Abstract
Reactive oxygen species (ROS) contribute to various models of hypertension, including deoxycorticosterone acetate (DOCA)-salt-induced hypertension. Recently, we have shown that ROS, generated by cytochrome P-450 1B1 (CYP1B1) from arachidonic acid, mediate vascular smooth muscle cell growth caused by angiotensin II. This study was conducted to determine the contribution of CYP1B1 to hypertension and associated pathophysiological changes produced by DOCA (30 mg/kg) given subcutaneously per week with 1% NaCl + 0.1% KCl in drinking water to uninephrectomized rats for 6 wk. DOCA-salt treatment increased systolic blood pressure (SBP). Injections of the selective inhibitor of CYP1B1, 2,3′,4,5′-tetramethoxystilbene (TMS; 300 μg/kg ip every 3rd day) initiated at the 4th week of DOCA-salt treatment normalized SBP and decreased CYP1B1 activity but not its expression in the aorta, heart, and kidney. TMS also inhibited cardiovascular and kidney hypertrophy, prevented the increase in vascular reactivity and endothelial dysfunction, and minimized the increase in urinary protein and K+ output and the decrease in urine osmolality, Na+ output, and creatinine clearance associated with DOCA-salt treatment. These pathophysiological changes caused by DOCA-salt treatment and associated increase in vascular superoxide production, NADPH oxidase activity, and expression of NOX-1, and ERK1/2 and p38 MAPK activities in the aorta, heart, and kidney were inhibited by TMS. These data suggest that CYP1B1 contributes to DOCA-salt-induced hypertension and associated pathophysiological changes, most likely as a result of increased ROS production and ERK1/2 and p38 MAPK activity, and could serve as a novel target for the development of agents like TMS to treat hypertension.
Keywords: cytochrome P-450 1B1 activity, reactive oxygen species, vascular reactivity, endothelial dysfunction, renal function
deoxycorticosterone acetate (DOCA), an analog of aldosterone with 1% salt in drinking water (DOCA-salt) causes angiotensin II (ANG II)-independent hypertension by promoting salt and water retention and an increase in plasma volume, resulting in decreased renin secretion and ANG II levels. Several other vasoactive agents, including catecholamines, vasopressin, and endothelin-1 (ET-1), have been shown to contribute to the development of DOCA-salt-induced hypertension (9–11, 38). These vasoconstrictor agents stimulate arachidonic acid (AA) release from phospholipids via activation of phospholipase A2 (3, 28, 47). AA metabolites generated via cyclooxygenase, prostaglandin (PG) E2 and I2, and cytochrome P-450 (CYP) epoxygenase (epoxyeicosatrienoic acids) contribute to antihypertensive mechanisms (18, 23, 34), whereas AA metabolites formed via cyclooxygenase, PG endoperoxide (PGH2), lipoxygenase [LO; (12-hydroxyeicosatetraenoic acid, 12-HETE)], and CYP4A ω-hydroxylase (20-HETE) contribute to prohypertensive mechanisms (27, 29#x2013;31, 34, 35, 44, 50, 54). LO inhibitors in renin-angiotensin-dependent models of hypertension and spontaneously hypertensive rats (SHR) (31, 43) and CYP4A inhibitors in SHR, ANG II, and DOCA-salt models of hypertension lower blood pressure (29, 30, 44).
AA metabolites generated by CYP4A have been reported to contribute to ANG II- and DOCA-salt-induced hypertension (1, 29, 30). AA metabolism is also associated with generation of reactive oxygen species (ROS), and its metabolite, 20-HETE, stimulates ROS production (24, 39). ROS have been implicated in the activation of p38 MAPK in vascular smooth muscle cells (VSMCs) and ERK1/2 in mesangial cells (15, 46, 49). Moreover, ROS produced in various models of hypertension, including the DOCA-salt model of hypertension, cause endothelial dysfunction and contribute to the development of hypertension (1, 17, 21, 36).
AA can also be metabolized by recombinant CYP1B1 into midchain and terminal HETEs, including 5-, 12-, 15-, and 20-HETE (6). Moreover, CYP1B1 is expressed in nonhepatic tissues, including cardiovascular tissues (8, 20, 56), and mediates ANG II-induced VSMC migration, proliferation, and hypertrophy by generating ROS from AA (51). These observations raised the possibility that HETEs and/or ROS generated by CYP1B1 might contribute to DOCA-salt-induced hypertension. To test this hypothesis, we examined the effect of 2,3′,4,5′-tetramethoxystilbene (TMS), a selective inhibitor of CYP1B1 (7), on DOCA-salt-induced hypertension in rats. The results of our study show that TMS normalizes blood pressure in DOCA-salt-induced hypertension and minimizes associated pathophysiological changes and that CYP1B1 contributes to this action of TMS.
MATERIALS AND METHODS
Materials
TMS and NG-nitro-l-arginine methyl ester (l-NAME) were obtained from Cayman Chemical (Ann Arbor, MI). DOCA, DMSO, (R)-(−)-phenylephrine hydrochloride (PE), ET-1 (human, porcine), acetylcholine chloride (ACh), sodium nitroprusside (SNP), indomethacin, NADPH, lucigenin, and the anti-β-actin antibody were purchased from Sigma Aldrich (St. Louis, MO). Dihydroethidium (DHE) was obtained from Molecular Probes (Eugene, OR). Anti-phospho-ERK1/2 MAPK and anti-phospho-p38 MAPK antibodies were obtained from Cell Signaling (Beverly, MA). The corresponding nonphosphorylated antibodies for these kinases and anti-NOX-1, anti-NOX-4, and anti-CYP1A1 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). CYP1B1 antibody was purchased from BD Biosciences (Woburn, MA).
DOCA-Salt-Induced Hypertension in Rats
All procedures were carried out in male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA; 250–350 g) according to the protocols approved by our Institutional Animal Care and Use Committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animals were kept at 24°C under controlled conditions of a 12:12-h light-dark cycle and humidity (50%) and were fed with standard rat chow (Ralston Purina, St. Louis, MO). All rats underwent left unilateral nephrectomy. Rats were anesthetized with ketamine (60 mg/kg ip) and xylazine (5 mg/kg ip), and a 2-cm left flank incision was made to ligate the left renal artery and vein and remove the left kidney. Animals treated with DOCA were injected subcutaneously with a suspension of DOCA in sesame oil (30 mg/kg per week, 0.2 ml) and received tap water containing 1% NaCl and 0.1% KCl for 6 wk. Uninephrectomized (UNX) control rats received vehicle without DOCA or salt. To study the effect of the CYP1B1 inhibitor TMS on DOCA-salt-induced hypertension, we administered TMS (300 μg/kg) or its vehicle, DMSO (100 μl), intraperitoneally every third day beginning at week 4 and measured systolic blood pressure (SBP) every week using a noninvasive tail-cuff method (Kent Scientific, Torrington, CT; model XBP 1000).
Measurement of CYP1B1 Activity
To determine the effect of TMS on CYP1B1 activity, we used the P450-Glo assay kit (Promega, Madison, WI) according to the manufacturer's instructions. After 6 wk of treatment, rats were anesthetized as described above, the left ventricle was punctured, and blood was flushed out by perfusion with cold saline (3 min). The heart, kidney, and thoracic aorta were isolated, cleaned of surrounding tissue, snap frozen in liquid N2, and stored at −80°C. Tissues were ground to a fine powder in liquid N2 and homogenized in ice-cold 0.1 mol/l potassium phosphate buffer (pH 7.4). After homogenization, samples were centrifuged at 10,000 g for 20 min at 4°C, and the supernatants were stored at −80°C. Protein concentration of the supernatants was determined using the Bradford method. A reaction mixture containing 20 μmol/l luciferin 6′-chloroethyl ether substrate with 0.1 mol/l potassium phosphate buffer (pH 7.4) was added to the samples containing equal amounts (500 μg) of protein and incubated at 37°C for 10 min. NADPH (final concentration 100 μmol/l) was added to the reaction mixture, which was further incubated at 37°C for 45 min. After incubation, luciferin detection reagent was added to the samples at a 1:1 ratio, mixed for 10 s, and incubated at room temperature for 20 min to stabilize the luminescent signal. Luminescence was measured using a luminometer (Turner Designs, Sunny Vale, CA; model TD-20/20) and expressed in relative luminescence units.
The effect of exogenous TMS on CYP1B1 activity was also determined. Before the supernatants were added to the reaction mixture, samples were incubated with 6 μmol/l TMS for 30 min at room temperature. After incubation, supernatants were added to the reaction mixture and the identical procedure to that described above was followed.
Measurement of Cardiac Hypertrophy: Heart Weight-to-Body Weight Ratio and Brain Natriuretic Peptide mRNA Expression by Quantitative RT-PCR
Heart weight-to-body weight ratio.
Animals were weighed at the completion of the experiment, anesthetized as described above, the left ventricle was punctured, and blood was flushed out by perfusion with cold saline (3 min). The heart was removed immediately and weighed, and left ventricles were rapidly frozen in liquid N2 to allow for subsequent measurement of brain natriuretic peptide (BNP) mRNA expression. Heart weight-to-body weight ratio (HW/BW, mg/g) was calculated as an indicator of cardiac hypertrophy.
Measurement of BNP mRNA expression measurement.
Total RNA was isolated from left ventricle homogenates using an RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer's instructions, and deoxyribonuclease was digested using an RNase-free DNase kit (Qiagen). Reverse transcription was performed using the Transcriptor First-Strand cDNA synthesis kit (Roche, Indianapolis, IN) with 300 ng of total RNA and 60 μmol/l random primer (Roche) in a 20-μl volume with time courses of 25°C for 10 min followed by 55°C for 30 min. Quantitative real-time PCR was performed in 96-well plates with a LightCycler (LC) 480 (Roche) using an LC480 Master Mix and a Universal Probe Library (UPL; Roche) at a concentration of 10 μmol/l with a final reaction volume of 10 μl with the following conditions: 95°C for 5 min for activation and 45 cycles of 95°C for 10 s, 60°C for 60 s, and 72°C for 10 s for amplification. After six endogenous control genes were tested, the β-tubulin gene was selected as an endogenous control for BNP. The sequences of primers and the relevant probes from the UPL used for BNP and β-tubulin are described in Table 1. All samples were analyzed in triplicate. The relative amount of mRNA content of the target gene was normalized to the endogenous control gene mRNA content in the same sample. Expression of BNP relative to β-tubulin in each sample was calculated on the basis of the ΔΔCt method, where ΔCt is the difference in threshold cycle (Ct) values between the target and endogenous control.
Table 1.
Sequences of primers and hybridization probes
| Identification | Sequence | Amplicon Length, bp |
|---|---|---|
| BNP | ||
| Forward | 5′-GTC AGT CGC TTG GGC TGT-3′ | 104 |
| Reverse | 5′-CAG AGC TGG GGA AAG AG-3′ | |
| UPL Probe | 5′-CTC TGC CT-3′ | |
| β-Tubulin | ||
| Forward | 5′-CAG AGC CAT TCT GGT GGA C-3′ | 112 |
| Reverse | 5′-GCC AGC ACC ACT CTG ACC-3′ | |
| UPL Probe | 5′-GAG CCT GG-3′ |
BNP, brain natriuretic peptide; UPL, universal probe library; bp, base pairs.
Measurement of Vascular Reactivity, Media-to-Lumen Ratio, and Endothelial Function
Vascular reactivity.
At the completion of the experiment, animals were anesthetized as described above, the thoracic aorta and superior mesenteric arteries were removed and cleaned of surrounding tissue, and rings ∼2 mm in length were mounted in a wire myograph system to measure changes in isometric tension (Danish Myo Technology, Aarhus, Denmark; model 610M). Rings were suspended and equilibrated in oxygenated (95% O2-5% CO2) Krebs buffer (pH 7.4; composition in mmol/l: 118 NaCl, 4.7 KCl, 25 NaHCO3, 1.2 MgSO4, 1.2 KH2PO4, 11.1 glucose, and 2.5 CaCl2·2H2O) at 37°C with an initial tension of 9 mN for thoracic aorta and 5 mN for superior mesenteric artery for 30 min. After equilibration, vessels were preconstricted with KCl (60 mmol/l) and washed three times with Krebs buffer. Cumulative concentration response curves to PE (10−9–10−5 mol/l) and ET-1 (10−12–10−8 mol/l) were obtained, and responses were measured as force of contraction in millinewtons (mN).
Measurement of media-to-lumen ratio.
After anesthetization, the thoracic aorta and mesenteric artery were isolated, surrounding tissues were removed, and arterial segments were incubated in 10% buffered formalin overnight, dehydrated with graded ethanol followed by xylene (1 h), and embedded in paraffin. The embedded arteries were cut into 5-μm sections with a Microm microtome (GMI, Ramsey, MN; model HM 315) and stained with hematoxylin and eosin. Sections were viewed with an Olympus inverted system microscope (Olympus America, Melville, NY; model IX50) and photographed with an Olympus digital camera (Olympus America; model DP71). Media thickness and lumen diameter were measured along the vessel wall with ImageJ 1.42 (http://rsb.info.nih.gov/nih-image; National Institutes of Health), and the media-to-lumen ratio was calculated.
Endothelium-dependent and -independent vasorelaxation.
After the vessels were constricted with PE (10−5 mol/l), responses to ACh (10−9–10−5 mol/l) were determined. The contribution of endothelium-derived hyperpolarizing factor (EDHF)-mediated relaxation was determined in the presence of indomethacin (10−5 mol/l) and l-NAME (10−4 mol/l) to rule out the formation of vasoactive prostanoids and nitric oxide, respectively. A combination of indomethacin and l-NAME was added to the myograph chamber ∼10 min before preconstriction of the vessels with PE. Endothelium-independent vasodilation was examined by adding increasing concentrations of SNP (10−9–10−5 mol/l) after the vessels had been constricted with PE (10−5 mol/l). Relaxations are expressed as a percentage of the constriction induced by PE.
Assessment of Kidney Hypertrophy and Function
Kidney weight (mg)-to-body weight (g) ratio was measured as an indicator of kidney hypertrophy. To assess renal function, we measured urine output, protein content, osmolality, Na+ and K+ concentrations, blood urea nitrogen (BUN), serum and urine creatinine, and creatinine clearance. For collection of urine samples, rats were individually placed in metabolic cages for 24 h. At the completion of the experiment, animals were anesthetized and perfused with saline via left ventricle puncture (3 min) as described above. The kidney was dissected free and weighed. Urine volume was calculated and analyzed for osmolality using a Vapro vapor pressure osmometer (model 5520; Wescor, South Logan, UT), protein content was determined using the standard Bradford method, and Na+ and K+ concentrations were measured using a flame photometer (Instrumentation Laboratory, Lexington, MA; model 443). Creatinine clearance was calculated to estimate glomerular filtration rate (GFR) by measuring creatinine concentration in serum and urine samples with a QuantiChrom creatinine assay kit (BioAssay Systems, Hayward, CA), and BUN was measured using a QuantiChrom urea assay kit (BioAssay Systems) according to the manufacturer's instructions.
Plasma Levels of 12- and 20-HETE
Plasma levels of 12-HETE and 20-HETE were determined using a 12(S)-HETE ELISA kit (Abnova, Taipei, Taiwan) and a 20-HETE ELISA kit (Detroit R&D, Detroit, MI), respectively, as recommended by the manufacturer.
Measurement of Vascular Superoxide Production
Thoracic aorta sections were stained with DHE as previously described (25). Fresh, unfixed aorta samples were placed in Tissue-Tek optimal cutting temperature compound embedding medium (Sakura Finetek USA, Torrance, CA) and frozen at −80°C. Frozen tissue segments were cut into 30-μm-thick sections with a cryostat (Bright Instrument, Huntingdon, UK; model OTF) and placed on a glass slide. Sections were equilibrated for 30 min at 37°C in PBS, and DHE (2 μmol/l) was topically applied to each tissue section and coverslipped. Sections were further incubated for 30 min in a light-protected humidified chamber at 37°C and rinsed in PBS. Fluorescence was detected using a 585-nm filter on an Olympus inverted system microscope (Olympus America; model IX50). Images were photographed using an Olympus digital camera (Olympus America; model DP71), and fluorescence was quantitated using ImageJ 1.42. Three regions of interest were selected on each section to measure fluorescence intensities, and the results were averaged. The mean fluorescence intensities were normalized to UNX control rats.
Measurement of DHE and 2-Hydroxyethidium by HPLC
To determine vascular superoxide production, we measured oxidation of DHE to 2-hydroxyethidium (2-OHE) using a fluorescence detector following separation by HPLC, as described previously (11), with slight modifications of the method. Frozen aortic rings were incubated in Krebs buffer containing 50 μmol/l DHE and 100 μmol/l NADPH for 15 min at 37°C. Vessels were then washed and further incubated in Krebs buffer for 1 h at 37°C. Vessels were then homogenized in 300 μl of cold methanol and filtered (0.22 μm), and DHE was separated from 2-OHE with a Schimadzu HPLC system using a C-18 reverse phase column (Sigma; Nucleosil 250, 4.5 mm). The mobile phase consisted of a gradient containing 60% acetonitrile and 0.1% trifluoroacetic acid, and 2-OHE was separated by a linear increase in acetonitrile concentration from 37 to 47% over 23 min at a flow rate of 0.5 ml/min. Production of 2-OHE was monitored by fluorescence detection at 580 (emission) and 480 nm (excitation). Data were analyzed using LC Solution Chromotography Data System software (Schimadzu, Kyoto, Japan).
Measurement of NADPH Oxidase Activity
NADPH oxidase activity was determined by measuring lucigenin-enhanced chemiluminescence, as described previously (52), with some modifications. The heart, kidney, and thoracic aorta were isolated, cleaned of surrounding tissue, and snap frozen in liquid N2. Tissues were ground to a fine powder in liquid N2, homogenized, and sonicated in lysis buffer containing protease inhibitors (20 mmol/l phosphate buffer, 1 mmol/l EGTA, 10 μg/ml aprotinin, 0.5 μg/ml leupeptin, 0.7 μg/ml pepstatin, 0.5 mmol/l phenylmethylsulfonyl fluoride, and 150 mmol/l sucrose). After sonication, samples were centrifuged at 3,000 g for 10 min at 4°C, and protein concentration of the supernatants was determined using the Bradford method. Reaction buffer containing 5 μmol/l lucigenin as the electron acceptor and 100 μmol/l NADPH as the substrate was added to the samples containing equal amounts of protein at a 1:1 ratio. Luminescence was measured every minute for 10 min with a luminometer (Turner Designs); buffer, which served as a blank, was subtracted from each reading, and activity was expressed in arbitrary units. To confirm the specificity of the assay, we also performed the reaction in the presence of the NADPH oxidase inhibitor apocynin (100 μmol/l).
Western Blot Analysis
The heart, kidney, and thoracic aorta were isolated, cleaned of surrounding tissue, snap frozen in liquid N2, and stored at −80°C. Tissues were ground to a fine powder in liquid N2 and homogenized in lysis buffer [1% IGEPAL CA-630, 25 mmol/l HEPES (pH 7.5), 50 mmol/l NaCl, 50 mmol/l NaF, 1 mmol/l Na3VO4, 1 mmol/l phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin] for 1 h. Insoluble particles were separated from lysates by centrifuging at 14,000 g for 15 min at 4°C, and protein content was determined using the Bradford method. Equal amounts of protein (30–60 μg) were resolved in SDS-polyacrylamide gels and then transferred to nitrocellulose membranes. After being blocked with milk in TBST buffer (20 mmol/l Tris, pH 7.6, 137 mmol/l NaCl, and 0.05% Tween) for 2 h at room temperature, membranes were incubated with primary antibodies at 1:300–1:10,000 dilutions overnight at 4°C. Membranes were then exposed to their respective secondary antibodies and conjugated with horseradish peroxidase at 1:1,000–1:1,500 dilutions for 2 h at room temperature. The immunoreactive proteins were detected using SuperSignal chemiluminescent substrate (Pierce, Rockford, IL). The density of the bands was analyzed using ImageJ 1.42 software. For confirmation of equal gel loading, membranes were stripped with Restore Western blot stripping buffer (Pierce) and reprobed with anti-β-actin antibody.
Statistical Analysis
Data were analyzed using ANOVA; the unpaired Student's t-test was used to determine the difference between two groups, and the Newman-Keuls test was used for multiple comparisons. The values of at least three different experiments are expressed as means ± SE. P values <0.05 are considered statistically significant.
RESULTS
TMS Prevents DOCA-Salt-Induced Blood Pressure Increase in Rats
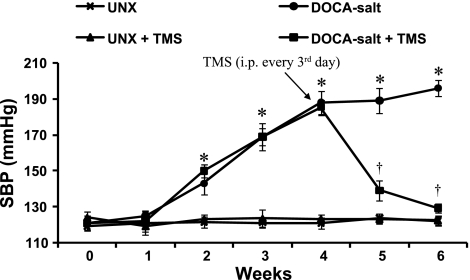
Administration of DOCA-salt to UNX rats for 6 wk increased SBP that returned to basal levels in rats treated with TMS (Fig. 1). TMS alone (Fig. 1) or its vehicle (DMSO) (data not shown) did not alter SBP.
Fig. 1.
2,3′,4,5′-Tetramethoxystilbene (TMS) prevents deoxycorticosterone acetate (DOCA)-salt-induced hypertension in rats. Uninephrectomized (UNX) rats were injected with DOCA (30 mg/kg per week, 0.2 ml) subcutaneously and given 1% NaCl and 0.1% KCl in tap water for 6 wk, and UNX control rats received vehicle but no DOCA or salt. TMS (300 μg/kg) was administrated intraperitoneally (ip) every 3rd day beginning from week 4, and systolic blood pressure (SBP) was measured every week by tail-cuff method. Data are means ± SE (n = 5). *P < 0.05, UNX vs. DOCA-salt. †P < 0.05, DOCA-salt vs. DOCA-salt + TMS.
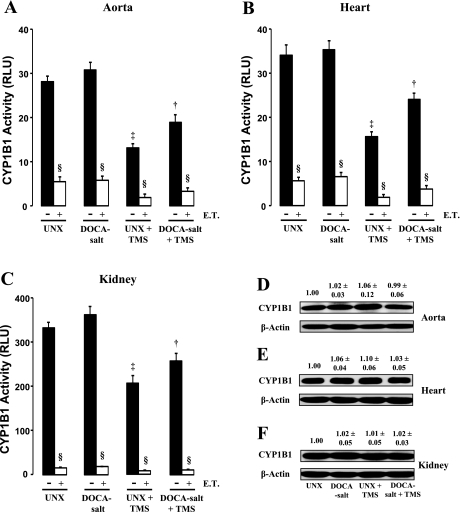
TMS Decreases CYP1B1 Activity in Aorta, Heart, and Kidney But Not Its Expression
DOCA-salt given to UNX rats did not alter CYP1B1 activity in the aorta, heart, or kidney (Fig. 2, A–C). Treatment with TMS decreased CYP1B1 activity in rats given DOCA-salt in all tissues studied (Fig. 2, A–C). TMS treatment also decreased CYP1B1 activity in UNX control rats (Fig. 2, A–C). CYP1B1 protein expression was not altered in any of the tissues examined from rats in the different treatment groups (Fig. 2, D–F). Addition of exogenous TMS to the incubation mixture further inhibited CYP1B1 activity in all treatment groups (Fig. 2, A–C).
Fig. 2.
TMS attenuates cytochrome P-450 1B1 (CYP1B1) activity without altering its expression. The effect of TMS on CYP1B1 activity in aorta (A), heart (B), and kidney (C) is shown. Tissues were isolated from rats in the various treatment groups and homogenized, and CYP1B1 activity was measured using the P450-Glo assay kit as recommended by supplier (materials and methods). Luminescence was measured using a luminometer and expressed as relative luminescence units (RLU). Samples were also incubated with exogenous TMS (E.T.; 6 μmol/l) for 30 min to determine any further effect of TMS on CYP1B1 activity. CYP1B1 protein expression was measured in the aorta (D), heart (E), and kidney (F) of animals from the various treatment groups by Western blot analysis as described in materials and methods. The blots were probed with anti-CYP1B1 antibody. Density of the bands was analyzed using ImageJ 1.42 software and expressed as the ratio of CYP1B1 to β-actin protein. Data are means ± SE (n = 3–5). ‡P < 0.05, UNX vs. UNX + TMS. †P < 0.05, DOCA-salt vs. DOCA-salt + TMS. §P < 0.05, E.T. vs. corresponding treatment group.
TMS Reduces Cardiac Hypertrophy Associated With DOCA-Salt-Induced Hypertension
Administration of DOCA-salt caused an increase in HW/BW and BNP mRNA expression, indicators of cardiac hypertrophy (Table 2). These increases were minimized by TMS treatment. TMS alone had no effect on either HW/BW or BNP mRNA levels in any of the treatment groups (Table 2).
Table 2.
TMS inhibits cardiac hypertrophy associated with DOCA-salt-induced hypertension in rats
| UNX | DOCA-Salt | UNX + TMS | DOCA-Salt + TMS | |
|---|---|---|---|---|
| BW, g | 494.0 ± 15.1 | 375.0 ± 15.9* | 496.4 ± 14.7 | 455.8 ± 16.1† |
| HW, mg | 1,484.0 ± 24.6 | 2,032.0 ± 81.3* | 1,426.0 ± 29.8 | 1,687.0 ± 27.0† |
| HW/BW, mg/g | 3.0 ± 0.1 | 4.4 ± 0.2* | 2.9 ± 0.2 | 3.6 ± 0.2† |
| BNP:β-tubulin mRNA, ΔΔCt | 1.0 | 6.5 ± 1.0* | 0.6 ± 0.1 | 1.4 ± 0.2† |
Data are means ± SE (n = 5). BNP data are displayed following normalization to uninephrectomized (UNX) control rat values. DOCA, deoxycorticosterone acetate; TMS, 2,3′,4,5′-tetramethoxystilbene; BW, body weight; HW, heart weight.
P < 0.05, UNX vs. DOCA-salt.
P < 0.05, DOCA-salt vs. DOCA-salt + TMS.
TMS Prevents Increases in Vascular Reactivity, Vascular Smooth Muscle Hypertrophy, and Endothelial Dysfunction in DOCA-Salt Hypertensive Rats
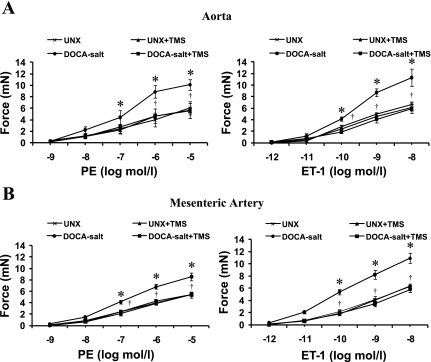
The contractile response to PE and ET-1 in aorta (Fig. 3A) and mesenteric arteries (Fig. 3B) was significantly higher in DOCA-salt-treated rats compared with vehicle-treated UNX rats. The increases in vascular reactivity to PE and ET-1 were prevented in both aorta and mesenteric arteries from animals treated with TMS (Fig. 3, A and B). TMS alone had no effect on the vascular response to these agents (Fig. 3, A and B). The increase in vascular reactivity of aorta and mesenteric arteries to PE and ET-1 was accompanied by an increase in media-to-lumen ratio in DOCA-salt treated rats, but not in animals that also received TMS; no change in media-to-lumen ratio was observed in rats treated with TMS alone (Table 3).
Fig. 3.
TMS prevents DOCA-salt-induced increase in vascular reactivity in rats. Concentration-response curves to phenylephrine (PE; 10−9–10−5 mol/l) and ET-1 (10−12–10−8 mol/l) in the aorta (A) and mesenteric arteries (B) from animals in each of the treatment groups were constructed. Data are means ± SE (n = 5). *P < 0.05, UNX vs. DOCA-salt. †P < 0.05, DOCA-salt vs. DOCA-salt + TMS.
Table 3.
TMS treatment prevents increased media-to-lumen ratio associated with DOCA-salt-induced hypertension in rats
| UNX | DOCA-Salt | UNX + TMS | DOCA-Salt + TMS | |
|---|---|---|---|---|
| Thoracic aorta | 6.67 ± 0.68 | 9.08 ± 0.36* | 5.78 ± 0.39 | 6.94 ± 0.68† |
| Mesenteric artery | 5.29 ± 0.12 | 7.06 ± 0.36* | 4.63 ± 0.46 | 5.22 ± 0.18† |
Data are means ± SE (n = 5).
P < 0.05, UNX vs. DOCA-salt.
P < 0.05, DOCA-salt vs. DOCA-salt + TMS.
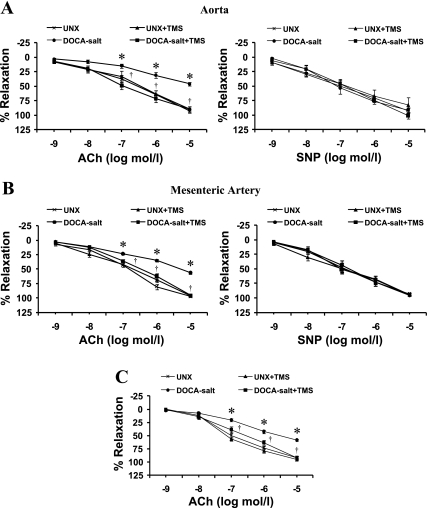
To determine the effect of TMS treatment on endothelial function in DOCA-salt-induced hypertension, we examined the response of ACh on aorta and mesenteric arteries preconstricted with PE. ACh caused relaxation of aorta (Fig. 4A) and mesenteric arteries (Fig. 4B) from UNX rats, but the relaxation was diminished in DOCA-salt-treated rats; this change was prevented by treatment with TMS. To determine the possible contribution of EDHF in ACh-induced relaxation, we studied the effect of ACh in the presence of indomethacin and l-NAME. In the presence of these agents, ACh caused relaxation of mesenteric arteries (Fig. 4C), but not aorta (data not shown), from UNX rats; relaxation was diminished in DOCA-salt-treated rats, but this decrease was prevented by TMS treatment (Fig. 4C). Relaxation responses to SNP were not altered in any of the treatment groups (Fig. 4, A and B). TMS alone did not alter the effect of any of these vasodilator agents (Fig. 4, A–C).
Fig. 4.
TMS prevents DOCA-salt-induced endothelial dysfunction in rats. Endothelial function was examined by measuring the response of the aorta (A) and mesenteric arteries (B) to increasing concentrations of ACh (10−9–10−5 mol/l) following constriction with PE (10−5 mol/l). Endothelium-independent vasodilation was examined by measuring the relaxation response to sodium nitroprusside (SNP; 10−9–10−5 mol/l) after the vessels were constricted with PE (10−5 mol/l). C: to determine the contribution of endothelium-derived hyperpolarizing factor (EDHF)-mediated relaxation, the response to ACh (10−9–10−5 mol/l) was determined in the presence of indomethacin (10−5 mol/l) and NG-nitro-l-arginine methyl ester (l-NAME; 10−4 mol/l). A combination of indomethacin and l-NAME was added to the myograph chamber ∼10 min before constriction with PE. Relaxation is expressed as a percentage of the contraction by PE. Data are means ± SE (n = 5). *P < 0.05, UNX vs. DOCA-salt. †P < 0.05, DOCA-salt vs. DOCA-salt + TMS.
TMS Reduces Kidney Hypertrophy and Minimizes Renal Dysfunction Induced by DOCA-Salt Administration
As shown in Table 4, administration of DOCA-salt caused an increase in kidney-to-body weight ratio, an index of renal hypertrophy, which was diminished by TMS treatment. Urine output, protein content, K+ concentration, BUN, and serum creatinine concentrations were increased, whereas urine osmolality, Na+ concentration, and creatinine clearance decreased in DOCA-salt hypertensive rats. The changes in these parameters were minimized by TMS treatment. TMS alone had no effect on any of these parameters.
Table 4.
TMS prevents kidney hypertrophy and reduces changes in renal function caused by DOCA-salt treatment
| UNX | DOCA-Salt | UNX + TMS | DOCA-Salt + TMS | |
|---|---|---|---|---|
| Kidney weight/body weight, mg/g | 5.3 ± 0.2 | 9.9 ± 0.2* | 5.1 ± 0.2 | 7.3 ± 0.4† |
| Urine output, ml/day | 26.4 ± 2.5 | 116.0 ± 7.3* | 22.9 ± 1.3 | 75.9 ± 8.0† |
| Proteinuria, mg/day | 30.0 ± 4.3 | 199.9 ± 33.6* | 26.6 ± 5.4 | 105.3 ± 24.5† |
| Osmolality, mosmol/kgH2O | 1,530.6 ± 97.6 | 675.0 ± 24.6 | 1,428.6 ± 96.4 | 951.8 ± 38.9† |
| Urinary [Na+], mmol/l | 137.8 ± 6.2 | 105.1 ± 5.0 | 140.2 ± 9.0 | 124.7 ± 4.9† |
| Urinary [K+], mmol/l | 75.2 ± 5.6 | 249.2 ± 32.0 | 82.5 ± 7.5 | 186.1 ± 9.8† |
| BUN, mg/dl | 54.4 ± 3.4 | 79.6 ± 8.1* | 54.2 ± 1.5 | 61.7 ± 4.5† |
| Serum creatinine, mg/dl | 0.5 ± 0.1 | 1.2 ± 0.1* | 0.5 ± 0.2 | 0.9 ± 0.1† |
| Creatinine clearance, ml/min | 2.3 ± 0.2 | 0.7 ± 0.1* | 2.2 ± 0.3 | 1.5 ± 0.2† |
Data are means ± SE (n = 5). BUN, blood urea nitrogen.
P < 0.05, UNX vs. DOCA-salt.
P < 0.05, DOCA-salt vs. DOCA-salt + TMS.
DOCA-Salt or TMS Does Not Alter Plasma Levels of 12- and 20-HETE
Plasma levels of 12- and 20-HETE have been reported to increase in SHR and in renovascular disease in humans (25, 36). As shown in Table 5, in rats with DOCA-salt-induced hypertension, plasma levels of these eicosanoids were not altered. TMS given alone or to DOCA-salt-treated animals also had no effect on plasma levels of 12- or 20-HETE.
Table 5.
DOCA-salt or TMS treatment does not alter plasma levels of 12- and 20-HETE
| UNX | DOCA-Salt | UNX + TMS | DOCA-Salt + TMS | |
|---|---|---|---|---|
| 12-HETE, ng/ml | 17.1 ± 1.1 | 17.1 ± 4.5 | 14.0 ± 3.2 | 14.7 ± 5.2 |
| 20-HETE, ng/ml | 10.1 ± 4.7 | 12.7 ± 5.3 | 8.8 ± 1.5 | 10.4 ± 5.1 |
Data are means ± SE (n = 5). HETE, hydroxyeicosatetraenoic acid.
TMS Inhibits Increase in Vascular Superoxide Production, NADPH Oxidase Activity and Protein Expression of NOX-1, But Not NOX-4, in DOCA-Salt-Induced Hypertension
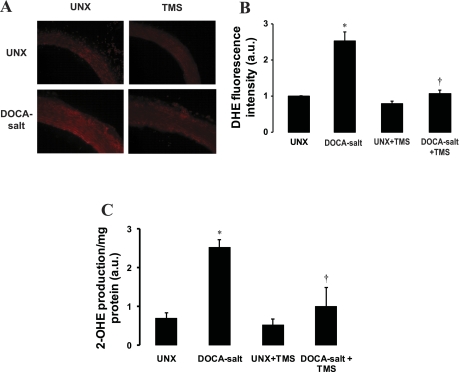
Vascular superoxide levels, as measured by 2-OHE fluorescence following conversion from DHE, were significantly greater in DOCA-salt-treated rats compared with UNX control rats (Fig. 5, A and B). TMS treatment abolished the increase in superoxide production in DOCA-salt-treated animals, and TMS alone did not alter superoxide levels compared with UNX control group (Fig. 5, A and B). To confirm the superoxide production detected by formation of 2-OHE from DHE by fluorescence microscopy, we used the HPLC method to separate 2-OHE from DHE and measured the fluorescence using a fluorescence detector. Fluorescence was greater in the aorta of DOCA-salt-treated rats than in UNX control rats that show more conversion of DHE to 2-OHE, which was inhibited by TMS (Fig. 5C). TMS alone had a minimal effect on basal conversion of DHE to 2-OHE (Fig. 5C).
Fig. 5.
TMS prevents the increase in DOCA-salt-induced vascular superoxide production. The effect of TMS on vascular superoxide production was determined in transverse sections of thoracic aorta from animals in each of the different treatment groups. A: representative fluorescent photomicrographs of sections determined by dihydroethidium (DHE) fluorescent staining (materials and methods). B: quantification of fluorescence. Fluorescence was detected using a 585-nm filter on an Olympus inverted system microscope. Images were photographed using an Olympus digital camera, and quantification of fluorescence was determined using ImageJ 1.42. Mean fluorescence intensities were normalized to values for UNX control rats and are expressed in arbitrary units (a.u.). C: vascular superoxide production measured by monitoring the conversion of DHE to 2-hydroxyethidium (2-OHE) using HPLC (materials and methods). Data are means ± SE (n = 3–5). *P < 0.05, UNX vs. DOCA-salt. †P < 0.05, DOCA-salt vs. DOCA-salt + TMS.
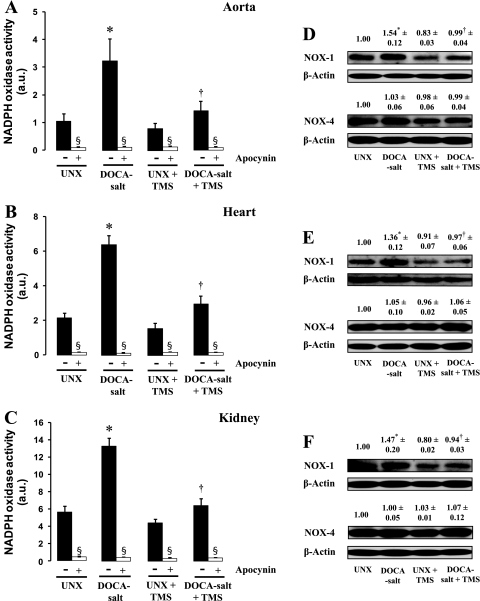
As was observed with vascular superoxide production via the DHE fluorescence method, DOCA-salt treatment was associated with an increase in NADPH oxidase activity in aorta, heart, and kidney (Fig. 6, A–C). The basal as well as the increase in NADPH oxidase activity was inhibited by apocynin (Fig. 6, A–C). DOCA-salt treatment in rats also increased protein expression of NADPH oxidase subtype 1 (NOX-1), but not NOX 4, in these tissues (Fig. 6, D–F). The increase in NADPH oxidase activity and NOX-1 protein expression in DOCA-salt-treated rats was reduced by TMS (Fig. 6, A–F). TMS alone had no effect on NADPH oxidase activity or NOX-1 expression in any of the treatment groups (Fig. 6, A–F).
Fig. 6.
TMS reduces the increase in NADPH oxidase activity and expression of NOX-1 associated with DOCA-salt-induced hypertension. A–C: effect of TMS treatment on NADPH oxidase activity in the aorta (A), heart (B), and kidney (C). NADPH oxidase activity was measured using lucigenin-enhanced chemiluminescence in the absence and presence of the NADPH oxidase inhibitor apocynin (100 μmol/l) (materials and methods). Luminescence was measured every minute for 10 min using a luminometer, and activity is expressed in arbitrary units. Data are means ± SE (n = 5). *P < 0.05, UNX vs. DOCA-salt. †P < 0.05, DOCA-salt vs. DOCA-salt + TMS. §P < 0.05, apocynin vs. corresponding treatment group. D–F: protein levels of NOX-1 and NOX-4 in the aorta (D), heart (E), and kidney (F) were determined by Western blot analysis as described in materials and methods (n = 3). Blots were probed with an anti-NOX-1 or anti-NOX-4 antibody. For confirmation of equal gel loading, membranes were reprobed with anti-β-actin antibody. Density of the bands was analyzed using ImageJ 1.42 software and is expressed as the ratio of NOX-1 or NOX-4 to β-actin protein.
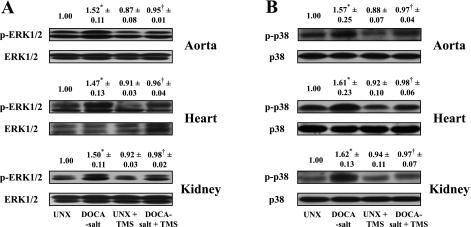
TMS Ameliorates DOCA-Salt-Induced Increase in the Activity of ERK1/2 and p38 MAPK in Aorta, Heart, and Kidney
To determine the effect of TMS on ERK1/2 and p38 MAPK activities in DOCA-salt-induced hypertension, the phosphorylation of these kinases in aorta, heart, and kidney was measured. DOCA-salt administration caused an increase in phosphorylation of both these kinases in all tissues studied (Fig. 7, A and B). DOCA-salt in combination with TMS treatment attenuated the increase in phosphorylation of ERK1/2 and p38 MAPK (Fig. 7, A and B), and TMS alone did not alter phosphorylation of these kinases (Fig. 7, A and B).
Fig. 7.
TMS treatment prevents the increase in ERK1/2 and p38 MAPK phosphorylation in rat aorta, heart, and kidney associated with DOCA-salt-induced hypertension. Representative Western blots show ERK1/2 (A) and p38 MAPK (B) phosphorylation (top) and protein expression of these kinases (bottom). Tissues were homogenized in lysis buffer, and after protein concentrations were measured, equal amounts of protein (30–60 μg) in each sample were subjected to SDS-PAGE and Western blot analysis (materials and methods). The blots were probed with anti-phospho-ERK1/2 (p-ERK1/2) or anti-phospho-p38 MAPK (p-p38) antibodies and their corresponding nonphosphorylated antibodies. Density of the bands was analyzed using ImageJ 1.42 software and is expressed as the ratio of phosphorylated to nonphosphorylated ERK1/2 and p38 MAPK. Data are means ± SE (n = 3). *P < 0.05, UNX vs. DOCA-salt. †P < 0.05, DOCA-salt vs. DOCA-salt + TMS.
DISCUSSION
This study provides the first evidence that CYP1B1 contributes to DOCA-salt-induced hypertension in UNX rats and associated pathophysiological changes in cardiovascular and renal function. This evidence is based on our demonstration that in DOCA-salt-induced hypertension in rats, TMS, a selective inhibitor of CYP1B1 (35), in doses that normalized SBP, 1) diminished cardiovascular hypertrophy, measured by an increase in HW/BW and expression of BNP mRNA and media-to-lumen ratio in the aorta and mesenteric arteries, respectively; 2) prevented the increase in the response of these vessels to PE and ET-1, as well as endothelial dysfunction; and 3) reduced kidney hypertrophy, as indicated by decreased kidney-to-body weight ratio, and minimized the increase in urine output, K+ excretion, and proteinuria and the decrease in Na+ excretion, osmolality, and GFR, as indicated by a reduction in creatinine clearance. The protective effects of TMS against DOCA-salt-induced hypertension and the associated pathophysiological changes were most likely due to decreased CYP1B1 activity in cardiovascular tissues. CYP1B1 was found to be highly expressed and constitutively active in aorta, heart, and kidney of rats. DOCA-salt-induced hypertension did not increase CYP1B1 expression or its activity. However, administration of TMS decreased CYP1B1 activity but not its expression in aorta, heart, and kidney of both UNX and DOCA-salt-treated rats. Although TMS normalized SBP, inhibited cardiovascular hypertrophy, and prevented the increase in vascular reactivity to vasoconstrictor agents and endothelial dysfunction, it did not totally block CYP1B1 activity. Because the assay we used also measures CYP1A1 activity, the remaining activity could result from CYP1A1, which is inhibited by much higher concentrations of TMS (3). However, this scenario is unlikely because CYP1A1 protein expression was not detected in significant amounts in aorta, heart, and kidney of normal animals. Moreover, DOCA-salt treatment did not alter CYP1A1 expression (unpublished data). Since addition of exogenous TMS in the assay mixture further inhibited CYP1B1 activity in the aorta, heart, and kidney, it appears that in our study, the doses of TMS that normalized SBP in DOCA-salt induced hypertension were insufficient to totally abolish CYP1B1 activity in these tissues. The plasma half-life and metabolism of TMS is currently not known, and whether TMS produces its effects directly and/or through one or more of its metabolites remains to be determined. The activity of CYP1B1 was much higher in kidney than in aorta and heart, and TMS, although minimized, did not completely prevent the changes in renal parameters produced by DOCA-salt-induced hypertension (Table 4). Further studies are required to determine whether more frequent treatment and/or higher doses of TMS completely prevent CYP1B1 activity and the impairment of kidney function caused by DOCA-salt-induced hypertension. Also, we cannot exclude the possibility that the effect of TMS to reduce renal and cardiac as well as vascular hypertrophy and an associated increase in vascular reactivity is the result of a decrease in blood pressure. In preliminary experiments (Jennings BL and Malik KU, unpublished observations), however, it was found that exposure to TMS (10 μmol/l) for 4 h reduced the constriction of isolated aorta and mesenteric rings to PE, ET-1, and ANG II from normotensive rats, suggesting that TMS may reduce vascular reactivity by inhibiting CYP1B1 activity.
The mechanism by which CYP1B1 contributes to DOCA-salt-induced hypertension could result from increased levels of catecholamines, vasopressin, and/or ET-1, which are known to stimulate phospholipase A2 activity and release AA (3, 28, 47). The latter fatty acid has been reported to be metabolized in vitro by CYP1B1 supersomes into midchain and terminal HETEs, including 12- and 20-HETE (6), which stimulate VSMC growth and have been implicated in hypertension (32, 48). Moreover, plasma levels of these HETEs are increased in SHR and in humans with renovascular disease (26, 37). However, in our study TMS did not alter the plasma levels of 12- or 20-HETE. Recently, we reported that in rat VSMCs, TMS or adenovirus CYP1B1 short hairpin (sh)RNA did not alter AA metabolism into HETEs (51). Moreover, treatment with TMS did not affect the conversion of AA to 12- and 20-HETE in the femoral arteries of rats (unpublished data). However, further studies are required to determine the effect of TMS on tissue levels of these HETEs in DOCA-salt-treated rats before one can totally exclude their role in the action of TMS to normalize blood pressure and minimize the pathophysiological changes associated with DOCA-salt-induced hypertension. AA metabolism has also been shown to be associated with generation of ROS (38) and activation of NADPH oxidase (39, 53), and ROS have been implicated in various models of hypertension including DOCA- or aldosterone-salt-induced hypertension and/or associated endothelial dysfunction (2, 17, 21, 36, 40, 45). Our recent demonstration in VSMCs that ANG II-induced ROS production is mediated by release of AA and its metabolism by the constitutively active CYP1B1 (51) raised the possibility that ROS generated via CYP1B1 from AA and/or other fatty acids by vasoactive agents might contribute to pathophysiological changes and consequently to DOCA-salt-induced hypertension. Supporting this view was our finding that the increase in aortic ROS production, decrease in endothelium-dependent relaxation to ACh but not endothelium-independent relaxation to SNP, and increase in vascular reactivity in the aorta and mesenteric artery were prevented by TMS. Moreover, the activity of NADPH oxidase and expression of NOX-1 in aorta, heart, and kidney was inhibited by TMS in DOCA-salt-induced hypertension. The relationship between CYP1B1 and NADPH oxidase and other ROS-producing systems is not known. We have reported that CYP1B1 supersomes can generate ROS from AA in vitro (51). In preliminary experiments in VSMCs, we have found that ANG II-induced increase in NADPH oxidase activity depends on cytosolic phospholipase A2 and CYP1B1 activity (41). Whether ROS and/or other lipid peroxidation products formed by AA and/or other fatty acid metabolism by CYP1B1 result in activation of NADPH oxidase and further generation of ROS remains to be determined. It has been reported that ROS can amplify their own production by activating NADPH oxidase or xanthine oxidase, increasing intracellular uptake of iron, and/or uncoupling endothelial nitric oxide synthase (4).
The increase in ROS production by CYP1B1 could promote cardiac, vascular, and renal hypertrophy in DOCA-salt-induced hypertension through activation of one or more signaling molecules including ERK1/2 or p38 MAPK (5, 19, 22). Supporting this view was our finding that DOCA-salt-induced hypertension was associated with increased activity of ERK1/2 and p38 MAPK in the aorta, heart, and kidney that was inhibited by TMS administration. Recently, we reported that ANG II- and AA-induced increases in ERK1/2 and p38 MAPK activity and VSMC growth are inhibited by TMS or in cells transduced with adenovirus CYP1B1 shRNA (51). Whether the effect of TMS to minimize the increase in urine output, proteinuria, and K+ excretion and the decrease in urinary osmolality, Na+ excretion, and GFR are also due to a decrease in ROS production and/or ERK1/2 and p38 MAPK activities as a result of CYP1B1 activity and the underlying mechanism remains to be established. The kidneys from UNX as well as DOCA-salt-treated rats exhibited higher basal CYP1B1 and NADPH oxidase activities than other tissues. However, kidneys from DOCA-salt-treated animals showed increased NADPH oxidase, but not CYP1B1 activity, compared with kidneys from UNX animals. Moreover, administration of TMS to DOCA-salt-treated rats, which partially inhibited CYP1B1 activity, abolished the increase in NADPH oxidase activity without affecting its activity in the kidney of control UNX animals. Therefore, it appears that the TMS-insensitive component of pathophysiological changes in renal function associated with DOCA-salt hypertension are either caused by ROS generated through other mechanisms or independent of ROS through genomic or nongenomic actions of DOCA on mineralocorticoid receptors (13).
Our finding that TMS reversed DOCA-salt-induced hypertension without complete inhibition of CYP1B1 activity and associated changes in renal function suggests that its effect to normalize blood pressure is only in part dependent on changes in kidney function. Whether TMS also affects the levels of catecholamines, vasopressin, and/or ET-1 (9–12, 38), which have been implicated in DOCA-salt hypertension, remains to be determined. Aldosterone and/or DOCA are known to increase blood pressure by exerting direct actions in the central nervous system and by increasing the activity of the renin-angiotensin and sympathetic nervous systems and ROS production (14, 55). Moreover, a recent study has shown that T cells (Th 17 cells), via generation of superoxides, contribute to DOCA-salt hypertension (16). Therefore, it is possible that CYP1B1, expressed in brain (33) and lymphocytes (42), via ROS generation might also contribute to DOCA-salt-induced hypertension.
In conclusion, this is the first study to demonstrate that CYP1B1 contributes to DOCA-salt-induced hypertension and associated cardiovascular and renal hypertrophy, endothelial dysfunction, increased vascular reactivity, and impaired renal function, most likely due to increased NADPH oxidase activity and ROS production and activation of ERK1/2 and p38 MAPK. Moreover, CYP1B1 could serve as a novel target for the development of agents such as TMS for the treatment of hypertension caused by mineralocorticoid excess and salt.
GRANTS
This project was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant R01-HL19134-35 (to K. U. Malik). S. Sahan-Firat was supported by the Scientific and Technical Research Council of Turkey. B. L. Jennings was supported, in part, by a fellowship provided by the Neuroscience Institute at the University of Tennessee Health Science Center.
DISCLAIMER
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NHBLI.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Bernd Meibohm and Josiah T. Ryman for assistance with analysis of samples using HPLC and Dr. David L. Armbruster for editorial assistance.
REFERENCES
- 1.Bao W, Behm DJ, Nerurkar SS, Ao Z, Bentley R, Mirabile RC, Johns DG, Woods TN, Doe CPA, Coatney RW, Ohlstein JF, Douglas SA, Willette RN, Yue TL. Effects of p38 MAPK inhibitor on angiotensin II-dependent hypertension, organ damage, and superoxide anion production. J Cardiovasc Pharmacol 49: 362–368, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension 38: 1107–1111, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bonventre JV, Swidler M. Calcium dependency of prostaglandin E2 production in rat glomerular mesangial cells. Evidence that protein kinase C modulates the Ca2+-dependent activation of phospholipase A2. J Clin Invest 82: 168–176, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai H. NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ Res 96: 818–822, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Cheng TH, Shih NL, Chen CH, Lin H, Liu JC, Chao HH, Liou JY, Chen YL, Tsai HW, Chen YS, Chen CF, Chen JJ. Role of mitogen-activated protein kinase pathway in reactive oxygen species-mediated endothelin-1-induced β-myosin heavy chain gene expression and cardiomyocyte hypertrophy. J Biomed Sci 12: 123–133, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Metabolism of retinoids and arachidonic acid by human and mouse cytochrome P450 1B1. Drug Metab Dispos 32: 840–847, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Chun YJ, Kim S, Kim D, Lee SK, Guengerich FP. A new selective and potent inhibitor of human cytochrome P450 1B1 and its application to antimutagenesis. Cancer Res 61: 8164–8170, 2001 [PubMed] [Google Scholar]
- 8.Conway DE, Sakurai Y, Weiss D, Vega JD, Taylor WR, Jo H, Eskin SG, Marcus CB, McIntire LV. Expression of CYP1A1 and CYP1B1 in human endothelial cells: regulation by fluid shear stress. Cardiovasc Res 81: 669–677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crofton JT, Share L, Shade RE, Lee-Kwon WJ, Manning M, Sawyer WH. The importance of vasopressin in the development and maintenance of DOC-salt hypertension in the rat. Hypertension 1: 31–38, 1979 [DOI] [PubMed] [Google Scholar]
- 10.de Champlain J, Bouvier M, Drolet G. Abnormal regulation of the sympathoadrenal system in deoxycorticosterone acetate-salt hypertensive rats. Can J Physiol Pharmacol 65: 1605–1614, 1987 [DOI] [PubMed] [Google Scholar]
- 11.Drolet G, Bouvier M, de Champlain J. Enhanced sympathoadrenal reactivity to haemorrhagic stress in DOCA-salt hypertensive rats. J Hypertens 7: 237–242, 1989 [DOI] [PubMed] [Google Scholar]
- 12.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol 287: C895–C902, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Fuller PJ, Young MJ. Mechanisms of mineralocorticoid action. Hypertension 46: 1227–1235, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Gómez-Sánchez EP. Central hypertensive effects of aldosterone. Front Neuroendocrinol 18: 440–462, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Gorin Y, Ricono JM, Wagner B, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Angiotensin II-induced ERK1/ERK2 activation and protein synthesis are redox-dependent in glomerular mesangial cells. Biochem J 381: 231–239, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II-induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong HJ, Hsiao G, Cheng TH, Yen MH. Supplementation with tetrahydrobiopterin suppresses the development of hypertension in spontaneously hypertensive rats. Hypertension 38: 1044–1048 2001 [DOI] [PubMed] [Google Scholar]
- 18.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov 8: 794–805, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim NH, Rincon-Choles H, Bhandri B, Choudry GG, Abboud HE, Gorin Y. Redox dependence of glomerular epithelial cell hypertrophy in response to glucose. Am J Physiol Renal Physiol 290: F741–F751, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Korashy HM, El-Kadi AOS. The role of aryl hydrocarbon receptor in the pathogenesis of cardiovascular diseases. Drug Metab Rev 38: 411–450, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madamanchi NR, Moon SK, Hakim ZS, Clark S, Mehrizi A, Patterson C, Runge MS. Differential activation of mitogenic signaling pathways in aortic smooth muscle cells deficient in superoxide dismutase isoforms. Arterioscler Thromb Vasc Biol 25: 950–956, 2005 [DOI] [PubMed] [Google Scholar]
- 23.McGiff JC. Prostaglandins, prostacyclin, thromboxanes. Annu Rev Pharmacol Toxicol 21: 479–509, 1981 [DOI] [PubMed] [Google Scholar]
- 24.Medhora M, Chen Y, Gruenloh S, Harland D, Bodiga S, Zielonka J, Gebremedhin D, Gao Y, Falck JR, Anjaiah S, Jacobs ER. 20-HETE increases superoxide production and activates NAPDH oxidase in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 294: L902–L911, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller FJ, Jr, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res 82: 1298–1305, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Minuz P, Jiang H, Fava C, Turolo L, Tacconelli S, Ricci M, Patrignani P, Morganti A, Lechi A, McGiff JC. Altered release of cytochrome P450 metabolites of arachidonic acid in renovascular disease. Hypertension 51: 1379–1385, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res 41: 175–193, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Muthalif MM, Benter IF, Uddin MR, Malik KU. Calcium/calmodulin-dependent protein kinase IIα mediates activation of mitogen-activated protein kinase and cytosolic phospholipase A2 in norepinephrine-induced arachidonic acid release in rabbit aortic smooth muscle cells. J Biol Chem 271: 30149–30157, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Muthalif MM, Benter IF, Khandekar Z, Gaber L, Estes A, Malik S, Parmentier JH, Manne V, Malik KU. Contribution of Ras GTPase/MAP kinase and cytochrome P450 metabolites to deoxycorticosterone-salt-induced hypertension. Hypertension 35: 457–463, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Muthalif MM, Karzoun NA, Gaber L, Khandekar Z, Benter IF, Saeed AE, Parmentier JH, Estes A, Malik KU. Angiotensin II-induced hypertension. Contribution of Ras GTPase/mitogen-activated protein kinase and cytochrome P450 metabolites. Hypertension 36: 604–609, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Nasjletti A. Arthur C. Corcoran Memorial Lecture. The role of eicosanoids in angiotensin-dependent hypertension. Hypertension 31: 194–200, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Reddy MA, Thimmalapura PR, Lanting L, Nadler JL, Fatima S, Natarajan R. The oxidized lipid and lipoxygenase product 12(S)-hydroxyeicosatetraenoic acid induces hypertrophy and fibronectin transcription in vascular smooth muscle cells via p38 MAPK and cAMP response element-binding protein activation. Mediation of angiotensin II effects. J Biol Chem 277: 9920–9928, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Rieder CRM, Ramsden DB, Williams AC. Cytochrome P450 1B1 mRNA in the human central nervous system. Mol Pathol 51: 138–142, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roman RJ. P-450 Metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Sacerdoti D, Escalante B, Abraham NG, McGiff JC, Levere RD, Schwartzman ML. Treatment with tin prevents the development of hypertension in spontaneously hypertensive rats. Science 243: 388–390, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Sánchez M, Galisteo M, Vera R, Villar IC, Zarzuelo A, Tamargo J, Pérez-Vizcaíno F, Duarte J. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J Hypertens 24: 75–84, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Sasaki M, Hori MT, Hino T, Golub MS, Tuck ML. Elevated 12-lipoxygenase activity in the spontaneously hypertensive rat. Am J Hypertens 10: 371–378, 1997 [PubMed] [Google Scholar]
- 38.Schiffrin EL, Sventek P, Li JS, Turgeon A, Reudelhuber T. Antihypertensive effect of an endothelin receptor antagonist in DOCA-salt spontaneously hypertensive rats. Br J Pharmacol 115: 1377–1381, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith RL, Weidemann MJ. Reactive oxygen production associated with arachidonic acid metabolism by peritoneal macrophages. Biochem Biophys Res Commun 97: 973–980, 1980 [DOI] [PubMed] [Google Scholar]
- 40.Somers MJ, Mavromatis K, Galis ZS, Harrison DG. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation 101: 1722–1728, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Song CY, Ghafoor HUB, Yaghini FA, Fang XR, Malik KU. Angiotensin II-induced reactive oxygen radical production by NAD(P)H oxidase is mediated by arachidonic acid metabolism via cytochrome P4501B1 in vascular smooth muscle cells (Abstract). FASEB J 24: 962.6, 2010 [Google Scholar]
- 42.Spencer DL, Masten SA, Lanier KM, Yang X, Grassman JA, Miller CR, Sutter TR, Lucier GW, Walker NJ. Quantitative analysis of constitutive and 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced cytochrome P450 1B1 expression in human lymphocytes. Cancer Epidemiol Biomarkers Prev 8: 139–146, 1999 [PubMed] [Google Scholar]
- 43.Stern N, Nozawa K, Golub M, Eggena P, Knoll E, Tuck ML. The lipoxygenase inhibitor phenidone is a potent hypotensive agent in the spontaneously hypertensive rat. Am J Hypertens 6: 52–58, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Su P, Kaushal KM, Kroetz DL. Inhibition of renal arachidonic acid ω-hydroxylase activity with ABT reduces blood pressure in the SHR. Am J Physiol Regul Integr Comp Physiol 275: R426–R438, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am J Pathol 161: 1773–1781, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Touyz RM, Cruzado M, Tabet F, Yao G, Salomon S, Schiffrin EL. Redox-dependent MAP kinase signaling by Ang II in vascular smooth muscle cells: role of receptor tyrosine kinase transactivation. Can J Physiol Pharmacol 81: 159–167, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Trevisi L, Bova S, Cargnelli G, Ceolotto G, Luciani S. Endothelin-1-induced arachidonic acid release by cytosolic phospholipase A2 activation in rat vascular smooth muscle via extracellular signal-regulated kinases pathway. Biochem Pharmacol 64: 425–431, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Uddin MR, Muthalif MM, Karzoun NA, Benter IF, Malik KU. Cytochrome P-450 metabolites mediate norepinephrine-induced mitogenic signaling. Hypertension 31: 242–247, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Viedt C, Soto U, Krieger-Brauer HI, Fei J, Elsing C, Kübler W, Kreuzer J. Differential activation of mitogen-activated protein kinases in smooth muscle cells by angiotensin II. Involvement of p22phox and reactive oxygen species. Arterioscler Thromb Vasc Biol 20: 940–948, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Wang JS, Singh H, Zhang F, Ishizuka T, Deng H, Kemp R, Wolin MS, Hintze TH, Abraham NG, Nasjletti A, Laniado-Schwartzman M. Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ Res 98: 962–969, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Yaghini FA, Song CY, Lavrentyev EN, Ghafoor HUB, Fang XR, Estes AM, Campbell WB, Malik KU. Angiotensin II-induced vascular smooth muscle cell migration and growth are mediated by cytochrome P450 1B1 dependent superoxide generation. Hypertension 55: 1461–1467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshida K, Kobayashi N, Ohno T, Fukushima H, Matsuoka H. Cardioprotective effect of angiotensin II type 1 receptor antagonist associated with bradykinin-endothelial nitric oxide synthase and oxidative stress in Dahl salt-sensitive hypertensive rats. J Hypertens 25: 1633–1642, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Zafari AM, Ushio-Fukai Minieri CA, Akers M, Lasségue B, Griendling KK. Arachidonic acid metabolites mediate angiotensin II-induced NADH/NADPH oxidase activity and hypertrophy in vascular smooth muscle cells. Antioxid Redox Signal 1: 167–179, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Zhang F, Chen CL, Qian JQ, Yan JT, Cianflone K, Xiao X, Wang DW. Long-term modifications of blood pressure in normotensive and spontaneously hypertensive rats by gene delivery of rAAV-mediated cytochrome P450 arachidonic acid hydroxylase. Cell Res 15: 717–724, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol 294: H1067–H1074, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Zhao W, Parrish AR, Ramos KS. Constitutive and inducible expression of cytochrome P450IA1 and P450IB1 in human vascular endothelial and smooth muscle cells. In Vitro Cell Dev Biol Anim 34: 671–673, 1998 [DOI] [PubMed] [Google Scholar]