Abstract
The mechanisms of sinoatrial node (SAN) dysfunction in heart failure (HF) remain unclear. We hypothesized that impaired rhythmic spontaneous sarcoplasmic reticulum Ca2+ release (Ca2+ clock) plays an important role in SAN dysfunction in HF. HF was induced in canine hearts by rapid ventricular pacing. The location of pacemaking sites was determined in vivo using computerized electrical mapping in acute open-chest preparations (normal, n = 3; and HF, n = 4). Isoproterenol (Iso, 0.2 μg·kg−1·min−1) infusion increased heart rate and shifted the pacemaking site to the superior SAN in all normal hearts. However, in failing hearts, Iso did not induce superior shift of the pacemaking site despite heart rate acceleration. Simultaneous optical recording of intracellular Ca2+ and membrane potential was performed in Langendorff-perfused isolated right atrium (RA) preparations from normal (n = 7) and failing hearts (n = 6). Iso increased sinus rate, enhanced late diastolic Ca2+ elevation (LDCAE), and shifted the pacemaking sites to the superior SAN in all normal but in none of the HF RAs. Caffeine (2 ml, 20 mmol/l) caused LDCAE and increased heart rate in four normal RAs but in none of the three HF RAs. Iso induced ectopic beats from lower crista terminalis in five of six HF RAs. These ectopic beats were suppressed by ZD-7288, a specific pacemaker current (If) blocker. We conclude that HF results in the suppression of Ca2+ clock, resulting in the unresponsiveness of superior SAN to Iso and caffeine. HF also increases the ectopic pacemaking activity by activating the If at the latent pacemaking sites in lower crista terminalis.
Keywords: calcium, sarcoplasmic reticulum, sympathetic nerve activity
sinoatrial node (SAN) remodeling and reduced SAN reserve occur frequently in patients with heart failure (HF) (20). However, the mechanisms of SAN dysfunction in HF remain unclear. The experimental and clinical studies in advanced HF have demonstrated widespread structural and electrophysiological remodeling of the atria (12, 21). In addition, HF is also known to result in the downregulation of the hyperpolarization-activated pacemaker current in the SAN (If) (23) but significant upregulation of the If in the right atrium (RA) (26). This differential remodeling was thought to underlie the mechanisms of SAN dysfunction and increased atrial arrhythmias in HF. However, multiple studies showed that the spontaneous diastolic depolarization in SAN occurs because of a synergistic interaction between the voltage clock mediated by voltage-sensitive membrane ion currents, such as If (2, 3), and Ca2+ clock mediated by rhythmic spontaneous sarcoplasmic reticulum (SR) Ca2+ release and Na+/Ca2+ exchanger current activation (10, 11, 13). Using simultaneous membrane potential (Vm) and intracellular Ca2+ (Cai2+) mapping, our laboratory recently demonstrated that the acceleration of the Ca2+ clock in the superior SAN plays an important role in SAN rate acceleration during β-adrenergic stimulation in an isolated, Langendorff-perfused RA preparation (8). HF is known to be associated with significant abnormalities of Cai2+ handling (1, 19), which may result in Ca2+ clock dysfunction. Consistent with this possibility, we showed that in ambulatory dogs, the increased sympathetic nerve discharges from the stellate ganglion is associated with abnormal heart rate responses, including tachybradycardia (17). The power spectral analysis of heart rate variability and autonomic nervous system activity demonstrated that the HF SAN is unable to respond properly to the tonic sympathetic activation in HF (18). We hypothesize that in HF, the impaired Ca2+ clock within the SAN works synergistically with reduced If to prevent SAN rate response to sympathetic stimulation, whereas the continued presence of If in the latent pacemaking sites is responsible for ectopic atrial activity. The purpose of the present study was to test these hypotheses in a canine model of pacing-induced HF.
MATERIALS AND METHODS
Canine model of pacing-induced HF.
All animal study protocols were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine and the Methodist Research Institute. We studied 23 adult mongrel dogs weighing 22 to 28 kg. The pacing-induced HF was produced in 10 dogs with the use of previously described techniques (17). In short, after anesthesia with isoflurane (1.5 to 2%), a pacemaker system was implanted to the right ventricular apex. After 2 wk, the pacemaker was activated to pace at 150 beats/min for 3 days, at 200 beats/min for 3 days, and at 250 beats/min for 3 wk to induce HF. Transthoracic echocardiography was performed at baseline and after cessation of rapid pacing to confirm that high-rate ventricular pacing induced HF. A two-dimensional short-axis view of the left ventricle was obtained at the level of the papillary muscles to estimate left ventricular ejection fraction. Left ventricular ejection fraction was 73.1 ± 2.9% at baseline and 35.0 ± 3.2% at HF (P < 0.001).
Computerized electrical mapping in vivo.
A Unemap computerized mapping system (Auckland, NZ) was used in the study (25). We used an electrode patch with 448 electrodes with 1-mm interelectrode distance covering a 1.5 × 2.7-cm area to study the effect of β-adrenergic stimulation at the earliest pacemaker sites in normal (n = 3) and HF (n = 4) dogs. We mapped the activation pattern of the SAN and surrounding RA according to methods previously published (25).
Langendorff-perfused canine isolated RA preparation.
We used isolated RAs from 10 normal dogs and 6 dogs with pacing-induced HF for optical mapping study. The heart was rapidly excised under general anesthesia, and the right coronary artery was perfused with 37°C Tyrode solution equilibrated with 95% O2-5% CO2 to maintain a pH of 7.4. The composition of Tyrode solution was (in mmol/l) 125 NaCl, 4.5 KCl, 0.25 MgCl2, 24 NaHCO3, 1.8 NaH2PO4, 1.8 CaCl2, and 5.5 glucose. The coronary perfusion pressure was regulated between 50 and 60 mmHg. Both ventricles and left atrium were removed, and all visible coronary artery branches were tied off. During optical recordings, contractility was inhibited by 10–17 μmol/l of blebbistatin, and the motion artifact was negligible even after isoproterenol (Iso) infusion (8). Pseudo-ECG was recorded with widely spaced bipolar RA electrodes using Iso-DAM8A (World Precision Instruments).
Assessment of SAN function in vitro.
Sinus node recovery time (SNRT) was determined by bipolar pacing with a programmable stimulator (Bloom Associates, Reading, PA) for 30 s at progressively shorter pacing cycle lengths (400, 350, and 300 ms) with the two electrodes placed near the SAN. The longest time interval from the last paced atrial depolarization to the first spontaneous sinus beat was recorded as the SNRT. The corrected SNRT was determined by subtracting an average of three sinus cycle lengths before the commencement of atrial pacing from the SNRT. The sinoatrial conduction time (SACT) was measured by the method described by Narulaet et al. (15): SACT = (return cycle length − basic cycle length)/2.
Dual Vm and Cai2+ recordings.
The simultaneous Vm and Cai2+ mapping was performed using previously described techniques (8). Briefly, the RAs were stained with Rhod-2 AM and RH237 (Molecular Probes, Eugene, OR) and excited with laser light at 532 nm. Fluorescence was collected using two cameras (MiCAM Ultima, BrainVision, Tokyo, Japan) at 1 ms/frame and 100 × 100 pixels with spatial resolution of 0.35 × 0.35 mm2/pixel for 2 s. After dual Vm and Cai2+ optical mapping of baseline spontaneous heart beats, pharmacological intervention was performed. The heart rate response to Iso (0.01, 0.03, 0.1, 0.3, and 1.0 μmol/l) was tested in normal and HF dog RAs. A bolus injection of 2 ml caffeine (20 mmol/l) within 1 s was performed in normal and HF dog RAs. ZD-7288 (3 μmol/l), a selective If blocker, was administered to evaluate the characteristic of Iso-induced ectopic beats in normal (n = 3) and HF (n = 5) dogs.
Data analysis.
The Cai2+ and Vm traces were normalized to their respective peak-to-peak amplitude for comparison in timing and morphology. The slope of enhanced late diastolic Ca2+ elevation (LDCAE) was measured by the previously described method (8).
Data were expressed as means ± SE. Statistical analysis was performed using paired Student's t-test or one-way analyses of variance (ANOVAs) with Bonferroni's post hoc analysis. The Pearson's χ2-tests were used to compare two categorical variables. The repeated-measure ANOVA model was used to compare Iso- or ZD-7288-induced heart rate response between control and HF dogs. A P value of ≤0.05 was considered significant.
RESULTS
Effects of β-adrenergic stimulation on SAN function in vivo.
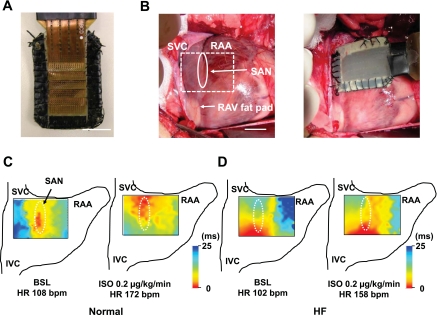
We used an electrode patch with 448 electrodes (Fig. 1A) to study the effect of β-adrenergic stimulation on the earliest pacemaker sites in normal (n = 3) and HF (n = 4) dogs. Examples of isochronal maps of electrical activation before and after Iso infusion in normal and HF dogs are shown in Fig. 1, C and D. The earliest pacemaker site is located on the middle (n = 2) or inferior (n = 1) SAN at baseline in normal dog hearts. β-Adrenergic stimulation with Iso infusion (0.2 μg·kg−1·min−1) shifted the earliest pacemaker site (red) to the superior SAN with heart rate acceleration in all three normal hearts (Fig. 1C). In all four failing hearts, the earliest pacemaker site was located on the inferior SAN at baseline, but treatment with Iso did not shift the early activation site to the superior SAN despite heart rate acceleration in any HF hearts (Fig. 1D). The distribution of earliest pacemaker site during Iso infusion showed a significant difference between normal and HF dogs (P = 0.008). Iso (0.02 μmol/l) significantly shifted the earliest pacemaker site upward in normal dogs compared with HF (8.6 ± 1.0 vs. 1.7 ± 0.7 mm, P = 0.002).
Fig. 1.
Computerized electrical mapping of sinoatrial node (SAN) activations in normal and heart failure (HF) dogs. A: picture of the 448-channel electrode patch. B: pictures of electrode location. Rectangle surrounded by dotted line (B, left) represents electrode patch location. C: isochronal maps at baseline (BSL; left) and during isoproterenol (Iso) perfusion (right) in normal dog. D: isochronal maps at BSL (left) and during Iso perfusion (right) in HF dog. SVC, superior vena cava; IVC, inferior vena cava; RAA, right atrial appendage; RAV, right atrial ventral; HR; heart rate; bpm, beats/min. Bars are 10 mm.
SAN dysfunction of HF dogs in vitro.
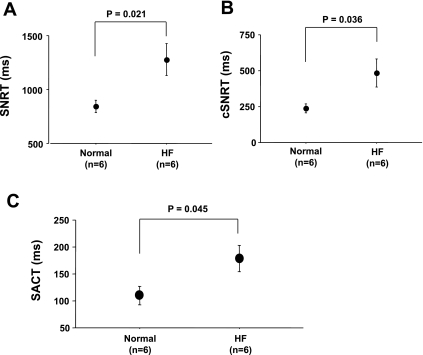
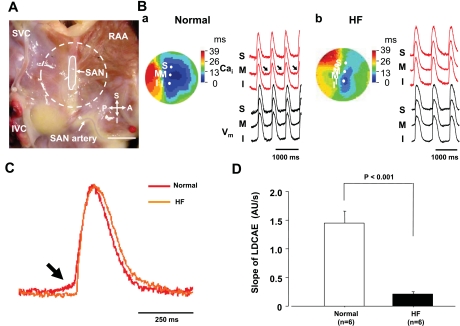
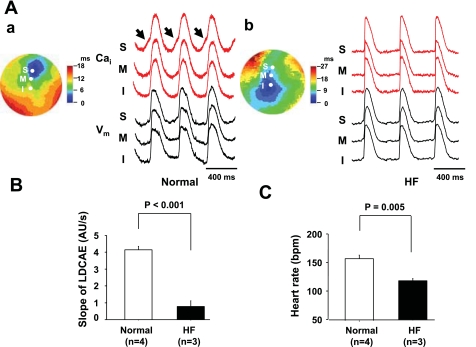
Figure 2 shows the assessment of SAN function in normal and HF RAs. The RAs of HF dogs had longer SNRT (1,277 ± 149 vs. 842 ± 57 ms, P = 0.021, Fig. 2A), and longer corrected SNRT (485 ± 97 vs. 238 ± 31 ms, P = 0.036, Fig. 2B) than those of normal dogs. SACT was also significantly longer in HF dogs than in normal dogs (179 ± 24 vs. 110 ± 17 ms, P = 0.045, Fig. 2C). Figure 3 shows the activation pattern on SAN and the surrounding RA during spontaneous sinus rhythm during baseline in normal and HF dogs. The pacemaking sites at baseline in RAs of normal dogs (n = 7) were located at the middle and inferior SANs in four and three dogs, respectively. All of RAs from HF dogs (n = 6) were the inferior SAN. Normal dogs showed small LDCAEs in the pacemaking sites at baseline, whereas the LDCAEs did not occur in any HF dogs [1.45 ± 0.20 vs. 0.11 ± 0.05 arbitrary units (AU)/s, P < 0.001, Fig. 3, C and D].
Fig. 2.
SAN function in normal and HF right atriums (RAs). A: sinus node recovery time (SNRT) in normal and HF RAs. B: correct sinus node recovery time (cSNRT) on normal and HF RAs. C: sinoatrial conduction time (SACT) on normal and HF RAs.
Fig. 3.
Activation patterns of SAN in normal and HF RAs. A: photo of isolated RA preparation showing the SAN and surrounding RA. Gray area is the SAN. Dotted circle corresponds to isochronal map field in B. B: activation pattern of the SAN and surrounding RA in normal (a) and HF (b) dogs. Color pictures show isochronal maps of membrane potential (Vm) propagation. Intracellular Ca2+ (Cai2+; red) and Vm (black) recordings from superior (S), middle (M), and inferior (I) SAN are presented. Arrows represent enhanced late diastolic Ca2+ elevation (LDCAE). C: magnified view of Cai2+ tracing at pacemaking site in SAN. Arrow shows difference between normal and HF in LDCAE. D: slope of LDCAE on pacemaking site in normal and HF dogs. Slope is significantly suppressed in HF (P < 0.001). P, posterior; A, anterior; AU, arbitrary units. Bar is 10 mm.
Impaired Ca2+ clock activation at superior SAN with β-adrenergic stimulation and caffeine injection in HF dogs.
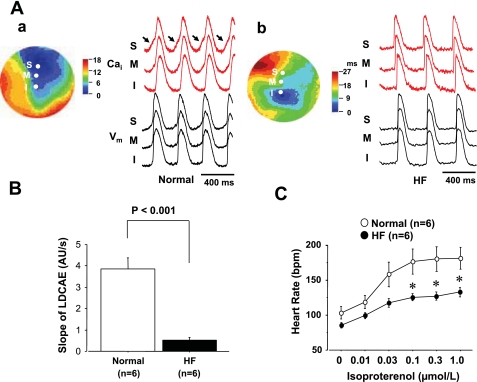
Figure 4A shows examples of activation patterns at Iso (0.1 μmol/l) infusion in normal and HF RAs. In a normal RA, a robust LDCAE (arrows in Cai2+ tracing of Fig. 4A,a) occurred at the pacemaking site of superior SAN. The earliest Vm activation site shifted to superior SAN after Iso infusion as shown in a previous study (8). This finding was consistently observed in all six normal RAs during Iso infusion. However, none of the six RAs from HF dogs showed LDCAE in superior SAN (Fig. 4A,b), and the earliest Vm activation site did not shift to superior SAN. The distribution of the pacemaking site during Iso infusion showed a significant difference between normal and HF RAs (P < 0.001). The slope of the LDCAE was significantly depressed in HF compared with normal dogs (0.52 ± 0.13 vs. 3.87 ± 0.50 AU/s at Iso 0.01 μmol/l, P < 0.001, Fig. 4B). Figure 4C shows the Iso-dose response curve of heart rate obtained from RAs of six normal and six HF dogs, respectively. In normal hearts, Iso dose-dependently increased heart rate. The heart rate response to Iso reduced significantly after the induction of HF (P < 0.001).
Fig. 4.
Effect of Iso on activation pattern of SAN in normal and HF RAs. A: activation patterns in normal and HF RAs during Iso perfusion. Isochronal map and Cai2+ (red) and Vm (black) tracings from S, M, and I SANs at Iso 0.1 μmol/l for normal (a) and HF (b) dogs. Arrows show robust LDCAEs in normal RA. B: slope of LDCAE at Iso 0.1 μmol/l in normal and HF RAs. Slope of LDCAE in superior SAN is significantly suppressed in HF (P < 0.001). C: effect of Iso to HR in normal and HF dogs. Iso-induced HR increase is impaired in RAs from HF dogs. *P < 0.05 vs. corresponding normal group.
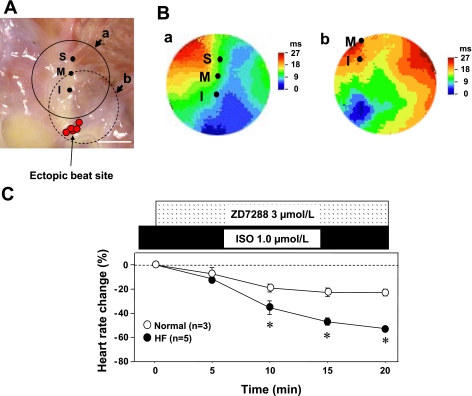
When Iso dosage was increased, Iso induced ectopic beats from the lower crista terminalis in five of six HF dogs (Fig. 5A) but none in the normal RAs. There were no LDCAEs in the ectopic beat sites. Figure 5C shows heart rate changes associated with If blocker (ZD-7288) treatment in normal and HF RAs. Iso induced ectopic beats in HF, and ZD-7288 (3 μmol/l) suppressed the rate of ectopic beats, resulting in bradycardia at 61 ± 3 beats/min (n = 5). ZD-7288 significantly reduced heart rate in HF RAs compared with that in normal RA (P < 0.001, Fig. 5C).
Fig. 5.
Iso-induced atrial ectopic pacemaker in HF RAs. A: locations of ectopic beats in HF RAs. Red circles show the sites of Iso-induced ectopic beats. All of Iso-induced ectopic beats occurred from the lower crista terminalis. Solid line and dotted circles correspond to isochronal map field of B,a and B,b, respectively. B: isochronal maps during ectopic beats appearance. C: change of HR with ZD-7288, pacemaker current (If) blocker, to Iso-induced ectopic beats in normal and HF dogs. ZD-7288 (3 μmol/l) significantly suppressed HR in HF compared with normal dogs (P < 0.001). *P < 0.01 vs. corresponding normal group. Bar is 10 mm.
Figure 6A,a shows the typical caffeine response in normal RA. Caffeine injection shifted the pacemaking site to superior SAN with concomitant LDCAE (arrows in Cai2+ tracing of Fig. 6A,a). This finding was consistently observed in all four normal RAs that were perfused with caffeine. In HF RAs, however, caffeine did not result in LDCAE compared with that in normal RA (0.78 ± 0.32 vs. 4.18 ± 0.20 AU/s, P < 0.001, Fig. 6B) and did not shift the pacemaking site upward in any of the three HF dogs (P = 0.03). Furthermore, the heart rate response to caffeine injection reduced significantly in HF RAs (118 ± 4 vs. 159 ± 7 beats/min, P = 0.005, Fig. 6C).
Fig. 6.
Effect of caffeine on activation pattern of SAN in HF dogs. A: effects of caffeine in normal and HF RAs. A,a: caffeine response in normal RA. Caffeine injection (20 mmol/l, 2-ml bolus given within 1 s) induces LDCAE in normal RA. Arrows show robust LDCAEs. A,b: caffeine responses in HF RA. There is no LDCAE. B: slope of LDCAE in normal and HF RAs. Caffeine-induced robust LDCAE is significantly suppressed in HF (P < 0.001). C: HR in normal and HF RAs after caffeine injection. Caffeine-induced HR increase is significantly suppressed in HF (P = 0.005).
DISCUSSION
The present study is the first that documents Ca2+ clock dysfunction in dogs with pacing-induced HF. The major findings of the present study include the following: 1) Iso-induced LDCAE was impaired in the superior SAN in HF; 2) response of LDCAE and heart rate to β-adrenergic stimulation are impaired in the superior SAN of failing hearts, suggesting a role for dysfunctional Ca2+ cycling and SAN function in HF; 3) caffeine also failed to increase the rate of SR Ca2+ release in the superior SAN in HF; and 4) Iso-induced ectopic beats from the lower crista terminalis worked as the leading pacemaker in HF. This ectopic pacemaker was suppressed by a specific If blocker. These findings indicate that a defective Ca2+ clock at the superior SAN is responsible for the unresponsiveness of SAN to sympathetic stimulation in HF. HF also increases the ectopic pacemaking activity by activating the If at the latent pacemaking sites in the lower crista terminalis.
Abnormal spontaneous SR Ca2+ release in HF.
Our data demonstrated that β-adrenergic stimulation induced lower heart rate responses in HF dogs than in normal dogs. We (18) previously reported that the spectral variables, indirect measures of autonomic nerve activity, correlated with left stellate ganglionated nerve activity at baseline but not during HF. These latter findings are consistent with diminished SAN responsiveness to autonomic modulation. The present study demonstrated a significantly reduced LDCAE at the pacemaking sites of SAN in HF compared with normal SAN, and the slope of the LDCAE during β-adrenergic stimulation was shallower in HF than in normal SANs. These results suggest that spontaneous SR Ca2+ release was impaired in SANs of HF dogs. In addition to Ca2+ clock malfunction, HF also impairs the membrane voltage clock by downregulating If (23) in the SAN. Zicha et al. (26) reported that hyperpolarization activated cyclic nucleotide-gated potassium channel 4 (HCN4), which encodes If, downregulates in the SAN in HF dogs. The suppression of heart rate acceleration in response to β-adrenergic stimulation in HF may be caused by If downregulation. However, Herrmann et al. (6) recently studied mice with deleted HCN4. They reported that HCN4 is not required to mediate the acceleration of the heart rate in response to β-adrenergic stimulation, although HCN4 is necessary for maintaining a stable cardiac rhythm. Their data suggest that HF-induced Ca2+ clock dysfunction is an important factor that limits SAN responsiveness to sympathetic stimulation.
Impaired response to caffeine.
Caffeine sensitizes the ryanodine receptor to activation, resulting in an increased SR Ca2+ release (24). The present study revealed that caffeine failed to increase Cai2+ in HF hearts, suggesting these findings may be explained by the depletion of SR Ca2+ content on the SR. Previous studies have reported the alterations in Ca2+ handling were found in failing myocardium (5). Several possible mechanisms could explain these phenomena. First, a decreased sarcoplasmic reticulum Ca2+-ATPase expression and function are present in HF and may result in a reduced SR Ca2+ accumulation (16). Second, an increased forward-mode Na+/Ca2+ exchanger current activity opposes SR Ca2+ accumulation (16). The decreased SR Ca2+ content may reduce spontaneous SR Ca2+ release, leading to Ca2+ clock dysfunction. Third, type-2 ryanodine receptor function is increased in HF, probably because of hyperphosphorylation and reduced FKBP12.6 binding (14). These changes may contribute to reduced SR Ca2+ content and further impair the Ca2+ handling in HF.
Iso-induced ectopic beats in HF.
Iso caused ectopic beats from the lower crista terminalis in HF dogs. Because these ectopic beats were suppressed by ZD-7288, If activation most likely underlied the increased ectopic activity. These results are consistent with our previous report that If is intimately involved in the generation of ectopic beats from the lower crista terminalis when the Ca2+ clock in SAN is impaired (22). Honjo et al. (7) reported that myocardial sleeves of pulmonary veins have the potential to generate spontaneous activity, and such arrhythmogenic activity is uncovered by the modulation of Cai2+ dynamics. In the lower crista terminalis area, the same mechanisms might have caused ectopic beats as well. HF decreases HCN4 expression at both mRNA and protein levels in SAN, whereas HCN4 is significantly upregulated in RA from the HF dog (26). The HCN4 upregulation in RA may play a role in atrial arrhythmogenesis in HF. Thus we suggest that the Ca2+ clock dysfunction and the HCN4 upregulation in atrium are associated with atrial ectopic beats in HF.
Clinical implications.
HF is associated with SAN dysfunction and increased ectopic pacemaker activity. Lower crista terminalis is a frequent origin of ectopic atrial tachycardia in patients without structural heart diseases (9) and is also a dominant ectopic pacemaking site in dogs with pacing-induced HF (4). Our data provide new insights into the combination of SAN dysfunction and increased ectopic pacemaking activity during sympathetic activation in patients with HF.
Conclusion.
SAN dysfunction in a canine model of HF is associated with Ca2+ clock malfunction, characterized by unresponsiveness of SAN to Iso and caffeine. These findings suggest reduced SR Ca2+ release in the superior SAN is a mechanism of SAN dysfunction in HF. HF also increases the ectopic pacemaking activity by activating the If at the latent pacemaking sites in lower crista terminalis.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grants P01-HL-78931, R01-HL-78932 and HL-71140; a Nihon Kohden/Saint Jude Medical Electrophysiology fellowship (to M. Maruyama); Medtronic-Zipes endowments (to P.-S. Chen); and an American Heart Association established investigator award (to S.-F Lin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Lei Lin and Nicole Courtney for assistance.
REFERENCES
- 1.Bers DM. Altered cardiac myocyte Ca regulation in heart failure. Physiology (Bethesda) 21: 380–387, 2006 [DOI] [PubMed] [Google Scholar]
- 2.DiFrancesco D. The pacemaker current (If) plays an important role in regulating SA node pacemaker activity. Cardiovasc Res 30: 307–308, 1995 [PubMed] [Google Scholar]
- 3.DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol 55: 455–472, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Fenelon G, Shepard RK, Stambler BS. Focal origin of atrial tachycardia in dogs with rapid ventricular pacing-induced heart failure. J Cardiovasc Electrophysiol 14: 1093–1102, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure. J Mol Cell Cardiol 34: 951–969, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Herrmann S, Stieber J, Stockl G, Hofmann F, Ludwig A. HCN4 provides a ‘depolarization reserve’ and is not required for heart rate acceleration in mice. EMBO J 26: 4423–4432, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honjo H, Boyett MR, Niwa R, Inada S, Yamamoto M, Mitsui K, Horiuchi T, Shibata N, Kamiya K, Kodama I. Pacing-induced spontaneous activity in myocardial sleeves of pulmonary veins after treatment with ryanodine. Circulation 107: 1937–1943, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Joung B, Tang L, Maruyama M, Han S, Chen Z, Stucky M, Jones LR, Fishbein MC, Weiss JN, Chen PS, Lin SF. Intracellular calcium dynamics and acceleration of sinus rhythm by beta-adrenergic stimulation. Circulation 119: 788–796, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalman JM, Olgin JE, Karch MR, Hamdan M, Lee RJ, Lesh MD. “Cristal tachycardias”: origin of right atrial tachycardias from the crista terminalis identified by intracardiac echocardiography. J Am Coll Cardiol 31: 451–459, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Lakatta EG, Maltsev VA, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart's pacemaker. Circ Res 106: 659–673, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakatta EG, Vinogradova T, Lyashkov A, Sirenko S, Zhu W, Ruknudin A, Maltsev VA. The integration of spontaneous intracellular Ca2+ cycling and surface membrane ion channel activation entrains normal automaticity in cells of the heart's pacemaker. Ann NY Acad Sci 1080: 178–206, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation 100: 87–95, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Maltsev VA, Lakatta EG. Dynamic interactions of an intracellular Ca2+ clock and membrane ion channel clock underlie robust initiation and regulation of cardiac pacemaker function. Cardiovasc Res 77: 274–284, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101: 365–376, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Narula OS, Shantha N, Vasquez M, Towne WD, Linhart JW. A new method for measurement of sinoatrial conduction time. Circulation 58: 706–714, 1978 [DOI] [PubMed] [Google Scholar]
- 16.O'Rourke B, Kass DA, Tomaselli GF, Kaab S, Tunin R, Marban E. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: experimental studies. Circ Res 84: 562–570, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Ogawa M, Zhou S, Tan AY, Song J, Gholmieh G, Fishbein MC, Luo H, Siegel RJ, Karagueuzian HS, Chen LS, Lin SF, Chen PS. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol 50: 335–343, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Piccirillo G, Ogawa M, Song J, Chong VJ, Joung B, Han S, Magri D, Chen LS, Lin SF, Chen PS. Power spectral analysis of heart rate variability and autonomic nervous system activity measured directly in healthy dogs and dogs with tachycardia-induced heart failure. Heart Rhythm 6: 546–552, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pogwizd SM, Bers DM. Calcium cycling in heart failure: the arrhythmia connection. J Cardiovasc Electrophysiol 13: 88–91, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Sanders P, Kistler PM, Morton JB, Spence SJ, Kalman JM. Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation 110: 897–903, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Sanders P, Morton JB, Davidson NC, Spence SJ, Vohra JK, Sparks PB, Kalman JM. Electrical remodeling of the atria in congestive heart failure: electrophysiological and electroanatomic mapping in humans. Circulation 108: 1461–1468, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Shinohara T, Joung B, Kim D, Maruyama M, Luk HN, Chen PS, Lin SF. Induction of atrial ectopic beats with calcium release inhibition: local hierarchy of automaticity in the right atrium. Heart Rhythm 7: 110–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verkerk AO, Wilders R, Coronel R, Ravesloot JH, Verheijck EE. Ionic remodeling of sinoatrial node cells by heart failure. Circulation 108: 760–766, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Vinogradova TM, Bogdanov KY, Lakatta EG. β-Adrenergic stimulation modulates ryanodine receptor Ca2+ release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ Res 90: 73–79, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Zhou S, Chang CM, Wu TJ, Miyauchi Y, Okuyama Y, Park AM, Hamabe A, Omichi C, Hayashi H, Brodsky LA, Mandel WJ, Ting CT, Fishbein MC, Karagueuzian HS, Chen PS. Nonreentrant focal activations in pulmonary veins in canine model of sustained atrial fibrillation. Am J Physiol Heart Circ Physiol 283: H1244–H1252, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Zicha S, Fernandez-Velasco M, Lonardo G, L'Heureux N, Nattel S. Sinus node dysfunction and hyperpolarization-activated (HCN) channel subunit remodeling in a canine heart failure model. Cardiovasc Res 66: 472–481, 2005 [DOI] [PubMed] [Google Scholar]