Abstract
Transplanted mesenchymal stem cells (MSC) release soluble factors that contribute to cardiac repair and vascular regeneration. We hypothesized that overexpression of GATA-4 enhances the MSC secretome, thereby increasing cell survival and promoting postinfarction cardiac angiogenesis. MSCs harvested from male rat bone marrow were transduced with GATA-4 (MSCGATA-4) using the murine stem cell virus retroviral expression system; control cells were either nontransduced (MSCbas) or transduced with empty vector (MSCNull). Compared with these control cells, MSCGATA-4 were shown by immunofluorescence, real-time PCR, and Western blotting to have higher expression of GATA-4. An increased expression of angiogenic factors in MSCGATA-4 and higher MSC resistance against hypoxia were observed. Human umbilical vein endothelial cells (HUVEC) treated with MSCGATA-4 conditioned medium exhibited increased formation of capillary-like structures and promoted migration, compared with HUVECs treated with MSCNull conditioned medium. MSCGATA-4 were injected into the peri-infarct region in an acute myocardial infarction model in Sprague-Dawley rats developed by ligation of the left anterior descending coronary artery. Survival of MSCGATA-4, determined by Sry expression, was increased at 4 days postengraftment. MSCGATA-4-treated animals showed significantly improved cardiac function as assessed by echocardiography. Furthermore, fluorescent microsphere and histological studies revealed increased blood flow and blood vessel density and reduced infarction size in MSCGATA-4-treated animals. We conclude that GATA-4 overexpression in MSCs increased both MSC survival and angiogenic potential in ischemic myocardium and may therefore represent a novel and efficient therapeutic approach for postinfarct remodeling.
Keywords: gene engineering, stem cell
cell-based therapeutics for heart disorders using autologous whole bone marrow (BM) and mesenchymal stem cells (MSCs) have been shown in both experimental and clinical settings to improve heart function, attenuate infarct size expansion, and contribute to myocardial regeneration (7, 16, 20, 24, 33). MSCs are particularly attractive therapeutic cells, given their capacity for multilineage differentiation and bioenergetic modulation (7, 33). Transdifferentiation into cardiomyocytes and into vascular lineage cells has been originally proposed as the principal mechanisms underlying their therapeutic action (13, 24, 35). Recently, it has been reported that the functional benefits observed after stem cell administration in animal models of cardiac injury might be related to secretion of soluble factors that, acting in a paracrine fashion, protect the heart, attenuate pathological ventricular remodeling, induce neovascularization, and promote regeneration (9, 16, 33, 36). However, the majority of transplanted MSCs die the first day following transplantation (22, 25).
GATA-4, a GATA zinc finger transcription factor family member, has been shown to regulate differentiation, growth, and survival of a wide range of cell types (11, 21). Recent evidence suggests that GATA-4 is also one of the antiapoptotic factors regulating cardiac myocyte survival (1, 15, 30). Apoptosis of cardiac myocytes induced by anthracyclines is associated with decreased GATA-4 expression, and restoration of GATA activity attenuated the apoptosis (15). Myocardial infarction (MI) significantly decreased the DNA-binding activity of GATA-4 (26). Rescuing GATA-4 activity prevented adverse postinfarction remodeling by promoting myocardial angiogenesis, antiapoptosis, and stem cell recruitment (26). Furthermore, cardioprotective agents such as hepatocyte growth factor can activate GATA-4 (17). These findings establish the involvement of GATA-4 in controlling cell survival and postinfarction remodeling.
Therapies employing both genes and stem cells hold promise for treatment of ischemic cardiovascular disease. In particular, MSCs are excellent carriers of therapeutic genes to the heart. We hypothesized that GATA-4 overexpression inhibits oxidative stress-induced injury to MSCs while also increasing MSC paracrine effects that promote postinfarction angiogenesis. Our results indicate that GATA-4 increases MSC survival and paracrine activity, which promotes neovascularization in the ischemic border zone and infarct area, thereby enhancing cardiac functional recovery.
MATERIALS AND METHODS
All protocols conform to the Guidelines for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH Publication No. 85-23, revised 1996) and were approved by the University of Cincinnati Animal Care and Use Committee.
Cell Culture and Transduction With GATA-4 Plasmid
MSCs were obtained from femurs and tibias of male Sprague-Dawley (SD) rats, as described previously (36). Cells were cultured with Iscove's modified Dulbecco's medium (IMDM) supplemented with 10% FBS and penicillin and streptomycin (100 U/ml and 100 μg/ml, respectively). The second passage of MSCs was used to transduce recombinant GATA-4. Retrovirus expressing GATA-4 was constructed using a murine stem cell virus (pMSCV) retroviral expression system (Clontech). Internal ribosome entry site-enhanced green fluorescent protein (IRES-EGFP) was cloned into pMSCV vectors at XhoI and EcoRI sites, and then GATA-4 was excised from pcDNA-GATA-4 (4) with HindIII and XhoI restriction enzymes and cloned into pMSCV-IRES-EGFP at BglII and SalI sites. GP2–293 cells (Clontech) were cotransfected with pMSCV-GATA-4-IRES-EGFP and pVSVG; control GP2-293 cells were cotransfected with pMSCV-IRES-EGFP and pVSVG. After 48 h, supernatants were filtered and incubated with MSCs in the presence of 10 μg/ml polybrene (Sigma) for 12 h. Stable GATA-4- and GFP-expressing clones were acquired by selecting with puromycin (3 μg/ml; Sigma) for 5 days and verified by immunostaining, quantitative real-time PCR, and Western blotting. Control cells (MSCNull) were transduced with GFP alone.
Quantitative Real-Time PCR
Total RNA from cells or tissues was isolated using the RNeasy mini kit (Qiagen). Complimentary DNA was synthesized in a 20-μl reaction mixture using SuperScript III First-Strand Synthesis for RT-PCR (Invitrogen). An aliquot of the cDNA was amplified using Taq DNA polymerase (2.5 units; Invitrogen) in the presence of 1 μM sense and antisense primers (Table 1). Quantitative real-time PCR was carried out on the iQ5 real-time system with iQ SYBR Supermix (Bio-Rad). Expression of each target mRNA relative to GAPDH was calculated under experimental and control conditions based on threshold cycle (Ct) as r = 2−Δ(ΔCt), where ΔCt = Ct target − Ct GAPDH and Δ(ΔCt) = ΔCt experimental − ΔCt control.
Table 1.
Sequence for each primer
| Primers | Sense | Antisense |
|---|---|---|
| GATA-4 | 5′-CTG TCA TCT CAC TAT GGG CA | 5′-CCA AGT CCG AGC AGG AAT TT |
| VEGF | 5′-ATT GAG ACC CTG GTG GAC | 5′-CCT ATG TGC TGG CTT TGG |
| IGF-1 | 5′-TCT GAG GAG GCT GGA GAT GT | 5′-GTT CCG ATG TTT TGC AGG TT |
| bFGF | 5′-CCA GTT GGT ATG TGG CAC TG | 5′-CAG GGA AGG GTT TGA CAA GA |
| GAPDH | 5′-ATG GGA GCT GGT CAT CAA C | 5′-CCA CAG TCT TCT GAG TGG CA |
Immunocytochemical Studies
Cells cultured on glass coverslips and heart sections were fixed in 4% paraformaldehyde, incubated with mouse monoclonal anti-sarcomeric α-actinin (Sigma) or with rabbit polyclonal anti-von Willebrand factor-VIII (vWF; Abcam) and anti-GATA-4 (Abcam). After a thorough washing, cells were incubated with fluorescently labeled secondary antibodies (Invitrogen). Nuclei were stained with 4′,6-diamino-2-phenylindole. Confocal images were obtained with a Leitz DMRBE fluorescence microscope equipped with TCS 4D confocal scanning attachments (Leica). Blood vessels positive for vWF were counted in both infarct and peri-infarct regions. At least 16 microscopic fields (×200) were randomly selected and counted in each treatment group (n = 4 animals/group). Blood vessel density was expressed as the number of vessels per microscopic surface area (0.74 mm2).
Electroimmunoblotting of GATA-4
Cells were washed in PBS and nuclear proteins extracted using NE-PER nuclear and cytoplasmic extraction reagents (Thermo Scientific) according to the supplier's protocol. Protein concentrations were quantified with DC protein assay reagent (Bio-Rad). Denatured nuclear protein (60 μg) was separated using 12% SDS-PAGE, transferred to nitrocellulose membrane (Bio-Rad), and incubated overnight at 4°C with GATA-4 antibody (Abcam). Membranes were then incubated for 1 h with horseradish peroxidase-conjugated secondary antibody at room temperature, washed, and developed with the ECL Plus kit (GE Healthcare). Blots were analyzed by densitometry with NIH Image software (AlphaEase FC, version 6.0.0).
Preparation of Concentrated Conditioned Medium
MSCs were trypsinized and seeded at 5 × 106 cells per 15-cm plate. After 24 h, regular culture medium was replaced with 15 ml of serum- and antibiotic-free IMDM. Twenty-four hours later, medium was collected, centrifuged (2,500 rpm for 3 min) to remove cell debris, transferred to ultrafiltration conical tubes (Amicon Ultra-15 with membranes selective for <5 kDa), and centrifuged (4,000 g for 30 min at 4°C) to concentrate the conditioned medium (CdM). The final concentration was adjusted with PBS to 100 times that of collected CdM. The concentrations of VEGF, IGF-1, and basic FGF in CdM were measured using ELISA (R&D Systems kit).
Capillary-Like Structure Formation
Human umbilical vein endothelial cells (HUVECs) were used for capillary morphogenesis assays in vitro. Tubulogenesis was induced using Matrigel (37). First, HUVECs were labeled with PKH67 cell tracker dye using PKH67 Green Fluorescent Cell Linker kit (Sigma) and following the manufacturer's instructions. Stained HUVECs (3 × 104) in 200 μl of medium (100 μl of fresh medium + 100 μl of CdM) were cultured in 24-well plates coated with 300 μl of Matrigel Basement Membrane Matrix (BD Biosciences). IGF-1-and/or VEGF-neutralizing antibodies (1:50) were added to CdMGATA-4 to a final concentration of 4,000 ng/ml. After 12 h of incubation, images were taken with an Olympus BX 41 microscope equipped with an Olympus digital camera, and capillary-like structures were quantified by measuring the cumulative tube length in five random microscopic fields (2.29 mm2).
Spheroid Sprouting
HUVEC spheroids were generated overnight in hanging-drop cultures consisting of 400 cells in EBM-2 medium with 2% FBS and 20% methylcellulose (Sigma) in nonadhesive round-bottom 96-well plates (14). Spheroids were then embedded in 3D Collagen Cell Culture System (Chemicon) for 24 h in the presence (CdM-fresh medium, 1:1) or absence of CdM. Images were taken with an Olympus BX 41 microscope and Olympus digital camera. The number of sprouts in each spheroid was determined by counting all clearly distinguishable sprouts that were >50 μm in length. Cumulative sprout length was calculated by measuring from the midpoint of the spheroid to the farthest migrating HUVEC and subtracting the radius of the spheroid (5).
Creation of MI Model
MI was created in 2- to 3-mo-old SD female rats by permanent ligation of the left anterior descending coronary artery (LAD). Animals were anesthetized with ketamine (100 mg/kg body wt ip) and xylazine (5 mg/kg body wt ip) and then mechanically ventilated. A left thoracotomy was performed at the fourth intercostal space using sterile technique. The heart was exposed and the LAD ligated with a 7-0 Ethicon suture just below the atrioventricular border. Immediately following LAD ligation, hearts were injected with medium (50 μl) or MSCbas, MSCNull, or MSCGATA-4 (1.5 × 106 cells/50 μl) along the ischemic border. Each heart received multiple injections (n = 5 on average) in the infarct border zone using a 27-gauge needle. The chest was then closed, and the animals were maintained on buprenorphine (0.5 mg/kg sc) for 24 h to alleviate pain. One sham surgery group was included; sham-operated animals underwent the same surgical procedure except without LAD ligation.
MSC survival in ischemic myocardium.
Animals were euthanized on day 4 post-MSC transplantation. Survival of male donor MSCs that expressed Sry gene in the female recipient hearts was assessed using real-time PCR. In addition, the number of MSCs was calculated according to the calibration curve, which showed the Ct of Sry gene expression against serially diluted MSCs (25).
Assessment of cardiac function by echocardiography.
An echocardiography study was performed 4 wk postligation using HDI 5000 SonoCT (Phillips) with a 15-MHz probe. Left ventricular (LV) parameters were obtained from two-dimensional images and M-mode interrogation in long-axis view. LV internal dimensions (LVID) were measured at both diastole (LVIDd) and systole (LVIDs). LV percent fractional shortening (FS) and LV ejection fraction (EF) were calculated as follows: FS = (LVIDd − LVIDs)/LVIDd ×100; EF = [(LVIDd)3 − (LVIDs)3]/(LVIDd)3 ×100.
Myocardial blood flow.
At 4 wk post-LAD ligation (and following the echocardiography study), measurements were performed to determine whether MSCGATA-4 increased coronary blood flow to the infarcted myocardium. A total volume of 200 μl (7.2 × 105) of 10-μm blue fluorescent microspheres were injected into the left atrium. After 1 min, a reference blood sample was withdrawn from the descending aorta at a rate of 1 ml/min. The heart was then excised and weighed. The border, infarcted, and normal regions were dissected and analyzed separately. Microspheres were extracted from blood and heart by 1.5-ml potassium hydroxide digestion, and fluorescence was measured with a 96-well plate reader. Myocardial blood flow (Qs) was calculated as Qs (ml·min−1·g−1) = (As/Ar)Qr (ml/min)/Wt (g), where Qr represents the withdrawal rate of the reference blood, As and Ar represent absorbance in sample tissue and reference blood, respectively, and Wt represents tissue weight (31). Blood flow in border and infarcted areas was expressed as a percentage of normal myocardium.
Determination of infarction size and LV anterior wall thickening.
The heart was cut into six slices of 1.5 mm each from the apex to the base. The sliced heart tissues were embedded in paraffin, and sections (5 μm thick) from slice 2 to slice 5 in each heart were mounted on microscopic glass slides and stained with Masson's trichrome. An image of the LV area of each slide was prepared using an Olympus BX41 microscope with a charge-coupled device camera (MagnaFire; Olympus). Infarct size was defined as the sum of the epicardial and endocardial infarct circumference divided by the sum of the total LV epicardial and endocardial circumferences using computer-based planimetry. LV anterior wall thickening was expressed as a percentage of interventricular septum thickness. Quantitative assessment of each parameter was performed using ImageJ analysis software (version 1.6065; NIH).
Statistical Analysis
In vivo data were obtained by an investigator blinded to the identity of the animal groups. In vitro experiments were carried out in triplicate, unless otherwise mentioned. Quantitative data are means ± SE. Differences were considered significant if the P value was <0.05. One-way analysis of variance (SigmaStat 3.1; Systat Software, San Jose, CA) with the Holm-Sidak method and/or Bonferroni correction (P < 0.017) was used to determine the significance of differences in multiple comparisons.
RESULTS
Characterization of MSCGATA-4
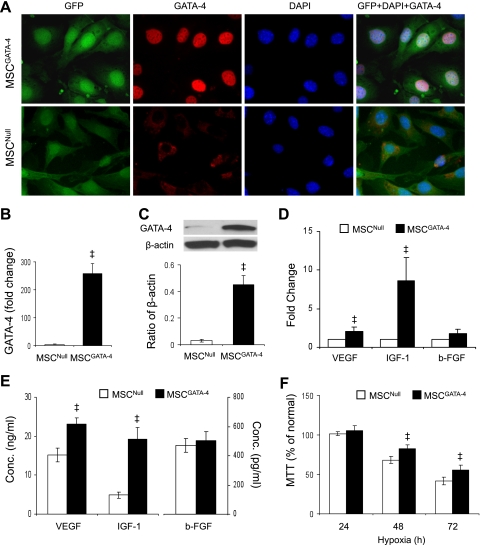
Retroviral-mediated transduction and expression of GATA-4/GFP bicistronic construct was confirmed by immunostaining, real-time PCR, and Western blotting. Whereas both MSCGATA-4 and MSCNull were GFP immunopositive, only MSCGATA-4 stained intensely for GATA-4 (Fig. 1A). Quantitative real-time PCR data indicated that expression of GATA-4 was 254-fold higher in MSCGATA-4 (Fig. 1B). MSCGATA-4 also exhibited higher levels of GATA-4 protein (Fig. 1C).
Fig. 1.
Characterization of GATA-4-transduced mesenchymal stem cells (MSCs). A: immunostaining of MSCs harvested from male rat bone marrow that were transduced with GATA-4 (MSCGATA-4) or empty vector (MSCNull; control). B: quantitative real-time PCR of GATA-4 expression. C: Western blot of GATA-4 and corresponding semiquantitative data. D: quantitative real-time PCR of VEGF, IGF-1, and basic FGF (b-FGF) in transduced MSCs. E: ELISA analysis of VEGF, IGF-1, and b-FGF in conditioned medium (CdM). F: (3,4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) intake by MSCs exposed to hypoxia for 24–72 h. ‡P < 0.05 vs. MSCNull.
Expression of IGF-1 and VEGF-A in MSC was significantly upregulated in MSCGATA-4 compared with MSCNull (Fig. 1D). To measure secretion of these growth factors, levels of IGF-1 and VEGF-A in CdM were assessed by ELISA and shown to be significantly increased in CdMGATA-4, but b-FGF was unchanged (Fig. 1E).
To study resistance of MSC to oxidative stress, MSCs were exposed to hypoxia for 24–72 h, and (3,4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) intake was assessed. Whereas MTT intake was not significantly different between MSCs exposed to hypoxia for 24 h and to normal culture, it was significantly reduced when MSCs were exposed to hypoxia for 48 or 72 h. Overexpression of GATA-4 partially prevented this reduction (Fig. 1F).
Angiogenesis In Vitro
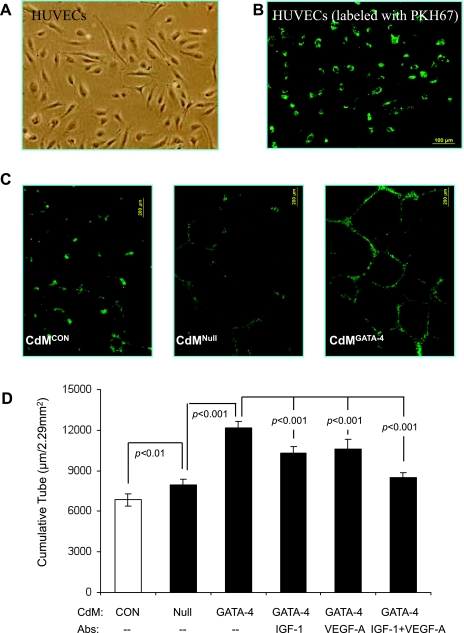
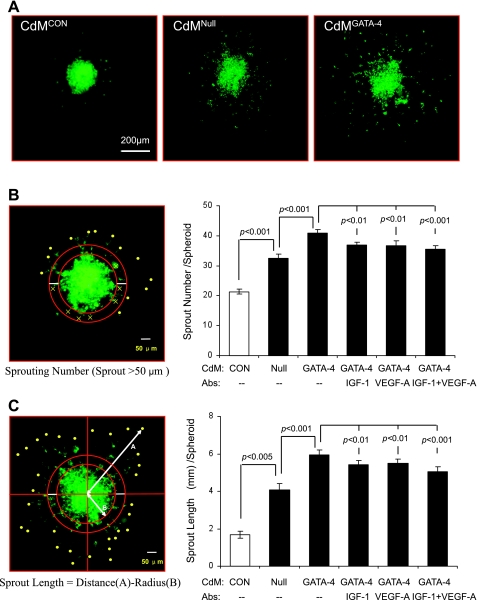
Angiogenesis was assessed by capillary-like tube formation and spheroid sprouting of HUVECs. Cultured HUVECs were labeled with PKH67 (Fig. 2, A and B), seeded into Matrigel, and then incubated with CdM for 12 h. Quantification of branch points per low-power microscopic field showed that the number of tubular structures was higher in cultures treated with CdMGATA-4 than in those treated with either CdMNull or control medium (Fig. 2C). This CdMGATA-4-stimulated increase in formation of capillary-like structures was diminished by treatment with neutralizing antibodies against IGF-1, VEGF-A, or both (Fig. 2D). The effect of MSCGATA-4 on HUVEC migration was investigated by measuring both the number of sprouts per spheroid and cumulative sprout length following 24-h CdMGATA-4 or CdMNull treatment (Fig. 3A). The methods for calculating these two spheroid parameters are described in materials and methods and illustrated in Fig. 3, B and C. The number of emerging capillary sprouts and the cumulative sprout length were both greater in CdMGATA-4-treated HUVECs. This CdMGATA-4-stimulated increase in HUVEC migration was diminished by treatment with neutralizing antibodies against IGF-1, VEGF-A, or both (Fig. 3, B and C).
Fig. 2.
CdM of MSCGATA-4 promotes capillary-like tube formation in vitro. A: human umbilical vein endothelial cell (HUVEC) morphology visualized by inverted microscopy. B: HUVECs were green after labeling with PKH67. C: representative images of capillary-like structures in MSCGATA-4 (CdMGATA-4)- or MSCNull (CDMNull)-conditioned medium or control medium (CdMCon). D: quantitative assessment of capillary-like tube formation under various CdM and neutralizing antibody conditions.
Fig. 3.
Effects of CdM on angiogenic sprouting of HUVECs. A: representative images of HUVEC spheroids stimulated with various CdM for 24 h. B and C show methodology and quantitative data for various CdM and neutralizing antibody conditions. B: sprout number per spheroid. C: sprout length per spheroid.
In Vivo Studies
It is well known that the sustained presence of genetically modified cells in heart can be harmful. Therefore, we used two controls, the original, basal MSC (MSCbas) and the transduction control (MSCNull), to exclude the possibility that viral transduction may impair the ability of MSC to repair myocardium. To facilitate the investigation of MSC morphology, we obtained MSCbas from BM of male rats expressing EGFP. MI was elicited by permanent ligation of the LAD in female SD rats. MI rats were placed in one of four groups: animals receiving either medium or MSCbas, MSCNull, or MSCGATA-4. Male MSCs and medium were transplanted immediately after development of MI. There were no deaths as a result of cell transplantation. There was also a fifth group, a sham control.
MSC survival in ischemic myocardium.
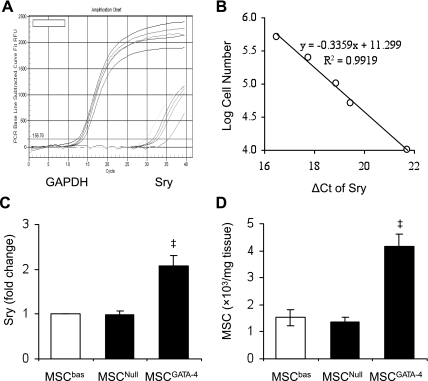
To measure the MSC survival in ischemic myocardium, we first established a calibration curve of the number of MSCs in recipient heart vs. Sry gene expression. Different amounts of male DNA (MSCs) were added to female DNA (rat heart tissue) as standards: 100, 50, 25, 10, 5, 1, and 0 × 104 MSCs/30 mg heart tissue. Ct values of Sry gene, which were obtained by subtracting the Ct of GAPDH, were plotted on a semilogarithmic scale of MSC number to get a balanced contribution of all reference dilutions (Fig. 4, A and B). Subsequently, four hearts per group were harvested 4 days after their respective treatment. Expression of Sry gene in peri-infarcted and infarcted myocardium was significantly higher in MSCGATA-4-transplanted hearts, compared with MSCNull or MSCbas (Fig. 4C). No Sry gene signal was observed in medium-control animals. The number of MSCs was calculated from the calibration curve. Overexpression of GATA-4 significantly increased MSC survival in ischemic myocardium (Fig. 4D).
Fig. 4.
Engraftment of MSCGATA-4 in ischemic myocardium 4 days posttransplantation. A: real-time PCR showing Sry gene fluorescence vs. amplification cycle number as well as GAPDH cycle number in samples containing serially diluted male MSC. B: calibration curve showing the ratio between threshold cycles (Ct) of Sry gene, which has been subtracted from the Ct of GAPDH and MSC number. C: expression of Sry gene in ischemic border and infarcted areas. D: number of MSCs in ischemic border and infarcted areas. ‡P < 0.05 vs. MSCNull.
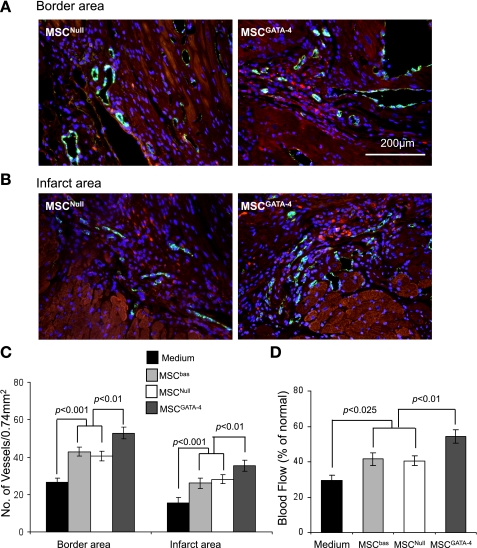
Angiogenesis and blood flow in ischemic myocardium.
The vascular structures in ischemic myocardium (n = 4 per group in 4 groups) were visualized by fluorescent immunostaining specific for vWF. A fluorescence-labeled endothelial cell (green), single or cluster, was counted as one microvessel. The vWF-positive microvessels showed significantly higher blood vessel density (number of capillaries/0.74 mm2) in both infarct and peri-infarct regions in MSCGATA-4-treated animals compared with MSCNull and MSCbas. Mean microvessel number did not differ between MSCbas- and MSCNull-treated animals (Fig. 5, A–C). To determine whether newly generated blood vessels translate to increased coronary blood flow to the infarcted myocardium, we determined functional status of blood vessels in infarcted heart using the fluorescent microsphere method for regional blood flow assessment. In fact, there was a significant decrease in blood flow to the infarcted and peri-infarcted areas, and MSCGATA-4 treatment increased it to about 70% of normal (Fig. 5D).
Fig. 5.
Histological analysis of von Willebrand factor (vWF)-positive microvessels and blood flow in risk areas 4 wk after injection of MSCs or medium in the ischemic border zone. A and B: representative photomicrographs are shown of vWF-positive cells in border and infarcted areas. C: quantitation of vWF-positive capillary density. D: blood flow in the ischemic area, including infarcted and border zones.
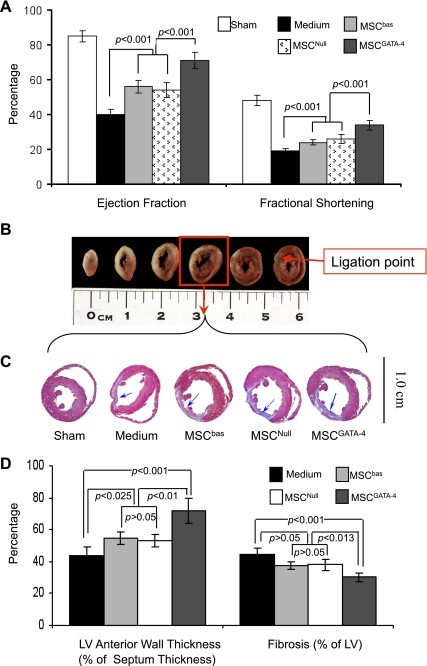
Cardiac function and infarct size.
Global heart function was assessed by echocardiography at 4 wk posttransplantation. EF and FS were significantly reduced in LAD animals compared with sham animals (EF: 43 ± 3 vs. 85 ± 4%, P < 0.001; FS: 18 ± 2 vs. 48 ± 3%, P < 0.001). Animals transplanted with MSCGATA-4 showed marked improvement in EF and FS compared with MSCbas or MSCNull (Fig. 6A).
Fig. 6.
MSCGATA-4 improves cardiac function and reduces infarction size by 4 wk postinjection. A: ejection fraction and fractional shortening calculated from M-mode echocardiograms. B: representative serial slices of heart that underwent left anterior descending coronary artery ligation at 2 days; slices are stained with 2,3,5-triphenyltetrazolium chloride, and the ischemic areas are pale. C: representative mid-left ventricular (LV) heart sections stained with Masson's trichrome. D: quantitative data of LV thickness and fibrosis.
Induction of MI in rats is a challenging procedure with significant variability. The infarct size and subsequent function are directly affected by risk zone size (burden of ischemia). To confirm the level of ligation, hearts were cut into six slices (1.5 mm thick per slice) at 2 days post-LAD and stained with 2,3,5-triphenyltetrazolium chloride in preliminary studies. Ligation points were distinct on the base slice in ischemic animals (Fig. 6B). Data for cardiac function and infarct size from animals without a clear ligation point in the base slice were excluded. MSCGATA-4-transplanted animals had significantly reduced infarct size and increased LV anterior wall thickness compared with medium controls or MSCNull- or MSCbas-transplanted groups (no infarction was observed in sham-operated animals) (Fig. 6, C and D). These data indicate that functional improvement in the MSCGATA-4 group is accompanied by a decrease in infarction size.
DISCUSSION
MSCs derived from adult BM have been proposed as a promising cell-based therapeutic for the improvement of infarcted heart function. The multiple trophic factors released by MSCs attenuated tissue injury, inhibited fibrotic remodeling, stimulated recruitment and proliferation of tissue stem cells, and reduced inflammatory oxidative stress (9, 36). We have shown presently that engraftment of MSCGATA-4 results in tissue preservation by increasing regional blood flow, reducing cell damage, and preventing fibrosis. All of these factors contributed to significantly enhanced systolic functional recovery, which is consistent with previous reports (7, 33). Our results are also in agreement with earlier findings suggesting that GATA-4-based gene transfer represents a novel and efficient therapeutic approach for the treatment of heart failure (26).
There are two main findings in the current study: 1) genetic modification of MSCs to overexpress GATA-4 resulted in their increased resistance to oxidative stress, and 2) MSCGATA-4 increased growth factor release, which promoted endothelial cell angiogenic response. The present study opens a new avenue to investigate whether stem cells engineered to express GATA-4 can be used as a therapy to restore cardiac function in infarcted heart.
GATA-4 Increases MSC Resistance to Ischemic Injury
Cell survival, particularly in an unfavorable environment such as ischemic cardiac tissue, depends on the ability of cells to overcome death triggers. To increase MSC survival in ischemic environment, we transduced antiapoptotic factor GATA-4 into MSC. No obvious difference was observed in MSC morphology between MSCGATA-4 and MSCNull. The expression of MEF-2C and CD31 in MSCGATA-4 was similar to that in our previous report (39). MSCGATA-4 still expressed one stem cell marker (c-kit). These results indicate that MSCGATA-4 retains a stem cell phenotype. However, overexpression of GATA-4 significantly increases MSC survival in a hypoxic and ischemic environment.
Our previous work indicates that the antiapoptotic capacity of mobilized progenitor cells is partially associated with upregulation of GATA-4 (3). Overexpression of GATA-4 upregulates expression of growth factors (e.g., VEGF and IGF-1) in MSC (Fig. 1, D and E). It has been reported that coinjection of VEGF and MSCs in ischemic hearts results in better cardiac function and MSC survival compared with injection of either alone (25). IGF-1 gene delivery has been combined with other growth factors to promote donor cell survival, engraftment, and differentiation as part of a multimodal therapy approach (12, 18). Hence, our research suggests that transduction of GATA-4 contributes to increased resistance of MSC to oxidative stress, possibly by upregulation of prosurvival and/or growth factors.
The survival rate of MSC was assessed based on expression of Sry gene in the ischemic border and infarct area. Sry gene-positive cells can be inferred to be of male origin, since the gene is located on the Y chromosome. Several laboratories have analyzed stem cell survival by evaluating Sry gene expression in LV or whole heart (23, 28, 34). However, expression of Sry gene in the entire LV was too low to be detected in our study. It has been reported by Dresske et al. (6) that the Sry gene-containing part was mainly localized in the LV adjacent to the infarcted area after MSCs were transplanted into ischemic border, whereas the right ventricle always remained negative. The Sry gene was also detected in the infarcted scar in 3 of 8 animals (6). We therefore harvested the border and infarcted area. To ensure that all implanted male MSCs were harvested, we collected enough heart tissue to cover the border zone and infarcted myocardium. Tissue obtained from each heart showed no significant difference in weight. To avoid uneven MSC distribution, we transplanted MSCs into the ischemic border by multiple injections (n = 5 on average).
The survival rate of MSC in ischemic myocardium was evaluated at 4 days posttransplantation. Although MSCs could be detected in myocardium by GFP or Y-chromosome FISH staining at 4 wk posttransplantation, it is very difficult to compare the difference in survival rate of various MSCs.
MSCGATA-4 Promotes Angiogenesis via Paracrine Effects
A prerequisite for successful tissue engineering is formation of a functional microvascular network. MSCGATA-4 transplantation recipients showed a marked reduction in infarct size and a significant increase in capillary density and local blood flow, suggesting that MSCGATA-4 contributes to angiogenesis. It has been demonstrated that intramuscularly injected MSCs and CdM from MSCs are both therapeutically effective for treating heart failure (8, 27), suggesting that paracrine factors of MSCs promote functional recovery of infarcted heart. In the current study, CdMGATA-4 added to HUVEC cultures significantly increased the number of capillary-like structures and spheroid sprouting. In ischemic myocardium, MSCs are able to differentiate into vascular endothelial cells and generate capillary-like structures (9). We cocultured MSCGATA-4 (and MSCNull) with endothelial cells for 1 wk in a dual-chamber system and found that expression of vWF was significantly upregulated in the MSCGATA-4 (3.9 ± 0.6 fold) compared with MSCNull (P < 0.05). These results correlated well with microvessel formation in ischemic myocardium.
Paracrine effects might act by humoral stimulation of preservation of preexisting cells and play a pivotal role in MSCGATA-4-mediated angiogenesis. This hypothesis is further supported by our data showing that MSCGATA-4 upregulated expression of several candidate factors, such as VEGF-A and IGF-1. The effects of IGF-1 and VEGF-A blockade are surprisingly similar, despite their different biological activities. Moreover, our microarray results indicate that overexpression of GATA-4 in MSC upregulates many other growth factor genes, such as bone morphogenetic protein 1 (7.6 ± 0.8 fold), β-nerve growth factor (5.5 ± 0.4 fold), and basic transcription factor 3 (5.5 ± 0.7 fold) (P < 0.05 vs. MSCNull), respectively. All of these factors may protect endothelial cells from ischemic injury and promote angiogenesis in the ischemic risk area. These data suggest that growth factor release from transplanted stem cells might represent another therapeutic strategy for cardiac repair (8, 32, 33).
In the current study, we did not identify which growth factors play a key role in MSCGATA-4-mediated blood vessel formation and upregulation of GATA-4-increased MSC survival. We did, however, observe that the effect of CdMGATA-4 on capillary formation was diminished by VEGF-A- and IGF-1-neutralizing antibodies. Although single growth factor therapeutic regimens have been attempted for FGF, hepatocyte growth factor (HGF), IGF, and VEGF with encouraging results (10, 17, 19, 29). MSC therapy is unique in that it engages functionally synergistic and redundant trophic factors (2, 9) that may be required for activation of endogenous stem cell repair mechanisms and a more sustained therapeutic effect.
Although it is unclear how overexpression of GATA-4 can increase the paracrine effect of MSC, it has been suggested that STAT transcription factors may play a role. GATA-4 and STAT cooperate at the transcriptional level to mediate signaling through JAK-STAT and PKC pathways (38). PKC phosphorylation enhances GATA-4 DNA-binding activity, and STAT-1 interacts with GATA-4 functionally and physically to synergistically activate growth factor-inducible promoters. Moreover, GATA factors are able to recruit STAT proteins to target promoters via GATA-binding sites, which are sufficient to support synergy (38). Thus STAT proteins can act as growth factor-inducible coactivators of tissue-specific transcription factors, and overexpression of GATA-4 in MSC may upregulate and increase growth factors secrete.
In conclusion, overexpression of GATA-4 resulted in upregulation of IGF-1 and VEGF in MSC, as well as enhanced survival of engrafted MSCs and promoted angiogenesis in ischemic myocardium. Transplantation of MSCGATA-4 ameliorated LV remodeling and improved LV function. These observations suggest that MSCs genetically modified to overexpress GATA-4 could significantly advance the efficacy of stem cell therapy.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL083236 (to M. Xu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge the input of Dr. M. Ashraf in the discussion.
REFERENCES
- 1.Aries A, Paradis P, Lefebvre C, Schwartz RJ, Nemer M. Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. Proc Natl Acad Sci USA 101: 6975–6980, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 98: 1076–1084, 2006. 16619257 [Google Scholar]
- 3.Dai Y, Ashraf M, Zuo S, Uemura R, Dai YS, Wang Y, Haider H, Li T, Xu M. Mobilized bone marrow progenitor cells serve as donors of cytoprotective genes for cardiac repair. J Mol Cell Cardiol 44: 607–617, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai YS, Markham BE. p300 Functions as a coactivator of transcription factor GATA-4. J Biol Chem 276: 37178–37185, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Dietrich F, Lelkes PI. Fine-tuning of a three-dimensional microcarrier-based angiogenesis assay for the analysis of endothelial-mesenchymal cell co-cultures in fibrin and collagen gels. Angiogenesis 9: 111–125, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Dresske B, El Mokhtari NE, Ungefroren H, Ruhnke M, Plate V, Janssen D, Siebert R, Reinecke A, Simon R, Fandrich F. Multipotent cells of monocytic origin improve damaged heart function. Am J Transplant 6: 947–958, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Feygin J, Mansoor A, Eckman P, Swingen C, Zhang J. Functional and bioenergetic modulations in the infarct border zone following autologous mesenchymal stem cell transplantation. Am J Physiol Heart Circ Physiol 293: H1772–H1780, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J 20: 661–669, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 103: 1204–1219, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haider H, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res 103: 1300–1308, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Jay PY, Bielinska M, Erlich JM, Mannisto S, Pu WT, Heikinheimo M, Wilson DB. Impaired mesenchymal cell function in Gata4 mutant mice leads to diaphragmatic hernias and primary lung defects. Dev Biol 301: 602–614, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanemitsu N, Tambara K, Premaratne GU, Kimura Y, Tomita S, Kawamura T, Hasegawa K, Tabata Y, Komeda M. Insulin-like growth factor-1 enhances the efficacy of myoblast transplantation with its multiple functions in the chronic myocardial infarction rat model. J Heart Lung Transplant 25: 1253–1262, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 103: 634–637, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Kern J, Steurer M, Gastl G, Gunsilius E, Untergasser G. Vasohibin inhibits angiogenic sprouting in vitro and supports vascular maturation processes in vivo. BMC Cancer 9: 284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y, Ma AG, Kitta K, Fitch SN, Ikeda T, Ihara Y, Simon AR, Evans T, Suzuki YJ. Anthracycline-induced suppression of GATA-4 transcription factor: implication in the regulation of cardiac myocyte apoptosis. Mol Pharmacol 63: 368–377, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 109: 1543–1549, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Kitta K, Day RM, Kim Y, Torregroza I, Evans T, Suzuki YJ. Hepatocyte growth factor induces GATA-4 phosphorylation and cell survival in cardiac muscle cells. J Biol Chem 278: 4705–4712, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Kofidis T, de Bruin JL, Yamane T, Balsam LB, Lebl DR, Swijnenburg RJ, Tanaka M, Weissman IL, Robbins RC. Insulin-like growth factor promotes engraftment, differentiation, and functional improvement after transfer of embryonic stem cells for myocardial restoration. Stem Cells 22: 1239–1245, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M, Ashare AB, Lathi K, Isner JM. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation 98: 2800–2804, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation 113: 1287–1294, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Molkentin JD. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem 275: 38949–38952, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Muller-Ehmsen J, Whittaker P, Kloner RA, Dow JS, Sakoda T, Long TI, Laird PW, Kedes L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol 34: 107–116, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Niagara MI, Haider H, Jiang S, Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res 100: 545–555, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature 410: 701–705, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Pons J, Huang Y, Takagawa J, Arakawa-Hoyt J, Ye J, Grossman W, Kan YW, Su H. Combining angiogenic gene and stem cell therapies for myocardial infarction. J Gene Med 11: 743–753, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Rysä J, Tenhunen O, Serpi R, Soini Y, Nemer M, Leskinen H, Ruskoaho H. GATA-4 is an angiogenic survival factor of the infarcted heart. Circ Heart Fail 3: 440–450, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol 296: H1888–H1897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheikh AY, Lin SA, Cao F, Cao Y, van der Bogt KE, Chu P, Chang CP, Contag CH, Robbins RC, Wu JC. Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium. Stem Cells 25: 2677–2684, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki G, Lee TC, Fallavollita JA, Canty JM., Jr Adenoviral gene transfer of FGF-5 to hibernating myocardium improves function and stimulates myocytes to hypertrophy and reenter the cell cycle. Circ Res 96: 767–775, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Suzuki YJ, Nagase H, Day RM, Das DK. GATA-4 regulation of myocardial survival in the preconditioned heart. J Mol Cell Cardiol 37: 1195–1203, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Takaba K, Jiang C, Nemoto S, Saji Y, Ikeda T, Urayama S, Azuma T, Hokugo A, Tsutsumi S, Tabata Y, Komeda M. A combination of omental flap and growth factor therapy induces arteriogenesis and increases myocardial perfusion in chronic myocardial ischemia: evolving concept of biologic coronary artery bypass grafting. J Thorac Cardiovasc Surg 132: 891–899, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, Matsuzaki M, Hamano K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol 291: H886–H893, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Tang YL, Zhao Q, Qin X, Shen L, Cheng L, Ge J, Phillips MI. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg 80: 229–236; discussion 236–237, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Terrovitis J, Lautamaki R, Bonios M, Fox J, Engles JM, Yu J, Leppo MK, Pomper MG, Wahl RL, Seidel J, Tsui BM, Bengel FM, Abraham MR, Marban E. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol 54: 1619–1626, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 100: II247–I256, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res 98: 1414–1421, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Venetsanakos E, Mirza A, Fanton C, Romanov SR, Tlsty T, McMahon M. Induction of tubulogenesis in telomerase-immortalized human microvascular endothelial cells by glioblastoma cells. Exp Cell Res 273: 21–33, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Paradis P, Aries A, Komati H, Lefebvre C, Wang H, Nemer M. Convergence of protein kinase C and JAK-STAT signaling on transcription factor GATA-4. Mol Cell Biol 25: 9829–9844, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu M, Wani M, Dai YS, Wang J, Yan M, Ayub A, Ashraf M. Differentiation of bone marrow stromal cells into the cardiac phenotype requires intercellular communication with myocytes. Circulation 110: 2658–2665, 2004 [DOI] [PubMed] [Google Scholar]