Abstract
Keratinocyte growth factor (KGF) has efficacy in several experimental models of lung injury; however, the mechanisms underlying KGF's protective effect remain incompletely understood. This study was undertaken to determine whether KGF augments barrier function in primary rat alveolar epithelial cells grown in culture, specifically whether KGF alters tight junction function via claudin expression. KGF significantly increased alveolar epithelial barrier function in culture as assessed by transepithelial electrical resistance (TER) and paracellular permeability. Fluorescence-activated cell sorting of freshly isolated type 1 (AT1) and type 2 (AT2) cells followed by quantitative real-time RT-PCR revealed that more than 97% of claudin mRNA transcripts in these cells were for claudins-3, -4, and -18. Using cultured AT2 cells, we then examined the effect of KGF on the protein levels of the claudins with the highest mRNA levels: -3, -4, -5, -7, -12, -15, and -18. KGF did not alter the levels of any of the claudins tested, nor of zona occludens-1 (ZO-1) or occludin. Moreover, localization of claudins-3, -4, -18, and ZO-1 was unchanged. KGF did induce a marked increase in the apical perijunctional F-actin ring. Actin depolymerization with cytochalasin D blocked the KGF-mediated increase in TER without significantly changing TER in control cells. Together, these data support a novel mechanism by which KGF enhances alveolar barrier function, modulation of the actin cytoskeleton. In addition, these data demonstrate the complete claudin expression profile for AT1 and AT2 cells and indicate that claudins-3, -4, and -18 are the primary claudins expressed in these cell types.
Keywords: perijunctional actin, actomyosin ring, acute respiratory distress syndrome, acute lung injury
disruption of the alveolar epithelial barrier is a hallmark of acute lung injury (59). Although alveolar barrier function relies on both endothelial and epithelial tight junctions, the epithelial barrier is much less permeable than the endothelial barrier (64). In fact, alveolar epithelial injury is sufficient for pulmonary edema formation, even in the presence of normal endothelial permeability (25). Furthermore, preservation of epithelial barrier function is a common mechanism by which keratinocyte growth factor (KGF) and other clinically relevant biological mediators provide protection in experimental lung injury (15, 18, 30, 38). However, despite the importance of epithelial integrity to outcomes for patients with clinical acute lung injury (34), the mechanisms of alveolar epithelial tight junction regulation remain incompletely understood.
Tight junctions ultimately determine epithelial paracellular permeability (43, 49), but many details about the nature of tight junctions between alveolar epithelial cells in health and disease are uncertain. For example, the specific tight junction proteins expressed by alveolar epithelial type 1 (AT1) and type 2 (AT2) cells have not been entirely elucidated (11, 12, 55), and whether AT1 and AT2 cells express similar junctional proteins has not been previously reported in detail. At baseline, multiple different cell-cell contacts exist in the alveolus, with AT1-AT1 and AT1-AT2 junctions being most abundant. During lung injury, AT2-AT2 junctions likely increase with proliferation of AT2 cells. With respect to paracellular permeability, the claudin family of tetraspan transmembrane proteins is foremost among tight junction constituents (51). Claudins demonstrate tissue-specific expression patterns that account for the different paracellular permeability characteristics of diverse epithelia (3). This is best exemplified in studies of the mouse nephron, where each claudin gene exhibits a unique pattern of expression along the nephron, and the segment-specific expression of different claudins regulates paracellular permeability properties (28). Previous work from our group has shown that a loss of claudins-3 and -4 in the alveolar epithelium impairs alveolar fluid clearance rates in mice and increases susceptibility to lung injury (65). Thus, a more complete understanding of the claudin contribution to paracelullar transport in the lung may have important clinical implications.
Considerable experimental data suggest that KGF may have clinical utility in preventing or ameliorating lung injury (5, 13, 32, 40, 53, 62, 67–69). Notably, recent data show that the protective effect of mesenchymal stem cells in experimental lung injury is attributable to KGF produced by the stem cells (30). KGF has pleiotropic effects in the lung (16, 58), and the mechanisms underlying KGF's protective effect remain incompletely understood. Although KGF is a potent mitogen for AT2 cells in vivo (50), this is unlikely to be the sole mechanism by which KGF attenuates lung injury (63). In fact, multiple non-mitogenic effects for KGF in the lung have been identified, including effects on ion transport (7, 48, 56). The effect of KGF on tight junction function and claudin protein expression has not been previously reported. In addition, KGF has been shown to modulate alveolar epithelial cell phenotype in vitro (8). Specifically, KGF appears to prevent freshly isolated AT2 cells from transdifferentiating toward a more AT1-like phenotype in culture (10, 23).
Therefore, the primary objective of this study was to further determine the mechanism for the protective effect of KGF on alveolar epithelial barrier function in primary alveolar epithelial cells grown in culture, specifically whether KGF alters tight junction function or claudin expression. Our hypothesis was that KGF treatment would augment barrier function through modulation of claudin expression. As claudins are the critical proteins regulating paracellular transport, a significant focus of this work was to determine which claudins are expressed in freshly isolated, purified populations of primary AT1 and AT2 cells and in cultured AT2 cells that have acquired a more AT1 cell-like phenotype. A second objective was to determine if cultured AT2 cells that adopt an AT1-like phenotype exhibit a different pattern of claudin expression or have different barrier properties from cells with a KGF-induced AT2-like phenotype. We show that KGF augments alveolar epithelial barrier tightness and that this effect is not dependent on cell proliferation. Contrary to our initial hypothesis, the barrier-enhancing effect of KGF is not due to alterations in claudin expression, but due to effects on the actin cytoskeleton. These data further show that AT1 and AT2 cells primarily express three claudins (-3, -4, and -18) and that changes in barrier tightness are not necessarily associated with changes in claudin expression in alveolar epithelial cells.
MATERIALS AND METHODS
Isolation of AT1 and AT2 cells by flow cytometry.
Animal protocols were approved by the local Institutional Animal Care and Use Committee and conform to National Institutes of Health animal use guidelines. Rats (150–200 g) were anesthetized with 4% isoflurane followed by an intraperitoneal injection of ketamine/xylazine (90/10 mg/kg body wt). Lungs were perfused with buffered saline containing heparin, removed en bloc, and lavaged with calcium-magnesium-free PBS containing 1 mM EGTA and 1 mM EDTA (pH 7.4) followed by RPMI containing HEPES. The lungs were instilled with elastase (Roche) for 20 min and minced. Resulting suspensions were filtered through membranes of decreasing porosity to obtain single cell suspensions. After filtration, cells were incubated with 50 μg/ml rat IgG (Sigma) and 50 μg/ml mouse IgG (Sigma) for 10 min at 4°C to block Fc receptor binding sites. Alexa 610 (Invitrogen)-conjugated anti-RT140, an antibody specific to AT1 cells, and anti-RT270, an antibody specific to AT2 cells (both kind gifts of Dr. Leland G. Dobbs, Univ. of California, San Francisco) were used to sort both cell types by flow cytometry as previously described (24). Alexa 488 anti-mouse IgG3 was used to visualize RT270. Differentially labeled rat AT1 and AT2 cells were sorted using FACS Aria (BD Biosciences), and results were analyzed using BD Diva version 6.1.3 (BD Biosciences). AT1 and AT2 cell purities were greater than 99% by immunofluorescence using anti-RT140 and -RT270.
Isolation and primary culture of rat type 2 alveolar epithelial cells.
For cell culture studies, alveolar epithelial type 2 cells were isolated from the lungs of pathogen-free male Sprague-Dawley rats as previously described (14, 22). Lungs were filtered and digested as above. Resulting suspensions were enriched for AT2 cells by differential adherence to IgG-coated plastic dishes. Unattached cells were collected and plated in DME-H21 supplemented with ciprofloxacin and 10% FBS at a density of 1.5 × 106 cells/cm2. Cell purity was >90% and cell viability was >95% (trypan blue exclusion). Cells were cultured for 5 days on 0.4-μm polycarbonate membrane Transwell permeable supports (Corning Costar). Cells in the treatment group were given media supplemented with 10 ng/ml KGF (Human Recombinant, Sigma) from the time of initial seeding consistent with previously published work (20). On day 4, serum-free media with or without KGF was added to both the apical and basal compartments.
Barrier function measurements.
Transepithelial electrical resistance (TER) of cells in medium cultured on permeable supports was measured using an ohmmeter (EVOM, World Precision Instruments) as previously described (18) (n = 9 biological replicates). To determine in vitro paracellular permeability to larger solutes, 10 μg/ml of the 0.5 kDa tracer pyranine (Sigma) was added to the apical compartment on day 5, and fluorescence was measured in a sample of medium from the basal compartment at 30 min at excitation and emission wavelengths of 485 and 527 nm, respectively (n = 7 biological replicates). Apparent permeability was calculated (37). Preliminary studies confirmed a linear correlation for pyranine flux between 0 and 2 h in alveolar epithelial cells grown with or without KGF (Supplemental Fig. 1; Supplemental material for this article is available online at the Journal website), consistent with previously published data for similar-sized molecules in primary rat alveolar epithelial cells (37). To investigate the role of the actin cytoskeleton in changes in barrier properties with KGF treatment, TER was measured after 15 min of treating cells with 0.1 μg/ml of the actin depolymerization agent cytochalasin D (Enzo Life Sciences) on day 5 (n = 4 biological replicates) (31, 47). For extracellular calcium depletion, TER was measured 15 min after changing the apical and basolateral chambers to serum-free media containing 1 mM EDTA and EGTA (54).
Determination of cell number.
To determine the effect of KGF on proliferation, cell number was determined as previously described (19). Isolated primary AT2 cells from rats (n = 4 biological replicates) were plated on 96-well tissue culture-treated plates at a seeding density identical to all Transwell experiments. A standard curve of increasing seeding density was also plated for each isolation. The cells were cultured and treated as for the in vitro permeability studies. At day 5, cells were washed with PBS and fixed in 70% ethanol for 10 min. Cells were then stained with 1% crystal violet (15 min) and thoroughly rinsed with tap water. The stain was extracted from cells by adding 100 μl of cell lysis buffer (1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, and 1% Nonidet P-40). Relative quantification was done by reading absorbance at 570 nm on a plate reader.
RNA isolation and quantitative real-time RT-PCR.
Total RNA was isolated from primary rat AT1 and AT2 cells sorted by flow cytometry (Qiagen) (n = 4 biological replicates). Additional DNase treatment was performed to minimize contamination with genomic DNA (Invitrogen). cDNA was synthesized using random hexamers (SuperScript III, Invitrogen). Quantitative real-time PCR using SYBR green (Applied Biosystems) was performed to amplify claudins-1, -2, -3, -4, -5, -6, -7, -8, -9, -10a, -10b, -11, -12, -14, -15, -16, -17, -18, -19, -20, -22, and -23 using primers as listed in Table 1. Specific products were confirmed by melt curve analysis. DNA standard curves were created for all claudins analyzed, α-tubulin, β-actin, and β2-microglobulin. The gene of interest was amplified and cloned into pCR2.1 (Invitrogen). The mass of one copy for each gene was calculated to estimate mass of plasmid required for specific number of copies in the standard curve. Copy number of the gene of interest was determined by comparison with the external DNA standard curve. Housekeeping genes β-actin, β2-microglobulin, and α-tubulin were amplified as internal controls, and all expression values were also normalized to the expression level of the housekeeping genes. This normalization of gene expression was done using GeNorm (52). For additional validation of the data, probes were used for the quantification of claudins-3, -4, -7, -12, -15, -18, -19, and α-tubulin (Table 1).
Table 1.
Primers and probes used for quantitative real-time PCR in freshly isolated, purified AT1 and AT2 cells
| Claudin | Forward Primer | Reverse Primer | Probe |
|---|---|---|---|
| Claudin-1 | CTGGGAGGTGCCCTACTTT | CCGCTGTCACACGTAGTCTT | |
| Claudin-2 | AACCTGGGCTCCAAAGAAAC | AATGGGTGGCAATTAGAGGA | |
| Claudin-3 | AGATGTACGACTCGCTGCTG | CTTGGCCGTCTCATCTTGT | CCTACTGGCAGCCTTCGGGC |
| Claudin-4 | CTCTCGCCTCCACGTTACTC | TCAGTCATCCTCGACACCAA | CGCCCGCGTTTCTGAGCACACCAGG |
| Claudin-5 | CAGGCTCTTGTGAGGACTTG | TGCCCTTTCAGGTTAGCAG | |
| Claudin-6 | CTGCAAATCTTGGGGATTGT | AGCAGCGAGTCGTACACCTT | |
| Claudin-7 | ATGCTCCTGGATTGGTCATC | CCTGCCCAGCCGATAAAG | AACCCCTTGACGCCCATGAATATT |
| Claudin-8 | CCCCGAGCATATACTCCAAAA | AGACGCTGCGTATTTCCTG | |
| Claudin-9 | GGCTGAGAGCTGTGAAGACC | AGCAGCGAGTCGTACACCTT | |
| Claudin-10a | CAGGGTCTGTGGATGAACTG | AGTCCTCTACACGCCTGGAT | |
| Claudin-10b | TCCACACTACCCACCGACTA | CAGGTTGGCAAAATAAGTGG | |
| Claudin-11 | AAGGGTTACCAGCGACAATG | AGAGACCCAACCCCTCAACT | |
| Claudin-12 | GCGACTCATCACATTCAACA | AGTCACTGCTTCCGTCATACC | CGGGCCTGTGGGTGAAGTGCGCCC |
| Claudin-14 | AAGCTCCCATTTGGTGAATG | AGGAAGCCTAGGAGCTGGAC | |
| Claudin-15 | AGCCACCTCTATCCCAATCC | CCAGGGCTGACATGAAGAA | TGGGGCTCAAGCCCCCTTGCGGG |
| Claudin-16 | AGCAAGCACGATGTGTTCAG | AGCTGGAATGCGATTGACTT | |
| Claudin-17 | GACCGCCAATATCATCATCC | TGACCTTCCTCTGGCTGTCT | |
| Claudin-18 | AAGGGCTCTGGAGGAGTTG | GCCCAGGATGGTGAAGTATG | CGTGCAACAGAGCTCGGGG |
| Claudin-19 | CTGTCAACGCCAGGTATGAA | TGCTGTTGGCTCTCTCAGG | TGGGCTGGCCATCCTGGGCGGT |
| Claudin-20 | TTTCATCCTGGCTGTGTCTG | TGCAGCTAAACATCCCAGTG | |
| Claudin-22 | GGAGGATGTGTGCTTCACTG | AGGAGAGCCTACCAAGTCCA | |
| Claudin-23 | GTTGCTGCTCAATCTCGTCA | CAGGCTGAGTCCCGAAGTAG | |
| α-Tubulin | GCCCTACAATTCCATCCTCA | CGCTCAATGTCGAGGTTTCT | GCACTCTGATTGTGCCTTCA |
| β-Actin | CATTGCTGACAGGATGCAGA | CTGGAAGGTGGACAGTGAGG | |
| β2-Microglobulin | TGACCGTGATCTTTCTGGTG | ATCTGAGGTGGGTGGAACTG |
All claudin mRNA levels were measured using Sybr Green. Levels of mRNA were also measured for some claudins using fluorescent detection probes.
Immunoblot.
After 5 days in culture, cells were harvested and lysed in complete cell lysis buffer (10 mM Tris·HCl, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, 1% Nonidet P-40, protease and phosphatase inhibitor cocktails, Sigma). Equal amounts of protein were resolved by SDS-PAGE under reducing conditions and blotted onto PVDF or nitrocellulose membranes. Membranes were blocked for 1 h at room temperature with 5% nonfat milk and probed for claudin-3, -4, -5, -7, -12, -15, -18, occludin, and ZO-1 (all from Invitrogen) overnight at 4°C. HRP-conjugated anti-mouse and anti-rabbit were used as secondary antibodies (Cell Signaling). β-Tubulin (Santa Cruz Biotechnology) was used as the loading control for densitometry analysis (ImageJ) in all conditions except for immunoblots of freshly isolated cells. In this instance, β-actin (Cell Signaling) was used as the loading control, and cells were harvested immediately after FACS analysis and sorting as described above.
Immunofluorescence staining.
Freshly isolated, unpurified cell suspensions were deposited onto glass slides using a Shandon Cytospin (Thermo Fisher Scientific). Samples were fixed in 4% paraformaldehyde, and antigen retrieval was done using citrate buffer at pH 6.0 (Dako Cytomation). Slides were incubated with 2% goat serum in all reactions to block nonspecific binding of antibodies. Cells were stained with antibodies against RT140, RT270, claudin-3, claudin-4, claudin-15, and claudin-18. Samples were incubated with primary antibody followed by the appropriate goat anti-mouse or goat anti-rabbit IgG Alexa 488-, Alexa 647-, or Alexa 594-secondary antibodies at 1:200 (Invitrogen). For cultured, primary rat AT2 cells, Transwells were fixed in ice-cold methanol:acetone (1:1) or room temperature 4% paraformaldehyde on day 5. Samples were permeabilized by exposure to 0.5% Triton X-100 in 1% BSA (EMD Chemicals) for 5 min and then blocked in 10% goat serum for 1 h. Transwells were incubated for 1 h at room temperature in primary antibodies against RT140, RT270, claudin-3, claudin-4, claudin-18, or ZO-1 followed by the appropriate goat anti-mouse or goat anti-rabbit IgG Alexa 488- or Alexa 647-secondary antibodies at 1:200. For filamentous actin detection, Transwells were stained with 1:40 Alexa 568 phalloidin (Invitrogen) for 1 h. All samples were mounted using Vectashield mounting media for fluorescence (Vector Labs). Confocal images were taken using LSM 510 Meta (Carl Zeiss) with EC Plan-Neofluar ×40 or Plan Apochromat ×63 oil lenses at a resolution of 1,024 × 1,024. Images were acquired using HAL-100 and LSM 510 Meta software. Epifluorescence images were obtained using Nikon Eclipse E600 (Nikon) with either a plan ×40 or ×20 objective. Images were acquired using Spot (RT Color Diagnostics Instruments) and Spot Advanced software. All images were processed using ImageJ or Photoshop (Adobe) and Illustrator (Adobe).
Statistics.
Differences between groups were compared using unpaired t-tests or ANOVA with post hoc Bonferroni correction for multiple comparisons. Each n represents cell isolation from a single rat. A P value ≤0.05 was considered significant. Data are reported as means ± SD or means ± SE where indicated.
RESULTS
Effect of KGF on barrier properties, cell phenotype, and proliferation.
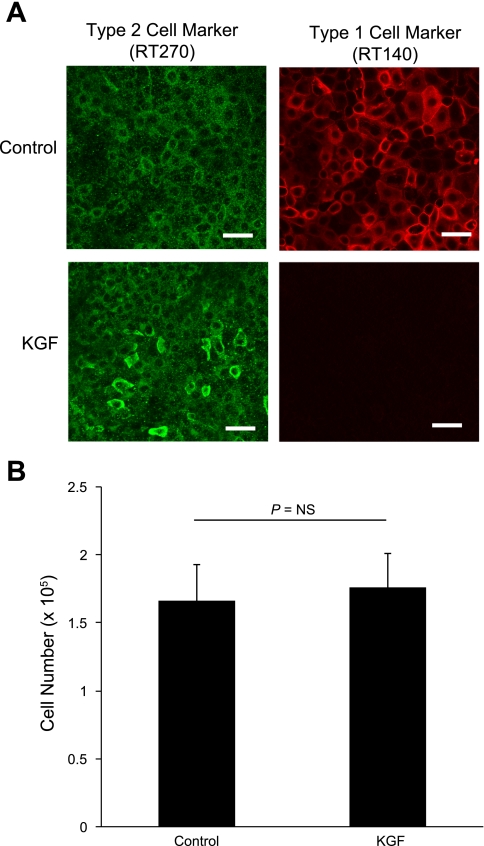
KGF (10 ng/ml) significantly increased TER in alveolar epithelial cell monolayers consistent with enhanced barrier function (710 ± 51 Ω·cm2 vs. 1,310 ± 130 Ω·cm2, n = 9 biological replicates, P < 0.001; Fig. 1A). In addition, KGF treatment produced a small but significant decrease in paracellular permeability to the 0.5 kDa tracer pyranine (apparent permeability, PAPP, 1.53 ± 0.13 vs. 1.11 ± 0.13 × 10−7 cm/s, n = 7 biological replicates, P < 0.05; Fig. 1B). These values were less than 1% of PAPP across cell-free Transwells. Consistent with previous reports (8), KGF-treated alveolar epithelial cells were more AT2-like as indicated by lack of RT140 staining after 5 days in culture (Fig. 2A). There was no difference in cell number with KGF treatment (1.67 ± 0.27 vs. 1.76 ± 0.26 × 105, n = 4 biological replicates, P = NS; Fig. 2B).
Fig. 1.
Effect of keratinocyte growth factor (KGF) on barrier function of alveolar epithelial cells. Freshly isolated alveolar epithelial type 2 (AT2) cells were cultured for 5 days in control media or media supplemented with 10 ng/ml KGF. A: transepithelial electrical resistance (TER) was significantly greater in KGF-treated cells. *P < 0.001 compared with control, n = 9 biological replicates. B: apparent permeability (PAPP) to the 0.5 kDa tracer molecule pyranine was significantly decreased in KGF-treated cells. *P < 0.05 compared with control, n = 7 biological replicates. These values were less than 1% of PAPP across cell-free Transwells. Data are expressed as means ± SE.
Fig. 2.
Effect of KGF administration on alveolar epithelial cell phenotype and proliferation. Freshly isolated AT2 cells were cultured for 5 days in control media or media supplemented with KGF. A: at day 5, cells were fixed and immunolabeled for the AT2 marker RT270 (green) and the AT1 marker RT140 (red). Although cells expressed RT270 under both conditions, KGF-treated cells were more AT2-like given the absence of RT140 staining. Scale bars, 50 μm. B: cell number was measured in confluent monolayers of control and KGF-treated cells at day 5 in culture. There was no difference in cell number between the 2 groups as measured by quantification of crystal violet staining (n = 4 biological replicates). Data are expressed as means ± SE.
Claudin expression profile in type 1 and type 2 alveolar epithelial cells.
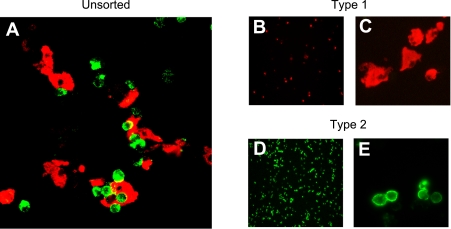
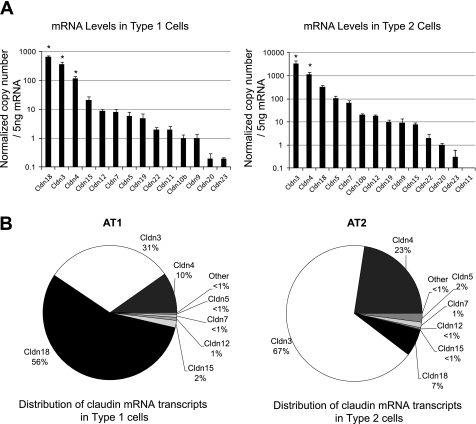
To determine the claudin expression profile in alveolar epithelial cells, we used fluorescence-activated cell sorting to isolate AT1 and AT2 cells with >99% purity (24) (Fig. 3) (n = 4 biological replicates). For all known claudins, mRNA expression was compared by quantitative real-time PCR using external standard curves such that mRNA copy numbers could be compared among genes. The mRNA levels for claudins-1, -2, -6, -8, -10a, -14, -16, and -17 were below the limits of detection in both cell types. AT1 cells expressed primarily claudins-18, -3, and -4 (P < 0.05 compared with all other claudins expressed in AT1 cells) with detectable levels of claudins-15, -12, -7, -5, -19, -22, -11, -10b, -9, -20, and -23 in decreasing order (Fig. 4A). In contrast, freshly isolated AT2 cells (Fig. 4A) expressed primarily claudins-3, -4, and -18, with detectable levels of claudins-5, -7, -10b, -12, -19, -9, -15, -22, -20, and -23 in decreasing order (P < 0.05 for claudins-3 and -4 compared with all other claudins expressed in AT2 cells). Claudin-11 was below the limit of detection in AT2 cells. The majority (97%) of claudin mRNA transcripts in both cells were for claudins-3, -4, and -18, although the relative amount of each of these was not the same (Fig. 4B). Comparison of claudin expression in both cell types together by analysis of variance followed by Bonferroni correction for multiple comparisons demonstrated that claudin-3 was the only transcript expressed at a statistically significantly different level between AT1 and AT2 cells with 10-fold more claudin-3 mRNA transcript in AT2 cells (P < 0.05). Probe-based and Sybr Green-based assays of mRNA expression yielded similar results.
Fig. 3.
Isolation of AT1 and AT2 cells by fluorescence-activated cell sorting. Lung cells liberated by digestion with elastase were stained with anti-RT140 (AT1 cell marker, red) and anti-RT270 (AT2 cell marker, green) as described in text. A: cytocentrifuged preparation of mixed cell population before FACS analysis and sorting showing AT1 (red) and AT2 (green) cells together. B–E: cytocentrifuged preparations of essentially pure (>99%) AT1 (B and C, red) and AT2 (D and E) cells after FACS analysis and sorting. Magnification, ×10 (B and D) and ×40 (A, C, and E).
Fig. 4.
Claudin mRNA expression levels in freshly isolated, purified AT1 and AT2 cells. To determine which claudins are expressed in these cell types, mRNA expression was compared in AT1 and AT2 cells for all known claudins using quantitative real-time PCR as described in text. A: AT1 cells (left) expressed primarily claudins-18, -3, and -4 (*P < 0.05 compared with all other claudins expressed in AT1 cells) with detectable levels of claudins-15, -12, -7, -5, -19, -22, -11, -10b, -9, -20, and -23 in decreasing order. AT2 cells (right) expressed primarily claudins-3, -4, and -18, with detectable levels of -5, -7, -10b, -12, -19, -9, -15, -22, -20, and -23 in decreasing order (*P < 0.05 for claudins-3 and -4 compared with all other claudins expressed in AT2 cells). B: >97% of claudin mRNA transcripts in AT1 (left) and AT2 (right) cells were for claudins-3, -4, and -18, although the relative amounts of each of these varied with claudin-18 being most highly expressed in AT1 cells and claudin-3 being the dominant transcript in AT2 cells. After analysis of variance followed by Bonferroni correction for multiple comparisons, claudin-3 was the only transcript expressed at a statistically significantly different level between AT1 and AT2 cells with 10-fold less claudin-3 in AT1 cells (P < 0.05). Data are expressed as means ± SE.
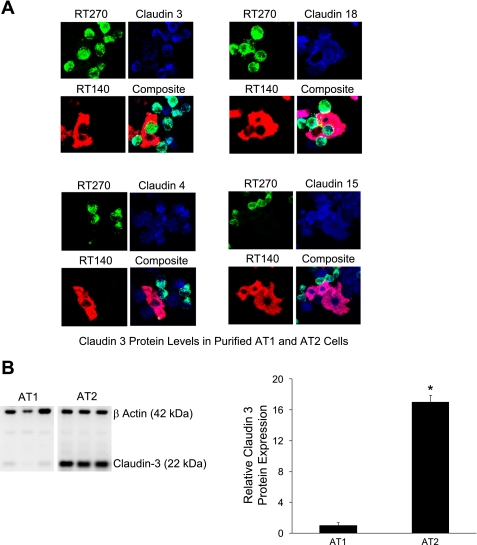
To assess claudin protein expression levels, immunofluorescence staining of freshly isolated, unpurified mixed whole lung cell preparations before flow cytometry was done (Fig. 5A). Consistent with the mRNA expression data, claudin-3 appeared more abundant in AT2 cells than AT1 cells. Claudins-4, -15, and -18 were detected in both AT1 and AT2 cells. As this immunofluorescence staining was done without fluorescence-activated cell sorting, cells negative for both AT1 and AT2 cell markers were present. Comprehensive immunoblot analysis was technically limited due to the quantity of material obtained from AT1 and AT2 cells after fluorescence-activated cell sorting and the limited availability of suitable antibodies. Thus, quantitative analysis of protein expression was not done on the freshly isolated cells. However, claudin-3, the only claudin with a statistically significantly different mRNA transcript level between AT1 and AT2 cells, was expressed at a 17-fold higher level in AT2 cells (P < 0.001, Fig. 5B) consistent with the mRNA expression data.
Fig. 5.
Claudin protein expression in AT1 and AT2 cells. A: cytocentrifuged preparations of lung cells before FACS analysis and sorting were stained for RT140 (red, lower left), RT270 (green, top left), claudin-3, -4, -15, or -18 (all blue, top right). Coexpression of specific claudins with RT140 and RT270 was also assessed (bottom right). Claudin-3 was primarily expressed in AT2 cells, although staining in AT1 cells was also detected. Claudins-4, -15, and -18 were expressed in AT1 and AT2 cells. Of note, cells negative for both AT1 and AT2 markers in the unpurified cell preparations stained positive for claudins-15, -4, and -3. Magnification, ×40 for all images. B: immunoblot analysis of claudin-3 in isolated, purified AT1 and AT2 cells after flow cytometry. β-actin was also labeled to normalize for total sample protein content. Claudin-3 protein levels were 17-fold greater in AT2 cells (*P < 0.001, n = 3 biological replicates). Data are expressed as means ± SE.
Effect of KGF on claudin expression and localization in cultured AT2 cells.
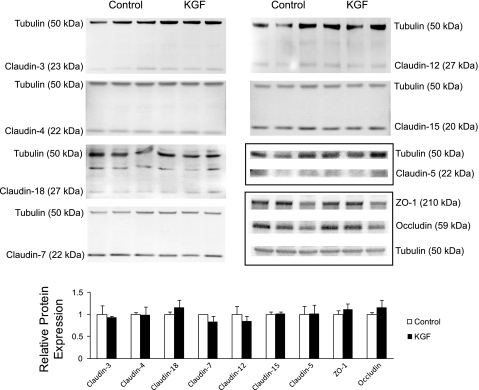
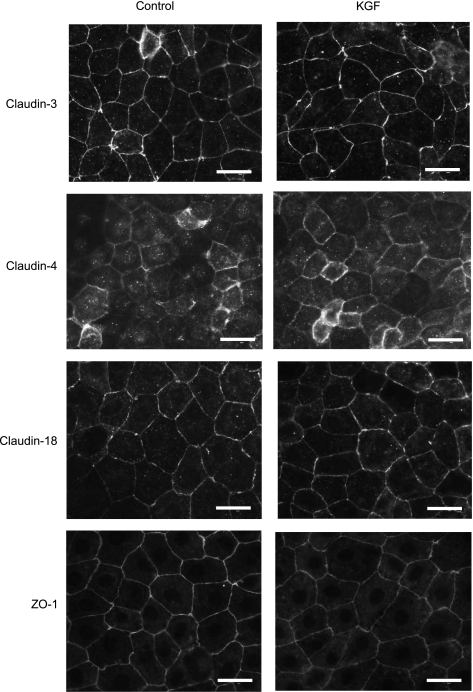
KGF did not significantly change the protein level of any tested tight junction proteins in cultured alveolar epithelial cells: claudins-3, -4, -5, -7, -12, -15, -18, occludin, and ZO-1 (n = 3 biological replicates, densitometry P = not significant for all groups; Fig. 6). These claudins were selected based on the results of the claudin expression profile determined in freshly isolated primary AT1 and AT2 cells (Fig. 4). There were also no differences in the localization of ZO-1 and claudins-3, -4, and -18 between control and KGF-treated cells (Fig. 7). All of these tight junction proteins were predominantly localized to the cell membrane at cell-cell contacts.
Fig. 6.
Effect of KGF administration on the expression of tight junction proteins in alveolar epithelial cells. Freshly isolated AT2 cells were cultured on permeable supports for 5 days in control media or media supplemented with KGF and then harvested and resolved by SDS-PAGE. Levels of claudin-3, -4, -5, -7, -12, -15, -18, zonula occludens (ZO)-1, and occludin were determined by immunoblot. Immunoblots were also labeled for β-tubulin to normalize for total sample protein content. Densitometry revealed no significant differences in expression levels of these tight junction proteins in KGF-treated cells compared with control (n = 3 biological replicates). Data are expressed as means ± SE.
Fig. 7.
Localization of tight junction proteins cultured for 5 days in the absence or presence of KGF. Freshly isolated AT2 cells were plated on permeable supports in control media or media supplemented with KGF, and then cultured for 5 days, fixed, and immunostained for claudin-3, claudin-4, claudin-18, and ZO-1. There were no differences in localization noted for these tight junction proteins in KGF-treated cells compared with control. All proteins were predominantly localized to the membrane at cell-cell contacts. Scale bars, 20 μm.
Mechanism of KGF effect on alveolar epithelial barrier properties.
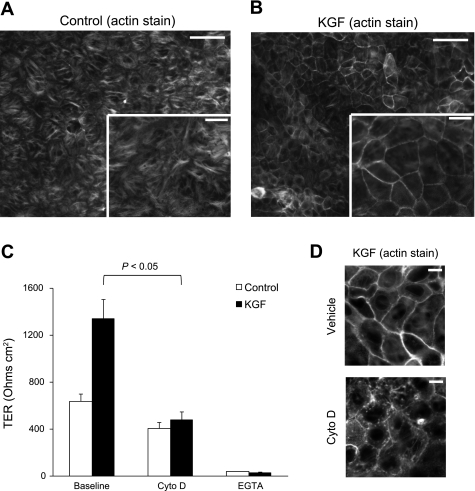
As changes in actin organization have been associated with changes in barrier permeability in epithelia (36, 47), the effect of KGF on the actin cytoskeleton was investigated. Cells cultured in the presence of KGF showed a marked increase in the apical perijunctional F-actin ring compared with controls (Fig. 8, A and B, and Supplemental Fig. 2). To determine if this change in cytoskeletal structure was associated with the KGF effect on TER, we treated the alveolar epithelial cell monolayers with the actin depolymerization agent cytochalasin D. Cytochalasin D completely abolished the barrier-enhancing effect of KGF on cultured AT2 cells without significantly decreasing TER in control cells (Fig. 8C). This decrease in TER in KGF-treated alveolar epithelial cells after cytochalasin D treatment was associated with disruption of the perijunctional F-actin ring (Fig. 8D). Moreover, tight junctions were still maintained after cytochalasin D treatment given the relatively preserved TER in control and KGF-treated alveolar epithelial cells compared with cells treated with calcium-free media, an intervention that results in the dissolution of tight junctions (Fig. 8C).
Fig. 8.
Effect of KGF administration on the actin cytoskeleton. Freshly isolated AT2 cells were plated on permeable supports in control media or media supplemented with KGF, and then cultured for 5 days, fixed, stained for F-actin with Alexa 568 phalloidin, and imaged under epifluorescence (A and B) or confocal microscopy (D). A and B: KGF-treated cells (B) demonstrated significant reorganization of the actin cytoskeleton with a marked increase in the apical perijunctional F-actin ring compared with control cells (A). Inset is higher magnification image (n = at least 4 biological replicates). Scale bars, 50 μm on ×20 image and 20 μm on inset ×40 image. C: actin depolymerization with cytochalasin D treatment decreased TER to control levels (P < 0.05) without a significant decrease in control TER (n = 4 biological replicates consisting of 4–6 technical replicates). Treatment of control and KGF-treated AT2 cells with calcium-free media resulted in near complete loss of TER. Data are expressed as means ± SE. D: the perijunctional F-actin ring in KGF-treated alveolar epithelial cells (top) was disrupted by treatment with cytochalasin D (bottom) (n = at least 4 biological replicates). Scale bars, 10 μm.
DISCUSSION
Contrary to our initial hypothesis, the major finding of this study is that KGF enhances alveolar epithelial tightness by altering the actin cytoskeleton rather than by changing claudin expression or trafficking. A second major finding is that AT1 and AT2 cells primarily express claudins-3, -4, and -18 at the mRNA level: these claudins account for 97% of transcripts in each cell type. However, the two cell types express these claudins in differing proportions. AT2 cells express 10-fold more claudin-3 mRNA and 17-fold more claudin-3 protein than AT1 cells. To our knowledge, this is the first report of the complete claudin transcript profile of primary AT1 and AT2 cells. A third finding is that although KGF contributes to the preservation of certain features of the AT2 phenotype in cultured AT2 cells, which normally adopt a more AT1-like phenotype, KGF does not alter claudin expression.
The preservation of intact alveolar epithelial barrier function is associated with improved clinical outcomes in patients with acute lung injury (34, 57). Successful recovery from lung injury requires the proliferation and differentiation of AT2 cells and formation of tight junctions (2, 33). KGF is a potent epithelial mitogen in vivo (50) that has been shown to ameliorate lung injury in multiple experimental models: bleomycin (13, 69), acid installation (67), hyperoxia (40), hydrogen peroxide (66), pseudomonas infection (53), radiation (69), and ventilator-induced lung injury (62). KGF also appears to be critical to the therapeutic capacity of human mesenchymal stem cells to restore alveolar epithelial fluid transport in acute lung injury (30). Although KGF induces AT2 cell proliferation in vivo, this effect has been less consistent in in vitro studies (4, 17, 20, 41). Alveolar epithelial type 2 cells have a low proliferative index when cultured in vitro (4, 21, 24), and our results are consistent with others that have found no increase in cell number following KGF treatment in vitro (20). KGF also has multiple non-mitogenic effects that are of potential importance (17). For instance, KGF upregulates active ion transport in alveolar epithelial cells by increasing Na+-K+-ATPase expression (7), which explains in part the ability of KGF to increase alveolar fluid clearance through non-mitogenic mechanisms (56). KGF also augments pulmonary surfactant production (48) and enhances alveolar epithelial repair (4). In this study, we demonstrate an additional mechanism by which KGF may preserve alveolar epithelial barrier function: modulation of tight junction function via changes in the actin cytoskeleton.
KGF promoted alveolar barrier tightness as measured by electrical resistance and permeability to a 0.5 kDa tracer. The increase in TER with KGF treatment in this study is particularly notable given that this growth factor is known to increase transcellular active ion transport in alveolar epithelial cells in culture (7) and in vivo (26). Although KGF is known to influence the actin cytoskeleton in different model systems (6, 9, 20, 35, 39, 42, 46, 60), previous studies have not reported that KGF increases TER in cultured AT2 cell via modulation of the actin cytoskeleton. However, Waters and colleagues (60) demonstrated that KGF pretreatment prevented hydrogen peroxide-induced disruption of the perijunctional F-actin ring in airway epithelia, thus preventing an increase in paracellular protein permeability. Savla and Waters (42) similarly demonstrated stabilization of the F-actin cytoskeleton with KGF treatment and resultant protection of airway epithelial barrier function after radiation exposure. Data from the present study extend these prior studies by showing that KGF treatment results in the formation of a more pronounced perijunctional actin ring and decreased permeability in cultured AT2 cells.
The importance of the F-actin cytoskeleton in influencing alveolar epithelial barrier function has been previously described but is not well characterized. For instance, although thrombin induces actin stress fiber formation and loss of barrier integrity in lung endothelial cells, it has the opposite effect in alveolar epithelial monolayers: increased perijunctional F-actin formation and an increase in TER (27). Importantly, soluble factors secreted by human mesenchymal stem cells, namely KGF, angiopoietin-1 (Ang1), and IL-1 receptor antagonist have recently been demonstrated to augment alveolar epithelial barrier function in models of acute lung injury and inflammation (15, 30, 38). Whereas the effect of KGF on paracellular permeability in these models is currently not well understood (30), Ang1 and human mesenchymal stem cells decreased epithelial permeability following cytomix-induced injury of cultured AT2 cells in part through cytoskeletal reorganization (15). Specifically, Ang1 promoted apical peripheral F-actin formation similar to the effect of KGF in our study. Therefore, modulation of the actin cytoskeleton may represent a common mechanism by which soluble factors secreted by human mesenchymal stem cells augment alveolar epithelial barrier function. As these cells are being studied for potential therapy in acute lung injury (61), the mechanism of cytoskeletal reorganization should be investigated further.
In many cell types, cytoskeletal disassembly disrupts barrier function, an effect mediated in part by the interaction of the apical junction complex and actin cytoskeleton (36, 44, 49). In this study, we used cytochalasin D, an actin depolymerization agent that leads to shortening and severing of existing microfilaments (45), to assess the contribution of increased perijunctional F-actin to KGF-induced increases in TER (47). Cytochalasin D treatment caused the TER of KGF-treated cells to return to baseline but did not significantly change TER in control cells, which had a much smaller perijunctional actin ring. This decrease in TER in KGF-treated alveolar epithelial cells was associated with disruption of the perijunctional F-actin ring as demonstrated by confocal microscopy. Furthermore, dissolution of existing tight junctions via extracellular calcium depletion resulted in near complete loss of TER in both groups. These data support the conclusion that the KGF-induced augmentation of barrier function is at least in part dependent on the changes in perijunctional actin.
Claudin expression in the alveolar epithelium can be regulated by multiple factors in the cell microenvironment, including the extracellular matrix (29) and proinflammatory cytokines (15). Given that KGF has been previously demonstrated to alter connexin expression and gap junctional communication in cultured alveolar epithelial cells (1), we hypothesized that KGF would also regulate claudin expression. However, KGF administration in vitro was not associated with changes in claudin expression in this study. An additional reason we elected to study the effects of KGF on tight junction structure and function was its ability to promote an AT2-cell like phenotype in culture. We hypothesized that maintaining a more AT2 cell-like phenotype in culture with KGF would allow modeling of the AT2 cell-cell contacts that potentially occur during acute lung injury. KGF did promote a more AT2-like phenotype as measured by absence of RT140 expression, but claudin expression was not affected. This is consistent with the inability of KGF to maintain all components of the AT2 phenotype (48). Cultured type 2 cells acquire many characteristics of type 1 cells, but are distinct from freshly isolated type 1 cells based on mRNA expression data (23).
Although the present studies identify an additional mechanism by which KGF may exert its beneficial effects in acute lung injury, another important aspect of this work is the complete description of the claudin mRNA expression profile in AT1 and AT2 cells. Claudins are essential in regulating paracellular permeability (51), and delineation of the claudin profile of the lung is necessary for the study of alveolar epithelial barrier function in acute lung injury. Previous studies are consistent with the findings of this study, but prior studies have not reported the complete claudin mRNA expression profile of primary type 1 and type 2 cells (12, 55). However, a limitation of this study is that protein levels for most claudins in freshly isolated AT1 and AT2 cells can only be inferred from mRNA levels. An experimental approach focused on detecting mRNA levels was selected given it provided the best ability to quantitatively compare expression for all claudins in the two cell types, even those for which suitable antibodies are not readily available. Comprehensive immunoblot analysis was also technically limited due to the quantity of material obtained from AT1 and AT2 cells after fluorescence-activated cell sorting. It is likely that there are some differences between claudin mRNA expression levels and actual protein levels in these cell types. However, for claudin-3, the only claudin with a statistically significantly different transcript level between AT1 and AT2 cells, the mRNA and protein data were consistent. In addition, immunostaining of mixed populations of AT1 and AT2 cells prior to FACS analysis and sorting supported the mRNA data on claudin expression levels. Moreover, that claudins-3, -4, and -18 make up more than 97% of the claudin transcripts in both AT1 and AT2 cells could potentially guide future experiments on paracellular transport in the lung. However, claudins expressed at low levels may have important effects on barrier properties that would not be predicted by their levels alone. The physiological consequences of higher claudin-3 levels in AT2 cells are uncertain; however, prior studies have shown that claudin-3 can bind with different claudins on opposing cell membranes, a quality not shared with all claudin family members (56).
In conclusion, we report an additional non-mitogenic mechanism by which KGF exerts a beneficial effect in acute lung injury, specifically enhanced alveolar barrier function through reorganization of the F-actin cytoskeleton that does not require a change in claudin expression. In addition, we describe the complete claudin mRNA expression profile in purified, freshly isolated rat AT1 and AT2 cells. These data will be important in future studies of the regulation of paracellular permeability in the alveolar epithelium during acute lung injury. There are important clinical implications to these findings. Because KGF shows promise as a potential therapeutic agent in acute lung injury, these data elaborate on the barrier-specific effects of this pleiotropic agent and identify what may be a common mechanism by which several barrier-enhancing mediators exert their effects. Further characterization of the barrier-enhancing properties of KGF independent from its other effects could lead to a targeted treatment of acute lung injury.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-088440.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Sheena Mundra and Xiaomin Liang for technical assistance.
REFERENCES
- 1.Abraham V, Chou ML, DeBolt KM, Koval M. Phenotypic control of gap junctional communication by cultured alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 276: L825–L834, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Adamson IY, Bowden DH. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest 30: 35–42, 1974 [PubMed] [Google Scholar]
- 3.Angelow S, Ahlstrom R, Yu AS. Biology of claudins. Am J Physiol Renal Physiol 295: F867–F876, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atabai K, Ishigaki M, Geiser T, Ueki I, Matthay MA, Ware LB. Keratinocyte growth factor can enhance alveolar epithelial repair by nonmitogenic mechanisms. Am J Physiol Lung Cell Mol Physiol 283: L163–L169, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Baba Y, Yazawa T, Kanegae Y, Sakamoto S, Saito I, Morimura N, Goto T, Yamada Y, Kurahashi K. Keratinocyte growth factor gene transduction ameliorates acute lung injury and mortality in mice. Hum Gene Ther 18: 130–141, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Boardman KC, Aryal AM, Miller WM, Waters CM. Actin re-distribution in response to hydrogen peroxide in airway epithelial cells. J Cell Physiol 199: 57–66, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Borok Z, Danto SI, Dimen LL, Zhang XL, Lubman RL. Na+-K+-ATPase expression in alveolar epithelial cells: upregulation of active ion transport by KGF. Am J Physiol Lung Cell Mol Physiol 274: L149–L158, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Borok Z, Lubman RL, Danto SI, Zhang XL, Zabski SM, King LS, Lee DM, Agre P, Crandall ED. Keratinocyte growth factor modulates alveolar epithelial cell phenotype in vitro: expression of aquaporin 5. Am J Respir Cell Mol Biol 18: 554–561, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Ceccarelli S, Cardinali G, Aspite N, Picardo M, Marchese C, Torrisi MR, Mancini P. Cortactin involvement in the keratinocyte growth factor and fibroblast growth factor 10 promotion of migration and cortical actin assembly in human keratinocytes. Exp Cell Res 313: 1758–1777, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Cheek JM, Evans MJ, Crandall ED. Type I cell-like morphology in tight alveolar epithelial monolayers. Exp Cell Res 184: 375–387, 1989 [DOI] [PubMed] [Google Scholar]
- 11.Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol 285: L1166–L1178, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Daugherty BL, Mateescu M, Patel AS, Wade K, Kimura S, Gonzales LW, Guttentag S, Ballard PL, Koval M. Developmental regulation of claudin localization by fetal alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 287: L1266–L1273, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Deterding RR, Havill AM, Yano T, Middleton SC, Jacoby CR, Shannon JM, Simonet WS, Mason RJ. Prevention of bleomycin-induced lung injury in rats by keratinocyte growth factor. Proc Assoc Am Physicians 109: 254–268, 1997 [PubMed] [Google Scholar]
- 14.Dobbs LG. Isolation and culture of alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 258: L134–L147, 1990 [DOI] [PubMed] [Google Scholar]
- 15.Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem 285: 26211–26222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finch PW, Rubin JS, Miki T, Ron D, Aaronson SA. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science 245: 752–755, 1989 [DOI] [PubMed] [Google Scholar]
- 17.Franco-Montoya ML, Bourbon JR, Durrmeyer X, Lorotte S, Jarreau PH, Delacourt C. Pulmonary effects of keratinocyte growth factor in newborn rats exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol 297: L965–L976, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Frank JA, Pittet JF, Wray C, Matthay MA. Protection from experimental ventilator-induced acute lung injury by IL-1 receptor blockade. Thorax 63: 147–153, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Frank JA, Wray CM, McAuley DF, Schwendener R, Matthay MA. Alveolar macrophages contribute to alveolar barrier dysfunction in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 291: L1191–L1198, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Galiacy S, Planus E, Lepetit H, Fereol S, Laurent V, Ware L, Isabey D, Matthay M, Harf A, d'Ortho MP. Keratinocyte growth factor promotes cell motility during alveolar epithelial repair in vitro. Exp Cell Res 283: 215–229, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Garat C, Kheradmand F, Albertine KH, Folkesson HG, Matthay MA. Soluble and insoluble fibronectin increases alveolar epithelial wound healing in vitro. Am J Physiol Lung Cell Mol Physiol 271: L844–L853, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Geiser T, Jarreau PH, Atabai K, Matthay MA. Interleukin-1β augments in vitro alveolar epithelial repair. Am J Physiol Lung Cell Mol Physiol 279: L1184–L1190, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez R, Yang YH, Griffin C, Allen L, Tigue Z, Dobbs L. Freshly isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. Am J Physiol Lung Cell Mol Physiol 288: L179–L189, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez RF, Allen L, Dobbs LG. Rat alveolar type I cells proliferate, express OCT-4, and exhibit phenotypic plasticity in vitro. Am J Physiol Lung Cell Mol Physiol 297: L1045–L1055, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorin AB, Stewart PA. Differential permeability of endothelial and epithelial barriers to albumin flux. J Appl Physiol 47: 1315–1324, 1979 [DOI] [PubMed] [Google Scholar]
- 26.Guery BP, Mason CM, Dobard EP, Beaucaire G, Summer WR, Nelson S. Keratinocyte growth factor increases transalveolar sodium reabsorption in normal and injured rat lungs. Am J Respir Crit Care Med 155: 1777–1784, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Kawkitinarong K, Linz-McGillem L, Birukov KG, Garcia JG. Differential regulation of human lung epithelial and endothelial barrier function by thrombin. Am J Respir Cell Mol Biol 31: 517–527, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 13: 875–886, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Koval M, Ward C, Findley MK, Roser-Page S, Helms MN, Roman J. Extracellular matrix influences alveolar epithelial claudin expression and barrier function. Am J Respir Cell Mol Biol 42: 172–180, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA 106: 16357–16362, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madara JL, Barenberg D, Carlson S. Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J Cell Biol 102: 2125–2136, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mason CM, Guery BP, Summer WR, Nelson S. Keratinocyte growth factor attenuates lung leak induced by alpha-naphthylthiourea in rats. Crit Care Med 24: 925–931, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Mason RJ. Biology of alveolar type II cells. Respirology 11Suppl: S12–S15, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis 142: 1250–1257, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Mehta PB, Robson CN, Neal DE, Leung HY. Keratinocyte growth factor activates p38 MAPK to induce stress fibre formation in human prostate DU145 cells. Oncogene 20: 5359–5365, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Meza I, Sabanero M, Stefani E, Cereijido M. Occluding junctions in MDCK cells: modulation of transepithelial permeability by the cytoskeleton. J Cell Biochem 18: 407–421, 1982 [DOI] [PubMed] [Google Scholar]
- 37.Morimoto K, Yamahara H, Lee VH, Kim KJ. Dipeptide transport across rat alveolar epithelial cell monolayers. Pharm Res 10: 1668–1674, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 104: 11002–11007, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oswari J, Matthay MA, Margulies SS. Keratinocyte growth factor reduces alveolar epithelial susceptibility to in vitro mechanical deformation. Am J Physiol Lung Cell Mol Physiol 281: L1068–L1077, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Panos RJ, Bak PM, Simonet WS, Rubin JS, Smith LJ. Intratracheal instillation of keratinocyte growth factor decreases hyperoxia-induced mortality in rats. J Clin Invest 96: 2026–2033, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panos RJ, Rubin JS, Csaky KG, Aaronson SA, Mason RJ. Keratinocyte growth factor and hepatocyte growth factor/scatter factor are heparin-binding growth factors for alveolar type II cells in fibroblast-conditioned medium. J Clin Invest 92: 969–977, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savla U, Waters CM. Barrier function of airway epithelium: effects of radiation and protection by keratinocyte growth factor. Radiat Res 150: 195–203, 1998 [PubMed] [Google Scholar]
- 43.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 286: C1213–C1228, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell 16: 3919–3936, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spector I, Shochet NR, Blasberger D, Kashman Y. Latrunculins–novel marine macrolides that disrupt microfilament organization and affect cell growth. I. Comparison with cytochalasin D. Cell Motil Cytoskeleton 13: 127–144, 1989 [DOI] [PubMed] [Google Scholar]
- 46.Steele IA, Edmondson RJ, Leung HY, Davies BR. Ligands to FGF receptor 2-IIIb induce proliferation, motility, protection from cell death and cytoskeletal rearrangements in epithelial ovarian cancer cell lines. Growth Factors 24: 45–53, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Stevenson BR, Begg DA. Concentration-dependent effects of cytochalasin D on tight junctions and actin filaments in MDCK epithelial cells. J Cell Sci 107: 367–375, 1994 [DOI] [PubMed] [Google Scholar]
- 48.Sugahara K, Rubin JS, Mason RJ, Aronsen EL, Shannon JM. Keratinocyte growth factor increases mRNAs for SP-A and SP-B in adult rat alveolar type II cells in culture. Am J Physiol Lung Cell Mol Physiol 269: L344–L350, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9: 799–809, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Ulich TR, Yi ES, Longmuir K, Yin S, Biltz R, Morris CF, Housley RM, Pierce GF. Keratinocyte growth factor is a growth factor for type II pneumocytes in vivo. J Clin Invest 93: 1298–1306, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol 68: 403–429, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viget NB, Guery BP, Ader F, Neviere R, Alfandari S, Creuzy C, Roussel-Delvallez M, Foucher C, Mason CM, Beaucaire G, Pittet JF. Keratinocyte growth factor protects against Pseudomonas aeruginosa-induced lung injury. Am J Physiol Lung Cell Mol Physiol 279: L1199–L1209, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Volberg T, Geiger B, Kartenbeck J, Franke WW. Changes in membrane-microfilament interaction in intercellular adherens junctions upon removal of extracellular Ca2+ ions. J Cell Biol 102: 1832–1842, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang F, Daugherty B, Keise LL, Wei Z, Foley JP, Savani RC, Koval M. Heterogeneity of claudin expression by alveolar epithelial cells. Am J Respir Cell Mol Biol 29: 62–70, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Folkesson HG, Jayr C, Ware LB, Matthay MA. Alveolar epithelial fluid transport can be simultaneously upregulated by both KGF and beta-agonist therapy. J Appl Physiol 87: 1852–1860, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 1376–1383, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol 282: L924–L940, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Waters CM, Savla U, Panos RJ. KGF prevents hydrogen peroxide-induced increases in airway epithelial cell permeability. Am J Physiol Lung Cell Mol Physiol 272: L681–L689, 1997 [DOI] [PubMed] [Google Scholar]
- 61.Weiss DJ, Kolls JK, Ortiz LA, Panoskaltsis-Mortari A, Prockop DJ. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc 5: 637–667, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Welsh DA, Summer WR, Dobard EP, Nelson S, Mason CM. Keratinocyte growth factor prevents ventilator-induced lung injury in an ex vivo rat model. Am J Respir Crit Care Med 162: 1081–1086, 2000 [DOI] [PubMed] [Google Scholar]
- 63.Werner S. Keratinocyte growth factor: a unique player in epithelial repair processes. Cytokine Growth Factor Rev 9: 153–165, 1998 [DOI] [PubMed] [Google Scholar]
- 64.Wiener-Kronish JP, Albertine KH, Matthay MA. Differential responses of the endothelial and epithelial barriers of the lung in sheep to Escherichia coli endotoxin. J Clin Invest 88: 864–875, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wray C, Mao Y, Pan J, Chandrasena A, Piasta F, Frank JA. Claudin-4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am J Physiol Lung Cell Mol Physiol 297: L219–L227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu KI, Pollack N, Panos RJ, Sporn PH, Kamp DW. Keratinocyte growth factor promotes alveolar epithelial cell DNA repair after H2O2 exposure. Am J Physiol Lung Cell Mol Physiol 275: L780–L787, 1998 [DOI] [PubMed] [Google Scholar]
- 67.Yano T, Deterding RR, Simonet WS, Shannon JM, Mason RJ. Keratinocyte growth factor reduces lung damage due to acid instillation in rats. Am J Respir Cell Mol Biol 15: 433–442, 1996 [DOI] [PubMed] [Google Scholar]
- 68.Yi ES, Salgado M, Williams S, Kim SJ, Masliah E, Yin S, Ulich TR. Keratinocyte growth factor decreases pulmonary edema, transforming growth factor-beta and platelet-derived growth factor-BB expression, and alveolar type II cell loss in bleomycin-induced lung injury. Inflammation 22: 315–325, 1998 [DOI] [PubMed] [Google Scholar]
- 69.Yi ES, Williams ST, Lee H, Malicki DM, Chin EM, Yin S, Tarpley J, Ulich TR. Keratinocyte growth factor ameliorates radiation- and bleomycin-induced lung injury and mortality. Am J Pathol 149: 1963–1970, 1996 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.