Abstract
This study evaluated endothelium-dependent vascular relaxation in response to acetylcholine (ACh) in isolated middle cerebral arteries (MCA) from Dahl salt-sensitive (Dahl SS) rats and three different congenic strains that contain a portion of Brown Norway (BN) chromosome 13 introgressed onto the Dahl SS genetic background through marker-assisted breeding. Two of the congenic strains carry a 3.5-Mbp portion and a 2.6-Mbp portion of chromosome 13 that lie on opposite sides of the renin locus, while the third contains a 2.0-Mbp overlapping region that includes the BN renin allele. While maintained on a normal salt (0.4% NaCl) diet, MCAs from Dahl SS rats and the congenic strains retaining the Dahl SS renin allele failed to dilate in response to ACh, whereas MCAs from the congenic strain carrying the BN renin allele exhibited normal vascular relaxation. In congenic rats receiving the BN renin allele, vasodilator responses to ACh were eliminated by nitric oxide synthase inhibition with NG-nitro-l-arginine methyl ester, angiotensin-converting enzyme inhibition with captopril, and AT1 receptor blockade with losartan. NG-nitro-l-arginine methyl ester-sensitive vasodilation in response to ACh was restored in MCAs of Dahl SS rats that received either a 3-day infusion of a subpressor dose of angiotensin II (3 ng·kg−1·min−1 iv), or chronic treatment with the superoxide dismutase mimetic tempol (15 mg·kg−1·day−1). These findings indicate that the presence of the Dahl SS renin allele plays a crucial role in endothelial dysfunction present in the cerebral circulation of the Dahl SS rat, even in the absence of elevated dietary salt intake, and that introgression of the BN renin allele rescues endothelium-dependent vasodilator responses by restoring normal activation of the renin-angiotensin system.
Keywords: renin-angiotensin system, vascular dysfunction, physiological genomics, vasodilation, angiotensin II, oxidant stress
studies in humans have shown that impaired endothelial function is a strong predictor of cardiovascular events, such as stroke, myocardial infarction, congestive heart failure, and sudden cardiac death, even in the absence of an elevation of arterial blood pressure (13, 15, 36, 44, 54). Long-term follow-up studies have shown that normotensive salt-sensitive individuals not only have a greater risk of developing hypertension than salt-resistant subjects, but also exhibit a higher mortality rate, even if they fail to develop hypertension (50).
Similar to salt-sensitive humans, the Dahl salt-sensitive (Dahl SS) rat develops a low-renin form of hypertension when fed a high-salt (HS) diet (4.0% NaCl) (5, 41). However, normotensive Dahl SS rats maintained on a normal-salt (NS) diet (0.4% NaCl) also exhibit a dramatic impairment in vascular relaxation that appears to be related to chronic exposure to low levels of circulating angiotensin II (ANG II) (8). For example, isolated middle cerebral arteries (MCAs) from Dahl SS rats fed a NS diet either fail to dilate or display a paradoxical vasoconstriction in response to acetylcholine (ACh), reduced Po2, and the stable prostacyclin analog iloprost (8). Previous studies indicate that vascular relaxation is restored when Dahl SS rats are given a continuous intravenous (iv) infusion of a subpressor dose of ANG II (3 ng·kg−1·min−1) for 3 days (9).
A relationship between plasma renin activity (PRA) and vascular relaxation mechanisms is further suggested by studies in SS-13BN/Mcwi consomic rats, in which chromosome 13 from the Brown Norway (BN) rat has been introgressed onto the Dahl SS genetic background through marker-assisted breeding (4). Although SS-13BN/Mcwi rats have only a 1.95% allelic variation from the parental Dahl SS strain (5, 24), normal vascular relaxation mechanisms to ACh and reduced Po2 that are lost in the Dahl SS strain are maintained in MCAs from the SS-13BN/Mcwi strain (8, 9). Also, in contrast to the parental Dahl SS rat, SS-13BN/Mcwi rats are able to maintain normal PRA and normal circulating ANG II levels when they are maintained on a NS diet, demonstrating that they can regulate their renin-angiotensin system normally (5). To further elucidate the role of the renin gene in contributing to these phenotypic differences, overlapping congenic strains were created that are focused around the region of chromosome that contains the renin gene.
The goal of the present study was to evaluate the vasodilator response to ACh in isolated MCAs of rats from the parental Dahl SS strain and three narrowed congenic strains having portions of BN chromosome 13, with and without the BN renin allele, introgressed onto the Dahl SS genetic background. The contribution of reduced ANG II levels and/or reduced activation of the angiotensin type 1 (AT1) receptor to impaired vascular relaxation in MCAs was further tested by evaluating vessel responses to ACh during angiotensin-converting enzyme (ACE) inhibition or AT1-receptor blockade with losartan in Ren1-BN rats (carrying the BN renin allele in the Dahl SS genetic background). Responses to ACh were also determined in Dahl SS rats receiving chronic iv infusion of a low-dose ANG II for 3 days to counteract the chronically low levels of circulating ANG II that exist in Dahl SS rats fed NS diet. The role of elevated vascular superoxide levels in contributing to the endothelial dysfunction present in Dahl SS fed a NS diet was also evaluated by chronically treating Dahl SS rats with the superoxide dismutase (SOD) mimetic tempol in the drinking water.
MATERIALS AND METHODS
General procedures.
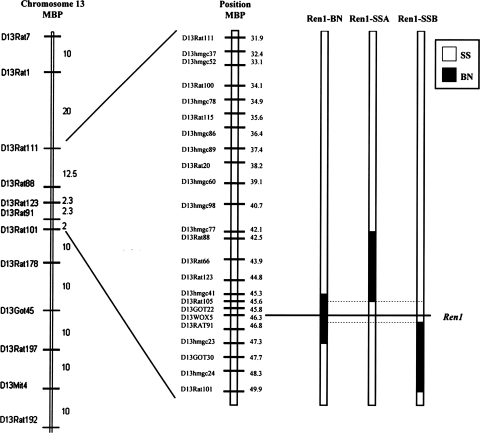
The present study was performed in male Dahl SS (Dahl SS/JHsd/Mcwi) rats and congenic strains derived from a cross between SS-13BN/Mcwi consomic rats and the Dahl SS rat. Congenic strains were developed by marker-assisted breeding, as previously described (33). The congenic strains used in this study are SS.BN-(D13hmgc41-D13hmgc23)/Mcwi (Ren1-BN strain), which has a 2.0-Mbp portion of BN chromosome 13 containing the BN renin allele, and two overlapping, narrowed congenic strains that retain the Dahl SS renin allele: SS.BN-(D13rat77-D13rat105) (Ren1-SSA strain), 3.5 Mbp in size, and SS.BN-(D13rat124-D13rat101) (Ren1-SSB strain), 2.6 Mbp in size (Fig. 1). Information about newly developed congenic strains and microsatellite markers is available through the Rat Genome Database (http://rgd.mcw.edu).
Fig. 1.
Map of genetic markers on rat chromosome 13. Solid segments represent the Brown Norway (BN) segments of chromosome 13 that were introgressed onto the Dahl salt-sensitive (SS) genetic background through marker-assisted breeding.
For all experiments, we used age-matched (9–14 wk old) male Dahl SS, Ren1-BN, Ren1-SSA, and Ren1-SSB rats that weighed 300–400 g. All animals were maintained on a NS (0.4% NaCl) diet (Dyets, Bethlehem, PA) since weaning, with drinking water ad libitum. Additional experimental groups (n = 5–8) were given either the ACE inhibitor captopril (100 mg·kg−1·day−1) in their drinking water for 3 days, the AT1-receptor antagonist losartan (20 mg·kg−1·day−1) in their drinking water for 7 days, or the SOD mimetic tempol (15 mg·kg−1·day−1) in their drinking water for 3 days. A separate group of Dahl SS rats maintained on 0.4% NaCl diet received a chronic iv infusion of a low dose of ANG II (3 ng·kg−1·min−1) for 3 days to counteract the chronically low ANG II levels present in Dahl SS rats fed NS diet, as previously described by Drenjancevic-Peric and Lombard (9). All experimental procedures were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Chronic blood pressure monitoring.
Mean arterial pressure was measured by radiotelemetry (Data Sciences, St. Paul, MN) in conscious 9-wk-old rats fed 0.4% NaCl diet (Dyets) since weaning. Telemetry transmitters (TA11PA-C40) were implanted subcutaneously (under isoflurane anesthesia), and the catheter was inserted into the abdominal aorta via the femoral artery. After 4 days of recovery, blood pressure was measured each morning between 9 AM and 12 PM, and the values obtained over the 3-h recording period were averaged every day for 3 consecutive days.
Isolated vessel preparation and vasodilator stimuli.
On the day of the experiment, animals were anesthetized with an intraperitoneal injection of a low dose of pentobarbital sodium (30 mg/kg; Ovation Pharmaceuticals, Lake Forest, IL) due to the sensitivity of the Dahl SS rat to the anesthetic (45). MCAs were isolated, cannulated with tapered glass micropipettes, and maintained at 37°C in a heated chamber for 1 h while they were perfused and superfused with physiological salt solution (PSS) and bubbled with a 21% O2/5% CO2/74% N2 gas mixture (8). The vessels were pressurized to 80 mmHg to simulate in vivo conditions, and internal diameters were measured via television microscopy. The response of the arteries to the endothelium-dependent vasodilator ACh (10−10-10−5 M) and the nitric oxide (NO) donor sodium nitroprusside (SNP; 10−10-10−4 M) was assessed by measuring vessel diameters during cumulative addition of the agonists to the tissue bath.
In experiments involving MCAs from Ren1-BN congenic rats, Dahl SS rats receiving an iv infusion of ANG II, or Dahl SS rats receiving chronic tempol treatment, the NO synthase (NOS) inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 100 μM) was added to the perfusate and superfusate for 20 min before the assessment of the vasodilator response to ACh to evaluate the role of NO in mediating vascular responses to ACh. In all experiments, maximum vessel diameter was determined by perfusing and superfusing the MCA with Ca2+-free PSS. Spontaneous resting tone (%) was calculated as follows: tone = [(Dmax − Dc)/Dmax] × 100, where Dmax is the diameter of the maximally relaxed vessel in Ca2+-free solution, and Dc is the resting control diameter in PSS.
Western blot analysis.
To evaluate the expression of the M3 muscarinic receptor, endothelial NOS (eNOS) and phospho-eNOS (S1177), Western blots were run with cerebral resistance arteries that were isolated from the ventral surface of the brain after the MCAs were removed for study (see above). After removal, the vessels were washed in PSS to remove blood and homogenized with a FastPrep-24 tissue and cell homogenizer (MP Biomedicals, Solon, OH) using cold homogenization buffer containing protease and phosphatase inhibitors and having the final composition (mM): 10.0 K2HPO4, 1.0 KH2PO4, 1.0 EDTA, and 250.0 sucrose. The protein concentration of the tissue homogenates was determined using the Coomassie Plus protein assay reagent (Thermo Fisher Scientific, Waltham, MA) and 5.0 μg of protein were loaded onto a 4–20% Biorad Criterion precast gel (Bio-Rad Laboratories, Hercules, CA) for separation by electrophoresis. Following electrophoretic separation, the protein was transferred onto a nitrocellulose membrane and blocked with 10% nonfat dry milk (NFDM) for 1 h. The membranes were then incubated overnight in 5% NFDM with the primary antibodies for either the M3 muscarinic receptor (Santa Cruz Biotechnology, Santa Cruz, CA; 1:200 dilution), phospho-eNOS (S1177) (BD Biosciences, San Jose, CA; 1:4,000 dilution), or β-actin (Sigma Aldrich, St Louis, MO; 1:25,000 dilution). The next day, the membranes were washed with Tris-buffered saline containing Tween-20 and incubated with the secondary antibodies in 5% NFDM for 2 h. After secondary antibody incubation, the membranes were washed with Tris-buffered saline-Tween-20, and protein bands were visualized using the SuperSignal West pico chemiluminescent substrate (Thermo Fisher Scientific). Protein bands were quantified using the UNSCAN-IT software (Silk Scientific, Orem, UT), and final expression was normalized to β-actin as follows: [(PD/PDβ-actin) × 100], where PD is the pixel density of the individual protein bands of interest, and PDβ-actin is the PD of the individual β-actin bands.
For evaluation of the degree of phosphorylation of eNOS at S1177, the antibodies for phospho-eNOS (S1177) were stripped from the membrane using a solution containing the following: 62.5 mM Tris·HCl, 2.5% sodium dodecyl sulfate, and 3.5 M 2-mercaptoethanol. Once the antibodies were removed, the membrane was washed and blocked with 5% NFDM for 1 h and incubated with a primary antibody for eNOS (BD Biosciences; 1:4,000 dilution) in 5% NFDM overnight. The next day, the membrane was washed and incubated with the secondary antibody in 5% NFDM for 2 h and visualized using the SuperSignal West pico chemiluminescent substrate (Thermo Fisher Scientific). Protein bands were quantified using the UNSCAN-IT software (Silk Scientific, Orem, UT), and final expression was normalized to total eNOS expression as follows: [(PDp-eNOS/PDeNOS) × 100], where PDp-eNOS is the PD of the individual phospho-eNOS (S1177) protein bands, and PDeNOS is the PD of the same eNOS bands.
Statistical analysis.
All data are presented as mean value ± SE. For comparisons of baseline vessel diameter, maximum vessel diameter, percent resting tone, and concentration-response curve values, differences between multiple groups were assessed using one-way ANOVA. Differences between individual means following ANOVA were evaluated using a post hoc Holm-Sidak test. For comparisons of diameter changes between two groups and for comparisons of Western blot analysis, an unpaired Student's t-test was used to evaluate significance. A P value of <0.05 was considered statistically significant.
RESULTS
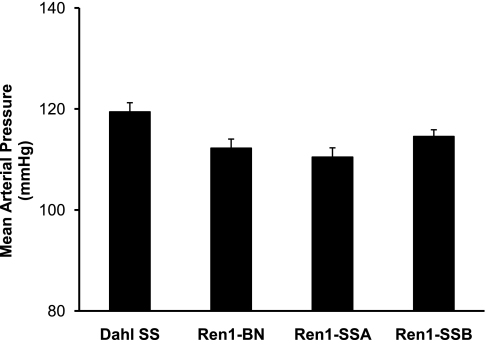
Baseline and maximum vessel diameters in the various experimental groups are summarized in Table 1. Except for l-NAME-treated MCAs from Dahl SS rats that received an ANG II infusion, there were no differences in vessel diameter or active tone (%) between the groups, showing that differences in vascular relaxation responses (see below) were not due to initial differences in resting diameter or active tone, i.e., preexisting constriction of the vessel. Conscious blood pressure measurements from the three congenic strains and the parental Dahl SS rat strain maintained on a NS diet are summarized in Fig. 2. Mean arterial blood pressures for all strains were in the normotensive range.
Table 1.
Diameters of middle cerebral arteries and active resting tone in middle cerebral arteries from the various experimental groups
| Experimental Group | Baseline Diameter, μm | n | Maximum Diameter, μm | n | Active Tone, % | n |
|---|---|---|---|---|---|---|
| Dahl SS | 146 ± 5.4 | 9 | 252 ± 3.4 | 9 | 42 ± 3.3 | 9 |
| Ren1-BN | 145 ± 3.5 | 10 | 248 ± 6.4 | 10 | 41 ± 3.2 | 10 |
| Ren1-SSA | 148 ± 4.4 | 6 | 260 ± 2.6 | 6 | 42 ± 1.9 | 6 |
| Ren1-SSB | 154 ± 6.6 | 5 | 260 ± 5.8 | 5 | 41 ± 1.8 | 5 |
| Ren1-BN + l-NAME | 131 ± 5.7 | 7 | 255 ± 5.2 | 7 | 49 ± 2.4 | 7 |
| Ren1-BN + captopril | 156 ± 3.9 | 7 | 245 ± 8.2 | 6 | 37 ± 2.7 | 6 |
| Dahl SS + captopril | 145 ± 8.4 | 6 | 245 ± 6.1 | 6 | 41 ± 3.3 | 6 |
| Ren1-BN + losartan | 152 ± 6.1 | 8 | 248 ± 7.1 | 7 | 40 ± 2.1 | 7 |
| Dahl SS + losartan | 147 ± 2.3 | 7 | 237 ± 6.6 | 7 | 38 ± 2.1 | 7 |
| Dahl SS-saline infused | 155 ± 8.6 | 6 | 249 ± 8.5 | 6 | 38 ± 2.1 | 6 |
| Dahl SS-ANG II infused | 157 ± 2.0 | 6 | 247 ± 7.8 | 5 | 37 ± 1.5 | 5 |
| Dahl SS-ANG II infused + l-NAME | 113 ± 4.2* | 5 | 246 ± 3.7 | 5 | 54 ± 2.2† | 5 |
| Ren1-BN + tempol | 148 ± 3.7 | 6 | 246 ± 10.5 | 6 | 39 ± 1.7 | 6 |
| Dahl SS + tempol | 133 ± 3.4 | 6 | 255 ± 2.9 | 5 | 48 ± 1.9 | 5 |
| Dahl SS + tempol+ l-NAME | 138 ± 10.1 | 6 | 246 ± 8.9 | 6 | 44 ± 3.3 | 6 |
Values are means ± SE; n, no. of animals. Percent active tone was calculated by subtracting the vessel baseline diameter from the maximum diameter, dividing the difference by the maximum diameter and multiplying by 100. SS, salt sensitive; BN, Brown Norway; l-NAME, NG-nitro-l-arginine methyl ester; ANG II, angiotensin II. *Significant difference (P < 0.05), Dahl SS-ANG II infused + l-NAME vs. all groups except Ren1-BN + l-NAME, Dahl SS + tempol, and Dahl SS + tempol + l-NAME. †Significant difference (P < 0.05), Dahl SS-ANG II infused + l-NAME vs. Ren1-BN + captopril, Dahl SS + losartan, Dahl SS-saline infused, and Dahl SS-ANG II infused.
Fig. 2.
Mean arterial blood pressures measured by radiotelemetry in conscious Dahl SS (n = 17), Ren1-BN (n = 13), Ren1-SSA (n = 11), and Ren1-SSB (n = 18) rats. Values are means ± SE in mmHg.
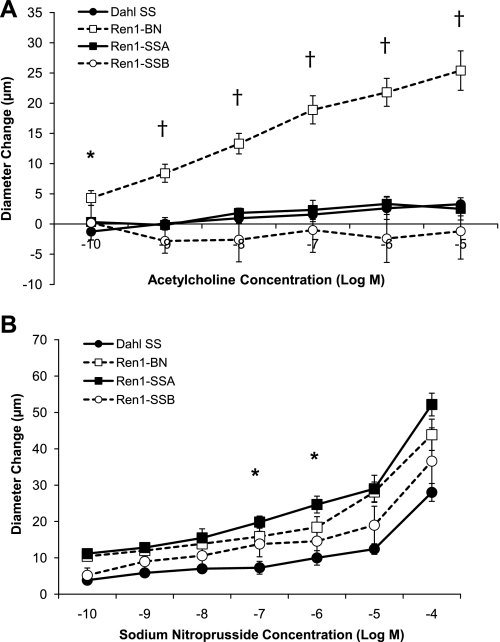
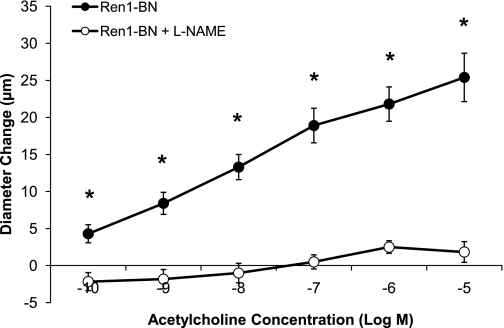
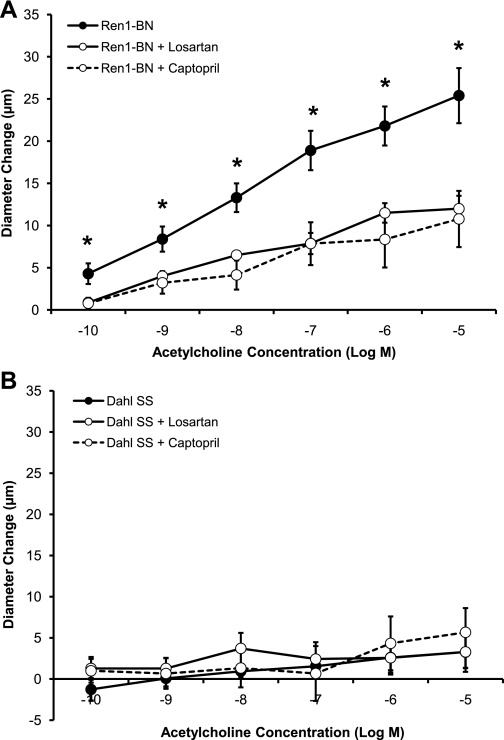
Figure 3 summarizes the response of isolated MCAs from the Dahl SS, Ren1-BN, Ren1-SSA, and Ren1-SSB rat strains to ACh (10−10-10−5 M) and SNP (10−10-10−4 M). Arteries from the Ren1-BN congenic strain, which contains the BN renin allele, dilated in a dose-dependent manner to ACh, whereas MCAs from the Dahl SS, Ren1-SSA, and Ren1-SSB strains, which retain the Dahl SS renin allele, did not (Fig. 3A). Arteries from all four rat strains dilated in response to SNP (Fig. 3B). The response to SNP was similar between groups, except for 10−7 M and 10−6 M SNP, where dilation of MCAs from Ren1-SSA rats was significantly greater than those of the Dahl SS parental strain. Addition of the NOS inhibitor l-NAME (100 μM) to the perfusate and superfusate eliminated the vasodilator response to ACh in Ren1-BN rats (Fig. 4), indicating that the response was NO dependent.
Fig. 3.
A: response of isolated middle cerebral arteries of Dahl SS (n = 9), Ren1-BN (n = 10), Ren1-SSA (n = 6), and Ren1-SSB (n = 5) rats to acetylcholine (10−10-10−5 M). *Significant difference (P < 0.05), Ren1-BN vs. Dahl SS. †Significant difference, Ren1-BN vs. Ren1-SSA, Ren1-SSB, and Dahl SS. B: response of isolated middle cerebral arteries of Dahl SS (n = 7), Ren1-BN (n = 7), Ren1-SSA (n = 6), and Ren1-SSB (n = 5) rats to sodium nitroprusside (10−10-10−4 M). *Significant difference (P < 0.05), Ren1-SSA vs. Dahl SS. Values are mean change in diameter from baseline ± SE in μm.
Fig. 4.
Response of isolated middle cerebral arteries of Ren1-BN rats (n = 10) to acetylcholine (10−10-10−5 M) ± NG-nitro-l-arginine methyl ester (l-NAME; 100 μM; n = 6) in the perfusate and superfusate. Values are mean change in diameter from baseline ± SE in μm. *Significant difference from l-NAME-treated arteries (P < 0.05). The Ren1-BN control response is replotted from Fig. 3.
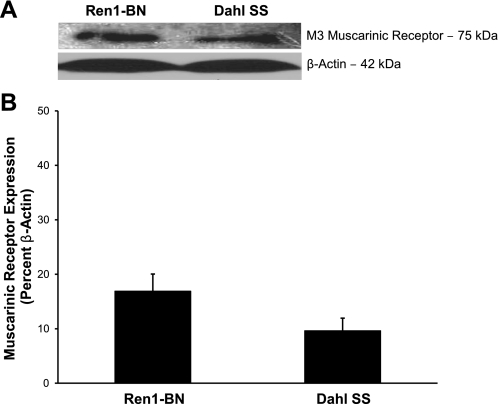
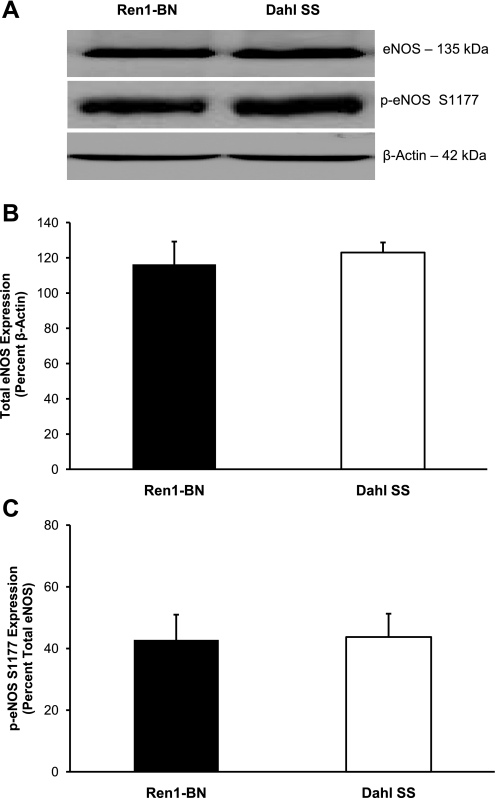
Expression of the M3 muscarinic receptor for ACh was similar in cerebral arteries from Ren1-BN congenic rats and Dahl SS rats (Fig. 5). Total eNOS expression and the degree of phosphorylation of eNOS at S1177 were also similar in cerebral arteries from Ren1-BN and Dahl SS rats (Fig. 6).
Fig. 5.
M3 muscarinic receptor expression in cerebral arteries of Ren1-BN (n = 5) and Dahl SS (n = 6) rats. A: representative Western blots of the M3 muscarinic receptor. B: summarized data are normalized to β-actin and expressed as means ± SE. There were no significant differences in muscarinic receptor expression in cerebral arteries of Ren1-BN and Dahl SS rats.
Fig. 6.
Endothelial nitric oxide synthase (eNOS) expression and activation in cerebral arteries of Ren1-BN (n = 8) and Dahl SS (n = 7) rats. A: representative Western blots of eNOS and phospho-eNOS (p-eNOS; S1177). B: summarized data for total eNOS expression are normalized to β-actin and expressed as means ± SE. C: summarized data for p-eNOS (S1177) expression are normalized to total eNOS and expressed as means ± SE. There were no significant differences in eNOS expression or phosphorylation at S1177 in cerebral arteries of Ren1-BN and Dahl SS rats.
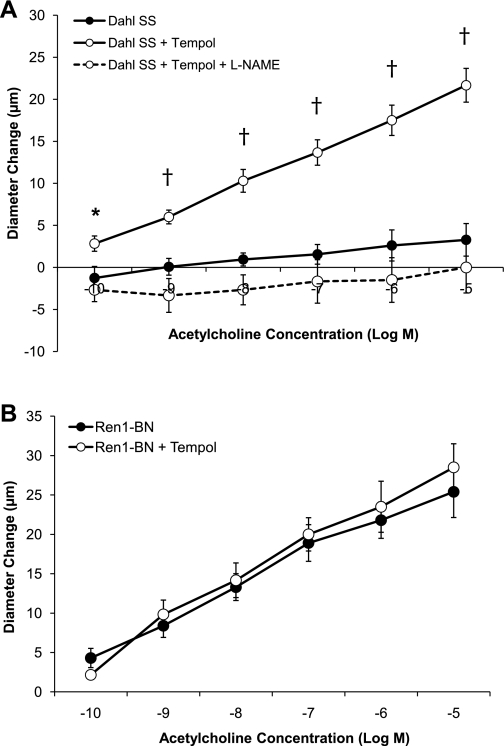
Chronic treatment of Dahl SS rats fed NS diet with the SOD mimetic tempol (15 mg·kg−1·day−1) restored the vasodilator response to ACh in isolated MCAs, and this response could be eliminated by preincubating the MCAs with l-NAME before the assessment of the vasodilator response to ACh (Fig. 7A). Chronic tempol treatment had no effect on the vasodilator response to ACh in isolated MCAs from Ren1-BN congenic rats (Fig. 7B).
Fig. 7.
A: response of isolated middle cerebral arteries of Dahl SS rats (n = 9) to acetylcholine (10−10-10−5 M) ± 7-day oral treatment with the SOD mimetic tempol (15 mg·kg−1·day−1; n = 6). Middle cerebral arteries from a separate group of tempol-treated Dahl SS rats were preincubated with l-NAME (100 μM; n = 6) for 20 min before the assessment of the vasodilator response to ACh. *Significant difference, Dahl SS + tempol vs. Dahl SS + tempol + l-NAME (P < 0.05). †Significant difference, Dahl SS + tempol vs. Dahl SS + tempol + l-NAME and Dahl SS. The Dahl SS control response is replotted from Fig. 3. B: response of isolated middle cerebral arteries of Ren1-BN rats (n = 9) to acetylcholine (10−10-10−5 M) ± 7-day oral treatment with the SOD mimetic tempol (15 mg·kg−1·day−1; n = 6). Tempol treatment had no effect on ACh-induced vasodilation in MCAs from Ren1-BN rats. The Ren1-BN control response is replotted from Fig. 3. Values are mean change in diameter from baseline ± SE in μm.
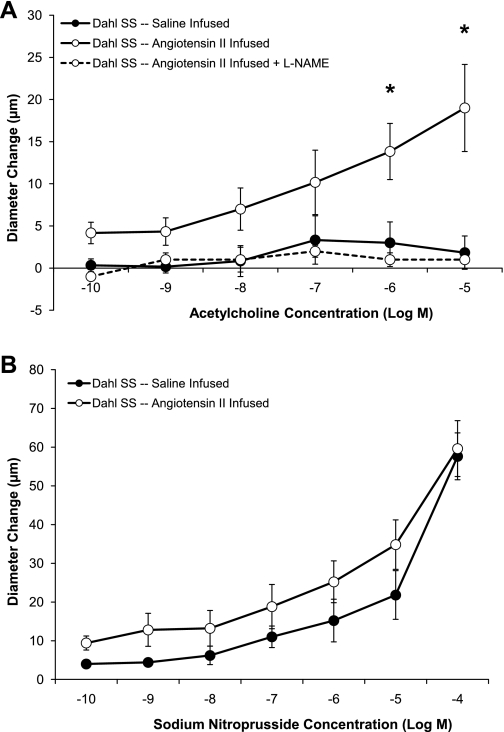
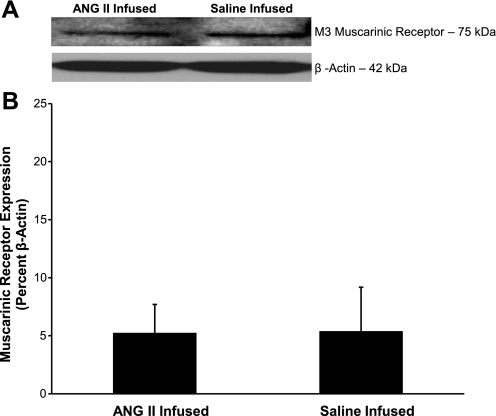
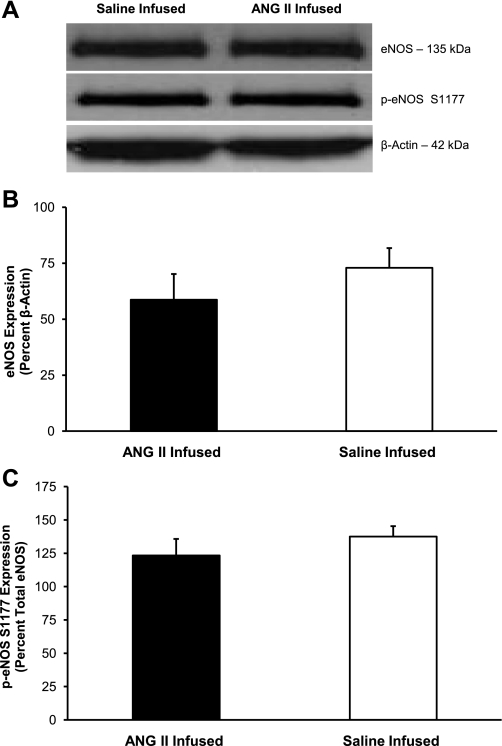
Figure 8 summarizes the response to ACh and SNP in MCAs from Dahl SS rats maintained on NS diet and receiving chronic iv infusion of a subpressor dose of ANG II for 3 days. In those experiments, vascular relaxation in response to ACh was restored in MCAs from Dahl SS rats receiving low-dose ANG II infusion, and SNP-induced dilation was slightly increased, although this was not significant. The ACh-induced dilation that was restored by low-dose ANG II infusion could be eliminated by preincubating the MCA with l-NAME, demonstrating that the response was NO dependent, similar to the ACh-induced vasodilator response observed in MCAs from Ren1-BN congenic rats. As shown in Figs. 9 and 10, respectively, chronic infusion of ANG II had no effect on M3 muscarinic receptor expression, eNOS expression, or phosphorylation of eNOS at S1177 in cerebral arteries of the Dahl SS rats.
Fig. 8.
A: response to acetylcholine (10−10-10−5 M) in isolated middle cerebral arteries from Dahl SS rats maintained on normal salt diet and receiving a chronic intravenous infusion of either ANG II (3 ng·kg−1·min−1; n = 6) or isotonic saline vehicle (n = 6). Middle cerebral arteries from a separate group of ANG II-infused Dahl SS rats were preincubated with l-NAME (100 μM; n = 6) before the assessment of the vasodilator response to ACh. *Significant difference, Dahl SS-ANG II infused vs. Dahl SS-saline infused and Dahl SS-ANG II infused + l-NAME (P < 0.05). B: response to the nitric oxide donor sodium nitroprusside (10−10-10−4 M) in isolated middle cerebral arteries from Dahl SS rats maintained on normal salt diet and receiving a chronic intravenous infusion of either ANG II (3 ng·kg−1·min−1; n = 5) or isotonic saline vehicle (n = 5). Values are mean change in diameter from baseline ± SE in μm.
Fig. 9.
M3 muscarinic receptor expression in cerebral arteries of Dahl SS rats that received an intravenous infusion of either ANG II (n = 4) or isotonic saline vehicle (n = 3). A: representative Western blots of the M3 muscarinic receptor. B: summarized data are normalized to β-actin and expressed as means ± SE. There were no significant differences in muscarinic receptor expression in cerebral arteries of ANG II or saline-infused Dahl SS rats.
Fig. 10.
eNOS expression and activation in cerebral arteries of Dahl SS rats that received an intravenous infusion of either ANG II (n = 7) or isotonic saline vehicle (n = 7). A: representative Western blots of eNOS and p-eNOS (S1177). B: summarized data for total eNOS expression are normalized to β-actin and expressed as means ± SE. C: summarized data for p-eNOS (S1177) expression are normalized to total eNOS and expressed as means ± SE. There were no significant differences in eNOS expression or phosphorylation at S1177 in cerebral arteries of ANG II or saline-infused Dahl SS rats.
Figure 11A shows the effect of oral administration of the ACE inhibitor captopril or the AT1-receptor antagonist losartan on the response to ACh in MCAs of Ren1-BN congenic rats. Vasodilator responses to ACh were significantly reduced in MCAs of Ren1-BN rats receiving either captopril or losartan treatment. By contrast, oral administration of captopril or losartan did not restore the vasodilator response to ACh in Dahl SS rats (Fig. 11B).
Fig. 11.
A: response of isolated middle cerebral arteries of Ren1-BN rats (n = 10) to acetylcholine (10−10-10−5 M) ± 3-day treatment with captopril (100 mg·kg−1·day−1; n = 7) or ± 7-day treatment with losartan (20 mg·kg−1·day−1; n = 8). *Significant difference (P < 0.05), Ren1-BN + losartan and Ren1-BN + captopril vs. Ren1-BN control. The Ren1-BN control response is replotted from Fig. 3. B: response of isolated middle cerebral arteries of Dahl SS rats (n = 9) to acetylcholine (10−10-10−5 M) ± 3-day treatment with captopril (100 mg·kg−1·day−1; n = 6) or ± 7-day treatment with losartan (20 mg·kg−1·day−1; n = 7). The Dahl SS control response is replotted from Fig. 3. Values are mean change in diameter from baseline ± SE in μm.
DISCUSSION
To evaluate the role of the renin gene in determining the different cardiovascular phenotypes observed in SS-13BN/MCWi consomic rats (5, 8, 9), novel congenic strains have recently been created that are focused around smaller regions of the portion of chromosome 13 that contain the renin gene. Studies using overlapping congenic strains containing the BN renin allele have shown that PRA is restored to normal (6) and that the angiogenic response to skeletal muscle stimulation that is lost due to low plasma ANG II levels in Dahl SS rats is rescued when the BN renin allele is introgressed into the Dahl SS genome (6).
The present study shows that the endothelium-dependent vasodilator response to ACh is restored in MCAs from the congenic strain containing a portion of BN chromosome 13 that includes the BN renin allele (Ren1-BN), but not in MCAs from the parental Dahl SS strain or congenic strains that retain the Dahl SS renin allele (Ren1-SSA, Ren1-SSB). Similar to the Dahl SS parental strain, both the congenic strains retaining the Dahl SS renin allele (as well as the Ren1-BN congenic rats) were normotensive on a NS diet, demonstrating that endothelial dysfunction in arteries of rats carrying the Dahl SS renin allele occurs before an elevation in blood pressure and in the absence of an increase in dietary salt intake. The observation that the NO donor SNP causes a dose-dependent dilation in strains carrying either the Dahl SS or BN renin allele (Fig. 3B) is important, because it demonstrates that the vascular smooth muscle cells of all the strains are sensitive to NO and are capable of relaxing in an endothelium-independent manner. In the present study, MCAs from the Ren1-SSA strain dilated more than those of the Dahl SS strain in response to 10−7 M and 10−6 M SNP. However, none of the strains carrying the Dahl SS renin allele dilated in response to ACh, showing that mechanisms other than altered sensitivity to NO (most likely increased levels of vascular oxidant stress; see below) are responsible for the loss of vascular relaxation in response to the endothelium-dependent vasodilator ACh in these strains.
The two control strains for the presence of the BN renin allele (Ren1-SSA and Ren1-SSB) contain portions of BN chromosome 13 that are cut off either just above (Ren1-SSA) or just below (Ren1-SSB) the BN renin allele (Fig. 1). A major strength of this experimental approach is that it allows us to localize genomic elements regulating vascular relaxation mechanisms with even more precision than possible with the SS-13BN/Mcwi consomic rat. However, it is important to recognize the possibility that either 1) another BN allele contained within the 2.0-Mbp segment of chromosome 13 introgressed onto the Dahl SS genetic background is responsible for restoring normal vascular relaxation responses in the Ren1-BN congenic strain; or 2) other BN alleles in the transferred chromosomal segments, excluding the Ren1 locus, inhibit vascular relaxation when introgressed into the Dahl SS genetic background in the Ren1-SSA or Ren1-SSB congenic strains. In light of our finding that vasodilator responses to ACh are rescued in Dahl SS rats that receive a chronic low-dose ANG II infusion (Fig. 8), the present results suggest that the loss of vascular relaxation in response to ACh in cerebral arteries of rat strains carrying the Dahl SS renin gene is due to endothelial dysfunction caused by exposure to chronically low levels of ANG II related to impaired function or regulation of the Dahl SS renin allele (1, 5, 20).
The occurrence of increased renin-angiotensin system activity and endothelial dysfunction in patients with diabetes, atherosclerosis, hypertension, and obesity is well known. In such disease states, treatment with ACE inhibitors and AT1-receptor antagonists has clearly been shown to restore endothelial function to normal in multiple vascular beds (16, 18, 27, 38, 39, 47). As a result, these treatments are often proposed as universally beneficial in improving endothelial function. However, AT1-receptor blockers and ACE inhibitors are less effective in treating low-renin forms of hypertension, especially in African Americans (2, 28, 43, 52, 53). In the present study, ACE inhibition and AT1-receptor blockade had no beneficial effect on endothelial function and vascular reactivity in Dahl SS rats (an accepted rodent model of low-renin hypertension), but led to a significant reduction in endothelium-dependent dilation to ACh in congenic rats carrying the BN renin allele. These observations are consistent with existing studies of normotensive Sprague-Dawley rats showing that ACE inhibition eliminates ACh-induced dilation of cremasteric arterioles (10, 11), and that AT1-receptor blockade impairs vascular relaxation mechanisms in Sprague-Dawley rats maintained on standard rodent chow (37). Taken together, these observations provide additional support for the hypothesis that ANG II, at physiological levels, plays an important role in maintaining normal vascular function, in contrast to the undisputed deleterious effects of high levels of ANG II that contribute to endothelial dysfunction in many pathophysiological states (12, 14, 17, 26, 32, 34, 40, 55).
A recent study by Reed and Coworkers (42) provides a striking example of differences in the effect of ANG II and AT1-receptor blockade under normal and pathological conditions. In that study, the authors found that AT1-receptor blockade with candesartan abrogated collateral zone blood flow following transient/repetitive ischemia in normotensive Wistar-Kyoto rats, while candesartan improved collateral zone flow in the myocardium of JCR rats, a rodent model of syndrome X characterized by elevated oxidant stress in the tissue. Moreover, infusion of a subpressor dose of ANG II increased collateral zone blood flow in Wistar-Kyoto rats, while a hypertensive dose of ANG II reduced collateral zone flow. That study clearly indicates that ANG II can have strikingly different effects under normal and pathological conditions. With that perspective in mind, the role of normal circulating levels of ANG II in maintaining endothelial function may become an especially important issue under conditions in which ANG II levels are suppressed, e.g., with elevated dietary salt intake, as suggested by the studies of Tzemos et al. (46), or in low-renin hypertension.
While the mechanisms by which chronically low plasma ANG II levels cause endothelial dysfunction in the cerebral circulation of the Dahl SS rat remain to be determined, recent studies have shown that the protective effect of ANG II to restore normal vascular relaxation mechanisms in Sprague-Dawley rats fed a HS diet requires activation of the EGF receptor and the ERK-1/2 pathway (29). Other studies suggest that low-dose ANG II infusion prevents the downregulation of the crucial antioxidant enzyme Cu/Zn SOD in cerebral arteries of normotensive Sprague-Dawley rats fed a HS diet (30). This finding is particularly significant in light of multiple studies showing that a HS diet increases oxidative stress in the vasculature and reduces antioxidant enzyme expression and activity (21–23, 56–58). Taken together, the results of this study and others (8, 9) suggest that elevated superoxide levels caused by chronically low ANG II levels are central to the loss of NO-dependent vasodilation in cerebral arteries of normotensive Dahl SS rats.
In addition to eliminating NO-dependent dilation to ACh, salt-induced ANG II suppression has been shown to lead to impaired relaxation to a variety of vasodilator stimuli, including reduced Po2 (also impaired in Dahl SS rats), other endothelium-dependent vasodilators (e.g., histamine), and endothelium-independent vasodilators, such as the GSα protein activator cholera toxin and the stable prostacylin analog iloprost (also impaired in Dahl SS rats) (8, 25, 48, 49). This widespread impairment of vascular relaxation to multiple vasodilator stimuli may involve, at least in part, oxidative damage to receptor-mediated signaling mechanisms. For example, Ca2+ signaling in response to muscarinic receptor activation is impaired in aortic endothelial cells of normotensive Sprague-Dawley rats fed a HS diet, but the reduction in the amplitude of receptor-mediated Ca2+ signaling in salt-fed rats can be prevented either by chronic low-dose ANG II infusion, or by chronic antioxidant treatment with tempol in the drinking water (56).
Perspectives.
Several human studies have shown that salt-sensitive individuals have endothelial dysfunction and reduced PRA compared with their salt-resistant counterparts (3, 19, 31, 35). Tzemos et al. (46) reported that salt loading impaired vascular endothelial function in young, healthy, normotensive subjects, and studies by Dickinson and coworkers (7) found that flow-mediated vasodilation improves with salt restriction in normotensive subjects, independent of any changes in resting blood pressure. Of particular interest, long-term follow-up studies in humans have shown that salt-sensitive humans not only have a greater likelihood of developing hypertension than salt-resistant counterparts, but also have a significantly higher long-term mortality rate, independent of hypertension (51). Given the relationship between endothelial dysfunction and the occurrence of cardiovascular events and death (13, 15, 36, 44, 54), it is attractive to postulate that reductions in dietary salt intake may be beneficial in preventing endothelial dysfunction, especially in salt-sensitive individuals, and that improvement of endothelial function by salt restriction may lower the incidence of such events. A novel perspective that emerges from the present study is the possible role of chronically low ANG II levels in contributing to endothelial dysfunction with elevated dietary salt intake, especially in low-renin forms of hypertension.
An additional contribution of the present experiments is to emphasize the value of the Dahl SS rat as an experimental model of endothelial dysfunction in salt-sensitive individuals, even in the absence of hypertension and elevated dietary salt intake. The present results indicate that narrowed congenic strains such as the Ren1-BN rat will provide a valuable experimental model for future studies to elucidate the mechanisms leading to endothelial dysfunction and cardiovascular disease, not only in salt-sensitive, low-renin forms of hypertension, but in salt-sensitive normotensive individuals as well.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-65289, HL-72920, HL-82798, and HL-92026, and the American Heart Association Midwest Affiliate Predoctoral Fellowship no. 0815532G.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Nadia Barreto, Matthew Hoffman, and Paul Graf for expert technical assistance.
REFERENCES
- 1.Amaral SL, Roman RJ, Greene AS. Renin gene transfer restores angiogenesis and vascular endothelial growth factor expression in Dahl S rats. Hypertension 37: 386–390, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Bakris GL, Smith DH, Giles TD, White WB, Davidai G, Weber MA. Comparative antihypertensive efficacy of angiotensin receptor blocker-based treatment in African-American and white patients. J Clin Hypertens (Greenwich) 7: 587–595, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bragulat E, de la Sierra A, Antonio MT, Coca A. Endothelial dysfunction in salt-sensitive essential hypertension. Hypertension 37: 444–448, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Cowley AW, Jr, Liang M, Roman RJ, Greene AS, Jacob HJ. Consomic rat model systems for physiological genomics. Acta Physiol Scand 181: 585–592, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Cowley AW, Jr, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension 37: 456–461, 2001 [DOI] [PubMed] [Google Scholar]
- 6.de Resende MM, Amaral SL, Moreno C, Greene AS. Congenic strains reveal the effect of the renin gene on skeletal muscle angiogenesis induced by electrical stimulation. Physiol Genomics 33: 33–40, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Dickinson KM, Keogh JB, Clifton PM. Effects of a low-salt diet on flow-mediated dilatation in humans. Am J Clin Nutr 89: 485–490, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Drenjancevic-Peric I, Lombard JH. Introgression of chromosome 13 in Dahl salt-sensitive genetic background restores cerebral vascular relaxation. Am J Physiol Heart Circ Physiol 287: H957–H962, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Drenjancevic-Peric I, Lombard JH. Reduced angiotensin II and oxidative stress contribute to impaired vasodilation in Dahl salt-sensitive rats on low-salt diet. Hypertension 45: 687–691, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Frisbee JC, Lombard JH. Short-term angiotensin converting enzyme inhibition reduces basal tone and dilator reactivity in skeletal muscle arterioles. Am J Hypertens 13: 389–395, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Frisbee JC, Weber DS, Lombard JH. Chronic captopril administration decreases vasodilator responses in skeletal muscle arterioles. Am J Hypertens 12: 705–715, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 74: 1141–1148, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation 106: 653–658, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Hanna IR, Taniyama Y, Szocs K, Rocic P, Griendling KK. NAD(P)H oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal 4: 899–914, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104: 2673–2678, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Higashi Y, Sasaki S, Nakagawa K, Ueda T, Yoshimizu A, Kurisu S, Matsuura H, Kajiyama G, Oshima T. A comparison of angiotensin-converting enzyme inhibitors, calcium antagonists, beta-blockers and diuretic agents on reactive hyperemia in patients with essential hypertension: a multicenter study. J Am Coll Cardiol 35: 284–291, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 112: 417–428, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Hornig B, Landmesser U, Kohler C, Ahlersmann D, Spiekermann S, Christoph A, Tatge H, Drexler H. Comparative effect of ace inhibition and angiotensin II type 1 receptor antagonism on bioavailability of nitric oxide in patients with coronary artery disease: role of superoxide dismutase. Circulation 103: 799–805, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Ishibashi K, Oshima T, Matsuura H, Watanabe M, Ishida M, Ishida T, Ozono R, Kajiyama G, Kanbe M. Effects of age and sex on sodium chloride sensitivity: association with plasma renin activity. Clin Nephrol 42: 376–380, 1994 [PubMed] [Google Scholar]
- 20.Jiang J, Stec DE, Drummond H, Simon JS, Koike G, Jacob HJ, Roman RJ. Transfer of a salt-resistant renin allele raises blood pressure in Dahl salt-sensitive rats. Hypertension 29: 619–627, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Lenda DM, Boegehold MA. Effect of a high salt diet on microvascular antioxidant enzymes. J Vasc Res 39: 41–50, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Lenda DM, Boegehold MA. Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation. Am J Physiol Heart Circ Physiol 282: H395–H402, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am J Physiol Heart Circ Physiol 279: H7–H14, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Liang M, Yuan B, Rute E, Greene AS, Olivier M, Cowley AW., Jr Insights into Dahl salt-sensitive hypertension revealed by temporal patterns of renal medullary gene expression. Physiol Genomics 12: 229–237, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Lombard JH, Sylvester FA, Phillips SA, Frisbee JC. High-salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol 284: H1124–H1133, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Luscher TF. Endothelial dysfunction: the role and impact of the renin-angiotensin system. Heart 84, Suppl 1: i20–i22, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancini GB, Henry GC, Macaya C, O'Neill BJ, Pucillo AL, Carere RG, Wargovich TJ, Mudra H, Luscher TF, Klibaner MI, Haber HE, Uprichard AC, Pepine CJ, Pitt B. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) Study. Circulation 94: 258–265, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, Hamburger RJ, Fye C, Lakshman R, Gottdiener J. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med 328: 914–921, 1993 [DOI] [PubMed] [Google Scholar]
- 29.McEwen ST, Balus SF, Durand MJ, Lombard JH. Angiotensin II maintains cerebral vascular relaxation via EGF receptor transactivation and ERK1/2. Am J Physiol Heart Circ Physiol 297: H1296–H1303, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McEwen ST, Schmidt JR, Somberg L, Cruz LL, Lombard JH. Time-course and mechanisms of restored vascular relaxation by reduced salt intake and angiotensin II infusion in rats fed a high-salt diet. Microcirculation 16: 220–234, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyoshi A, Suzuki H, Fujiwara M, Masai M, Iwasaki T. Impairment of endothelial function in salt-sensitive hypertension in humans. Am J Hypertens 10: 1083–1090, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 90: E58–E65, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Moreno C, Kaldunski ML, Wang T, Roman RJ, Greene AS, Lazar J, Jacob HJ, Cowley AW., Jr Multiple blood pressure loci on rat chromosome 13 attenuate development of hypertension in the Dahl S hypertensive rat. Physiol Genomics 31: 228–235, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Nakashima H, Suzuki H, Ohtsu H, Chao JY, Utsunomiya H, Frank GD, Eguchi S. Angiotensin II regulates vascular and endothelial dysfunction: recent topics of Angiotensin II type-1 receptor signaling in the vasculature. Curr Vasc Pharmacol 4: 67–78, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Overlack A, Ruppert M, Kolloch R, Gobel B, Kraft K, Diehl J, Schmitt W, Stumpe KO. Divergent hemodynamic and hormonal responses to varying salt intake in normotensive subjects. Hypertension 22: 331–338, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 104: 191–196, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Phillips SA, Lombard JH. Chronic AT1 receptor blockade alters the mechanisms mediating hypoxic dilation in middle cerebral arteries. J Cardiovasc Pharmacol 46: 706–712, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Prasad A, Husain S, Quyyumi AA. Abnormal flow-mediated epicardial vasomotion in human coronary arteries is improved by angiotensin-converting enzyme inhibition: a potential role of bradykinin. J Am Coll Cardiol 33: 796–804, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Prasad A, Tupas-Habib T, Schenke WH, Mincemoyer R, Panza JA, Waclawin MA, Ellahham S, Quyyumi AA. Acute and chronic angiotensin-1 receptor antagonism reverses endothelial dysfunction in atherosclerosis. Circulation 101: 2349–2354, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rapp JP, Wang SM, Dene H. A genetic polymorphism in the renin gene of Dahl rats cosegregates with blood pressure. Science 243: 542–544, 1989 [DOI] [PubMed] [Google Scholar]
- 42.Reed R, Kolz C, Potter B, Rocic P. The mechanistic basis for the disparate effects of angiotensin II on coronary collateral growth. Arterioscler Thromb Vasc Biol 28: 61–67, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Saunders E, Weir MR, Kong BW, Hollifield J, Gray J, Vertes V, Sowers JR, Zemel MB, Curry C, Schoenberger J. A comparison of the efficacy and safety of a beta-blocker, a calcium channel blocker, and a converting enzyme inhibitor in hypertensive blacks. Arch Intern Med 150: 1707–1713, 1990 [PubMed] [Google Scholar]
- 44.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 101: 1899–1906, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Stekiel TA, Contney SJ, Bosnjak ZJ, Kampine JP, Roman RJ, Stekiel WJ. Chromosomal substitution-dependent differences in cardiovascular responses to sodium pentobarbital. Anesth Analg 102: 799–805, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Tzemos N, Lim PO, Wong S, Struthers AD, MacDonald TM. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension 51: 1525–1530, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Wassmann S, Hilgers S, Laufs U, Bohm M, Nickenig G. Angiotensin II type 1 receptor antagonism improves hypercholesterolemia-associated endothelial dysfunction. Arterioscler Thromb Vasc Biol 22: 1208–1212, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Weber DS, Lombard JH. Elevated salt intake impairs dilation of rat skeletal muscle resistance arteries via ANG II suppression. Am J Physiol Heart Circ Physiol 278: H500–H506, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Weber DS, Lombard JH. Angiotensin II AT1 receptors preserve vasodilator reactivity in skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol 280: H2196–H2202, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Weinberger MH. Salt sensitivity is associated with an increased mortality in both normal and hypertensive humans. J Clin Hypertens (Greenwich) 4: 274–276, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 37: 429–432, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Weir MR, Gray JM, Paster R, Saunders E. Differing mechanisms of action of angiotensin-converting enzyme inhibition in black and white hypertensive patients. The Trandolapril Multicenter Study Group. Hypertension 26: 124–130, 1995 [DOI] [PubMed] [Google Scholar]
- 53.Weir MR, Saunders E. Pharmacologic management of systemic hypertension in blacks. Am J Cardiol 61: 46H–52H, 1988 [DOI] [PubMed] [Google Scholar]
- 54.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 42: 1149–1160, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Yan C, Kim D, Aizawa T, Berk BC. Functional interplay between angiotensin II and nitric oxide: cyclic GMP as a key mediator. Arterioscler Thromb Vasc Biol 23: 26–36, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Zhu J, Drenjancevic-Peric I, McEwen S, Friesema J, Schulta D, Yu M, Roman RJ, Lombard JH. Role of superoxide and angiotensin II suppression in salt-induced changes in endothelial Ca2+ signaling and NO production in rat aorta. Am J Physiol Heart Circ Physiol 291: H929–H938, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. J Vasc Res 44: 382–390, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Zhu J, Mori T, Huang T, Lombard JH. Effect of high-salt diet on NO release and superoxide production in rat aorta. Am J Physiol Heart Circ Physiol 286: H575–H583, 2004 [DOI] [PubMed] [Google Scholar]