Abstract
Surfactant protein A (SP-A) plays an important role in the maintenance of lung lipid homeostasis. Previously, an SP-A receptor, P63 (CKAP4), on type II pneumocyte plasma membranes (PM) was identified by chemical cross-linking techniques. An antibody to P63 blocked the specific binding of SP-A to pneumocytes and the ability of SP-A to regulate surfactant secretion. The current report shows that another biological activity of SP-A, the stimulation of surfactant uptake by pneumocytes, is inhibited by P63 antibody. cAMP exposure resulted in enrichment of P63 on the cell surface as shown by stimulation of SP-A binding, enhanced association of labeled P63 antibody with type II cells, and promotion of SP-A-mediated liposome uptake, all of which were inhibited by competing P63 antibody. Incubation of A549 and type II cells with SP-A also increased P63 localization on the PM. The phosphatidylinositol 3-kinase (PI3-kinase) signaling pathway was explored as a mechanism for the transport of this endoplasmic reticulum (ER)-resident protein to the PM. Treatment with LY-294002, an inhibitor of the PI3-kinase pathway, prevented the SP-A-induced PM enrichment of P63. Exposure of pneumocytes to SP-A or cAMP activated Akt (PKB). Blocking either PI3-kinase or Akt altered SP-A-mediated lipid turnover. The data demonstrate an important role for the PI3-kinase-Akt pathway in intracellular transport of P63. The results add to the growing body of evidence that P63 is critical for SP-A receptor-mediated interactions with type II pneumocytes and the resultant regulation of surfactant turnover.
Keywords: lung, phospholipid, plasma membrane, lipid turnover
alveolar type II cells produce and secrete lung surfactant, the complex mixture of phospholipids and proteins that functions to reduce surface tension and prevents collapse of the alveoli during expiration. These specialized cells store surfactant components in a membrane-bound lamellar body. Surfactant protein A (SP-A), the most abundant surfactant protein present in the lung surfactant, belongs to the mammalian C-type lectin family of proteins (28, 35). In monomeric form, it is a 28- to 32-kDa hydrophilic protein that organizes into trimers and can further oligomerize into higher order forms (35). The primary structure of the protein contains a collagen-like region connected by a neck region to a globular carbohydrate-recognition domain. SP-A has a calcium-dependent activity that can: bind to dipalmitoylphosphatidylcholine (DPPC), the major phospholipid present in the lung; increase the aggregation and molecular order of phospholipids; bind to peroxiredoxin 6 and regulate its phospholipase A2 activity (59); and, in conjunction with SP-B, promote tubular myelin formation in lung surfactant (30, 37). As a member of the collectin family of host defense proteins, SP-A also contributes to innate pulmonary immunity (56). In fact, SP-A may have multiple roles in respiratory physiology, as related protein forms are present in all vertebrate classes, including most primitive amphibious fish (48).
Either a deficit or an excess of alveolar surfactant can impair lung function. Hence, the quantity of extracellular surfactant must be rigorously controlled. Both SP-A and DPPC are internalized and recycled by type II pneumocytes, and evidence is accumulating that SP-A plays a role in the clathrin-mediated surfactant lipid uptake pathway (2). Although mice deficient in SP-A demonstrate fairly normal surfactant metabolism, they are unable to regulate the rate of phospholipid uptake in response to physiological stimuli (25). In a detailed analysis of the pathways of surfactant clearance, we demonstrated that a major portion of the removal of surfactant phospholipid from the lung in wild-type mice took place through an SP-A/clathrin-coated pit/receptor-mediated pathway that was enhanced on secretagogue treatment (2). In the absence of SP-A, the gene-targeted mice were able to maintain normal basal rates of uptake through compensatory use of a nonclathrin/actin-dependent mechanism. However, this pathway was unable to respond to the challenges of secretagogue stimulation or hyperventilation (25). SP-A binding and uptake by the lung and type II cells in culture occur via a receptor-mediated clathrin-coated pit pathway (25, 40). Thus our attention has been focused on the characterization of the SP-A receptor responsible for SP-A-mediated clearance of surfactant phospholipid.
Several potential type II cell receptors for SP-A have been isolated and partially characterized (12, 29, 45, 47). Recently, we have identified an SP-A-type II cell surface binding protein, P63, using chemical cross-linking techniques (22). The P63/CKAP4 protein is a 63-kDa nonglycosylated, type II transmembrane protein oriented with the shorter (106 amino acids) NH2 terminus of the protein in the cytoplasm, whereas the larger COOH-terminal portion of the protein is exposed to the lumen of the endomembrane compartment of the endoplasmic reticulum (ER) (41, 44). For the population of P63 on the plasma membrane, the COOH terminus would be exposed to the alveolar space. P63 is a cytoskeleton-linking membrane phosphoprotein and is a resident of a membrane network interposed between the rough ER and Golgi apparatus, the ERGIC (41, 42, 51). It has been suggested that P63 mediates the direct association between ER membranes and microtubules through the cytoplasmic portion of the protein and, thereby, plays a role in mitosis (51). Although the original studies of P63 described it as an ER-resident protein (43), it is now known to be expressed on the surface of vascular smooth muscle cells where it acts as a receptor for tissue plasminogen activator, on bladder epithelial cells as a receptor for the frizzled-8 protein-related antiproliferative factor (APF) (13, 38), and on the plasma membrane of type II pneumocytes as a receptor for SP-A (3, 22).
In type II cells, specific protein-to-protein interactions between SP-A and the candidate SP-A receptor, P63, have been confirmed by chemical cross-linking, coimmunoprecipitation, and colocalization of the two proteins in the same intracellular compartments (22). In addition, antibody to P63 interfered with one of the biological activities of SP-A, the ability to block surfactant phospholipid secretion. These data are consistent with a physiological function of P63 in surfactant turnover (22). In our recent detailed study of the interactions of SP-A with type II cells, we found that antibody to P63 interfered with the specific calcium-dependent binding of SP-A (3). Additionally, siRNA directed against P63 lowered cellular levels of P63 protein and reduced both SP-A binding and biological activity (3). Taken together, the results are consistent with the conclusion that the transmembrane protein P63 is a cell surface receptor for SP-A and thus must move from the ER to the plasma membrane for its physiological role.
The surface density of the SP-A receptor on type II cells is enhanced through secretagogue exposure (9). We hypothesized that exposure of the pneumocytes to the surfactant protein receptor ligand would also serve to initiate trafficking of the receptor to the plasma membrane. This hypothesis is consistent with results using other cell systems showing that incubation with ligands, and with SP-A in particular, can cause translocation of intracellular receptor proteins to the cell surface. SP-A exposure augments the plasma membrane expression of the scavenger receptor A on alveolar macrophages (31) and of the complement subcomponent (C1q) receptor protein (C1qR) on the monocytic cell line, U937 (33). The current study explores the role of secretagogues and of SP-A exposure in the intracellular transport of the ER-resident protein, P63, to the plasma membrane and the possible mechanism involved. The phosphatidylinositol 3-kinase (PI3-kinase) signaling pathway has been implicated in the translocation of many proteins (21, 34). The hypothesis for involvement of this pathway in P63 translocation was attractive in view of a previous report from White and Strayer (53) that incubation of type II cells with SP-A resulted in the activation of PI3-kinase. Thus we explored the role of the PI3-kinase signaling pathway in the forward transport of P63 from intracellular stores to the plasma membrane. To avoid confusion with another protein in the literature, the transcription factor p63, we have used a capital p for P63 (CKAP4).
EXPERIMENTAL PROCEDURES
Cell Culture
Type II pneumocytes.
All animal protocols adhered to the guidelines from the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Pennsylvania Animal Care and Use Committee. Type II cells were isolated from pathogen-free adult male Sprague-Dawley rat lungs as described previously (10, 11, 16). Briefly, perfused lungs were digested with elastase, minced, and filtered. Macrophages were removed from the cell preparation by plating on IgG-coated petri dishes. The nonadherent cells were pelleted, resuspended in MEM with 10% FBS, and plated on either plastic 35-mm dishes (Costar, Cambridge, MA) or 12-mm inserts of Transwell microporous membranes (3-μm pore size; Costar). After overnight culture and removal of nonadherent cells, >95% of cells attached to the dish were type II cells.
A549 cells.
A549 human lung adenocarcinoma cells were from the American Type Culture Collection (Rockville, MD) and were grown in MEM (Invitrogen) medium containing 10% FBS and 1% antibiotics.
Purification and Labeling of SP-A and Antibodies
Native human SP-A was isolated from the bronchoalveolar lavage (BAL) fluids of patients with alveolar proteinosis. SP-A was purified according to the method of Hawgood et al. (23) using 1-butanol and β-d-glucopyranoside extraction, dialysis, and microconcentration, as previously described (1). The purity of the SP-A preparation was monitored by SDS-PAGE (32). Endotoxin levels were 0.5 pg/μg SP-A protein (Limulus Amebocyte Lysate Test; Lonza, Walkersville, MD). SP-A, antibody to P63, and nonimmune IgG were iodinated using IODO-GEN (Pierce, Rockford, IL) with directions provided by Pierce. The iodinated protein was dialyzed against Tris buffer and used within 3 wk. The specific activity of the 125I-labeled proteins ranged from 150 to 600 dpm/ng protein, and >95% was trichloroacetic acid-precipitable.
Production of P63 Antibody and Analysis of P63 Structure
The P63 protein antibody was produced by Strategic Biosolutions (Newark, DE) and recognized P63 protein in rat type II cells and human A549 cells, as described previously (3). The primary structure of P63 was analyzed using the ScanProsite software obtained from the ExPASy proteomics web site (http://ca.expasy.org) (20).
Immunofluorescence Confocal Microscopy
Freshly isolated rat type II cells were grown on glass coverslips for 24 h and were treated with SP-A or left untreated. Cells were then rinsed with PBS and stained with wheat germ agglutinin Alexa 594 at 1 μg/ml for 15 min at room temperature and washed three times with PBS. Cells were fixed with 2% paraformaldehyde for 20 min, washed, and incubated with P63 antibody overnight at 4°C. Next, the cells were washed and incubated for 1 h with the Alexa 488-labeled secondary antibody, washed again, mounted, and viewed by confocal microscopy.
Isolation of Plasma Membrane Proteins
Plasma membranes were isolated from type II and A549 cells as described earlier (10, 18, 22). Briefly, cells were suspended in 0.32 M sucrose in cold HEPES-Tris buffer, pH 7.4, and sonicated. Cell lysates were layered over a discontinuous sucrose gradient (0.5, 0.7, 0.9, and 1.2 M sucrose) and centrifuged (40,000 g, 1 h, 4°C). The plasma membrane fraction was collected from the 0.9–1.2 M sucrose interface, diluted to 0.32 M sucrose with HEPES-Tris buffer, and centrifuged again (95,000 g, 30 min). The plasma membrane pellet was resuspended in PBS containing 0.1% Triton X-100, pH 7.4, and vortexed. Aliquots were stored at −80°C.
Biotinylation of Plasma Membrane
Before or after incubation with SP-A (1 μg/ml, 3 h), A549 cell surface proteins were isolated using Pierce Cell Surface Protein Isolation Kit (Thermo Scientific, Rockford, IL) according to the manufacturer's protocol. Cells were washed twice with ice-cold PBS, and the Sulfo-NHS-Biotin solution, which is cell membrane-impermeable, was added in PBS for 30 min at 4°C with intermediate agitation. Quenching Solution (500 μl) was added, and the cells were scraped from the dish and centrifuged in Tris-buffered saline (TBS; 25 mM Tris·HCl, pH 7.2, and 150 mM NaCl). HEN buffer [HEPES (Sigma, St. Louis, MO), 25 mM EDTA, and 0.1 mM Neocuproine (Sigma)] with 1% SDS and protease inhibitors was added to the cell pellet, and the cells were sonicated. Neutralization buffer and 500 μl of streptavidin-agarose beads were added followed by incubation at room temperature with end-over-end rotation overnight. Unbound proteins were removed by centrifugation (5 times). The biotinylated proteins bound to the streptavidin-agarose beads were eluted using SDS-PAGE sample buffer containing dithiothreitol and heating at 95°C for 10 min. The samples were centrifuged and analyzed by SDS-PAGE and Western blotting.
SDS-PAGE and Western Blotting
Cell and plasma membrane protein content were measured using the Bradford protein assay (Bio-Rad Laboratories, Hercules, CA) with IgG as the protein standard (7). Plasma membranes and whole cell lysates were resolved on 12% Tris-glycine gels (Invitrogen) by SDS-PAGE under reducing conditions (32). Proteins were electrophoretically transferred to nitrocellulose membrane using Bio-Rad semidry apparatus (49, 50). The membrane was blocked with 5% nonfat dry milk in TBS at room temperature for 1 h on a shaking platform. Membranes were incubated overnight with a predetermined dilution of the primary antibody at 4°C in 5% milk TBST buffer (TBS with 0.1% Tween 20). After washing with TBST, blots were incubated with the indicated horseradish peroxidase-labeled secondary antibodies (Amersham Biosciences, Arlington Heights, IL). When membranes were reprobed, blots were stripped by incubating the membrane for 20 min at room temperature in the antibody stripping solution (Chemicon, Temecula, CA). Blots were rinsed in TBST, blocked in TBST-4% milk, and reprobed with a second antibody. Visualization of the protein bands on Western blots used either enhanced chemiluminescence (ECL) Plus (Amersham Pharmacia Biotech, Piscataway, NJ) or the Odyssey procedure, performed according to the manufacturer's instructions (LI-COR Bioscience, Lincoln, NE) and scanned on the Odyssey infrared scanner. Semiquantitative evaluation of levels of proteins used computer-assisted densitometric scanning (ImageJ software). In all experiments, the control and experimental groups shown as representative Western blots are from the same gel from the same experiment. In some cases, the gel lanes were cut and rearranged for clarity of presentation.
Western blot analysis was carried out using the following primary antibodies: polyclonal rabbit P63 antisera produced against human recombinant P63 expressed in Escherichia coli (3); anti-caveolin-1 and anti-flotillin-1 (BD Transduction Laboratories, Lexington, KY); and monoclonal anti-pSer473 Akt and polyclonal anti-Akt (Cell Signaling Technology, Boston, MA).
Uptake of Liposomes by Type II Cells
Unilamellar liposomes were prepared from lipids using the molar ratios of 0.75 phosphatidylcholine (PC) [⅔ DPPC and \]⅓ egg PC], 0.15 cholesterol, and 0.1 egg phosphatidylglycerol (Avanti, Birmingham, AL) with added [methyl-3H]choline-labeled DPPC (New England Nuclear, Boston, MA) as described previously (1). When SP-A was added to the lipid, light vortexing was used, and the preparation sat on ice for 1 h before use. Type II cells plated on Transwell membranes were used for these experiments. One set of cells was treated with 8-bromoadenosine-cAMP (0.1 mM) for 1 h. Some cells were pretreated for 15 min with inhibitors of the PI3-kinase pathway (LY-294002, 10 μM, Calbiochem, La Jolla, CA, and wortmannin, 50–500 nM, Sigma) or pretreated for 30 min with either a pan-inhibitor of PKC (chelerythrine chloride, 2 μM, an inhibitor of all PKC isoenzymes; Calbiochem) or an inhibitor of Akt (Akt Inhibitor VIII, 10 μM, an inhibitor of Akt1/2; Calbiochem) before cAMP stimulation. Liposomes were added to the cells for 2 h, the media were removed, and the Transwell membranes with cells attached were moved to clean dishes for the harvest to reduce background counts. The cells were harvested from the membranes with trypsin treatment, and the cells were solvent-extracted (6). Uptake of liposomes was quantitated as trypsin-insensitive cell-associated 3H-dpm and was calculated using the specific activity of the liposomes adjusted to the content of DPPC.
PC Secretion
Isolated type II cells were incubated with 0.5 μCi/dish [methyl-3H]choline for 18 h to label cellular phospholipids. Next, the cells were washed and incubated for 30 min. One set of cells was harvested to serve as a time 0 control. The remaining cells either were or were not treated with ATP (1 mM; Sigma) for 1 h. Some cells were pretreated with purified SP-A (0.1 μg/ml, 15 min) and/or the following: an inhibitor of the PI3-kinase pathway (LY-294002, 10 μM, 15 min, or wortmannin, 300 or 500 nM), an inhibitor of PKC (chelerythrine chloride, 2 μM, an inhibitor of all PKC isozymes, 30 min), and/or an inhibitor of Akt (Akt Inhibitor VIII, 10 μM, 30 min) before ATP stimulation. The media were collected and centrifuged to remove detached cells, methanol was added to the cell monolayer, and the cells were scraped from the dish. Lipids were extracted from the cells and media using the Bligh and Dyer method (6). The amount of phospholipid secretion was calculated as the percentage of lipid counts per minute (cpm) in the medium relative to the total cpm of lipid present in the cells and the medium.
Binding of SP-A or Antibodies to Type II Cells
Type II cells on Transwell membranes were preincubated at 37°C with or without PI3-kinase inhibitor (LY-294002) for 15 min followed by addition of cAMP (0.1 mM) to some samples for 25 min. The cells were moved to the cold (4°C) and incubated with 125I-labeled proteins for 1 h followed by washing, lysis with 0.2 N of NaOH, and measurement of cpm.
Statistics
Data are reported as means ± SE. Statistical comparisons were performed with SigmaStat (Jandel Scientific) using a standard t-test. Results were considered statistically significantly different at P values of <0.05.
RESULTS
P63 Antibody Inhibited Secretagogue-Stimulated Uptake of Liposomes Containing SP-A
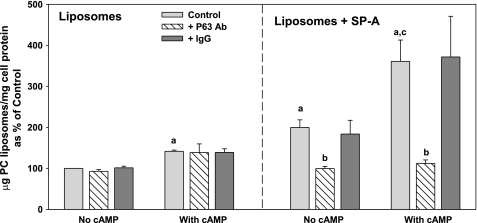
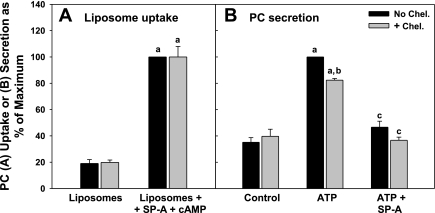
cAMP treatment enhances the uptake of phospholipid liposomes by type II cells in culture and in the isolated lung, whereas the presence of SP-A on the liposomes augments this effect (1, 2). To determine the role of P63, antibody to P63 was used in competition experiments to block the SP-A-mediated internalization of liposomes labeled with 3H-DPPC. Type II cells on Transwell membranes were incubated with 3H-DPPC liposomes (45 μg/ml) for 2 h with or without exposure to 0.1 mM cAMP. SP-A (5 μg/ml) was added to some of the liposome preparations. Figure 1, left, shows that the increase in liposome uptake by type II cells augmented by cAMP exposure was not affected by the P63 antibody. However, cellular internalization of liposomes that contained SP-A (Fig. 1, right) was blocked by the anti-P63 antibody under basal or cAMP-stimulated conditions. The presence of nonimmune IgG (5 μg/ml) was without effect on liposome endocytosis (Fig. 1).
Fig. 1.
Surfactant protein A (SP-A)-enhanced uptake of 3H-dipalmitoylphosphatidylcholine (DPPC)-labeled phospholipid liposomes by type II cells is inhibited by antibody (Ab) to P63. Type II pneumocytes were plated on Transwell membranes. Some cells were exposed to cAMP (0.1 mM) for 1 h without or with anti-P63 Ab or IgG (5 μg/ml) added for an additional 15 min. Next, the cells were incubated with 3H-DPPC-labeled liposomes [45 μg phosphatidylcholine (PC)/ml] without SP-A (Liposomes) or with SP-A (5 μg/ml; Liposomes + SP-A) for 2 h. The cells were removed from the membranes with trypsin, and were solvent-extracted. Uptake was measured as trypsin-insensitive 3H-PC disintegrations per minute per milligram cell protein. The data are means ± SE, n = 3–12 experiments performed in duplicate or triplicate. Control is the amount of liposome uptake that occurred without SP-A or cAMP. Control = 1.8 ± 0.3 μg PC/mg cell protein, n = 12. a, Significant difference from Control (liposomes alone, no cAMP); b, significant difference from Liposomes + SP-A without Ab; c, significant difference from Liposomes + SP-A without Ab or cAMP.
Translocation of P63 in Response to SP-A Treatment
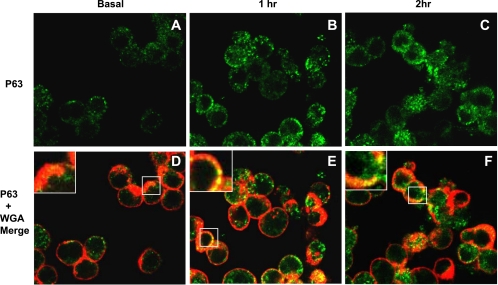
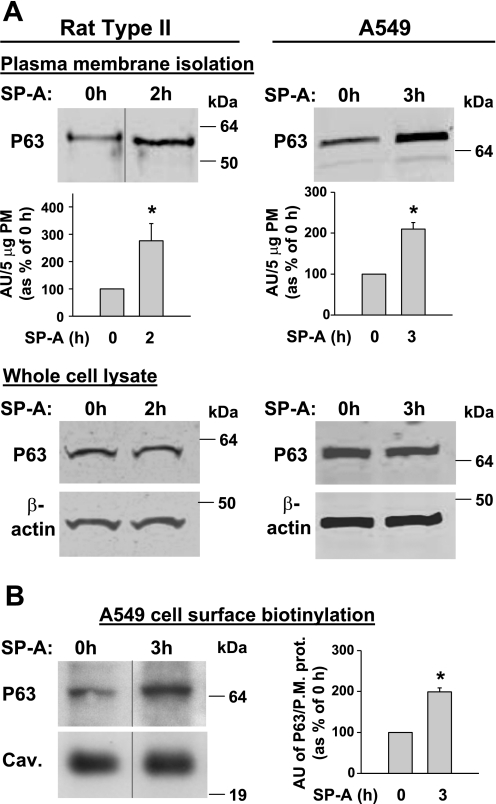
Since many agonists influence distribution of their respective receptors (14), we hypothesized that SP-A would regulate the movement of P63 from the ER to the plasma membrane in lung epithelial cells. To address this question, rat type II cells were treated with SP-A (1 μg/ml), fixed without permeabilization, and stained with P63 antibody to view the surface distribution of P63. Since high-affinity binding of SP-A to type II cells saturates at ∼1 μg SP-A/ml, this concentration of SP-A was used in this and several subsequent studies (3, 57). Identification of the cell surface was achieved by counterstaining the cells with wheat germ agglutinin, a plasma membrane marker. Figure 2 shows that, in response to SP-A exposure, there was a time-dependent increase in the cell surface expression of P63 that colocalized with wheat germ agglutinin. The enhanced levels of P63 in the plasma membrane could be visualized after 1 h of incubation with SP-A (Fig. 2, B and E) and remained elevated for 2 h (Fig. 2, C and F). For confirmation, Western blot analysis was performed using P63 antibody and plasma membrane proteins purified from untreated and SP-A-treated (1 μg/ml, 2 h) type II cells. Densitometric quantitation of identical gels revealed a 2.8-fold enrichment (P = 0.019; n = 6) in P63 in the plasma membranes after incubation with SP-A (Fig. 3A, top left). To support the data using another cell type, plasma membranes were isolated from similarly treated A549 cells, a human lung adenocarcinoma cell line that has been used as a model system for type II pneumocytes. A549 cells have P63 protein by Western blot and have demonstrated high-affinity SP-A binding that is inhibitable by P63 antibody (3). A549 cells demonstrated a 2.1-fold enrichment (P = 0.003; n = 3) of P63 in the plasma membranes after 3 h of SP-A exposure (Fig. 3A, top right). To ascertain whether SP-A exposure caused an increase in the expression levels of P63, we used whole cell lysates from control and SP-A-treated type II cells and A549 cells (Fig. 3A, bottom left and right). β-Actin was used as a loading control. Western blot analysis demonstrated that SP-A did not increase the levels of P63 protein in either cell type within the time period tested. To support the data of P63 plasma membrane enrichment by SP-A, A549 cell surface proteins were isolated by protein biotinylation using a membrane-impermeable biotin ester. The biotinylated proteins were bound to streptavidin beads, separated by SDS-PAGE, and subjected to Western blot techniques. Caveolin or flotillin were analyzed as markers for plasma membrane and as loading controls. Figure 3B demonstrates that P63 was located on the cell surface, and incubation with SP-A for 3 h enriched the P63 surface content. Quantification of the data shows a significant 2-fold increase in the level of P63 cell surface expression after SP-A exposure.
Fig. 2.
SP-A induced translocation of P63 to the cell surface in type II cells. Rat type II cells were treated with SP-A for up to 2 h, fixed, left unpermeabilized, and stained for immunofluorescence with P63 Ab (A–C, green) and wheat germ agglutinin Alexa 594 (WGA; red). D–F show merged images of cells stained with P63 Ab and WGA. Magnification, ×60. Insets in D–F are enlargements of areas enclosed in boxes. Samples stained with nonimmune Ab (data not shown) were black.
Fig. 3.
P63 enrichment in the cell surface after exposure to SP-A. A: plasma membranes (PM) and whole cell lysates. Type II cells and A549 cells were incubated with SP-A (1 μg/ml) for 2 or 3 h, respectively. Representative blots shown are of isolated PM fractions (5-μg protein) or whole cell lysates (25-μg protein) subjected to Western blot analysis using P63 Ab. β-Actin Ab was used as a loading control for the cell lysates. Protein bands were visualized using the Odyssey scanner. Bar graphs quantitate the density of the PM P63 protein bands as the means ± SE for type II cell (n = 6) or A549 cell (n = 3) experiments and are expressed as arbitrary units (AU) per 5 μg PM protein as a percentage of the P63 present without SP-A exposure (0-h control = 100%). *P < 0.02 relative to 0 h. Molecular masses of rat P63 and human P63 were ∼63 and ∼66 kDa, respectively. B: A549 cell surface biotinylation. Representative immunoblot of surface biotinylated P63 precipitated with streptavidin beads (left) and quantitation of multiple gels (right). A549 cells were incubated with SP-A (1 μg/ml) for 3 h. The cells were placed at 4°C, and the cell surface proteins were labeled with a membrane-impermeable biotinylation reagent. Membrane proteins were isolated using streptavidin beads, subjected to gel electrophoresis, and probed with anti-P63 Ab. Blots were also probed with anti-flotillin (data not shown) or anti-caveolin (shown) Ab as cell membrane protein markers and loading controls. Protein bands were visualized using enhanced chemiluminescence (ECL). The bar graph quantitates the density of the P63 protein band relative to either caveolin (Cav.) or flotillin (P.M. prot. = PM protein caveolin or flotillin) in AU and expressed as a percentage of the P63 present without SP-A exposure. Data were similar using either caveolin or flotillin. Data are means ± SE, n = 4. *P < 0.001 relative to 0 h. In some immunoblots, samples from the same gel were cut as indicated and rearranged for clarity.
These results indicate that exposure to SP-A can cause trafficking of P63 from its ER localization to the cell surface in lung epithelial cells.
Inhibition of PI3-Kinase Pathway Alters SP-A Biological Activity
To determine whether the PI3-kinase signaling pathway plays a role in the movement of P63 protein to the cell surface, LY-294002 (10 μM) or wortmannin was used to block the kinase activity in type II cells before secretagogue exposure. These compounds, through different mechanisms, specifically block the activation of PI3-kinase and thus inhibit the phosphorylation of target proteins (60). Blocking the kinase pathway should obstruct the intracellular trafficking of P63 to the plasma membrane and hence the biological activities of SP-A.
Liposome uptake.
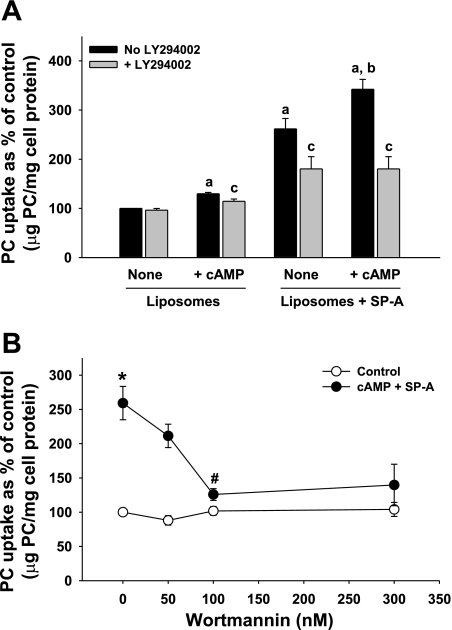
Type II cells were incubated with 3H-DPPC liposomes (45 μg PC/ml) without or with SP-A (5 μg/ml) in the presence or absence of cAMP (0.1 mM) for 2 h. As seen in Fig. 4A, both secretagogue treatment and the presence of SP-A on the liposomes enhanced liposome uptake by the pneumocytes, as shown earlier in Fig. 1. Under basal conditions in the absence of cAMP, preincubation in LY-294002 (10 μM) had no effect on the uptake of liposomes that did not contain SP-A. There was only a minor effect on the incorporation of these liposomes in the presence of cAMP (Fig. 4A, Liposomes). The predominant effect of LY-294002 was on liposomes that contained SP-A. Exposure to the agent blocked the SP-A-mediated augmentation of liposome incorporation by type II cells under both basal and cAMP-stimulated conditions (Fig. 4A, Liposomes + SP-A).
Fig. 4.
Phosphatidylinositol 3-kinase (PI3-kinase) inhibitors block the biological activity of SP-A on PC liposome uptake. A: LY-294002. Type II cells plated on Transwell membranes were incubated without LY-294002 (No LY294002) or with LY-294002 (10 μM) for 15 min, with or without cAMP (0.1 mM) for 1 h. 3H-DPPC-labeled liposomes (45 μg PC/ml) were added without (Liposomes) or with SP-A at 5 μg/ml (Liposomes + SP-A) for 2 h. The cells were harvested with trypsin. Data are means ± SE of duplicate dishes in 3 experiments. a, Significant difference from liposomes alone, no additions; b, significant difference from Liposomes + SP-A, no cAMP (None); c, significant difference from no LY-294002. P < 0.05; n = 3. B: wortmannin. Type II cells plated on Transwell membranes were incubated without wortmannin or with wortmannin (50–300 nM) for 15 min, with or without cAMP (0.1 mM) for 1 h. 3H-DPPC-labeled liposomes (45 μg PC/ml) were added without SP-A (Control, no cAMP and no SP-A added to liposomes) or with SP-A at 5 μg/ml (cAMP and SP-A with liposomes) for 2 h. The cells were harvested with trypsin. Data are means ± SE or means ± range of duplicate or triplicate dishes in 2 (Control, wortmannin at 50 nM) to 5 experiments. *Significant difference from Control; #significant difference from cAMP + SP-A, no wortmannin, P < 0.05, n = 3–5.
Blocking the kinase pathway with wortmannin also interfered with the promotion of liposome uptake by SP-A. Wortmannin is a specific, covalent, and irreversible inhibitor of PI3-kinase. In experiments performed as described for LY-294002, wortmannin (50–300 nM) demonstrated a concentration-dependent inhibition of the cAMP (0.1 mM)-stimulated uptake of surfactant liposomes containing SP-A into type II cells (Fig. 4B).
Secretion.
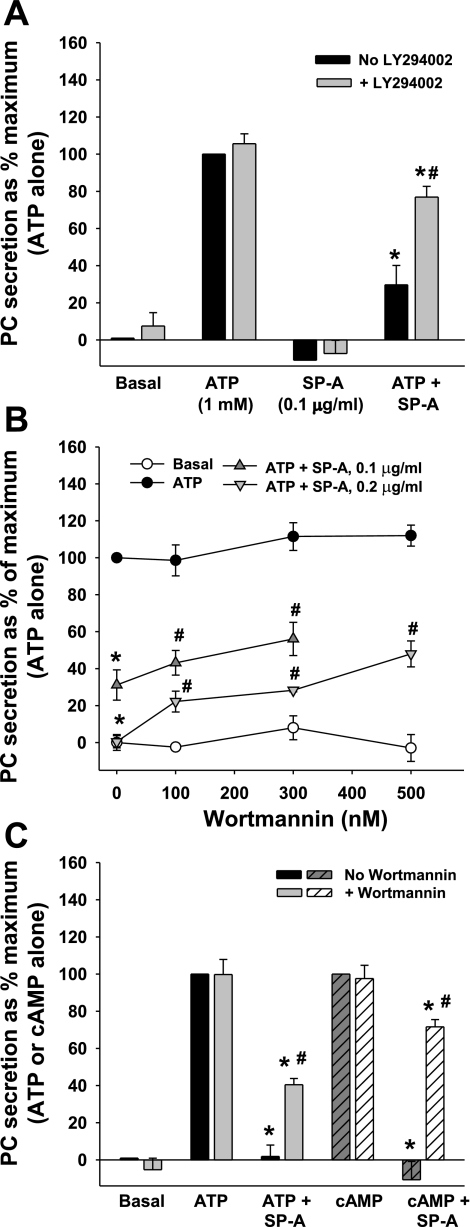
SP-A is a potent inhibitor of surfactant secretion from type II cells, and this effect is receptor-mediated (3, 22, 35). To examine whether blocking PI3-kinase-mediated trafficking of P63 would affect this biological activity of SP-A, secretion studies were performed. Type II cells were incubated with [3H]choline overnight to label PC. The cells then were treated with 10 μM LY-294002 for 15 min. Some cell cultures had ATP (1 mM), SP-A (0.1 μg/ml), or both added. ATP is a well-known secretagogue that stimulates PC secretion from type II cells (4, 39). The LY-294002 compound had very little effect on the basal levels or ATP-induced PC secretion by type II cells (Fig. 5A). In secretagogue-stimulated cells that were treated with SP-A, PC release was reduced over two-thirds to 30 ± 10% of maximum (ATP-stimulated) as shown previously (Ref. 4 and for review, Refs. 35, 58). However, pretreatment of the cells with LY-294002 attenuated the inhibitory effect of SP-A as PC secretion was significantly restored to 77 ± 6% of maximum values (mean ± SE, n = 3).
Fig. 5.
Inhibition of PI3-kinase partially reverses the ability of SP-A to inhibit PC secretion. A: LY-294002, ATP, cells on dishes. Type II cells were incubated with [3H]choline overnight to label PC. Cells were washed and incubated without or with 1 mM ATP. LY-294002 (10 μM) and SP-A (0.1 μg/ml) were added at 30 and 15 min, respectively, before the addition of ATP. Secretion was measured over 1 h. Data are means ± SE of duplicate or triplicate samples from 3 experiments. *Significant difference from ATP; #significant difference from ATP + SP-A, no LY-294002. P < 0.05; n = 3. B: wortmannin, ATP, cells on dishes. Type II cells were plated on plastic dishes and incubated with [3H]choline overnight to label PC. After 24 h, the cells were washed and incubated without or with 1 mM ATP. Wortmannin (0–500 nM) and SP-A (at either 0.1 or 0.2 μg/ml) were added at 30 and 15 min, respectively, before the addition of ATP. Secretion was measured over 1 h. Data are means ± SE of duplicate or triplicate samples from 4 to 6 experiments. Control values for each experiment were subtracted, and data are expressed as a percentage of maximum stimulation by 1 mM ATP (100%). Control or ATP-stimulated PC secretion is 2.2 ± 0.3 or 5.5 ± 0.4% (mean ± SE, n = 6), respectively. *Significant difference from ATP; #significant difference from ATP + SP-A, P < 0.05; n = 3. C: wortmannin, ATP or cAMP, cells on membranes. Cells were plated on Transwell membranes. After 24 h, the cells were washed and incubated without or with 1 mM ATP or 0.1 mM cAMP. Wortmannin (300 nM) and SP-A (at 0.1 μg/ml) were added at 30 and 15 min, respectively, before the addition of ATP or cAMP. Secretion was measured over 1 h. Data are means ± SE of duplicate or triplicate samples from 3 to 7 experiments. Control values for each experiment are subtracted, and data are expressed as a percentage of maximum stimulation by 1 mM ATP or cAMP (100%). Control or ATP-stimulated PC secretion was 2.5 ± 0.2 or 4.7 ± 0.3%, respectively (mean ± SE, n = 3). Control or cAMP-stimulated PC secretion was 2.8 ± 0.6 or 5.2 ± 1.1% (mean ± SE, n = 4), respectively. *Significant difference from secretagogue-stimulated values; #significant difference from ATP + SP-A or cAMP + SP-A, P < 0.05; n = 3 or 4.
In addition, the ability of SP-A to block ATP-stimulated PC secretion was significantly reversed by preincubation of type II cells plated on plastic dishes and preincubated with 100–500 nM wortmannin (Fig. 5B). The extent of effect was dependent on the concentration of wortmannin or SP-A used. Figure 5C compares the effects of 300 nM wortmannin on secretion from type II cells on Transwell membranes and stimulated with either ATP or cAMP. Again, blocking PI3-kinase significantly blunted the biological activity of SP-A. However, neither LY-294002 nor wortmannin completely reversed the SP-A inhibitory effect on PC secretion, and neither returned surfactant secretion to secretagogue-stimulated levels. Nevertheless, inhibition of PI3-kinase activation significantly interfered with the receptor/ligand-dependent biological activities of SP-A as the augmentation of liposome uptake was prevented, and the SP-A-dependent inhibition of secretagogue-induced surfactant secretion was diminished.
Inhibition of the PI3-Kinase Pathways Abrogates the Secretagogue and SP-A Enhancement of P63 Cell Surface Density
cAMP.
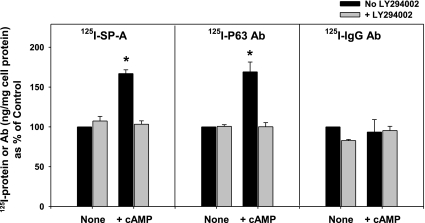
Previous work had demonstrated that the ability of secretagogues to enhance the binding of iodinated SP-A to type II cells occurred via the receptor protein, P63, presumably by increasing receptor density on the plasma membrane (3). To directly measure changes in cell surface levels of P63, the antibody to P63 was labeled with 125I, and binding studies were performed using untreated cells or 125I-labeled nonimmune IgG as controls. Type II cells plated on Transwell membranes were incubated with the secretagogue cAMP (0.1 mM, 37°C). The cells were placed in the cold (4°C), and 125I-SP-A (0.5 μg/ml) or 125I-antibodies were added. After 1 h, the cells were harvested and analyzed. As shown previously (3), cAMP (0.1 mM) treatment significantly increased 125I-SP-A binding by 67 ± 5% (Fig. 6, left). With secretagogue exposure, 125I-P63 antibody cell association was enhanced to the same extent as SP-A (69 ± 12% over control values; Fig. 6, middle). The inhibitor of PI3-kinase activity, LY-294002, was used to test whether the secretagogue-mediated process occurred through this pathway. Thus the cells were pretreated with LY-294002 (10 μM, 37°C), and binding studies were performed (4°C). As seen in Fig. 6, the enhanced binding of 125I-SP-A by cAMP is blocked by pretreatment with LY-294002 as was the cell association of iodinated anti-P63 antibody. Cell association of labeled nonimmune IgG did not change under any conditions (Fig. 6, right).
Fig. 6.
cAMP-stimulated increase in binding of SP-A or anti-P63 Ab is blocked by LY-294002 treatment. Type II cells plated on Transwell membranes were incubated without (no LY-294002) or with [+ LY-294002 (10 μM)] for 15 min followed by cAMP (0.1 mM) or no addition for 25 min at room temperature. The cells were placed in the cold (4°C), and 125I-SP-A (0.5 μg/ml), 125I-anti-P63 Ab (1.5 μg/ml), or 125I-IgG (1.5 μg/ml) were added for 1 h. The cells were harvested with 2 N of NaOH and counted. Data are means ± SE of triplicate dishes in 3 experiments and expressed as %control. Control values for SP-A, P63 Ab, and nonimmune IgG were 56 ± 13, 14 ± 3, and 22 ± 6 ng/mg cell protein, respectively. *Significant difference from all other samples. P < 0.05; n = 3.
SP-A.
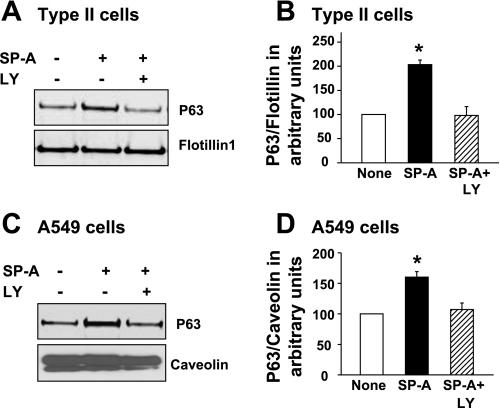
To establish that the PI3-kinase pathway also was active in the intracellular transport of P63 protein due to SP-A exposure, LY-294002 was used to inhibit the kinase activity in type II and A549 cells. Following treatment, the plasma membranes were isolated by sucrose density centrifugation, and P63 protein content was determined by densitometric analysis of Western blots. Blocking activation of PI3-kinase prevented the SP-A-induced increase in the amount of P63 protein in plasma membranes from both type II cells (Fig. 7, A and B) and A549 cells (Fig. 7, C and D).
Fig. 7.
Blocking the PI3-kinase pathway prevents PM enrichment of P63. Type II cells (A and B) or A549 cells (C and D) were incubated without additions (None) or with SP-A (1 μg/ml) without or with LY-294002 (LY; 10 μM) for either 2 h (type II cells) or 3 h (A549 cells). The cells were harvested, and PM were isolated. Representative Western blots (A and C) are shown (5 μg total membrane protein/lane). B and D: densitometric analysis of total membrane P63 protein. Data are means ± SE, n = 4 (type II cells) or n = 3 (A549 cells). *Significantly different from both None and SP-A + LY, P < 0.05.
SP-A and cAMP Exposure Causes Activation of the Ser/Thr Kinase, Akt
To gain further insight into the signaling mechanisms involved in P63 transport, we examined PI3-kinase downstream target kinases, PKC and Akt (PKB; for review, Ref. 8). We tested the SP-A-mediated processes that were dependent on P63 as defined by the ability of antibody to P63 to block either secretagogue-stimulated uptake of SP-A-liposome complexes (Fig. 1) or SP-A-inhibited surfactant secretion (22). Chelerythrine chloride (2 μM), a pan-PKC inhibitor that acts on the catalytic domain, had no significant effect (100.0 ± 8.0% of maximum, mean ± SE, n = 3) on the cAMP-induced uptake of SP-A-containing liposomes into type II cells, as shown in Fig. 8A. With regard to surfactant secretion, chelerythrine chloride (2 μM) slightly reduced (by 18%) the ATP-stimulated PC secretion from type II cells (Fig. 8B). Some effect was expected as ATP activates PKC to induce surfactant exocytosis. However, chelerythrine chloride did not reverse the inhibitory effect of SP-A on ATP-enhanced surfactant release (Fig. 8B).
Fig. 8.
The PKC inhibitor, chelerythrine chloride (Chel.), does not affect SP-A/cAMP enhancement of PC liposome uptake (A) or SP-A inhibition of ATP-stimulated PC secretion (B). A: type II cells plated on Transwell membranes were incubated without chelerythrine chloride (No Chel.) or with Chel. (2 μM) for 15 min and without or with cAMP (0.1 mM) for 1 h. 3H-DPPC-labeled liposomes (45 μg PC/ml) were added without (Liposomes) or with SP-A at 5 μg/ml (Liposomes + SP-A) for 2 h. The cells were harvested with trypsin. Data are means ± SE of duplicate or triplicate dishes in 3 experiments and expressed as percentage of maximum (Liposomes + SP-A + cAMP). Maximum liposome PC uptake (Liposomes + SP-A + cAMP) was 3.7 ± 0.3 μg PC/mg cell protein, n = 3. a, Significant difference from Liposomes alone, no additions. P < 0.05; n = 3. B: type II cells were incubated with [3H]choline overnight to label PC. Cells were washed and incubated without or with 1 mM ATP. Chel. (2 μM) and SP-A (0.2 μg/ml) were added at 30 and 15 min, respectively, before the addition of ATP. Secretion was measured over 1 h. Data are means ± SE of duplicate or triplicate samples from 3 experiments expressed as percentage of maximum (ATP alone). Control and maximum (ATP-stimulated) PC secretion values were 2.6 ± 0.5 and 7.4 ± 1.0%, respectively (mean ± SE, n = 3). a, Significant difference from Control; b, significant difference from ATP, no Chel.; c, significant difference from ATP. P < 0.05; n = 3.
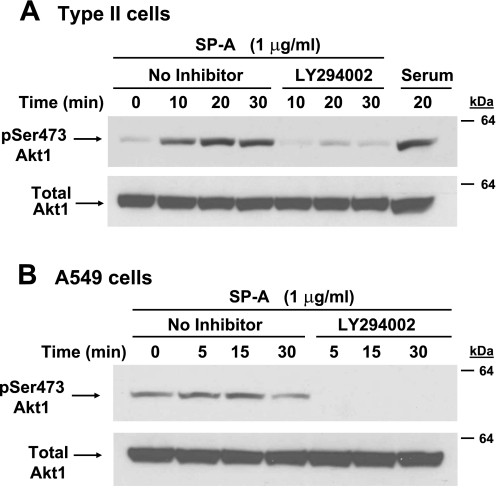
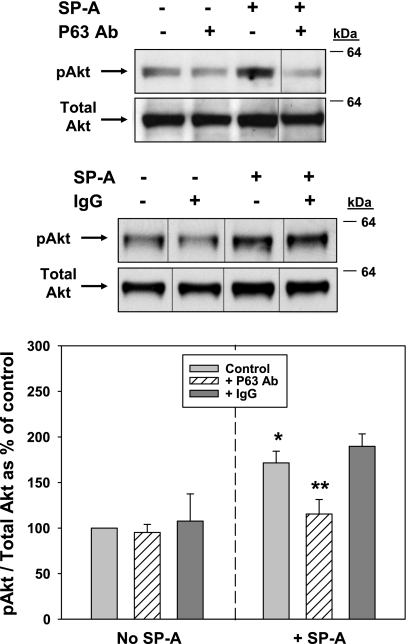
Since the PKC inhibitor did not affect the biological activity of SP-A, we turned our attention to Akt. It is well-known that phosphorylation of Akt on serine residue 473 leads to its activation. Therefore, type II cells (Fig. 9A) and A549 cells (Fig. 9B) were treated with 1 μg/ml SP-A, and cell lysates were subjected to Western blot analysis using the anti-pSer473 Akt antibody. Antibody to Akt protein was used for normalization. Both cell types tested demonstrated a rapid and robust increase in Akt phosphorylation within 15–20 min of SP-A exposure (Fig. 9, A and B). Stimulation of PI3-kinase activity is immediately upstream of Akt activation. The LY-294002 compound specifically blocks the activation of PI3-kinase and thus inhibits the phosphorylation of Akt (60). LY-294002 (10 μM) added to type II cells (Fig. 9A) or A549 cells (Fig. 9B) before SP-A exposure effectively inhibited the activation of Akt within 5–10 min. The ability to stimulate Akt was dependent on SP-A/P63 interactions as the addition of antibody to P63 in competition studies prevented SP-A-induced Akt phosphorylation, whereas the same concentration of nonimmune IgG had no effect (Fig. 10).
Fig. 9.
Incubation of cells with SP-A activates the PI3-kinase-Akt pathway. A and B: serum-starved (24 h) type II cells (A) or A549 cells (B) were treated without (No Inhibitor) or with LY-294002 (10 μM) for 45 min and then incubated with SP-A (1 μg/ml) for 0–30 min. The cells were lysed, and whole cell lysates (25 μg/lane) were analyzed by Western blot using Ab to pSer473 Akt (top). Membranes were stripped and reprobed with Akt Ab (Total Akt1; bottom). Incubation with 10% serum for 20 min served as the positive control. This experiment was repeated 3 times for each cell type with similar results.
Fig. 10.
Activation of Akt by SP-A is inhibited by Ab to P63. Type II cells were serum-starved (4 h) and either were not (Control) or were preincubated with Ab against P63 (25 μg protein/ml, top set of gels) or nonimmune IgG (25 μg protein/ml, bottom set of gels) for 15 min before the addition of SP-A (1 μg/ml, 20 min). The cells were lysed and analyzed by Western blot as in Fig. 9. Samples from the same gel were cut as indicated and rearranged for clarity. Graph shows quantitation of gels in AU expressed as a percentage of control, no additions (mean ± SE, n = 3–8). *Significant difference from No SP-A Control; **significant difference from + SP-A control, P < 0.05.
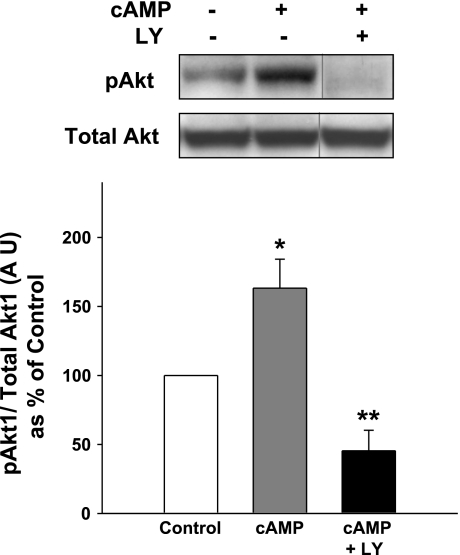
Next, the effect of cAMP on Akt phosphorylation in type II cells plated on Transwell membranes was examined. Akt was partially activated in the pneumocytes under these culture conditions, but addition of cAMP further increased Akt phosphorylation by ∼1.7-fold, as shown in Fig. 11. The activation of Akt was substantially inhibited by a prior exposure of the cells to LY-294002.
Fig. 11.
Incubation of type II cells on Transwell membranes with cAMP activates the PI3-kinase-Akt pathway. Serum-starved (5 h) type II cells were not (Control) or were preincubated with LY (10 μM). Some samples then were incubated with cAMP (cAMP + LY; 0.1 mM) for 10 min. The cells were lysed, and whole cell lysates (25 μg/lane) were analyzed by Western blot using pSer473 Akt Ab (pAkt; top). Membranes were stripped and reprobed with Akt Ab (Total Akt1; bottom). Samples from the same gel were cut as indicated and rearranged for clarity. Graph shows quantitation of gels in AU expressed as a percentage of control, no additions (mean ± SE, n = 4). *Significant difference from Control; **significant difference from No SP-A Control and cAMP, P < 0.05.
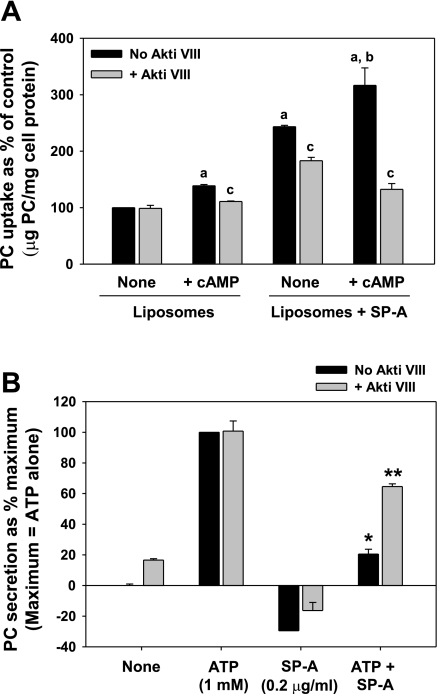
The Akt inhibitor, Akt Inhibitor VIII (Akti VIII), is a pleckstrin homology domain-specific selective inhibitor of Akt activity with no effect against PKC. Preincubation of type II cells with Akti VIII served to produce a strikingly similar inhibition of SP-A biological activity as that caused by LY-294002 exposure. Again, the predominant effect was on the inhibition of SP-A-stimulated liposome uptake under both basal and cAMP-stimulated conditions (Fig. 12A). Furthermore, the ability of SP-A to block ATP-enhanced secretion was reversed with Akti VIII (Fig. 12B). Like LY-294002, Akti VIII had no effect on ATP-stimulated secretion. Thus the data indicate an important role for the PI3-kinase-Akt pathway in the trafficking of P63 on stimulation.
Fig. 12.
The Akt inhibitor, Akt Inhibitor VIII (Akti VIII), blocks the biological activity of SP-A on PC liposome uptake (A) and type II cell secretion (B). A: type II cells plated on Transwell membranes were incubated without Akti VIII (No Akti VIII) or with Akti VIII (10 μM) for 15 min with or without cAMP (0.1 mM) for 1 h. 3H-DPPC-labeled liposomes (45 μg PC/ml) were added without (Liposomes) or with SP-A at 5 μg/ml (Liposomes + SP-A) for 2 h. The cells were harvested with trypsin. Data are means ± SE of duplicate or triplicate dishes in 3–6 experiments. None, no cAMP; a, significant difference from Liposomes alone, no additions; b, significant difference from Liposomes + SP-A, None (no cAMP); c, significant difference from no Akti VIII. P < 0.05; n = 3–6. B: type II cells were incubated with [3H]choline overnight to label PC. Cells were washed and incubated without or with 1 mM ATP. Akti VIII (10 μM) and SP-A (0.2 μg/ml) were added at 30 and 15 min, respectively, before the addition of ATP. Secretion was measured over 1 h. Data are means ± SE of duplicate or triplicate samples from 3 experiments. *Significant difference from ATP; **significant difference from ATP + SP-A, no Akti VIII. P < 0.05; n = 3.
DISCUSSION
Evidence continues to accumulate in support of the role of P63 on pneumocytes as a receptor for SP-A (3). In earlier work, P63 was shown to be critically involved in the ability of SP-A to block phospholipid secretion from type II cells (3, 22). Here, we show that a second biological activity of SP-A, the SP-A-stimulated uptake of surfactant liposomes into type II cells, also is mediated by P63. The presence of competing P63 antibody blocked lipid uptake under both basal and secretagogue-stimulated conditions. Secretagogue-mediated enhancement of SP-A binding to type II cells is well-documented (3, 9, 10), and our recent data implicate a role for P63 protein (3). The current work directly demonstrates the enrichment of P63 on the cell surface on exposure to cAMP using iodinated P63 antibody. The ligand of P63, SP-A, also is shown to stimulate the plasma membrane expression of P63. Finally, evidence is presented that the PI3-kinase-Akt signaling pathway plays a role in the intracellular transport of P63 to the plasma membrane.
To explore mechanisms involved in the plasma membrane enrichment of P63 by stimuli, our first goal was to determine whether an increase in P63 synthesis was responsible. SP-A has been shown to enhance transcription of SP-B through the PI3-kinase pathway (46). Western blot analysis of whole cell lysates from A549 and type II cells treated with SP-A did not show a difference in band intensity, indicating that SP-A treatment did not alter the total expression levels of P63 in these cell types. In an earlier study of SP-A receptors on pneumocytes, we determined that blocking protein synthesis with cycloheximide also had no effect on the increase in SP-A binding stimulated by secretagogues (9). Furthermore, the response to stimuli is rapid. The increase in secretagogue-dependent SP-A binding to type II cells occurs within 20 min (10). Thus there is no evidence that the enhancement of P63 synthesis is responsible for the increased cell surface P63 expression.
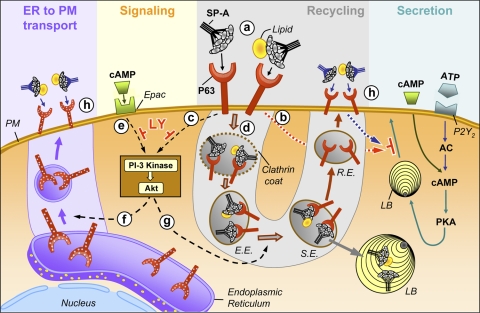
Figure 13 depicts our working model for the regulation of P63 on the surface of type II pneumocytes. At “a,” SP-A and SP-A/lipid bind to P63 on the surface of type II cells. As a result, the release of surfactant from lamellar bodies at the cell surface is blocked (“b”), and the PI3-kinase signaling pathway is activated (“c”). The SP-A-lipid-P63 complex is internalized via the classic clathrin-mediated endocytosis pathway into early endosomes (“d”). The latter process provides the mechanism for the SP-A-mediated enhancement of surfactant lipid uptake. The ligand-receptor complex moves to a sorting endosome where SP-A/lipid continues to the lamellar body and P63 enters the recycling endosome (RE) pathway for transport back to the cell surface. Activation of Akt by SP-A-P63 (“c”) or cAMP-exchange protein directly activated by cAMP (Epac) interactions (“e”) results in the forward transport of P63 from the ER to the plasma membrane (“f”) and the stimulation of the recycling pathway (“g”). This process would serve to regulate levels of P63 on the cell surface (“h”) to maintain the biological activity of SP-A.
Fig. 13.
Model for the turnover of P63 on the surface of type II cells. SP-A binds to P63 on the PM of type II cells (“a”) resulting in the: blockage of basal or stimulated surfactant secretion (“b”); initiation of a signaling cascade involving PI3-kinase and Akt (“c”); and internalization of the SP-A-P63 complex via a clathrin-mediated pathway (“d”). PI3-kinase is also stimulated by cAMP interaction with Epac, the exchange protein directly activated by cAMP (“e”). Activation of Akt results in the forward transport of P63 in the endoplasmic reticulum (ER) to the PM (“f”) and the stimulation of P63 recycling (“g”). The end result of the latter 2 pathways is the presence of a constant or elevated level of P63 on the cell surface necessary to maintain SP-A-mediated lipid uptake (“h”) and block further lamellar body (LB) exocytosis (“h”). E.E., early endosome; S.E., sorting endosome; R.E., recycling endosome; AC, adenyl cyclase.
It is now established that SP-A is internalized via a clathrin-coated pit pathway (40), and earlier work demonstrated colocalization of SP-A and P63 in the early endosome intracellular compartment (22). The subsequent fate of SP-A is not completely clear. In studies using an intact lung, SP-A instilled in the trachea is subsequently recovered from lamellar bodies, a route that would probably involve passage through a sorting endosome, as shown in our model (17, 19, 24). On the other hand, studies using type II cells in culture found that endocytosed SP-A was recycled to the cell surface (55). It is quite possible that both pathways are used by SP-A. P63 likely dissociates from SP-A, probably in the sorting endosome compartment as is the case with other ligand-receptor pathways since P63 does not strongly colocalize with lamellar body membranes (22) but was found with Rab11, a marker for the RE (S. R. Bates and N. Gupta, unpublished observations).
We evaluated the effects of SP-A on the cell surface expression of its receptor, P63, since other protein ligands have been shown to have the ability to regulate their respective receptors (14). Importantly, SP-A can cause translocation of intracellular proteins to the cell surface. For example, SP-A exposure augments the cell surface expression of C1qR protein on the monocytic cell line, U937 (33), and both the scavenger receptor A (31) and the mannose receptor (5, 26) on alveolar macrophages. Immunofluorescent microscopy shown here using nonpermeabilized type II cells demonstrated enhanced cell surface distribution of P63 in SP-A-exposed cells as documented by an increase in colocalization of P63 with wheat germ agglutinin, a plasma membrane marker. Furthermore, plasma membranes purified from SP-A-treated type II cells and A549 cells showed enrichment in P63. Finally, biotinylation of cell surface proteins determined that P63 was on the cell surface and confirmed that the levels of P63 were increased twofold after SP-A exposure.
An additional stimulus, exposure to cAMP, also resulted in an increase in P63 protein cell surface density. cAMP has been shown to increase the levels of SP-A binding to type II cells in this work as well as elsewhere (9), an effect that was due to binding to P63 as the enhanced binding of SP-A was blocked by antibody to P63 (3). cAMP also increased the cell association of anti-P63 antibody to the cells indicative of an increase in P63 on the plasma membrane. The effect of the other secretagogue used in this study, ATP, on pneumocytes is limited to the enhancement of lipid secretion with little effect on SP-A binding (9). The effects of cAMP are mediated by two pathways, the cAMP-dependent PKA and the relatively newly described cAMP receptor Epac (15). The cAMP-stimulated increase in the levels of P63 on the plasma membrane may occur through the latter pathway since the effect of cAMP on P63 enrichment in the plasma membrane (as measured by increased cell association of 125I-P63 antibody and 125I-SP-A to pneumocytes) is blocked by an inhibitor of PI3-kinase. It is well-known that cAMP stimulates PC secretion through PKA, but surfactant secretion was unaffected by the inhibitors of PI3-kinase.
In light of the fact that cAMP has been shown to activate the PI3-kinase-Akt pathway through Epac (36) and that White and Strayer (53) had previously demonstrated that PI3-kinase activity rapidly increases on incubation of type II cells with SP-A, we explored the possibility that the PI3-kinase signaling pathway played a role in the cAMP/SP-A-dependent enrichment of P63 on the cell surface. The contribution of the PI3-kinase signaling pathway was assessed using the highly specific PI3-kinase inhibitors LY-294002 and wortmannin. Blocking the PI3-kinase phosphorylation process with these agents interfered with both the SP-A/P63- and cAMP-stimulated phenomena. The cAMP-stimulated surface increase in P63 was prevented by LY-294002 as measured by the reduction in both SP-A binding and P63 antibody association to type II cells. Furthermore, the ability of SP-A to increase the expression of P63 protein in plasma membranes of both type II cells and A549 cells also was blocked by the inhibitory agent, LY-24002. Presumably through prevention of P63 recovery on the plasma membrane, LY-294002 or wortmannin preexposure reversed the biological activity of SP-A to stimulate liposome uptake into type II cells. In addition, inhibitor treatment diminished the inhibitory effect of SP-A on surfactant secretion. Both processes have been shown to be dependent on SP-A-receptor interactions (for review, Ref. 35).
Phosphoinositide-dependent protein kinase-1 (PDK1) is downstream from PI3-kinase and activates both PKC and Akt kinase via phosphorylation (8). A survey of the amino acid sequences of P63 homologs (20) determined that the cytosolic domain of P63 contains several possible PKC phosphorylation motifs (51). However, the pan-PKC inhibitor, chelerythrine chloride, had no effect on SP-A-mediated regulation of surfactant turnover. On the other hand, White and Strayer (54) previously demonstrated that treatment of type II and H441 cells with bovine SP-A resulted in phosphorylation of Akt. The present work showed that exposure to human SP-A results in the activation of Akt in type II and A549 cells that was inhibited by a prior exposure to the PI3-kinase inhibitor LY-294002. In addition, cAMP activated Akt in type II cells on Transwell membranes, an effect that was inhibited by LY-294002. Studies in other systems also have reported similar effects of cAMP on Akt activation (27, 52). A role for Akt in the intracellular movement of P63 was confirmed using the potent inhibitor of Akt kinase activity, Akt Inhibitor VIII. Preincubation of pneumocytes with this compound blocked SP-A biological activity on liposome uptake and surfactant secretion to a similar extent as was seen with pretreatment with LY-294002, the PI3-kinase inhibitor. The downstream targets of Akt that are involved in P63 subcellular trafficking remain to be identified but should affect both the forward transport of P63 from the ER to the plasma membrane (Fig. 13, “f”) and the recycling pathway (Fig. 13, “g”).
The interaction between the two proteins blocks both the basal secretion of surfactant from lamellar bodies and the enhanced secretion that results from the stimulation of PKA by the secretagogues ATP or cAMP (Fig. 13, “b”). The mechanism involved in this process remains unclear but may involve SP-A mediation of the opening of the lamellar body fusion pore with the plasma membrane or alterations in the cytoskeleton (4). Inhibitors of the PI3-kinase pathways partially interfered with the ability of SP-A to block ATP- and cAMP-stimulated secretion but demonstrated differences in potency in the partial reversal of the SP-A effect. Variables that influenced results included cell substrate, secretagogue used, concentration of SP-A, and concentration and type of PI3-kinase inhibitor used. Possible explanations for the lack of complete reversal of SP-A-dependent inhibition of PC secretion by PI3-kinase inhibitors are that: 1) low levels of P63 on the cell surface are sufficient for SP-A to block secretion; 2) once surfactant secretion is blocked, replenishment of P63 on the cell surface is only partially necessary to retain the inhibitory effect of SP-A; 3) reversal requires longer than the 1-h time period of the experiment; or 4) mechanisms other than the PI3-kinase-Akt pathway may participate.
The concentrations of SP-A necessary to alter lipid uptake and secretion from type II cells differ considerably. However, both processes depend on the interaction of SP-A with P63 since antibody to P63 interferes with these biological effects of SP-A, as demonstrated here and elsewhere (3, 22). For example, as little as 0.1 μg/ml SP-A will reduce ATP-stimulated surfactant secretion, whereas 5–10 μg of SP-A/ml are necessary for the enhancement of liposome uptake by type II pneumocytes (1). The latter may be explained by the assumption that SP-A associates with the entire surface of the 100-nm phospholipid liposome. Thus there are steric considerations, and only a fraction of the SP-A would be positioned to bind to both the liposome and the P63 on the cell plasma membrane. Hence, with SP-A-lipid complexes, the concentration of SP-A must be considerably increased to obtain a comparable amount of SP-A binding to P63 on the cell surface of type II cells as would occur in the lipid-free conditions used in the experiments that measured the regulation of surfactant secretion by SP-A.
The physiological significance of interaction of SP-A and P63 in the intact lung would be as follows. Binding of SP-A to P63 results in three outcomes: 1) the inhibition of surfactant secretion; 2) the promotion of a clathrin-mediated uptake of SP-A and its associated surfactant lipid; and 3) the initiation of a PI3-kinase signaling cascade resulting in the restoration or enrichment of P63 receptor levels at the plasma membrane. We hypothesize that these three processes are used by the type II cells in the lung to control the amount of extracellular surfactant. SP-A is constantly being secreted and endocytosed. When excess SP-A is present and bound to receptors or a physiological stimulus such a stretching occurs, the signaling pathway is launched. With internalization of the SP-A-P63 complex, recovery of levels of P63 receptors on the surface is necessary. The receptors are recruited from the ER or from recycling endosomes to the cell surface to facilitate the further uptake of SP-A and lipid and inhibit secretion of more surfactant with SP-A. When levels of SP-A are low or there is no other stimulation, P63 remains in the ER, recycling is low, inhibition of secretion diminishes, and lipid uptake is lessened resulting in greater release of surfactant and expansion of the levels of extracellular SP-A. Since SP-A is associated with surfactant lipid, as it presumably is in the normal alveolus, regulation of surfactant lipid would predominantly follow that of SP-A. Thus the physiological role of SP-A is to serve as a mediator for the regulation of surfactant levels in the alveolar space.
To conclude, a growing body of evidence indicates that receptor-mediated SP-A binding to type II cells via clathrin-coated pits makes a major contribution to the clearance of surfactant from the alveolar space of the lung (2). Thus the need emerges to characterize the SP-A receptor that would control the biological activity of this surfactant protein. Several candidate SP-A binding proteins have been reported earlier, but their role in lipid turnover is unclear (for review, Ref. 28). Integrating our new findings with previous data provides continuing evidence that P63 is a receptor for SP-A. SP-A-P63 interactions were shown to affect the biological processes of SP-A, specifically the inhibition of surfactant release (3, 22) and the augmentation of surfactant lipid uptake. Two stimuli triggered the expression of P63 on the cell surface, exposure to SP-A and incubation with secretagogues (3, 9, 22). Whether both stimuli use identical pathways remains to be determined, although each was inhibited by blocking the PI3-kinase signaling pathway. Evidence indicated that Akt was the downstream kinase responsible, although the translocation mechanism is not clear as yet. Considered together, these results espouse the concept that the transmembrane protein P63 (CKAP4) is an SP-A receptor. In addition, our findings support a role for the PI3-kinase-Akt system in the transport process that retains or increases the concentration of P63 on the plasma membrane. Altering the cell surface expression of P63 remains an attractive option for controlling surfactant homeostasis.
GRANTS
This research was supported by the National Heart, Lung, and Blood Institute Grant HL-19737.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Ling Gao and Daniel S. Gonder for excellent technical support.
Preliminary results of portions of this work were presented at the 2006, 2007, and 2008 American Thoracic Society meetings in San Diego, CA, San Francisco, CA, and Toronto, Canada, respectively, as well as the 2009 and 2010 Experimental Biology Meetings in New Orleans, Los Angeles, and Anaheim, CA, respectively.
REFERENCES
- 1.Bates SR, Dodia C, Fisher AB. Surfactant protein A regulates uptake of pulmonary surfactant by lung type II cells on microporous membranes. Am J Physiol Lung Cell Mol Physiol 267: L753–L760, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Bates SR, Dodia C, Tao JQ, Fisher AB. Surfactant protein-A plays an important role in lung surfactant clearance: evidence using the surfactant protein-A gene-targeted mouse. Am J Physiol Lung Cell Mol Physiol 294: L325–L333, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bates SR, Kazi AS, Tao JQ, Yu KJ, Gonder DS, Feinstein SI, Fisher AB. Role of P63 (CKAP4) in binding of surfactant protein-A to type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 295: L658–L669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates SR, Tao JQ, Notarfrancesco K, DeBolt K, Shuman H, Fisher AB. Effect of surfactant protein A on granular pneumocyte surfactant secretion in vitro. Am J Physiol Lung Cell Mol Physiol 285: L1055–L1065, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Beharka AA, Gaynor CD, Kang BK, Voelker DR, McCormack FX, Schlesinger LS. Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J Immunol 169: 3565–3573, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959 [DOI] [PubMed] [Google Scholar]
- 7.Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of proteins utilizing the principle of protein dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 8.Cantley LC. The phosphoinositide 3-kinase pathway. Science 296: 1655–1657, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Bates SR, Fisher AB. Secretagogues increase the expression of surfactant protein A receptors on lung type II cells. J Biol Chem 271: 25277–25283, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Fisher AB, Strayer DS, Bates SR. Mechanism for secretagogue-induced surfactant protein A binding to lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 275: L38–L46, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Chinoy MR, Dodia C, Fisher AB. Increased surfactant internalization by rat type II cells cultured on microporous membranes. Am J Physiol Lung Cell Mol Physiol 264: L300–L307, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Chroneos ZC, Abdolrasulnia R, Whitsett JA, Rice WR, Shepherd VL. Purification of a cell-surface receptor for surfactant protein A. J Biol Chem 271: 16375–16383, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Conrads TP, Tocci GM, Hood BL, Zhang CO, Guo L, Koch KR, Michejda CJ, Veenstra TD, Keay SK. CKAP4/p63 is a receptor for the frizzled-8 protein-related antiproliferative factor from interstitial cystitis patients. J Biol Chem 281: 37836–37843, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Corps AN, Brown KD. Ligand-receptor interactions involved in the stimulation of Swiss 3T3 fibroblasts by insulin-like growth factors and insulin. Biochem J 252: 119–125, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.deRooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396: 474–477, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Dobbs LG, Gonzalez R, Williams MC. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis 134: 141–145, 1986 [DOI] [PubMed] [Google Scholar]
- 17.Fisher AB, Dodia C, Chander A. Alveolar uptake of lipid and protein components of surfactant. Am J Physiol Lung Cell Mol Physiol 261: L334–L340, 1991 [DOI] [PubMed] [Google Scholar]
- 18.Fisher AB, Dodia C, Chander A, Kleinzeller A. Transport of choline by plasma membrane vesicles from lung-derived epithelial cells. Am J Physiol Cell Physiol 263: C1250–C1257, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Fisher AB, Dodia C, Ruckert P, Tao JQ, Bates SR. Pathway to lamellar bodies for surfactant protein A. Am J Physiol Lung Cell Mol Physiol 299: L51–L58, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 31: 3784–3788, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill DJ, Teo H, Sun J, Perisic O, Veprintsev DB, Vallis Y, Emr SD, Williams RL. Structural studies of phosphoinositide 3-kinase-dependent traffic to multivesicular bodies. Biochem Soc Symp 74: 47–57, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Gupta N, Manevich Y, Kazi AS, Tao JQ, Fisher AB, Bates SR. Identification and characterization of p63 (CKAP4/ERGIC-63/CLIMP-63), a surfactant protein A binding protein, on type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 291: L436–L446, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Hawgood S, Benson BJ, Hamilton RL., Jr Effects of surfactant-associated proteins and calcium ions on the structure and surface activity of lung surfactant lipids. Biochemistry 24: 184–190, 1985 [DOI] [PubMed] [Google Scholar]
- 24.Ikegami M, Ueda T, Purtell J, Woods E, Jobe A. Surfactant protein A labeling kinetics in newborn and adult rabbits. Am J Respir Cell Mol Biol 10: 413–418, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Jain D, Dodia C, Bates SR, Hawgood S, Poulain FR, Fisher AB. SP-A is necessary for increased clearance of alveolar DPPC with hyperventilation or secretagogues. Am J Physiol Lung Cell Mol Physiol 284: L759–L765, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Kabha K, Schmegner J, Keisari Y, Parolis H, Schlepper-Schaeffer J, Ofek I. SP-A enhances phagocytosis of Klebsiella by interaction with capsular polysaccharides and alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 272: L344–L352, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Kagawa T, Varticovski L, Sai Y, Arias IM. Mechanism by which cAMP activates PI3-kinase and increases bile acid secretion in WIF-B9 cells. Am J Physiol Cell Physiol 283: C1655–C1666, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, Bernal AL, Reid KB, Madan T, Chakraborty T. Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol 43: 1293–1315, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Kresch MJ, Christian C, Lu H. Isolation and partial characterization of a receptor to surfactant protein A expressed by rat type II pneumocytes. Am J Respir Cell Mol Biol 19: 216–225, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Kuroki Y, Akino T. Pulmonary surfactant protein A (SP-A) specifically binds dipalmitoylphosphatidylcholine. J Biol Chem 266: 3068–3073, 1991 [PubMed] [Google Scholar]
- 31.Kuronuma K, Sano H, Kato K, Kudo K, Hyakushima N, Yokota S, Takahashi H, Fujii N, Suzuki H, Kodama T, Abe S, Kuroki Y. Pulmonary surfactant protein A augments the phagocytosis of Streptococcus pneumoniae by alveolar macrophages through a casein kinase 2-dependent increase of cell surface localization of scavenger receptor A. J Biol Chem 279: 21421–21430, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970 [DOI] [PubMed] [Google Scholar]
- 33.Malhotra R, Haurum J, Thiel S, Sim RB. Interaction of C1q receptor with lung surfactant protein A. Eur J Immunol 22: 1437–1445, 1992 [DOI] [PubMed] [Google Scholar]
- 34.Martin TF. Phosphoinositides as spatial regulators of membrane traffic. Curr Opin Neurobiol 7: 331–338, 1997 [DOI] [PubMed] [Google Scholar]
- 35.McCormack FX. Structure, processing and properties of surfactant protein A. Biochim Biophys Acta 1408: 109–131, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Mei FC, Qiao J, Tsygankova OM, Meinkoth JL, Quilliam LA, Cheng X. Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem 277: 11497–11504, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Poulain FR, Allen L, Williams MC, Hamilton RL, Hawgood S. Effect of surfactant apoproteins on liposome structure: implications for tubular myelin structure. Am J Physiol Lung Cell Mol Physiol 262: L730–L739, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Razzaq TM, Bass R, Vines DJ, Werner F, Whawell SA, Ellis V. Functional regulation of tissue plasminogen activator on the surface of vascular smooth muscle cells by the type-II transmembrane protein p63 (CKAP4). J Biol Chem 278: 42679–42685, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Rooney SA. Regulation of surfactant secretion. In: Lung Surfactant: Cellular and Molecular Processing, edited by Rooney SA, Landes RG. Austin, TX: Landes Bioscience, 1998, p. 139–155 [Google Scholar]
- 40.Ryan RM, Morris RE, Rice WR, Ciraolo G, Whitsett JA. Binding and uptake of pulmonary surfactant protein (SP-A) by pulmonary type II epithelial cells. J Histochem Cytochem 37: 429–440, 1989 [DOI] [PubMed] [Google Scholar]
- 41.Schweizer A, Ericsson M, Bachi T, Griffiths G, Hauri HP. Characterization of a novel 63 kDa membrane protein. Implications for the organization of the ER-to-Golgi pathway. J Cell Sci 104: 671–683, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Schweizer A, Fransen JA, Matter K, Kreis TE, Ginsel L, Hauri HP. Identification of an intermediate compartment involved in protein transport from endoplasmic reticulum to Golgi apparatus. Eur J Cell Biol 53: 185–196, 1990 [PubMed] [Google Scholar]
- 43.Schweizer A, Rohrer J, Hauri HP, Kornfeld S. Retention of p63 in an ER-Golgi intermediate compartment depends on the presence of all three of its domains and on its ability to form oligomers. J Cell Biol 126: 25–39, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schweizer A, Rohrer J, Jeno P, DeMaio A, Buchman TG, Hauri HP. A reversibly palmitoylated resident protein (p63) of an ER-Golgi intermediate compartment is related to a circulatory shock resuscitation protein. J Cell Sci 104: 685–694, 1993 [DOI] [PubMed] [Google Scholar]
- 45.Strayer DS. Identification of a cell membrane protein that binds alveolar surfactant. Am J Pathol 138: 1085–1095, 1991 [PMC free article] [PubMed] [Google Scholar]
- 46.Strayer DS, Korutla L. Activation of surfactant protein-B transcription: signaling through the SP-A receptor utilizing the PI3 kinase pathway. J Cell Physiol 184: 229–238, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Strayer DS, Yang S, Jerng HH. Surfactant protein A-binding proteins. Characterization and structures. J Biol Chem 268: 18679–18684, 1993 [PubMed] [Google Scholar]
- 48.Sullivan LC, Daniels CB, Phillips ID, Orgeig S, Whitsett JA. Conservation of surfactant protein A: evidence for a single origin for vertebrate pulmonary surfactant. J Mol Evol 46: 131–138, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tran H, Pankov R, Tran SD, Hampton B, Burgess WH, Yamada KM. Integrin clustering induces kinectin accumulation. J Cell Sci 115: 2031–2040, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Vedrenne C, Klopfenstein DR, Hauri HP. Phosphorylation controls CLIMP-63-mediated anchoring of the endoplasmic reticulum to microtubules. Mol Biol Cell 16: 1928–1937, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Webster CR, Anwer MS. Role of the PI3K/PKB signaling pathway in cAMP-mediated translocation of rat liver Ntcp. Am J Physiol Gastrointest Liver Physiol 277: G1165–G1172, 1999 [DOI] [PubMed] [Google Scholar]
- 53.White MK, Strayer DS. Surfactant protein A regulates pulmonary surfactant secretion via activation of phosphatidylinositol 3-kinase in type II alveolar cells. Exp Cell Res 255: 67–76, 2000 [DOI] [PubMed] [Google Scholar]
- 54.White MK, Strayer DS. Survival signaling in type II pneumocytes activated by surfactant protein-A. Exp Cell Res 280: 270–279, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Wissel H, Lehfeldt A, Klein P, Muller T, Stevens PA. Endocytosed SP-A and surfactant lipids are sorted to different organelles in rat type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 281: L345–L360, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 5: 58–68, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Wright JR, Borchelt JD, Hawgood S. Lung surfactant apoprotein SP-A (26–36 kDa) binds with high affinity to isolated alveolar type II cells. Proc Natl Acad Sci USA 86: 5410–5414, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wright JR, Dobbs LG. Regulation of pulmonary surfactant secretion and clearance. Annu Rev Physiol 53: 395–414, 1991 [DOI] [PubMed] [Google Scholar]
- 59.Wu YZ, Manevich Y, Baldwin JL, Dodia C, Yu K, Feinstein SI, Fisher AB. Interaction of surfactant protein A with peroxiredoxin 6 regulates phospholipase A2 activity. J Biol Chem 281: 7515–7525, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Yao R, Cooper GM. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science 267: 2003–2006, 1995 [DOI] [PubMed] [Google Scholar]