Abstract
Multiple, perhaps interactive, mechanisms participate in the linkage between increased neural activity and cerebral vasodilation. In the present study, we assessed whether neural activation-related pial arteriolar dilation (PAD) involved interactions among adenosine (Ado) A2 receptors (A2Rs), large-conductance Ca2+-operated K+ (BKCa) channels, and inward rectifier K+ (Kir) channels. In rats with closed cranial windows, we monitored sciatic nerve stimulation (SNS)-induced PAD in the absence or presence of pharmacological blockade of A2Rs (ZM-241385), ecto-5′-nucleotidase (α,β-methylene-adenosine diphosphate), BKCa channels (paxilline), and Kir channels (BaCl2). Individually, these interventions led to 53–66% reductions in SNS-induced PADs. Combined applications of these blockers led to little or no further repression of SNS-induced PADs, suggesting interactions among A2Rs and K+ channels. In the absence of SNS, BaCl2 blockade of Kir channels produced 52–80% reductions in Ado and NS-1619 (BKCa channel activator)-induced PADs. In contrast, paxilline blockade of BKCa channels was without effect on dilations elicited by KCl (Kir channel activator) and Ado suffusions, indicating that Ado- and NS-1619-associated PADs involved Kir channels. In addition, targeted ablation of the superficial glia limitans was associated with a selective 60–80% loss of NS-1619 responses, suggesting that the BKCa channel participation (and paxilline sensitivity) derived largely from channels within the glia limitans. Additionally, blockade of either PKA or adenylyl cyclase caused markedly attenuated pial arteriolar responses to SNS and, in the absence of SNS, responses to Ado, KCl, and NS-1619. These findings suggested a key, possibly permissive, role for A2R-linked cAMP generation and PKA-induced K+ channel phosphorylation in somatosensory activation-evoked PAD.
Keywords: neurovascular coupling, phosphorylation, adenylyl cyclase, cAMP-dependent protein kinase, cGMP-dependent protein kinase
neurovascular coupling is often defined as the cerebral hemodynamic changes associated with local neuronal (synaptic) activation. In the brain, enhanced synaptic activity results in the dilation of parenchymal arterioles in the vicinity of activated neurons, with transmission of the vasodilating signal involving astrocytes (12, 13, 29). In the cerebral cortex, that astrocytic influence also appears to include the upstream dilation of pial arterioles, thereby permitting more blood to reach the dilated downstream parenchymal arteriolar segments that lie in the vicinity of activated neurons (34, 35). As a prime example of this phenomenon, the somatosensory activation paradigm, sciatic nerve stimulation (SNS), results in the rapid dilation of pial arterioles overlying the hindlimb region of the somatosensory cortex (18, 30, 35). This occurs despite the general absence of direct contacts from the underlying neurons to the pial arterioles. The process of upstream dilation also occurs in peripheral vascular beds and involves signaling via vascular myoendothelial pathways (8). In contrast, the endothelium does not appear to participate in SNS-evoked pial arteriolar dilations (35).
Recent findings from our laboratory have indicated that astrocytes and the glia limitans play an essential role in signaling pial arterioles to dilate after SNS (35). Furthermore, results from preliminary studies have revealed important roles for large-conductance Ca2+-operated K+ (BKCa) channels and inward rectifier K+ (Kir) channels in the process of SNS-related pial arteriolar dilation (25, 37). Based on findings obtained in brain slices and, more recently, in vivo (5, 6), it is thought that increased neuronal activity gives rise to increases in intracellular Ca2+ in nearby astrocytes that may spread to additional astrocytes, ultimately promoting the opening of BKCa channels on astrocytic endfeet (27) in contact with arterioles. The K+ released to the extracellular space through these BKCa channels can interact with Kir channels on arteriolar smooth muscle, leading to smooth muscle cell K+ efflux, hyperpolarization, and relaxation. It has also been postulated that the interastrocytic Ca2+ “wave” may be linked to ATP release to the extracellular space. The extracellular ATP can interact with metabotropic purinergic receptors on neighboring astrocytes, giving rise to Ca2+ release from stores (and propagation of the Ca2+ wave) or be hydrolyzed to adenosine (Ado) via ecto-nucleotidases (36). The Ado formed in this process appears to play a major role in SNS-induced pial arteriolar dilation, as reported by Meno et al. (18), who found that the vasodilating response was substantially repressed in the presence of a selective Ado A2 receptor blocker, ZM-241385.
However, a large overlap in Ado- and K+ channel-related contributions to the pial arteriolar responses elicited by SNS was indicated in the studies cited above. This led us to suspect that an interaction between Ado and K+ channels is involved in SNS-induced pial arteriolar relaxation. Adenosine-induced pial arteriolar dilation has been attributed to the activation of Gs-linked smooth muscle Ado A2 receptors (see Ref. 12). These receptors are known to be coupled to adenylyl cyclase (AC) activation and increased cAMP generation. In addition, increased cAMP, via the activation of PKA, has been linked to enhanced function of BKCa and Kir channels on vascular smooth muscle cells (for a review, see Ref. 11).
Based on the above considerations, we initially hypothesized, and subsequently confirmed, that exposure to selective blockers of A2 receptors (ZM-241385), Kir channels (BaCl2), and BKCa channels (paxilline) was associated with a nonadditive (overlapping) attenuation in SNS-induced pial arteriolar dilations. It seemed likely that the overlap in the contributions from the two K+ channels would, at least in part, relate to recent findings pointing to a coupled role for astrocytic endfoot BKCa channels and smooth muscle Kir channels (5, 6) in neurally evoked dilation of cerebral arterioles. Thus, in the context of the present study, the opening of BKCa channels in the glia limitans releases K+ to the extracellular space. That K+ then increases the conductance of Kir channels on pial arteriolar smooth muscle, promoting vascular relaxation. This model favors the segregation of Kir and BKCa channels to sites on pial vascular smooth muscle and the glia limitans, respectively. Accordingly, based on such a model, experiments were designed to address the following hypotheses: 1) Ba2+-related blockade of Kir channels would not only attenuate K+-induced pial arteriolar dilations and SNS-induced dilations but also (under resting conditions) BKCa agonist-induced dilations as well; 2) conversely, BKCa blockade, in the absence of SNS, would likely have little or no influence on K+-induced (Kir channel-mediated) pial arteriolar relaxation; and 3) using an established technique for selective glia limitans ablation (34, 35), we would anticipate, based on the aforementioned Kir/BKCa channel “segregation model,” that BKCa channel opener-induced dilations would be impaired, whereas K+ responses would be unaffected. The final hypothesis addressed in this investigation was that the overlap between A2 receptor, BKCa channel, and Kir channel blockade effects on SNS was also a function of A2 receptor-linked AC activation and subsequent PKA-mediated phosphorylation of BKCa and/or Kir channels, thereby enhancing the vasodilatory functions of these channels. To that end, we examined the effects of PKA and AC blockade on SNS-, Ado-, K+-, and BKCa opener-induced pial arteriolar dilations.
METHODS
Animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Illinois (Chicago, IL). Female Sprague-Dawley rats (250–300 g) were used in this study.
Cranial window preparation.
After anesthesia induction with isoflurane, rats were endotracheally intubated and mechanically ventilated. Surgery was performed under continuous anesthesia with 2.0% isoflurane-70% N2O-30% O2. Bilateral femoral arterial and venous catheters were placed for systemic arterial pressure monitoring, arterial blood gas measurement, and intravenous drug infusion. Rats were then prepared for the placement of a closed cranial window. The surgical procedures for closed cranial window installation have been described in previous reports from our laboratory (35, 38). Briefly, a craniotomy (10 mm in diameter) was performed, and the underlying dura was carefully removed, keeping the sagittal sinus intact. A glass cranial window (11-mm diameter, 1.5 mm thick) equipped with three ports (inflow, outflow, and intracranial pressure monitoring) was fixed to the skull with cyanoacrylate gel (3M, St. Paul, MN). After the cranial window placement, isoflurane was discontinued, and a 10 μg/kg iv fentanyl bolus was given. The rat was then maintained on 70% N2O-30% O2-fentanyl (25 μg/kg/h iv) thereafter. Artificial cerebrospinal fluid (aCSF), at 37°C, which was equilibrated with 10% O2-5% CO2-balance N2, was suffused at 0.5 ml/min through the space under the window. Body temperature was maintained at 37°C with a servocontrol heating pad, and mean arterial blood pressure and intracranial pressure were continuously monitored during the experiment. Arterial blood samples were taken at 60-min intervals for arterial Po2, arterial Pco2, and pH analysis using a blood gas/pH analyzer (GEM Premier 3000, Instrumentation Laboratory, Lexington, MA). Arterial Po2 (≥100 mmHg), arterial Pco2 (32–40 mmHg), and pH (7.35–7.44) were maintained throughout the study.
Neuronal activation model.
Neural activation was induced by SNS. Briefly, the contralateral sciatic nerve was dissected in the region of the sciatic notch. The proximal end was placed in a silver ring electrode, which was insulated with silicone rubber. The nerve was electrically stimulated for 20 s with square-wave pulses (current: 0.7 mA, frequency: 2 Hz, and pulse duration: 0.1 ms) using a stimulator (Stimulator HC, ML155, AD Instruments, Sydney, Australia). Somatosensory evoked potentials generated during SNS were recorded from the somatosensory cortex, first in the absence and then in the presence of the pharmacological blockers listed below. Bipolar recordings were made from stainless steel screws fastened to the skull, with a reference placed in the occipital bone. N1P1 amplitude differences were acquired and analyzed using Powerlab 4/30 and Labchart 7 (AD Instruments). Electrophysiological signals were digitized at 2 kb/s and bandpass filtered (0.1–1 kHz).
Evaluation of pial arteriolar responses.
Pial arterioles (30–50 μm in diameter) located in the contralateral hindlimb projection area of the somatosensory cortex (∼2 mm caudal and lateral to the bregma) were selected for study. Arteriolar responses were observed using a Nikon microscope equipped with an epi-illumination darkfield system. Images were captured using a digital video camera (CoolSNAP ES, Fryer, Huntley, IL), projected on a computer monitor, and saved for subsequent diameter measurements using MetaMorph software (Universal Imaging, Downingtown, PA). In each individual experiment, diameter values from three segments of the selected arterioles were obtained and averaged. Pial arteriolar reactivity was expressed as the percent change in arteriolar diameter relative to the initial baseline. In all experiments, the initial diameter measurements were made 1 h after isoflurane and 45 min after the initiation of the drug-free aCSF suffusion. Hypercapnia (Pco2 ≈ 70 mmHg) was imposed to test pial arteriolar reactivity. Only those vessels displaying adequate responses to CO2 (reactivity > 1.0, calculated as the percentage of diameter increase/mmHg Pco2 change) were selected for this study. However, only two animals were excluded according to this criterion.
Experimental groups and rationale.
Rats were divided into five experimental groups based on several interrelated hypotheses. Group 1 addressed the hypothesis that Ado, as well as Kir and BKCa channels, participate in SNS-induced pial arteriolar dilations in a nonadditive manner. Thus, selective inhibitors of Ado A2 receptors, Ado generation from AMP, Kir channels, and BKCa channels were topically applied, individually or in combination, to examine the roles of Ado, Kir channels, and BKCa channels in neural activation-evoked vasodilations. Group 2 was devised to better clarify the interactions among Ado receptors, BKCa channels, and Kir channels. To that end, in the absence of SNS, we examined 1) whether Kir channel blockade (with 100 μM BaCl2) attenuated Ado- and BKCa channel opener-evoked dilations, 2) whether BKCa blockade (with 10 μM paxilline) also attenuated Ado and K+ (Kir channel)-mediated pial arteriolar relaxations, and 3) whether Ado A2 receptor blockade (with 10 μM ZM-241385) would have any influence on pial arteriolar dilations elicited by K+ and NS-1619. Group 3 addressed the hypothesis that the combined, but nonadditive, dependence of SNS-induced pial arteriolar dilations on Ado A2 receptors and BKCa/Kir channels relates to A2 receptor-associated activation of AC and the subsequent PKA-linked phosphorylation of BKCa and Kir channels, potentiating their K+ extrusion function. Group 3 was separated into three subgroups. Subgroups 1 and 2 examined the effects of PKA inhibition (using two chemically dissimilar blockers) on SNS-, Ado-, NS-1619-, and K+-induced pial arteriolar diameter increases. In subgroup 3, we used a selective AC blocker in the place of PKA inhibitors. In group 4, since cAMP may cross-activate PKG (see Refs. 14 and 24), we tested the involvement of PKG in SNS- and Ado-induced pial arteriolar dilation using a selective PKG blocker. Finally, in group 5, we endeavored to obtain evidence of the importance of astrocytic endfoot localization of BKCa channels to the pial arteriolar dilations elicited by BKCa activation. To that end, we monitored NS-1619 responses in the absence and presence of glia limitans ablation using a 24-h topical exposure to the selective gliotoxin l-α-aminoadipic acid (l-AAA). Control rats were given aCSF vehicle in the place of l-AAA. The validity of this strategy has been established in a number of previous reports from our laboratory as well as others (see, e.g., Refs. 15, 34, and 35).
Experimental protocols.
In group 1, rats were assigned to seven subgroups, defined by the specific pharmacological blocker used. These were 1) an A2 receptor antagonist, ZM-241385 (10 μM); 2) an ecto-5′-nucleotidase blocker [α,β-methylene-adenosine diphosphate (AOPCP); 300 μM]; 3) a Kir channel blocker, BaCl2 (100 μM); 4) a BKCa channel blocker, paxilline (10 μM); 5) a combination of ZM-241385 (10 μM) and BaCl2 (100 μM); 6) a combination of ZM-241385 (10 μM) and paxilline (10 μM); and 7) combined application of BaCl2 (100 μM) and paxilline (10 μM). Before the application of these pharmacological agents, pial arteriolar responses to SNS were obtained. After the restoration of baseline pial arteriolar diameter (∼20 min), a suffusion of one or two of the aforementioned four blockers was then initiated. Forty-five minutes later, measurement of the pial arteriolar response to SNS was repeated.
Group 2 experiments were designed to reveal interactions between Ado, Kir channels, and BKCa channels. To that end, we measured pial arteriolar diameter changes during exposure to Ado (10 and 100 μM), KCl (6 and 12 mM), and NS-1619 (10 and 50 μM). Between topical applications of the above vasodilators, baseline pial arteriolar diameters were restored by a 20-min suffusion of drug-free aCSF. Subsequently, a suffusion of BaCl2 (100 μM), paxilline (10 μM), or ZM-241385 (10 μM) was initiated. After 45 min, pial arteriolar responses to the above vasodilators were reassessed.
In group 3, subgroups 1 and 2, two selective and efficacious, but chemically dissimilar, inhibitors of PKA, Rp-8-CPT-cAMP (8-CPT; 10 μM) and KT-5720 (1 μM), were used to detect whether Ado-linked, PKA-mediated phosphorylation of BKCa and Kir channels plays a role in SNS-evoked pial arteriolar dilations. Thus, pial arteriolar responses to SNS, Ado, KCl, and NS-1619 were evaluated first in the absence and then in the presence of a PKA inhibitor. The sequence of suffusions was as follows: drug-free aCSF (45 min) → KCl (6 or 12 mM) → drug-free aCSF (20 min)→ NS-1619 (10 or 50 μM) suffusion → drug-free aCSF (20 min) → Ado (10 or 100 μM) → drug-free aCSF (20 min) → SNS (20 s)→ drug-free aCSF (20 min) → 8-CPT (10 μM, 30 min) or KT-5720 (1 μM, 30 min) → repeat KCl, NS-1619, and Ado suffusions as well as SNS. In group 3, subgroup 3, we sought to obtain further evidence to support the role of Ado-linked, PKA-mediated phosphorylation in SNS-evoked pial arteriolar dilation by examining a key upstream component in that pathway, namely, AC. The suffusion sequence was similar to subgroups 1 and 2 except that the PKA blockers were replaced by MDL-12330A (5 μM), a selective AC inhibitor, and there was a suffusion of the selective AC activator forskolin (1 and 10 μM), which was added after the initial 45-min drug-free aCSF suffusion.
Group 4 was designed to examine whether the expected Ado-linked AC activation and increased cAMP generation, in association with SNS, might also activate PKG. The study sequence for these evaluations was as follows: drug-free aCSF (45 min) → PKG activator 8-pCPT-cGMP (10 or 50 μM) → drug-free aCSF (20 min) → Ado (10 or 100 μM) → drug-free aCSF (20 min) → SNS (20 s) → drug-free aCSF (20 min) → selective PKG inhibitor Rp-8-Br-PET-cGMP (8-PET; 10 μM, 30 min) → repeat vasodilator suffusions and SNS. No changes in pial arteriolar responses to SNS, Ado, KCl, and NS-1619 were seen in time controls, where the above pharmacological inhibitor interventions were replaced with drug-free aCSF solutions (data not shown).
Group 5 experiments involved evaluations of pial arteriolar responses in vehicle- versus l-AAA-treated rats. As described in previous reports (34, 35), 300 μl of an aCSF vehicle or a 2 mM solution of l-AAA were injected under the cranial window 24 h before study. This protocol has been previously shown to selectively damage the superficial glia limitans without altering evoked cortical neuronal electrical responses or affecting endothelium-dependent and -independent vascular responses elicited by topical applications of ACh and a nitric oxide (NO) donor, respectively (e.g., Ref. 34). These experiments, in the present study, were performed in two stages. In the first stage, we only evaluated pial arteriolar reactivities to topical applications of the BKCa activator NS-1619 (10 and 50 μM). The results of these initial measurements indicated that NS-1619-induced dilation was indeed repressed in l-AAA- versus vehicle-treated rats (n = 4 rats/group). We then performed a second stage of experiments, where we endeavored to determine the specificity of the diminished NS-1619 vasodilatory responses. Thus, we compared (in vehicle- vs. l-AAA-treated rats, n = 4 rats/group) pial arteriolar responses to suffusions of Ado (10 and 100 μM), KCl (6 and 12 mM), and NS-1619 (10 and 50 μM). Since NS-1619 responses in the presence of l-AAA were similarly repressed (relative to vehicle) with both protocols, the NS-1619 results were pooled.
The agents AOPCP, paxilline, NS-1619, forskolin, 8-PET, and l-AAA were obtained from Sigma-Aldrich (St. Louis, MO). ZM-241385 was purchased from Tocris (Ellisville, MO). 8-CPT and 8-pCPT-cGMP were purchased from Biolog Life Science Institute. KT-5720 and MDL-12330A were purchased from Calbiochem (La Jolla, CA). All chemicals except for AOPCP and 8-pCPT-cGMP were prepared using DMSO and then diluted 1:1,000 in aCSF before use; AOPCP and 8-pCPT-cGMP were dissolved in aCSF. All concentrations are expressed as final molar concentrations suffused under the cranial window. Values are presented as means ± SE. Statistical comparisons of arteriolar diameter percent change values within groups were made using one-way repeated-measures ANOVA combined with post hoc Tukey analysis. Analyses of diameter changes between groups were made using an unpaired t-test. P values of <0.05 were considered as significant in all statistical tests.
RESULTS
In all experiments, the physiological variables were within normal limits. Thus, arterial Po2 values were maintained above 100 mmHg in all rats studied, whereas arterial Pco2, pH, and mean artertial blood pressure were 32–40 mmHg, 7.35–7.45, and 100–130 mmHg, respectively, over the course of the experiments. No significant differences were observed when initial and final values were compared in all groups.
Involvement of Ado, BKCa channels, and Kir channels in SNS-induced pial arteriolar dilations.
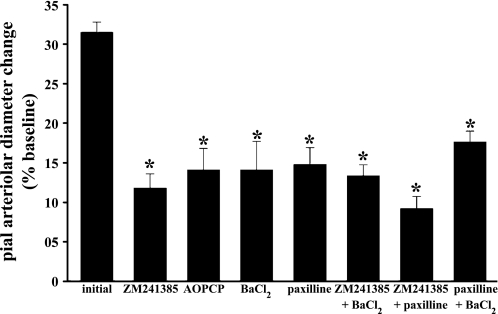
SNS was accompanied by a rapid dilation of pial arterioles, with the peak increase (31.9 ± 1.8% diameter increase over baseline value in the presence of drug-free aCSF; Fig. 1) occurring ∼30 s after the onset of SNS. That response was reversible and reproducible, as repeat exposures to SNS (time controls) evoked virtually identical vasodilations. Significantly reduced arteriolar responses to SNS were observed after the topical application of ZM-241385, AOPCP, BaCl2, and paxilline (Fig. 1). The magnitude of the inhibitor effects on SNS-associated pial arteriolar responses were similar, with reductions from control responses ranging from 55% to 64% when SNS responses were examined in the presence of the four agents tested individually (Fig. 1). These results suggested that Ado, Kir channels, and BKCa channels contribute nonadditively to pial arteriolar dilations during SNS. That conclusion was strengthened by the additional findings showing a limited or no further loss of SNS-evoked pial arteriolar dilations in the presence of combined inhibitor applications (Fig. 1). N1P1 amplitude differences recorded during SNS in the absence and then in the presence of the above blockers were virtually identical in magnitude (data not shown). Others (16, 18) have reported similar findings, at least with respect to ZM-241385 and BaCl2.
Fig. 1.
Sciatic nerve stimulation (SNS)-induced pial arteriolar dilation in the absence or presence of topical applications of the A2 receptor blocker ZM-241385 (10 μM), the ecto-5′-nucleotidase inhibitor α,β-methylene-adenosine diphosphate (AOPCP; 300 μM), the inward rectifier K+ (Kir) channel blocker BaCl2 (100 μM), and the large-conductance Ca2+-operated K+ (BKCa) channel blocker paxilline (10 μM). The effects of paired suffusions of ZM-241385, paxilline, and/or BaCl2 were also evaluated. Pial arteriolar responses are expressed as percent changes from the baseline diameter value. For each agent (or combination) tested, an initial pial arteriolar response to SNS was measured. A second response was then obtained after 45-min suffusion of the blocker(s). Initial values for each subgroup were found not to be statistically different and therefore were grouped together (initial n = 40). Values are means ± SE; n values for the drug treatment groups were as follows: ZM-241386, n = 6; AOPCP, n = 5; BaCl2, n = 6; paxilline, n = 9; ZM-241385 + paxilline, n = 6; ZM-241385 + BaCl2, n = 5; and paxilline + BaCl2, n = 4. *P < 0.05 vs. initial values.
Effects of Kir channel, BKCa channel, or A2 receptor blockade on K+-, NS-1619-, and Ado-induced dilations.
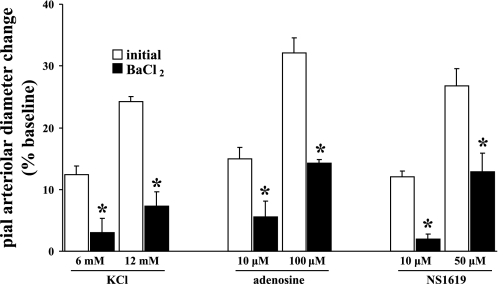
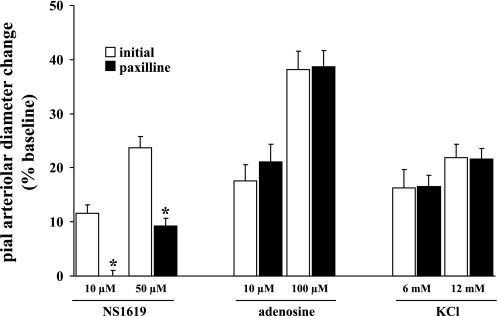
Cortical suffusions of Ado (10 and 100 μM), NS-1619 (10 and 50 μM), and KCl (6 and 12 mM) were accompanied by dose-dependent dilations of pial arterioles, which were subsequently blunted (by 56–70%) in the presence of 100 μM BaCl2 (Fig. 2). On the other hand, suffusion of 10 μM paxilline blunted dilations induced by the selective BKCa channel opener NS-1619. However, Ado and KCl reactivities were unaltered (Fig. 3). Similarly, in the presence of the A2 antagonist ZM-241385 (10 μM), Ado-induced dilations were virtually lost, as one might expect, whereas KCl- and NS-1619-evoked dilations were not significantly altered (Fig. 4). These findings suggest that Kir channel activation represents an important component of the pial arteriolar dilations that occur in the presence of increased Ado and during the activation of BKCa channels. Subsequent findings (see below) imply that the potentiating effect of Ado may arise from PKA-associated phosphorylations, presumably targeted to Kir (and possibly BKCa) channels. On the other hand, loss of Ado (A2) receptor function only affects dilations associated with increased Ado, for example, during SNS (see Fig. 1) and topical applications of Ado (Fig. 4).
Fig. 2.
Effect of the selective Kir channel blocker BaCl2 (100 μM) on pial arteriolar dilations induced by the selective Kir channel opener KCl (6 and 12 mM), adenosine (Ado; 10 and 100 μM), and the selective BKCa channel opener NS-1619 (10, 50 μM). Open and solid bars represent arteriolar responses in the absence and presence of BaCl2, respectively. Values are means ± SE; n = 6 rats/group. *P < 0.05 vs. initial values.
Fig. 3.
Effect of the BKCa channel blocker paxilline (10 μM) on NS-1619 (10 and 50 μM)-induced, Ado (10 and 100 μM)-induced, and KCl (6 and 12 mM)-induced pial arteriolar dilations. Open and solid bars represent arteriolar responses in the absence and presence of paxilline, respectively. Values are means ± SE; n = 6 rat/group. *P < 0.05 vs. initial values.
Fig. 4.
Effect of the selective A2 receptor antagonist ZM-241385 (10 μM) on pial arteriolar dilations induced by Ado (10 and 100 μM), the selective Kir channel opener KCl (6 and 12 mM), and the selective BKCa channel opener NS-1619 (10 and 50 μM). Open and solid bars represent arteriolar responses in the absence and presence of ZM-241385, respectively. Values are means ± SE; n = 6 rats/group. *P < 0.05 vs. initial values.
Role of PKA-dependent phosphorylation in SNS-induced pial arteriolar dilations.
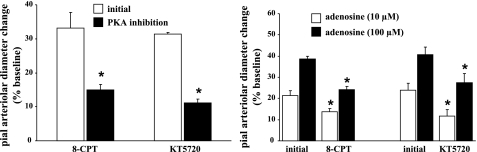
Application of the selective PKA blockers 8-CPT and KT-5720 attenuated SNS-related pial arteriolar dilations by 58% and 65%, respectively (Fig. 5, left). Similar results were observed with dilations elicited by Ado, although the degree of reduction was somewhat smaller (30–50%; Fig. 5, right). These findings suggest that SNS-evoked pial arteriolar relaxation is mediated, at least partially, through Ado receptor-dependent PKA activation. We next examined whether vasodilations linked to Kir and BKCa channel opening are influenced by PKA-mediated phosphorylations. As shown in Fig. 6, left, pial arteriolar dilation responses to KCl (6 and 12 mM) were reduced by 42–50% in the presence of 8-CPT. In the presence of the other PKA blocker, KT-5720, pial arteriolar dilations elicited by KCl were diminished by 58–69% (Fig. 6, left). A similar inhibitory effect on pial arteriolar dilations was observed when the effects of these two PKA blockers on NS-1619-induced responses were examined. Thus, 8-CPT suffusion was accompanied by a 46–68% attenuation in the response to NS-1619 (10 and 50 μM; Fig. 6, right), whereas in the presence of KT-5720 there was a 57–71% reduction in the pial arteriolar dilations elicited by NS-1619 (Fig. 6, right). Since these data were obtained in the absence of SNS, the results would seem to reflect the importance of baseline PKA-linked phosphorylation to the pial arteriolar dilations associated with direct activation of Kir and BKCa channels. It is interesting to note that K+- and NS-1619-induced dilations were not affected by Ado A2 receptor blockade imposed under baseline conditions (Fig. 4). This would appear to indicate that baseline PKA-linked Kir and BKCa channel phosphorylations were associated PKA-activating pathways other than A2 receptor-linked cAMP generation.
Fig. 5.
Effects of selective PKA inhibitors [10 μM Rp-8-CPT-cAMP (8-CPT) and 1 μM KT-5720] on SNS-induced (left) and Ado-induced (right) pial arteriolar dilations. Values are means ± SE; n = 6 rats in the 8-CPT-treated group and 4 rats in the KT-5720-treated group. *P < 0.05 vs. initial responses.
Fig. 6.
Role of PKA-dependent phosphorylation in pial arteriolar dilations elicited by topically applied openers of Kir channels [KCl (6 and 12 mM); left panel] and BKCa channels [NS-1619 (10 and 50 μM); right]. These evaluations were performed in the absence (initial) and presence of the PKA blockers 8-CPT (10 μM) and KT-5720 (1 μM). Values are means ± SE; n = 6 rats in the 8-CPT-treated groups and 4 rats in the KT-5720-treated groups. *P < 0.05 vs. initial responses.
Role of AC in the regulation of pial arteriolar responses.
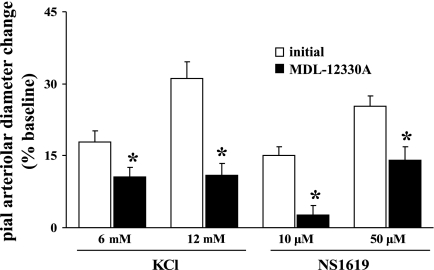
Increased PKA activity largely arises from AC activation and intracellular cAMP accumulation. Given the Ado A2 receptor dependence of the SNS-evoked dilations of pial arterioles, and in consideration of A2 receptor-Gs protein linkage to AC activation, we examined the effects of a topically applied selective AC inhibitor, MDL-12330A (5 μM), on SNS-, Ado-, KCl-, and NS-1619-induced pial arteriolar dilations. In conjunction with those evaluations, we tested the AC-blocking efficacy of 5 μM MDL-12330A by monitoring pial arteriolar responses to the selective AC activator forskolin (1 and 10 μM) in the absence and presence of MDL-12330A. Thus, forskolin-induced dilations were diminished by 65–66% (Fig. 7, left). Similar to the effects seen with PKA inhibitors, significant reductions in pial arteriolar responses to SNS (by 85%; Fig. 7, left), Ado (by 53–55%; Fig. 7, right), KCl (by 40–65%; Fig. 8), and NS-1619 (by 44–83%; Fig. 8) were observed in the presence of the AC blocker.
Fig. 7.
Influence of the adenylyl cyclase inhibitor MDL-12330A (5 μM) on forskolin- and SNS-induced (left) and Ado-induced (right) pial arteriolar dilations. Measurements were obtained before (open bars) and in the subsequent presence of (solid bars) MDL-12330A. Values are means ± SE; n = 5 rats/group. *P < 0.05 vs. initial responses.
Fig. 8.
Pial arteriolar dilations evoked by topical application of the Kir channel opener KCl and the BKCa channel opener NS-1619 in the absence (open bars) and presence (filled bars) of the adenylyl cyclase blocker MDL-12330A (5 μM). Values are means ± SE; n = 5 rats/group. *P < 0.05 vs. initial responses.
Involvement of PKG in SNS- and Ado-induced pial arteriolar dilations.
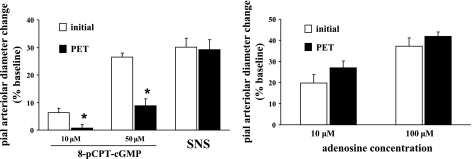
To test whether PKG may have been activated, in addition to PKA, by increased intracellular levels of cAMP (24), we added a separate experimental group where a selective PKG inhibitor, 8-PET, was used to examine the involvement of PKG activation in both SNS- and Ado-induced pial arteriolar dilations. The PKG activator 8-pCPT-cGMP was also introduced to confirm the PKG-blocking efficacy of 8-PET on PKG. In the presence of 8-PET, 8-pCPT-cGMP-induced dilations were significantly attenuated (by 67–90%; Fig. 9, left). However, no changes in either SNS or Ado-induced dilations were observed (Fig. 9, left and right).
Fig. 9.
Effect of PKG inhibition [via 10 μM Rp-8-Br-PET-cGMP (PET)] on pial arteriolar responses to the PKG activator 8-pCPT-cGMP (10 and 50 μM) and SNS-induced pial arteriolar dilations (left) as well as dilations elicited by Ado (10 and 100 μM; right). Measurements were obtained before (open bars) and in the presence of (solid bars) PET. Values are means ± SE; n = 6 rats/group. *P < 0.05 vs. initial responses.
Astrocytic endfoot (glia limitans) localization of BKCa channels: role in the vasodilation elicited by NS-1619.
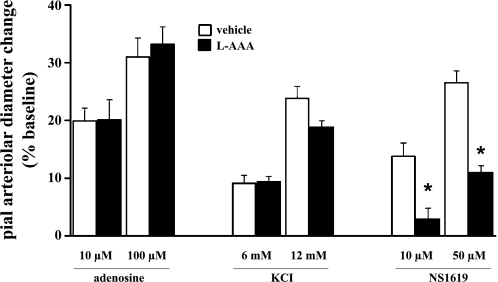
In these experiments, we examined the dose-related responses to suffusion of the selective BKCa channel opener NS-1619 in rats given topical applications of the gliotoxin l-AAA or aCSF vehicle 24 h before study. For comparison, responses to KCl (6 and 12 mM) and Ado (10 and 100 μM) were also monitored. As shown in Fig. 10, compared with control animals, ablation of the superficial glia limitans was accompanied by a significant 60–80% loss of pial arteriolar reactivity to NS-1619. No significant differences in K+ and Ado responses were observed when l-AAA and vehicle-treated rats were compared (Fig. 10). Thus, in contrast to Kir channel and Ado receptor contributions, these findings indicate that a substantial fraction of the BKCa channel dependence of SNS-evoked pial arteriolar dilation involves BKCa channels residing in the glia limitans. This observation is in accord with the model proposed by Nelson and coworkers (29), whereby the BKCa-linked release of K+ from the astrocytic endfeet (in the present case, the glia limitans) interacted with Kir channels on neighboring arteriolar smooth muscle cells, leading to smooth muscle relaxation.
Fig. 10.
Pial arteriolar dilations elicited by topical applications of Ado (10 and 100 μM), KCl (6 and 12 mM), and the BKCa channel opener NS-1619 (10 and 50 μM) in rats 24 h after topical application of the selective gliotoxin l-α-aminoadipic acid (l-AAA) or artificial cerebrospinal fluid (aCSF) vehicle. It should be noted that the data comparing l-AAA- and vehicle-treated rats were collected using two separate experimental protocols. In one case, only NS-1619 responses were monitored. In the other case, responses to Ado, KCl, and NS-1619 were measured sequentially. Since NS-1619 responses in the presence of l-AAA were similarly repressed (relative to vehicle) with both protocols, NS-1619 results were pooled. Thus, with the Ado and KCl data, n = 4 animals for vehicle and l-AAA treatments; for NS1619 data, n = 8 rats animals for vehicle and l-AAA treatments. Values are means ± SE. *P < 0.05 vs. pial arteriolar dilation in the vehicle-treated group.
DISCUSSION
There were several key findings in this study. First, evidence pointed to multiple factors contributing, in a nonadditive fashion, to the pial arteriolar dilations accompanying increased synaptic activity in the somatosensory cortex. These factors included Ado (via an interaction with A2 receptors), BKCa channels, and Kir channels. Second, pharmacological blockade of Kir channels not only limited SNS-evoked pial arteriolar dilations but also markedly attenuated dilations elicited by Ado and the BKCa channel opener NS-1619. On the other hand, whereas BKCa channel blockade did repress SNS-induced dilations, it had no effect on arteriolar responses linked to Ado and Kir channel opening. This suggests that dilations induced by Ado and opening of BKCa channels require the participation of Kir channels, whereas dilations associated with Ado increases and Kir channel opening do not require BKCa channel contributions. Third, findings from rats treated with the gliotoxin l-AAA revealed that the BKCa dependency in this study largely derived from BKCa channels within the superficial glia limitans. Finally, the A2 receptor-dependent linkage to SNS-evoked pial arteriolar dilations appears to be a function of AC activation, increased cAMP, and PKA-mediated phosphorylation of BKCa and/or Kir channels.
The heavy reliance on pharmacological approaches in addressing the hypotheses guiding this study represents a potential limitation. Concerns regarding the selectivity and efficacy of the agents used must be given consideration when attempting to draw conclusions from the data obtained. Indeed, owing to the overlapping (nonadditive) vascular effects of the various blockers, one must take additional measures to ensure that those overlapping effects are not due to off-target actions. With respect to PKA influences, we used a number of approaches. First, we used two different PKA blockers that are chemically dissimilar and whose inhibitory actions involve interactions at separate sites on PKA. That is, KT-5720 acts as a competitive inhibitor at the ATP-binding site (10) and 8-CPT occupies the cAMP binding site of the PKA regulatory subunit (2). Second, the doses of the two PKA blockers that were used in the present investigation (1 and 10 μM for KT-5720 and 8-CPT, respectively) have been shown to be equally effective in preventing Ado-evoked increases in Kir currents in rabbit coronary artery smooth muscle cells (28). Third, a selective AC blocker was applied, at a dose that eliminated most of the pial arteriolar response to the putative AC activator forskolin. Nevertheless, irrespective of the blocker applied, whether targeting PKA or AC, similar reductions in pial arteriolar responses to SNS, Ado, and the K+ channel openers were observed. These corroborative findings therefore provide some validation of the pharmacological interventions related to cAMP/PKA.
With the other pharmacological inhibitors, we used dose values taken from the literature (e.g., Refs. 4, 9, 17, 20, and 31). The dose-response actions of those inhibitors were subsequently tested in pilot studies to confirm their effectiveness in attenuating pial arteriolar responses to appropriate activator/agonists. In most instances (e.g., examinations of Kir channels, BKCa channels, Ado receptors, and PKG function), we included activator/agonist dose-response measurements, as part of the experimental sequence, to confirm inhibitor efficacy. In other cases (e.g., 300 μM AOPCP), we relied on the literature (1) and pilot data (36). In choosing the inhibitor doses applied in the present investigation, we often took the more cautious approach of favoring selectivity over magnitude of response repression. As such, in most cases, the percent inhibition of activator/agonist responses was <100%, ranging from 60–100%. Accordingly, since we did not achieve complete inhibition of pial arteriolar dilations in all cases, we would speculate that the effects observed may actually underestimate the contribution of a given target.
However, for the three principal targets in this study (Kir channels, BKCa channels, and Ado receptors), some caution and additional comments are warranted. First, although Ba2+ (at ≤100 μM) is considered to be highly selective toward Kir channels, in contrast to other K+ channels (i.e., BKCa, ATP-sensitive K+, and voltage-gated K+ channels) found in vascular smooth muscle cells, it is not necessarily selective among the various Kir channel subtypes. In the brain, there are at least two principal subtypes one might consider. Thus, Kir2.1 and Kir4.1 are differentially concentrated in vascular smooth muscle and astrocytes, respectively (19, 39). How that distribution may have influenced present findings remains unclear, although blockade of both channels could negatively impact on signaling within the vascular and astrocytic elements of the neurovascular unit. Second, paxilline is purported to be the most efficacious antagonist of the BKCa opener NS-1619 (7). The selectivity and efficacy of the 10 μM dose of paxilline used in the present investigation were supported by its ability to substantially attenuate NS-1619 responses without affecting vasodilations elicited by Ado or Kir channel activation (Fig. 3). Furthermore, the marked and selective loss of NS-1619 responses in association with glia limitans ablation represent compelling evidence in support of a key role for glia limitans BKCa channels. Third, the 10 μM dose of ZM-241385 used in the present study, while being associated with a nearly complete loss of Ado responses, was in excess of the accepted dose for A2A selectivity over A2B receptors (22). Yet, since both receptors are linked to AC activation, our conclusions regarding the role of Ado need not be altered.
Despite their limitations, these selective pharmacological tools did permit us to gain some insights into the sites of interaction and cross-talk. Thus, Kir channel blockade was not only associated with the expected repression of K+-induced dilations but was also accompanied by a marked attenuation in pial arteriolar dilations elicited by Ado and the BKCa channel opener NS-1619 (Fig. 2). The most likely explanation for the NS-1619 finding arises from work published by Nelson and coworkers (e.g., see Refs. 3 and 29), whereby K+ released through astrocytic endfoot BKCa channels (27) activated Kir2.1 channels in adjacent arteriolar smooth muscle. Although the findings of Nelson et al. were derived from parenchymal tissue, where arterioles are largely enveloped by astrocytic endfoot processes, those results still apply in the present context, since pial arterioles overlie a bed of glial endfeet, the glia limitans (35). The segregation of BKCa and Kir2.1 channels to sites on the superficial glia limitans and pial vascular smooth muscle, respectively, can account for the present findings showing that Ba2+-induced Kir channel blockade had a marked effect on NS-1619-associated dilations, whereas paxilline-mediated blockade of BKCa channels had no effect on Kir channel-related dilations elicited by 6–12 mM KCl (Fig. 3). Additional evidence supporting the importance of glia limitans BKCa channels in the pial arteriolar dilation elicited by the BKCa channel opener is shown in Fig. 10. Thus, topical exposure to the highly astrocyte-selective gliotoxin l-AAA, which ablates the superficial aspects of the glia limitans without damage to pial arterioles or neurons (see Refs. 34 and 35), was accompanied by a marked attenuation in pial arteriolar responses to NS-1619. This indicates that NS-1619-induced dilations largely arise from interactions with BKCa channels within the glia limitans compared with vascular smooth muscle. Evidence that the BKCa channel dependency (and paxilline sensitivity) of neurovascular coupling is linked to astrocytes over cerebral vascular smooth muscle can be found in a recent report by Girouard et al. (6).
Another key issue addressed in the present investigation related to the apparent interdependence of Ado A2 receptors, Kir channels, and BKCa channels in SNS-induced pial arteriolar dilations. Thus, we examined whether the process responsible for that interdependence derived from Ado A2 receptor-linked AC activation, giving rise to PKA-mediated phosphorylation of BKCa channels (32) and Kir channels, enhancing their function (11). In support of the above, we found that both PKA and AC blockade were associated with a substantial attenuation in pial arteriolar responses to SNS and Ado suffusion. In additional evaluations, we observed no contributions from PKG, suggesting that the cAMP effects only occurred through PKA activation (Fig. 9) and not through cross-activation of PKG (see Refs. 14 and 33). In addition, the lack of a PKG inhibitor influence on SNS-induced pial arteriolar dilations indicates that the NO/cGMP/PKG pathway did not contribute to this response. This is consistent with previous findings showing no effect of NO synthase blockade on SNS-evoked pial arteriolar dilations in rats (21). Somewhat surprisingly, significant reductions in the vasodilating responses to the Kir and BKCa channel activators were also observed in the presence of PKA and AC blockade (Figs. 6 and 8). This would seem to indicate the presence of a basal, perhaps permissive, PKA influence on Kir and BKCa channel function (and pial arteriolar tone) that is sensitive to AC/PKA blockade even in the absence of increased activity via the Ado-AC-PKA pathway. Thus, some basal level of phosphorylation may need to be present for Kir and BKCa channels to open in response to their activators. That “permissive” function may account for the similarity in the pial vascular effects of PKA/AC blockade when SNS- and Ado-induced dilations (high cAMP/PKA activity) to the arteriolar responses accompanying the K+ channel activators under conditions of lower cAMP/PKA activity were compared.
In conclusion, the present findings have shown that enhanced synaptic activity in the somatosensory cortex leads to dilation of overlying pial arterioles via a variety of interdependent mechanisms. Those mechanisms involve sites both within the glia limitans (35) and the pial arterioles themselves. Thus, increased neuronal activity within the underlying neuropil is thought to give rise to an interastrocytic Ca2+ wave whose propagation is, at least partly, dependent on Ca2+-linked ATP release and an interaction with metabotropic purinergic P2Y receptors, with subsequent Ca2+ release from intracellular storage sites. That Ca2+ can not only promote further ATP release but also activate outward K+ currents via BKCa channels on the glia limitans (for reviews, see Refs. 23 and 26). Additionally, the released ATP can be converted to Ado via abundant ecto-nucleotidases (36). The increased extracellular Ado can then interact with A2 receptors on sites within the glia limitans and on pial arteriolar smooth muscle, resulting in PKA activation and phosphorylation of BKCa and Kir channels, respectively. We speculate that the channel phosphorylation may have a permissive role toward K+ channels. In other words, a basal level of phosphorylation may need to be present to permit the channels to respond to their activators.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-088259 (to D. A. Pelligrino) and American Heart Association Grant 0635337N (to H. Xu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Agostinho P, Caseiro P, Rego AC, Duarte EP, Cunha RA, Oliveira CR. Adenosine modulation of d-[3H]aspartate release in cultured retina cells exposed to oxidative stress. Neurochem Int 36: 255–265, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Dostmann WR, Taylor SS, Genieser HG, Jastorff B, Doskeland SO, Ogreid D. Probing the cyclic nucleotide binding sites of cAMP-dependent protein kinases I and II with analogs of adenosine 3′,5′-cyclic phosphorothioates. J Biol Chem 265: 10484–10491, 1990 [PubMed] [Google Scholar]
- 3.Dunn KM, Nelson MT. Potassium channels and neurovascular coupling. Circ J 74: 608–616, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunwiddie TV, Diao LH, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J Neurosci 17: 7673–7682, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci 9: 1397–1403, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci USA 107: 3811–3816, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gribkoff VK, Lum-Ragan JT, Boissard CG, Post-Munson DJ, Meanwell NA, Starrett JE, Jr, Kozlowski ES, Romine JL, Trojnacki JT, Mckay MC, Zhong J, Dworetzky SI. Effects of channel modulators on cloned large-conductance calcium-activated potassium channels. Mol Pharmacol 50: 206–217, 1996 [PubMed] [Google Scholar]
- 8.Gustafsson F, Holstein-Rathlou N. Conducted vasomotor responses in arterioles: characteristics, mechanisms and physiological significance. Acta Physiol Scand 167: 11–21, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Kanu A, Leffler CW. Carbon monoxide and Ca2+-activated K+ channels in cerebral arteriolar responses to glutamate and hypoxia in newborn pigs. Am J Physiol Heart Circ Physiol 293: H3193–H3200, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kase H, Iwahashi K, Nakanishi S, Matsuda Y, Yamada K, Takahashi M, Murakata C, Sato A, Kaneko M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun 142: 436–440, 1987 [DOI] [PubMed] [Google Scholar]
- 11.Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res 44: 65–81, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol 100: 307–317, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci 32: 160–169, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Leffler CW, Fedinec AL, Parfenova H, Jaggar JH. Permissive contributions of NO and prostacyclin in CO-induced cerebrovascular dilation in piglets. Am J Physiol Heart Circ Physiol 289: H432–H438, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Leffler CW, Parfenova H, Fedinec AL, Basuroy S, Tcheranova D. Contributions of astrocytes and CO to pial arteriolar dilation to glutamate in newborn pigs. Am J Physiol Heart Circ Physiol 291: H2897–H2904, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leithner C, Royl G, Offenhauser N, Fuchtemeier M, Kohl-Bareis M, Villringer A, Dirnagl U, Lindauer U. Pharmacological uncoupling of activation induced increases in CBF and CMRO2. J Cereb Blood Flow Metab 30: 311–322, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Ye K, Blad CC, den Dulk H, Brouwer J, IJzerman AP, Beukers MW. ZM241385, DPCPX, MRS1706 are inverse agonists with different relative intrinsic efficacies on constitutively active mutants of the human adenosine A2B receptor. J Pharmacol Exp Ther 320: 637–645, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Meno JR, Crum AV, Winn HR. Effect of adenosine receptor blockade on pial arteriolar dilation during sciatic nerve stimulation. Am J Physiol Heart Circ Physiol 281: H2018–H2027, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Nagelhus EA, Mathiisen TM, Ottersen OP. Aquaporin-4 in the central nervous system: cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience 129: 905–913, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Nakahata K, Kinoshita H, Tokinaga Y, Ishida Y, Kimoto Y, Dojo M, Mizumoto K, Ogawa K, Hatano Y. Vasodilation mediated by inward rectifier K+ channels in cerebral microvessels of hypertensive and normotensive rats. Anesth Analg 102: 571–576, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Ngai AC, Meno JR, Winn HR. l-NNA suppresses cerebrovascular response and evoked potentials during somatosensory stimulation in rats. Am J Physiol Heart Circ Physiol 269: H1803–H1810, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Ongini E, Dionisotti S, Gessi S, Irenius E, Fredholm BB. Comparison of CGS 15943, ZM 241385 and SCH 58261 as antagonists at human adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol 359: 7–10, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Paulson OB, Hasselbalch SG, Rostrup E, Knudsen GM, Pelligrino D. Cerebral blood flow response to functional activation. J Cereb Blood Flow Metab 30: 2–14, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelligrino DA, Wang Q. Cyclic nucleotide crosstalk and the regulation of cerebral vasodilation. Prog Neurobiol 56: 1–18, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Pelligrino DA, Xu HL. Ca2+-activated K+ (BKCa) channels in the rat glia limitans may the source of the K+ that activates pial arteriolar smooth muscle Kir channels during increased neuronal activation in vivo (Abstract). Soc Neurosci Abstr: 862.2, 2007 [Google Scholar]
- 26.Pelligrino DA, Xu HL, Vetri F. Caffeine and the control of cerebral hemodynamics. J Alzheimers Dis 20, Suppl 1: S51–S62, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price DL, Ludwig JW, Mi H, Schwarz TL, Ellisman MH. Distribution of rSlo Ca2+-activated K+ channels in rat astrocyte perivascular endfeet. Brain Res 956: 183–193, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Son YK, Park WS, Ko JH, Han J, Kim N, Earm YE. Protein kinase A-dependent activation of inward rectifier potassium channels by adenosine in rabbit coronary smooth muscle cells. Biochem Biophys Res Commun 337: 1145–1152, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Straub SV, Nelson MT. Astrocytic calcium signaling: the information currency coupling neuronal activity to the cerebral microcirculation. Trends Cardiovasc Med 17: 183–190, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vetri F, Menicucci D, Lapi D, Gemignani A, Colantuoni A. Pial arteriolar vasomotion changes during cortical activation in rats. Neuroimage 38: 25–33, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Walker JP, Barbato JC, Koch LG. Cardiac adenosine production in rat genetic models of low and high exercise capacity. Am J Physiol Regul Integr Comp Physiol 283: R168–R173, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Wellman GC, Santana LF, Bonev AD, Nelson MT. Role of phospholamban in the modulation of arterial Ca2+ sparks and Ca2+-activated K+ channels by cAMP. Am J Physiol Cell Physiol 281: C1029–C1037, 2001 [DOI] [PubMed] [Google Scholar]
- 33.White RE, Kryman JP, ElMowafy AM, Han GC, Carrier GO. cAMP-dependent vasodilators cross-activate the cGMP-dependent protein kinase to stimulate BKCa channel activity in coronary artery smooth muscle cells. Circ Res 86: 897–905, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Xu HL, Koenig HM, Ye S, Feinstein DL, Pelligrino DA. Influence of the glia limitans on pial arteriolar relaxation in the rat. Am J Physiol Heart Circ Physiol 287: H331–H339, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Xu HL, Mao L, Ye S, Paisansathan C, Vetri F, Pelligrino DA. Astrocytes are a key conduit for upstream signaling of vasodilation during cerebral cortical neuronal activation in vivo. Am J Physiol Heart Circ Physiol 294: H622–H632, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Xu HL, Pelligrino DA. ATP release and hydrolysis contribute to rat pial arteriolar dilatation elicited by neuronal activation. Exp Physiol 92: 647–651, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Xu HL, Vetri F, Pelligrino DA. An iberiotoxin-insensitive large-conductance Ca2+-activated K+ (BKCa) channel may contribute to pial arteriolar dilations during increased neuronal activity in rats (Abstract). Soc Neurosci Abstr: 862.1, 2007 [Google Scholar]
- 38.Xu HL, Ye S, Baughman VL, Feinstein DL, Pelligrino DA. The role of the glia limitans in ADP-induced pial arteriolar relaxation in intact and ovariectomized female rats. Am J Physiol Heart Circ Physiol 288: H382–H388, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL. Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K+ current in K+-mediated vasodilation. Circ Res 87: 160–166, 2000 [DOI] [PubMed] [Google Scholar]