ameliorated via interventions that regulate the LTA4 hydrolase biology. Our study also highlighted possible inflammatory cell types and downstream genes that will need to be observed closely to evaluate the effects of these interventions on alveolar structures. This establishes the LTA4 hydrolase as a noteworthy site for future investigations designed to evaluate the disease susceptibility, disease progression, and therapeutic utility in pulmonary emphysema.
GRANTS
This work was supported by the Flight Attendant Medical Research Institute (to Y. M. Shim), by the Virginia Thoracic Society (biomedical research grant to Y. M. Shim), and by National Institutes of Health Grants K08-HL-91127 (to Y. M. Shim) and HL-98526, HL-66027, and CA-87879 (to R. M. Strieter).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Avis I, Hong SH, Martinez A, Moody T, Choi YH, Trepel J, Das R, Jett M, Mulshine JL. Five-lipoxygenase inhibitors can mediate apoptosis in human breast cancer cell lines through complex eicosanoid interactions. FASEB J 15: 2007–2009, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Avis IM, Jett M, Boyle T, Vos MD, Moody T, Treston AM, Martinez A, Mulshine JL. Growth control of lung cancer by interruption of 5-lipoxygenase-mediated growth factor signaling. J Clin Invest 97: 806–813, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailie MB, Standiford TJ, Laichalk LL, Coffey MJ, Strieter R, Peters-Golden M. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumonia in association with decreased alveolar macrophage phagocytic and bactericidal activities. J Immunol 157: 5221–5224, 1996 [PubMed] [Google Scholar]
- 4.Beeh KM, Kornmann O, Buhl R, Culpitt SV, Giembycz MA, Barnes PJ. Neutrophil chemotactic activity of sputum from patients with COPD: role of interleukin 8 and leukotriene B4. Chest 123: 1240–1247, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Busse W. The role and contribution of leukotrienes in asthma. Ann Allergy Asthma Immunol 81: 17–26; quiz 26–19, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Byrum RS, Goulet JL, Snouwaert JN, Griffiths RJ, Koller BH. Determination of the contribution of cysteinyl leukotrienes and leukotriene B4 in acute inflammatory responses using 5-lipoxygenase- and leukotriene A4 hydrolase-deficient mice. J Immunol 163: 6810–6819, 1999 [PubMed] [Google Scholar]
- 7.Dahlen SE, Hedqvist P, Hammarstrom S, Samuelsson B. Leukotrienes are potent constrictors of human bronchi. Nature 288: 484–486, 1980 [DOI] [PubMed] [Google Scholar]
- 8.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol 83: 64–70, 2008 [DOI] [PubMed] [Google Scholar]
- 9.De Vooght V, Vanoirbeek JA, Haenen S, Verbeken E, Nemery B, Hoet PH. Oropharyngeal aspiration: an alternative route for challenging in a mouse model of chemical-induced asthma. Toxicology 259: 84–89, 2009 [DOI] [PubMed] [Google Scholar]
- 10.DiGiovanni FA, Ellis R, Wattie J, Hirota JA, Southam DS, Inman MD. Concurrent dual allergen exposure and its effects on airway hyperresponsiveness, inflammation and remodeling in mice. Dis Model Mech 2: 275–282, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinosa K, Bosse Y, Stankova J, Rola-Pleszczynski M. CysLT1 receptor upregulation by TGF-beta and IL-13 is associated with bronchial smooth muscle cell proliferation in response to LTD4. J Allergy Clin Immunol 111: 1032–1040, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Ford-Hutchinson AW, Bray MA, Doig MV, Shipley ME, Smith MJ. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature 286: 264–265, 1980 [DOI] [PubMed] [Google Scholar]
- 13.Fretland DJ, Widomski DL, Anglin CP, Penning TD, Yu S, Djuric SW. Leukotriene B4-induced granulocyte trafficking in guinea pig dermis. Effect of second-generation leukotriene B4 receptor antagonists, SC-50605 and SC-51146. Inflammation 17: 353–360, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Friedrich EB, Tager AM, Liu E, Pettersson A, Owman C, Munn L, Luster AD, Gerszten RE. Mechanisms of leukotriene B4–triggered monocyte adhesion. Arterioscler Thromb Vasc Biol 23: 1761–1767, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Goetzl EJ, Pickett WC. Novel structural determinants of the human neutrophil chemotactic activity of leukotriene B. J Exp Med 153: 482–487, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grumelli S, Corry DB, Song LZ, Song L, Green L, Huh J, Hacken J, Espada R, Bag R, Lewis DE, Kheradmand F. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. Plos Med 1: e8, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill AT, Campbell EJ, Bayley DL, Hill SL, Stockley RA. Evidence for excessive bronchial inflammation during an acute exacerbation of chronic obstructive pulmonary disease in patients with alpha(1)-antitrypsin deficiency (PiZ). Am J Respir Crit Care Med 160: 1968–1975, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350: 2645–2653, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Hubbard RC, Fells G, Gadek J, Pacholok S, Humes J, Crystal RG. Neutrophil accumulation in the lung in alpha 1-antitrypsin deficiency. Spontaneous release of leukotriene B4 by alveolar macrophages. J Clin Invest 88: 891–897, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishizawa K, Kubo H, Yamada M, Kobayashi S, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett 556: 249–252, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Izquierdo JL, Almonacid C, Parra T, Perez J. [Systemic and lung inflammation in 2 phenotypes of chronic obstructive pulmonary disease]. Arch Bronconeumol 42: 332–337, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Janelle MF, Doucet A, Bouchard D, Bourbonnais Y, Tremblay GM. Increased local levels of granulocyte colony-stimulating factor are associated with the beneficial effect of pre-elafin (SKALP/trappin-2/WAP3) in experimental emphysema. Biol Chem 387: 903–909, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Jonasson S, Hedenstierna G, Hedenstrom H, Hjoberg J. Comparisons of effects of intravenous and inhaled methacholine on airway physiology in a murine asthma model. Respir Physiol Neurobiol 165: 229–236, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Jonasson S, Hjoberg J, Hedenstierna G, Basu S. Allergen-induced formation of F2-isoprostanes in a murine asthma model identifies oxidative stress in acute airway inflammation in vivo. Prostaglandins Leukot Essent Fatty Acids 80: 1–7, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Kostikas K, Gaga M, Papatheodorou G, Karamanis T, Orphanidou D, Loukides S. Leukotriene B4 in exhaled breath condensate and sputum supernatant in patients with COPD and asthma. Chest 127: 1553–1559, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Malmsten CL, Palmblad J, Uden AM, Radmark O, Engstedt L, Samuelsson B. Leukotriene B4: a highly potent and stereospecific factor stimulating migration of polymorphonuclear leukocytes. Acta Physiol Scand 110: 449–451, 1980 [DOI] [PubMed] [Google Scholar]
- 27.March TH, Cossey PY, Esparza DC, Dix KJ, McDonald JD, Bowen LE. Inhalation administration of all-trans-retinoic acid for treatment of elastase-induced pulmonary emphysema in Fischer 344 rats. Exp Lung Res 30: 383–404, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Martin EL, Sheikh TA, Leco KJ, Lewis JF, Veldhuizen RA. Contribution of alveolar macrophages to the response of the TIMP-3 null lung during a septic insult. Am J Physiol Lung Cell Mol Physiol 293: L779–L789, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Martin TR, Pistorese BP, Chi EY, Goodman RB, Matthay MA. Effects of leukotriene B4 in the human lung. Recruitment of neutrophils into the alveolar spaces without a change in protein permeability. J Clin Invest 84: 1609–1619, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mikell Paige MSS, Dorothy Bunyan A, Shim YM. HPLC quantification of 5-hydroxyeicosatetraenoic acid in human lung cancer tissues. Biomed Chromatogr 23: 817–821, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Mulgrew AT, Taggart CC, McElvaney NG. Alpha-1-antitrypsin deficiency: current concepts. Lung 185: 191–201, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Nakano R, Oka M, Nakamura T, Fukuda M, Kawabata S, Terashi K, Tsukamoto K, Noguchi Y, Soda H, Kohno S. A leukotriene receptor antagonist, ONO-1078, modulates drug sensitivity and leukotriene C4 efflux in lung cancer cells expressing multidrug resistance protein. Biochem Biophys Res Commun 251: 307–312, 1998 [DOI] [PubMed] [Google Scholar]
- 33.O'Byrne PM, Postma DS. The many faces of airway inflammation. Asthma and chronic obstructive pulmonary disease. Asthma Research Group. Am J Respir Crit Care Med 159: S41–S63, 1999 [PubMed] [Google Scholar]
- 34.Palmblad J, Malmsten CL, Uden AM, Radmark O, Engstedt L, Samuelsson B. Leukotriene B4 is a potent and stereospecific stimulator of neutrophil chemotaxis and adherence. Blood 58: 658–661, 1981 [PubMed] [Google Scholar]
- 35.Park SJ, Wiekowski MT, Lira SA, Mehrad B. Neutrophils regulate airway responses in a model of fungal allergic airways disease. J Immunol 176: 2538–2545, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care 46: 798–825, 2001 [PubMed] [Google Scholar]
- 37.Pillow JJ, Korfhagen TR, Ikegami M, Sly PD. Overexpression of TGF-alpha increases lung tissue hysteresivity in transgenic mice. J Appl Physiol 91: 2730–2734, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Saetta M, Di Stefano A, Maestrelli P, Turato G, Ruggieri MP, Roggeri A, Calcagni P, Mapp CE, Ciaccia A, Fabbri LM. Airway eosinophilia in chronic bronchitis during exacerbations. Am J Respir Crit Care Med 150: 1646–1652, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Sharma R, Singhal SS, Wickramarachchi D, Awasthi YC, Awasthi S. RLIP76 (RALBP1)-mediated transport of leukotriene C4 (LTC4) in cancer cells: implications in drug resistance. Int J Cancer 112: 934–942, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Shim YM, Zhu Z, Zheng T, Lee CG, Homer RJ, Ma B, Elias JA. Role of 5-lipoxygenase in IL-13-induced pulmonary inflammation and remodeling. J Immunol 177: 1918–1924, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Teckman JH, Lindblad D. Alpha-1-antitrypsin deficiency: diagnosis, pathophysiology, and management. Curr Gastroenterol Rep 8: 14–20, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Turino GM. The origins of a concept: the protease-antiprotease imbalance hypothesis. Chest 122: 1058–1060, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Wardlaw AJ, Hay H, Cromwell O, Collins JV, Kay AB. Leukotrienes, LTC4 and LTB4, in bronchoalveolar lavage in bronchial asthma and other respiratory diseases. J Allergy Clin Immunol 84: 19–26, 1989 [DOI] [PubMed] [Google Scholar]
- 44.Weibel ER. Principles and methods for the morphometric study of the lung and other organs. Lab Invest 12: 131–155, 1963 [PubMed] [Google Scholar]
- 45.Weibel ER, Hsia CC, Ochs M. How much is there really? Why stereology is essential in lung morphometry. J Appl Physiol 102: 459–467, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Wenzel SE, Larsen GL, Johnston K, Voelkel NF, Westcott JY. Elevated levels of leukotriene C4 in bronchoalveolar lavage fluid from atopic asthmatics after endobronchial allergen challenge. Am Rev Respir Dis 142: 112–119, 1990 [DOI] [PubMed] [Google Scholar]
- 47.Woolhouse IS, Bayley DL, Stockley RA. Sputum chemotactic activity in chronic obstructive pulmonary disease: effect of alpha(1)-antitrypsin deficiency and the role of leukotriene B(4) and interleukin 8. Thorax 57: 709–714, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wozel G, Blasum C, Winter C, Gerlach B. Dapsone hydroxylamine inhibits the LTB4-induced chemotaxis of polymorphonuclear leukocytes into human skin: results of a pilot study. Inflamm Res 46: 420–422, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Zheng T, Zhu Z, Wang Z, Homer RJ, Ma B, Riese RJ, Jr, Chapman HA, Jr, Shapiro SD, Elias JA. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest 106: 1081–1093, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest 103: 779–788, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu Z, Ma B, Zheng T, Homer RJ, Lee CG, Charo IF, Noble P, Elias JA. IL-13-induced chemokine responses in the lung: role of CCR2 in the pathogenesis of IL-13-induced inflammation and remodeling. J Immunol 168: 2953–2962, 2002 [DOI] [PubMed] [Google Scholar]