Abstract
Once excess liquid gains access to air spaces of an injured lung, the act of breathing creates and destroys foam and thereby contributes to the wounding of epithelial cells by interfacial stress. Since cells are not elastic continua, but rather complex network structures composed of solid as well as liquid elements, we hypothesize that plasma membrane (PM) wounding is preceded by a phase separation, which results in blebbing. We postulate that interventions such as a hypertonic treatment increase adhesive PM-cytoskeletal (CSK) interactions, thereby preventing blebbing as well as PM wounds. We formed PM tethers in alveolar epithelial cells and fibroblasts and measured their retractive force as readout of PM-CSK adhesive interactions using optical tweezers. A 50-mOsm increase in media osmolarity consistently increased the tether retractive force in epithelial cells but lowered it in fibroblasts. The osmo-response was abolished by pretreatment with latrunculin, cytochalasin D, and calcium chelation. Epithelial cells and fibroblasts were exposed to interfacial stress in a microchannel, and the fraction of wounded cells were measured. Interventions that increased PM-CSK adhesive interactions prevented blebbing and were cytoprotective regardless of cell type. Finally, we exposed ex vivo perfused rat lungs to injurious mechanical ventilation and showed that hypertonic conditioning reduced the number of wounded subpleural alveolus resident cells to baseline levels. Our observations support the hypothesis that PM-CSK adhesive interactions are important determinants of the cellular response to deforming stress and pave the way for preclinical efficacy trials of hypertonic treatment in experimental models of acute lung injury.
Keywords: alveolar epithelial cell, acute lung injury, interfacial stress, osmotic response, cytoprotection
the syndrome of ventilator-induced lung injury (VILI) contributes to the morbidity and mortality of critically ill patients (25). The clinical manifestations of the syndrome are indistinguishable from those of all-cause acute lung injury and at their core reflect a mechanotransduction event, i.e., the effects of deforming stress on cell and tissue injury, remodeling, and repair. The complex topographical distributions of lung mechanical properties, and hence of parenchymal stress and strain together with the numerous and nuanced injury manifestations, make it very difficult to establish mechanistic cause and effect relationships on the scale of interest, e.g., that of individual cells. Our research, therefore, focuses on a very specific mechanotransduction event, namely physical stress-related epithelial cell wounding and repair, which we believe contributes to the pathogenesis VILI.
In the current study, we present a body of work in which we have explored the effects of hypertonic exposure on cell wounding in experimental models ranging from individual cells to rodent VILI preparations. The experiments were not designed to test the preclinical efficacy of an intervention, but rather to explore a cell biophysical mechanism. The research is motivated by a mind experiment (Fig. 1) that considers a cell to be a fluid-filled sponge [cytosol and cytoskeleton (CSK)] covered by a relatively impermeable membrane [the plasma membrane (PM) or lipid bilayer]. The membrane sticks to the sponge but can be separated from it (analogous to cell blebbing) in response to a compressive stress. Separation occurs when the hydrostatic liquid pressure (of the cytosol) exceeds the adhesive energy between the sponge (the CSK biopolymer network) and the (plasma) membrane. Because sponge and liquid have different elastic moduli, an externally imposed shape change causes a redistribution of the liquid relative to the solid elements of the structure, i.e., a phase separation (4), and is, in turn, the source of the hydrostatic transmembrane pressure. Once the (plasma) membrane is deprived of its underlying solid support (that of the subcortical CSK), it is more likely to yield and rupture. We postulate that interventions, which 1) increase the adhesive interactions between PM and CSK, and/or 2) reduce the hydrostatic pressure head at the PM-CSK interface, will prove cell-protective. We show that hypertonic cell conditioning is such an intervention.
Fig. 1.
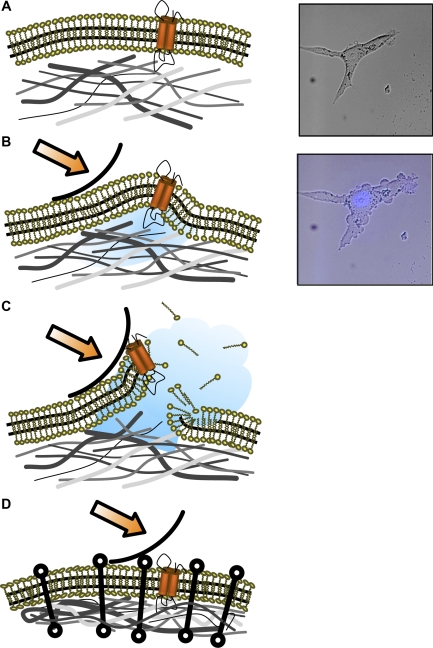
The pressure gradient paradigm of cellular injury. As deformation of the epithelial cell occurs, a pressure gradient at the wavefront (arrow) develops and moves across the surface in a manner similar to a rolling pin (A and B). Initially, plasma membrane (PM) lipid-cytoskeletal (CSK) protein interactions carry most of the load. As the compressive force increases a phase separation of PM from the underlying CSK, a bleb forms (B). With continued deformation, hydraulic force increases PM lateral tension in the blebbed area, which may exceed lytic tension, leading to PM disruption (C). Cytoprotection may occur under conditions of increased PM lipid-CSK protein adhesive interactions such as hypertonic exposure (D, compare with A–C). Right: actual experimental observations of a 3T3 fibroblast before (A) and immediately after (B) exposure to interfacial stress. Propidium iodide (PI) negative staining [4′,6′-diamidino-2-phenylindole (DAPI)-stained nucleus, blue] in B demonstrates absence of PM wounding and confirms cell viability.
MATERIALS AND METHODS
Culture of A549, Swiss 3T3, and freshly harvested rat alveolar epithelial cells.
The use of rodents for lung harvest and cell isolation was approved by the Mayo Clinic animal care and use committee. Sprague-Dawley rat alveolar epithelial cell (AEC) type 2 (AT2) cells were isolated using a modified protocol based on the method of Dobbs et al. (5). Day 2 cultures were used for all AT2 experiments. Rat AEC type 1 (AT1) cells were isolated by immunoselection for T1α as recently described (23). A549 and Swiss 3T3 were cultured to confluency in F-12K and DMEM media, respectively, on either glass coverslips or culture dishes (Fisher Scientific, Pittsburgh, PA) under conditions of 21% O2-10% CO2, and the media contained 10% FBS and 1% penicillin-streptomycin.
Optical trapping experiments.
All cells were grown on optical grade glass culture dishes (0.17-mm Delta T; Fisher Scientific) under conditions of 21% O2-10% CO2. On the day of experiment, dishes were washed twice with culture media and then reincubated in either F-12K or DMEM media without FBS plus the experimental probes as appropriate: 1 μM cytochalasin D, 1 mM EGTA (Sigma, St. Louis, MO), 500 nM latrunculin A (Biomol, Plymouth Meeting, PA), or hyper- (340 mOsm) or hypo- (240 mOsm) HEPES-buffered MEM (HMEM).
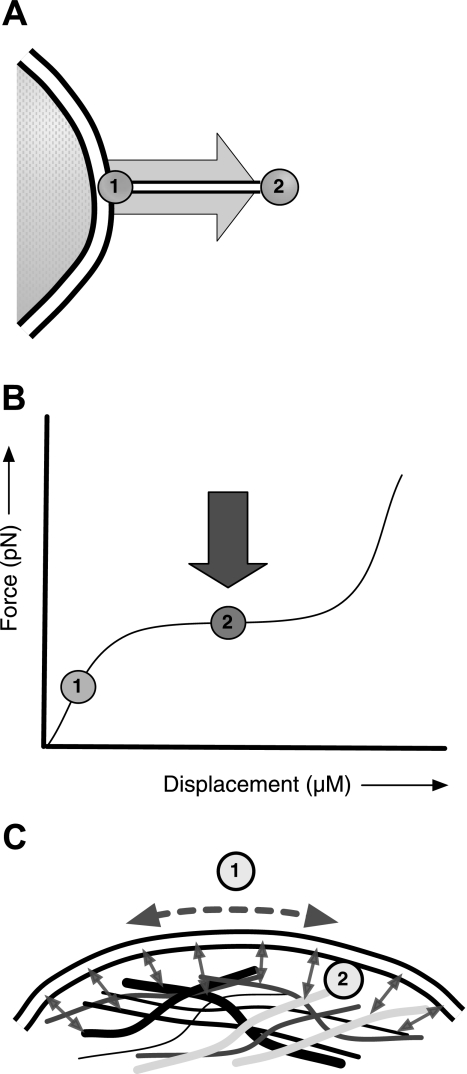
A single cell from each plate was selected, and a 1-μm concavalin A-coated silica bead was optically trapped, brought into apposition with the PM, and withdrawn slowly to pull a lipid tether of ∼5-μm length (Fig. 2A). Concavalin A binds to mannosyl groups of glycolipids and glycoproteins. These are embedded in the PM, do not readily associate with actin or actin-associated proteins, and therefore serve as virtual hooks for pulling the lipid bilayer off the supporting subcortical CSK. The displacement of the tethered bead relative to the center of the trap was measured with a four-quadrant photodiode-coupled interferometer providing a real-time readout of tether retractive force (Fig. 2B) as described previously by Guilford et al. (8). Force measurements were made at a fixed tether length (“2” and dark gray arrow in Fig. 2B), whereas medium osmolarity was increased or decreased by 50 mOsm. A representative force tracing during such an experiment is shown in Supplemental Fig. S1 (available in the data supplement online at the AJP-Lung Cellular and Molecular Physiology web site). Final osmolarity was confirmed by vapor pressure osmometry of the culture media.
Fig. 2.
Optical trap lipid tether measurements. A: a lipid tether is formed when a coated bead is brought into apposition with the PM of the cell (“1”) and withdrawn (“2”). B: during an experiment, retractive tension (force, in piconewtons) and displacement (in micrometers) are measured and plotted in real-time. The bead is manipulated such that the plateau phase of the tether is determined, and once a quasi-steady state is achieved (“2”), an osmotic intervention is administered (dark gray arrow). C: important biophysical determinants of lipid tether retractive force include 1) PM lateral tension, and 2) membrane lipid-CSK protein (i.e., adhesive) interactions.
CSK rearrangement in response to osmotic pressure.
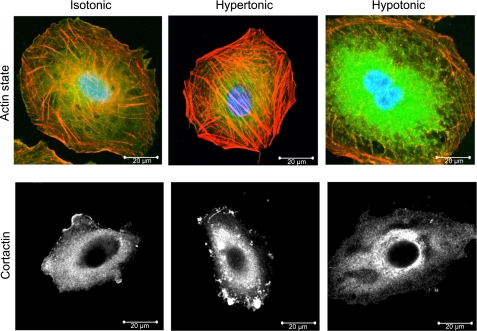
A549 and AT2 were exposed to osmotic pressures ranging from 150 to 450 mOsm. F-actin, G-actin, and cortactin were fluorescently labeled with 20 μM rhodamine phalloidin (Sigma), dextran-DNase I-conjugated Alexa Fluor 488 (Molecular Probes/Invitrogen, Carlsbad, CA), and 1 μg/μl cortactin monoclonal antibody (Upstate/Millipore, Billerica, MA), respectively. Nuclei were stained with DAPI (Sigma). Imaging was performed under ×100 objective fluorescence microscopy (Fig. 3).
Fig. 3.
A549 cell CSK rearrangement in response to osmotic pressure. Top: hypertonic treatment increases subcortical F-actin polymerization as demonstrated by rhodamine phalloidin staining (top row, middle panel, red; compare with control left panel), whereas G-actin, stained green by DNase I, dominates the response to hypotonic exposure (top row, right panel). DAPI-stained nuclei appear blue. Scale bar = 20 μm, magnification = ×100. Bottom: hypertonic conditioning promotes peripheral cortactin translocation (bottom row, middle panel, white densities) compared with isotonic (left) or hypotonic (right) exposure.
Interfacial stress model of injury.
Cells were plated on glass coverslips, cultured to confluency, and subsequently placed into a custom fabricated microfluidic chamber based on a design modified from Bilek et al. (1). The chamber was mounted on a fluorescence-capable microscope and filled with isotonic (290 mOsm), hypotonic (250 mOsm), or hypertonic (340 mOsm) HMEM solution containing propidium iodide (PI) to measure PM integrity and Hoechst 34580 dye to aid in counting of cell nuclei. A syringe pump (New Era Pump Systems, Farmingdale, NY) was used to inject or withdraw air into/from the chamber at a constant rate so that the air-liquid interface (gas bubble) advanced at a velocity of 2 mm/s.
Fluorescence microscopy was performed before and after each bubble run to quantify the fraction of PI-positive cells (Fig. 4A). Cells that lost adherence and were washed away by the interfacial force were assumed injured and PI-positive for reporting purposes. We verified independently that analyses restricted to adherent cells yielded the same conclusions (Supplemental Table S1). Statistical comparisons were made using one-way ANOVA and post hoc t-tests for paired observations.
Fig. 4.
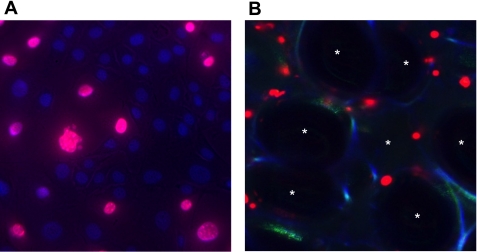
A: fluorescence image of an A549 cell monolayer 5 s after exposure to interfacial stress (a gas bubble) in a microchannel. Wounded cells may be identified by their red nuclear PI fluorescence. The nuclei of uninjured cells appear blue on account of Hoechst 34580 staining. B: confocal image of subpleural alveoli in a rat lung subjected to injurious mechanical ventilation. Individual alveolar spaces are labeled with asterisks. Alveolar walls may be identified by their blue autofluorescence. PI-stained nuclei (red) indicate transient or permanent loss of cellular PM integrity resulting from injurious mechanical ventilation.
Isolated perfused rat lung preparation.
As previously described (6), rat lungs were excised, the trachea, right ventricular outflow tract, and the left atrium were cannulated, and the vasculature was perfused using a recirculating pump. The preparation was subsequently placed into an environmentally controlled chamber, and the lungs were mechanically ventilated for 20 min with tidal volumes between 35 and 40 ml/kg and zero positive end-expiratory pressure (PEEP). Groups of eight lungs were randomized to PI-containing Krebs perfusates titrated to osmotic pressures of 250 mOsm (hypotonic), 300 mOsm (isotonic), or 350 mOsm (hypertonic), respectively. A fourth group was perfused with a hypertonic urea solution (349 mOsm). Additional groups of eight rat lungs each were pretreated with hypotonic, isotonic, or hypertonic saline aerosols. At the end of each experiment, subpleural regions of the living (unfixed) lungs were imaged with confocal microscopy, and the number of PI-positive alveolus resident cells were counted (Fig. 4B and Table 1). The number of PI-positive cells per alveolus defined a cell injury index (CI), which served as surrogate effect endpoint. We established in several prior and associated control studies that lungs subjected to noninjurious mechanical ventilation have few if any PI-positive cells. All data are presented as means ± SD. ANOVA was used to test for differences between groups. Statistical significance was accepted at P < 0.05.
Table 1.
Lung injury indices in isolated, perfused rat lungs
| Isotonic, 290 mosM | Hypertonic, 340 mosM | Mannitol, 350 mosM | Urea, 349 mosM | Hypotonic, 240 mosM | |
|---|---|---|---|---|---|
| Perfusate | 0.25 ± 0.04 | 0.13 ± 0.02* | 0.09 ± 0.05* | 0.30 ± 0.03 | 0.31 ± 0.06 |
| Aerosol | 0.34 ± 0.14 | 0.19 ± 0.10* | 0.40 ± 0.25 |
Values are lung injury indices ± SE, n = 8 per group. Isolated, perfused rat lungs perfused or nebulized with hypertonic solutions had significantly fewer injured cells than lungs conditioned with any other solution.
P < 0.05 vs. isotonic. Please see materials and methods for complete details.
RESULTS
Osmotic stress alters PM-CSK adhesive interactions.
We measured osmotic stress-induced changes in the retractive force of PM lipid tethers of epithelial and mesenchymal cells using a prototype optical trapping system. The technique and its underlying assumptions had been developed and discussed in a series of papers by Raucher et al. (13–17), who demonstrated that PM tether tension is dominated by adhesive interactions between lipid bilayer and the underlying CSK as opposed to by stress in the plane of the PM. Figure 2 shows a schematic of our experimental approach (A and B) as well as the biophysical determinants of lipid tether retractive force (C).
In A549 cells, a 50-mOsm increase in extracellular medium osmolarity (isotonic = 290 mOsm) led to a visible change in cell size accompanied by a 42% increase in tether retractive force from 14.7 ± 1.0 to 26.2 ± 5.2 pN at 1 min (n = 10; P < 0.01). Conversely, a 50 mOsm decrease in extracellular medium osmolarity resulted in cell swelling accompanied by a 34% force reduction from 14.4 ± 1.4 to 9.4 ± 1.6 pN (n = 7; P < 0.05). Response patterns similar to those observed in A549 cells were demonstrated in freshly harvested rat AT2 (Fig. 5A). In AT2, the transition to a hypertonic milieu caused a 41% increase in lipid tether retractive force to 25.1 ± 1.6 pN (n = 8; P < 0.01) by 1 min, whereas it decreased 5.0 ± 2.1 pN from 14.4 ± 1.4 to 9.4 ± 1.2 pN (n = 6; P < 0.05) or 58% under hypotonic conditions (n = 4; P < 0.05). Rat AT1 cells responded similarly to AT2 wherein a hypertonic exposure resulted in a 39% (n = 6; P < 0.02) increase, and hypotonic a 22% (n = 4; P = 0.053) decrease in force from baseline.
Fig. 5.
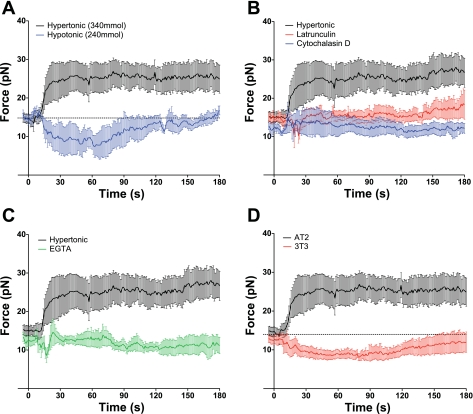
Lipid tether retractive force measurements in primary rat alveolar epithelial type 2 (AT2) cells exposed to 50-mOsm changes in osmotic pressure. Data are presented as means and SE. A: lipid tether retractive force increased after a hypertonic challenge (340 mOsm, black) and decreased following hypotonic (240 mOsm, blue) exposure. B: CSK disruption by 1 μM cytochalasin D (blue) and 500 nM latrunculin A (red) abolished the osmotic pressure-mediated increase in force (black). C: extracellular calcium chelation by 10 mM EGTA (green) abolished the osmotic-mediated increase in force (black). D: Swiss 3T3 fibroblasts (red) and epithelial rat AT2 (black) differ in their lipid tether force response to a hypertonic challenge. Although an increase in osmotic pressure raised the retractive force in epithelial cells, the same stimulus reduced tether force in mesenchymal cells.
Given the changes in epithelial cell volume, the corresponding changes in tether retractive force would be difficult to reconcile with a cell mechanics model, in which the lipid bilayer of the undeformed cell is an important stress-bearing structure. Therefore, we investigated the influence of the CSK polymerization state on the recoil response of the tether to osmotic stress. Consistent with prior reports on hypertonically challenged neutrophils (18) and sucrose-perfused alveolar capillaries (19), exposure of A549 cells to hypertonic media was associated with the translocation of cortactin to the subcortical CSK and consequent F-actin nucleation and assembly (Fig. 3) (21). Conversely, hypotonic cell swelling was associated with remodeling from F- to G-actin.
To test whether osmotic stress-related CSK remodeling influences adhesive interactions with the PM lipid bilayer, we exposed AT2 cells (Fig. 5, B and C) to cytochalasin D, latrunculin, or EGTA. These agents attenuated the increase in force to hypertonic challenge by 85 ± 35, 71 ± 23, and 94 ± 44%, respectively (n = 6; P < 0.01). Similar reductions (n = 4; P < 0.05) were observed in AT1 cells preincubated with either latrunculin (88 ± 35%) or EGTA (111 ± 23%), again supporting similarities in osmotic response across epithelial cell lines.
Because our observations on epithelial cells contradict earlier reports of the osmotic stress responses of Swiss 3T3 fibroblasts (14), we made measurements of tether mechanics in these mesenchymal cells as well. In contrast to AEC, exposure of 3T3 fibroblasts to a 50-mOsm increase in medium osmolarity (Fig. 5D) resulted in a 28% decrease in membrane retractive force from 12.5 ± 1.2 to 9.0 ± 1.1 pN (P < 0.03; n = 6), whereas the transition to a hypoosmotic environment was associated with an increase in tether retractive force. This observation suggests that the biophysical responses of PM and CSK to osmotic stress are cell type-specific. Although we have yet to delineate the responsible molecular mechanisms for the divergent osmotic stress responses, they do provide an opportunity to test our hypothesis, which is general and should apply to all cells, in both cell species.
CSK-PM adhesive force is negatively correlated with the probability of PM wounding by interfacial stress.
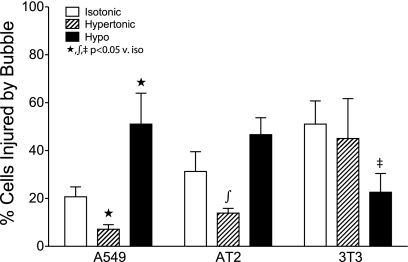
A549 and freshly harvested rat AT2 cells were cultured to confluency on glass coverslips and then placed in a custom fabricated microfluidics chamber on a fluorescence-enabled microscope. Cells were exposed to a compressive interfacial stress associated with the passage of an air bubble through the chamber and across the monolayer at a defined rate (1). PI was used as a marker of PM integrity, and cells were imaged before and immediately after the bubble pass to determine cellular injury rates (Fig. 4A). Under isotonic (290 mOsm) conditions, the stress associated with a single sweep of an air-liquid interface at a rate of 2 mm/s caused PM disruptions in 20.7 ± 4.2% (n = 20) of A549, in 31.3 ± 8.2% (n = 5) of AT2, and in 51.9 ± 9.0% (n = 14) of 3T3 cells, respectively (Fig. 6). Ten-minute conditioning with hypertonic (340 mOsm) HMEM reduced the number of injured A549 cells by 65% from 20.7 ± 4.2 to 7.1 ± 1.9% (n = 10; P < 0.01) and that of AT2 by 55% from 31.3 ± 8.3 to 13.9 ± 2.0% (n = 5; P < 0.05). In contrast, incubating 3T3 fibroblasts with hypertonic media had no significant effect on interfacial stress-related injury (51.9 ± 9.0 vs. 45.0 ± 16.7%, n = 12), whereas a hypotonic (240 mOsm) exposure reduced injury to 22.6 ± 7.9% (n = 11).
Fig. 6.
Effects of osmotic conditioning on the probability of cell injury by interfacial stress. Confluent A549, AT2, and 3T3 cell monolayers were exposed to a single bubble pass in a microchannel, and the fraction of wounded cells was measured under 3 osmotic stress conditions (PI-positive/total cells per ×10 image field; see Fig. 4A). Data are presented as means and SE. When conditioned in hypertonic media (dashed bars), monolayers of the epithelial A549 and AT2 cells contained significantly fewer injured cells than monolayers conditioned with isotonic (white bars) or hypotonic media (Hypo; black bars). In contrast to epithelial cells, in mesenchymal 3T3 cells there was no significant difference in injury rates between isotonic (v. iso) and hypertonic media, whereas hypotonic exposure significantly reduced the number of injured cells.
Hypertonic conditioning preserves alveolar cell integrity in ex vivo ventilated and perfused rat lungs.
Ex vivo perfused and mechanically ventilated rat lungs were assessed for alveolus resident cell injury using confocal imaging (Fig. 4B) as previously described (6). Compared with noninjured controls, injurious mechanical ventilation caused a substantial increase in the number of wounded, and hence PI-positive, subpleural cells. Table 1 details the responses of isolated perfused rat lungs to injurious ventilation and the effects of varying osmotic exposures on them. Lungs perfused with hypertonic Krebs solutions or mannitol had significantly fewer injured cells (CI of 0.13 ± 0.02 and 0.09 ± 0.05) than lungs conditioned with either isotonic or hypotonic perfusates (0.25 ± 0.04 and 0.31 ± 0.06, respectively). In contrast, hypertonic perfusates containing urea were not cell-protective.
Osmoprotective effects were also demonstrated in lungs following hypertonic aerosol delivery. Lungs that had been pretreated with hypertonic saline aerosols had significantly fewer PI-positive cells compared with those treated with either normal saline or distilled water, as reflected in injury index values of 0.34 ± 0.14 vs. 0.40 ± 0.25 and 0.20 ± 0.11, respectively (P < 0.01).
DISCUSSION
In the current study, we focus on a specific mechanotransduction event that we believe contributes to the pathogenesis of VILI: interfacial stress-related wounding of small airway and alveolar epithelial cells. We view the cell as a cytosol-soaked CSK sponge covered by a relatively impermeable lipid bilayer (Fig. 1) and, in turn, show a hyperosmotic model exploit as both proof of concept and a possible cell-protective intervention (Fig. 1D). To establish the importance of PM-CSK interactions on cell mechanics and interfacial stress responses, we conducted lipid tether recoil force measurements at baseline, during small step changes in milieu osmolarity, and under states of altered CSK organization (Fig. 5B) and free water activity (Fig. 5C). Across all experimental conditions, the relative adhesion of PM lipids to the underlying CSK proteins dominated epithelial cell responses. Hypertonic exposure-induced reductions in cell volume and water activity were associated with increases in tether recoil, whereas hypotonic cell swelling had the opposite effect. This indicates that in AEC, the resting tension in the plane of PM must be relatively low. However, this may not be the case in Swiss 3T3 cells because in them the PM tether recoil force decreased following a hypertonic challenge, as if a decrease in cell volume unloaded a tensed PM lipid bilayer. Regardless of the specific mechanism, the divergent, cell-specific osmotic responses represented an opportunity to test the general validity of our cell mechanics model and overarching hypothesis.
Once we knew that we could measure and manipulate the PM-CSK adhesive interactions, we investigated their importance toward membrane wounding (6). Using an experimental approach originally described by Bilek et al. (1) and Kay et al. (10), we confirmed the group's observation that the tension of an advancing air-liquid interface in a microchannel is sufficiently large to deform and wound epithelial lining cells. This injury mechanism is thought to explain lung damage associated with the cyclic recruitment and derecruitment (“opening and collapse”) of unstable units (7, 9, 12). Not only were we able to show that the compressive stress of an advancing air-liquid interface produces cell blebbing (Fig. 1, right; Supplemental Movie S1), but also we confirmed the model prediction that osmotic manipulation of PM-CSK adhesion strength is correlated with the probability of cell injury. Accordingly, hypertonic protection against cell injury was confined to epithelial cells and was not observed in 3T3 fibroblasts.
Models of the partially fluid or foam-filled airways have largely focused on the determinants of interfacial stress, whereby the cells of the airway wall were treated as homogenous elastic solids. However, this simplifying assumption ignores small-scale variability in cellular microstructure and does not account for the movement of liquid (cytosol) relative to the solid CSK when the cell is exposed to deforming stress. As elegantly argued by Charras (3) and Mitchison et al. (11), who considered mechanisms in spontaneously blebbing cells, it is more helpful to view the cell as a liquid (cytosol)-soaked sponge (CSK) that is coated with a wrinkled, relatively stiff, and impermeable lipid bilayer. The solid components are represented by a network of interconnected biopolymers and organelles and are bathed in a viscous liquid containing water, ions, small molecules, and soluble proteins. The theory of poroelasticity (2), which had been introduced in geophysics to predict hydraulic stresses in fluid infiltrated rocks, is vastly superior to viscoelasticity as a model of such a two-phase structure.
Deforming stress, irrespective of if it is generated internally by a contracting CSK or by an external surface pressure gradient as may be the case in a fluid-filled small airway, produces cytosolic pressure gradients, which drive a phase separation between cytosol and the CSK network (Fig. 1, B and C, and right). A stress sufficient to deform the cell amounts to squeezing water from a sponge. If the deformed sponge (i.e., CSK) is covered by a stiff, loosely adherent, wrinkled, and mostly impermeable membrane (i.e., the PM), then there is a threshold hydraulic pressure, which separates the two structures, and liquid accumulates between them (i.e., a bleb forms; see Supplemental Movie S1). Once a bleb has formed, stress in the plane of the membrane is transferred from the subcortical CSK to the lipid bilayer, which, in turn, is likely to experience lytic tension.
The probability of phase separation and bleb formation depends largely on the fraction, i.e., the relative spacing between solid elements, and the adhesive energy between PM and subcortical CSK. Every one of these factors is influenced by hypertonic cell conditioning. At least in AEC, exposure to hypertonic media initiates a polymerization response of the subcortical CSK (Fig. 3), which is, in part, mediated and aided by the translocation of nucleation factors such as cortactin and ARP2/3 (24). Consequently, the cell becomes stiffer, i.e., the bulk elastic modulus of the drained solid phase increases. The loss of free water activity associated with cell shrinkage raises the viscosity of the liquid phase and, combined with the decreased fluid fraction (solid phase crowding), increases the hydraulic resistance to cytosol flow (27). As a result, the deformation-associated phase separation, and with it the pressure at the PM-CSK interface, are apt to decrease. As our PM tether tension responses in AEC suggest, the increased adhesive interactions between PM lipids and CSK proteins increase the lytic tension at that interface opposing bleb formation.
The results of our bubble studies (Fig. 6) validate this mechanism of injury and warrant further investigation of a potential hypertonic protective effect to this type of wounding. The rate of A549 and AT2 cell injury caused by a single bubble pass were reduced by approximately half in samples pretreated with hypertonic media. Coupled with the lipid tether data, it appears that hypertonic treatment of AEC decreases cellular free water activity, invariably leading to an increase in PM-CSK adhesive interactions, reducing the probability of bleb formation, and thereby protecting epithelial cells from injury by deforming stress.
The response of epithelial cells to hypotonic media was not as robust. The fact that a hypertonic milieu is cell-protective does not mean that hypotonic cell swelling necessarily predisposes epithelial cells to PM stress failure. It is true that hypotonic cell swelling was associated with a decrease in PM-CSK adhesion. However, it was also associated with a breakdown of the central F-actin network (Fig. 3). This is bound to alter the stress distribution within the cell and may turn a tensegrity structure into one akin to a fluid-filled shell. Rather than stress being distributed throughout the cell by discrete solid elements (22) in a G-actin- and cytosol-filled cell, the remaining subcortical actin fiber network may simply counterbalance a hydrostatic pressure (20). This could fundamentally alter the force transmission and the effects of deforming stress on the PM-CSK interface. This may also explain why some authors have proposed that interventions that decrease cell stiffness may be cytoprotective (26).
Finally, in an effort to translate our basic cellular biophysical measurements into physiologically relevant animal model systems, we engaged in proof-of-concept hypertonic treatment protocols in isolated, perfused rat lungs and in mechanically ventilated rats. The findings (Table 1) support the predictive model established in cell culture. Rat lungs ventilated under injurious settings while simultaneously receiving either hypertonic saline or mannitol perfusions experienced a dramatic reduction in cell injury rate as measured by PI-positive cells per lung field under confocal microscopy at the end of each experiment (Fig. 4B). A similar protection was observed when the lung was exposed to hypertonic saline nebulization during injurious ventilation. Lungs perfused with urea did not receive protection from injury in the same manner as those infused with hyperosmotic mannitol or saline. This finding is reconcilable when the differences in cellular response to these agents are considered. Because of a rapid diffusion and equilibration, cells exposed to urea lack a robust change in cellular volume or free water activity and thereby experience little compensatory regulation, CSK remodeling, and associated changes in PM-CSK adhesive interactions. In short, our model predicts that the PM-CSK characteristics of cells exposed to urea ought more closely resemble those of an isotonic rather than a hypertonic exposed cell, and our injury findings are consistent with those predictions.
Summary.
We have shown that relatively small increases in medium osmolarity have cell-protective effects in experimental models of wounding relevant to VILI and have suggested an attractive mechanism of action, namely the prevention of bleb formation in cells exposed to deforming stress. We have provided experimental evidence from PM tether mechanics, interfacial stress injury, and isolated perfused rat lung models to support this paradigm and have uncovered potentially important phenotypic differences in cellular response to osmotic exposure that deserve further investigation with regard to wounding and repair mechanism as well as therapeutic applicability.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-63178 and the Mayo Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1.Bilek AM, Dee KC, Gaver DP., 3rd Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol 94: 770–783, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Biot MA. Mechanics of deformation and acoustic propagation in porous media. J Appl Physiol 33: 1482–1498, 1962 [Google Scholar]
- 3.Charras GT. A short history of blebbing. J Microsc 231: 466–478, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Charras GT, Coughlin M, Mitchison TJ, Mahadevan L. Life and times of a cellular bleb. Biophys J 94: 1836–1853, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobbs LG, Gonzalez R, Williams MC. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis 134: 141–145, 1986 [DOI] [PubMed] [Google Scholar]
- 6.Gajic O, Lee J, Doerr CH, Berrios JC, Myers JL, Hubmayr RD. Ventilator-induced cell wounding and repair in the intact lung. Am J Respir Crit Care Med 167: 1057–1063, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Ghadiali SN, Gaver DP. Biomechanics of liquid-epithelium interactions in pulmonary airways. Respir Physiol Neurobiol 163: 232–243, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guilford WH, Dupuis DE, Kennedy G, Wu J, Patlak JB, Warshaw DM. Smooth muscle and skeletal muscle myosins produce similar unitary forces and displacements in the laser trap. Biophys J 72: 1006–1021, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain M, Sznajder JI. Bench-to-bedside review: distal airways in acute respiratory distress syndrome. Crit Care 11: 206, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kay SS, Bilek AM, Dee KC, Gaver DP., 3rd Pressure gradient, not exposure duration, determines the extent of epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol 97: 269–276, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Mitchison TJ, Charras GT, Mahadevan L. Implications of a poroelastic cytoplasm for the dynamics of animal cell shape. Semin Cell Dev Biol 19: 215–223, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naire S, Jensen OE. Epithelial cell deformation during surfactant-mediated airway reopening: a theoretical model. J Appl Physiol 99: 458–471, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Raucher D, Sheetz MP. Cell spreading and lamellipodial extension rate is regulated by membrane tension. J Cell Biol 148: 127–136, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raucher D, Sheetz MP. Characteristics of a membrane reservoir buffering membrane tension. Biophys J 77: 1992–2002, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raucher D, Sheetz MP. Membrane expansion increases endocytosis rate during mitosis. J Cell Biol 144: 497–506, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raucher D, Sheetz MP. Phospholipase C activation by anesthetics decreases membrane-cytoskeleton adhesion. J Cell Sci 114: 3759–3766, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Raucher D, Stauffer T, Chen W, Shen K, Guo S, York JD, Sheetz MP, Meyer T. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell 100: 221–228, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Rizoli SB, Rotstein OD, Parodo J, Phillips MJ, Kapus A. Hypertonic inhibition of exocytosis in neutrophils: central role for osmotic actin skeleton remodeling. Am J Physiol Cell Physiol 279: C619–C633, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Safdar Z, Yiming M, Grunig G, Bhattacharya J. Inhibition of acid-induced lung injury by hyperosmolar sucrose in rats. Am J Respir Crit Care Med 172: 1002–1007, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spagnoli C, Beyder A, Besch S, Sachs F. Atomic force microscopy analysis of cell volume regulation. Phys Rev E Stat Nonlin Soft Matter Phys 78: 031916, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thirone AC, Speight P, Zulys M, Rotstein OD, Szaszi K, Pedersen SF, Kapus A. Hyperosmotic stress induces Rho/Rho kinase/LIM kinase-mediated cofilin phosphorylation in tubular cells: key role in the osmotically triggered F-actin response. Am J Physiol Cell Physiol 296: C463–C475, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang N, Naruse K, Stamenovic D, Fredberg JJ, Mijailovich SM, Tolic-Norrelykke IM, Polte T, Mannix R, Ingber DE. Mechanical behavior in living cells consistent with the tensegrity model. Proc Natl Acad Sci USA 98: 7765–7770, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, Hubmayr RD. Type I alveolar epithelial phenotype in primary culture. Am J Respir Cell Mol Biol (July8, 2010). doi:10.1165/rcmb.2009-0359OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weed SA, Karginov AV, Schafer DA, Weaver AM, Kinley AW, Cooper JA, Parsons JT. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J Cell Biol 151: 29–40, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 369: 1553–1564, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Yalcin HC, Hallow KM, Wang J, Wei MT, Ou-Yang HD, Ghadiali SN. Influence of cytoskeletal structure and mechanics on epithelial cell injury during cyclic airway reopening. Am J Physiol Lung Cell Mol Physiol 297: L881–L891, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Zhou EH, Trepat X, Park CY, Lenormand G, Oliver MN, Mijailovich SM, Hardin C, Weitz DA, Butler JP, Fredberg JJ. Universal behavior of the osmotically compressed cell and its analogy to the colloidal glass transition. Proc Natl Acad Sci USA 106: 10632–10637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.