Abstract
Cell models of ischemic preconditioning (IPC) indicate nitric oxide (NO) is involved in protection accruing during reoxygenation but disagree whether it acts through PKG. Using a more relevant intact heart model, we studied isolated rabbit hearts subjected to 30-min coronary artery occlusion/120-min reperfusion. We previously found protection from PKG activator 8-(4-chlorophenylthio)-guanosine 3′,5′-cyclic monophosphate (CPT-cGMP) at reperfusion was blocked by A2b adenosine receptor (A2bAR), ERK, or phosphatidylinositol 3-kinase (PI3-kinase) blockers. In this investigation A2bAR agonist BAY 60-6583 or CPT-cGMP at reperfusion reduced infarction comparably to IPC. Their protection was abrogated by Nω-nitro-l-arginine methyl ester (l-NAME), suggesting a PKG-independent NO synthase in IPC's mediator pathway downstream of PKG and A2bAR. NO donor S-nitroso-N-acetyl-d,l-penicillamine (SNAP) at reperfusion also protected. This protection was not blocked by PI3-kinase inhibitor wortmannin or ERK antagonist PD-98059, suggesting NO acted downstream of these kinases. Protection from SNAP was not affected by mitochondrial ATP-sensitive K+ channel closer 5-hydroxydecanoate, PKC antagonist chelerythrine, reactive oxygen species scavenger N-2-mercaptopropionylglycine, or soluble guanylyl cyclase antagonist 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ). Absence of ODQ effect indicated NO was acting independently of PKG. BAY 58-2667, a soluble guanylyl cyclase activator, was protective, and l-NAME blocked its infarct-sparing effect, indicating a second signaling event dependent on NO generation but independent of PKG. SB216763, a blocker of glycogen synthase kinase-3β (GSK-3β), decreased infarct size, and its infarct-sparing effect was not affected by l-NAME, suggesting GSK-3β acted downstream or independently of NO. Hence, NO signaling occurs in IPC's mediator pathway downstream of Akt and ERK, and its protection is independent of PKG.
Keywords: myocardial infarction; Nω-nitro-l-arginine methyl ester; nitric oxide; protein kinase G; preconditioning; reperfusion injury; S-nitroso-N-acetyl-d,l-penicillamine
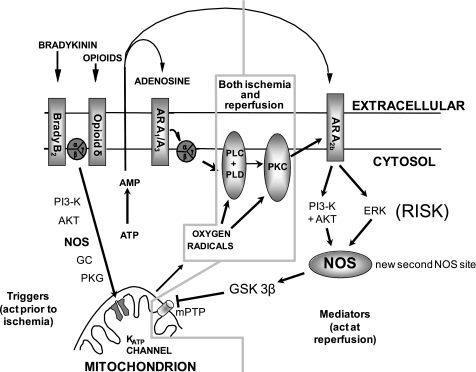
ischemic preconditioning (IPC) and postconditioning protect the heart through signal transduction pathways that are very similar to each other. Insight into IPC's signaling pathway has revealed a number of feasible strategies for conferring protection that are just beginning to be explored in the clinical setting (19, 31, 37). Unfortunately, there is still much about the protective mechanism and its signaling pathway that remains unknown. Over the past decade we have attempted to map the signal transduction steps in our rabbit heart model and have paid particular attention to the relative position of the signaling elements. Our strategy has focused on stimulation of the pathway at some intermediate point, such as PKC, with phorbol ester and then pharmacologically blocking another known element. If the protection is lost, then the blocked step must have been downstream of the stimulated one (12). The signaling of IPC can be been divided into two phases: a preischemic trigger phase and a mediator phase that occurs in early reperfusion (Fig. 1). In the trigger phase, transient activation causes a protected phenotype that persists for one to several hours (hence the term trigger), and most of the steps in the trigger phase have been well documented (47). Among those steps are the nitric oxide synthase (NOS)-guanylyl cyclase-PKG cascade, which couples bradykinin (34), muscarinic receptors (23), and presumably opioid receptors to mitochondrial ATP-sensitive potassium channels (mKATP) and PKC.
Fig. 1.
Proposed signaling scheme for ischemic preconditioning. Note that in the schematic, nitric oxide synthase (NOS) signals through protein kinase G (PKG) upstream in the trigger pathway but acts in a PKG-independent manner downstream in the mediator pathway at reperfusion. The role of glycogen synthase kinase-3β (GSK-3β) is controversial, but if it is in the pathway, then it must be downstream of the newly described NOS site. AR, adenosine receptor; PI3-K, phosphatidylinositol 3-kinase; PLC, phospholipase C; PLD, phospholipase D; ERK, extracellular signal-regulated kinase; GC, guanylyl cyclase; PKC, protein kinase C; mKATP, mitochondrial ATP-dependent K+ channel; mPTP, mitochondrial permeability transition pore; RISK, reperfusion injury survival kinases.
The mediator pathway must be activated in the first minute of reperfusion and is much less well understood. Activation of the reperfusion injury survival kinases (RISK), which include phosphatidylinositol 3-kinase (PI3-kinase), Akt, and ERK, are clearly required for IPC's protection during the mediator phase in rabbit (43) and rat hearts (17). Recent studies using a pig heart model, however, have failed to show such involvement (42), suggesting that this mechanism may not be common to IPC in all species. We recently proposed that the low-affinity A2b adenosine receptor (A2bAR) is responsible for activating RISK during the mediator phase in rabbit hearts (24, 36). We found that PKC somehow lowers the threshold for A2bAR signaling so that endogenous adenosine released by ischemic myocardium can activate these receptors (24). As noted above, NO is involved in triggering IPC's protection by activating PKG, which then opens mKATP, leading to ultimate activation of PKC through redox signaling (34). When we activated PKG with a cell-permeant cGMP analog, 8-(4-chlorophenylthio)-guanosine 3′,5′-cyclic monophosphate (CPT-cGMP), at the end of an ischemic insult, infarct size was reduced (25). Blocking studies showed that the pathway involved PKC, A2bAR, and RISK. However, in pilot studies for this project we noted that Nω-nitro-l-arginine methyl ester (l-NAME) could also block protection from CPT-cGMP or an A2bAR agonist given at reperfusion. One possible explanation would be that two NO steps exist in IPC's signaling pathway: one upstream of A2bAR in the trigger pathway that is PKG dependent and one downstream in the mediator pathway that is PKG independent. That hypothesis is supported by several recent studies in which NO directly protected isolated mitochondria from injury in a simulated ischemia model (9, 20). Additionally, PKG-independent S-nitrosylation of proteins has been proposed as a possible mechanism of IPC's protection (44). However, other recent studies using cell-based models have reported that NO protects mitochondria in cardiomyocytes by a PKG-dependent mechanism (9, 50). We therefore decided to conduct experiments using a more clinically relevant whole heart model to test whether a PKG-independent NO step could be demonstrated in IPC's mediator pathway.
We employed two important tools to evaluate the role of NO in the mediator pathway. The NO donor S-nitroso-N-acetyl-d,l-penicillamine (SNAP) was assumed to mimic NOS's production of NO in our hearts, and the NOS inhibitor l-NAME was assumed to block NO production. We tested the ability of selective blockers of known steps in IPC's signaling pathway to abort protection from SNAP given just before reperfusion. Conversely, we stimulated IPC's pathway at various known sites and tested whether l-NAME could block the protection. Using this approach, we attempted to pinpoint the location of NOS in the mediator pathway.
METHODS
Isolated heart model.
All animal care satisfied published guidelines (33), and procedures were approved by institutional committees. New Zealand White rabbits of either sex weighing 2–3 kg were anesthetized with pentobarbital sodium (30 mg/kg) and ventilated with 100% oxygen. Hearts were exposed through a left thoracotomy, and a suture was passed around a branch of the left coronary artery. The heart was removed and perfused on a Langendorff apparatus with modified Krebs-Henseleit bicarbonate buffer that contained (in mM) 118.5 NaCl, 24.7 NaHCO3, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 2.5 CaCl2, and 10.0 glucose. The buffer was gassed with 95% O2-5% CO2. A fluid-filled latex balloon was inserted into the left ventricle to measure pressure. All hearts were allowed to equilibrate for 20 min before the protocol was started.
Protocol for infarct studies.
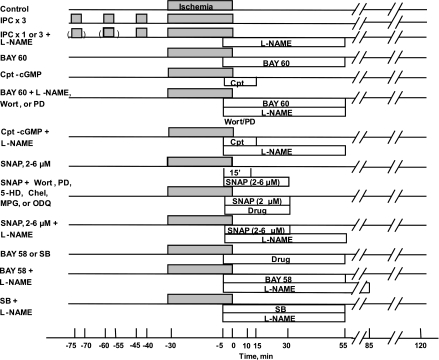
Twenty-nine groups of hearts were studied (Fig. 2). Three control groups are included. Because of the extended period over which the experiments were conducted, contemporary control groups were evaluated for the BAY 58-2667 (BAY 58) and SB216763 (SB) experiments (see below). All hearts were subjected to 30 min of regional ischemia and 120 min of reperfusion. Control hearts received no treatment. Ischemic preconditioning was accomplished with three cycles (IPC × 3) of 5 min of regional ischemia and 10 min of reperfusion before the prolonged ischemia. In groups 5 and 6, the effect of 60 min of l-NAME (200 μM) starting 5 min before reperfusion was examined in hearts preconditioned with either one or three cycles of IPC. Hearts in group 7 were treated with BAY 60-6583 (BAY 60), a selective A2bAR agonist (24) (300 nM), for 1 h beginning 5 min before the onset of reperfusion, and those in group 8 were treated with CPT-cGMP (10 μM) for 20 min, again starting 5 min before reperfusion. Hearts in groups 9 and 10 were treated with either BAY 60 or CPT-cGMP, as well as a simultaneous infusion of l-NAME, as described above. Hearts in groups 11 and 12 were treated with BAY 60, as described above, as well as 20-min infusions of either the PI3-kinase inhibitor wortmannin (Wort; 100 nM) or the MEK1/2, and therefore ERK1/2, antagonist PD-98059 (PD; 10 μM) starting 5 min before reperfusion. Hearts in groups 13 and 14 were treated with SNAP (2 μM) for either 15 or 35 min beginning 5 min before release of the coronary occlusion. Hearts in groups 15–20 were exposed to SNAP for 35 min, as described above, and were simultaneously treated with Wort, PD, the mKATP channel closer 5-hydroxydecanoate (5-HD; 200 μM), the PKC antagonist chelerythrine (Chel; 2.8 μM), the reactive oxygen species (ROS) scavenger N-2-mercaptopropionylglycine (MPG; 300 μM), or 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; 2 μM), a potent antagonist of soluble guanylyl cyclase, also for 35 min. Hearts in groups 21–23 were treated with 2, 4, or 6 μM SNAP for 35 min and l-NAME for 60 min. Hearts in groups 24 and 25 received SNAP alone for 35 min at 4 and 6 μM. Hearts in groups 26 and 27 were treated with the highly selective activator of soluble guanylyl cyclase BAY 58 (50 nM) for 1 h beginning 5 min before the onset of reperfusion either alone or with l-NAME. For these experiments the l-NAME infusion started 5 min before the onset of reperfusion and continued for 90 min. Hearts in groups 28 and 29 were treated with SB, a blocker of glycogen synthase kinase-3β (GSK-3β; 1 μM) either alone or with l-NAME for 1 h commencing 5 min before the onset of reperfusion. All antagonists, Wort (51, 53), PD (51), 5-HD (35), Chel (24), MPG (11, 29), ODQ (53), and l-NAME (34, 51), were administered at doses that had previously successfully aborted pre- and/or postconditioning's protective effect in our isolated rabbit heart model.
Fig. 2.
Experimental protocols. BAY 60, BAY 60-6583; Chel, chelerythrine; 5-HD, 5-hydroxydecanoic acid; IPC, ischemic preconditioning; l-NAME, Nω-nitro-l-arginine methyl ester; MPG, N-2-mercaptopropionylglycine; ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; PD, PD-98059; SB, SB216763; Wort, wortmannin. S-nitroso-N-acetyl-d,l-penicillamine (SNAP) was present alone at 2 μM for either 15 or 35 min, and doses of 4 and 6 μM were infused for 35 min.
Measurement of infarct size.
At the end of the experiment, the coronary artery was reoccluded, and 2- to 9-μm fluorescent microspheres (Microgenics, Fremont, CA) were infused to delineate the ischemic zone (region at risk) as the area of tissue without fluorescence. The heart was cut into 2-mm-thick slices that were incubated in 1% triphenyltetrazolium chloride in sodium phosphate buffer (pH 7.4) at 37°C for 10 min. The slices were immersed in 10% formalin to preserve the tissue. The risk zone was identified by illuminating slices with ultraviolet light. The areas of infarct and risk zone were determined by planimetry of each slice, and volumes were calculated by multiplying each area by slice thickness and summing them for each heart. Infarct size is expressed as a percentage of the risk zone.
Materials.
BAY 58 and BAY 60 were gifts from Thomas Krahn (Bayer HealthCare, Wuppertal, Germany). All other pharmacological agents were purchased from Sigma Aldrich (St. Louis, MO). Either distilled water or DMSO was used to dissolve the drugs and to prepare stock solutions. The final DMSO concentration was kept below 0.1%.
Statistics.
All data are means ± SE. One-way analysis of variance with the Student-Newman-Keuls post hoc test was performed on baseline hemodynamic and infarct variables, including risk zone and infarct size. The significance of differences between the infarct and risk zone plots for any two groups was determined by analysis of covariance. P < 0.05 was considered significant.
RESULTS
Hemodynamics.
There was no difference in baseline heart rate, developed pressure, or coronary flow among the 29 groups studied (data not shown). Of course, during coronary occlusion both developed pressure and coronary flow fell significantly in all groups; heart rates hardly changed. None of the drugs administered 5 min before ischemia had any effect on hemodynamics. After reperfusion, there were increases in both developed pressure and coronary flow, although neither parameter returned to preocclusion levels.
Infarct studies.
As shown in Table 1 average body weight in some groups was significantly higher than that seen in the control group. This was a reflection of the duration of this study and the changing size of available animals. In general, higher heart weights went along with increased body weights. Importantly, however, risk zone sizes were not significantly different among groups, although there was a tendency for risk zones to be smaller in SNAP + ODQ and SB + l-NAME.
Table 1.
Infarct data
| n | Body Weight, kg | Heart Weight, g | Risk Zone, cm3 | Infarct Zone, cm3 | Infarct/Risk Zone, % | |
|---|---|---|---|---|---|---|
| Control 1 | 9 | 2.1 ± 0.1 | 7.0 ± 0.1 | 1.24 ± 0.10 | 0.43 ± 0.04 | 34.4 ± 2.2 |
| Control 2 | 6 | 2.2 ± 0.0 | 8.3 ± 0.3 | 1.29 ± 0.09 | 0.43 ± 0.06 | 33.0 ± 3.2 |
| Control 3 | 4 | 2.4 ± 0.0 | 8.2 ± 0.1 | 1.42 ± 0.07 | 0.58 ± 0.06 | 40.6 ± 2.2 |
| BAY 60 | 6 | 2.2 ± 0.0 | 6.8 ± 0.1 | 1.11 ± 0.07 | 0.13 ± 0.02‡ | 11.9 ± 1.4‡ |
| IPC × 3 | 4 | 2.0 ± 0.1 | 7.2 ± 0.2 | 1.17 ± 0.09 | 0.13 ± 0.03‡ | 10.9 ± 1.9‡ |
| IPC × 1 + l-NAME | 6 | 2.2 ± 0.1 | 7.6 ± 0.4 | 1.21 ± 0.09 | 0.41 ± 0.07 | 33.0 ± 3.1 |
| IPC × 3 + l-NAME | 8 | 2.2 ± 0.1 | 7.6 ± 0.3 | 1.25 ± 0.08 | 0.35 ± 0.05 | 28.4 ± 3.7 |
| BAY 60 + l-NAME | 6 | 2.3 ± 0.0 | 7.0 ± 0.1 | 1.41 ± 0.11 | 0.52 ± 0.07 | 36.0 ± 2.5 |
| BAY 60 + Wort | 6 | 2.3 ± 0.0 | 7.3 ± 0.2 | 1.43 ± 0.05 | 0.47 ± 0.03 | 32.5 ± 1.4 |
| BAY 60 + PD | 6 | 2.4 ± 0.0 | 7.3 ± 0.3 | 1.31 ± 0.16 | 0.40 ± 0.04 | 30.6 ± 1.4 |
| CPT-cGMP | 8 | 2.0 ± 0.1 | 7.1 ± 0.2 | 1.35 ± 0.10 | 0.25 ± 0.07† | 17.0 ± 3.3‡ |
| CPT-cGMP + l-NAME | 6 | 2.1 ± 0.1 | 7.9 ± 0.2 | 1.32 ± 0.11 | 0.42 ± 0.08 | 31.1 ± 3.5 |
| SNAP 2 μM (15 min) | 6 | 2.8 ± 0.1‡ | 8.1 ± 0.1‡ | 1.50 ± 0.13 | 0.48 ± 0.06 | 31.6 ± 1.7 |
| SNAP 2 μM (35 min) | 8 | 2.4 ± 0.0 | 7.2 ± 0.1 | 1.34 ± 0.10 | 0.17 ± 0.02‡ | 12.5 ± 1.6‡ |
| SNAP 4 μM (35 min) | 6 | 2.1 ± 0.0 | 7.1 ± 0.4 | 1.20 ± 0.07 | 0.12 ± 0.04‡ | 9.7 ± 2.4‡ |
| SNAP 6 μM (35 min) | 6 | 2.2 ± 0.1 | 7.5 ± 0.2 | 1.12 ± 0.09 | 0.15 ± 0.03‡ | 13.2 ± 1.6‡ |
| SNAP 2 μM + Wort | 6 | 2.4 ± 0.0 | 7.0 ± 0.1 | 1.34 ± 0.09 | 0.20 ± 0.05‡ | 14.5 ± 2.5‡ |
| SNAP 2 μM + PD | 6 | 2.7 ± 0.0‡ | 8.3 ± 0.1‡ | 1.40 ± 0.18 | 0.26 ± 0.07† | 17.2 ± 2.3‡ |
| SNAP 2 μM +5-HD | 6 | 2.4 ± 0.0 | 7.7 ± 0.2 | 1.22 ± 0.10 | 0.16 ± 0.02‡ | 12.7 ± 1.4‡ |
| SNAP 2 μM + Chel | 6 | 2.7 ± 0.1‡ | 8.2 ± 0.1‡ | 1.29 ± 0.09 | 0.17 ± 0.02‡ | 13.2 ± 1.2‡ |
| SNAP 2 μM + MPG | 6 | 2.5 ± 0.1* | 7.6 ± 0.2 | 1.42 ± 0.10 | 0.23 ± 0.04† | 16.0 ± 1.7‡ |
| SNAP 2 μM + ODQ | 6 | 2.5 ± 0.0‡ | 8.9 ± 0.1‡ | 1.06 ± 0.15 | 0.11 ± 0.02‡ | 10.1 ± 1.2‡ |
| SNAP 2 μM + l-NAME | 6 | 2.2 ± 0.1 | 7.8 ± 0.3 | 1.41 ± 0.18 | 0.46 ± 0.08 | 32.4 ± 2.7 |
| SNAP 4 μM + l-NAME | 6 | 2.3 ± 0.1 | 7.6 ± 0.2 | 1.22 ± 0.06 | 0.15 ± 0.03‡ | 12.3 ± 2.3‡ |
| SNAP 6 μM + l-NAME | 6 | 2.3 ± 0.1 | 7.9 ± 0.1 | 1.21 ± 0.04 | 0.17 ± 0.03‡ | 13.6 ± 2.0‡ |
| BAY 58 | 6 | 2.4 ± 0.0 | 8.2 ± 0.1 | 1.29 ± 0.08 | 0.24 ± 0.03† | 18.8 ± 2.3‡ |
| BAY 58 + l-NAME | 6 | 2.4 ± 0.0 | 8.1 ± 0.1 | 1.43 ± 0.08 | 0.54 ± 0.05 | 37.7 ± 2.0 |
| SB | 10 | 2.3 ± 0.1 | 8.0 ± 0.6* | 1.13 ± 0.14 | 0.14 ± 0.04* | 14.5 ± 2.6‡ |
| SB + l-NAME | 6 | 1.9 ± 0.1* | 6.9 ± 0.3 | 1.07 ± 0.08 | 0.07 ± 0.01‡ | 5.9 ± 0.7‡ |
Values are means ± SE; n = no. of hearts. Control 1 is control group for all hearts except for those treated with SB216763 (SB; Control 2) or BAY 58-2667 (BAY 58; Control 3). A new control group was generated if more than 3 mo had elapsed between study groups. S-nitroso-N-acetyl-d,l-penicillamine (SNAP) was infused for 15 min in only 1 group; in all other groups it was infused for 35 min.
BAY 60, BAY 60-6583; Chel, chelerythrine; CPT-cGMP, 8-(4-chlorophenylthio)-guanosine 3′,5′-cyclic monophosphate; 5-HD, 5-hydroxydecanoate; IPC, ischemic preconditioning; l-NAME, Nω-nitro-l-arginine methyl ester; MPG, N-2-mercaptopropionylglycine; ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; PD, PD-98059; Wort, wortmannin.
P < 0.025;
P < 0.005;
P < 0.001, statistical significance of difference from respective control.
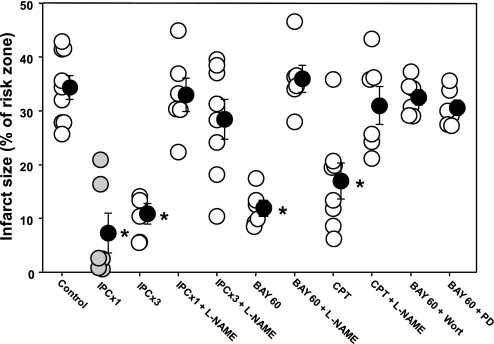
Infarct size in control rabbits averaged 34.4 ± 2.2% of the risk zone in control group 1, 40.9 ± 2.2% in control group 2, and 33.0 ± 3.2% in control group 3 (see Figs. 3, 6, and 7). There was no difference between these three control groups. As expected, three cycles of IPC resulted in marked protection in the four hearts tested, which is similar to what is seen with only one cycle of IPC (43) (Fig. 3). l-NAME administered at reperfusion handily blocked the infarct-sparing effect of one cycle of IPC. We also tried l-NAME against three cycles of IPC, a more robust preconditioning stimulus (46). l-NAME still blocked protection (Fig. 3). Because l-NAME was only present at reperfusion, these data clearly demonstrate that there must be an NO step in the mediator pathway.
Fig. 3.
Infarct size as a percentage of risk zone for control group 1 rabbits and those treated with either 1 or 3 cycles of IPC (IPC × 1 or 3), BAY 60, or 8-(4-chlorophenylthio)-cGMP (CPT) alone or with coadministered l-NAME, Wort, or PD. Open circles represent individual hearts, and solid circles indicate group means ± SE; shaded circles for IPC × 1 represent historical data (43). Whereas IPC, BAY 60, and CPT were individually very protective, l-NAME aborted that protection. Wort and PD each also blocked BAY 60's protective effect. *P < 0.001.
Fig. 6.
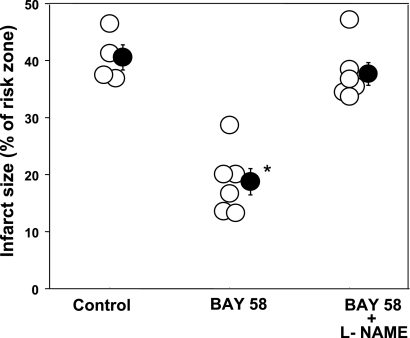
Infarct size as a percentage of risk zone for control group 3 rabbits and those treated with BAY 58-2667 (BAY 58) alone or simultaneously with l-NAME. Open circles represent individual hearts, and solid circles indicate group means ± SE. BAY 58 was protective, and this protection was completely blocked by l-NAME. *P < 0.001.
Fig. 7.
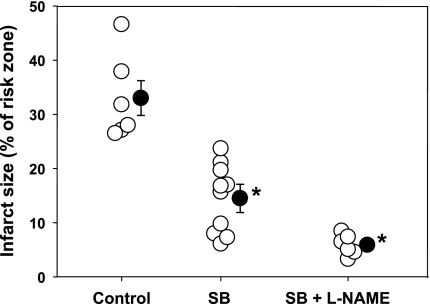
Infarct size as a percentage of risk zone for control group 2 rabbits and those treated with SB alone or simultaneously with l-NAME. Open circles represent individual hearts, and solid circles indicate group means ± SE. SB was protective, and this protection was not affected by l-NAME. *P < 0.001.
The highly selective A2bAR agonist BAY 60 was very protective (infarction 11.9 ± 1.4% of risk zone, P < 0.001), and, as anticipated in light of the previous findings with 5'-(N-ethylcarboxamido)adenosine (NECA) (51), l-NAME completely aborted this protection (Fig. 3). Wort and PD had similar effects on BAY 60's infarct-sparing effect, indicating RISK is downstream of A2bAR. NO acts in many pathways by activating guanylyl cyclase to make the PKG activator cGMP. The cell-permeant cGMP analog CPT-cGMP at reperfusion mimics IPC's protection and was found to do so in an A2bAR-dependent manner (25). l-NAME completely blocked protection from CPT-cGMP as well (Fig. 3), indicating that the NOS site that l-NAME blocked is not followed by a PKG step.
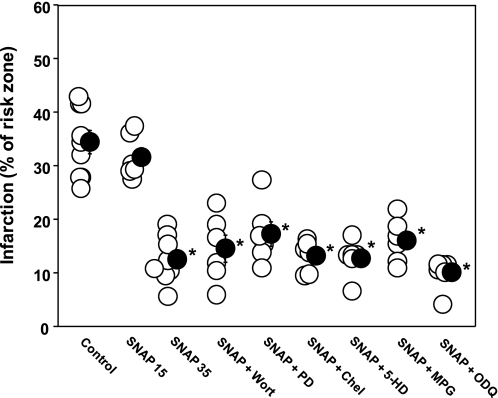
Because these data implied that there was a signaling step downstream of the adenosine A2bAR that involved NOS, we explored this pathway by administering SNAP and a series of antagonists of known components of IPC's protective pathway. As shown in Fig. 4, infusing SNAP for only 15 min was not protective, but infusing it for 35 min was. Antagonists of PI3-kinase (Wort), ERK (PD), or PKC (Chel), the mKATP channel blocker (5-HD), or the ROS scavenger MPG had no effect on SNAP's protection, yet all of the above had blocked protection from the cGMP analog CPT-cGMP when given at reperfusion (25). We previously demonstrated that NO produced in IPC's trigger phase protects by activating guanylyl cyclase to make cGMP (38). However, the guanylyl cyclase antagonist ODQ did not block SNAP's infarct-sparing ability (Fig. 4). This observation again indicated that NO was protecting through a PKG-independent pathway.
Fig. 4.
Infarct size as a percentage of risk zone for control group 1 rabbits and those treated with 2 μM SNAP for either 15 (SNAP 15) or 35 min (SNAP 35) and rabbits treated simultaneously with the 35-min SNAP infusion and Wort, PD, Chel, 5-HD, MPG, or ODQ. Open circles represent individual hearts, and solid circles indicate group means ± SE. The longer infusion of SNAP was protective, and this protection could not be abrogated by any of the antagonists (Wort, PD, Chel, 5-HD, ODQ), the reactive oxygen species scavenger (MPG), or the KATP channel closer (5-HD). *P < 0.001.
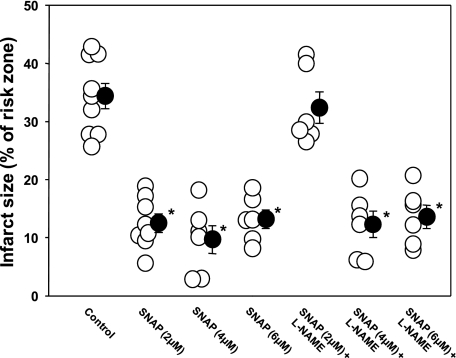
Our experimental design depends on SNAP being strictly an NO donor and l-NAME being strictly a NOS inhibitor. We devised a simple test of their specificity. If l-NAME could block protection from SNAP, then one of them must have a nonspecific effect. Figure 5 shows the results. SNAP was tested at three different doses, 2, 4, and 6 μM, given over 35 min in both the presence and absence of l-NAME. When the two were combined in experiments in which the standard SNAP concentration of 2 μM was employed, protection was in fact absent. However, when the concentration of SNAP was increased to 4 or 6 μM, protection could not be blocked by l-NAME. The failure of SNAP to protect at 2 μM in the presence of l-NAME was presumably because the loss of endogenous NO in the presence of l-NAME put the tissue NO level below the threshold needed for protection. We concluded that l-NAME could not block protection from SNAP, indicating that our tools were indeed selective.
Fig. 5.
SNAP was administered at 3 different doses in the presence and absence of l-NAME. l-NAME blocked the protection from low-dose but not high-dose SNAP. Loss of protection at the low dose of SNAP was presumably due to the loss of endogenous NO, which would have been additive with that from SNAP. Open circles represent individual hearts, and solid circles indicate group means ± SE. *P < 0.001.
We additionally evaluated the effect of l-NAME on the protection seen after activation of soluble guanylyl cyclase with BAY 58. As expected, the latter significantly decreased infarct size (Fig. 6). l-NAME completely blocked this protective effect, indicating that PKG activation protects in a NO-dependent manner. Again, that indicates that NO's protection must be independent of PKG. It should be mentioned that in a previous study, l-NAME failed to block protection from BAY 58 in an isolated rat heart (21). l-NAME increased infarct size, but its effect failed to achieve statistical significance.
Inhibition of GSK-3β through phosphorylation is thought to be one of the very distal signal transduction events in IPC's signaling pathway (18). As has previously been demonstrated (15), SB, an antagonist of GSK-3β, was very protective when administered at reperfusion (Fig. 7). l-NAME had no effect on SB's protection, implying that NO is acting upstream of GSK-3β.
In addition to calculating infarct/risk zone ratios (Table 1 and Figs. 3–7), we constructed plots of infarct size vs. risk zone to ensure that the linear relationships and regressions for allegedly protected groups were significantly different from those of the control groups. Indeed, relationships for IPC × 3, BAY 60, CPT-cGMP, 2–6 μM SNAP for 35 min, SNAP + Wort, SNAP + PD, SNAP + 5-HD, SNAP + Chel, SNAP + MPG, SNAP + ODQ, 4 and 6 μM SNAP + l-NAME, SB, SB + l-NAME, and BAY 58 were significantly different from the respective control group (data not shown).
DISCUSSION
Our current results suggest that exogenous NO can induce powerful cardioprotective signaling when administered at the beginning of reperfusion and that endogenous NO production is involved in the signal transduction events associated with the mediator phase of both IPC and pharmacological postconditioning. Although NO's involvement in IPC's trigger pathway where it opens mKATP channels in a PKG-dependent manner (10) is well known, we also uncovered a second NO step that is activated at reperfusion and is located downstream of all known cytoplasmic signaling elements in the pathway other than perhaps GSK-3β. Our results suggest that this second NO site protects in a PKG-independent fashion. These findings lead us to propose the scheme presented in Fig. 1, where NOS appears in both the trigger and mediator pathways.
Several previous studies had indicated that elevation of cardiac cGMP at reperfusion would protect the heart, implicating PKG's involvement in these cardioprotective interventions (8). We found that the PKG activator CPT-cGMP given at reperfusion produces protection, but it does so by stimulating the distal portion of the trigger pathway (see Fig. 1), since its protection can be blocked by a mKATP blocker, ROS scavenger, PKC blocker, A2bAR blocker, or inhibitors of RISK (25). Therefore, it appeared logical that giving NO to the heart at reperfusion should protect by activating guanylyl cyclase ahead of PKG and that this protection would also be vulnerable to all of the above blockers. Some observations seemed to support this assumption as well. Statins upregulate NOS activity predominantly by posttranscriptional mechanisms and increase eNOS mRNA stability (26, 27); they protect the heart when administered at reperfusion, and this protection is aborted by l-NAME (48) as well as the RISK antagonist Wort (2, 48). Inhibitors of phosphodiesterase-5, such as sildenafil and vardenafil, enhance NO-driven cGMP accumulation. These drugs, too, are cardioprotective when administered at reperfusion (30, 39). Predictably, their cardioprotective effect is aborted by the guanylyl cyclase inhibitor ODQ and the PKG antagonist KT 5823 (30) as well as 5-HD (39). BAY 58, an NO-independent guanylyl cyclase activator, had a postconditioning effect in both rabbit and rat hearts (21), and protection was aborted by either the direct PKG antagonist KT 5823 or the mKATP blocker 5-HD. The atrial (ANP) and brain (BNP) natriuretic peptides activate particulate guanylyl cyclase, and they, too, are very protective when administered at reperfusion (4, 52). All of the above findings would suggest that increasing NO at reperfusion should protect and should do so by stimulating guanylyl cyclase.
However, there were also unexplained observations. Natriuretic peptides should activate PKG independently of NOS, but the protective effect of BNP was still abrogated by l-NAME (4). A2b antagonists block protection from CPT-cGMP (25), and yet the protection from BAY 60 in the present study could be blocked by l-NAME, as could that from BAY 58. Protection from the A2bAR agonist NECA was also blocked by l-NAME (51). All of these seemingly discrepant observations would best be explained by a second NOS site deep in the mediator pathway that acts independently of cGMP and PKG. Had NO's postconditioning effect been dependent on PKG, then SNAP and CPT-cGMP should have behaved identically to the inhibitors, but they did not. We therefore concluded that PKG or A2bAR activation leads to NO generation that protects the reperfused heart in a PKG-independent fashion.
Burley and Baxter (4) reported that a 15-min infusion of SNAP at reperfusion had no effect, and they concluded exogenous NO was not cardioprotective. In the current study we reproduced their observation in our rabbit heart model with a 15-min infusion of SNAP (Fig. 4). However, we have noted on multiple occasions that agonists that are protective at reperfusion usually must be infused for longer than 15 min. We have interpreted this to mean that it is necessary to continue the signaling that keeps mitochondrial permeability transition pores closed until the cell is sufficiently recovered to perform without external support (43, 49). Additionally, kinetics may have been involved. Although exposure of bovine chromaffin cells to SNAP for 15 min produced maximal cGMP production (13), NO production by human ciliary smooth muscle cells peaked only after a 30-min exposure to SNAP (6). At 15 min, the increase was ∼2.9-fold over the basal level. That prompted us to test a longer SNAP infusion of 35 min, which did salvage ischemic myocardium. We used SNAP in an earlier study and found that its protection of the rabbit heart could be blocked by either a mKATP blocker or a radical scavenger (38). Giricz et al. (14) protected rat hearts by pretreating them with SNAP, and that protection was blocked by either of the PKG inhibitors KT 5823 or Rp-8pCPT-PET-cGMPs, again showing that NO clearly triggers protection via PKG. However, in those two studies SNAP was given as a preconditioning pulse, where it would have activated only the trigger pathway, and was not present at reperfusion. Because SNAP was not present at reperfusion, those results are not discordant with the present ones.
Xu et al. (50) observed in isolated rat cardiomyocytes that adenosine stimulates NO production by activating A2 adenosine receptors, and this increased production could be aborted by l-NAME. Although this observation is consistent with our data, the authors also noted that adenosine can prevent H2O2-induced depolarization of mitochondrial membrane potential, protection which was partially blocked by l-NAME and the PKG inhibitor KT 5823. This partial blockade by KT 5823 implies a PKG-dependent component in the mechanism. Unfortunately, their protocol used authentic adenosine, which is not receptor selective, and it is unclear whether it protected by first conditioning A2b receptors through the traditional trigger pathway (22), which does involve a PKG-dependent step, or by directly activating naive A2b adenosine receptors and triggering signaling, which according to the present data should be unaffected by a PKG blocker.
The most likely NOS responsible for the mediator NO release in these studies is endothelial NOS (eNOS), because signal transduction pathways do not regulate inducible NOS (iNOS). Probably the most serious challenges to the present findings are the recent studies done in eNOS knockout mice. Two strains of eNOS knockout mice have been designed, one developed at the University of North Carolina (UNC) and the other at Harvard. Of course, in eNOS knockout mice the absence of eNOS should affect both the trigger and mediator phases and block any pre- or postconditioning protection. As expected, the UNC knockouts could not be preconditioned with a single cycle of ischemia (3). Unexpectedly, however, these hearts could be protected if multiple cycles were employed. Current evidence indicates that IPC's trigger pathway involves three receptors. Bradykinin and opioid receptors couple to PKC through a pathway that includes eNOS, whereas the adenosine A1/A3 receptors couple to PKC directly through the phospholipases. Multiple cycles of IPC would produce more adenosine that could bypass the trigger NO step and restore protection in the face of NOS blockade of that early step (8). However, if our theory of a mediator NO step were accurate, then the absence of eNOS at reperfusion should have blocked protection in these eNOS knockouts, and salvage should have been impossible even with multiple preconditioning cycles. Indeed, in the present study multiple cycles of IPC could not overcome l-NAME's NOS blockade at reperfusion. This conundrum appeared to be resolved by Sharp et al. (40), who found smaller infarcts in UNC eNOS-deficient mice than in wild-type mice, and this was attributed to a compensatory elevation of iNOS. The latter would have substituted for the absence of eNOS at reperfusion, resulting in salvage in Bell and Yellon's eNOS knockout mice (3). The Harvard knockout strain had no compensatory increase of iNOS, and those hearts had bigger infarcts and could not be protected with IPC (40). These data support our hypothesis of eNOS acting in both the trigger and mediator phases.
However, the above observations were challenged by Guo et al. (16), who studied both UNC and Harvard strains in in situ preparations. Infarcts were equivalent in wild-type mice and both knockout stains. IPC with either one or six preconditioning cycles of ischemia protected UNC hearts, and the authors found no increase in iNOS in these hearts. These observations argue against the need for eNOS in either the trigger or mediator phase. Unfortunately, there was no attempt to precondition the Harvard knockout mice. Finally, in contrast to our observation that l-NAME blocked IPC's protection in rabbits (8), Guo et al. (16) noted that injecting the NOS inhibitor Nω-nitro-l-arginine into wild-type mice before IPC failed to block its protection, yet NOS should have been blocked in both the trigger and mediator phases. They concluded that, at least in the mouse heart, NO was not involved in IPC's protection.
The source of the discrepancy among the above three studies in mice is unclear, since all studies were done in respected and established laboratories. Infarct studies in mice are technically challenging, however, and methodological differences between laboratories are probably involved. The present study was done in a different species, rabbit, but supports the earlier observations of Bell and Yellon (3) and Sharp et al. (40). Our study may be criticized because it relies completely on pharmacological tools, but, as can be seen, genetically altered mouse models do not always resolve the issues either. One possible explanation for the confusion with these eNOS knockout studies is that neuronal NOS (nNOS), which is also expressed in the heart (1), could have been the source of cardioprotective NO.
Inherent assumptions used in the interpretation of our data are that SNAP protects by liberating NO and that l-NAME blocks protection by blocking NOS. The one test we could devise to test that assumption was to combine the two in the infarct model. l-NAME should not block SNAP's protection in this scheme. In our initial attempts protection was absent, but then we reasoned that l-NAME would block all endogenous NO production so that a higher concentration of SNAP might be needed. Indeed, when we doubled and tripled the SNAP concentration, protection was evident, suggesting that neither SNAP nor l-NAME had any influential nonspecific effects that would preclude their use in these investigations.
Of course, NO can also be produced by nonenzymatic chemical reactions. Zweier et al. (54) reported that NO could be generated in the ischemic heart by direct reduction of nitrite in the hypoxic, acidotic milieu encountered under such conditions, and this route of NO formation was not blocked by NOS inhibitors. In our experiments l-NAME did block protection, thus implying a critically important enzymatic source of NO.
NO can cause posttranslational modification of target proteins independently of cGMP and PKG by nitrosylation of their thiol groups (S-nitrosylation). In fact, many proteins in an IPC heart become S-nitrosylated (44). Estrogen receptor agonists protect ischemic hearts against infarction and S-nitrosylate many proteins, and l-NAME blocks both effects (28). S-nitrosylation of the L-type calcium channel has received much attention as a possible mechanism of IPC's protection (45), but many other mitochondrial proteins are also nitrosylated by IPC (44). When S-nitrosylated, mitochondrial complex I produces fewer free radicals (41), possibly attenuating formation of mitochondrial permeability transition pores, the putative end-effectors of IPC's protection. Accordingly, NO attenuated ROS production and matrix Fe2+ and preserved aconitase activity in reoxygenated isolated mitochondria (20). Costa and Garlid (9) reported that SNAP inhibited transition pore formation in isolated rat mitochondria, but in that model a PKC inhibitor blocked the protection. In the present study inhibition of PKC had no effect on SNAP's protection. S-nitrosylation of the phosphatase PTEN inhibits it (7), which could protect the heart by augmenting PI3-kinase and Akt activity. However, the latter possibility seems unlikely, since Wort did not affect SNAP's protection in the present study either. The nitrosylating agent S-nitroso-2-mercaptopropionyl glycine given at reperfusion mimics IPC's protection in mouse heart (32), and a PKG-independent action of NO has been proposed to inhibit permeability transition pore formation in mitochondria after IPC [for review see Burwell and Brooks (5)]. In summary, it is not difficult to imagine how NO could protect the heart through a PKG-independent mechanism.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grant HL-20468.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O'Rourke B, Rodriguez ER, Huang PL, Lima JAC, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 416: 337–340, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Bell RM, Yellon DM. Atorvastatin, administered at the onset of reperfusion, and independent of lipid lowering, protects the myocardium by up-regulating a pro-survival pathway. J Am Coll Cardiol 41: 508–515, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Bell RM, Yellon DM. The contribution of endothelial nitric oxide synthase to early ischaemic preconditioning: the lowering of the preconditioning threshold. An investigation in eNOS knockout mice. Cardiovasc Res 52: 274–280, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Burley DS, Baxter GF. B-type natriuretic peptide at early reperfusion limits infarct size in the rat isolated heart. Basic Res Cardiol 102: 529–541, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Burwell LS, Brookes PS. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia-reperfusion injury. Antioxid Redox Signal 10: 579–599, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Carreiro S, Anderson S, Gukasyan HJ, Krauss A, Prasanna G. Correlation of in vitro and in vivo kinetics of nitric oxide donors in ocular tissues. J Ocul Pharmacol Ther 25: 105–112, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Carver DJ, Gaston B, deRonde K, Palmer LA. Akt-mediated activation of HIF-1 in pulmonary vascular endothelial cells by S-nitrosoglutathione. Am J Respir Cell Mol Biol 37: 255–263, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen MV, Yang XM, Downey JM. Nitric oxide is a preconditioning mimetic and cardioprotectant and is the basis of many available infarct-sparing strategies. Cardiovasc Res 70: 231–239, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Costa ADT, Garlid KD. Intramitochondrial signaling: interactions among mitoKATP, PKCε, ROS, and MPT. Am J Physiol Heart Circ Physiol 295: H874–H882, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa ADT, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, Critz SD. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res 97: 329–336, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Dost T, Cohen MV, Downey JM. Redox signaling triggers protection during the reperfusion rather than the ischemic phase of preconditioning. Basic Res Cardiol 103: 378–384, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Downey JM, Krieg T, Cohen MV. Mapping preconditioning's signaling pathways: an engineering approach. Ann NY Acad Sci 1123: 187–196, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Ferrero R, Rodríguez-Pascual F, Miras-Portugal MT, Torres M. Comparative effects of several nitric oxide donors on intracellular cyclic GMP levels in bovine chromaffin cells: correlation with nitric oxide production. Br J Pharmacol 127: 779–787, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giricz Z, Görbe A, Pipis J, Burley DS, Ferdinandy P, Baxter GF. Hyperlipidaemia induced by a high-cholesterol diet leads to the deterioration of guanosine-3′,5′-cyclic monophosphate/protein kinase G-dependent cardioprotection in rats. Br J Pharmacol 158: 1495–1502, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross ER, Hsu AK, Gross GJ. GSK3β inhibition and KATP channel opening mediate acute opioid-induced cardioprotection at reperfusion. Basic Res Cardiol 102: 341–349, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Guo Y, Li Q, Wu WJ, Tan W, Zhu X, Mu J, Bolli R. Endothelial nitric oxide synthase is not necessary for the early phase of ischemic preconditioning in the mouse. J Mol Cell Cardiol 44: 496–501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol 288: H971–H976, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Role of glycogen synthase kinase-3β in cardioprotection. Circ Res 104: 1240–1252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, Seguchi O, Myoishi M, Minamino T, Ohara T, Nagai Y, Nanto S, Watanabe K, Fukuzawa S, Hirayama A, Nakamura N, Kimura K, Fujii K, Ishihara M, Saito Y, Tomoike H, Kitamura S. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet 370: 1483–1493, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Korge P, Ping P, Weiss JN. Reactive oxygen species production in energized cardiac mitochondria during hypoxia/reoxygenation: modulation by nitric oxide. Circ Res 103: 873–880, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krieg T, Liu Y, Rütz T, Methner C, Yang XM, Dost T, Felix SB, Stasch JP, Cohen MV, Downey JM. BAY 58–2667, a nitric oxide-independent guanylyl cyclase activator, pharmacologically post-conditions rabbit and rat hearts. Eur Heart J 30: 1607–1613, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Krieg T, Qin Q, McIntosh EC, Cohen MV, Downey JM. ACh and adenosine activate PI3-kinase in rabbit hearts through transactivation of receptor tyrosine kinases. Am J Physiol Heart Circ Physiol 283: H2322–H2330, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Krieg T, Qin Q, Philipp S, Alexeyev MF, Cohen MV, Downey JM. Acetylcholine and bradykinin trigger preconditioning in the heart through a pathway that includes Akt and NOS. Am J Physiol Heart Circ Physiol 287: H2606–H2611, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Kuno A, Critz SD, Cui L, Solodushko V, Yang XM, Krahn T, Albrecht B, Philipp S, Cohen MV, Downey JM. Protein kinase C protects preconditioned rabbit hearts by increasing sensitivity of adenosine A2b-dependent signaling during early reperfusion. J Mol Cell Cardiol 43: 262–271, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuno A, Solenkova NV, Solodushko V, Dost T, Liu Y, Yang XM, Cohen MV, Downey JM. Infarct limitation by a protein kinase G activator at reperfusion in rabbit hearts is dependent on sensitizing the heart to A2b agonists by protein kinase C. Am J Physiol Heart Circ Physiol 295: H1288–H1295, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 97: 1129–1135, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem 273: 24266–24271, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Lin J, Steenbergen C, Murphy E, Sun J. Estrogen receptor-β activation results in S-nitrosylation of proteins involved in cardioprotection. Circulation 120: 245–254, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Yang XM, Iliodromitis EK, Kremastinos DT, Dost T, Cohen MV, Downey JM. Redox signaling at reperfusion is required for protection from ischemic preconditioning but not from a direct PKC activator. Basic Res Cardiol 103: 54–59, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maas O, Donat U, Frenzel M, Rütz T, Kroemer HK, Felix SB, Krieg T. Vardenafil protects isolated rat hearts at reperfusion dependent on GC and PKG. Br J Pharmacol 154: 25–31, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miura T, Miki T. Limitation of myocardial infarct size in the clinical setting: current status and challenges in translating animal experiments into clinical therapy. Basic Res Cardiol 103: 501–513, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Nadtochiy SM, Burwell LS, Ingraham CA, Spencer CM, Friedman AE, Pinkert CA, Brookes PS. In vivo cardioprotection by S-nitroso-2-mercaptopropionyl glycine. J Mol Cell Cardiol 46: 960–968, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Research Council Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press, 1996 [Google Scholar]
- 34.Oldenburg O, Qin Q, Krieg T, Yang XM, Philipp S, Critz SD, Cohen MV, Downey JM. Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am J Physiol Heart Circ Physiol 286: H468–H476, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Pain T, Yang XM, Critz SD, Yue Y, Nakano A, Liu GS, Heusch G, Cohen MV, Downey JM. Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ Res 87: 460–466, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Philipp S, Yang XM, Cui L, Davis AM, Downey JM, Cohen MV. Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res 70: 308–314, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, André-Fouët X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med 359: 473–481, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Qin Q, Yang XM, Cui L, Critz SD, Cohen MV, Browner NC, Lincoln TM, Downey JM. Exogenous NO triggers preconditioning via a cGMP- and mitoKATP-dependent mechanism. Am J Physiol Heart Circ Physiol 287: H712–H718, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Salloum FN, Takenoshita Y, Ockaili RA, Daoud VP, Chou E, Yoshida K, Kukreja RC. Sildenafil and vardenafil but not nitroglycerin limit myocardial infarction through opening of mitochondrial KATP channels when administered at reperfusion following ischemia in rabbits. J Mol Cell Cardiol 42: 453–458, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharp BR, Jones SP, Rimmer DM, Lefer DJ. Differential response to myocardial reperfusion injury in eNOS-deficient mice. Am J Physiol Heart Circ Physiol 282: H2422–H2426, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med 204: 2089–2102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, Schulz R, Heusch G. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res 104: 15–18, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Solenkova NV, Solodushko V, Cohen MV, Downey JM. Endogenous adenosine protects preconditioned heart during early minutes of reperfusion by activating Akt. Am J Physiol Heart Circ Physiol 290: H441–H449, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res 101: 1155–1163, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C, Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel α1 subunit and reduced ischemia/reperfusion injury. Circ Res 98: 403–411, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Tanno M, Tsuchida A, Nozawa Y, Matsumoto T, Hasegawa T, Miura T, Shimamoto K. Roles of tyrosine kinase and protein kinase C in infarct size limitation by repetitive ischemic preconditioning in the rat. J Cardiovasc Pharmacol 35: 345–352, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Tissier R, Cohen MV, Downey JM. Protecting the acutely ischemic myocardium beyond reperfusion therapies: are we any closer to realizing the dream of infarct size elimination? Arch Mal Coeur Vaiss 100: 794–802, 2007 [PubMed] [Google Scholar]
- 48.Wolfrum S, Dendorfer A, Schutt M, Weidtmann B, Heep A, Tempel K, Klein HH, Dominiak P, Richardt G. Simvastatin acutely reduces myocardial reperfusion injury in vivo by activating the phosphatidylinositide 3-kinase/Akt pathway. J Cardiovasc Pharmacol 44: 348–355, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Xu Z, Downey JM, Cohen MV. Timing and duration of administration are crucial for antiinfarct effect of AMP 579 infused at reperfusion in rabbit heart. Heart Dis 5: 368–371, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Xu Z, Park SS, Mueller RA, Bagnell RC, Patterson C, Boysen PG. Adenosine produces nitric oxide and prevents mitochondrial oxidant damage in rat cardiomyocytes. Cardiovasc Res 65: 803–812, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Yang XM, Krieg T, Cui L, Downey JM, Cohen MV. NECA and bradykinin at reperfusion reduce infarction in rabbit hearts by signaling through PI3K, ERK, and NO. J Mol Cell Cardiol 36: 411–421, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Yang XM, Philipp S, Downey JM, Cohen MV. Atrial natriuretic peptide administered just prior to reperfusion limits infarction in rabbit hearts. Basic Res Cardiol 101: 311–318, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Yang XM, Philipp S, Downey JM, Cohen MV. Postconditioning's protection is not dependent on circulating blood factors or cells but involves adenosine receptors and requires PI3-kinase and guanylyl cyclase activation. Basic Res Cardiol 100: 57–63, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med 1: 804–809, 1995 [DOI] [PubMed] [Google Scholar]