Abstract
The adenine nucleotide hypothesis postulates that the ATP released from red blood cells is broken down to ADP and AMP in coronary capillaries and that ATP, ADP, and AMP act on purinergic receptors on the surface of capillary endothelial cells. Purinergic receptor activation initiates a retrograde conducted vasodilator signal to the upstream arteriole that controls coronary blood flow in a negative feedback manner. A previous study (M. Farias 3rd, M. W. Gorman, M. V. Savage, and E. O. Feigl, Am J Physiol Heart Circ Physiol 288: H1586–H1590, 2005) demonstrated that coronary venous plasma ATP concentration increased during exercise and correlated with coronary blood flow. The present experiments test the adenine nucleotide hypothesis by examining the balance between oxygen delivery (via coronary blood flow) and myocardial oxygen consumption during exercise before and after purinergic receptor blockade. Dogs (n = 7) were chronically instrumented with catheters in the aorta and coronary sinus and a flow transducer around the circumflex coronary artery. During control treadmill exercise, myocardial oxygen consumption increased and the balance between oxygen delivery and myocardial oxygen consumption fell as indicated by a declining coronary venous oxygen tension. Blockade of P1 and P2Y1 purinergic receptors combined with inhibition of nitric oxide synthesis significantly decreased the balance between oxygen delivery and myocardial oxygen consumption compared with control. The results support the hypothesis that ATP and its breakdown products ADP and AMP are part of a negative feedback control mechanism that matches coronary blood flow to myocardial oxygen consumption at rest and during exercise.
Keywords: ATP, ADP, AMP, purinergic receptors, feedback control, dog
during resting conditions, the myocardium extracts ∼80% of the oxygen delivered by coronary blood flow. Thus any increase in myocardial oxygen consumption, as occurs during exercise, must be accompanied by an increase in coronary blood flow to deliver the needed oxygen. Adrenergic vasodilator feedforward control of coronary blood flow as proposed by Miyashiro and Feigl (30) has been demonstrated during exercise (8, 10, 22). However, there is a residual coronary vasodilation after adrenergic β-receptor blockade that indicates the presence of a local metabolic negative feedback mechanism controlling coronary blood flow during exercise. The present study examines the hypothesis that ATP and its breakdown products ADP and AMP act as transmitters in a negative feedback manner, controlling coronary blood flow during exercise.
ATP is released from red blood cells when they are in a low oxygen-tension environment and hemoglobin oxygen saturation is low (13). As myocardial oxygen consumption increases during exercise, the coronary capillary oxygen tension falls, stimulating the release of ATP from red blood cells. The postulate is that ATP and its breakdown products ADP and AMP act on purinergic receptors on the luminal surface of the capillary endothelial cells to initiate a retrograde conducted response that dilates the upstream arteriole, thus increasing flow in a feedback manner.
A previous study demonstrated that coronary venous plasma ATP concentration increased during exercise and correlated with coronary blood flow (15). The present paper is a critical test of the adenine nucleotide hypothesis of coronary blood flow control during exercise using purinergic receptor-blocking agents. Blockade of P1 and P2Y1 purinergic receptors combined with inhibition of nitric oxide synthesis decreased the balance between oxygen delivery and myocardial oxygen consumption during rest and exercise in support of the adenine nucleotide hypothesis.
MATERIALS AND METHODS
All animal experiments were conducted according to protocols approved by the Institutional Animal Care and Use Committee of the University of Washington.
Acute Closed-Chest Coronary Perfusion Preparation
Male dogs (23–27 kg) were premedicated with morphine (80 mg sc) and anesthetized with α-chloralose (100 mg/kg iv). Additional morphine (40 mg im) was given after 1 h, and chloralose (500 mg iv) was given approximately each hour. The animals were ventilated and given intravenous NaHCO3 to maintain blood gases within normal limits. Aortic pressure was measured via a fluid-filled catheter introduced via the right femoral artery. Without opening the chest, a stainless steel cannula was advanced via the right carotid artery and wedged into the circumflex coronary artery (41). The circumflex coronary artery was perfused with heparinized (750 U/kg iv) blood from the left femoral artery. A servo-controlled pump (32) maintained circumflex coronary artery pressure constant at 100 mmHg while circumflex blood flow was measured with an inline ultrasonic transit-time flow transducer (Transonics, Ithaca, NY). Dogs were treated with ibuprofen (12.5 mg/kg iv) to retard pump-induced white cell activation and propranolol (1 mg/kg iv) to prevent tachycardia. Intracoronary bolus drug injections were made just proximal to the coronary cannula lumen. At the conclusion of experiments additional anesthetic was given, and the circumflex artery perfusion territory was stained with intracoronary crystal violet dye just before euthanasia with intravenous KCl. The stained myocardium was cut out and weighed. Coronary blood flow was normalized per gram of perfused myocardium.
Intracoronary ATP Dose-Response Experiments
The purpose of the acute closed-chest experiments was to determine the purinergic receptor types involved in adenine nucleotide coronary vasodilation using selective purinergic agents in the canine coronary circulation. A baseline ATP dose-response curve was obtained via bolus intracoronary ATP injections (50 μl, Hamilton syringe) at increasing concentrations. The response to each injection was defined as the peak increase in mean coronary blood flow above baseline. Coronary flow was allowed to return to baseline after each injection. To avoid irreversible changes in the preparation, ATP doses resulting in coronary flows greater than approximately five times baseline flow were not administered. Two dose-response curves were obtained in each animal. In experiments where the additive effect of multiple blocking agents was tested, one or two purinergic receptor antagonists were administered before the first ATP dose-response curve.
Conscious Exercising Dog Preparation
Experiments were performed on seven purpose-bred adult mongrel dogs (25–30 kg) taught to run on a motorized treadmill. The surgical procedures have been previously described by Tune et al. (43). Briefly, under isoflurane anesthesia, a left thoracotomy was performed in the fourth intercostal space. A modified Seldinger technique was used to implant a polyurethane catheter into the descending thoracic aorta to obtain arterial blood samples and pressure. A second polyurethane catheter for coronary venous blood sampling was placed in the coronary sinus via an incision in the right atrial appendage. A flow transducer was placed around the circumflex coronary artery. Antibiotic (cefazolin, 20 mg/kg iv) was administered 1 h before surgery and again during surgery. Pain was controlled via an intercostal nerve block in the second to sixth intercostal spaces (bupivacaine, up to 2 mg/kg) before incision. Postoperative pain was controlled with oxymorphone (1 mg/kg im), buprenorphine (0.02 mg/kg sc), and fentanyl (75 μg/h transdermal patch for 3 days). Dogs also received carprofen (2.2 mg/kg po) twice daily for 5 days after surgery. Beginning 24 h after surgery, dogs received daily doses of aspirin (81 mg po) and clopidogrel (75 mg po) to help maintain catheter patency. The arterial catheter was flushed daily and filled with heparin (1,000 U/ml). The coronary venous catheter was flushed continuously with heparinized saline (5 U/ml, 2 ml/h) via a balloon pump (I-Flow, Lake Forest, CA) stored in a jacket worn by the dogs (AKCMA, Hawthorne, CA). Initial experiments were conducted after a recovery period of at least 10 days following surgery.
Flow and Pressure Measurement
Coronary blood flow was continuously measured throughout the experimental protocol with an ultrasonic transit-time perivascular flow transducer (Transonics) calibrated before implantation. After experiments were completed, the animals were euthanized with pentobarbital sodium, and the circumflex artery perfusion territory was dyed with India ink. The weight of the dyed tissue was used to calculate coronary blood flow per gram of perfused myocardium. Arterial pressure was measured using a 3F catheter tip manometer (Millar Instruments, Houston, TX) inserted into the arterial catheter. Data were digitized and recorded continuously using Windaq software (Akron, OH).
Blood Sampling
Arterial and coronary venous blood samples were collected simultaneously during rest and exercise in heparinized glass syringes that were immediately sealed and placed in ice. The samples were analyzed for hydrogen ion concentration, carbon dioxide tension, and oxygen tension with an Instrumentation Laboratories Gem Premier 3000 blood gas analyzer (Waltham, MA). Arterial and coronary venous oxygen content was determined using the fuel-cell method (Total O2X; Hospex, Chestnut Hill, MA). Myocardial oxygen consumption (in μl O2·min−1·g−1) was calculated by multiplying mean coronary blood flow per gram of perfused tissue by the arterial-coronary venous difference in oxygen content. Hemoglobin saturation was calculated from the measured pH, Pco2, and Po2 using the formula for dog blood developed by Reeves et al. (37).
Exercise Protocol
Coronary blood flow, arterial pressure, and heart rate were continuously measured while the dogs were standing in a sling and during two levels of treadmill exercise, 1) 3 mph, 5% grade and 2) 4 mph, 10% grade in ascending order. The exercise periods were continued beyond the time when heart rate and coronary blood flow became stable. During the stable hemodynamic period, simultaneous arterial and coronary venous samples were drawn as exercise continued. Coronary blood flow, heart rate, and mean arterial pressure were averaged during 20–30 s of this stable period. The average exercise period was 2.7 min. The animals were allowed to rest between each exercise level.
Each dog performed an exercise study under control conditions (vehicle administration only) and during purinergic blockade on separate days. Purinergic blockade consisted of treatment with P1 and P2Y1 receptor antagonists and a nitric oxide synthesis inhibitor. Blood samples were drawn under resting conditions before and after drug or vehicle administration, as well as during exercise.
Drugs
ATP (A7699; Sigma, St. Louis, MO) was dissolved in isotonic saline for intracoronary injections. Purinergic P1 receptor responses were inhibited with 8-phenyltheophylline (P2276, Sigma) (8-PT, 3 mg/kg iv) dissolved in 1.5 ml of equal parts 1 N NaOH, ethanol, and propylene glycol and diluted to 25 ml with saline. P2Y1 receptors were inhibited with 2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-biphosphate (2159; Tocris, Ellisville, MO) (MRS 2500, 0.1 mg/kg iv) (4). MRS 2500 was dissolved in isotonic saline. Nitric oxide synthesis was inhibited by l-nitroarginine (N5501; Sigma) (LNA, 35 mg/kg iv) dissolved in warmed saline and infused through a 0.22-μm syringe tip filter. The weak but highly selective P2Y2 agonist uridine 5′-tetraphosphate δ-phenyl ester (triethyl ammonium salt) (MRS 2768) (25) was dissolved in saline for intracoronary injections. MRS 2768 was provided by the Molecular Recognition Section, NIDDK National Institutes of Health. The same drugs with the same doses were used in the exercise experiments and the acute coronary ATP dose-response studies.
Data Analyses
Plots of coronary venous oxygen tension vs. myocardial oxygen consumption were analyzed using linear regression followed by analysis of covariance. Values presented in tables and text are means ± SE. Logarithmic ATP dose-response plots were fit to sigmoidal dose-response curves with variable Hill slope using GraphPad Prism (San Diego, CA) software. The baseline was fixed at zero and the maximum increase at 3.5 ml·min−1·g−1. The Hill slope was variable but constrained to be the same before and after addition of the ATP antagonist. Thus addition of the antagonist produced a parallel shift in the ATP dose-response curve in each experiment. The magnitude of the rightward shift (log units) for each antagonist was tested for statistical significance using the paired t-test.
RESULTS
Intracoronary ATP Dose-Response Experiments
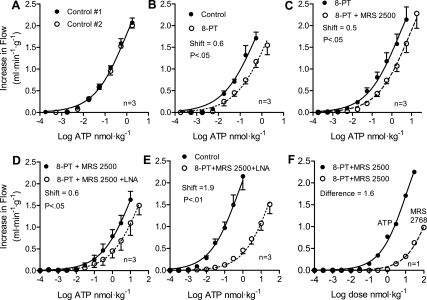
The baseline flows are given in Table 1. Time-control ATP dose-response experiments demonstrated no change in nucleotide responses from the first curve to the second (Fig. 1A). In this case the rightward shift was 0.05 ± 0.03 log units (P = NS). Addition of the P1 receptor antagonist 8-PT shifted the nucleotide responses by 0.60 ± 0.05 log units (Fig. 1B, P < 0.05). This demonstrates that AMP and/or adenosine contribute to the ATP response. Addition of the P2Y1 receptor antagonist MRS 2500 after 8-PT shifted the curve by an additional 0.53 ± 0.11 log units (Fig. 1C, P < 0.05), demonstrating a P2Y1 component to nucleotide-induced vasodilation. Addition of the nitric oxide synthesis inhibitor LNA after pretreatment with 8-PT and MRS 2500 shifted the curve rightward by an additional 0.56 ± 0.07 log units (Fig. 1D, P < 0.05). Compared with control nucleotide responses, combined purinergic blockade with 8-PT + MRS 2500 + LNA shifted the responses rightward by 1.90 ± 0.16 log units (Fig. 1E, P < 0.01). After pretreatment with 8-PT to block P1 and MRS 2500 to block P2Y1 purinergic receptors, the weak but highly specific P2Y2 agonist MRS 2768 produced coronary vasodilation in a dose-dependent manner (Fig. 1F). This indicates the presence of P2Y2 receptors in the canine coronary circulation.
Table 1.
Baseline blood flows
| First Dose Response | Second Dose Response | |
|---|---|---|
| Fig. 1A | 0.47 ± 0.01 | 0.52 ± 0.04 |
| Fig. 1B | 0.48 ± 0.02 | 0.51 ± 0.01 |
| Fig. 1C | 0.52 ± 0.08 | 0.47 ± 0.05 |
| Fig. 1D | 0.48 ± 0.01 | 0.35 ± 0.03 |
| Fig. 1E | 0.61 ± 0.04 | 0.41 ± 0.04 |
Values are means ± SE in milliliter per minute per gram, n = 3 in every case.
Fig. 1.
A: intracoronary ATP injections were used as a source of adenine nucleotides to produce coronary vasodilation. Time controls demonstrate the stability of the preparation. B: purinergic P1 receptor blockade with 8-phenyltheophylline (8-PT) partially inhibits the increase in coronary blood flow attributable to intracoronary injections of ATP. According to the present ATP hypothesis, the P1 receptor component of the vasodilation is primarily due to AMP from the intravascular breakdown of ATP to AMP. C: blocking P2Y1 receptors with MRS 2500 after pretreatment with P1 receptor blockade (8-PT) demonstrates the P2Y1 receptor component of ATP- and/or ADP-mediated coronary vasodilation. D: addition of l-nitroarginine (LNA) inhibition of nitric oxide synthesis after prior P1 (8-PT) and P2Y1 (MRS 2500) receptor blockade illustrates the nitric oxide component of ATP, ADP, and AMP coronary vasodilation. E: P1 (8-PT) and P2Y1 (MRS 2500) purinergic receptor blockade combined with nitric oxide synthesis inhibition with LNA produced a useful blockade of nucleotide coronary vasodilation. This is the combination used in the chronic exercising dog experiments. F: following pretreatment with 8-PT to block purinergic P1 receptors and MRS 2500 to block P2Y1 receptors, nucleotides still produce coronary vasodilation, indicating that additional purinergic receptors are involved. The highly specific but not very potent P2Y2 receptor agonist MRS 2768 produces coronary vasodilation. This suggests that ATP and/or ADP and AMP may also be acting via P2Y2 receptors.
Exercise Experiments
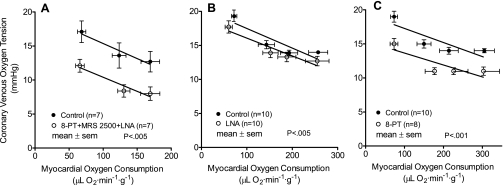
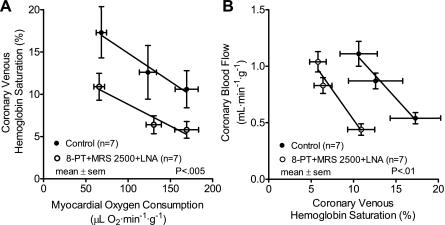
Results of the exercise experiments are presented in Table 2. Coronary venous oxygen tension is plotted vs. myocardial oxygen consumption in Fig. 2. Coronary venous oxygen tension is an index of the balance between myocardial oxygen supply and consumption. Purinergic P1 and P2Y1 receptor blockade combined with inhibition of nitric oxide synthesis lowered coronary venous oxygen tension at rest and during exercise, resulting in a parallel downward shift in the plot (Fig. 2A, P < 0.005). Earlier studies in this laboratory using the same exercising dog model have examined the effects of LNA alone (44) and 8-PT alone (43) on coronary venous oxygen tension. These results are presented in Fig. 2, B and C, for comparison. The highest myocardial oxygen consumption levels are lower in Fig. 2A than in Fig. 2, B and C, because, after purinergic blockade, dogs were unable to run at the highest intensity level used previously. The exercise limitation secondary to purinergic receptor blockade could be due to effects in the central nervous system, lungs, heart, or skeletal muscle. If the limitation is due to coronary vascular effects it suggests the importance of adenine nucleotides in cardiac performance during exercise. Purinergic blockade combined with inhibition of nitric oxide synthesis produced a parallel downward shift in the plot of coronary venous hemoglobin oxygen saturation vs. myocardial oxygen consumption (Fig. 3A, P < 0.005). The leftward shift in the relation between coronary blood flow and coronary venous hemoglobin oxygen saturation in Fig. 3B (P < 0.01) after purinergic blockade combined with inhibition of nitric oxide synthesis represents a decreased negative feedback control.
Table 2.
Hemodynamic and metabolic variables at rest and during graded treadmill exercise
| Exercise |
||||
|---|---|---|---|---|
| Rest | Rest + Drug | Level 1 | Level 2 | |
| Coronary Blood Flow, ml·min−1·g−1 | ||||
| Vehicle | 0.55 ± 0.05 | 0.54 ± 0.05 | 0.87 ± 0.07 | 1.11 ± 0.11 |
| Purinergic Blockade | 0.58 ± 0.05 | 0.44 ± 0.05 | 0.83 ± 0.07 | 1.04 ± 0.09 |
| Myocardial O2 Consumption, μl O2·min−1·g−1 | ||||
| Vehicle | 76 ± 9 | 68 ± 6 | 124 ± 10 | 169 ± 14 |
| Purinergic Blockade | 79 ± 7 | 66 ± 6 | 130 ± 9 | 169 ± 14 |
| Mean Aortic Pressure, mmHg | ||||
| Vehicle | 108 ± 6 | 108 ± 6 | 112 ± 11 | 140 ± 29 |
| Purinergic Blockade | 105 ± 6 | 123 ± 5 | 116 ± 4 | 123 ± 7 |
| Heart Rate, beats/min | ||||
| Vehicle | 106 ± 8 | 111 ± 6 | 167 ± 5 | 199 ± 4 |
| Purinergic Blockade | 119 ± 4 | 74 ± 4 | 138 ± 6 | 170 ± 8 |
| Arterial Hydrogen Ion Concentration, nM | ||||
| Vehicle | 38 ± 1.6 | 40 ± 0.9 | 38 ± 0.8 | 38 ± 1.1 |
| Purinergic Blockade | 39 ± 0.7 | 33 ± 0.5 | 31 ± 1.3 | 33 ± 1.0 |
| Coronary Venous Hydrogen Ion Concentration, nM | ||||
| Vehicle | 43 ± 0.9 | 44 ± 0.7 | 42 ± 0.8 | 44 ± 1.2 |
| Purinergic Blockade | 42 ± 0.5 | 38 ± 0.4 | 37 ± 0.9 | 39 ± 0.9 |
| Arterial Carbon Dioxide Tension, mmHg | ||||
| Vehicle | 33 ± 1 | 34 ± 1 | 31 ± 1 | 30 ± 1 |
| Purinergic Blockade | 35 ± 1 | 26 ± 1 | 24 ± 2 | 26 ± 1 |
| Coronary Venous Carbon Dioxide Tension, mmHg | ||||
| Vehicle | 45 ± 1 | 46 ± 1 | 42 ± 1 | 45 ± 1 |
| Purinergic Blockade | 45 ± 1 | 38 ± 1 | 38 ± 1 | 39 ± 1 |
| Arterial Oxygen Tension, mmHg | ||||
| Vehicle | 90 ± 2 | 88 ± 1 | 87 ± 3 | 90 ± 5 |
| Purinergic Blockade | 86 ± 1 | 99 ± 2 | 97 ± 3 | 89 ± 2 |
| Arterial Hemoglobin Saturation, % | ||||
| Vehicle | 95 ± 0.4 | 95 ± 0.2 | 95 ± 0.5 | 95 ± 0.7 |
| Purinergic Blockade | 95 ± 0.5 | 97 ± 0.3 | 97 ± 0.2 | 96 ± 0.3 |
| Coronary Venous Oxygen Tension, mmHg | ||||
| Vehicle | 16 ± 2 | 17 ± 2 | 14 ± 2 | 13 ± 1 |
| Purinergic Blockade | 15 ± 1 | 12 ± 1 | 8 ± 1 | 8 ± 1 |
| Coronary Venous Hemoglobin Saturation, % | ||||
| Vehicle | 15.6 ± 3.3 | 17.3 ± 3.0 | 12.6 ± 3.2 | 10.6 ± 2.2 |
| Purinergic Blockade | 13.7 ± 1.5 | 10.9 ± 1.5 | 6.4 ± 1.1 | 5.8 ± 1.0 |
| Arterial Oxygen Content, ml O2/dl blood | ||||
| Vehicle | 17.1 ± 1.1 | 16.5 ± 0.6 | 16.9 ± 0.7 | 17.6 ± 0.6 |
| Purinergic Blockade | 17.0 ± 0.6 | 17.4 ± 0.7 | 17.2 ± 0.8 | 17.3 ± 0.7 |
| Coronary Venous Oxygen Content, ml O2/dl blood | ||||
| Vehicle | 3.2 ± 0.7 | 3.6 ± 0.5 | 2.5 ± 0.6 | 2.1 ± 0.4 |
| Purinergic Blockade | 3.1 ± 0.3 | 2.2 ± 0.3 | 1.1 ± 0.3 | 0.9 ± 0.2 |
| Hematocrit. % | ||||
| Vehicle | 39 ± 3 | 39 ± 2 | 39 ± 2 | 39 ± 2 |
| Purinergic Blockade | 39 ± 2 | 39 ± 2 | 39 ± 2 | 39 ± 2 |
Values are means ± SE. Results from 7 dogs treated with vehicle or purinergic blockade [l-nitroarginine (LNA) + 8-phenyltheophylline (8-PT) + 2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-biphosphate (MRS 2500)]. All dogs were studied under both conditions.
Fig. 2.
A: purinergic P1 (8-PT) and P2Y1 (MRS 2500) receptor blockade combined with nitric oxide synthesis inhibition (LNA) decreased the balance between oxygen delivery and myocardial oxygen consumption as indicated by the coronary venous oxygen tension. These data support the hypothesis that ATP and its breakdown products ADP and AMP are part of a negative feedback mechanism controlling coronary blood flow during rest and exercise. B: for comparison, data from Tune et al. (44) illustrate that inhibition of nitric oxide synthesis alone with LNA has a modest effect on oxygen balance during rest and exercise. C: for comparison, data from Tune et al. (43) demonstrate that purinergic P1 receptor blockade alone with 8-PT reduces the balance between oxygen delivery and myocardial oxygen consumption during rest and exercise. The present interpretation of the data in C is that the effect is attributable to inhibition of AMP acting on P1 receptors.
Fig. 3.
A: relation between myocardial oxygen consumption and coronary venous hemoglobin oxygen saturation before and after P1 (8-PT) and P2Y1 (MRS 2500) purinergic receptor blockade combined with inhibition of nitric oxide synthesis (LNA). Inhibiting the adenine nucleotide feedback mechanism resulted in decreasing coronary venous hemoglobin oxygen saturation during rest and exercise, which supports the nucleotide hypothesis. B: relation between coronary venous hemoglobin oxygen saturation and coronary blood flow before and after P1 (8-PT) and P2Y1 (MRS 2500) purinergic receptor blockade combined with inhibition of nitric oxide synthesis (LNA) during rest and exercise. Note that very similar coronary flows were obtained after blockade but only at lower hemoglobin oxygen saturations than control. The leftward shift represents the decrease in negative feedback control. Thus more hemoglobin oxygen desaturation is required to obtain the same flow after blockade.
DISCUSSION
The major finding of the present experiments is that blockade of P1 and P2Y1 purinergic receptors combined with inhibition of nitric oxide synthesis depresses the balance between oxygen delivery and myocardial oxygen consumption at rest and during exercise. The data support the hypothesis that ATP and its breakdown products ADP and AMP are part of a negative feedback control mechanism that helps match oxygen delivery to myocardial oxygen consumption via control of coronary blood flow. The coronary venous oxygen tension in Fig. 2 represents the balance of oxygen delivery to the myocardium and oxygen consumption by the myocardium. A depression of the relation shown in Fig. 2 indicates an inhibition of the negative feedback control mechanism and an increased error signal. As predicted by the adenine nucleotide hypothesis, the leftward shift in Fig. 3B demonstrates that coronary venous oxyhemoglobin saturation must fall further to obtain flows similar to control after purinergic P1 and P2Y1 receptor blockade combined with inhibition of nitric oxide synthesis.
Outline of the Adenine Nucleotide Hypothesis
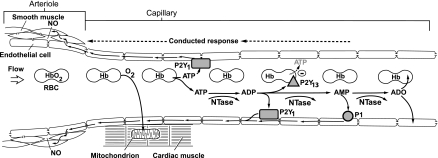
A sensor is required for a negative feedback control mechanism, and hemoglobin is the oxygen sensor in the adenine nucleotide hypothesis. The hemoglobin oxygen saturation in coronary venous capillaries reflects the balance between oxygen delivery and oxygen consumption at the local microvascular unit. Thus the controlled variable in the nucleotide hypothesis is the coronary venous hemoglobin oxygen saturation. On the basis of the work of Ellsworth and colleagues (13) the assumption is that red blood cells are the primary source of ATP. However, the ATP could be released by any cell type in the heart. Even white blood cells release ATP when activated (14). The hypothesis is diagrammed in Fig. 4. The initial element in the hypothesis is that, when oxygen is unloaded from hemoglobin, deoxyhemoblogin facilitates the release of ATP from red blood cells. The ATP then activates purinergic P2Y1 receptors on capillary endothelial cells, which results in a retrograde conducted signal that produces vasodilation of the upstream arteriole. Nitric oxide is involved, presumably in the endothelial cell to vascular smooth muscle transmission. ATP in the plasma is broken down by nucleotidases in the plasma and on the surface of endothelial cells to ADP, AMP, and adenosine. ADP acts on endothelial P2Y1 receptors to produce a retrograde conducted vasodilator response. ADP also acts on P2Y13 receptors on red blood cells to inhibit the further release of ATP from red blood cells. AMP acts on endothelial P1 receptors to initiate a conducted vasodilator response. Adenosine is avidly taken up by endothelial and red blood cells to enter the purine salvage pathway and replenish ATP.
Fig. 4.
Schematic diagram of the adenine nucleotide hypothesis of negative feedback coronary control. Oxygen (O2) is released from hemoglobin (Hb) in coronary capillaries in response to myocardial oxygen consumption. Deoxyhemoglobin facilitates the release of ATP from red blood cells (RBC) that acts on endothelial purinergc P2Y1 receptors to initiate a retrograde conducted response via gap junctions connecting endothelial cells. The conducted signals dilate the upstream arteriole smooth muscle cells via gap junctions and the release of nitric oxide (NO). Nucleotidases (NTase) in the plasma and on the surface of endothelial cells break ATP down to ADP, which activates P2Y1 receptors. ADP also activates P2Y13 receptors on the surface of RBC to inhibit the further release of ATP from RBC. ADP is broken down to AMP, which activates P1 receptors to generate a retrograde conducted vasodilator response. AMP is degraded to adenosine (ADO) that is rapidly taken up by RBC and endothelial cells.
The Evidence for the Sequential Steps in the Adenine Nucleotide Hypothesis
ATP release from red blood cells.
Ellsworth et al. (13) have recently reviewed the evidence that red blood cells release ATP when in a low oxygen environment. Although the mechanism whereby deoxyhemoglobin stimulates ATP release from red blood cells has not been identified, the observation of ATP release has been repeated many times. There is evidence that ATP exits red blood cells via pannexin channels, which are like half of gap-junction connexins but do not connect two cells together (42).
ATP and/or ADP activation of P2Y1 receptors.
The evidence for ATP and/or ADP stimulating P2Y1 receptors is given in Fig. 1C. After pretreatment with the purinergic P1 receptor blocker 8-PT, the addition of the P2Y1 receptor blocking agent MRS 2500 significantly shifts the nucleotide coronary vasodilation dose-response curve to the right. The present experiments do not distinguish the relative importance of ATP and ADP in the P2Y1 response. ADP is also a potent coronary vasodilator (47) and acts via P2Y1 receptors (23, 36).
AMP activation of P1 receptors.
Inhibition of AMP-induced coronary vasodilation has been shown using P1 receptor antagonists (3, 19). The effect was not due to AMP being degraded to adenosine (which also acts on P1 receptors). Pretreatment with adenosine deaminase to inactivate adenosine does not prevent AMP-induced coronary vasodilation or its inhibition by P1 receptor blockade (23). Furthermore, treatment with α,β-methylene ADP to inhibit AMP conversion to adenosine does not reduce AMP responses (19, 33).
Purinergic P1 receptors, AMP, and adenosine.
Purinergic P1 receptor blockade with 8-PT during exercise decreases the balance between oxygen delivery and myocardial oxygen consumption as shown in Fig. 2C. The data in Fig. 2C are from Tune et al. (43), where 8-PT was used to test the role of adenosine in exercise coronary vasodilation. Although it is well established that adenosine is released from the myocardium during pathological hypoxia or ischemia (10, 16, 17), Tune et al. (43) found that cardiac interstitial adenosine concentrations do not reach the vasodilator threshold during normal physiological exercise. Other studies using adenosine receptor antagonists or adenosine deaminase have reached a similar conclusion that, in the absence of cardiac hypoxia, adenosine levels are below the threshold for coronary vasodilation (6, 9, 10, 27, 38). On the basis of these earlier adenosine studies, it is concluded that the effects of purinergic blockade in the present experiments are not attributable to adenosine (although adenosine receptors are also blocked). In 2000 Tune et al. (43) could not explain the downward shift with 8-PT shown in Fig. 2C and ascribed it to an unknown additional action beyond blocking adenosine vasodilation. The present interpretation is that the previously unexplained action of 8-PT is attributable to blockade of AMP acting on P1 receptors as described by the present hypothesis.
Purinergic P2Y1 receptors.
Although exercise experiments were not done using MRS 2500 alone, it may be possible to estimate the effect of P2Y1 blockade. At the maximum myocardial oxygen consumption reached in the present experiments (169 μl O2·min−1·g−1, Table 2), the decrease in coronary venous oxygen tension attributable to LNA treatment alone (Fig. 2B) is 1.2 mmHg. Subtracting this from the coronary venous oxygen tension at the same myocardial oxygen consumption during 8-PT treatment alone, 12.4 mmHg (Fig. 2C), yields an estimated coronary venous oxygen tension of 11.2 mmHg. The measured coronary venous oxygen tension during 8-PT + LNA + MRS 2500 when myocardial oxygen consumption was 169 μl O2·min−1·g−1 (Table 2) was 8 mmHg. The additional drop of ∼3 mmHg may be attributable to blockade of P2Y1 receptors.
Conducted response.
Vascular endothelial cells are coupled together and to adjacent vascular smooth muscle cells via gap junctions. This permits signals generated in the capillaries to be conducted retrograde to the upstream feed arteriole where flow is controlled. There is a rapid component to conducted responses (18) and a slow component (7).
In microvascular preparations, the application of ATP inside small arterioles (11, 12, 29), outside capillaries (12), or inside venules (5) produces retrograde conducted vasodilator responses. Thus ATP released from red blood cells in small arterioles and capillaries where oxygen is unloaded from hemoglobin provides adenine nucleotides that are capable of generating a signal that is conducted to the upstream arteriole.
Nitric oxide.
Nitric oxide (NO) is an endothelium-derived relaxing factor that traverses the interstitial space between endothelial cells and vascular smooth muscle cells. Endothelial cells release nitric oxide in response to shear stress from flowing blood (1) and in response to other agonists (28). Thus nitric oxide is not specific to the present nucleotide hypothesis. Inhibition of nitric oxide synthesis decreases nucleotide-conducted vasodilation in microcirculation preparations (5, 29). The effect of inhibiting nitric oxide synthesis with LNA during exercise is shown in Fig. 2B. There is a modest but significant decrease in the balance between oxygen supply and myocardial oxygen consumption at rest and during exercise. The present experiments do not demonstrate where nitric oxide is involved in the causal pathway of vasodilation. It is assumed that nitric oxide is involved in the transmission of the vasodilator signal between endothelial cells and vascular smooth muscle cells as illustrated in Fig. 4.
Criteria for a Feedback Transmitter
In 1983 Feigl (16) suggested the following six criteria for a feedback transmitter controlling coronary blood flow.
1. The proposed transmitter is released under appropriate conditions, and it can be recovered from the tissue under those conditions.
This criterion was satisfied in a previous paper by Farias et al. (15), where plasma ATP concentrations were measured during exercise in dogs. The coronary venous ATP concentrations increased with increasing levels of exercise intensity and correlated well with coronary blood flow. Plasma concentrations of ADP and AMP probably increased as well but were below the detection limit of the assay.
2. Transmitter substance artificially infused into the target tissue should faithfully mimic physiological activation.
Coronary vasodilation due to ATP was first reported by Gillespie in 1934 (20), has been repeated many times (16), and is shown again in Fig. 1. The coronary vasodilator actions of ADP and AMP are discussed above.
3. The biochemical apparatus for production of the proposed transmitter is present in the tissue in an appropriate location.
Red blood cells make ATP by glycolysis (2). The mechanism whereby ATP is released from red blood cells when oxygen tension is low has been reviewed by Ellsworth et al. (13).
4. A mechanism for inactivation and/or uptake of the transmitter is present at an appropriate location in the tissue.
ATP in plasma is rapidly broken down to ADP, AMP, and adenosine by nucleotidases in the plasma and on the surface of endothelial and red blood cells (21, 35, 48). Adenosine is subsequently taken up by endothelial cells, leukocytes, and red blood cells, where it enters the purine salvage pathway. (26, 34). Wang et al. (46) demonstrated that the release of ATP from red blood cells is inhibited by ADP acting on P2Y13 purinergic receptors on the red cell surface. This is a nice adaptation because it lessens the loss of ATP from red blood cells as they transverse the venous circulation.
5. The action of various inhibitors and blocking agents on synthesis, release, target-organ receptor function, or transmitter inactivation should have effects consistent with the hypothesis: blocking agents should give the same effect whether the transmitter is released physiologically or artificially applied.
This is the subject of the present study, where blockade of P1 and P2Y1 purinergic receptors combined with inhibition of nitric oxide synthesis lowered oxygen delivery via coronary blood flow in relation to myocardial oxygen consumption (Figs. 2 and 3). The leftward shift of the relation between coronary blood flow and coronary venous hemoglobin oxygen saturation shown in Fig. 3B represents the decrease in negative feedback control produced by purinergic P1 and P2Y1 receptor blockage combined with inhibition of nitric oxide synthesis.
6. Quantitative studies should indicate that the amount and time course of transmitter released under physiological conditions is appropriate to give the indicated effect.
Although a previous study demonstrated that coronary venous ATP concentration increased during exercise and correlated well with coronary blood flow (15), this criterion has not yet been tested. Red blood cells fold up like partially closed umbrellas as they squeeze through capillaries (40). The concentration of ATP and its breakdown products is unmeasured in the tight space between red blood cells and purinergic receptors on the surface of capillary endothelial cells.
Feedforward Vs. Feedback Control
Feedforward control is also called parallel control, yoked control, or open-loop control. A typical example is when two variables are controlled by a common input. In the coronary circulation, parallel feedforward control has been demonstrated, where β-adrenoceptor activation on cardiac cells increases myocardial oxygen consumption and simultaneously dilates coronary vascular smooth muscle cells via β-adrenoceptor activation (8, 10, 22, 30). Another example of feedforward open-loop control is the hydrogen peroxide hypothesis (39). According to this hypothesis cardiac mitochondria generate superoxide in proportion to the rate of oxygen consumption. Superoxide is converted to hydrogen peroxide that diffuses to coronary arterioles to produce vasodilation. In this way coronary blood flow is coupled to myocardial oxygen consumption without an oxygen sensor.
In a negative feedback control system there is a sensor for the regulated variable that is used to generate an error signal. In the adenine nucleotide hypothesis hemoglobin in coronary capillaries acts as an oxygen sensor.
The disadvantage of feedforward control is that small discrepancies will accumulate over time and may lead to very large errors. This is because there is no self-checking component in feedforward control. The disadvantage of negative feedback control is that there is a tradeoff between accuracy, stability, and response time. The higher the feedback gain, the faster the response and the smaller the error signal. However, increasing gain beyond a stability threshold leads to oscillations and ultimately to dynamic instability. However, Miyashiro and Feigl (31) modeled the coronary circulation and found that a combination of adrenergic feedforward and local metabolic feedback control resulted in a rapid and accurate response without oscillations. The adenine nucleotide negative-feedback control revealed in the present experiments plus the previously documented adrenergic feedforward control (8, 10, 22) demonstrates combined control of coronary blood flow during exercise.
In the adenine nucleotide hypothesis the regulated variable is the coronary venous hemoglobin oxygen saturation, which reflects that balance between oxygen delivery and myocardial oxygen consumption at the local microvascular unit level. The prediction of the adenine nucleotide hypothesis is that blocking purinergic vasodilation will decrease the balance between oxygen delivery and consumption as demonstrated in Fig. 2A. More specifically it is that purinergic blockade will increase the feedback error signal (deoxyhemoglobin saturation) to obtain similar flows per minute (Fig. 3B) or flow per heartbeat before and after blockade.
Other Purinergic Receptors
The results in Fig. 1, C, D, and F, indicate that combined blockade of P1 and P2Y1 purinergic receptors incompletely inhibits coronary vasodilation attributable to adenine nucleotides. This suggests that other purinergic receptors are involved. Because the additional receptors may act through nitric oxide as a downstream mediator, LNA was added to produce a more effective purinergic blockade (Fig. 1D).
Following pretreatment with the P1 blocker 8-PT plus the P2Y1 blocker MRS 2500, the highly selective but weak P2Y2 receptor agonist MRS 2768 produces coronary vasodilation in a dose-dependent manner (Fig. 1F). This indicates the presence of P2Y2 receptors in the canine coronary circulation. The result is in agreement with the work of Wang et al. (45), who found expression of P2Y1 and P2Y2 purinergic receptors in endothelial cells from human umbilical veins. The relative potency difference between ATP and MRS 2768 (1.6 log units) at the P2Y2 receptor is within expected range. The EC50 for ATP acting on P2Y2 receptors is 0.085 ± 0.012 μM (24), and the EC50 for MRS 2768 is 1.89 ± 1.07 μM (25). These results do not establish that ATP and/or ADP and AMP cause coronary dilation by activating P2Y2 receptors but do suggest that P2Y2 receptors may be involved in nucleotide coronary vasodilation. Unfortunately adequate P2Y2 receptor blocking agents are not presently available to test the role of P2Y2 receptors in exercise coronary vasodilation.
Conclusion
The present data demonstrate that purinergic receptor blockade decreases the balance between oxygen delivery and myocardial oxygen consumption during rest and exercise (Figs. 2 and 3). A previous study showed that coronary venous ATP concentration increases during exercise (15). Taken together, these studies provide evidence that the nucleotide ATP and its breakdown products ADP and AMP are part of a negative feedback system controlling coronary blood flow.
Previous research demonstrated adrenergic feedforward coronary vasodilation during exercise (8, 10, 22). Thus there is evidence for combined feedforward and feedback control of coronary blood flow. This is not surprising for such a vital function. Although these feedforward and feedback control mechanisms have been identified, this may not be a complete description, as further research may reveal additional mechanisms that are active during normal exercise.
GRANTS
This work was supported by NIH grant RO1 HL 82781 (E. O. Feigl) and NIH Intramural Research Program NIDDK (K. A. Jacobson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Boo YC, Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. Am J Physiol Cell Physiol 285: C499–C508, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Brewer GJ, Kruckeberg WC, Westover CJ, Oberman HA. Erythrocyte metabolism. Prog Clin Biol Res 11: 5–19, 1976 [PubMed] [Google Scholar]
- 3.Burnstock G. A basis of distinguishing two types of purinergic receptors. In: Membrane Receptors For Drugs And Hormones: A Multidisciplinary Approach, edited by Bolis L, Straub R. New York: Raven, 1978, p. 107–118 [Google Scholar]
- 4.Cattaneo M, Lecchi A, Ohno M, Joshi BV, Besada P, Tchilibon S, Lombardi R, Bischofberger N, Harden TK, Jacobson KA. Antiaggregatory activity in human platelets of potent antagonists of the P2Y 1 receptor. Biochem Pharmacol 68: 1995–2002, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins DM, McCullough WT, Ellsworth ML. Conducted vascular responses: communication across the capillary bed. Microvasc Res 56: 43–53, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Dole WP, Yamada N, Bishop VS, Olsson RA. Role of adenosine in coronary blood flow regulation after reductions in perfusion pressure. Circ Res 56: 517–524, 1985 [DOI] [PubMed] [Google Scholar]
- 7.Domeier TL, Segal SS. Electromechanical and pharmacomechanical signalling pathways for conducted vasodilatation along endothelium of hamster feed arteries. J Physiol 579: 175–186, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncker DJ, Stubenitsky R, Verdouw PD. Autonomic control of vasomotion in the porcine coronary circulation during treadmill exercise: evidence for feed-forward beta-adrenergic control. Circ Res 82: 1312–1322, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Duncker DJ, Stubenitsky R, Verdouw PD. Role of adenosine in the regulation of coronary blood flow in swine at rest and during treadmill exercise. Am J Physiol Heart Circ Physiol 275: H1663–H1672, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 88: 1009–1086, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Duza T, Sarelius IH. Conducted dilations initiated by purines in arterioles are endothelium dependent and require endothelial Ca2+. Am J Physiol Heart Circ Physiol 285: H26–H37, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol 269: H2155–H2161, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology (Bethesda) 24: 107–116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res 99: 1100–1108, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Farias M, 3rd, Gorman MW, Savage MV, Feigl EO. Plasma ATP during exercise: possible role in regulation of coronary blood flow. Am J Physiol Heart Circ Physiol 288: H1586–H1590, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Feigl EO. Coronary physiology. Physiol Rev 63: 1–205, 1983 [DOI] [PubMed] [Google Scholar]
- 17.Feigl EO. Berne's adenosine hypothesis of coronary blood flow control. Am J Physiol Heart Circ Physiol 287: H1891–H1894, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Figueroa XF, Chen CC, Campbell KP, Damon DN, Day KH, Ramos S, Duling BR. Are voltage-dependent ion channels involved in the endothelial cell control of vasomotor tone? Am J Physiol Heart Circ Physiol 293: H1371–H1383, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Fleetwood G, Gordon JL. Purinoceptors in the rat heart. Br J Pharmacol 90: 219–227, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillespie JH. The biological significance of the linkages in adenosine triphosphoric acid. J Physiol 80: 345–359, 1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon JL. Extracellular ATP: effects, sources and fate. Biochem J 233: 309–319, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorman MW, Tune JD, Richmond KN, Feigl EO. Feedforward sympathetic coronary vasodilation in exercising dogs. J Appl Physiol 89: 1892–1902, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Gorman MW, Ogimoto K, Savage MV, Jacobson KA, Feigl EO. Nucleotide coronary vasodilation in guinea pig hearts. Am J Physiol Heart Circ Physiol 285: H1040–H1047, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson KA, Costanzi S, Ivanov AA, Tchilibon S, Besada P, Gao ZG, Maddileti S, Harden TK. Structure activity and molecular modeling analyses of ribose- and base-modified uridine 5′-triphosphate analogues at the human P2Y2 and P2Y4 receptors. Biochem Pharmacol 71: 540–549, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko H, Carter RL, Cosyn L, Petrelli R, de CS, Besada P, Zhou Y, Cappellacci L, Franchetti P, Grifantini M, Van CS, Harden TK, Jacobson KA. Synthesis and potency of novel uracil nucleotides and derivatives as P2Y2 and P2Y6 receptor agonists. Bioorg Med Chem 16: 6319–6332, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolassa N, Plank B, Turnheim K. pH and temperature dependence of adenosine uptake in human erythrocytes. Eur J Pharmacol 52: 345–351, 1978 [DOI] [PubMed] [Google Scholar]
- 27.Kroll K, Feigl EO. Adenosine is unimportant in controlling coronary blood flow in unstressed dog hearts. Am J Physiol Heart Circ Physiol 249: H1176–H1187, 1985 [DOI] [PubMed] [Google Scholar]
- 28.Luscher TF, Richard V, Tschudi M, Yang ZH, Boulanger C. Endothelial control of vascular tone in large and small coronary arteries. J Am Coll Cardiol 15: 519–527, 1990 [DOI] [PubMed] [Google Scholar]
- 29.McCullough WT, Collins DM, Ellsworth ML. Arteriolar responses to extracellular ATP in striated muscle. Am J Physiol Heart Circ Physiol 272: H1886–H1891, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Miyashiro JK, Feigl EO. Feedforward control of coronary blood flow via coronary beta-receptor stimulation. Circ Res 73: 252–263, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Miyashiro JK, Feigl EO. A model of combined feedforward and feedback control of coronary blood flow. Am J Physiol Heart Circ Physiol 268: H895–H908, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Mohrman DE. A servo-controlled roller pump for cardiovascular research. Am J Physiol Heart Circ Physiol 238: H269–H274, 1980 [DOI] [PubMed] [Google Scholar]
- 33.Moody CJ, Meghji P, Burnstock G. Stimulation of P1-purinoceptors by ATP depends partly on its conversion to AMP and adenosine and partly on direct action. Eur J Pharmacol 97: 47–54, 1984 [DOI] [PubMed] [Google Scholar]
- 34.Pearson JD, Carleton JS, Hutchings A, Gordon JL. Uptake and metabolism of adenosine by pig aortic endothelial and smooth-muscle cells in culture. Biochem J 170: 265–271, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearson JD, Gordon JL. Nucleotide metabolism by endothelium. Annu Rev Physiol 47: 617–627, 1985 [DOI] [PubMed] [Google Scholar]
- 36.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998 [PubMed] [Google Scholar]
- 37.Reeves RB, Park JS, Lapennas GN, Olszowka AJ. Oxygen affinity and Bohr coefficients of dog blood. J Appl Physiol 53: 87–95, 1982 [DOI] [PubMed] [Google Scholar]
- 38.Saito D, Steinhart CR, Nixon DG, Olsson RA. Intracoronary adenosine deaminase reduces canine myocardial reactive hyperemia. Circ Res 49: 1262–1267, 1981 [DOI] [PubMed] [Google Scholar]
- 39.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol 26: 2614–2621, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Skalak R, Branemark PI. Deformation of red blood cells in capillaries. Science 164: 717–719, 1969 [DOI] [PubMed] [Google Scholar]
- 41.Smith FD, D'Alecy LG, Feigl EO. Cannula-tip coronary blood flow transducer for use in closed-chest animals. J Appl Physiol 37: 592–595, 1974 [DOI] [PubMed] [Google Scholar]
- 42.Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, Sprague RS. Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol 299: H1146–H1152, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tune JD, Richmond KN, Gorman MW, Olsson RA, Feigl EO. Adenosine is not responsible for local metabolic control of coronary blood flow in dogs during exercise. Am J Physiol Heart Circ Physiol 278: H74–H84, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Tune JD, Richmond KN, Gorman MW, Feigl EO. Role of nitric oxide and adenosine in control of coronary blood flow in exercising dogs. Circulation 101: 2942–2948, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Karlsson L, Moses S, Hultgardh-Nilsson A, Andersson M, Borna C, Gudbjartsson T, Jern S, Erlinge D. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol 40: 841–853, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Olivecrona G, Gotberg M, Olsson ML, Winzell MS, Erlinge D. ADP acting on P2Y13 receptors is a negative feedback pathway for ATP release from human red blood cells. Circ Res 96: 189–196, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Winbury MM, Papierski DH, Hemmer ML, Hambourger WE. Coronary dilator action of the adenine-ATP series. J Pharmacol Exp Ther 109: 255–260, 1953 [PubMed] [Google Scholar]
- 48.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol 362: 299–309, 2000 [DOI] [PubMed] [Google Scholar]