Abstract
Bardet-Biedl syndrome (BBS) is a genetically heterogeneous, autosomal-recessive disorder associated with several clinical features including obesity, hypertension, and cardiovascular abnormalities. BBS proteins play an important role in the function of cilia, a mechanosensory organelle in endothelial cells, but whether these proteins are directly involved in the regulation of vascular function is unclear. Here, we show that Bbs genes (1–12) are expressed in endothelial and smooth muscle cell lines and tissues enriched in endothelial (lung) and smooth muscle (stomach) cells as well as the aorta. Next, we used aortic rings to examine the vascular function of two BBS mouse models that recapitulate the human phenotype, namely Bbs2−/− (obese and normotensive) and Bbs6−/− (obese and hypertensive) mice. Interestingly, the endothelium-dependent relaxation (induced by ACh) was significantly enhanced in Bbs2−/− but not Bbs6−/− mice. In contrast, the endothelium-independent relaxation (induced by sodium nitroprusside) was unaltered in both BBS mouse models. In addition, the contractile responses to serotonin and endothelin-1 were attenuated in Bbs2−/− but not Bbs6−/− mice. Of note, the NO-producing enzymes (eNOS and iNOS) were upregulated in the aorta of Bbs2−/− but not Bbs6−/− mice. On the other hand, the expression level of membrane subunits of NADPH oxidase (p22phox and p47phox) in the aorta was decreased in Bbs2−/− mice but increased in Bbs6−/− mice. In conclusion, these data implicate Bbs genes in the regulation of vascular function and demonstrate that disrupting Bbs2 and Bbs6 genes affect differentially the vascular function.
Keywords: obesity, hypertension, vascular reactivity
bardet-biedl syndrome (BBS) is a genetically heterogeneous disorder characterized by obesity, learning disabilities, polydactyly, renal abnormalities, hypogonadism, and retinopathy (34). Hypertension and cardiovascular disease are also common in BBS patients (20). The BBS results from mutations in several genes, 12 described thus far, that encode proteins that are critical for the structure and function of cilia (33). In keeping with this, highly conserved homologs of BBS proteins are found in ciliated organisms but not in nonciliated species (19, 22). In addition, Bbs gene products were shown to localize to the centriolar satellites of centrosomes and basal bodies of primary cilia where they interact with components of the dynein transport machinery of intraflagellar transport (17, 30).
Recent studies provide important clues on how BBS proteins are involved in ciliary function and why mutations in any Bbs gene lead to the same phenotype. Indeed, 7 BBS proteins (BBS1, BBS2, BBS4, BBS5, BBS7, BBS8, and BBS9) were found to form a core complex, known as the BBSome, which functions as a unit that mediates protein/vesicle trafficking to cilia (23). On the other hand, 3 chaperonin-like BBS proteins (BBS6, BBS10, and BBS12) form a complex in combination with other chaperonin proteins of the CCT/TRiC family and mediate the assembly of the BBSome (28).
The development of animal models of BBS has allowed a remarkable progress in understanding the specific role of BBS proteins in various tissues and to gain mechanistic insight into the pathophysiology of this syndrome. Of note, BBS mouse models exhibit major components of the human phenotype including obesity, retinal degeneration, and neurological deficits (10, 13, 22, 24). We (10, 26) also demonstrated that Bbs4- and Bbs6-null mice have elevated blood pressure, whereas Bbs1 M390R knockin and Bbs2 knockout mice do not.
Disruption of Bbs genes in the neurons appears to affect the membrane localization of several receptors including the leptin receptor (4, 29). Consequently, loss of BBS proteins in the hypothalamic neurons perturbs the mechanisms that control energy balance leading to obesity (26, 29). On the other hand, inactivation of Bbs genes in retinal tissue leads to mislocalization of rhodopsin, which precedes apoptotic death of the photoreceptors and retinal degeneration (24). Despite this progress, there are a large number of cell types and tissues in which the role of BBS proteins is still undermined.
The key role of Bbs genes in ciliary function led us to hypothesize that BBS proteins are critically involved in the regulation of vascular function. The expression of Bbs genes in the endothelial and smooth muscle cells as well as the vasculature was examined. In addition, to clarify the role of Bbs genes in the control of vascular functions, we evaluated the vascular effects of deleting Bbs2 or Bbs6 genes in mice.
MATERIALS AND METHODS
Drugs and reagents.
ACh, A-23187, sodium nitroprusside (SNP), KCl, and 5-HT were obtained from Sigma-Aldrich and dissolved in physiological saline solution. Prostaglandin F2α (PGF2α; Lutalyse; Pfizer Animal Health) was supplied by the University of Iowa Pharmacy. Endothelin-1 (ET-1) was purchased from Peninsula Laboratories and dissolved in physiological saline. All concentrations are stated as the final concentrations in the organ chamber. We used antibodies against phosphorylated endothelial nitric oxide synthase (phospho-eNOS; Ser1177) and inducible nitric oxide synthase (iNOS) both from Cell Signaling Technology, total eNOS (Santa Cruz Biotechnology), and β-actin (Abcam). Secondary rabbit antibody was purchased from Santa Cruz Biotechnology. Enhanced chemiluminescence (ECL) detection kit was purchased form Amersham GE Healthcare. Smooth muscle cell basal medium and endothelial cell basal medium were from Lonza.
Animals.
Generation of Bbs2−/− and Bbs6−/− mice and genotyping were described previously (13, 24). Littermate wild-type mice were used as controls. All mice were fed standard mouse chow (LM-485; Teklad Premier Laboratory Diets) and water ad libitum. Care of the mice used in the experiments met the standards set forth by the National Institutes of Health (NIH) in their guidelines for the care and use of experimental animals. All procedures were approved by the Animal Care and Use Committee of the University of Iowa.
Aortic ring preparations.
Aortic rings were prepared for measurements of contraction and relaxation as described previously (5, 6). In brief, 4- to 7-mo-old Bbs2−/− and Bbs6−/− mice and littermate controls were given a lethal dose of pentobarbital (50–100 mg/mouse ip), and the thoracic aorta was quickly removed and placed in Krebs buffer containing the following (in mmol/l): 118.3 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 11 glucose. Connective tissue and remaining fat in the adventitia were removed, and the vessel was cut into four equal, straight pieces of 4–5 mm in length. Vascular rings were suspended in a myograph chamber (Multi Wire Myograph System Model 610M; Danish Myo Technology) containing 5 ml of oxygenated Krebs solution maintained at 37°C. The rings were connected to a force transducer via two built-in steel hooks to measure isometric tension (contraction and relaxation). Resting tension was increased stepwise to reach the final tension of 0.5 g, and the rings were allowed to equilibrate for 45 min. This amount of resting tension is optimal for contraction in these arteries as we (5, 6) have shown previously. After an initial precontraction (submaximally, 40–50% of maximum) with PGF2α (10–50 μmol/l) and relaxation with ACh (10−4 M) and SNP (10−4 M) to establish the integrity and functionality of the vessel, dose-response curves were preformed. Vessels were contracted submaximally with PGF2α as described above. After a stable contraction plateau was reached, dose-response curves were obtained for ACh and SNP (10 nM to 0.3 μM). Contractile response in the aorta to ET-1 (0.1 nM to 0.3 μM), KCl (4–160 mM), and 5-HT (10 nM to 0.3 μM) in a dose-responsive manner were examined. A maximum of five dose-response curves were performed per segment.
Gene expression assays.
Expression of Bbs genes was examined in cultured mouse brain microvascular endothelial (BMVEC) and smooth muscle (BMSMC) cells as well as in the aorta, lung, and stomach of wild-type mice using a standard RT-PCR assay. BMVEC and BMSMC (obtained from Dr. Steven Moore, University of Iowa) were grown in endothelial cell basal medium and smooth muscle cell basal medium, respectively, as described previously (9). Mice were killed by CO2 asphyxiation, and the tissues were dissected, freeze-clamped, and stored at −80°C. Total RNA was purified by TRIzol method from 100-mm dishes of BMVEC and BMSMC or from 50-mg tissues. cDNA was amplified by reverse transcriptase II (Invitrogen). The following PCR conditions were used: step 1, 95°C, 5 min; step 2, 95°C, 30 s, 55°C, 30 s, 72°C, 1 min, 30 cycles; step 3, 72°C, 10 min. Real-time RT-PCR was carried out with SYBR Green (Bio-Rad) using primers shown in Table 1. Samples were analyzed in 2% agarose gels.
Table 1.
PCR primers used for gene expression studies
| Gene | Sense Primer | Antisense Primer |
|---|---|---|
| Bbs1 | 5′-TTGTCTGTGCAGTCACTCAG-3′ | 5′-CGTCCTCATCTGCCAGGTT-3′ |
| Bbs2 | 5′-ATGCTGCTGCCTGTGTTCACT-3′ | 5′-TGCAGAGCACAGTTTCCACC-3′ |
| Bbs3 | 5′-CCACAATCATTAACAAGCTGA-3′ | 5′-CTGACATGTCAAACACTGTA-3′ |
| Bbs4 | 5′-ACTCAGGTTCCTGCATCTGTT-3′ | 5′-CACTGTGCAGTTGGTCTTGTG-3′ |
| Bbs5 | 5′-GGAGCATGTGTATGATAAGAT-3′ | 5′-TCCTCCACTCTGCTGAGAGCTT-3′ |
| Bbs6 | 5′-AGGACGATACCACTGAGCTGG-3′ | 5′-CCTCAGACCTCACAG ACTCTT-3′ |
| Bbs7 | 5′-GTCTTAGCACTCATAATGGA-3′ | 5′-TCAAAGCTGTCAACACACAAG-3′ |
| Bbs8 | 5′-CTATCACTAGCTCATCTGGAAG-3′ | 5′-ACATCGTTTTCATGGTGGAGAA-3′ |
| Bbs9 | 5′-CCGTGCTGCAAGTGGAAGTC-3′ | 5′-GGTTTCCATGTTCCACATTTC-3′ |
| Bbs10 | 5′-CTACCAGAGGTTGCTTCACA-3′ | 5′-AGAGTGAGGTATAAATGCACATG-3′ |
| Bbs11 | 5′-TGTCACCTGTGATGCTGAAG-3′ | 5′-CTTCATTCTCAGAGAAGAAGTGG-3′ |
| Bbs12 | 5′-GGACAGATAATAGCCACTGG-3′ | 5′-CTTGCTGCAGACACAGACTCG-3′ |
| M3R | 5′-ACACACACACAGAGACTCT CC-3′ | 5′-CATTGTGACTCTCTGACATAG-3′ |
| β-Actin | 5′-CATCCTCTTCCTCCCTGGAGAAGA-3′ | 5′-ACAGGATTCCATACCCAAGAAGGAAGG-3′ |
Western blotting.
Bbs2−/− and Bbs6−/− mice and littermate controls (4–7 mo old) were killed with CO2 asphyxiation, and the thoracic aorta was quickly removed. Proteins were extracted by homogenizing the aorta in tissue lysate buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM NaF, 5 mM EDTA, 1% Triton X-100, 2 mM sodium orthovanadate, and Roche cocktail protease inhibitor tablet). Twenty-microgram protein samples were subjected to SDS-PAGE, electrotransferred on a polyvinylidene fluoride membrane, and then probed with primary antibodies at the following concentrations: phospho-eNOS (Ser1177, 1:1,000), eNOS (1:1,000), iNOS (1:1,000), and β-actin (1:10,000), followed by a secondary anti-rabbit antibody (1:10,000). Protein expression was visualized with ECL detection kit.
Data analysis.
All data are expressed as means ± SE. Western blot data were quantified using NIH ImageJ. SigmaStat (Systat Software) was used to analyze the data. For concentration-response curves, comparisons were made with two-way repeated-measures ANOVA followed by a Tukey test or t-test when appropriate. The remaining data were compared by one-way ANOVA followed by Tukey post hoc test. P < 0.05 was considered significant.
RESULTS
Bbs genes are expressed in the vasculature.
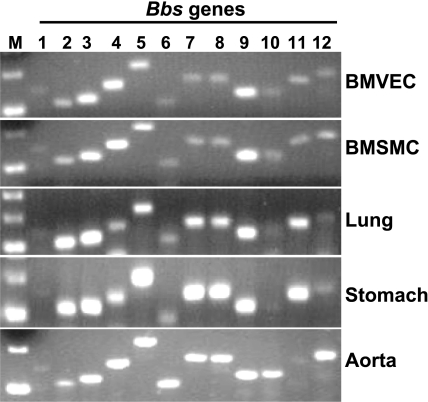
To address the potential role of Bbs genes in the vascular function, we first tested whether Bbs genes are expressed in the cells that form the vasculature (smooth muscle and endothelial cells). As shown in Fig. 1, the 12 Bbs genes are expressed with various intensities in cultured endothelial (BMVEC) as well as smooth muscle (BMSMC) cells. These Bbs genes were also expressed with different degrees in the tissues enriched in endothelial (lung) and smooth muscle (stomach) cells as well as in a blood vessel, the aorta (Fig. 1).
Fig. 1.
Bbs genes are expressed in the vasculature. Analysis of the expression of 12 established Bbs genes in mouse brain microvascular endothelial (BMVEC) and smooth muscle (BMSMC) cells and wild-type mice tissues including lung, stomach, and aorta by RT-PCR. M, marker.
Endothelial-dependent relaxation is differentially altered in BBS mice.
To characterize the role of Bbs genes in the control of vascular function, we performed vascular studies in the aorta of Bbs2−/−, Bbs6−/−, and wild-type control mice. Wild-type littermates of Bbs2−/− and Bbs6−/− mice did not exhibit any difference and, therefore, were combined and used as controls throughout the studies described here. Bbs2−/− and Bbs6−/− mice were obese as indicated by the increased body weight and fat mass (Supplemental Fig. S1, available in the data supplement online at the AJP-Heart and Circulatory Physiology web site). We (26) have previously shown that whereas the obese Bbs6−/− are hypertensive, the obese Bbs2−/− are normotensive.
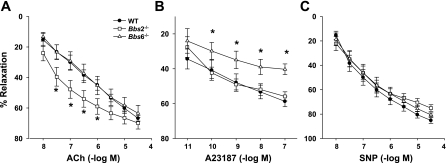
Aortic rings of obese normotensive Bbs2−/− mice showed a significantly enhanced relaxation to ACh, but the maximal relaxations were not different compared with wild-type controls (Fig. 2A). The obese hypertensive Bbs6−/− mice had a normal response to ACh (Fig. 2A). To test the possibility that alterations in the muscarinic receptors may contribute to the enhanced vasodilatory response to ACh, we used quantitative RT-PCR to compare the expression level of the muscarinic receptor subtype (M3R) that mediates the vascular effects of ACh (2, 18) in the aorta of BBS and control mice. Consistent with our findings, the expression levels of M3R were significantly increased in Bbs2−/− but not Bbs6−/− mice (Supplemental Fig. S2).
Fig. 2.
Comparison of vascular relaxation between Bbs2−/−, Bbs6−/−, and wild-type control mice (WT). Vasodilatation to endothelial-specific dilator ACh (A), endothelial- and NO-specific ionophore A-23187 (B), and endothelial-independent NO donor sodium nitroprusside (SNP; C) were tested in aortic rings from Bbs2−/− and Bbs6−/− mice and wild-type littermate controls. *P < 0.05 vs. wild-type mice (n ≥ 8).
In contrast to ACh responses, the endothelium-dependent relaxations induced by the calcium ionophore A-23187 were significantly impaired in Bbs6−/−, but not Bbs2−/−, mice (Fig. 2B). On the other hand, the aortic response to an endothelium-independent stimulus (SNP) was unchanged in both BBS mouse models (Fig. 2C) suggesting that the changes in the endothelium-dependent relaxations are not due to smooth muscle dysfunction.
Contractile responses are markedly decreased in BBS mice.
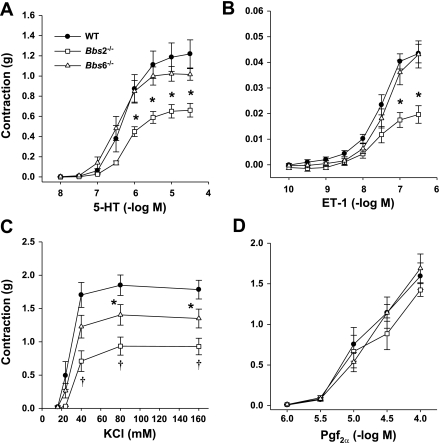
To test whether the ability of the vessel to contract is also altered in BBS mice, we compared the effect of several vasoactive substances on the aorta of Bbs2−/− and Bbs6−/− mice and littermate controls. Strikingly, the aortic rings of Bbs2−/− mice showed a significantly attenuated contractile response to ET-1, serotonin (5-HT), and KCl (Fig. 3, A–C). The vasoconstrictions induced by KCl were also significantly attenuated in Bbs6−/− mice relative to wild-type controls but less compared with Bbs2−/− mice (Fig. 3C). In contrast, there was no difference in the responses induced by PGF2α (Fig. 3D) indicating that the changes in the contractile responses are selective.
Fig. 3.
Characterization of contractile vascular responses in Bbs2−/−, Bbs6−/−, and wild-type control mice. Contractions were induced in aortic rings from Bbs2−/− and Bbs6−/− mice and wild-type littermate controls using receptor-dependent agonists including serotonin (5-HT; A), endothelin-1 (ET-1; B), and prostaglandin F2α (PGF2α; D) or the receptor-independent constrictor potassium chloride (KCl; C). *P < 0.05 vs. wild-type mice, †P < 0.05 vs. wild-type and Bbs2−/− mice (n ≥ 8).
Absence of structural alterations in the vasculature of BBS mice.
We examined the possibility that structural changes may contribute to the alterations in vascular reactivity in BBS mice. For this, we analyzed the vascular cross-section area, inner and outer diameter, and wall thickness of BBS mice carotids. However, there was no significant difference in all of these parameters between Bbs2−/−, Bbs6−/−, and wild-type controls (Supplemental Fig. S3).
Contrasting molecular changes in the vasculature of BBS mice.
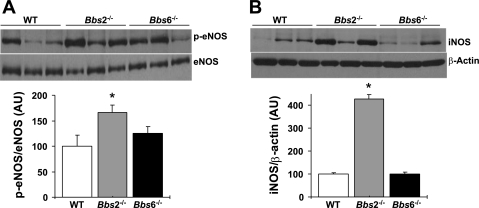
To gain insight into the molecular mechanisms that account for vascular changes in BBS mice, we examined potential alterations in the levels of NO-producing enzymes in the vasculature of BBS mice. Given that eNOS is considered as a major source of NO in the vascular wall, we measured the activity level (phosphorylation of Ser1177) of this enzyme in the aorta. As shown in Fig. 4A, compared with wild-type control littermates, aortas of Bbs2−/− animals exhibited marked increase in phospho-eNOS levels (normalized to total eNOS), whereas Bbs6−/− mice showed only a modest (not statistically significant) increase. Of note, normalization of phospho-eNOS to β-actin, instead of total eNOS, yielded similar results (wild-type control mice, 100 ± 9%; Bbs2−/− mice, 162 ± 9%; and Bbs6−/− mice, 117 ± 13%). Next, we compared the expression (protein level) of another NO-producing enzyme, iNOS, between BBS and control mice. The iNOS level was significantly larger (∼4-fold) in the aortas of Bbs2−/− but not Bbs6−/− mice (Fig. 4B). Such upregulation in the NO-producing enzymes in Bbs2−/− is consistent with the exaggerated ACh-induced vasorelaxation in this mouse model.
Fig. 4.
Expression level of NO-producing enzymes in the aorta of Bbs2−/−, Bbs6−/−, and wild-type littermate control mice. Levels of phosphorylated endothelial nitric oxide synthase (p-eNOS) and eNOS (A) and inducible nitric oxide synthase (iNOS) and β-actin (B) were measured by Western blot. Representative Western blot are shown on the top, and quantification results on the bottom. *P < 0.05 vs. wild-type mice (n ≥ 6). AU, arbitrary units.
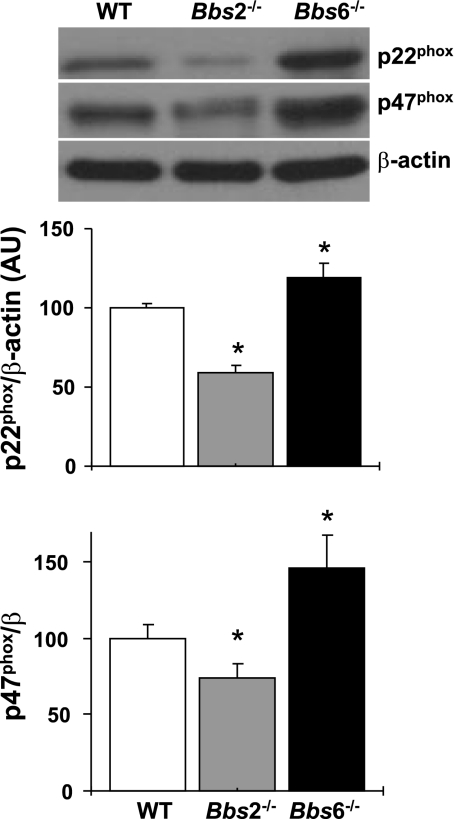
Oxidative stress is an important causative factor of endothelial dysfunction and is thought to play a major role in the occurrence and complications of the metabolic syndrome (1, 12, 27). Therefore, we evaluated the expression (protein level) of membrane subunits of NADPH oxidase (p22phox and p47phox) in the aorta of BBS mice. Of note, whereas the expression of p22phox and p47phox was decreased in Bbs2−/− mice, the expression of these proteins was significantly increased in Bbs6−/− mice (Fig. 5).
Fig. 5.
Expression level of membrane subunits of NADPH oxidase in the aorta of Bbs2−/−, Bbs6−/−, and wild-type littermate control mice. Protein levels of p22phox and p47phox were measured in aortas by Western blot, and β-actin was used as loading control. Representative Western blots are shown on the top, and quantification results on the bottom. *P < 0.05 vs. wild-type mice (n ≥ 6).
DISCUSSION
We describe several new findings in the present study. First, we show that Bbs genes are expressed in the endothelial and smooth muscle cells, the tissues enriched in these cells, and the aorta. Second, we found that despite obesity, two mouse models of BBS show no defect in vasodilatation. In fact, the hypertensive Bbs6−/− showed normal endothelial function, whereas the normotensive Bbs2−/− model showed increased endothelial-dependent vascular relaxation. Third, aortic rings from both Bbs2−/− and Bbs6−/− mice exhibited significant reduction in the contractile responses induced by various stimuli. More specifically, Bbs2−/− showed decreased contractile response to serotonin, ET-1, and KCl, whereas Bbs6−/− exhibited a significant decrease in the contraction induced by KCl but no changes in the response to ET-1. Finally, we found contrasting changes in the expression of key molecular pathways involved in vascular regulation in the aorta of Bbs2−/− and Bbs6−/− mice. These findings demonstrate that Bbs2 and Bbs6 genes alter differentially the vascular tone in mice.
Despite the presence of obesity in both mouse models studied, Bbs2−/− and Bbs6−/− mice showed contrasting vascular changes. However, the enhanced endothelial function (as indicated by the effect of ACh) and reduced contractile responses in Bbs2−/− mice are consistent with the lack of hypertension in this mouse model (26). On the other hand, the vascular changes we described in Bbs6−/− mice are in line with the presence of hypertension in this model. These changes in vascular reactivity may contribute to the difference in arterial pressure in these two mouse models.
Similar to our findings in mice, in humans several BBS genotypes including BBS6 were found to be accompanied by hypertension except for patients with BBS2 who remain normotensive (20). In addition, variants of Bbs6, but not Bbs2, gene have been reported to increase the risk of raised blood pressure in common obese individuals (3). Thus elucidation of the mechanisms that protects some obese patients from the development of endothelial dysfunction and hypertension as in BBS2 will help understand the pathophysiology of obesity-associated vascular abnormalities and high blood pressure.
We identified several molecular changes that may account for the contrasting vascular changes in BBS mice. We show that aorta of Bbs2−/− mice exhibit higher expression levels of the muscarinic receptor subtype (M3) that mediates the endothelium-dependent relaxation caused by ACh (2, 18). This finding is consistent with enhanced ACh-induced vasodilatation in this mouse model. We further show increased levels of eNOS phosphorylation in position Ser1177 in the aorta of Bbs2−/− mice. Although regulation of eNOS activity is complex, phosphorylation of Ser1177 of eNOS is known to enhance the activity of the enzyme leading to an elevation of NO production (11, 15). Bbs2−/− mice also exhibited a large (∼4-fold) increase in iNOS expression in the aorta. Elevated iNOS expression was previously reported in the aorta of diet-induced obese mice (25). The expression profile of NO-producing enzymes in Bbs2−/− mice may contribute to improved endothelial function and reduced receptor-mediated contractile responses in this mouse model. NO is critical for vascular relaxation and considered as the major endothelium-derived relaxing factor in the conduit vessels such as the aorta (14, 32). Release of NO by the vascular endothelium causes the vascular smooth muscle to relax by activating soluble guanylate cyclase, increasing the cGMP concentration, and activating the cGMP-dependent protein kinase G, resulting in vasodilation (21, 32). However, an increase in iNOS such as that observed in the aorta of Bbs2−/− mice may promote inflammation (7, 8). Such an inflammatory state may offset the beneficial effects of higher eNOS leading to normal arterial pressure in this mouse model.
The expression level of NADPH oxidase, a key reactive oxygen species-producing enzyme, is a critical determinant of vascular function (14, 16). Of note, vascular NADPH oxidase appears to be a major source of increased oxidative stress in obesity (31). We found that the expression level of membrane subunits of NADPH oxidase (p22phox and p47phox) in the aorta was decreased in the normotensive Bbs2−/− mice but increased in the hypertensive Bbs6−/− animals.
We recognize several limitations to the present studies that need to be addressed. First, vascular functions were examined ex vivo. Whether the changes we found using aortic rings sustain in vivo remain to be determined. Further studies are also needed to assess the relevance of the vascular reactivity to the control of arterial pressure in BBS mice. Second, we performed molecular assays using RNA and proteins obtained from the whole vessel. The contributions of the various cell types that form the vessel to the changes we report require further investigation. However, we demonstrated that Bbs genes are expressed in the cells that are critical for the control of vascular tone (smooth muscle and endothelial cells). Third, although some of the molecular alterations we found appear consistent with the variations in vascular reactivity and arterial pressure in the two BBS mouse models, additional studies are needed to functionally link the alterations in the expression of eNOS, iNOS, and subunits of NADPH oxidase to the changes in vascular reactivity and arterial pressure. Finally, additional studies are also required to determine the mechanisms by which loss of Bbs2 and Bbs6 genes lead to contrasting molecular changes in the vasculature.
In conclusion, our data implicate Bbs genes in the regulation of vascular tone and demonstrate that disrupting Bbs2 and Bbs6 genes alter differentially the vascular function in mice. The vascular changes may contribute to the contrasting blood pressure effects caused by mutations in these Bbs genes.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-084207. A. M. Beyer was supported by NIH National Research Service Award (NRSA) Research Fellowship HL-07121. V. C. Sheffield is an investigator of the Howard Hughes Medical Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Frank Faraci for the critical reading of the manuscript.
REFERENCES
- 1.Ando K, Fujita T. Metabolic syndrome and oxidative stress. Free Radic Biol Med 47: 213–218, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Beny JL, Nguyen MN, Marino M, Matsui M. Muscarinic receptor knockout mice confirm involvement of M3 receptor in endothelium-dependent vasodilatation in mouse arteries. J Cardiovasc Pharmacol 51: 505–512, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Benzinou M, Walley A, Lobbens S, Charles MA, Jouret B, Fumeron F, Balkau B, Meyre D, Froguel P. Bardet-Biedl syndrome gene variants are associated with both childhood and adult common obesity in French Caucasians. Diabetes 55: 2876–2882, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci USA 105: 4242–4246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyer AM, Baumbach GL, Halabi CM, Modrick ML, Lynch CM, Gerhold TD, Ghoneim SM, de Lange WJ, Keen HL, Tsai YS, Maeda N, Sigmund CD, Faraci FM. Interference with PPAR gamma signaling causes cerebral vascular dysfunction, hypertrophy, and remodeling. Hypertension 51: 867–871, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyer AM, de Lange WJ, Halabi CM, Modrick ML, Keen HL, Faraci FM, Sigmund CD. Endothelium-specific interference with peroxisome proliferator activated receptor gamma causes cerebral vascular dysfunction in response to a high-fat diet. Circ Res 103: 654–661, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borutaite V, Moncada S, Brown GC. Nitric oxide from inducible nitric oxide synthase sensitizes the inflamed aorta to hypoxic damage via respiratory inhibition. Shock 23: 319–323, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Chauhan SD, Seggara G, Vo PA, MacAllister RJ, Hobbs AJ, Ahluwalia A. Protection against lipopolysaccharide-induced endothelial dysfunction in resistance and conduit vasculature of iNOS knockout mice. FASEB J 17: 773–775, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Collins XH, Harmon SD, Kaduce TL, Berst KB, Fang X, Moore SA, Raju TV, Falck JR, Weintraub NL, Duester G, Plapp BV, Spector AA. ω-Oxidation of 20-hydroxyeicosatetraenoic acid (20-HETE) in cerebral microvascular smooth muscle and endothelium by alcohol dehydrogenase 4. J Biol Chem 280: 33157–33164, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Davis RE, Swiderski RE, Rahmouni K, Nishimura DY, Mullins RF, Agassandian K, Philp AR, Searby CC, Andrews MP, Thompson S, Berry CJ, Thedens DR, Yang B, Weiss RM, Cassell MD, Stone EM, Sheffield VC. A knockin mouse model of the Bardet-Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc Natl Acad Sci USA 104: 19422–19427, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Dobrian AD, Davies MJ, Schriver SD, Lauterio TJ, Prewitt RL. Oxidative stress in a rat model of obesity-induced hypertension. Hypertension 37: 554–560, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Fath MA, Mullins RF, Searby C, Nishimura DY, Wei J, Rahmouni K, Davis RE, Tayeh MK, Andrews M, Yang B, Sigmund CD, Stone EM, Sheffield VC. Mkks-null mice have a phenotype resembling Bardet-Biedl syndrome. Hum Mol Genet 14: 1109–1118, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Fulton D, Gratton JP, Mccabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonk AM, Houben AJ, de Jongh RT, Serne EH, Schaper NC, Stehouwer CD. Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology 22: 252–260, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Kim JC, Badano JL, Sibold S, Esmail MA, Hill J, Hoskins BE, Leitch CC, Venner K, Ansley SJ, Ross AJ, Leroux MR, Katsanis N, Beales PL. The Bardet-Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nat Genet 36: 462–470, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Lamping KG, Wess J, Cui Y, Nuno DW, Faraci FM. Muscarinic (M) receptors in coronary circulation: gene-targeted mice define the role of M2 and M3 receptors in response to acetylcholine. Arterioscler Thromb Vasc Biol 24: 1253–1258, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Li JB, Gerdes JM, Haycraft CJ, Fan YL, Teslovich TM, May-Simera H, Li HT, Blacque OE, Li LY, Leitch CC, Lewis RA, Green JS, Parfrey PS, Leroux MR, Davidson WS, Beales PL, Guay-Woodford LM, Yoder BK, Stormo GD, Katsanis N, Dutcher SK. Comparative and basal genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell 117: 541–552, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Moore SJ, Green JS, Fan YL, Bhogal AK, Dicks E, Fernandez BA, Stefanelli M, Murphy C, Cramer BC, Dean JC, Beales PL, Katsanis N, Bassett AS, Davidson WS, Parfrey PS. Clinical and genetic epidemiology of Bardet-Biedl syndrome in Newfoundland: a 22-year prospective, population-based, cohort study. Am J Med Genet A 132: 352–360, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murad F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med 355: 2003–2011, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Mykytyn K, Mullins RF, Andrews M, Chiang AP, Swiderski RE, Yang BL, Braun T, Casavant T, Stone EM, Sheffield VC. Bardet-Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc Natl Acad Sci USA 101: 8664–8669, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129: 1201–1213, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Nishimura DY, Fath M, Mullins RF, Searby C, Andrews M, Davis R, Andorf JL, Mykytyn K, Swiderski RE, Yang BL, Carmi R, Stone EM, Sheffield VC. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci USA 101: 16588–16593, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noronha BT, Li JM, Wheatcroft SB, Shah AM, Kearney MT. Inducible nitric oxide synthase has divergent effects on vascular and metabolic function in obesity. Diabetes 54: 1082–1089, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, Weiss R, Nishimura DY, Sheffield VC. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest 118: 1458–1467, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism 55: 928–934, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Seo S, Baye LM, Schulz NP, Beck JS, Zhang QH, Slusarski DC, Sheffield VC. BBS6, BBS10, and BBS12 form a complex with CCT/TRiC family chaperonins and mediate BBSome assembly. Proc Natl Acad Sci USA 107: 1488–1493, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo SJ, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet 18: 1323–1331, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science 313: 629–633, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Sonta T, Inoguchi T, Tsubouchi H, Sekiguchi N, Kobayashi K, Matsumoto S, Utsumi H, Nawata H. Evidence for contribution of vascular NAD(P)H oxidase to increased oxidative stress in animal models of diabetes and obesity. Free Radic Biol Med 37: 115–123, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 196: 193–222, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Yen HJ, Tayeh MK, Stone EM, Sheffield VC, Slusarski DC. Bardet-Biedl syndrome genes are important in retrograde intracellular trafficking and Kupffer's vesicle cilia function. Hum Mol Genet 15: 667–677, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Zaghloul NA, Katsanis N. Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. J Clin Invest 119: 428–437, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]