Abstract
Several studies have implicated gamma-herpesviruses, particularly Epstein-Barr virus (EBV), in the progression of idiopathic pulmonary fibrosis. The data presented here examine the possible role that EBV plays in the potentiation of this disease by evaluating the pulmonary response to expression of the EBV lytic transactivator protein Zta. Expression of Zta in the lungs of mice via adenovirus-mediated delivery (Adv-Zta) produced profibrogenic inflammation that appeared most pronounced by day 7 postexposure. Relative to mice exposed to control GFP-expressing adenovirus (Adv-GFP), mice exposed to Adv-Zta displayed evidence of lung injury and a large increase in inflammatory cells, predominantly neutrophils, recovered by bronchoalveolar lavage (BAL). Cytokine and mRNA profiling of the BAL fluid and cells recovered from Adv-Zta-treated mice revealed a Th2 and Th17 bias. mRNA profiles from Adv-Zta-infected lung epithelial cells revealed consistent induction of mRNAs encoding Th2 cytokines. Coexpression in transient assays of wild-type Zta, but not a DNA-binding-defective mutant Zta, activated expression of the IL-13 promoter in lung epithelial cells, and detection of IL-13 in Adv-Zta-treated mice correlated with expression of Zta. Induction of Th2 cytokines in Zta-expressing mice corresponded with alternative activation of macrophages. In cell culture and in mice, Zta repressed lung epithelial cell markers. Despite the profibrogenic character at day 7, the inflammation resolves by 28 days postexposure to Adv-Zta without evidence of fibrosis. These observations indicate that the EBV lytic transactivator protein Zta displays activity consistent with a pathogenic role in pulmonary fibrosis associated with herpesvirus infection.
Keywords: cytokines, T-helper phenotype, alternative macrophage activation
idiopathic pulmonary fibrosis (IPF) is an interstitial lung disease of unknown etiology that is characterized by fibroblastic foci and excessive extracellular matrix deposition in the patchy pathological pattern known as usual interstitial pneumonia (54). Classically, IPF is diagnosed in people over age 50 with a mean age of 66 years old and a median survival between 2–5 years (1, 14). The clinical progression of IPF can be characterized as either chronic or acute. The chronic manifestation of this disease displays a steady and progressive decline in respiratory function over years, while acute exacerbation of the disease is characterized by rapid decreases in lung function over 1 or 2 mo (15, 80).
Several lines of evidence suggest that viral infection contributes to the pathogenesis of IPF. The connection between active and/or latent herpesvirus infections has been demonstrated through serological analysis of patients with IPF (81) and by the detection of viral DNA in lung epithelial cells of IPF patients (19, 20, 75, 78). Animal models support the paradigm connecting herpesvirus infections with specific T-helper 2 (Th2) cytokine profiles and alternative macrophage activation (13, 60, 83) as cofactors for the development of pulmonary fibrosis (80). Moreover, recent evidence indicates that herpesvirus promotes epithelial-mesenchymal transition, a process that occurs during fibrosis (49, 69).
One possible way that Epstein-Barr virus (EBV), a member of the herpesvirus family, may act as an agent that contributes to IPF is by activating expression of cellular genes involved in the inflammatory response. A rearrangement of the EBV genome (WZhet) that occurs in 61% of lung biopsies from IPF patients positive for EBV DNA activates constitutive expression of the viral transcription factor Zta (31). Zta triggers the switch from latent to lytic infection by forming a DNA binding homodimer and activating transcription of associated target genes (68). Zta also interacts with several key cellular transcriptional regulatory proteins that affect inflammation including NF-κB (61), p53 (53), and the histone acetylase CREB-binding protein (2). Zta-mediated regulation of cellular genes involved in the inflammatory process such as IL-8 (27), IL-13 (79), and TGF-β (11, 27) has also been demonstrated.
Here we have investigated the possible role of Zta in lung inflammation and IPF by delivering a replication-defective Zta-expressing recombinant adenovirus (Adv-Zta) to in vitro and in vivo model systems. This approach reveals the complexity of the cross talk between cell types in an animal and benefits from the ability to individually assess the contribution of immune and epithelial cells. The analysis of Adv-Zta-infected mice and lung epithelial cells suggests that Zta regulates expression of cellular genes that promote profibrogenic inflammation and differentiation.
MATERIALS AND METHODS
Experimental animals.
Wild-type C57BL/6 mice were derived from breeding pairs in the Tulane vivarium or obtained from Jackson Laboratories (Bar Harbor, ME). All mice were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility according to National Institutes of Health guidelines. All animal protocols received review and approval from the Institutional Animal Care and Use Committee. Mice were maintained on a 12-h light/12-h dark schedule and given food and water ad libitum.
Plasmids.
pGL-3 control luciferase and pRL-TK luciferase were purchased from Promega. p2X(ZIIIB)BG luciferase, pRCCMV, and pRCCMV-Zta were described previously (42). An IL-13 promoter reporter construct (−1172C/luc) (10) was provided by Donata Vercelli (Univ. of Arizona). An E-cadherin luciferase reporter plasmid (pGL2Basic-EcadK1, Addgene plasmid 19290) was provided by Eric Fearon (Univ. of Michigan). pRCCMV-Zta-S186D was prepared by site-directed mutagenesis of the parent plasmid pRCCMV-Zta using the following primers: forward 5′-caagaatcgggtggctgacagaaaatgccgggcc-3′, reverse 5′-ggcccggcattttctgtcagccacccgattctt-3′. Mutagenic PCR reactions were set up by combining 50 ng of forward and 50 ng of reverse primers with 4 μl 5× HF buffer, 0.60 μl DMSO, 0.4 μl of dNTPs (10 mM stock), 100 ng of parent plasmid, 0.4 units of Phusion polymerase (New England Biolabs, Ipswich, MA), and nuclease-free water to a final volume of 20 μl. PCR reactions were performed using the following cycling conditions: 98°C for 30 s followed by 25 cycles of 98°C for 10 s, 45°C for 30 s, 72°C for 5 min, followed by final elongation at 72°C for 10 min. The pRCCMV-Zta parental vector was then restriction digested with DpnI. The resulting product was then cloned into Escherichia coli MAX Efficiency DH5α (Invitrogen, Carlsbad, CA). The S186D mutation was verified by automated DNA sequencing. All plasmid constructs were purified by double banding on cesium chloride density gradients as described (4).
Adenovirus culture and titer.
An adenovirus construct containing the EBV BZLF1 gene encoding the transcriptional activator Zta (referred to as Adv-Zta) was obtained from Shannon Kenney (Univ. of Wisconsin-Madison). The GFP-expressing adenovirus (Adv-GFP) was provided by Jay Kolls (Louisiana State Univ. School of Medicine).
Adenovirus was propagated using media collected from 293 cells infected with adenovirus essentially as described (33). After CsCl centrifugation, the purified virus band was collected between the CsCl layers, and the CsCl was removed by passing the virus suspension over an Econo-Pac 10 DG desalting column (Bio-Rad, Hercules, CA) equilibrated in virus storage buffer (150 mM NaCl, 20 mM HEPES, pH 7.8). The virus concentration was estimated using a spectrophotometer A260 to give approximate particles/ml. Sterile glycerol was then added to the final adenovirus preparation to give a final concentration of 10% glycerol in virus storage buffer. The titer of the adenovirus was determined by performing plaque assays to assess plaque-forming units/ml (pfu/ml).
Adenovirus exposure.
For adenovirus exposure, a fresh aliquot of virus (Adv-Zta or Adv-GFP) was defrosted and diluted to 1 × 108 pfu into 50 μl of PBS. Mice were anesthetized using isoflurane, and the virus or PBS vehicle alone was administered at 1 × 108 pfu/animal by oropharyngeal aspiration as described (38). After adenovirus exposure, the mice were allowed to recover and then weighed daily until death.
Bronchoalveolar lavage.
Bronchoalveolar lavage (BAL) was performed on mice as outlined (22). Total recovered lavage fluid ranged from 3.1 to 3.9 ml. The first lavage sample was centrifuged at 1,500 g for 5 min at 4°C to pellet the cells and was then aliquoted and stored at −70°C. The cells from the first lavage sample were then resuspended in 500 μl of lavage buffer and combined with lavages two to five. The total cell count was recorded by mixing 10 μl of the resuspended cells 1:1 with Trypan blue (MP Biomedicals, Solon, OH) and counted on a Bright-Line Hemacytometer. Cells (5 × 104) were then cytospun onto slides. The slides were allowed to dry, stained with Hema 3 (Fisher Scientific, Pittsburgh, PA), and allowed to dry. The slides were then dipped in xylene and mounted using permount (Fisher Scientific). Differential cell counts were performed on 100 cells/sample by an investigator who was unaware of the identity of the samples.
Protein and cytokine analyses.
Cytokine profiling of BAL samples was performed on a Luminex Bio-Plex 200 system using the Bio-Plex Mouse Cytokine 23-Plex Panel (Bio-Rad). Briefly, BAL samples were defrosted on ice, and 0.5% (wt/vol) BSA was added as a carrier protein before sample loading onto the supplied filter plate. Cytokine standards were reconstituted in lavage buffer (described above) supplemented with 0.5% BSA and diluted to produce the broad range standard curve as outlined in the Bio-Plex instruction manual. All subsequent steps were conducted as outlined in the Bio-Plex cytokine assay instruction manual. Cytotoxicity was measured by detection of enzymatic activity of lactate dehydrogenase in BAL fluid using the BioVision LDH-cytotoxicity assay kit II (BioVision, Mountain View, CA). Protein concentrations in BAL samples were determined using the BCA protein assay kit (Pierce, Rockford, IL) by regression analysis of a standard curve constructed using BSA diluted in lavage buffer. Equal volumes of BAL fluid were analyzed for MMP-9 by gelatin zymography using precast Novex zymogram gels (Invitrogen). Active and total TGF-β levels in the BAL fluid were determined by ELISA (R&D Systems, Minneapolis, MN) with and without prior acidification of the samples, respectively.
Histology.
Lungs were prepared by tying off the right lung with surgical suture followed by removal and snap freezing in liquid nitrogen. The trachea was then exposed and intubated using a 20-gauge 1.25-inch catheter secured in place with surgical suture. The left lung was then inflated at 30-cm pressure with 10% (wt/vol) formalin (Sigma-Aldrich, St. Louis, MO) for 20 min before removal. The left lung was then incubated overnight in 10% (wt/vol) formalin and then transferred into PBS the following day. The fixed left lung was embedded in paraffin, and 5-μm sections were stained to reveal anatomic details. Trichrome staining was performed using the Gomori One Step Trichrome Stain Kit (Newcomersupply, Middleton, WI) according to the supplier's instructions. Images were captured on an Olympus BX50 microscope equipped with a Pixera Pro 600 ES camera using Pixera studio software.
Immunohistochemistry.
Deparaffinized and rehydrated sections were stained with primary antibodies for 1 h in a humid chamber using the following concentrations in antibody diluent (PBS with 1% BSA): 20 μg/ml arginase I (V-20; Santa Cruz Biotechnology, Santa Cruz, CA); 25 μg/ml iNOS/NOS Type II (BD Biosciences, San Jose, CA); and 2 μg/ml IL-13 (Santa Cruz Biotechnology). Samples were washed 3× in antibody diluent and then incubated with antibody diluent containing 0.55 μg/ml biotin-SP-conjugated AffiniPure donkey anti-rabbit IgG or 0.65 μg/ml biotin-SP-conjugated AffiniPure donkey anti-goat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). Samples were then washed 3× in antibody diluent and then incubated for 1 h with 0.5 μg/ml peroxidase-conjugated streptavidin (Jackson ImmunoResearch Laboratories) followed by three washes in PBS and one in 50 mM Tris·HCl, pH 7.4. The samples where then incubated for ∼10 min in freshly prepared DAB staining solution [0.93 mM 3,3′-diaminobenzidine tetrahydrochloride, 50 mM Tris·HCl, pH 7.4, 0.006% (vol/vol) H2O2]. The reaction was stopped by washing 2× in deionized H2O before counterstaining for 20 s in Gill's hematoxylin #3; 20 s 0.52 M acetic acid; 30 s deionized H2O; 40 s bluning reagent (1.4% saturated LiCO solution); 30 s deionized H2O. After counterstaining, the samples were dehydrated using an ethanol step gradient and then washed in xylene before mounting with permount. IL-13 positivity was determined by counting the number of stained cells/field at ×400 magnification.
Immunofluorescence.
Epifluorescent staining of lung sections for Zta and GFP was performed using the following primary antibodies: anti-Epstein-Barr Virus-Zebra (Argene, Varilhes, France; diluted 1:100), anti-green fluorescent protein rabbit IgG fraction (Invitrogen; diluted 1:200) or rabbit anti-surfactant protein C (Millipore; diluted 1:2,000) or goat anti-CC10 (Santa Cruz Biotechnology; diluted 1:800) in PBS + Ca + Mg containing 5% donkey serum and 0.5% Triton X-100 and 0.14 μg/ml DAPI. Secondary donkey Alexa Fluor antibodies (594 or 488, Invitrogen) were diluted 1:1,000 in the same solution. Images were obtained on a Zeiss Axioplan II microscope (Carl Zeiss, Thornwood, NY) and processed as previously described (26) or on an Olympus BX60 fluorescent microscope using Magnafire image acquisition software. For E-cadherin immunofluorescence, A549 cells were grown in chamber slides (Lab-Tek II) to near confluence and switched to low (0.5%) serum for 1 day before infection with Adv-Zta or Adv-GFP at a multiplicity of infection of 20 in 0.2 ml of PBS for 1 h. After infection, the cells were returned to culture in DMEM and 0.5% serum. After 3 days, the infected cells were rinsed three times with PBS and fixed by incubating with 4% paraformaldehyde in PBS at room temperature for 10 min followed by three washes with PBS and storage at 4°C. The fixed cells were permeabilized with 0.2 ml of PBS plus 0.2% Triton X-100 for 5 min. After blocking with PBS, 0.1% Triton, and 5% BSA for 1 h, the slides were rinsed three times with PBS and incubated overnight at 4°C in 100 μl of PBS plus 1% BSA with mouse anti-E-cadherin antibody (1:50, Santa Cruz Biotechnology). The primary antibody was removed, and the slides were washed in 200 μl of PBS plus 0.1% Triton three times for 5 min each. Then, the slides were incubated in 100 μl of PBS plus 0.1% Triton including Alexa Fluor 594 donkey anti-mouse antibody (1:1,000, Invitrogen) and 0.14 μg/ml DAPI at room temperature for 1 h. After washing with 200 μl of PBS plus 0.1% Triton three times for 5 min each, the slides were rinsed with water and mounted with a coverslip using Prolong Gold antifade (Invitrogen). Images were captured using an Olympus BX60 fluorescent microscope and Magnafire image acquisition software.
Cell culture.
The immortalized mouse lung type II cell line, C10, was obtained from L. M. Anderson (NCI) and originally isolated from BALB/C mice (50). C10 mouse lung epithelial cells were cultured in CMRL supplemented with 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin. When 10-cm plates of cells were ∼75% confluent, the media was removed and replaced with 3 ml of CMRL containing 1.5 × 108 pfu of Adv-Zta, Adv-GFP, or PBS (moi = 20). The cells were then placed back in the incubator and shaken every 15 min for 1 h. At the end of 1 h, the media was replaced with fresh CMRL supplemented with 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin. The following day, media was removed and stored at −70°C, and the cells were trypsinized, washed, and stored as a pellet at −70°C until further use. Survival of adenovirus-infected C10 cells was determined by MTT assays in a microtiter plate format as described by the assay supplier (Promega).
RNA isolation and quantitative RT-PCR.
Total RNA used for assessing the levels of mRNA transcripts was isolated from frozen, adenovirus-treated C10 cell pellets using an RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer's protocol and stored at −80°C until further use.
Total RNA prepared by Qiagen RNeasy column extraction was DNase I (Invitrogen) treated and reverse transcribed using SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen) according to the manufacturer's instructions. After DNase treatment, samples were then cooled on ice for 1 min and split into two reaction tubes [1 for cDNA synthesis reaction and 1 for no-reverse transcriptase (negative) control]. Two reaction master mixes, one containing SuperScript III reverse transcriptase/RNaseOUT enzyme mix and one without, were prepared. The appropriate volume of reaction mix was added to each reaction tube, and samples were incubated in a thermocycler at the following conditions: 10 min at 25°C, 50 min at 50°C, and 5 min at 85°C. Reactions were stored at −80°C.
Transcript expression levels were determined in Adv-Zta-, Adv-GFP-, and PBS-treated C10 cells by performing real-time quantitative PCR using the mouse Th-17 for autoimmunity and inflammation RT2 profiler PCR array platform (SA Biosciences, Frederick, MD). The SuperScript III-generated cDNA, diluted 2.5× in nuclease-free TE buffer (Promega), was added to a master mix containing 50% Platinum SYBR-Green qPCR SuperMix-UDG (Invitrogen), 10% Fluorescein-NIST Traceable Standard (stock solution, 0.5 mM, Invitrogen), 4% diluted cDNA, and 36% UltraPure DNase/RNase free distilled water (Invitrogen). This solution was applied to the PCR array 96-well plate containing primer sets, 25 μl/well. Thermal cycling was conducted on a BioRad MyiQ cycler for 10 min at 95°C followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. Dissociation (melting) curve analyses were performed at the end of the qPCR run. Samples from three separate infections were tested. Results obtained from the PCR reactions were imported into the PCR Array Data Analysis Web Portal (http://www.superarray.com/pcrarraydataanalysis.php) for data analysis using the ΔΔCt method. The results were plotted by the software in tabular, heat map, and scatter plot formats.
For isolation of total RNA from lavage samples, total cells collected from BAL fluid (total vol 1.9–3.1 ml) were concentrated by centrifugation at 1,000 g for 10 min and stored at −80°C as a cell pellet. Lavage cell pellets were defrosted on ice, and mRNA was extracted as above for C10 cell pellets with the exception that 350 μl of RLT lysis buffer was used for total RNA extraction and 250 ng of total RNA was used for first-strand synthesis as described above. The quantity and quality of each individual cDNA sample was validated by qPCR using primers for 36B4 (74) and 18S using the following primer pair (18s-reverse 5′GTCGGGAGTGGGTAATTTGC3′; 18S-forward 5′GAGGGAGCCTGAGAAACGG3′). Transcript expression levels were then determined in Adv-Zta- and Adv-GFP-treated BAL cells by combining equal cDNA amounts and performing real-time quantitative PCR using the mouse Th-17 for autoimmunity and inflammation RT2 profiler PCR array platform (SA Bioscience). The SuperScript III-generated cDNA was diluted in nuclease-free water and then added to an equal amount of 2× iQ SYBR® Green Supermix (Bio-Rad). This solution was applied to the PCR array 96-well plate containing primer sets, 25 μl/well.
Transient expression assays.
One day before transfection, DMEM containing 1 × 105 C10 lung epithelial cells was plated in a 24-well plate without antibiotics. The cells were transfected with Lipofectamine 2000 reagent as recommended by the supplier (Invitrogen). Briefly, a DNA mix containing 0.2 μg of the test luciferase reporter, 0.1 μg of the reference luciferase reporter (pRL-TK-luciferase), 0.1 μg of the Zta expression plasmid or empty vector control, and 0.4 μg of sheared salmon sperm DNA was prepared in 150 μl of Optimem (Invitrogen). The Lipofectamine 2000 mix (48 μl Optimem and 2 μl Lipofectamine 2000) was added to the DNA mix followed by a 20-min incubation of the resulting transfection mixture at room temperature. The media on the cells was replaced with the 200-μl transfection mixture, and the cells were returned to the incubator. After 5 h, the transfection mix was removed, and 0.5 ml of DMEM, 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin was added. After 48 h, the transfected cells were processed for luciferase assays with the Dual Luciferase Reporter Assay System as specified by the supplier (Promega). The results with the test luciferase reporter were normalized to the reference luciferase reporter.
Statistical analysis.
Unless otherwise indicated, all data are presented as means ± SE. Unpaired two-tailed t-tests were performed using Prism 4.0 software (Graphpad, La Jolla, CA). The results were considered statistically significant at P < 0.05.
RESULTS
Adenovirus-mediated transient expression of Zta in the lungs of mice.
To determine the effects of Zta expression in the lung, recombinant adenoviruses expressing either Zta (Adv-Zta) or GFP (Adv-GFP) were administered to mice by oropharyngeal aspiration at 1 × 108 pfu. As a control, the PBS vehicle was administered to mice similarly. Treated mice were monitored for daily weight changes. The combined weight change data from two separate experiments showed that mice infected with the Zta-expressing adenovirus displayed a transient decrease (mean daily weight change ± SE of −0.10+/−0.12 g; n = 27) in weight on day 7 that was not observed in Adv-GFP-exposed animals (mean daily weight change ± SE of 0.29+/−0.07 g; n = 28). Subsequent analyses of the adenovirus-treated mice focused on day 7 postexposure.
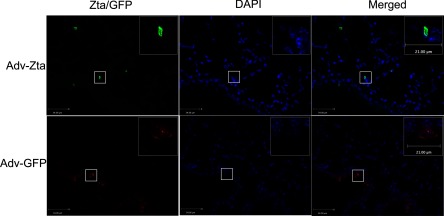
Both Zta and GFP expression were assayed in adenovirus-exposed animals by immunofluorescence microscopy of lung sections. In Adv-Zta-exposed animals, Zta colocalized with DAPI staining of lung epithelial cell nuclei (Fig. 1) indicating that Zta expression is consistent with previously observed nuclear localization (87). Zta-positive immunostaining appeared diffusely in epithelial cells of the lung parenchyma while detection of Zta in the airways appeared sporadic. Zta became detectable by day 3 postexposure and appeared more widespread by day 7 with a decline to low, but still detectable, levels in some mice, by day 14. Mice exposed to Adv-GFP showed prominent GFP immunofluorescence in epithelial cells of the airways in addition to the lung parenchyma (Fig. 1). GFP staining appeared to be primarily cytoplasmic with some diffuse nuclear staining.
Fig. 1.
Immunofluorescent detection of Zta in the lungs of exposed mice. Histological sections from formalin-fixed, paraffin-embedded lung tissue of mice at day 7 postexposure were incubated with an antibody specific for Zta (top, left) or GFP (bottom, left). Visualization of antibody binding (green for Zta and red for GFP) was with a fluorescently tagged second antibody. The 2 right panels show blue nuclear DAPI staining of the same area. The boxed areas in the low-magnification images (×400) are shown magnified in the top, right corner inset (×630). The bar indicates 34 and 21 μm in the low- and high-power images, respectively.
Promotion of lung injury and inflammation by Adv-Zta.
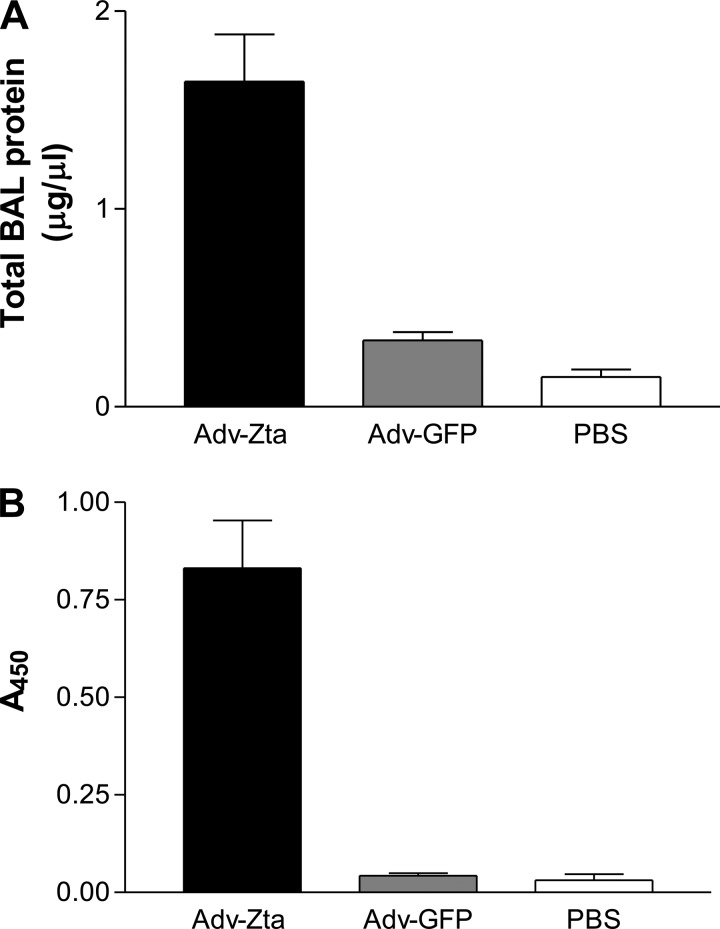
BAL fluid was collected from mice in the three exposure groups on day 7 postexposure. Measurements of total protein and of the intracellular enzyme lactate dehydrogenase (LDH), indicators of mucosal permeability and cell lysis, respectively (18), were performed on the BAL fluid. Administration of the Adv-GFP control vector increased the protein concentration in the BAL fluid approximately twofold relative to the protein concentration in BAL fluid from mice exposed to PBS, whereas the mice treated with Adv-Zta displayed a greater than 10-fold increase in protein concentration relative to the PBS-exposed control mice (Fig. 2A). LDH levels in the BAL fluid from Adv-Zta-treated mice were approximately 20 times higher than those in the BAL fluid from Adv-GFP-treated mice, which were comparable to the PBS control LDH levels (Fig. 2B). These data suggest that Zta expression in the lung promoted injury. To test effects of Zta on survival in cell culture, murine lung epithelial (C10) cells growing in microtiter wells were infected with Adv-Zta or Adv-GFP at increasing moi and were monitored for survival by MTT assay. Adv-GFP up to a moi of 200 did not alter survival of C10 through 48 h. Adv-Zta infection up to a moi of 200 did not alter survival of C10 cells over 24 h, but cells infected at a moi ≥50 did not survive longer than 48 h (data not shown).
Fig. 2.
Evidence of lung injury after Adv-Zta treatment. Bronchoalveolar lavage (BAL) fluid was prepared from mice harvested on day 7 postexposure to Adv-Zta, Adv-GFP, or vehicle (PBS). The graphs display mean values ± SE for mice treated with Adv-Zta (black bars; n = 9), Adv-GFP (gray bars; n = 10), and PBS (white bars; n = 4). A: BAL protein levels. The protein concentration in the BAL fluid as determined by bicinchoninic acid protein assay for the indicated treatment is shown. Adv-Zta vs. Adv-GFP, P < 0.0001; Adv-GFP vs. PBS, P = 0.0539. B: levels of lactate dehydrogenase (LDH) activity in the BAL fluid from treated mice. The BAL fluid from treated mice was assayed for LDH by colorimetric assay in a microtiter plate format. The graph shows LDH activity (A450) in the BAL fluid from mice in the indicated treatment group. Adv-Zta vs. Adv-GFP, P = 0.0003; Adv-GFP vs. PBS, P = 0.3217.
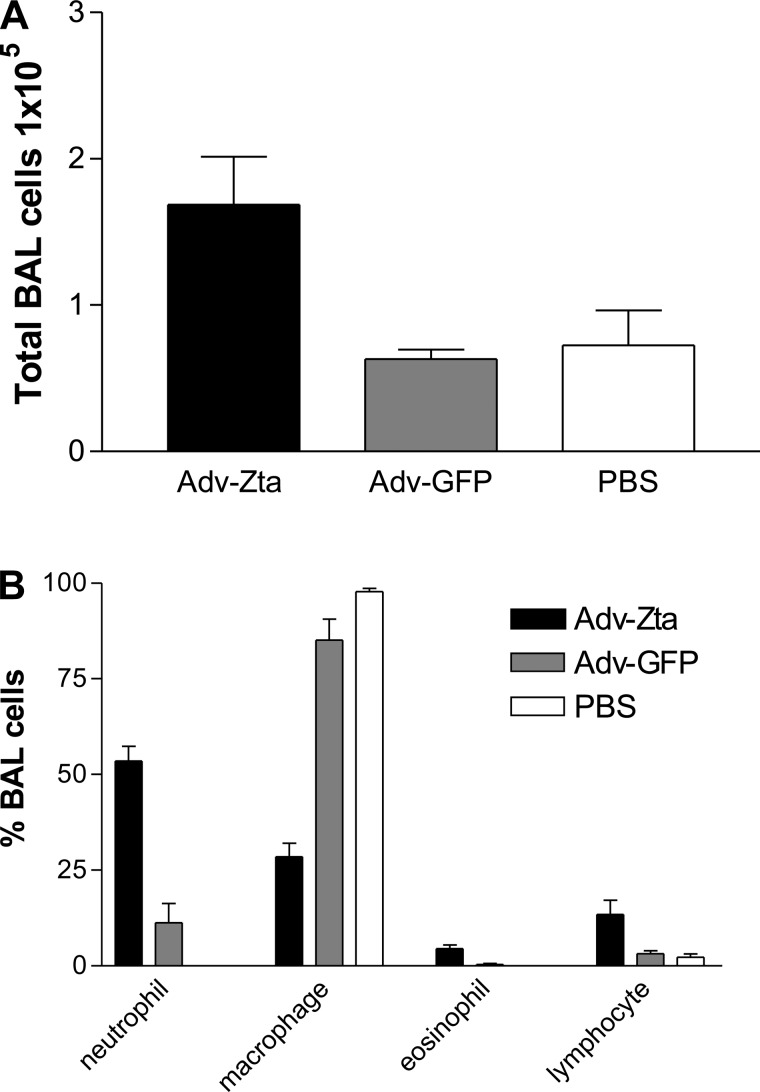
The cells recovered in the BAL fluid from exposed mice were evaluated to assess lung inflammation. The total number of cells in the BAL fluid from Adv-GFP-treated mice was comparable to that in the PBS-treated control mice (Fig. 3A). Adv-Zta induced a greater than twofold increase in total BAL cells relative to the total cell number in the BAL fluid from PBS-treated mice. The composition of inflammatory cells in the BAL fluid was determined by differential cell staining (Fig. 3B). The BAL fluid from PBS-treated control mice contained primarily macrophages. A small increase in neutrophils (∼10% of total cells) appeared in mice treated with Adv-GFP. Treatment with Adv-Zta caused much greater recruitment of neutrophils into the lung (>50% of total cells) with modest increases in eosinophils and lymphocytes. Although Adv-GFP prompted an inflammatory response, Adv-Zta produced more pronounced acute lung inflammation.
Fig. 3.
Enhanced inflammation in Adv-Zta-treated mice. BAL fluid was prepared from mice harvested on day 7 postexposure to Adv-Zta, Adv-GFP, or vehicle (PBS). A: total cell counts recovered by BAL. The graph shows the total number (mean ± SE) of live cells (trypan blue exclusion) as determined by direct counting with a hemacytometer in the BAL fluid recovered from mice in the indicated treatment groups. Adv-Zta, black bars, n = 9; Adv-GFP, gray bars, n = 10; PBS, white bars, n = 4. Adv-Zta vs. Adv-GFP, P = 0.0003; Adv-GFP vs. PBS, P = 0.7234. B: differential cell counts of BAL cells. Cytospin samples of the cells recovered by BAL were stained with Diffquik. Cells were visualized by microscopy, and 100 cells were counted from each sample. The graph shows the percentage of the indicated cell type (mean ± SE) in the cells recovered from the BAL fluid from mice treated with Adv-Zta, black bars, n = 9; Adv-GFP, gray bars, n = 10; and PBS, white bars, n = 4. Neutrophil cell count Adv-Zta vs. Adv-GFP, P < 0.0001; lymphocyte cell count Adv-Zta vs. Adv-GFP, P = 0.0535.
Resolution of lung inflammation in Adv-Zta-treated mice.
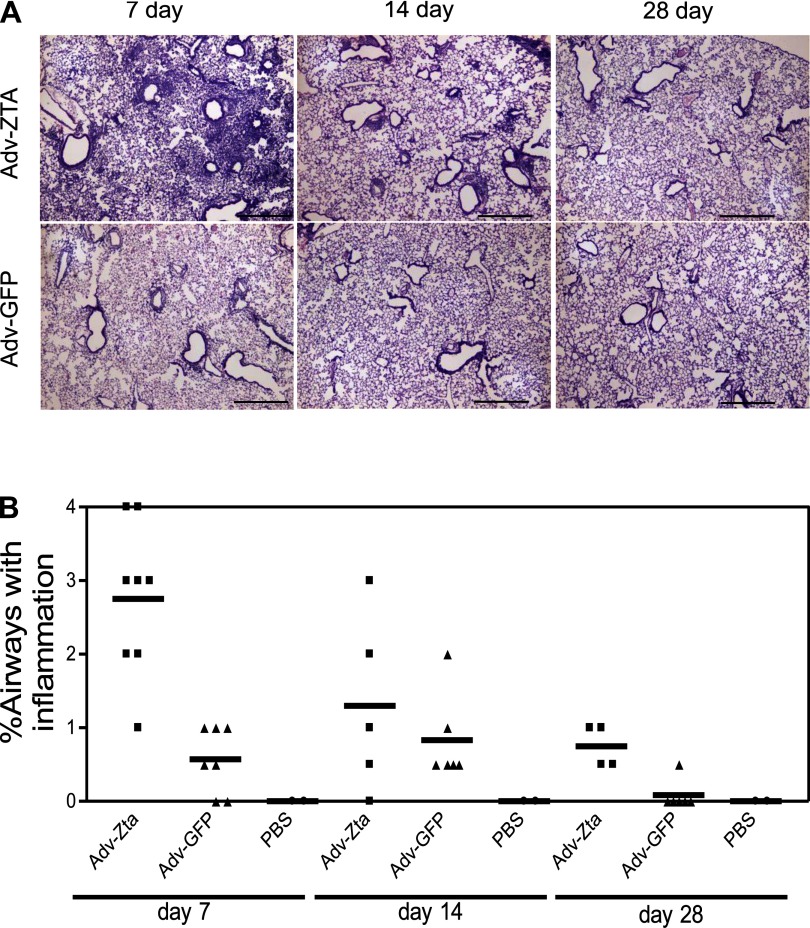
To determine if the inflammation induced by Adv-Zta progresses to fibrosis, lung sections from mice harvested on days 7, 14, and 28 postexposure were examined for fibrotic changes. On day 7 postexposure, Adv-Zta-treated mice displayed a massive influx of inflammatory cells in the alveoli and surrounding tissue (Fig. 4A). This inflammatory response in Adv-Zta-treated mice was markedly pronounced compared with the inflammatory response displayed in Adv-GFP-treated mice on day 7 (Fig. 4A). Histopathological examination of lung sections from these mice over the 28-day period indicated that the inflammatory response gradually diminished in both adenovirus-exposed groups (Fig. 4, A and B). Trichrome staining of lung sections from mice on day 28 postexposure confirmed no differences in collagen deposition in Adv-Zta- vs. Adv-GFP-exposed mice (Supplemental Fig. 1; Supplemental material for this article is available online at the Journal website). These data indicate that the lung inflammation induced by Adv-Zta resolves by day 28 postexposure with restoration of normal lung architecture.
Fig. 4.
Resolution of lung inflammation in Adv-Zta-treated mice. A: mice were treated with 1 × 108 pfu of the indicated adenovirus and killed at various times postexposure. The right lung of each mouse was fixed by intratracheal perfusion of 10% neutral buffered formalin. Hematoxylin and eosin (H&E)-stained lung sections from paraffin-embedded lung tissue of Adv-Zta (top) or Adv-GFP (bottom)-treated mice at 7 (left), 14 (middle), and 28 (right) days postexposure are shown. Scale bar represents 0.5 mm. B: histopathological assessment of lung inflammation at various times postexposure to adenovirus. H&E-stained lung sections shown in A from each mouse in the various treatment groups processed at the indicated times postexposure were evaluated for inflammation with the observer unaware of the sample identity. Scores for Adv-Zta (squares)-, Adv-GFP (triangles)-, and PBS (circles)-treated mice were assigned according to the percentage of airways associated with an influx of inflammatory cells (0 = 0–5%; 1 = 5–10%; 2 = 10–20%; 3 = 20–40%; 4 >40%).
Characterization of Zta-induced lung inflammation.
To investigate the specific mediators associated with the inflammatory response observed in adenovirus-exposed mice, we utilized a multiplex sandwich-based ELISA to simultaneously measure the level of 23 individual cytokines in each BAL sample (Supplemental Table 1). From the cytokine profiles for each set of mice (Adv-Zta, Adv-GFP, and PBS exposed), we selected cytokines to serve as phenotypic markers for the classic T-helper (Th) cell subsets (Table 1). As expected, the BAL fluid from PBS-treated mice displayed low-to-undetectable levels of the inflammatory markers (Table 1). Treatment with Adv-GFP increased most of these inflammatory cytokines with interferon-γ (Th1) and IL-6 (Th17) showing the most notable increases (Table 1). Consistent with the more prominent inflammatory response described above, the BAL fluid from mice treated with Adv-Zta displayed generally increased levels of inflammatory cytokines relative to those of the Adv-GFP-treated mice with some important differences. The Th17 and Treg markers were elevated in Adv-Zta-treated mice relative to their levels in Adv-GFP-treated mice. Interferon-γ levels were decreased in Adv-Zta-treated mice, which contrasted with the general increase of Th2 cytokine levels in Adv-Zta-treated mice relative to that in the Adv-GFP-treated animals. Although IL-12 (p40) is a subunit that is shared by IL-12 and IL-23 (66), the low levels of the IL-12 unique subunit, IL-12 (p70), suggest that the abundance of IL-12 (p40) is primarily due to overexpression of IL-23. These observations suggest that the phenotype of lung inflammation established by adenovirus delivery to mice shifts from a Th1/Th17 bias to a Th2/Th17 type response due to the expression of the EBV Zta protein.
Table 1.
Cytokine levels in BAL samples from adenovirus-treated and PBS control mice
| Th Phenotype | Cytokine | PBS | Adv-GFP | Adv-Zta |
|---|---|---|---|---|
| 1 | IFN-γ | 0.98 ± 0.2 (3) | 113.23 ± 47.9 (10) | 38.54 ± 5.0 (9) |
| IL-12 (p70)* | 1.37 ± 0.0 (1) | 1.31 ± 0.3 (10) | 11.38 ± 1.9 (9) | |
| 2 | IL-4 | 0.97 ± 0.1 (4) | 1.64 ± 0.3 (10) | 3.81 ± 0.3 (9) |
| IL-5 | 0.46 ± 0.0 (2) | 1.84 ± 0.7 (10) | 16.77 ± 3.1 (9) | |
| IL-9 | 8.01 ± 2.9 (3) | 8.03 ± 1.7 (9) | 21.21 ± 3.0 (9) | |
| IL-13 | 8.76 ± 6.1 (3) | 17.47 ± 5.4 (10) | 76.56 ± 14.2 (9) | |
| Treg | IL-10 | 1.63 ± 0.0 (1) | 7.03 ± 2.3 (5) | 42.06 ± 3.6 (9) |
| TGF-β | 208.45 ± 9.8 (4) | 215.04 ± 7.6 (10) | 334.11 ± 25.3 (9) | |
| 17 | IL-6 | 0.84 ± 0.2 (4) | 42.32 ± 28.7 (10) | 613.18 ± 76.6 (9) |
| IL-12 (p40)* | 15.22 ± 2.9 (4) | 102.25 ± 23.0 (10) | 284.27 ± 18.6 (9) | |
| IL-17 | 1.70 ± 0.5 (4) | 18.69 ± 8.3 (10) | 85.31 ± 31.3 (9) | |
| KC | 4.45 ± 1.0 (4) | 61.23 ± 19.3 (10) | 194.82 ± 33.6 (9) |
Average cytokine concentrations are expressed in pg/ml and given with the calculated SE. Sample sizes (n) are in parentheses.
IL-12 (p70) is unique to IL-12, whereas IL-12 (p40) is shared by IL-12 and IL-23.
To determine the source of inflammatory cytokines in Adv-Zta-treated mice, we quantified in an array format the mRNA levels in cells, predominantly inflammatory cells, isolated from the lavage fluid (Supplemental Table 2). To accommodate interanimal variation, cDNAs from the lavaged cells of four Adv-Zta- or five Adv-GFP-treated mice were combined for the measurement. The results were expressed as fold change (Adv-Zta/Adv-GFP) mRNA levels in BAL cells from Adv-Zta-treated mice relative to those from Adv-GFP-treated mice (Table 2). Levels of BAL cell mRNAs encoding Th1 cytokines in Adv-Zta-treated mice were comparable to those in Adv-GFP-treated mice despite the threefold reduction of interferon-γ in the BAL fluid in Adv-Zta-treated mice relative to Adv-GFP-treated mice. Cells in the BAL fluid appeared to account for some of the enhanced expression of Th2 cytokines in Adv-Zta-treated mice as well as the increased IL-10 levels, a Treg cytokine. The most notable disparity in the comparison was the 14.5-fold increase in IL-6 levels in the BAL fluid of Adv-Zta-treated mice compared with the 29.5-fold reduction in IL-6 mRNA that occurred in the BAL cells. Thus, cytokines expressed in infiltrating inflammatory cells partially account for the inflammatory milieu that develops in Adv-Zta-exposed mice.
Table 2.
Ratios (Adv-Zta/Adv-GFP) of BAL cytokine levels, mRNA levels in lung epithelial cells, and mRNA levels in BAL cells after virus infection
| Th Phenotype | Cytokine/mRNA | BAL Cytokine Levels | C10 mRNA | BAL Cell mRNA |
|---|---|---|---|---|
| 1 | IFN-γ | 0.34 | 3.00 | 1.10 |
| IL-12 (p70)† | 8.68 | 6.14* | 2.79* | |
| 2 | IL-4 | 2.32 | 5.52 | 1.28 |
| IL-5 | 9.11 | 5.55 | 2.01 | |
| IL-9 | 2.64 | — | — | |
| IL-13 | 4.38 | 6.86 | 3.16 | |
| Treg | IL-10 | 5.98 | 1.41 | 5.31 |
| TGF-β | 1.55 | 1.07 | −1.16 | |
| 17 | IL-6 | 14.48 | 1.04 | −29.45 |
| IL-12 (p40)/IL-12B | 2.78 | 10.79 | 1.55 | |
| IL-17/IL-17a | 4.56 | 1.08 | 3.56 | |
| KC/Cxcl 1 | 3.18 | −12.71 | 1.2 |
Ratios of the mean values for Adv-Zta/Adv-GFP for each of the indicated assays. Cytokine and BAL cell mRNA levels were determined on day 7 after infection. Dashes indicate not determined.
IL-23, α-subunit p19 transcript.
IL-12 (p70) is unique to IL-12, whereas IL-12 (p40) is shared by IL-12 and IL-23.
M2 alveolar macrophage activation in Adv-Zta-treated mice.
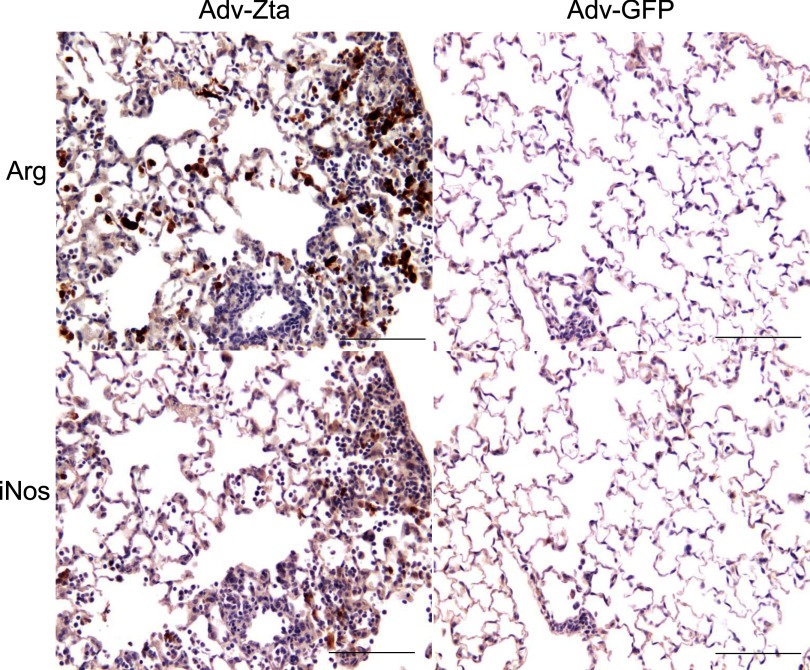
Macrophages recruited during lung inflammation can differentiate into distinct phenotypes with specialized functions in wound healing, host defense, or immune regulation (62). Exposure to Th2 cytokines promotes alternative macrophage activation, the M2 subtype, which stimulates wound healing and fibrogenesis (62). Immunohistochemical detection of inducible nitric oxide synthase (iNOS) for classically activated macrophages (M1) or of arginase for alternatively activated macrophages (M2) distinguishes the macrophage activation status (55). Immunostaining of serial lung sections for the iNOS and arginase I markers was performed to determine macrophage activation status at day 7, 14, and 28 postexposure to Adv-Zta, Adv-GFP, or PBS (Fig. 5). Assessment of the most inflamed areas in each section revealed few M1- or M2-positive macrophages in PBS-treated mice (not shown). Adv-Zta-treated mice displayed predominantly M2 macrophages that peaked at day 7 postexposure (Fig. 5), remained detectable at day 14, and returned to baseline by day 28 (Supplemental Fig. 2). M1 or M2 macrophages in lung sections from Adv-GFP-treated animals remained at low levels similar to that of the PBS control treatments (Fig. 5). The appearance of M2 macrophages is consistent with the Th2 cytokine bias in the Adv-Zta-treated mice described above.
Fig. 5.
Alternative activation of macrophages by Zta. Macrophage activation status was determined by immunostaining histological sections of formalin-fixed, paraffin-embedded lung tissue for iNOS (M1) or arginase (M2). Brown staining marked cells positive for arginase (top) or iNOS (bottom). Adjacent lung sections from Adv-Zta (left)- and Adv-GFP (right)-treated mice killed on day 7 are shown. The scale bar indicates 100 μm.
Induction of Th2 cytokine expression in mouse lung epithelial cells by Zta.
The primary target for Adv-Zta-induced injury and associated inflammation is the lung epithelium. Since Zta functions primarily as a transcription factor, any changes in cytokine expression would be expected to occur to a large extent at the mRNA level. After 24 h of infection, total RNA was extracted from adenovirus-exposed C10 cells and used for cDNA synthesis followed by RT-PCR array to determine levels of mRNAs encoding selected cytokines. Supplemental Table 3 shows the ratio of the mRNA level measured in Adv-Zta-infected C10 cells relative to Adv-GFP-infected C10 cells as the average fold change (with corresponding P value) as determined in three independent experiments. Table 2 compares the Adv-Zta/Adv-GFP mRNA levels in C10 cells to the Adv-Zta/Adv-GFP BAL cytokine levels. Induction of Th1 mRNAs by Adv-Zta in C10 cells did not correlate with the reduced (interferon-γ) or low [IL-12 (p40); Table 1] cytokine levels in Adv-Zta-treated mice. The source of the Treg cytokine, IL-10, appeared to be primarily inflammatory cells, as Adv-Zta-infected lung epithelial cells showed little increase in IL-10 mRNA levels. Of the Th17 cytokines, Adv-Zta induced only the mRNA encoding an IL-23 subunit in C10 cells. Since IL-23 is upstream of the other Th17 cytokines, IL-23 induction in lung epithelial cells by Adv-Zta could account for the strong Th17 inflammatory response in Adv-Zta-treated mice. Increases in Th2 cytokines in the BAL fluid of Adv-Zta-treated mice appeared consistent with elevated expression of the mRNAs encoding Th2 cytokines in Adv-Zta-infected lung epithelial cells. These data agree with the postulate that Zta expression in lung epithelial cells initiates inflammation through enhanced Th2 cytokine expression with variable effects on markers of other T-helper phenotypes.
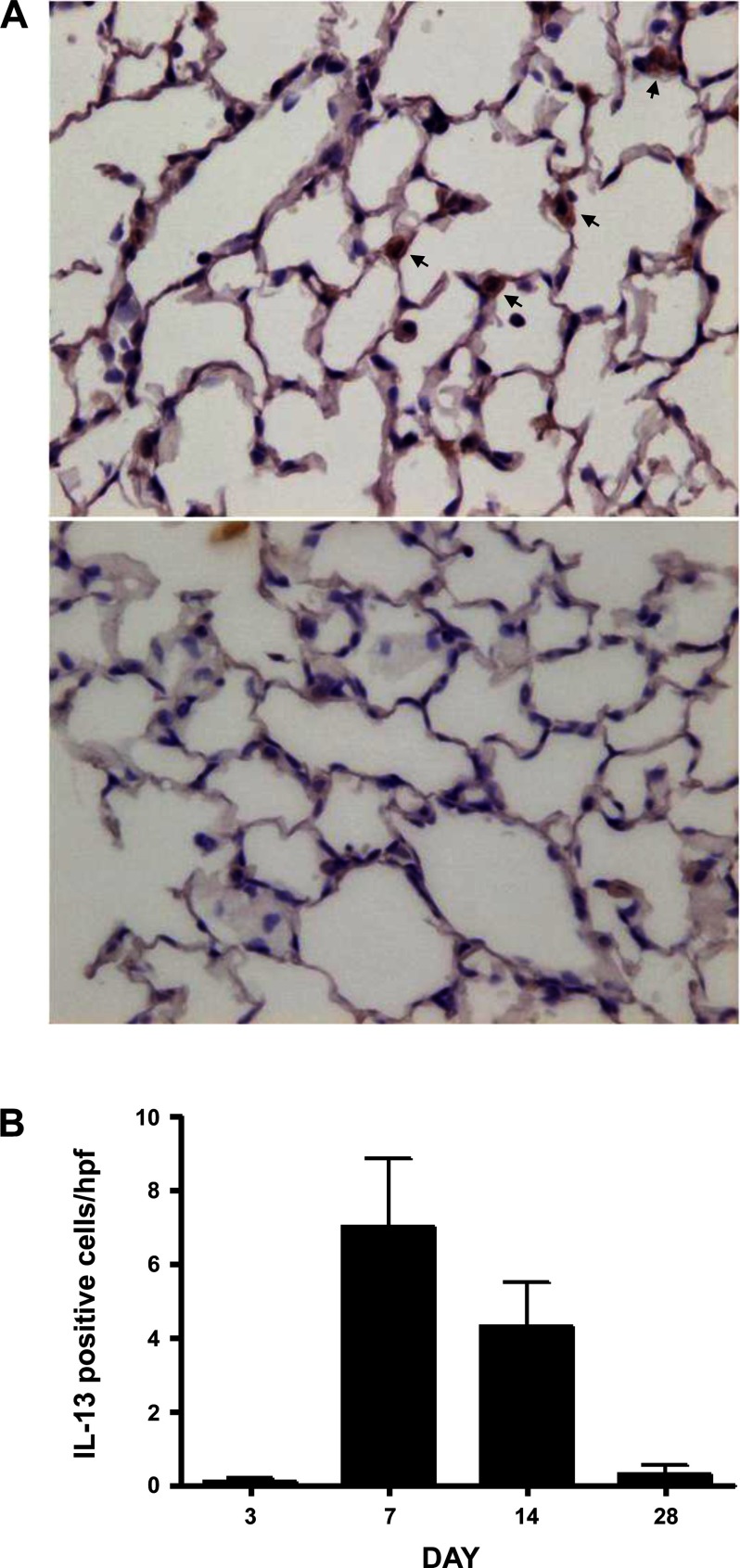
Expression of Th2 cytokines, in particular IL-13, correlates with pulmonary fibrosis in interferon-γ receptor knockout mice chronically infected with murine γ-herpesvirus 68 (60). Levels of IL-13 mRNA increased greater than sixfold in Adv-Zta-infected C10 cells relative to that in Adv-GFP-infected cells (Table 2). To determine if lung epithelial cells are a source of IL-13 expression in vivo, we performed immunohistochemical analyses of lung sections from Zta-expressing mice. Bronchiolar and alveolar epithelial cells appeared positive for IL-13 expression (Fig. 6A). Moreover, detection of IL-13 peaked at day 7 postexposure, which coincided with peak Zta expression (Fig. 6B).
Fig. 6.
Induction of IL-13 expression by Zta. IL-13 expression was determined in lung sections by immunohistochemical staining. A: brown staining IL-13-positive cells (arrows) were observed in Adv-Zta-treated animals. B: quantification of IL-13 immunostaining in Adv-Zta-treated mice. Counting 10 randomly selected high-powered fields (excluding large airways) per section (×400) for IL-13-positive cells showed that IL-13 staining was highest at day 7 post-Adv-Zta exposure and gradually decreased over a 28-day period. The differences between columns are statistically significant by nonparametric ANOVA (P < 0.0001).
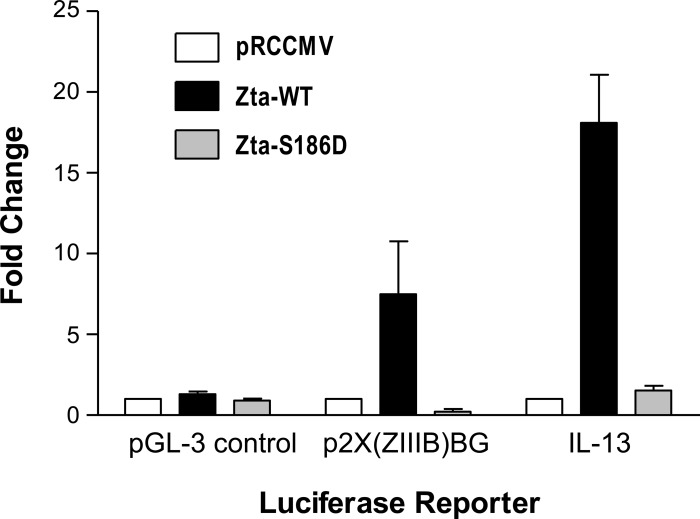
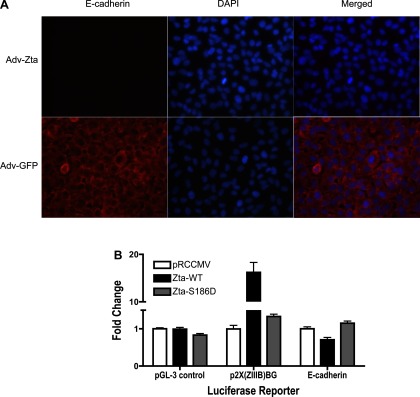
To address the mechanism of Th2 cytokine activation in lung epithelial cells by Zta, we performed cotransfection assays with an IL-13 promoter-luciferase reporter construct and Zta expression plasmids in C10 cells. In control transfections, expression of wild-type Zta produced little effect relative to the empty expression vector (pRCCMV) upon luciferase expression from the pGL-3 control plasmid, but activated luciferase expression seven- to eightfold from the positive control reporter p2X(ZIIIB)BG, a plasmid with two Zta-responsive elements upstream of the luciferase reporter (Fig. 7). Coexpression of wild-type Zta activated luciferase expression from the IL-13 promoter ∼18-fold. Expression of a Zta protein bearing a point mutation that disrupts DNA binding [Zta-S186D (7)] prevented activation of the IL-13 promoter-luciferase reporter and p2X(ZIIIB)BG. Similarly, wild-type Zta, but not Zta-S186D, activated IL-13 luciferase in A549 cells, a human lung adenocarcinoma cell line (data not shown). Additional experiments indicated that cis-acting sequences that mediate transcriptional activation of IL-13 promoter by Zta in B lymphocytes (79) contribute to activation of the IL-13 promoter in lung epithelial cells (data not shown). These data suggest that at least one of the mechanisms whereby Zta polarizes the immune response to the Th2 phenotype is through transcriptional activation of Th2 cytokine expression in lung epithelial cells.
Fig. 7.
Activation of the IL-13 promoter in lung epithelial cells by Zta. Mouse lung epithelial cells (C10 cells) in a 24-well plate were cotransfected with 0.2 μg of a luciferase reporter construct and 0.1 μg of a wild-type (Zta-WT, black bars) or mutant Zta (Zta-S186D, gray bars)-expressing plasmid or empty vector (pRCCMV, white bars). To normalize for variation in transfection efficiency, 0.1 μg pRL-TK-renilla luciferase (Promega) was included in the transfection mixture. After 48 h, firefly and renilla luciferase activities were determined (Promega). The negative control reporter (pGL-3 control; Promega) contains SV40 promoter and enhancer sequences directing expression of firefly luciferase. The positive control reporter [p2X(ZIIIB)BG (42)] contains Zta-responsive elements upstream of firefly luciferase. The IL-13 luciferase reporter [−1112C/luc (9)] contains human IL-13 promoter sequences directing expression of firefly luciferase. For each luciferase reporter construct, the ratio of firefly luciferase to renilla luciferase in the presence of empty vector (pRCCMV) was normalized to 1. The graph shows the mean fold change ± SE in firefly/renilla luciferase activity from 3 independent transfection assays performed in duplicate for the indicated firefly luciferase reporter construct. Induction of IL-13-luciferase by wild-type Zta relative to empty vector (pRCCMV) or mutant Zta (Zta-S186D) is significant (P = 0.0003 and P = 0.0004, respectively, by unpaired t-test with Welch correction).
Induction of additional fibrogenesis markers by Zta.
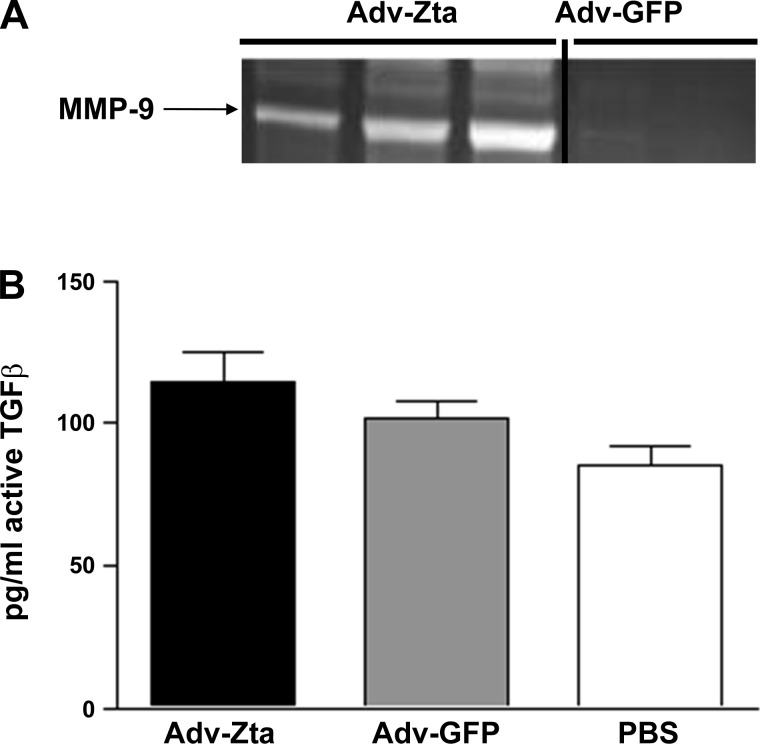
MMP-9 levels are elevated in patients with pulmonary fibrosis and correlate with enhanced neutrophilic inflammation in usual interstitial pneumonia (77). Furthermore, ablation of the MMP-9 gene reduces IL-13-dependent lung remodeling (40). Cells recovered by BAL of Adv-Zta-treated mice displayed greater than 10-fold elevated MMP-9 mRNA levels than that in mice treated with Adv-GFP (Supplemental Table 2). Moreover, Adv-Zta treatment of C10 cells induced MMP-9 mRNA levels greater than 20-fold (Supplemental Table 3). Consistent with these observations, analyses of BAL fluid by zymography demonstrated elevated levels of MMP-9 in Adv-Zta treated relative to that in mice treated with Adv-GFP (Fig. 8A). Previous work showed that induction of MMP-9 by IL-13 promoted activation of TGF-β (41). However, despite the high levels of MMP-9, levels of TGF-β in Zta-treated mice increased only modestly (Fig. 8B). Thus, detection of MMP-9 in the BAL fluid suggests a mechanism for destruction of the basement membrane and access of inflammatory cells to the alveolar space without significant increases in TGF-β activation.
Fig. 8.
Induction of MMP-9 and activation of TGF-β by Zta. A: assessing MMP-9 levels in BAL fluid from adenovirus-exposed mice. Equal volumes of BAL fluid from Adv-Zta- (n = 3) and Adv-GFP-treated (n = 2) mice on day 7 postexposure were analyzed by gelatin zymography for MMP-9 activity. B: measurement of active TGF-β in the BAL fluid of mice in the 3 treatment groups. The graph shows the concentration of active TGF-β in pg/ml in the BAL fluid recovered from mice on day 7 postexposure to Adv-Zta, Adv-GFP, or PBS.
Herpesvirus-induced pulmonary fibrosis has been associated with epithelial-mesenchymal transition (EMT) (49, 69), the process by which differentiated epithelial cells convert into cells possessing a mesenchymal phenotype (84). To determine if Zta contributes to EMT, we infected human A549 lung epithelial cells with Adv-Zta, then stained the Zta-expressing cells for E-cadherin. Loss of E-cadherin, which mediates cell adhesion between adjacent epithelial cells, is an early event in EMT (84). A549 cells infected with Adv-Zta did not express E-cadherin, whereas control Adv-GFP-infected A549 cells retained E-cadherin (Fig. 9A). In coexpression assays with C10 cells, wild-type Zta, but not the S186D mutant defective for DNA binding, repressed expression of luciferase from a cotransfected E-cadherin promoter-luciferase reporter (Fig. 9B). As described above, control assays with Zta-responsive [p2X(ZIIIB)BG] and Zta-unresponsive (pGL-3 control) reporters indicated the specificity of E-cadherin promoter repression by Zta. These observations, Zta-mediated repression of the E-cadherin promoter, and the loss of E-cadherin in Zta-expressing lung epithelial cells, are consistent with the concept that Zta induces some aspects of EMT.
Fig. 9.
Repression of E-cadherin expression in lung epithelial cells by Zta. A: immunofluorescent detection of E-cadherin in A549 cells after infection with recombinant adenoviruses. A549 cells grown in chamber slides were infected with Adv-Zta (top) or Adv-GFP (bottom) at 20 moi. Three days postinfection, the cells were processed for immunofluorescent detection of E-cadherin (left). Cells were visualized with DAPI nuclear stain (middle). The merged images (right) demonstrate that Adv-Zta represses expression of E-cadherin in A549 cells (top), whereas Adv-GFP does not (bottom). B: repression of the E-cadherin promoter in lung epithelial cells by Zta. Mouse lung epithelial cells (C10) were cotransfected with firefly luciferase reporter constructs [pGL-3 control (negative control), p2X(ZIIIB)BG (positive control), and E-cadherin (pGL2Basic-EcadK1, test)], Zta-expressing plasmids [pRCCMV (empty vector), Zta-WT (wild-type Zta), and Zta-S186D (mutant Zta)], and pRL-TK-luciferase as an internal transfection efficiency control (see Fig. 7). The graph shows the mean fold change ± SE in firefly/renilla luciferase activity from 3 independent transfection assays performed in duplicate for the indicated firefly luciferase reporter construct. For each reporter plasmid, the mean firefly/renilla luciferase ratio in the presence of empty vector (pRCCMV) was normalized to 1. Induction of the 2X(ZIIIB)BG promoter and repression of the E-cadherin promoter by wild-type Zta is significant (P < 0.0001 and P = 0.0041).
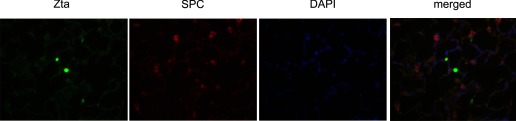
Induction of EMT in alveolar epithelial cells is associated with repression of surfactant protein C (SP-C) expression (52). We examined lung sections from Adv-Zta-exposed mice for coexpression of SP-C and Zta by coimmunofluorescence. Green nuclear staining identified Zta-expressing cells in the parenchyma, whereas cells positive for SP-C displayed red cytoplasmic staining (Fig. 10). However, cells positive for both SP-C or the M2 macrophage marker arginase (Supplemental Fig. 4) and Zta could not be detected. In contrast, Zta-positive cells in the bronchioles appeared positive for both Clara cell secretory protein (CCSP) and Zta (Supplemental Fig. 3). These observations are consistent with the deduction that Zta expression in alveolar epithelial cells represses SPC expression. Zta-mediated repression of an epithelial marker in vivo agrees with promotion of at least some aspects of EMT.
Fig. 10.
Discordant expression of Zta and surfactant protein C (SP-C) in the lung parenchyma of Adv-Zta-treated mice. Expression of Zta in Adv-Zta-treated mice on day 7 postexposure was detected in the lung parenchyma by immunofluorescence (green, left). Immunostaining of the same area for SP-C (red) and counterstaining with DAPI (blue) are shown (2 middle). The merged image (right) demonstrates that Zta and SP-C expression do not coincide.
DISCUSSION
Various agents of lung injury including gastroesophageal reflux, genetic abnormality, inhaled pollutants, autoimmunity, and viral infection have been proposed as the etiologic agents of IPF (16, 47, 54, 73). This study explores links between IPF and herpesviruses [(46, 51, 78, 89) reviewed in Ref. 80] by examining the potential of the EBV lytic transactivator protein, Zta, to modulate cellular inflammation in the lung. Consistent with a possible role in pulmonary fibrosis, Zta induced an influx of inflammatory cells, dominated by neutrophils, into the lungs of mice. According to the IPF clinical research network, the presence of neutrophils in the BAL fluid of IPF patients is a morphological characteristic of an acute exacerbation of IPF (15). Characterization of the inflammatory cytokine profiles in Zta-expressing mice revealed elevated Th2 and Th17 responses. Upon adenovirus transduction, the mouse lung epithelial cells are the predominant site of transgene expression (44, 88). Thus, induction of Th2 cytokines in vivo by Zta is consistent with Zta-mediated induction of mRNAs encoding Th2 cytokines in lung epithelial cells in vitro. As shown recently in B cells (79), we show that in lung epithelial cells Zta transcriptionally activates expression of the Th2 cytokine IL-13. Consistent with this observation, detection of IL-13 in Adv-Zta-treated mice correlates temporally with detection of Zta. Activation of Th2 cytokine expression by Zta in lung epithelial cells could account for the observed alternative activation of macrophages and elevated levels of MMP-9 in Adv-Zta-treated mice. Expression of Th2 cytokines and M2 macrophage polarization occurs in IPF (30, 58) and has been demonstrated in mouse models of pulmonary fibrosis induced by murine γ-herpesvirus 68 (58, 60). Moreover, EMT has been reported in MHV68-infected murine lung epithelial cells (69). The data presented here suggest that Zta expression in lung epithelial cells contributes to this pathogenic signature.
Epithelial injury is commonly observed in IPF (17, 73) and may be an early event in disease pathogenesis (5). Inhibition of alveolar epithelial apoptosis reduces lung fibrosis in experimental models (35, 36). The limited Zta expression pattern relative to GFP in virally infected mice and the elevated levels of LDH in Zta-expressing mice suggest that Zta induces epithelial injury. Cell death does not appear to be a direct effect of Zta expression, as Adv-Zta reduced the viability of lung epithelial cell cultures only at a high moi, and Zta expression could be detected in Adv-Zta-treated mice as long as 2 wk (data not shown). Zta expression induces a neutrophil influx, which may promote alveolar epithelial cell apoptosis (35). Neutrophilic inflammation appears to play a role in acute exacerbations of IPF (6, 15, 47). Lytic replication of EBV increases alveolar epithelial cell apoptosis (48), but our data do not directly implicate Zta in the process.
Epithelial cells are involved in the characteristic imbalance of profibrogenic Th2 cytokines observed in IPF (73). IL-13 is a profibrogenic Th2 cytokine that is found at elevated levels in IPF patients (24). Interferon-γ knockout mice infected with murine herpesvirus (MHV68) develop lung fibrosis and display elevated levels of IL-13 (58). Similar to effects of Zta on IL-13 expression in B cells (79), Zta transcriptionally activates the IL-13 gene in lung epithelial cells. IL-13 promotes collagen synthesis and fibroblast proliferation in vitro and in vivo (28, 72), and IL-13 inhibition may reduce lung inflammation and remodeling (8).
IL-13 can induce TGF-β1 (21, 41) and CCL2 (also referred to as monocyte chemoattractant protein-1, MCP-1) (90), and interactions between the three mediators appear to be central to fibrogenesis in IPF (63). Given that IL-13 is directly activated by Zta in lung epithelial cells, it is interesting to speculate that Zta may exert multiple effects by inducing cross talk between immune and epithelial cells. This could possibly be the case where direct induction of IL-13 by Zta in lung epithelial cells sets up a signal exchange between the epithelium and the immune system, consequently triggering the observed >100-fold increase in mRNA expression of CCL2 in BAL fluid cells and the subsequent increase in CCL2 protein in BAL fluid (Supplemental Table 4). Furthermore, this exchange could possibly account for the expression of both Th2 and Th17 markers. The slight TGF-β1 increase does not suggest strong dependence on Zta expression like the large increase in CCL2. CCL2 reduces PGE2 production by alveolar epithelial cells (57) thereby diminishing PGE2-mediated suppression of fibroblast migration, proliferation, and activation (32, 39, 67). Overexpression of CCL2 occurs in IPF (3, 10, 29) and correlates with disease severity (76). In the mouse model of herpesvirus-induced lung fibrosis, antiviral treatments reduce Th2 cytokine and CCL2 levels (59). Inhibition of CCL2 by ablation of its receptor, CCR2, retards epithelial chemotaxis (12) and attenuates fibrosis in mice induced by overexpression of IL-13 (90) or by FITC or by bleomycin (56). Thus, Zta expression in the lung stimulates expression of two important mediators of pulmonary fibrosis, IL-13 by lung epithelial cells and CCL2/MCP-1 by infiltrating inflammatory cells.
Th2 cytokine profiles predominate in IPF (71, 82). The presence of Th2 cytokines potentiates another characteristic of IPF (58), alternative activation of alveolar macrophages (62). Expression of Zta in mice induces arginase expression in macrophages, a marker for alternative activation (25). Alternatively activated macrophages (M2) advance fibrogenesis by secreting fibronectin, matrix metalloproteinases, and profibrotic growth factors (23, 65). Alternative macrophage activation occurs in the mouse model of herpesvirus-induced lung fibrosis (58), which can be reduced by antiviral treatment (59).
EBV can replicate in the lower respiratory tract (46), and expression of Zta occurs during lytic replication of EBV (68). Controlling EBV replication has stabilized lung function in a limited number of IPF patients (78). Preventing herpesvirus replication blocks MMP-9 expression and restricts the development of pulmonary fibrosis in a mouse model of herpesvirus-induced lung fibrosis (59). Since inhibiting virus replication reduces Zta expression in EBV-infected alveolar epithelial cells (48), antifibrotic effects of virus replication inhibitors could be related to repression of Zta. However, replication inhibitors are not likely to block Zta expression from the WZhet rearranged EBV genome that occurs frequently in IPF (31).
Our data suggest that activation of Th2 cytokine expression in lung epithelial cells accounts for the initial phase of Zta-induced lung inflammation. This cytokine profile promotes alternate activation of infiltrating inflammatory cells, which elicits lung injury and secretes high levels of CCL-2. In patients with IPF, this inflammation and injury likely resolves with TGF-β activation and associated scarring, but these later events do not occur in Adv-Zta-treated mice. Despite the profibrogenic phenotype, Zta-induced inflammation resolves without fibrosis by day 28. Even 10-fold higher amounts of Adv-Zta than that described here fail to elicit a lasting fibrotic change (our unpublished observation). TGF-β, a key mediator of lung fibrosis (85), is only modestly increased in Zta-expressing mice and is essentially unaltered in Adv-Zta-infected lung epithelial cells. Lytic infection of alveolar epithelial cell cultures with EBV significantly enhances TGF-β expression, but the lack of potential binding sites for Zta in the TGF-β promoter sequence suggests that the induction is not a direct effect of Zta (48). Despite a large increase in active MMP-9 levels in Adv-Zta-treated mice (Fig. 8A), additional results show that Adv-Zta treatment of mice only modestly enhances (<15% increase relative to Adv-GFP; Fig. 8B) TGF-β activation. Lung fibrosis resulting from MMP-9-dependent activation of TGF-β on IL-13 overexpression has been demonstrated (41), but a failure to engage this TGF-β activation pathway in Adv-Zta-treated mice likely factors into the absence of fibrosis by day 28. Perhaps the strong Th17 response in Adv-Zta-treated mice prevents activation of TGF-β by MMP-9 and promotes resolution of the Th2-biased inflammation without scarring.
Infection of murine lung epithelial cell cultures with MHV68 promotes EMT (69). We show here that Zta represses expression of lung epithelial markers in vitro (E-cadherin) and in vivo (SP-C). Thus, Zta expression appears to initiate EMT, but our efforts to demonstrate expression of mesenchymal characteristics in Zta-expressing lung epithelial cells have been unsuccessful. We conclude that Zta can initiate early aspects of EMT, e.g., loss of differentiation markers, but the process is incomplete.
The association of herpesviruses with IPF has not been universally confirmed (34, 89). Immunosuppression due to corticosteroid therapy, a common treatment for IPF, could account for the detection of herpesviruses and other viruses in IPF patients (37, 54). However, polyomaviruses, which also can be activated by immunosuppression, are not detected in IPF patients (70). An association of interstitial lung disease with herpesvirus infections in horses (83, 86) and a cat (45) lends credibility to similar associations in humans.
The enhanced inflammatory response and distinct inflammatory phenotype displayed by Adv-Zta-treated mice relative to those treated with Adv-GFP indicate that Zta expression is a determining factor in the response. It cannot be ruled out that the adenovirus vehicle influences the inflammatory response exacerbated by the expression of Zta. Previous infection with a murine gamma-herpesvirus alters the subsequent infection response on adenovirus challenge (64).
The in vitro data indicate that the inflammatory response to Adv-Zta in vivo is governed, at least in part, by Zta-mediated transcriptional activation of Th2 cytokine expression in the lung epithelium. Elevated expression of Th2 cytokines likely correlates with M2 macrophage activation. This profibrogenic inflammatory response is observed in IPF and animal models of the disease. Together, these results support a mechanism whereby herpesviruses may contribute to lung fibrosis or exacerbation of the disease by altering the normal immune response.
GRANTS
This work was supported by Louisiana Education Quality Support Fund (2005-08)-RD-A-36 from the Louisiana State Board of Regents (G. F. Morris) and National Heart, Lung, and Blood Institute Grant RO1-HL-083901 (J. A. Lasky). J. F. Guenther received support from T32-HL-O7973 (J. A. Lasky) and matching funds from the Tulane Cancer Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Shannon Kenney (Univ. of Wisconsin-Madison) for Adv-Zta and Donata Vercelli (Univ. of Arizona) for the IL-13 luciferase plasmid IL-13-2666.
REFERENCES
- 1. American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement: American Thoracic Society and European Respiratory Society. Am J Respir Crit Care Med 161: 646–664, 2000. [DOI] [PubMed] [Google Scholar]
- 2. Adamson AL, Kenney S. The Epstein-Barr virus BZLF1 protein interacts physically and functionally with the histone acetylase CREB-binding protein. J Virol 73: 6551–6558, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antoniades HN, Neville-Golden J, Galanopoulos T, Kradin RL, Valente AJ, Graves DT. Expression of monocyte chemoattractant protein 1 mRNA in human idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA 89: 5371–5375, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: Wiley & Sons, 1994. [Google Scholar]
- 5. Barbas-Filho JV, Ferreira MA, Sesso A, Kairalla RA, Carvalho CR, Capelozzi VL. Evidence of type II pneumocyte apoptosis in the pathogenesis of idiopathic pulmonary fibrosis/usual interstitial pneumonia. J Clin Pathol 54: 132–138, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beeh KM, Beier J, Kornmann O, Buhl R. Neutrophilic inflammation in induced sputum of patients with idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 20: 138–143, 2003. [PubMed] [Google Scholar]
- 7. Bhende PM, Seaman WT, Delecluse HJ, Kenney SC. BZLF1 activation of the methylated form of the BRLF1 immediate-early promoter is regulated by BZLF1 residue 186. J Virol 79: 7338–7348, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blease K. Therapeutics targeting IL-13 for the treatment of pulmonary inflammation and airway remodeling. Curr Opin Investig Drugs 9: 1180–1184, 2008. [PubMed] [Google Scholar]
- 9. Cameron L, Webster RB, Strempel JM, Kiesler P, Kabesch M, Ramachandran H, Yu L, Stern DA, Graves PE, Lohman IC, Wright AL, Halonen M, Klimecki WT, Vercelli D. Th2 cell-selective enhancement of human IL13 transcription by IL13–1112C>T, a polymorphism associated with allergic inflammation. J Immunol 177: 8633–8642, 2006. [DOI] [PubMed] [Google Scholar]
- 10. Car BD, Meloni F, Luisetti M, Semenzato G, Gialdroni-Grassi G, Walz A. Elevated IL-8 and MCP-1 in the bronchoalveolar lavage fluid of patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am J Respir Crit Care Med 149: 655–659, 1994. [DOI] [PubMed] [Google Scholar]
- 11. Cayrol C, Flemington EK. Identification of cellular target genes of the Epstein-Barr virus transactivator Zta: activation of transforming growth factor beta igh3 (TGF-beta igh3) and TGF-beta 1. J Virol 69: 4206–4212, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christensen PJ, Du M, Moore B, Morris S, Toews GB, Paine R., 3rd Expression and functional implications of CCR2 expression on murine alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 286: L68–L72, 2004. [DOI] [PubMed] [Google Scholar]
- 13. Cohn LA, Norris CR, Hawkins EC, Dye JA, Johnson CA, Williams KJ. Identification and characterization of an idiopathic pulmonary fibrosis-like condition in cats. J Vet Intern Med 18: 632–641, 2004. [DOI] [PubMed] [Google Scholar]
- 14. Collard HR, King TE, Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 168: 538–542, 2003. [DOI] [PubMed] [Google Scholar]
- 15. Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE, Jr, Lasky JA, Loyd JE, Noth I, Olman MA, Raghu G, Roman J, Ryu JH, Zisman DA, Hunninghake GW, Colby TV, Egan JJ, Hansell DM, Johkoh T, Kaminski N, Kim DS, Kondoh Y, Lynch DA, Muller-Quernheim J, Myers JL, Nicholson AG, Selman M, Toews GB, Wells AU, Martinez FJ. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 176: 636–643, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doran P, Egan JJ. Herpesviruses: a cofactor in the pathogenesis of idiopathic pulmonary fibrosis? Am J Physiol Lung Cell Mol Physiol 289: L709–L710, 2005. [DOI] [PubMed] [Google Scholar]
- 17. Drakopanagiotakis F, Xifteri A, Polychronopoulos V, Bouros D. Apoptosis in lung injury and fibrosis. Eur Respir J 32: 1631–1638, 2008. [DOI] [PubMed] [Google Scholar]
- 18. Drent M, Cobben NA, Henderson RF, Jacobs JA, Wouters EF, van Dieijen-Visser MP. BAL fluid LDH activity and LDH isoenzyme pattern in lipoid pneumonia caused by an intravenous injection of lamp oil. Eur Respir J 9: 2416–2418, 1996. [DOI] [PubMed] [Google Scholar]
- 19. Ebrahimi B, Dutia BM, Brownstein DG, Nash AA. Murine gammaherpesvirus-68 infection causes multi-organ fibrosis and alters leukocyte trafficking in interferon-gamma receptor knockout mice. Am J Pathol 158: 2117–2125, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Egan JJ, Woodcock AA, Stewart JP. Viruses and idiopathic pulmonary fibrosis. Eur Respir J 10: 1433–1437, 1997. [DOI] [PubMed] [Google Scholar]
- 21. Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13[alpha]2 receptor is involved in induction of TGF-[beta]1 production and fibrosis. Nat Med 12: 99, 2006. [DOI] [PubMed] [Google Scholar]
- 22. Graham RM, Friedman M, Hoyle GW. Sensory nerves promote ozone-induced lung inflammation in mice. Am J Respir Crit Care Med 164: 307–313, 2001. [DOI] [PubMed] [Google Scholar]
- 23. Gratchev A, Guillot P, Hakiy N, Politz O, Orfanos CE, Schledzewski K, Goerdt S. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein betaIG-H3. Scand J Immunol 53: 386–392, 2001. [DOI] [PubMed] [Google Scholar]
- 24. Hancock A, Armstrong L, Gama R, Millar A. Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am J Respir Cell Mol Biol 18: 60–65, 1998. [DOI] [PubMed] [Google Scholar]
- 25. Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, Pearce EJ, Wynn TA. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of l-arginine metabolism. J Immunol 167: 6533–6544, 2001. [DOI] [PubMed] [Google Scholar]
- 26. Honer zu Bentrup K, Ramamurthy R, Ott CM, Emami K, Nelman-Gonzalez M, Wilson JW, Richter EG, Goodwin TJ, Alexander JS, Pierson DL, Pellis N, Buchanan KL, Nickerson CA. Three-dimensional organotypic models of human colonic epithelium to study the early stages of enteric salmonellosis. Microbes Infect 8: 1813–1825, 2006. [DOI] [PubMed] [Google Scholar]
- 27. Hsu M, Wu SY, Chang SS, Su IJ, Tsai CH, Lai SJ, Shiau AL, Takada K, Chang Y. Epstein-Barr virus lytic transactivator Zta enhances chemotactic activity through induction of interleukin-8 in nasopharyngeal carcinoma cells. J Virol 82: 3679–3688, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ingram JL, Antao-Menezes A, Mangum JB, Lyght O, Lee PJ, Elias JA, Bonner JC. Opposing actions of Stat1 and Stat6 on IL-13-induced upregulation of early growth response-1 and platelet-derived growth factor ligands in pulmonary fibroblasts. J Immunol 177: 4141–4148, 2006. [DOI] [PubMed] [Google Scholar]
- 29. Iyonaga K, Takeya M, Saita N, Sakamoto O, Yoshimura T, Ando M, Takahashi K. Monocyte chemoattractant protein-1 in idiopathic pulmonary fibrosis and other interstitial lung diseases. Hum Pathol 25: 455, 1994. [DOI] [PubMed] [Google Scholar]
- 30. Joshi BH, Hogaboam C, Dover P, Husain SR, Puri RK. Role of interleukin-13 in cancer, pulmonary fibrosis, and other TH2-type diseases. Vitam Horm 74: 479–504, 2006. [DOI] [PubMed] [Google Scholar]
- 31. Kelly BG, Lok SS, Hasleton PS, Egan JJ, Stewart JP. A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 166: 510–513, 2002. [DOI] [PubMed] [Google Scholar]
- 32. Kohyama T, Ertl RF, Valenti V, Spurzem J, Kawamoto M, Nakamura Y, Veys T, Allegra L, Romberger D, Rennard SI. Prostaglandin E(2) inhibits fibroblast chemotaxis. Am J Physiol Lung Cell Mol Physiol 281: L1257–L1263, 2001. [DOI] [PubMed] [Google Scholar]
- 33. Kolls J, Peppel K, Silva M, Beutler B. Prolonged and effective blockade of tumor necrosis factor activity through adenovirus-mediated gene transfer. Proc Natl Acad Sci USA 91: 215–219, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Konishi K, Gibson KF, Lindell KO, Richards TJ, Zhang Y, Dhir R, Bisceglia M, Gilbert S, Yousem SA, Song JW, Kim DS, Kaminski N. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 180: 167–175, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuwano K, Hagimoto N, Kawasaki M, Yatomi T, Nakamura N, Nagata S, Suda T, Kunitake R, Maeyama T, Miyazaki H, Hara N. Essential roles of the Fas-Fas ligand pathway in the development of pulmonary fibrosis. J Clin Invest 104: 13–19, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuwano K, Kunitake R, Maeyama T, Hagimoto N, Kawasaki M, Matsuba T, Yoshimi M, Inoshima I, Yoshida K, Hara N. Attenuation of bleomycin-induced pneumopathy in mice by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol 280: L316–L325, 2001. [DOI] [PubMed] [Google Scholar]
- 37. Kuwano K, Nomoto Y, Kunitake R, Hagimoto N, Matsuba T, Nakanishi Y, Hara N. Detection of adenovirus E1A DNA in pulmonary fibrosis using nested polymerase chain reaction. Eur Respir J 10: 1445–1449, 1997. [DOI] [PubMed] [Google Scholar]
- 38. Lakatos HF, Burgess HA, Thatcher TH, Redonnet MR, Hernady E, Williams JP, Sime PJ. Oropharyngeal aspiration of a silica suspension produces a superior model of silicosis in the mouse when compared to intratracheal instillation. Exp Lung Res 32: 181–199, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lama V, Moore BB, Christensen P, Toews GB, Peters-Golden M. Prostaglandin E2 synthesis and suppression of fibroblast proliferation by alveolar epithelial cells is cyclooxygenase-2-dependent. Am J Respir Cell Mol Biol 27: 752–758, 2002. [DOI] [PubMed] [Google Scholar]
- 40. Lanone S, Zheng T, Zhu Z, Liu W, Lee CG, Ma B, Chen Q, Homer RJ, Wang J, Rabach LA, Rabach ME, Shipley JM, Shapiro SD, Senior RM, Elias JA. Overlapping and enzyme-specific contributions of matrix metalloproteinases-9 and -12 in IL-13-induced inflammation and remodeling. J Clin Invest 110: 463–474, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, Senior RM, Elias JA. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med 194: 809–821, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lin Z, Yin Q, Flemington E. Identification of a negative regulatory element in the Epstein-Barr virus Zta transactivation domain that is regulated by the cell cycle control factors c-Myc and E2F1. J Virol 78: 11962–11971, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu X, Yan Z, Luo M, Engelhardt JF. Species-specific differences in mouse and human airway epithelial biology of recombinant adeno-associated virus transduction. Am J Respir Cell Mol Biol 34: 56–64, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Love DN. Feline herpesvirus associated with interstitial pneumonia in a kitten. Vet Rec 89: 178–181, 1971. [DOI] [PubMed] [Google Scholar]
- 46. Lung ML, Lam WK, So SY, Lam WP, Chan KH, Ng MH. Evidence that respiratory tract is major reservoir for Epstein-Barr virus. Lancet 1: 889–892, 1985. [DOI] [PubMed] [Google Scholar]
- 47. Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? Eur Respir J 30: 835–839, 2007. [DOI] [PubMed] [Google Scholar]
- 48. Malizia AP, Keating DT, Smith SM, Walls D, Doran PP, Egan JJ. Alveolar epithelial cell injury with Epstein-Barr virus upregulates TGFbeta1 expression. Am J Physiol Lung Cell Mol Physiol 295: L451–L460, 2008. [DOI] [PubMed] [Google Scholar]
- 49. Malizia AP, Lacey N, Walls D, Egan JJ, Doran PP. CUX1/Wnt signaling regulates epithelial mesenchymal transition in EBV infected epithelial cells. Exp Cell Res 315: 1819–1831, 2009. [DOI] [PubMed] [Google Scholar]
- 50. Malkinson AM, Dwyer-Nield LD, Rice PL, Dinsdale D. Mouse lung epithelial cell lines–tools for the study of differentiation and the neoplastic phenotype. Toxicology 123: 53–100, 1997. [DOI] [PubMed] [Google Scholar]
- 51. Manika K, Alexiou-Daniel S, Papakosta D, Papa A, Kontakiotis T, Patakas D, Antoniadis A. Epstein-Barr virus DNA in bronchoalveolar lavage fluid from patients with idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 24: 134–140, 2007. [PubMed] [Google Scholar]
- 52. Maniscalco WM, Sinkin RA, Watkins RH, Campbell MH. Transforming growth factor-beta 1 modulates type II cell fibronectin and surfactant protein C expression. Am J Physiol Lung Cell Mol Physiol 267: L569–L577, 1994. [DOI] [PubMed] [Google Scholar]
- 53. Mauser A, Saito S, Appella E, Anderson CW, Seaman WT, Kenney S. The Epstein-Barr virus immediate-early protein BZLF1 regulates p53 function through multiple mechanisms. J Virol 76: 12503–12512, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Meltzer EB, Noble PW. Idiopathic pulmonary fibrosis. Orphanet J Rare Dis 3: 8, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164: 6166–6173, 2000.10843666 [Google Scholar]
- 56. Moore BB, Paine R, III, Christensen PJ, Moore TA, Sitterding S, Ngan R, Wilke CA, Kuziel WA, Toews GB. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol 167: 4368–4377, 2001. [DOI] [PubMed] [Google Scholar]
- 57. Moore BB, Peters-Golden M, Christensen PJ, Lama V, Kuziel WA, Paine R, 3rd, Toews GB. Alveolar epithelial cell inhibition of fibroblast proliferation is regulated by MCP-1/CCR2 and mediated by PGE2. Am J Physiol Lung Cell Mol Physiol 284: L342–L349, 2003. [DOI] [PubMed] [Google Scholar]
- 58. Mora AL, Torres-Gonzalez E, Rojas M, Corredor C, Ritzenthaler J, Xu J, Roman J, Brigham K, Stecenko A. Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis. Am J Respir Cell Mol Biol 35: 466–473, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mora AL, Torres-Gonzalez E, Rojas M, Xu J, Ritzenthaler J, Speck SH, Roman J, Brigham K, Stecenko A. Control of virus reactivation arrests pulmonary herpesvirus-induced fibrosis in IFN-gamma receptor-deficient mice. Am J Respir Crit Care Med 175: 1139–1150, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mora AL, Woods CR, Garcia A, Xu J, Rojas M, Speck SH, Roman J, Brigham K, Stecenko AA. Lung infection with gamma-herpesvirus induces progressive pulmonary fibrosis in Th2 biased mice. Am J Physiol Lung Cell Mol Physiol 289: L711–L721, 2005. [DOI] [PubMed] [Google Scholar]
- 61. Morrison TE, Kenney SC. BZLF1, an Epstein-Barr virus immediate-early protein, induces p65 nuclear translocation while inhibiting p65 transcriptional function. Virology 328: 219–232, 2004. [DOI] [PubMed] [Google Scholar]
- 62. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Murray LA, Argentieri RL, Farrell FX, Bracht M, Sheng H, Whitaker B, Beck H, Tsui P, Cochlin K, Evanoff HL, Hogaboam CM, Das AM. Hyper-responsiveness of IPF/UIP fibroblasts: interplay between TGF[beta]1, IL-13 and CCL2. Int J Biochem Cell Biol 40: 2174, 2008. [DOI] [PubMed] [Google Scholar]
- 64. Nguyen Y, McGuffie BA, Anderson VE, Weinberg JB. Gammaherpesvirus modulation of mouse adenovirus type 1 pathogenesis. Virology 380: 182–190, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Noel W, Raes G, Hassanzadeh Ghassabeh G, De Baetselier P, Beschin A. Alternatively activated macrophages during parasite infections. Trends Parasitol 20: 126–133, 2004. [DOI] [PubMed] [Google Scholar]
- 66. Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13: 715–725, 2000. [DOI] [PubMed] [Google Scholar]
- 67. Pan T, Mason RJ, Westcott JY, Shannon JM. Rat alveolar type II cells inhibit lung fibroblast proliferation in vitro. Am J Respir Cell Mol Biol 25: 353–361, 2001. [DOI] [PubMed] [Google Scholar]
- 68. Petosa C, Morand P, Baudin F, Moulin M, Artero JB, Muller CW. Structural basis of lytic cycle activation by the Epstein-Barr virus ZEBRA protein. Mol Cell 21: 565–572, 2006. [DOI] [PubMed] [Google Scholar]
- 69. Pozharskaya V, Torres-Gonzalez E, Rojas M, Gal A, Amin M, Dollard S, Roman J, Stecenko AA, Mora AL. Twist: a regulator of epithelial-mesenchymal transition in lung fibrosis. PLoS One 4: e7559, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Procop GW, Kohn DJ, Johnson JE, Li HJ, Loyd JE, Yen-Lieberman B, Tang YW. BK and JC polyomaviruses are not associated with idiopathic pulmonary fibrosis. J Clin Microbiol 43: 1385–1386, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rottoli P, Magi B, Perari MG, Liberatori S, Nikiforakis N, Bargagli E, Cianti R, Bini L, Pallini V. Cytokine profile and proteome analysis in bronchoalveolar lavage of patients with sarcoidosis, pulmonary fibrosis associated with systemic sclerosis and idiopathic pulmonary fibrosis. Proteomics 5: 1423–1430, 2005. [DOI] [PubMed] [Google Scholar]
- 72. Saito A, Okazaki H, Sugawara I, Yamamoto K, Takizawa H. Potential action of IL-4 and IL-13 as fibrogenic factors on lung fibroblasts in vitro. Int Arch Allergy Immunol 132: 168–176, 2003. [DOI] [PubMed] [Google Scholar]
- 73. Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc 3: 364–372, 2006. [DOI] [PubMed] [Google Scholar]
- 74. Shan B, Yao TP, Nguyen HT, Zhuo Y, Levy DR, Klingsberg RC, Tao H, Palmer ML, Holder KN, Lasky JA. Requirement of HDAC6 for transforming growth factor-beta1-induced epithelial-mesenchymal transition. J Biol Chem 283: 21065–21073, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stewart JP, Egan JJ, Ross AJ, Kelly BG, Lok SS, Hasleton PS, Woodcock AA. The detection of Epstein-Barr virus DNA in lung tissue from patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 159: 1336–1341, 1999. [DOI] [PubMed] [Google Scholar]
- 76. Suga M, Iyonaga K, Ichiyasu H, Saita N, Yamasaki H, Ando M. Clinical significance of MCP-1 levels in BALF and serum in patients with interstitial lung diseases. Eur Respir J 14: 376–382, 1999. [DOI] [PubMed] [Google Scholar]
- 77. Suga M, Iyonaga K, Okamoto T, Gushima Y, Miyakawa H, Akaike T, Ando M. Characteristic elevation of matrix metalloproteinase activity in idiopathic interstitial pneumonias. Am J Respir Crit Care Med 162: 1949–1956, 2000. [DOI] [PubMed] [Google Scholar]
- 78. Tang YW, Johnson JE, Browning PJ, Cruz-Gervis RA, Davis A, Graham BS, Brigham KL, Oates JA, Jr, Loyd JE, Stecenko AA. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol 41: 2633–2640, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tsai SC, Lin SJ, Chen PW, Luo WY, Yeh TH, Wang HW, Chen CJ, Tsai CH. EBV Zta protein induces the expression of interleukin-13, promoting the proliferation of EBV-infected B cells and lymphoblastoid cell lines. Blood 114: 109–118, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vannella KM, Moore BB. Viruses as co-factors for the initiation or exacerbation of lung fibrosis. Fibrogen Tissue Rep 1: 2, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Vergnon JM, Vincent M, de Thé G, Mornex JF, Weynants P, Brune J. Cryptogenic fibrosing alveolitis and Epstein-Barr virus: an association? Lancet 2: 768–771, 1984. [DOI] [PubMed] [Google Scholar]
- 82. Wallace WA, Ramage EA, Lamb D, Howie SE. A type 2 (Th2-like) pattern of immune response predominates in the pulmonary interstitium of patients with cryptogenic fibrosing alveolitis (CFA). Clin Exper Immunol 101: 436–441, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Williams KJ, Maes R, Del Piero F, Lim A, Wise A, Bolin DC, Caswell J, Jackson C, Robinson NE, Derksen F, Scott MA, Uhal BD, Li X, Youssef SA, Bolin SR. Equine multinodular pulmonary fibrosis: a newly recognized herpesvirus-associated fibrotic lung disease. Vet Pathol 44: 849–862, 2007. [DOI] [PubMed] [Google Scholar]
- 84. Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc 3: 377–382, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol 2: 103–121, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wong DM, Belgrave RL, Williams KJ, Del Piero F, Alcott CJ, Bolin SR, Marr CM, Nolen-Walston R, Myers RK, Wilkins PA. Multinodular pulmonary fibrosis in five horses. J Am Vet Med Assoc 232: 898–905, 2008. [DOI] [PubMed] [Google Scholar]
- 87. Xue SA, Lu QL, Poulsom R, Karran L, Jones MD, Griffin BE. Expression of two related viral early genes in Epstein-Barr virus-associated tumors. J Virol 74: 2793–2803, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yang Y, McKerlie C, Borenstein SH, Lu Z, Schito M, Chamberlain JW, Buchwald M. Transgenic expression in mouse lung reveals distinct biological roles for the adenovirus type 5 E1A 243- and 289-amino-acid proteins. J Virol 76: 8910–8919, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zamo A, Poletti V, Reghellin D, Montagna L, Pedron S, Piccoli P, Chilosi M. HHV-8 and EBV are not commonly found in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 22: 123–128, 2005. [PubMed] [Google Scholar]
- 90. Zhu Z, Ma B, Zheng T, Homer RJ, Lee CG, Charo IF, Noble P, Elias JA. IL-13-induced chemokine responses in the lung: role of CCR2 in the pathogenesis of IL-13-induced inflammation and remodeling. J Immunol 168: 2953–2962, 2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.