Abstract
After a myocardial infarction (MI), an episode of ischemia-reperfusion (I/R) can result in a greater impairment of left ventricular (LV) regional function (LVRF) than that caused by an initial I/R episode in the absence of MI. Membrane type-I matrix metalloproteinase (MT1-MMP) proteolytically processes the myocardial matrix and is upregulated in LV failure. This study tested the central hypothesis that a differential induction of MT1-MMP occurs and is related to LVRF after I/R in the context of a previous MI. Pigs with a previous MI [3 wk postligation of the left circumflex artery (LCx)] or no MI were randomized to undergo I/R [60-min/120-min left anterior descending coronary artery (LAD) occlusion] or no I/R as follows: no MI and no I/R (n = 6), no MI and I/R (n = 8), MI and no I/R (n = 8), and MI and I/R (n = 8). Baseline LVRF (regional stroke work, sonomicrometry) was lower in the LAD region in the MI group compared with no MI (103 ± 12 vs. 188 ± 26 mmHg·mm, P < 0.05) and remained lower with peak ischemia (35 ± 8 vs. 88 ± 17 mmHg·mm, P < 0.05). Using a novel interstitial microdialysis method, MT1-MMP was directly measured and was over threefold higher in the LCx region and over twofold higher in the LAD region in the MI group compared with the no MI group at baseline. MT1-MMP fluorogenic activity was persistently elevated in the LCx region in the MI and I/R group but remained unchanged in the LAD region. In contrast, no changes in MT1-MMP occurred in the LCx region in the no MI and I/R group but increased in the LAD region. MT1-MMP mRNA was increased by over threefold in the MI region in the MI and I/R group. In conclusion, these findings demonstrate that a heterogeneous response in MT1-MMP activity likely contributes to regional dysfunction with I/R and that a subsequent episode of I/R activates a proteolytic cascade within the MI region that may contribute to a continued adverse remodeling process.
Keywords: membrane type-I matrix metalloproteinase, matrix metalloproteinases
a myocardial ischemic event can be described as tissue hypoxia resulting from pathologically decreased blood flow triggering a cascade of deleterious events, resulting in cellular injury and death. Restoration of blood flow to the ischemic tissue, defined as reperfusion, carries with it the risk for additional myocardial and vascular injury (12). These events, collectively termed ischemia-reperfusion (I/R) injury, are a major source of morbidity and mortality (23). With severe I/R injury, significant myocyte cell death can occur, resulting in a myocardial infarction (MI), and a preexisting MI superimposed upon another I/R event results in an increased risk of major adverse outcomes, suggesting that the two events may be additive when sequential (20). Indeed, it has been estimated that ∼40% of those patients presenting with an acute coronary syndrome have a history of a previous MI (16, 20). However, the contributory mechanisms that make the myocardium more vulnerable to this second I/R episode remain poorly understood. It has also been established that changes occur in the myocardium remote from the site of initial MI, including the upregulation of bioactive molecules such as endothelin and PKC (17, 33). These same bioactive molecules have been shown to activate proteolytic pathways in the post-MI myocardium (25). Therefore, the central hypothesis of the present study was that the subsequent I/R event occurs in a myocardium where signaling pathways and proteases are primed to respond and is associated with an exacerbation of left ventricular (LV) regional dysfunction.
A major proteolytic pathway evoked by MI and I/R events is a family of proteases known as matrix metalloproteinases (MMPs) (8). Previous studies (5, 45) have shown that the induction of specific MMP types occurs in the myocardium after MI and I/R events. A recently identified class of MMPs is membrane type MMPs (MMPs). Membrane type MMPs are tethered to the cell membrane and serve a diverse range of proteolytic functions, including the degradation of local extracellular matrix (46) and activation of other MMPs (41). One of the best-characterized and prototypical membrane type MMPs is membrane type-I MMP (MT1-MMP), which has been reported to be increased in abundance and activity in the context of I/R (5). Moreover, studies (3, 6) have established that MT1-MMP is induced by upstream bioactive molecules such as PKC and endothelin. However, whether and to what degree MT1-MMP is induced within the remote region before I/R, and whether it would be increased in magnitude after the I/R event, remain to be demonstrated. Therefore, this study sought to measure MT1-MMP activity in a system amplified by the context of a second I/R event. Accordingly, the specific objectives of this project were threefold. First, we wanted to characterize the relationship between regional function and MT1-MMP activity after an initial MI. Second, we sought to use a porcine model of I/R in the context of a previous MI to examine regional and temporal changes in specific interstitial proteolytic pathways in vivo. A fundamental event after a MI is the activation of profibrotic signaling pathways, which includes the transforming growth factor (TGF)-β pathway (8, 25). In vitro evidence has emerged that MT1-MMP may play a role in the release of TGF and, ultimately, increased the transcription of fibrillar collagens (8, 15, 36). Accordingly, the third objective of this study was to more carefully examine the relationship between MT1-MMP induction and TGF-β signaling pathways and fibrillar collagen expression.
METHODS
Chronic instrumentation.
All animals were treated and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Revised 1996), and all protocols were approved by the Medical University of South Carolina's Institutional Animal Care and Use Committee. Yorkshire pigs (castrated males, weight: 35 kg, Hambone Farms, Orangeburg, SC) were randomized to one of four different groups: no MI and no I/R (−MI,−I/R group, n = 6), no MI and I/R (−MI,+I/R group, n = 8), MI and no I/R (+MI,−I/R group, n = 8), and MI and I/R (+MI,+I/R group, n = 8). Briefly, pigs assigned to receive a MI underwent sedation with intramuscular ketamine (22 mg/kg), placement of an intravenous cannula in an ear vein, and endotracheal intubation followed by the initiation of mechanical ventilation. A stable plane of anesthesia was then maintained with isoflurane (1%, 3 l/min O2, Baxter Healthcare), and a maintenance infusion of lactated Ringer solution (10 ml/kg) was begun. A sterile left thoracotomy was then performed, and a small pericardiotomy was made, exposing the left circumflex coronary artery (LCx). Ligation of obtuse marginal artery 1 and/or 2 was performed using a 4−0 silk suture. Creation of the MI was confirmed by ECG changes and the visualization of tissue ischemic changes. This method has been demonstrated to create an infarct involving 22% of the LV mass in previously published reports from this laboratory (21, 49). The pericardium was then irrigated and approximated, the thoracotomy was closed, and the pleural space was evacuated of air. Animals receiving a MI were then allowed to recover for 21 days.
Acute instrumentation.
Animals with prior MI and those without prior MI underwent identical instrumentation to assess LV regional function and to measure interstitial MMP activity. After animals had been sedated with diazepam (200 mg po), anesthesia was induced with sufentanyl (2 μg/kg iv, Baxter Healthcare) and etomidate (0.3 mg/kg iv, Bedford Laboratories). After endotracheal intubation, mechanical ventilation was initiated, and a stable anesthetic plane was achieved with morphine sulfate (3 mg·kg−1·h−1 iv, Elkins-Sinn) and isoflurane (1%, 3 l/min O2, Baxter Healthcare). Maintenance intravenous fluids (150 ml/h, lactated Ringer solution) and lidocaine HCl (1 mg/h iv, Elkins-Sinn) were administered throughout the protocol.
An 8-Fr introducer with a side arm was placed in the right carotid artery for blood pressure measurements and subsequent placement of the LV pressure/infusion catheter. A previously calibrated microtipped transducer catheter with a side port for infusion and a pigtail (7.5-Fr, Millar Instruments, Houston, TX) was advanced through the hemostatic sheath and positioned in the LV. A venous line (7-Fr) was placed in the right external jugular vein for fluid administration. After an intravenous bolus of magnesium chloride (1 g, Luitpold Pharmaceuticals) had been delivered, a median sternotomy was performed, the pericardial sac was opened, and any adhesions were cleared in the case of prior MI induction. A pair of piezoelectric crystals (2 mm, Sonometrics) was positioned against the LV anterior free wall endocardial surface to measure segmental wall motion (18). Specifically, the region in which the crystals were placed was between left anterior descending coronary artery (LAD) diagonals 2 and 3, termed the “LAD region” for the purposes of the study, as shown in Fig. 1. These sonomicrometry crystals were specifically placed in a viable myocardial region that would be eventually targeted to undergo I/R. Pressure waveforms and crystal signals were digitized on a computer for subsequent analysis at a sampling frequency of 1 kHz (PONEMAH, Data Sciences, St. Paul, MN). Microdialysis probes (see Microdialysis for details) were inserted in the LAD region and in the region of the LCx, which was the MI region for animals randomized to groups with previous MI. After instrumentation and a 30-min equilibration period, baseline measurements were recorded. These measurements included heart rate, aortic pressure, LV end-diastolic and peak pressure, and regional segmental shortening.
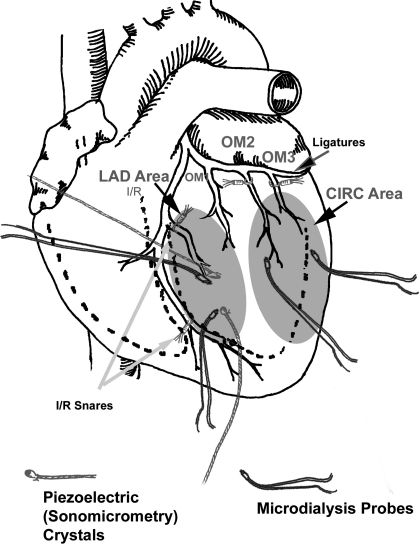
Fig. 1.
Surgical schematic drawing indicating the left circumflex coronary artery (CIRC) area, where the initial myocardial infarction (MI) was created in pigs assigned to +MI groups, as well the left anterior descending coronary artery (LAD) area remote from the initial MI site, where ischemia-reperfusion (I/R) was induced in pigs assigned to +I/R groups. Also shown are the sites where piezoelectric crystals were placed in the LAD (targeted for I/R) area as well as microdialysis probes, which were placed in both the CIRC and LAD areas.
Microdialysis.
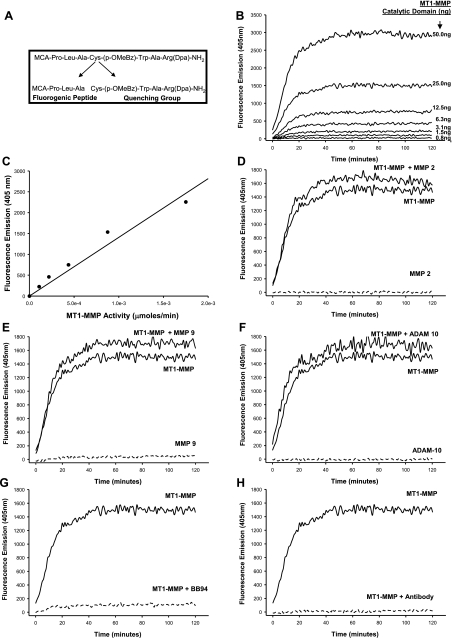
A sterile microdialysis probe containing a 4-mm-long membrane (20 kDa, CMA/Microdialysis, North Chelmsford, MA) was placed into the LV midmyocardium in two regions: the main region served by the first and second obtuse marginal branches of the LCx (LCx region) and the region served by the diagonal branches of the LAD (LAD region). These microdialysis probes have been successfully placed in the LV of animals and patients previously to measure local bioactive signaling and MMP activity (5, 6, 22, 38). Moreover, it has been demonstrated that this surgical approach for placement of the microdialysis probe was not associated with alterations in blood flow or extravasation of red blood cells, did not change local bioactive peptide levels, and was not associated with an acute inflammatory response (5, 6). Both the inflow and outflow ports of the microdialysis probe were connected to sterile tubing (PEEK, 0.12-mm inner diameter) and then connected to a computer-controlled microinfusion syringe pump (5 μl/min, Bioanalytical Systems, West Layfayette, IN). The infusion solution used in the microdialysis system contained a MT1-MMP-specific fluorogenic substrate (60 μM, catalog no. 444258, Calbiochem) that would only yield a detectable change in ultraviolet emission when proteolytically processed at a specific amino acid sequence, as shown in Fig. 2A (22, 23). MT1-MMP specific proteolysis of the substrate was determined through a series of in vitro experiments. The substrate [60 μM, enzymatic efficiency (kcat/Km) = 7.3 × 105 M−1·s−1 for MT1-MMP] was incubated with increasing levels of the recombinant catalytic domain of MT1-MMP (0.8–50.0 ng, specific activity: hydrolysis of ≥140 μmol substrate/min at 37°C, catalog no. 475935, Calbiochem). The in vitro substrate-MT1-MMP catalytic domain reaction was allowed to proceed at 37°C for 2 h, and fluorescence of the cleaved substrate was measured (FLUOstar, BMG Laboratories) at an emission/excitation wavelength of 405/330 nm. A robust increase in fluorescence, indicating specific cleavage of the MT1-MMP substrate, occurred in the presence of the MT1-MMP catalytic domain (Fig. 2B). The fluorescent signal was calibrated in terms of MT1-MMP activity (μmol/min) using the linear portion of the fluorescence emission-concentration curve (y = 6.95 × 10−7x, r2 = 0.991, P < 0.001), shown in Fig. 2C. To more carefully evaluate the specificity of the substrate to MT1-MMP-mediated proteolysis, the substrate was incubated with different recombinant proteases in the presence and absence of the MT1-MMP recombinant domain (25 ng; Fig. 2, D–F). When the substrate was incubated alone with the catalytic domain of MMP-2 (125 ng/ml, catalog no. SE-237, Enzo Life Sciences, Plymouth Meeting, PA; Fig. 2D), no detectable signal was obtained, but the signal was fully restored with the addition of the MT1-MMP recombinant catalytic domain. When a similar experiment was performed for MMP-9 (125 ng/ml, catalog no. SE-244, Enzo Life Sciences; Fig. 2E), a minimal signal was detected alone, but a slight additive signal was observed when the substrate was incubated with both catalytic domains for MMP-9 and MT1-MMP. These findings suggest that MMP-9 may have a small (∼10%) contribution to the overall MT1-MMP proteolytic-mediated fluorogenic signal. In the presence of A disintegrin and a metalloprotease (ADAM)-10, there was no significant fluorescent substrate cleavage with ADAM-10 (Fig. 2F). Coincubation of the substrate and the MT1-MMP catalytic domain in the presence of the broad-spectrum MMP inhibitor BB-94 (6.25 nM, British Biotech) or a custom-designed antibody against MT1-MMP (against peptide 90–120 of MT1-MMP, Open Biosystems, Huntsville, AL) resulted in a complete suppression of the fluorescent signal, as shown in Fig. 2, G and H, respectively.
Fig. 2.
A: structure of the membrane type-I matrix metalloproteinase (MT1-MMP) fluorogenic substrate with arrows indicating the site of specific MMP proteolysis. A series of in-vitro experiments was performed to validate and confirm the specificity of the fluorogenic MT1-MMP substrate used in the microdialysis protocol. B: the substrate was incubated with increasing levels of the recombinant catalytic domain of MT1-MMP, and the reaction was allowed to proceed at 37°C for 2 h. A robust increase in fluorescence, indicating specific cleavage of the MT1-MMP substrate, occurred in the presence of the MT1-MMP catalytic domain. C: fluorescence measurements were plotted against MT1-MMP activity (in μmol/min) of the catalytic domain standards to express fluorescence in terms of MT1-MMP activity. D–F: when the substrate was incubated with the catalytic domain of MMP-2 (D), MMP-9 (E), or A disintegrin and a metalloprotease (ADAM)-10 (F), no fluorescence was detected. However, when the recombinant MT1-MMP catalytic domain was introduced into these reactions, a fluorescent signal was evident. G and H: coincubation of the substrate and the MT1-MMP catalytic domain in the presence of the broad-spectrum MMP inhibitor BB-94 (G) or an antibody against MT1-MMP (H) resulted in the suppression of all fluorescent activity.
This laboratory has previously established that the interstitial space surrounding the microdialysis probe is a closed compartment system, resulting in this quenched MMP fluorogenic substrate equilibrating within with the interstitial space, and that, therefore, the microdialysate fluorescence will reflect MT1-MMP activity in vivo (24, 40). Steady-state interstitial fluorescence of the MT1-MMP substrate was achieved in ∼10 min, but equilibration of this system was performed for 30 min before any measurements. A series of critical validation procedures was performed for the combined use of this MT1-MMP substrate and the microdialysis system. First, an in vitro microdialysis model was used (5, 22), where a recombinant active MT1-MMP construct (MT1-MMP catalytic domain, 7.8–125.0 ng/ml, catalog no. 475935, Calbiochem) was present in the surrounding fluid compartment. The entire microdialysis system was protected from ambient light, and the dialysate was collected into chilled amber tubes and immediately processed for fluorimetry (280/360 nm). The MT1-MMP fluorescent signal was converted to units of enzyme activity using the approach described above.
I/R protocol.
After 30 min of equilibration, pigs randomized into groups undergoing I/R had snares placed around the diagonal arteries serving the LAD region. Regional ischemia was then induced by tightening the snares. Because this region of the LV does not support a major myocardial conduction pathway, snare tightening did not result in atrioventricular block or refractory arrhythmogenesis. A previous study (44) has demonstrated that this site of coronary occlusion in pigs creates an area at risk of 38% of the LV mass. Microdialysis samples and hemodynamic readings were taken at baseline and every 30 min during ischemia (60 min of total ischemia time) and reperfusion (120 min total). Pigs randomized to groups not undergoing I/R received identical microdialysis sampling and hemodynamic recordings. All microdialysis samples were kept on ice until the protocol was complete. On completion, 100 μl of each dialysate sample were added to 96-well polystyrene plates (Nalge Nunc), and fluorescence was measured (FLUOstar, BMG Labtechnologies). At the conclusion of the protocol, isoflurane anesthesia was increased to 5%, and with a full surgical plane of anesthesia maintained, the heart was harvested. The LV was dissected and placed in ice-cold lactated Ringer solution. The base of the LV, which encompassed the LCx region targeted for MI, was sliced into a ring and frozen. LV rings from pigs with MI were subsequently processed for triphenyltetrazolium chloride staining to determine MI size, as previously described (21, 48).
Real-time PCR analysis.
The MI region is a metabolically active site with respect to fibrosis, and the TGF-β signaling pathway is likely key factor in this process (25). TGF-β is held inactive in the interstitial space by latency TGF-β-binding protein (LTBP)-1 (15). MT1-MMP has been shown to cleave LTBP-1 in vitro (36), leading to TGF-β release and the induction of profibrotic molecules such as collagens I and III. Accordingly, the relationship between MT1-MMP, LTBP-1, TGF-β receptor I (TGFR-I), procollagen type I, and procollagen type III mRNA levels were quantified. LV myocardial homogenates were subjected to RNA extraction (RNeasy Fibrous Tissue Mini Kit, Qiagen, Valencia, CA), and the quantity and quality of the RNA were determined (Experion Automated Electrophoresis System, Bio-Rad Laboratories, Hercules, CA). RNA (1 μg) was reverse transcribed to generate cDNA (iScript cDNA Synthesis Kit, Bio-Rad). cDNA was amplified with gene-specific primer/probe sets (TaqMan Universal PCR Master Mix, catalog no. 4364321, Applied Biosystems, Foster City, CA) using single-color real-time PCR (MyiQ, Bio-Rad). The specific TaqMan primer/probe sets (Applied Biosystems) were as follows: MT1-MMP (catalog no. Ss03394431_m1), collagen type I (catalog no. Ss03373340_m1), collagen type III (catalog no. Ss03375691_g1), TGFR-I (Integrated DNA Technology, custom sequence: forward 5′-AGGCCGTACTGGCTCTTTACAACA-3′, reverse 5′-AGCCAGAGGCGGACTACTACGCCAA-3′, and probe 5′-/56-FAM-TTGGTTGCCGCTTTCCACCATTAG-3BHQ-1/-3′), LTBP-1 (Integrated DNA Technology, custom sequence: forward 5′-TGGGTTATACGGTTTCTGGC-3′, reverse 5′-GGTTGAGTGTTCTTTGGCTTG-3′, and probe 5′/56-FAM-TGATGGATTGGCCTGGGTCTATGAAC-3IABkFQ-3′), and 18S rRNA (eukaryotic 18S rRNA, catalog no. Hs99999901_s1, Applied Biosystems). Negative controls were run to verify the absence of genomic DNA contamination (reverse transcription control) and the absence of overall DNA contamination in the PCR system and working environment (template control). The real-time PCR fluorescence signal was converted to cycle times (Ct) and normalized to the 18S signal.
Zymography.
Relative MMP-2 and MMP-9 levels were determined by gelatin zymography as previously described (5, 21). Purified human MMP-2 and MMP-9 (catalog no. CC073, Chemicon) were included on each gel to provide internal standards. The zymograms were digitized, and levels of all analytes were quantitated (Gel Pro Analyzer, Media Cybernetics) by two-dimensional integrated optical density.
Computations and data analysis.
LV regional function (LVRF) was assessed using stroke work and segmental shortening calculations based on piezoelectric crystal data. Stroke work was calculated using the area of the LV pressure-segment length loop, as previously described (19, 48). Segmental shortening was computed as the percent difference between the segment lengths measured at LV end diastole and end systole. Baseline LVRF, hemodynamic data, and MT1-MMP fluorogenic activity were initially compared between the non-MI and previous MI groups using two-sample t-tests. LVRF and hemodynamic data at subsequent time points were compared with baseline values by ANOVA. Where significant changes were found by ANOVA, a Bonferroni t-test was used for specific comparisons between groups. MI sizes were calculated as a percentage of the LV ring perimeter and were compared between MI groups using a two-sample t-test. In addition to comparing the absolute MT1-MMP enzyme activity, the absolute change from baseline was computed for each value as well as the percent change from the relative baseline value. For the mRNA and MMP measurements, the individual mRNA levels were normalized to the referent control (−MI,−I/R group) values, and comparisons were then performed using a t-test with a null hypothesis set to 100. Relative mRNA values across treatment groups and regions were compared by an adjusted t-test (module prcomp, STATA). Statistical analyses were performed using STATA statistical software (STATA Intercooled version 8.0). Results are presented as means ± SE. Values of P < 0.05 were considered to be statistically significant.
RESULTS
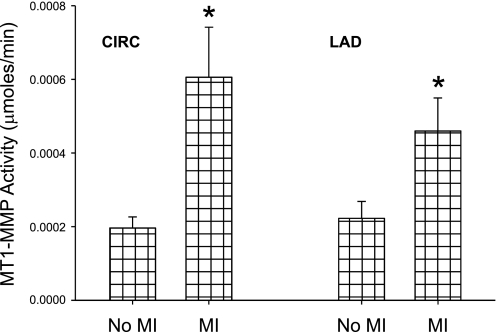
Hemodynamic data at baseline for non-MI and previous MI pigs is shown in Table 1. Heart rate, LV end-diastolic pressure, and mean arterial pressure were elevated in animals with a previous MI compared with those without previous MI at baseline. The elevated heart rate and mean arterial pressure were likely due to increased neurohormonal stimulation, specifically from efferent activation (28). The mean pulse pressure, which is proportional to forward stroke volume, was not different between pigs with previous MI and those without previous MI at baseline. Furthermore, no animal with previous MI showed any clinical signs of overt heart failure, such as edema or cyanosis. Therefore, this preparation did not appear to evoke an acute hemodynamic compromise. The mean MI size in absolute terms was 18.8 ± 1.3% and was not different between the groups (P = 0.48). This is ∼3% smaller than in previous studies (21, 49) and likely due to MI scar contracture as well as hypertrophy of the viable myocardium (25). LVRF, as measured by regional stroke work, was decreased at baseline in animals with previous MI, as shown in Fig. 3. MT1-MMP activity was over threefold higher in the MI group compared with the non-MI group at baseline in the LCx (initial MI) region and twofold higher in the LAD (targeted for I/R) region, as shown in Fig. 4. Therefore, interstitial MT1-MMP activation was increased within the infarct and viable myocardial regions after an initial MI.
Table 1.
Baseline hemodynamics and LV regional function in pigs without previous MI versus pigs with previous MI
| No MI | MI | |
|---|---|---|
| Heart Rate, beats/min | 90 ± 4 | 100 ± 4* |
| LV Pressures, mmHg | ||
| Peak | 111 ± 2 | 118 ± 3 |
| End diastole | 13 ± 1 | 19 ± 1* |
| Aortic Pressures, mmHg | ||
| Mean | 83 ± 2 | 90 ± 2* |
| Pulse | 36 ± 3 | 32 ± 3 |
Values are means ± SE; sample size was n = 14 animals in the no myocardial infarction (MI) group and 16 animals in the MI group. LV, left ventricular.
P < 0.05.
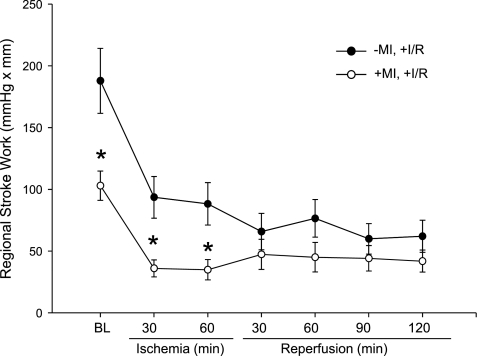
Fig. 3.
Regional stroke work in pigs without and with previous MI undergoing I/R. Stroke work was decreased at baseline (BL) in animals with previous MI and remained decreased through peak ischemia compared with animals without previous MI. Values are means ± SE; sample size was n = 8 in the −MI,+I/R group and +MI,+I/R group. *P < 0.05 vs. the respective −MI,+I/R group value.
Fig. 4.
MT1-MMP fluorogenic activity at baseline. In both the CIRC (initial MI) and LAD (targeted for I/R) regions, baseline MT1-MMP activity was elevated in the animals with previous MI compared with the animals without previous MI. Values are means ± SE; sample size was n = 14 in the no MI group and n = 16 in the MI group. *P < 0.05 vs. the no MI group.
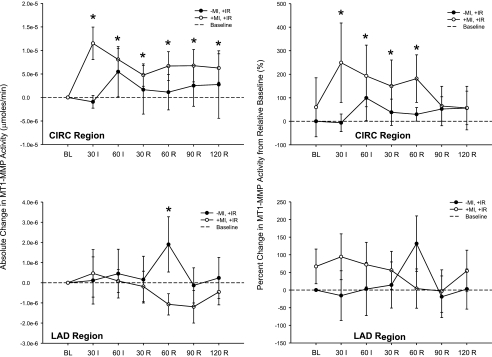
Hemodynamic parameters remained unchanged over the course of the protocol for animals assigned to groups that did not undergo I/R. For groups that underwent I/R, LVRF remained lower in previous MI pigs at peak ischemia compared with non-MI pigs, as shown in Fig. 3. MT1-MMP activity (Fig. 5) was significantly higher in the MI group compared with the non-MI group over the entire I/R interval within the LCx region (irrespective of time, F value: 4.02, P = 0.047). With respect to absolute changes in MT1-MMP activity across time, MT1-MMP activity was significantly higher in the MI group throughout the ischemia and reperfusion interval. In the LAD region, however, MT1-MMP activity was increased at reperfusion in the I/R group only. If these data were considered as percent changes from relative baseline values, then a similar trend occurred. Specifically, MT1-MMP fluorogenic activity was significantly increased in the LCx region during the ischemic episode and the early part of the reperfusion period in the previous MI group.
Fig. 5.
Continuous MT1-MMP activity computed as a function of baseline in the CIRC (targeted for MI) region (top) and LAD (targeted for I/R) region (bottom) in pigs with and without previous MI undergoing I/R. MT1-MMP activity was elevated in the CIRC (MI) region in the +MI,+I/R group but unchanged in the LAD (I/R) region. In contrast, no changes in MT1-MMP activity occurred in the CIRC region in the −MI,+I/R group, but activity increased in the LAD (I/R) region at 60 min of reperfusion. Values are means ± SE; sample size was n = 8 in the −MI,+I/R group and +MI,+I/R group. *P < 0.05 vs. the respective baseline.
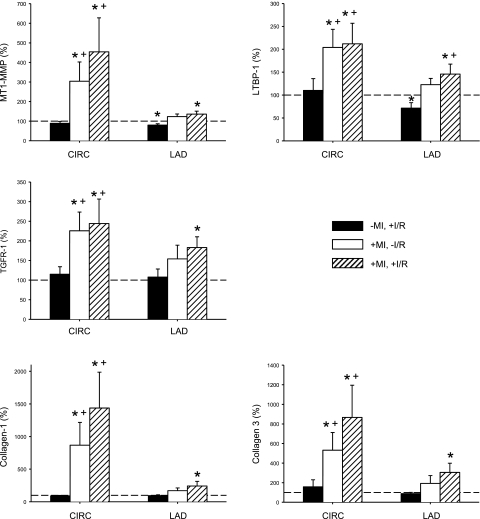
Myocardial mRNA levels in the LCx and LAD are shown in Fig. 6. MT1-MMP mRNA levels were increased by over threefold in the LCx region in both previous MI groups. This was in contrast to the LAD region, where MT1-MMP mRNA levels were only increased in the +MI,+I/R group. Relative mRNA levels for LTBP-1 and TGFR-I were significantly higher within the LCx region in both MI groups and were significantly increased within the LAD region in the +MI,+I/R group. Relative mRNA levels for procollagen I and III were increased within the LAD region in both groups with a preexisting MI and were higher in the LAD region in the +MI,+I/R group.
Fig. 6.
Relative mRNA values for MT1-MMP, latency transforming growth factor (TGF)-β-binding protein (LTBP)-1, TGF-β receptor 1 (TGFR-I), collagen type I, and collagen type III at the completion of the I/R protocol were determined by real-time PCR. MT1-MMP mRNA levels were increased by over threefold in the CIRC region in both MI groups but were only increased in the LAD region in the +MI,+I/R group. LTBP-1 and TGFR-I mRNA levels followed a similar pattern, where levels were increased in the LAD region only in the group with a previous MI (+MI,+I/R group). This pattern was seen again in the collagen type I and III mRNA levels. This indicates that the LAD region in the groups that sustained a previous MI was primed to respond to a subsequent I/R episode through transcriptional upregualtion. The dashed line indicates the referent control (−MI,−I/R group), which was set to 100%. *P < 0.05 vs. the referent control (100%); +P < 0.05 vs. the −MI,+I/R group.
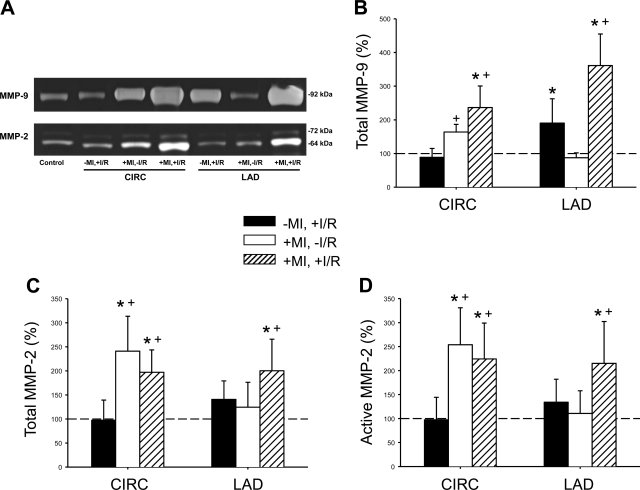
To more carefully examine the relative degree of induction of the gelatinase class of MMPs, quantitative zymography was performed. Representative zymograms for MMP-9 and size-fractionated MMP-2, indicative of the latent (72 kDa) and active (64 kDa) forms, are shown in Fig. 7A. Clear lytic bands, corresponding to MMP-9 and MMP-2, aligning with recombinant MMP-9/MMP-2 standards were present in all samples. Within the MI region but without a second I/R episode (+MI,−I/R group), relative MMP-9 levels were increased from referent control values (Fig. 7B) and increased to an equivalent degree in both the LCx and LAD regions after a second I/R episode (+MI,+I/R group). In the I/R-only group (−MI,+I/R group), MMP-9 levels increased in the targeted I/R region, consistent with an acute reperfusion injury response. With respect to total MMP-2 levels (summation of latent and active forms), MMP-2 levels were increased within the established MI region, with no changes in total levels after I/R (Fig. 7C). Relative MMP-2 levels increased in the targeted I/R region in the +MI,+I/R group. The active form of MMP-2 demonstrated a similar trend, where active MMP-2 increased by over twofold in the +MI,+I/R group (Fig. 7D).
Fig. 7.
A: representative zymograms showing lytic bands corresponding to MMP-9 (88 kDa) and the latent (72 kDa) and active (64 kDa) forms of MMP-2. MMP-9 levels were higher in the CIRC region in both MI groups. In the LAD region, MMP-9 levels (B) were increased in both I/R groups, regardless of the presence of a previous MI. Total MMP-2 levels (C; summation of latent and active forms) and active MMP-2 (64-kDa band; D) were higher within the CIRC region of both groups with previous MI compared with both groups without MI. The dashed line indicates the referent control (−MI,−I/R group), which was set to 100%. *P < 0.05 vs. the referent control (100%); +P < 0.05 vs. the −MI,+I/R group.
DISCUSSION
A MI commonly results from a period of prolonged ischemia followed by reperfusion. A second I/R episode in the context of a previous MI can result in deleterious clinical consequences (20). Specifically, in a clinical observational study (20), increased LV dilation and dysfunction occurred after a second I/R episode. The underlying contributory pathways by which the LV myocardium is increased in susceptibility to a second I/R event are yet to be established. Previous clinical and large animal studies (5, 6, 26) have demonstrated metabolic abnormalities in the myocardial region remote from the initial MI as well as abnormalities in the extracellular matrix. In particular, the extracellular family of proteases, MMPs, have been shown to be altered within the viable remote myocardium as well as in the MI region itself after an established MI (1, 5, 6, 45). Specifically, the novel transmembrane protein MT1-MMP, which is a pleiotropic molecule with diverse functions (10, 47), is increased with an acute I/R event (5). However, to what degree MT1-MMP is influenced by I/R in the context of an established MI is yet to be determined. Accordingly, the present study used a clinically relevant large animal model of MI and I/R with regional function measurements and interstitial MT1-MMP activity interrogation via microdialysis techniques to test the hypothesis that there is a persistent induction of MT1-MMP post-MI that is amplified by a second I/R event. The novel findings of the present study are threefold. First, there was a significant increase in interstitial MT1-MMP in the MI and remote region post-MI, which was associated with regional dysfunction. Second, a robust increase in MT1-MMP occurred in the previous MI region after a second event of I/R. Third, increased expression of components of the profibrotic cascade, TGF-β as well as fibrillar collagen, were selectively increased with a preexisting MI and a second I/R event. These findings demonstrate that a heterogeneous response in MT1-MMP activity potentially contributes to regional dysfunction with I/R and that a subsequent episode of I/R activates a proteolytic cascade within the MI region and a profibrotic response within the I/R region that can promulgate a more pronounced and adverse myocardial remodeling process.
Previous studies (21, 48) using a global MMP pharmacological inhibitor have demonstrated a cause-effect relationship between MMPs and adverse LV remodeling post-MI. However, the specific MMPs that are upregulated and causative in this particular setting are as yet unidentified. Several studies have described an associative link between the induction of the transmembrane protein MT1-MMP and the context of myocardial ischemia. Wilson et al. (45) demonstrated region- and type-specific induction of MMPs in a sheep model of post-MI LV remodeling and reported a >300% increase in MT1-MMP in the MI region. In addition, Deschamps et al. (5) demonstrated a >50% increase in MT1-MMP abundance after I/R in a pig model. Overexpression of MT1-MMP in mice has been demonstrated to cause LV remodeling, myocardial fibrosis, and reduced survival post-MI (36). Furthermore, a robustly increased abundance of MT1-MMP has been reported in clinical entities such as dilated cardiomyopathy (35). Of great import, however, is that these previous studies analyzed MT1-MMP levels in tissue homogenates using in vitro assay systems. Given that MT1-MMP is membrane bound and trafficked to the membrane, in vitro measurements of MT1-MMP levels do not necessarily imply biological activity. The present study analyzed interstitial MT1-MMP biological activity in vivo through a validated microdialysis approach, thereby directly measuring interstitial MT1-MMP activity in the I/R and post-MI context.
Using in vitro assays, it has been established that MT1-MMP is involved in a number of biological signaling and protein modification pathways (14, 25, 27, 41, 46). With regard to biological signaling, MT1-MMP performs a complex array of regulatory processes, including the degradation of several extracellular matrix components as well as participating in the activation of biological molecules (7, 14, 25, 36, 41–43). Specifically, MT1-MMP has been shown to convert membrane-bound TNF-α to its soluble form, therefore creating a ligand for cognate TNF-α receptors (7). As discussed below, previous studies (36, 42) have demonstrated TGF-β is indirectly proteolytically processed by MT1-MMP.
MT1-MMP plays an important role in the posttranslational modification of other MMP types. In particular, it has been established that the primary mechanism by which pro-MMP-2, a ubiquitous protein within the myocardium, is activated involves MT1-MMP (7). Pro-MMP-2 is activated by the collaborative efforts of MT1-MMP and tissue inhibitor of metalloprotein (TIMP)-2, initiating a cascade of cell surface protease activity (27, 42). Therefore, MT1-MMP activity can lead to diverse events dependent on the substrates available, with a heterogeneity of responses. This is relevant to the present study for two reasons. First, MT1-MMP activity was increased in both the myocardium remote from the previous MI and within the MI region at baseline. Yet this increased MT1-MMP activity likely resulted in very different outcomes, given the differing substrate portfolios of each region. Concurrently, increased MT1-MMP activity in the MI region itself is suggestive of collagen turnover and potential scar instability. Second, the baseline elevation of MT1-MMP activity in the remote region (which was targeted for I/R) indicates that this region had been primed by the previous MI. This heterogeneity was underscored by the relative levels in the active form of MMP-2. Since the predominant pathway for proteolytic processing of latent MMP-2 to the 64-kDa active form is by MT1-MMP (14, 27), changes in the relative abundance of active MMP-2 would potentially serve as an index for MT1-MMP substrate processing. In the present study, increased latent MMP-2, a putative substrate for MT1-MMP, was present within the MI region and was associated with higher levels of the proteolytically processes (active MMP-2). The present study demonstrated, through the use of a fluorogenic peptide, that increased MT1-MMP proteolytic activity occurred within this MI region and was associated with fourfold higher mRNA levels. Taken together, these findings support the concept that persistent MT1-MMP induction and activity occurs within the MI region, which may then play a relevant biological role with a subsequent episode of I/R.
In regard to the cellular source of the MT1-MMP, previous studies by this laboratory have performed immunohistochemical analyses of MT1-MMP, localizing MT1-MMP to both the sarcolemmal surface of myoctes and demonstrating a strong colocalization of MT1-MMP and α-smooth muscle actin, suggesting expression by myocardial myofibroblasts. Specifically, the MI region is known to contain myofibroblasts (2), which have been reported to produce increased levels of MT1-MMP in cardiac disease states (39). Therefore, in the viable myocardium remote from the initial MI, myocytes were likely the source of MT1-MMP, whereas within the MI region itself, which is robustly populated by fibroblasts/myofibroblasts, is a potential source of MT1-MMP (36, 39). However, it cannot be discounted that other cell types, such as macrophages, could have also contributed to the MT1-MMP signal post-MI, and future histochemical localization studies will be necessary to directly address this issue. Subsequently, in the present study, a second I/R event in the remote region resulted in further increased MT1-MMP activity in the MI region. This is potentially due to the cellular phenotype transformation that has taken place within the MI region. This, in turn, could exacerbate scar instability in the context of a second I/R event. This indicates that the MI itself is a biologically active region susceptible to potentially deleterious structural and functional changes associated with subsequent I/R episodes.
While six different membrane-type MMPs have been described to date, MT1-MMP is the prototypical and most examined member of this subfamily since its discovery over a decade ago (34). One aspect that makes MT1-MMP unique among the MMP family is that it is activated intracellularly through a furin-dependent pathway and has full proteolytic capabilities upon membrane insertion (47). The membrane-anchored position of MT1-MMP putatively allows more localized and efficient collagen degradation at the cell surface compared with other secreted MMPs (11). Therefore, the spatial orientation of MT1-MMP in the membrane is sensitive to disruption by tissue homogenization for in vitro assays. This point underscores the rationale of the present study, which used a microdialysis system to measure intact MT1-MMP activity. Direct measurements of interstitial MT1-MMP activity demonstrated significantly increased proteolysis under steady-state conditions in both the myocardium remote from the previous MI (i.e., the region targeted for a second I/R event) and within the previous MI itself with respect to referent normal controls. The present study demonstrated elevated MT1-MMP mRNA levels within the MI region, and, therefore, a likely mechanism for the heightened MT1-MMP activity that occurred in the context of a previous MI was due to increased transcription. Bioactive molecules, oxidative stress, and mechanical signals, all which are operative in the post-MI context, are likely contributory factors for inducing MT1-MMP (3, 6, 29, 30). Specifically, MT1-MMP activity/abundance is increased by exposure to TNF-α, IL-6, and the endothelin/PKC pathway (3, 6, 29). Upregulation of TNF-α, IL-6, and the endothelin/PKC pathway has been reported after MI (25). In addition, increased mechanical strain can induce MT1-MMP promoter activity (30). Given the heterogeneity of the post-MI myocardium, it is highly likely that mechanical stress/strain patterns are consequently altered in this context, potentially inducing MT1-MMP. In the present study, MT1-MMP mRNA levels increased to the greatest degree within the I/R region when superimposed upon a previous MI. These unique findings would suggest that a “priming effect” had occurred within the remote viable myocardium after a previous MI, which resulted in a more robust induction of MT1-MMP transcription with a subsequent I/R event.
In light of the fact that fibrosis is a structural milestone after MI, and evidence has suggested a potential intersection between MT1-MMP and the profibrotic TGF-β signaling pathway, specific determinants of the TGF-β pathway and collagen expression were examined after MI and a second I/R episode. TGF-β is synthesized as an inactive precursor bound to LTBP-1 through disulfide bonds and a cysteine-rich motif found within LTBP-1 (15). Proteolytic processing of LTBP-1 and the subsequent full activation and release of TGF into the interstitium is a critical step in this profibrotic signaling pathway. Through in vitro and ex vivo approaches, evidence for a mechanistic link between MT1-MMP proteolytic processing of LTBP-1 has emerged (36, 42). In the present study, increased MT1-MMP mRNA and activity were accompanied by increased mRNA levels of the putative MT1-MMP substrate LTBP-1. Since TGF-β and LTBP-1 are commonly expressed in a stoichiometric fashion, this would suggest that a greater amount of the TGF-β-LTBP-1 complex is present within the MI region, which would be proteolytically processed by MT1-MMP and result in the activation of the TGFR pathway. Indeed, increased TGFR-I and fibrillar collagen I and III mRNA levels occurred within the MI region, which would be consistent with heightened activation of the TGF-β pathway. One of the more novel findings from the presents study was that increased mRNA levels for LTBP-1, TGFR-I, and fibrillar collagens were increased within the I/R region in the context of a previous MI. These observations would suggest that a rapid induction of the profibrotic pathway occurred in this context. While associative, these findings would suggest that a greater degree of matrix remodeling would have occurred within this region, which would have potentially adverse consequences with respect to matrix remodeling in the post-I/R period.
The present study also examined regional mechanical function in parallel with interstitial MT1-MMP activity. Under steady-state conditions, decreased regional stroke work in the previous MI group paralleled the relative 50% increase in MT1-MMP activity in the same region. Thus, in the context of a previous MI, there is a directly proportional inverse relationship between increased MT1-MMP activity and decreased regional function. Potential mechanisms for the impaired regional contractility may reflect the functional multiplicity of this membrane-bound protease. For example, MT1-MMP can induce cytokines, which are capable of indirectly and directly decreasing myocyte contractile function (25). Specifically, TNF-α and IL-6 can attenuate contractility through altered myofilament/Ca2+ dynamics (50). The membrane-bound position of MT1-MMP enables focal degradation of cell surface components, such as integrins (31). Integrins, the cell surface receptors that mediate myocyte adhesion to the extracellular matrix, can be altered through the direct proteolytic activity of MT1-MMP (31). Integrins play an important role in cardiac performance, and disruption of normal integrin function has been linked to reduced cardiac contractility (13). In the present study, animals with and without previous MI were hemodynamically stable at baseline. The I/R event decreased regional stroke work proportionally in both the previous MI group and no MI group. Regional stroke work remained depressed in both groups throughout reperfusion, consistent with the phenomenon of myocardial stunning. Interestingly, there was no further induction of MT1-MMP activity in the I/R region of the previous MI group compared with the non-MI group, implying a differential regulation of transcriptional and translational events in the distinct regions.
The present study is not without limitations. The animals within the study were all castrated males, and therefore we were unable to examine the effects of sex on the results in each group. Sex could be an important factor in this study, as hormones have been shown to influence MT1-MMP. Specifically, an upregulation of MT1-MMP levels in vascular smooth muscle cells has been demonstrated in the presence of estrogen (9). Furthermore, activation of a membrane-bound estrogen receptor has recently been demonstrated to reduce infarct size in rats after I/R (4). Further studies using male and female pigs will be needed to evaluate the influence of sex on MT1-MMP activity in the post-MI and I/R context. While in vitro validation studies (5, 6) have demonstrated that this peptide is cleaved in a concentration-dependent and relatively specific manner by MT1-MMP, it cannot be ruled out that other proteases may have contributed to the fluorogenic signal measured in vivo. For example, the in vitro experiments performed in the present study identified that gelatinases, such as MMP-9, may contribute to a small degree to the overall fluorogenic signal. Thus, future studies using different fluorogenic constructs as well as specifically interrupting MT1-MMP activity in vivo will be necessary to improve the specificity of this substrate-microdialysis approach. This leads to the final important limitation of the present study, in that a direct cause-and-effect relationship between MT1-MMP and regional dysfunction was not established. The present study used a large animal model, which would allow for reasonable recapitulation of the changes in LV geometry and regional function that would occur after a MI in humans. Thus, while this large animal model provided a means by which to interrogate the myocardial interstitium using microdialysis and a MT1-MMP fluorogenic substrate, this obviates the opportunity to use a transgenic approach to modify MT1-MMP levels directly. Using a transgenic knockout approach, it has been demonstrated that global MT1-MMP deletion causes a severe phenotype and developmental defects in collagen turnover and death within the first month of life (10). This laboratory (37) has recently reported that targeted myocardial overexpression of MT1-MMP in mice was associated with significant exacerbation of post-MI remodeling and increased fibrosis, likely mediated through the LTBP-1/TGF-β axis. While nonselective MMP inhibition has been demonstrated to alter the course of LV remodeling and regional dysfunction in this porcine model of MI (19, 21), a specific MT1-MMP pharmacological approach that could be deployed in this context has yet to be realized. Thus, the present study demonstrated an association between MT1-MMP induction and activation with a preexisting MI and a second episode of I/R to that of regional dysfunction; future studies using a targeted and specific modulation of MT1-MMP would be necessary to establish a direct cause-and-effect relationship. Nevertheless, the present study demonstrated a heterogeneity in MT1-MMP interstitial activity post-MI that was affected by a subsequent I/R episode, and these proteolytic effects may influence LV regional structure and function.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-57952, HL-59165, HL-95608, and HL-78825 and by a Veterans Affairs Health Administration Merit Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Blom AS, Mukherjee R, Pilla JJ, Lowry AS, Yarbrough WM, Mingoia JT, Hendrick JW, Stroud RE, McLean JE, Affuso J, Gorman RC, Gorman JH, 3rd, Acker MA, Spinale FG. Cardiac support device modifies left ventricular geometry and myocardial structure after myocardial infarction. Circulation 112: 1274–1283, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res 65: 40–51, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Coker ML, Doscher MA, Thomas CV, Galis ZS, Spinale FG. Matrix metalloproteinase synthesis and expression in isolated LV myocyte preparations. Am J Physiol Heart Circ Physiol 277: H777–H787, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol 297: H1806–H1813, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deschamps AM, Yarbrough WM, Squires CE, Allen RA, McClister DM, Dowdy KB, McLean JE, Mingoia JT, Sample JA, Mukherjee R, Spinale FG. Trafficking of the membrane type-1 matrix metalloproteinase in ischemia and reperfusion: relation to interstitial membrane type-1 matrix metalloproteinase activity. Circulation 111: 1166–1174, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Deschamps AM, Zavadzkas J, Murphy RL, McLean JE, Jeffords L, Saunders S, Sheats N, Stroud R, Beck C, Spinale FG. Interruption of endothelin signaling modifies membrane type-1 matrix metalloproteinase activity during ischemia and reperfusion. Am J Physiol Heart Circ Physiol 294: H875–H883, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.d'Ortho MP, Will H, Atkinson S, Butler G, Messent A, Gavrilovic J, Smith B, Timpl R, Zardi L, Murphy G. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur J Biochem 250: 751–757, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest 106: 55–62, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grandas OH, Mountain DJ, Kirkpatrick SS, Rudrapatna VS, Cassada DC, Stevens SL, Freeman MB, Goldman MH. Effect of hormones on matrix metalloproteinases gene regulation in human aortic smooth muscle cells. J Surg Res 148: 94–99, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 99: 81–92, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Hotary K, Allen E, Punturieri A, Yana I, Weiss SJ. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol 149: 1309–1323, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jennings RB, Murry CE, Steenbergen C, Jr, Reimer KA. Development of cell injury in sustained acute ischemia. Circulation 82: II2, 1990 [PubMed] [Google Scholar]
- 13.Keller RS, Shai SY, Babbitt CJ, Pham CG, Solaro RJ, Valencik ML, Loftus JC, Ross RS. Disruption of integrin function in the murine myocardium leads to perinatal lethality, fibrosis, and abnormal cardiac performance. Am J Pathol 158: 1079–1090, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knäuper V, Will H, López-Otin C, Smith B, Atkinson SJ, Stanton H, Hembry RM, Murphy G. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem 271: 17124–17131, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Koli K, Saharinen J, Hyytiäinen M, Penttinen C, Keski-Oja J. Latency, activation, and binding proteins of TGF-β. Microsc Res Tech 52: 354–362, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J; American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121: e46–e215, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Loennechen JP, Støylen A, Beisvag V, Wisløff U, Ellingsen O. Regional expression of endothelin-1, ANP, IGF-1, and LV wall stress in the infarcted rat heart. Am J Physiol Heart Circ Physiol 280: H2902–H2910, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Lu L, Xu Y, Greyson CR, Ursell PC, Schwartz GG. Non-elastic deformation of myocardium in low-flow ischemia and reperfusion: ultrastructure-function relations. J Mol Cell Cardiol 31: 1157–1169, 1999 [DOI] [PubMed] [Google Scholar]
- 19.McElmurray JH, 3rd, Mukherjee R, New RB, Sampson AC, King MK, Hendrick JW, Goldberg A, Peterson TJ, Hallak H, Zile MR, Spinale FG. Angiotensin-converting enzyme and matrix metalloproteinase inhibition with developing heart failure: comparative effects on left ventricular function and geometry. J Pharmacol Exp Ther 291: 799–811, 1999 [PubMed] [Google Scholar]
- 20.Motivala AA, Tamhane U, Ramanath VS, Saab F, Montgomery DG, Fang J, Kline-Rogers E, May N, Ng G, Froehlich J, Gurm H, Eagle KA. A prior myocardial infarction: how does it affect management and outcomes in recurrent acute coronary syndromes? Clin Cardiol 31: 590–596, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Baskin JM, Deschamps AM, Lowry AS, Escobar GP, Lucas DG, Yarbrough WM, Zile MR, Spinale FG. Myocardial infarct expansion and matrix metalloproteinase inhibition. Circulation 107: 618–625, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Multani M, Ikonomidis JS, Kim PY, Miller EA, Payne KJ, Mukherjee R, Dorman BH, Spinale FG. Dynamic and differential changes in myocardial and plasma endothelin in patients undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg 129: 584–590, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 88: 581–609, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumann U, Kubota H, Frei K, Ganu V, Leppert D. Characterization of Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2, a fluorogenic substrate with increased specificity constants for collagenases and tumor necrosis factor converting enzyme. Anal Biochem 328: 166–173, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res 94: 1543–1553, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Ohte N, Narita H, Iida A, Wakami K, Asada K, Fukuta H, Kato T, Hyano J, Kimura G. Impaired myocardial oxidative metabolism in the remote normal region in patients in the chronic phase of myocardial infarction and left ventricular remodeling. J Nucl Cardiol 16: 73–81, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Osenkowski P, Toth M, Fridman R. Processing, shedding, and endocytosis of membrane type 1-matrix metalloproteinase (MT1-MMP). J Cell Physiol 200: 2–10, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Parrish DC, Alston EN, Rohrer H, Nkadi P, Woodward WR, Schütz G, Habecker BA. Infarction-induced cytokines cause local depletion of tyrosine hydroxylase in cardiac sympathetic nerves. Exp Physiol 95: 304–314, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajavashisth TB, Xu XP, Jovinge S, Meisel S, Xu XO, Chai NN, Fishbein MC, Kaul S, Cercek B, Sharifi B, Shah PK. Membrane type 1 matrix metalloproteinase expression in human atherosclerotic plaques: evidence for activation by proinflammatory mediators. Circulation 99: 3103–3109, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Ruddy JM, Jones JA, Stroud RE, Mukherjee R, Spinale FG, Ikonomidis JS. Differential effects of mechanical and biological stimuli on matrix metalloproteinase promoter activation in the thoracic aorta. Circulation 120, Suppl 11: S262–S268, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seiki M. Membrane-type 1 matrix metalloproteinase: a key enzyme for tumor invasion. Cancer Lett 194: 1–11, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Shuros AC, Salo RW, Florea VG, Pastore J, Kuskowski MA, Chandrashekhar Y, Anand IS. Ventricular preexcitation modulates strain and attenuates cardiac remodeling in a swine model of myocardial infarction. Circulation 116: 1162–1169, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Simonis G, Dahlem MH, Hohlfeld T, Yu X, Marquetant R, Strasser RH. A novel activation process of protein kinase C in the remote, non-ischemic area of an infarcted heart is mediated by angiotensin-AT1 receptors. J Mol Cell Cardiol 35: 1349–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Sohail A, Sun Q, Zhao H, Bernardo MM, Cho JA, Fridman R. MT4-(MMP17) and MT6-MMP (MMP25): a unique set of membrane-anchored matrix metalloproteinases: properties and expression in cancer. Cancer Metastasis Rev 27: 289–302, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation 102: 1944–1949, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Spinale FG, Escobar GP, Mukherjee R, Zavadzkas JA, Saunders SM, Jeffords LB, Leone AM, Beck C, Bouges S, Stroud RE. Cardiac-restricted overexpression of membrane type-1 matrix metalloproteinase in mice: effects on myocardial remodeling with aging. Circ Heart Fail 2: 351–360, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spinale FG, Escobar GP, Mukherjee R, Zavadzkas JA, Saunders SM, Jeffords LB, Leone AM, Bouges S, Stroud RE, Dobrucki LW, Sinusas AJ. Cardiac restricted over-expression of membrane type-1 matrix metalloproteinase in mice: effects on myocardial remodeling following myocardial infarction. J Biol Chem 285: 30316–30327, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spinale FG, Koval CN, Deschamps AM, Stroud RE, Ikonomidis JS. Dynamic changes in matrix metalloproteinase activity within the human myocardial interstitium during ischemia reperfusion. Circulation 118: S16–S23, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spruill LS, Lowry AS, Stroud RE, Squires CE, Mains IM, Flack EC, Beck C, Ikonomidis JS, Crumbley AJ, McDermott PJ, Spinale FG. Membrane-type-1 matrix metalloproteinase transcription and translation in myocardial fibroblasts from patients with normal left ventricular function and from patients with cardiomyopathy. Am J Physiol Cell Physiol 293: C1362–C1373, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Stack MS, Gray RD. Comparison of vertebrate collagenase and gelatinase using a new fluorogenic substrate peptide. J Biol Chem 264: 4277–4281, 1989 [PubMed] [Google Scholar]
- 41.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem 270: 5331–5338, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Tatti O, Vehviläinen P, Lehti K, Keski-Oja J. MT1-MMP releases latent TGF-β1 from endothelial cell extracellular matrix via proteolytic processing of LTBP-1. Exp Cell Res 314: 2501–2514, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Toth M, Chvyrkova I, Bernardo MM, Hernandez-Barrantes S, Fridman R. Pro-MMP-9 activation by the MT1-MMP/MMP-2 axis and MMP-3: role of TIMP-2 and plasma membranes. Biochem Biophys Res Commun 308: 386–395, 2003 [DOI] [PubMed] [Google Scholar]
- 44.van der Pals J, Koul S, Götberg MI, Olivecrona GK, Ugander M, Kanski M, Otto A, Götberg M, Arheden H, Erlinge D. Apyrase treatment of myocardial infarction according to a clinically applicable protocol fails to reduce myocardial injury in a porcine model. BMC Cardiovasc Disord 10: 1, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson EM, Moainie SL, Baskin JM, Lowry AS, Deschamps AM, Mukherjee R, Guy TS, St John-Sutton MG, Gorman JH, 3rd, Edmunds LH, Jr, Gorman RC, Spinale FG. Region- and type-specific induction of matrix metalloproteinases in post-myocardial infarction remodeling. Circulation 107: 2857–2863, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Woessner JF., Jr MMPs and TIMPs–an historical perspective. Mol Biotechnol 22: 33–49, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Yana I, Weiss SJ. Regulation of membrane type-1 matrix metalloproteinase activation by proprotein convertases. Mol Biol Cell 11: 2387–2401, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yarbrough WM, Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Escobar GP, Joffs C, Lucas DG, Crawford FA, Jr, Spinale FG. Matrix metalloproteinase inhibition modifies left ventricular remodeling after myocardial infarction in pigs. J Thorac Cardiovasc Surg 125: 602–610, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Yarbrough WM, Mukherjee R, Escobar GP, Hendrick JW, Sample JA, Dowdy KB, McLean JE, Mingoia JT, Crawford FA, Jr, Spinale FG. Modulation of calcium transport improves myocardial contractility and enzyme profiles after prolonged ischemia-reperfusion. Ann Thorac Surg 76: 2054–2061, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Yokoyama T, Vaca L, Rossen RD, Durante W, Hazarika P, Mann DL. Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. J Clin Invest 92: 2303–2312, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]