Abstract
Although transmural heterogeneity of action potential duration (APD) is established in single cells isolated from different tissue layers, the extent to which it produces transmural gradients of repolarization in electrotonically coupled ventricular myocardium remains controversial. The purpose of this study was to examine the relative contribution of intrinsic cellular gradients of APD and electrotonic influences to transmural repolarization in rabbit ventricular myocardium. Transmural optical mapping was performed in left ventricular wedge preparations from eight rabbits. Transmural patterns of activation, repolarization, and APD were recorded during endocardial and epicardial stimulation. Experimental results were compared with modeled data during variations in electrotonic coupling. A transmural gradient of APD was evident during endocardial stimulation, which reflected differences previously seen in isolated cells, with the longest APD at the endocardium and the shortest at the epicardium (endo: 165 ± 5 vs. epi: 147 ± 4 ms; P < 0.05). During epicardial stimulation, this gradient reversed (epi: 162 ± 4 vs. endo: 148 ± 6 ms; P < 0.05). In both activation sequences, transmural repolarization followed activation and APD shortened along the activation path such that significant transmural gradients of repolarization did not occur. This correlation between transmural activation time and APD was recapitulated in simulations and varied with changes in intercellular coupling, confirming that it is mediated by electrotonic current flow between cells. These data suggest that electrotonic influences are important in determining the transmural repolarization sequence in rabbit ventricular myocardium and that they are sufficient to overcome intrinsic differences in the electrophysiological properties of the cells across the ventricular wall.
Keywords: action potential remodeling, electrophysiology
transmural differences in cellular electrophysiology are known to exist in mammalian ventricular myocardium (13, 47). In studies of isolated ventricular myocytes, endocardial cells display an action potential duration (APD) that is consistently longer than that of epicardial cells (9, 26, 28, 51). Furthermore, ventricular M cells with distinct electrophysiological characteristics have been identified in the midmyocardium of some mammals, including rabbit (26), dog (40), and human (10, 19). At slow stimulation rates, M cells display an APD that is much longer than cells isolated from either epi- or endocardial regions. The dominant influence of these cellular electrophysiological heterogeneities in intact myocardium has been suggested by studies using microelectrode recordings from three regions on the transmural surface of isolated canine ventricular wedge preparations (49). Epicardial and endocardial regions were found to repolarize before the midmyocardium, due to the significant population of M cells in the ventricular wall. The resultant transmural repolarization gradients dictated the characteristics of the voltage signal recorded across the isolated ventricular wall, analogous to the ECG T wave. In contrast, a number of studies (15, 42, 19) in human hearts have suggested that under physiological conditions, electrotonic influences abolish transmural electrical gradients during repolarization. These data question the importance of transmural gradients of repolarization in the ECG T wave, the origin of which remains controversial after decades of investigation (30, 32). Understanding the balance between intrinsic repolarization characteristics and electrotonic influences in ventricular myocardium is therefore central to our understanding of cardiac electrophysiology.
The aim of the current study was to examine the relative contribution of cellular heterogeneity and cellular coupling to transmural repolarization. We hypothesized that the effect of different activation sequences on transmural APD would depend on the relative balance of intrinsic cellular differences and electrotonic synchronization of repolarization. We therefore examined the effect of activation sequence on transmural APD using wide-field optical imaging techniques in the rabbit left ventricular (LV) free wall. Voltage signals from hundreds of sites across the transmural surface were mapped simultaneously during different activation sequences. The experimental results were recapitulated using a mathematical model of rabbit ventricular myocardium, which was then used to examine the effects of variations in electrotonic coupling on transmural patterns of APD during different activation sequences. The data support a dominant role for electrotonic influences and suggest that intrinsic cellular differences play a relatively minor role in determining the transmural repolarization sequence in rabbit ventricular myocardium.
METHODS
Ethical approval.
This work conforms to the standards set out in the UK Animals (Scientific Procedures) Act 1986. The work was approved by the University of Glasgow Ethical Review Committee and carried out under UK Project Licence No. PPL60/3538.
Experimental preparation.
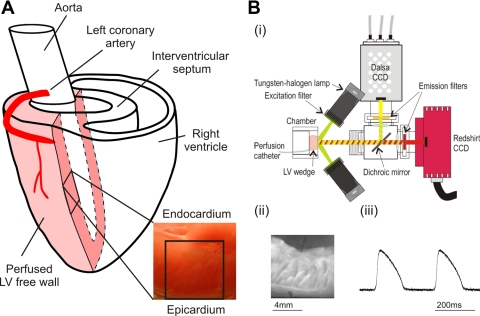
Male New Zealand white rabbits (n = 8) were killed with an intravenous injection of 100 mg/kg pentobarbital sodium. Hearts were excised and placed in chilled Tyrode solution, and the left coronary artery was cannulated and perfused with oxygenated Tyrode solution maintained at pH 7.4 and 37°C. The cannula was oversewn to maintain a stable position while perfusion pressure was monitored. The atria and interventricular septum were removed, and any cut vessels were sutured. Perfused LV wedge preparations were dissected (Fig. 1A) and loaded with voltage-sensitive dye (100 μl of 1 mg/ml RH237; Molecular Probes, Eugene, Oregon). Underperfused areas were removed, and a microtome blade was used to produce a clean transmural imaging surface. The preparation was mounted in a custom-built chamber, with the transmural surface in the midportion of the LV orientated for optical imaging (Fig. 1A, inset). Then, 15 mmol/l 2,3-butanedione monoxime (Sigma-Aldrich) were used to eliminate motion artifact during optical recordings. The effects on conduction velocity and repolarization in rabbit ventricular myocardium at this concentration are minor (24). Two Ag/AgCl disc electrodes were placed in the center of the preparation, one on the epicardial side and the other on the endocardial side, and were used to record a pseudo-ECG from the chamber. Two platinum bipolar stimulating electrodes were placed at opposite points on the endo- and epicardial edges of the transmural surface. The preparation was paced at a cycle length of 350 ms with a 2-ms pulse at twice diastolic threshold. Pacing was carried out for at least 30 s from each position before optical recording. In each case, the stimulation site was maintained for 5 min, and the optical measurements were repeated at the end of this period. This protocol was used to ensure steady-state electrophysiological measurements and avoided the induction of the ventricular electrical remodeling that can occur after 1 h of altered activation pattern (25). In a subset of experiments, the preparation was repositioned within the chamber to allow epicardial imaging. The midportion of the ventricle was imaged, and the epicardial edge of the transmural surface was orientated at the top of the imaging window, such that an area of epicardium including the edge of the cut surface was imaged. Electrode positions were maintained during epicardial imaging.
Fig. 1.
Experimental setup. A: perfused left ventricular (LV) wedge preparation. B: optical mapping system (i) with a fluorescence image of the transmural surface (ii) and transmural optical action potentials (APs; iii). CCD, charge-coupled device.
Optical mapping.
An optical mapping system (Fig. 1B, i), was used to record transmural optical action potentials (APs). Light from four 75-W tungsten-halogen lamps passed through interference filters (525 ± 15 nm) onto the transmural surface. Light was collected through a lens and split with a dichroic mirror at 630 nm. The longer wavelength portion passed through a long pass emission filter (>695 nm) and was focused onto a charge-coupled device (CCD) camera (RedShirt) yielding a transmural fluorescence image (Fig. 1B, ii). The CCD camera was set up to image an array of 26 × 26 square pixels at a sampling rate of 5 kHz. For the current study, the optical magnification gave a field width of 8.5 mm, resulting in a single pixel width of 327 μm. Examples of single pixel transmural optical APs are given in Fig. 1B, iii. The shorter wavelength light portion was focused onto a second CCD camera (Dalsa) that was used to acquire plain images of the preparation.
Data analysis.
Data analysis was performed using custom software. Activation time (AT) was determined at the steepest upstroke of the optical AP and repolarization time (RT) as the time at 90% repolarization. APD at 90% repolarization (APD90) was calculated by RT − AT. Rise time was calculated as the time between 10 and 90% of the AP upstroke. Transmural patterns of AT, RT, and APD90 were sampled between the stimulus points perpendicular to the activation isochrones. All data are expressed as means ± se. Groups of data were compared using a Student's t-test (paired where appropriate), or where more than two groups were compared, a repeated-measures ANOVA with Tukey-Kramer multiple comparisons testing. To compare APD90 at multiple sites during endo- and epicardial stimulation a two-way ANOVA was used, in which stimulation site was considered a categorical variable and position a continuous variable.
Modeling.
Computer simulations were performed using a detailed electrophysiological model of the rabbit ventricular AP (39). Transmural propagation of APs was computed in a rectangular slab of rabbit ventricular myocardium (1.0 × 1.0 × 0.5 cm) using the monodomain cable equation. Linear transmural fiber rotation was incorporated with a total fiber rotation of 120° over the thickness of the slab (44). A transmural gradient in the conductance of the transient outward current was incorporated to mimic earlier experimental results (13), with maximal conductance on the epicardial surface and a gradual transmural decrease down to 84% on the endocardium. Both endo- and epicardial point stimulations at a cycle length of 350 ms were simulated. Optical signals at the transmural surface were computed from a 0.5 × 0.5 cm field-of-view using a previously developed model of photon transport based on the diffusion equation with partial flux boundary conditions (3, 22). The optical reduced scattering (μs′) and absorption (μa) coefficients were μs′ = 1.38/mm and μa = 0.52/mm at the excitation wavelength and μs′ = 0.87/mm and μa = 0.1/mm at the emission wavelength of the voltage-sensitive dye (22). The monodomain equation was solved using a forward finite-difference scheme with a spatial resolution of 0.1 mm and an adaptive time step varying between 0.005 and 0.001 ms. This approach has previously been shown to produce quantitatively similar transmural conduction velocity (CV) and APD data to bidomain models (8, 36). All simulations were performed on a parallel cluster consisting of 32 AMD Athlon MP2200+ processors running at 1.8 GHz. The message passing interface library and a “domain-slicing” algorithm were used to parallelize the code (51). The computations of the optical signals were performed on a desktop with an Intel Dual Core CPU running at 2.13GHz. This final procedure simply acted as a form of spatial filtering and did not change the direction of computed APD gradients.
RESULTS
Transmural optical APs.
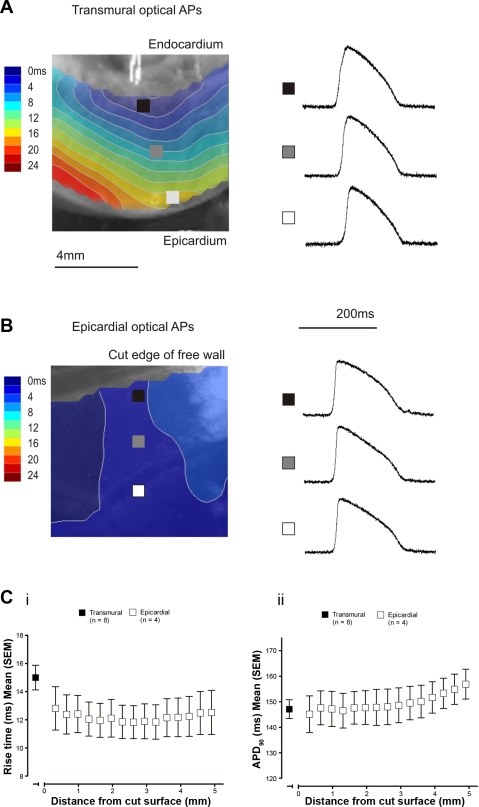
Figure 2 shows a comparison between optical APs recorded from the transmural and epicardial surfaces during endocardial stimulation. Examples from the subendocardial, midmyocardial, and subepicardial regions of the transmural surface are shown in Fig. 2A, with epicardial APs sampled at similar spacing (Fig. 2B). There was no difference in the apex-base positioning of the epicardial sampling points. ATs are shown by the superimposed contour maps. AT progressed from the endocardial stimulating electrode as a planar wave across the transmural surface, whereas near synchronous activation of the epicardial surface was observed. Figure 2C shows optical AP rise time (i) and APD90 (ii), comparing those recorded from the epicardial region of the transmural surface with those recorded from the epicardial surface at progressively increasing distances away from the cut surface. There were no significant differences in rise time or APD90 of optical APs recorded from the cut surface when compared with those recorded from the epicardial surface (Table 1).
Fig. 2.
Characteristics of transmural optical APs. A: transmural surface with activation isochrones during endocardial stimulation. Optical APs sampled from the subendocardium (black square), midmyocardium (grey square), and subepicardium (white square) are shown. B: epicardial surface with activation isochrones during endocardial stimulation and optical APs sampled at equivalent spacing to those on the transmural surface. C: optical AP rise time (i) and APD90 (ii) recorded from the subepicardial region of the transmural surface and the epicardium at increasing distances from the cut surface.
Table 1.
Optical action potential characteristics
| Transmural (n = 8) | Epicardial (n = 4) | P Value | |
|---|---|---|---|
| Rise time, ms | 15.0 ± 0.9 | 12.5 ± 1.3 | 0.13 |
| APD90, ms | 147.1 ± 3.7 | 152.7 ± 6.1 | 0.46 |
Values are means ± SE. APD90, action potential duration at 90% repolarization.
Transmural patterns of APD are modified by activation sequence.
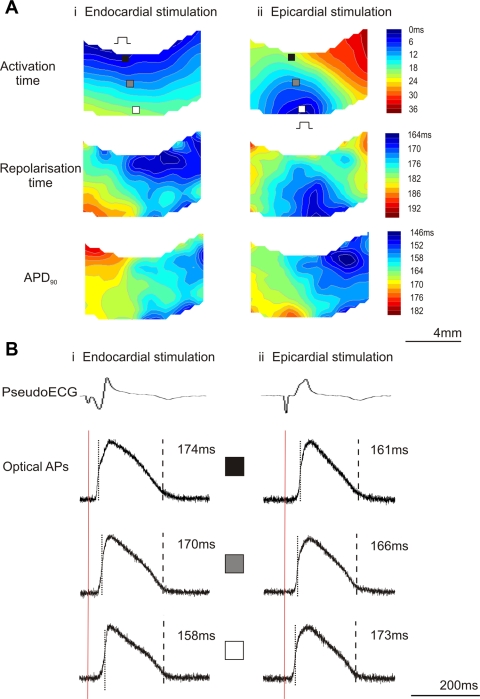
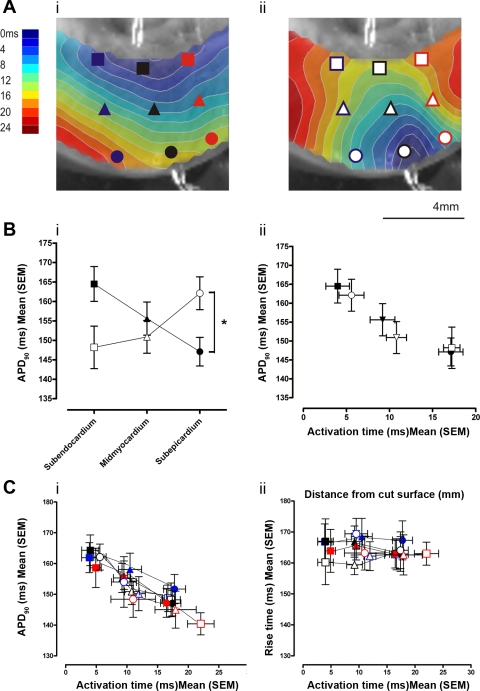
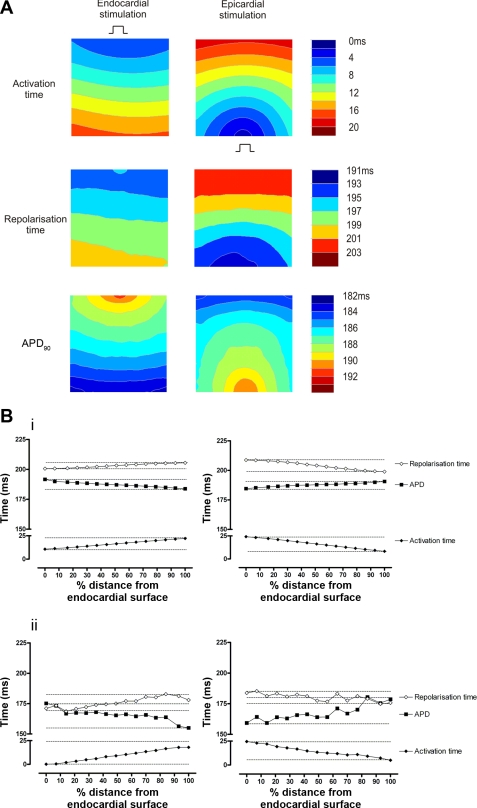
Contour maps showing transmural patterns of activation, repolarization, and APD90 during endo- and epicardial stimulation from one experiment are shown in Fig. 3A. Transmural optical APs sampled from the subendocardium, midmyocardium, and subepicardium, along with the pseudo-ECGs, are shown in Fig. 3B. During endocardial stimulation, a nearly planar transmural activation front was seen, whereas during epicardial stimulation the wavefront was semicircular. Mean transmural CV was greater during endocardial than epicardial stimulation (30.0 ± 0.9 vs. 24.6 ± 1.2 cm/s; P < 0.005). Patterns of RT were more complex but appeared to follow patterns of activation. Transmural dispersion of repolarization was not different between endocardial and epicardial stimulation (1.77 ± 0.36 vs. 1.10 ± 0.31 ms/mm2; P > 0.05). Significant transmural gradients of APD90 were evident during both activation sequences. The transmural sampling sites for determination of mean APD90 are indicated in Fig. 4A and were the same for each activation sequence. As shown in Fig. 4B, i, during endocardial-to-epicardial activation, the longest APD90 was found at the subendocardium (165 ± 5 ms), with a progressive shortening of APD90 at the midmyocardium (156 ± 4 ms) and the subepicardium (147 ± 4 ms). During a reversal of activation sequence (epicardium-to-endocardium), the longest APD90 values were found in the subepicardium (162 ± 4 ms), with progressively shorter APD90 values at the midmyocardium (151 ± 4 ms) and subendocardium (148 ± 6 ms; P < 0.05). This pattern of activation time and APD remained stable during continued pacing of either endo- or epicardial sites for up to 5 min. As shown in Fig. 4B, ii, an inverse relationship between AT and APD90 was apparent. Within the limited area of ventricle sampled, apicobasal gradients of APD90 did not exceed transmural gradients. There were no significant apicobasal or transmural gradients in RT during either activation sequence. As shown in Fig. 4C, i, the same inverse relationship between AT and APD90 existed whether the data were sampled from the apical, mid, or basal free wall. In contrast, as shown in Fig. 4C, ii, RT from all sampled areas remained constant across a range of ATs. There was no difference between mean APD90 recorded from early activated epicardial regions compared with subepicardial regions during endo- (145.8 ± 5.0 vs. 147.1 ± 3.7 ms) or epicardial stimulation (160.1 ± 7.2 vs. 162.1 ± 4.2 ms).
Fig. 3.
Transmural APs during endocardial and epicardial stimulation. A: contour maps of transmural activation time (AT), repolarization time (RT), and action potential duration at 90% repolarization (APD90) during endocardial (i) and epicardial (ii) stimulation. B: pseudoECG and single-pixel optical AP traces during endocardial (i) and epicardial (ii) stimulation. Optical APs were sampled from the pixels indicated: subendocardium (black), midmyocardium (grey), and subepicardium (white). Stimulus (red line), AT (dotted line), and RT (dashed line) and APD90 are given.
Fig. 4.
Relationship between activation time and action potential duration. A: isochronal maps of transmural AT superimposed on a plain image of the transmural surface with optical AP sampling points for the mid LV free wall (black), compared with basal (blue) and apical (red) LV. B: mean APD90 in the subendocardium (■, □), midmyocardium (▴, ▵), and subepicardium (●, ○) during endocardial (■, ▴, ●) and epicardial stimulation (□, ▵, ○), plotted against transmural position (i) and AT (ii). *P < 0.05. C: APD90 (i) and RT (ii) from each area plotted as a function of AT.
In a subset of experiments (n = 4), the effect of activation sequence on APs recorded from the epicardial surface was examined. Epicardial pixels were partitioned into 1 ms steps of AT from the earliest epicardial activation during epicardial stimulation. Mean APD90 during endo- and epicardial stimulation was then compared for each step. In the earliest activated epicardial pixels, there was no difference between epicardial APD90 and subepicardial APD90 during endo- (145.8 ± 5.0 vs. 147.1 ± 3.7 ms; P > 0.05) or epicardial stimulation (160.1 ± 7.2 vs. 162.1 ± 4.2 ms; P > 0.05). Pixels activated within the first 7 ms of the epicardial stimulus had a significantly longer APD90 than those recorded during endocardial stimulation. The differences in epicardial APD90 were not significant in APs from later activated epicardial pixels.
Modulation of APD90 by activation sequence in simulations.
The experimental data suggest an electrotonic effect of activation sequence on APD90 in myocardial tissue that results in synchronization of RT in space. This was examined using a mathematical model incorporating intrinsic transmural gradients of APD90. Contour maps generated from simulations of the transmural surface are shown in Fig. 5A. The isochronal maps of AT show wavefront configurations with similar characteristics to those observed experimentally. The graphs in Fig. 5B detail the transmural patterns of AT, RT, and APD90 during endo- and epicardial stimulation and compare the data from simulations (Fig. 5B, i) with the experimental data (Fig. 5B, ii). Transmural patterns of activation, repolarization, and APD90 derived from simulated optical APs during endo- and epicardial stimulation are shown in Fig. 5B, i. During endocardial stimulation, there is a progressive increase in AT from endocardium-to-epicardium. RT follows AT, but the overall transmural gradient in RT is less than in AT. Therefore, as the activation wavefront travels from endocardium-to-epicardium, APD90 becomes progressively shorter. During epicardial stimulation there is a progressive increase in AT from epicardium-to-endocardium, with a corresponding increase in RT that is of smaller magnitude than that in AT, meaning that APD90 again shortens along the activation path. Similar relationships among AT, RT, and APD90 were observed in the optical measurements from the transmural surface (Fig. 5B, ii).
Fig. 5.
Comparison of data from experiments and simulations. A: contour maps of transmural AT, RT, and APD90 during endo- and epicardial stimulation from simulations. B: transmural AT, RT, and APD90 from a line of pixels perpendicular to the activation isochrones plotted as a function of distance from the subendocardium during endo- and epicardial stimulation from modeled (i) and experimental (ii) data. Experimental data are taken from the example shown in Fig. 3.
Pharmacological modulation of electrotonic coupling.
In a subset of experiments, the stimulation protocols were repeated in the presence of the uncoupler carbenoxolone (50 μM; n = 4) and the connexin 43 potentiator rotigaptide (1 μM; n = 4). Carbenoxolone reduced transmural CV during both activation sequences [endocardial stimulation 31.2 ± 1.5 vs. 18.5 ± 1.5 cm/s (P < 0.01) and epicardial stimulation 25.8 ± 1.4 vs. 16.9 ± 1.6 cm/s (P < 0.001)]. Rotigaptide produced an increase in transmural CV during epicardial (21.4 ± 1.6 vs. 29.4 ± 1.7 cm/s; P < 0.05) but not endocardial stimulation (33.4 ± 2.8 vs. 37.6 ± 1.3 cm/s). Pharmacological modulation of coupling had no significant effects on transmural gradients or absolute values of APD90.
Correlation of activation time and APD90 and the effect of variations in electrotonic coupling.
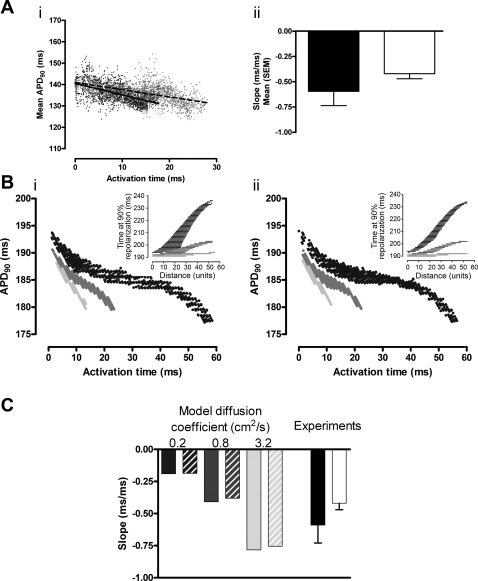
Linear regression between AT and APD90 for each transmural pixel (Fig. 6A) showed an inverse correlation between AT and APD90 (endocardial stimulation −0.59 ± 0.14 and epicardial stimulation −0.42 ± 0.05). The difference between the mean regression slope for endo- and epicardial stimulation was not significant. These measurements suggest that for a 10-ms increase in activation time across the wall of the ventricle, the APD shortens by ∼5 ms due to electrotonic interaction. The effect of the gap junction inhibitor carbenoxolone was to reduce conduction velocity by 40% without a significant change in APD90, thus reducing the gradient of the relationship between AT and APD90.. If electrotonic influences were significant, they would be expected to alter APD and contribute to the altered slope. For example, increasing the conduction velocity (with rotigaptide) would be expected to steepen this relationship and reduce the range of transmural APDs due to increased electronic interaction. The spread of the experimental data precluded detection of small changes in APD, but the model allowed the theoretical effect of intercellular resistance to be assessed.
Fig. 6.
Effect of change in coupling. A, i: APD90 as a function of AT for each transmural pixel in a single experiment with linear regression lines during endocardial (black dots, solid line: r2 = 0.29; P < 0.0001) and epicardial stimulation (grey dots, dashed line: r2 = 0.34; P < 0.0001). A, ii: mean regression slope during endocardial (black) and epicardial stimulation (white, P > 0.05). B: APD90 as a function of AT using modeled data with variation in diffusion coefficient (0.2, 0.8, and 3.2 cm2/s) during endocardial (i) and epicardial (ii) stimulation. Insets: relationship between RT and distance from the stimulation site. C: linear regression slopes for APD90 vs. AT from experimental data and modeled data (endocardial stimulation in solid bars and epicardial stimulation in hatched bars).
Under control conditions, the model predicted a monotonic change in APD with activation time. Despite the departure from linearity at low values of diffusion coefficient due to boundary effects, a linear correlation was used to quantify changes in gradient. Under control conditions, the gradient of the relationships between AT and APD90 were −0.4 and −0.39 for endocardial and epicardial stimulation, respectively, i.e., similar to the measured values. Variations in electrotonic coupling were then imposed by changing the diffusion coefficient in the model. The diffusion coefficient was varied over a 16-fold range, producing conduction velocities of 50–200% of the baseline values. The relationship between AT and APD90 for each diffusion coefficient is shown in Fig. 6B, i for endocardial stimulation and Fig. 6B, ii for epicardial stimulation; insets show how the repolarization time varies with distance from stimulus site, independent of conduction velocity. The clear differences in gradient, and hence electrotonus, are a result of changing diffusion coefficient. As shown in Fig. 6C, when compared with baseline values, a fourfold reduction in diffusion coefficient is sufficient to produce a 50% reduction in transmural conduction velocity, and this was associated with an approximate halving of the slope of the regression line between AT and APD90 (−0.19 for both endocardial and epicardial stimulation). Reduction of intracellular coupling had a small effect on absolute values of APD90, prolonging the initial APD90 by ∼2% and resulted in an increase in the overall APD gradient. An increase in the diffusion coefficient in the model resulted in an increase in the slope of the regression line (to −0.78 for endocardial stimulation and −0.75 for epicardial stimulation), a decrease in initial APD90 by <2%, and a decrease in the overall APD gradient. If changes in intercellular coupling caused alterations of APD90 of the order of ∼2%, these would not be detected experimentally due to the variation in APD90 values across the transmural area (see Fig. 6A).
DISCUSSION
Transmural imaging.
In the current study, the electrical characteristics of the cut surface of rabbit LV myocardium were studied to investigate the effects of activation sequence on transmural electrophysiology. Similar preparations from canine and rabbit hearts have been used by other investigators in previous studies (48–50). Despite the damage generated at the cut surface, normal APs can be recorded, both optically and using microelectrodes, from the immediate subsurface layer. Yan et al. (48) used activation-recovery intervals (ARIs) recorded from intramural plunge electrodes in the intact ventricular wall to verify that the repolarization characteristics measured on the cut surface were representative of those within the preparation. In the current study, optical measurements from the cut surface revealed AP characteristics comparable to those recorded from the undamaged epicardial surface, and there were no differences in epicardial APs recorded adjacent to and distant from the cut surface. However, uncoupling in experimental preparations such as these does appear to be associated with a lengthening of APD and an increasing of APD dispersion (35), as demonstrated in a recent comparison of ARI and refractory period (RP) in vivo with those recorded following dissection to a wedge preparation (45). In the current study, a perfused free wall wedge preparation was used, which has a relatively lower cut surface-to-tissue ratio than other wedge preparations and which may minimize differences in coupling between the preparation and the intact heart. In addition, the AP characteristics recorded in the current study, both in terms of rise time and APD90, are similar to those previously recorded from the epicardial surface of Langendorff-perfused rabbit hearts (6, 46), suggesting that the electrophysiological characteristics of the underlying tissue are not significantly affected by the presence of the cut surface.
Optical rise times.
The rise time of optical APs (∼10–15 ms) is a function of the rise time of the cellular AP (∼1–2 ms) and blurring due to nonsynchronous activation across the pixel area and within the depth of the sensitive volume (18). As previously described, optical measurements derived from wide-field systems, such as the one used in the current study, can sample fluorescence from a significant depth of focus. Estimates based on the optical properties of the myocardium suggest that 80% of the signal originates from the first 700 μm (4). As these values are well within the space constant of rabbit myocardium, it is unlikely that dramatic differences in electrophysiology exist within the sampled volume.
Transmural activation wavefronts.
Endo- and epicardial point stimulation was used in the current study. Previous studies (11, 46) comparing right atrial (RA) with endocardial point stimulation have shown similar epicardial activation patterns, implying a similar transmural activation sequence. Comparable transmural wavefront configurations to those seen in the current study have previously been observed in canine ventricular myocardium (34) and were linked to reduced connexin 43 expression in the subepicardium (33). In the current study, the different wavefront configurations seen in the experiments were reproduced in the model, in which the Purkinje network was not present, suggesting that they arise, at least in part, from changes in fiber rotation and coupling across the transmural surface. In the current study, transmural CV during epicardial stimulation was found to be significantly slower than during endocardial stimulation, as was also reported in canine ventricular myocardium (34).
Transmural APD gradient.
In the current study, a significant transmural gradient of APD90 was evident at physiological temperature and stimulation frequency. The transmural gradient was of the order of 15 ms, in keeping with previous recordings from rabbit ventricular myocardium (50). Studies (1) in canines have suggested a complex transmural APD profile, with midmyocardial APD prolongation, but we observed a monotonic transmural APD relation. The lack of midmyocardial APD90 prolongation seen here is in agreement with data recorded from microelectrode impalements in a rabbit ventricular wedge preparation (50). Although M cells were reported in cells isolated from rabbit midmyocardium (26), their contribution to the electrophysiology of the intact myocardium at physiological rates is slight.
Effect of activation sequence on APD90 and repolarization.
In these experiments, a gradient from the longest APD at the subendocardium to the shortest at the subepicardium was seen during endocardial stimulation, and this pattern reversed on epicardial stimulation. This suggested that, regardless of transmural region, early activated sites have a longer APD. The similarity of the correlation between AT and APD90 across the whole transmural surface during both endo- and epicardial stimulation suggests that differences in wavefront curvature or an apicobasal APD gradient did not significantly contribute to the patterns of APD90 observed. The relationship between AT and APD90 suggests that for every 10-ms increment in activation time APD90 shortens by ∼5 ms and that this occurs over a distance of ∼3 mm due to electrotonic interaction. Carbenoxolone-induced reduction in conduction velocity (by ∼40%) was not associated with significant changes in APD90, corresponding well with the very small changes in absolute APD90 observed as a result of altered intercellular coupling in the computational model. Thus the dominant effect of uncoupling is to diminish the electronic effect on APD90, rather than to reveal the underlying differences in regional electrophysiology.
An inverse relationship between AT and RP on the epicardial surface has previously been observed during physiological activation sequences. In open-chest dogs, epicardial ARI and monophasic APs were longer during ventricular epicardial stimulation than RA pacing (17). The RP of extracellular electrograms has been reported to be shorter when stimulation was distant to the measurement site compared with stimulation at the measurement site (43). Over a distance of 4–6 mm, the change in RT was always less than the change in AT, indicating a shortening of APD over this distance. Gotoh et al. (20) identified a progressive shortening of APD from the stimulus site in the transverse axis, but not in the longitudinal axis, resulting in synchronization of RT. More recently, optical imaging techniques have allowed recording of detailed spatial patterns of repolarization. Efimov et al. (11) compared epicardial activation and repolarization patterns during RA and endo- and epicardial stimulation in guinea-pig LV. Epicardial repolarization spread anisotropically, mirroring fiber orientation in the ventricular epicardium, and repolarization patterns were unchanged during different activation sequences, implying that modulation of APD had occurred.
In contrast to observations on the epicardial surface, direct modulation of transmural APD by activation sequence has not previously been described. Indeed, increased transmural heterogeneity of APD and the resultant dispersion of repolarization have been reported as important factors in arrhythmogenesis under a range of pathophysiological circumstances (2). In experiments using intracellular microelectrodes to record from the endo- and epicardium of rabbit ventricular wedge preparations, no difference in APD was detected between endo- and epicardial stimulation at a cycle length of 2,000 ms (27). Similar results have been reported in canine ventricular wedge preparations (14). The authors conclude that transmural differences in APD were independent of electrotonic load and likely to be due to differences in cellular repolarization characteristics. In both cases, stimulating electrodes were placed at the center of the wedge preparation and APs were recorded from the transmural (cut) surface, which was at a distance of least 5 mm from the stimulus. Electrotonic modulation of APD is greatest close to the stimulus, which may explain the absence of an observed electrotonic effect in these studies. However, in the current study, cycle length and the orientation of the electrodes were different: stimulating electrodes were placed within 1 mm of the cut surface on either epicardial or endocardial sites, ensuring that the transmural activation pattern was directly dictated by the stimulus.
The measurements in the current study were performed after short intervals of altered activation sequence to examine the immediate effects of changes in electrotonic load, rather than the effects associated with ventricular electrical remodeling, which are significant after ∼60 min after alteration of the activation pattern and have been well characterized in mammalian ventricles (23, 25).
Simulations support electrotonic modulation of transmural APD.
Simulations using various electrophysiological models of cardiac APs have shown that APD shortens as it propagates away from the stimulus site both on the epicardial surface (37) and transmurally (41). Furthermore, the boundaries of the medium can also modulate the spatial patterns of repolarization and APD (31). Recently, Sampson and Henriquez (38) investigated the electrotonic modulation of APD in two different electrophysiological models representing mouse and guinea-pig ventricular APs. Their simulation showed that besides intrinsic heterogeneity and geometry, the differences in underlying transmembrane ionic currents, and hence the stability of repolarization to an external perturbation, played an important role in the electrotonic modulation of APD. In our simulations using a detailed description of the rabbit ventricular AP with a modest intrinsic transmural gradient in the transient outward current, we found that APD was strongly modulated by activation sequence. We have performed similar simulations using the guinea-pig Luo-Rudy model (12) in which we found that APD modulation was less prominent, thus confirming the results of Sampson and Henriquez (38) on model dependency.
Implications for interpretation of the ECG T wave.
The concordance of the polarity of the QRS complex and T wave of the ECG is thought to arise when spatial spread of depolarization and repolarization occurs in opposite directions (7, 29). Apicobasal gradients of APD are known to exist in mammalian ventricle and may be important in the inscription of the T wave. In the current study, transmural repolarization followed activation and tended to occur with a faster time course, resulting in minimal transmural dispersion of RT. Apicobasal gradients of APD over a relatively small distance did not exceed those seen in the transmural plane. Clearly, the absolute magnitude of apex-base repolarization differences in the intact heart may be greater, as they occur over a larger distance than that sampled here. Overall, the data suggest that isolated transmural gradients of repolarization may not be solely responsible for the ECG T wave.
Implications for human hearts.
In the current study, electrotonic effects in both experiments and simulations were sufficient to reverse APD gradients and therefore minimize any repolarization gradient over a distance of ∼5 mm. The available data suggest that such effects may have a significant influence on the time course of repolarization over relatively small distances from the stimulus site. An inverse relationship between AT and ARI has been demonstrated on the endocardial (5, 21) and epicardial (16) surfaces of normal human LV in vivo. Whether electrotonic influences are able to counteract the effect of an abnormal transmural activation sequence in the human ventricle remains unclear. The implications of the current study for pathological states also merit further investigation. The modeled data (Fig. 6B) suggest that the range of APD increases as coupling is reduced, raising the possibility that pathological uncoupling may increase dispersion of repolarization and thereby enhance susceptibility to arrhythmias, as has previously been suggested in a rabbit model of heart failure (47).
Conclusions.
In this study, transmural APD90 was recorded during endocardial and epicardial stimulation. The results demonstrate that transmural gradients of APD90 are modified by activation sequence. As distance from the site of stimulation increases, APD90 shortens, resulting in relatively synchronous transmural repolarization. The results are consistent with the effect of electrotonic current flow between cells modulating the repolarization phase of the AP and suggest that, in intact tissue, electrotonic modulation of APD dominates the effects of intrinsic transmural differences in cellular repolarization characteristics.
GRANTS
R. C. Myles, F. L. Burton, and S. M. Cobbe were funded by the British Heart Foundation. O. Bernus was funded by the Engineering and Physical Sciences Research Council.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Antzelevitch C, Sicouri S, Litovsky SH, Lukas A, Krishnan SC, Di Diego JM, Gintant GA, Lui DW. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M-cells. Circ Res 69: 1427–1449, 1991 [DOI] [PubMed] [Google Scholar]
- 2.Antzelevitch C, Shimizu W, Yan GX, Sicouri S, Weissenburger J, Nesterenko VV, Burashnikov A, Di Diego J, Saffitz J, Thomas GP. The M-cell: its contribution to the ECG and to normal and abnormal electrical function of the heart. J Cardiovasc Electrophysiol 10: 1124–1152, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Bernus O, Wellner M, Mironov SF, Pertsov AM. Simulation of voltage-sensitive optical signals in three-dimensional slabs of cardiac tissue: application to transillumination and coaxial imaging methods. Phys Med Biol 50: 215–229, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Bishop MJ, Gavaghan DJ, Trayanova NA, Rodriguez B. Photon scattering effects in optical mapping of propagation and arrhythmogenesis in the heart. J Electrocardiol 40, Suppl 6: S75–S80, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauhan VS, Downar E, Nanthakumar K, Parker JD, Ross HJ, Chan W, Picton P. Increased ventricular repolarization heterogeneity in patients with ventricular arrhythmia vulnerability and cardiomyopathy. Am J Physiol Heart Circ Physiol 290: H79–H86, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Choi BR, Burton F, Salama G. Cytosolic Ca2+ triggers early afterdepolarizations and Torsade de Pointes in rabbit hearts with type 2 long QT syndrome. J Physiol 543: 615–631, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen I, Giles W, Noble D. Cellular basis for the T-wave of the electrocardiogram. Nature 262: 657–661, 1976 [DOI] [PubMed] [Google Scholar]
- 8.Colli Franzone P, Pavarino LF, Taccardi B. Simulating patterns of excitation, repolarization and action potential duration with cardiac Bidomain and Monodomain models. Math Biosci 197: 35–66, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Dilly KW, Rossow CF, Votaw VS, Meabon JS, Cabarrus JL, Santana LF. Mechanisms underlying variations in excitation-contraction coupling across the mouse left ventricular free wall. J Physiol 572: 227–241, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drouin E, Charpentier F, Gauthier C, Laurent K, Le Marec H. Electrophysiologic characteristics of cells spanning the left ventricular wall of human heart: evidence for presence of M-cells. J Am Coll Cardiol 26: 185–192, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Efimov IR, Huang DT, Rendt JM, Salama G. Optical mapping of repolarization and refractoriness from intact hearts. Circulation 90: 1469–1480, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Faber GM, Rudy Y. Action potential and contractility changes in [Na+]i-overloaded cardiac myocytes: a simulation study. Biophys J 78: 2392–2404, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fedida D, Giles WR. Regional variations in action potentials and transient outward current in myocytes isolated from rabbit left ventricle. J Physiol 442: 191–209, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fish JM, Di Diego JM, Nesterenko V, Antzelevitch C. Epicardial activation of left ventricular wall prolongs QT interval and transmural dispersion of repolarization: implications for biventricular pacing. Circulation 109: 2136–2142, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Franz MR, Bargheer K, Rafflenbeul W, Haverich A, Lichtlen PR. Monophasic action potential mapping in human subjects with normal electrocardiograms: direct evidence for the genesis of the T-wave. Circulation 75: 379–386, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Franz MR, Swerdlow CD, Liem LB, Schaefer J. Cycle length dependence of human action potential duration in vivo. J Clin Invest 82: 972–979, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furukawa Y, Miyazaki T, Miyoshi S, Moritani K, Ogawa S. Anisotropic conduction prolongs ventricular repolarization and increases its spatial gradient in the intact canine heart. Jpn Circ J 64: 287–294, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Girouard SD, Laurita KR, Rosenbaum DS. Unique properties of cardiac action potentials recorded with voltage-sensitive dyes. J Cardiovasc Electrophysiol 7: 1024–1038, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Glukhov AV, Fedorov VV, Lou Q, Ravikumar VK, Kalish PW, Schuessler RB, Moazami N, Efimov IR. Transmural dispersion of repolarization in failing and nonfailing human ventricle. Circ Res 106: 981–991, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gotoh M, Uchida T, Mandel WJ, Fishbein MC, Chen PS, Karagueuzian HS. Cellular graded responses and ventricular vulnerability to reentry by a premature stimulus in isolated canine ventricle. Circulation 95: 2141–2154, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Hanson B, Sutton P, Elameri N, Gray M, Critchley H, Gill JS, Taggart P. Interaction of activation-repolarization coupling and restitution properties in humans. Circ Arrhythmia Electrophysiol 2: 162–170, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Hyatt CJ, Zemlin CW, Smith RM, Matiukas A, Pertsov AM, Bernus O. Reconstructing subsurface electrical wave orientation from cardiac epi-fluorescence recordings: Monte Carlo vs. diffusion approximation. Opt Express 16: 13758–13772, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Janse MJ, Sosunov EA, Coronel R, Opthof T, Anyukhovsky EP, de Bakker JM, Plotnikov AN, Shlapakova IN, Danilo P, Jr, Tijssen JG, Rosen MR. Repolarization gradients in the canine left ventricle before and after the induction of short-term cardiac memory. Circulation 112: 1711–1718, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Kettlewell S, Walker NL, Cobbe SM, Burton FL, Smith GL. The electrophysiological and mechanical effects of 2,3-butane-dione monoxime and cytocholasin-D in the Langendorff perfused rabbit heart. Exp Physiol 89: 163–172, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Libbus I, Rosenbaum DS. Transmural action potential changes underlying ventricular electrical remodelling. J Cardiovasc Electrophysiol 14: 394–402, 2002 [DOI] [PubMed] [Google Scholar]
- 26.McIntosh MA, Cobbe SM, Smith GL. Heterogeneous changes in action potential and intracellular Ca2+ in left ventricular myocyte sub-types from rabbits with heart failure. Cardiovasc Res 45: 397–409, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Medina-Ravell VA, Lankipalli RS, Yan GX, Antzelevitch C, Medina-Malpica NA, Medina-Malpica OA, Droogan C, Kowey PR. Effect of epicardial or biventricular pacing to prolong QT interval and increase transmural dispersion of repolarization. Circulation 107: 740–746, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Näbauer M, Beuckelmann DJ, Uberfuhr P, Steinbeck G. Regional differences in current density and rate-dependent properties of the transient outward current in subepicardial and subendocardial myocytes of human left ventricle. Circulation 93: 168–177, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Noble D, Cohen I. The interpretation of the T-wave of the electrocardiogram. Cardiovasc Res 12: 13–27, 1978 [DOI] [PubMed] [Google Scholar]
- 30.Opthof T, Coronel R, Janse MJ. Is there a significant transmural gradient in repolarization time in the intact heart?: repolarization gradients in the intact heart. Circ Arrhythmia Electrophysiol 2: 89–96, 2009 [DOI] [PubMed] [Google Scholar]
- 31.van Oosterom A, Jacquemet V. The effect of tissue geometry on the activation recovery interval of atrial myocytes. Physica D 238: 962–968, 2009 [Google Scholar]
- 32.Patel C, Burke JF, Patel H, Gupta P, Kowey PR, Antzelevitch C, Yan GX. Is there a significant transmural gradient in repolarization time in the intact heart?: Cellular basis of the T-wave: a century of controversy. Circ Arrhythmia Electrophysiol 2: 80–88, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poelzing S, Akar FG, Baron E, Rosenbaum DS. Heterogeneous connexin43 expression produces electrophysiological heterogeneities across ventricular wall. Am J Physiol Heart Circ Physiol 286: H2001–H2009, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Poelzing S, Dikshteyn M, Rosenbaum DS. Transmural conduction is not a two-way street. J Cardiovasc Electrophysiol 16: 455, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Poelzing S. Are electrophysiologically distinct M-cells a characteristic of the wedge preparation? Heart Rhythm 6: 1035–1037, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Potse M, Dubé B, Richer J, Vinet A, Gulrajani RM. A Comparison of monodomain and bidomain reaction-diffusion models for action potential propagation in the human heart. IEEE Trans Biomed Eng 53: 2425–2435, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Qu Z. Dynamical effects of diffusive cell coupling on cardiac excitation and propagation: a simulation study. Am J Physiol Heart Circ Physiol 287: H2803–H2812, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Sampson KJ, Henriquez CS. Electrotonic influences on action potential duration dispersion in small hearts: a simulation study. Am J Physiol Heart Circ Physiol 289: H350–H360, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Shannon TR, Wang F, Puglisi J, Weber C, Bers DM. A mathematical treatment of integrated Ca dynamics within the ventricular myocyte. Biophys J 87: 3351–3371, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sicouri S, Fish J, Anzelevitch C. Distribution of M-cells in the canine ventricle. J Cardiovasc Electrophysiol 5: 824–837, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Taccardi B, Punske BB, Sachse F, Tricoche X, Colli-Franzone P, Pavarino LF, Zabawa C. Intramural activation and repolarization sequences in canine ventricles. J Electrocardiol 38: 131–137, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Taggart P, Sutton PMI, Opthof T, Coronel R, Trimlett R, Pugsley W, Kallis P. Transmural repolarization in the left ventricle in humans during normoxia and ischaemia. Cardiovasc Res 50: 454–462, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Toyoshima H, Burgess MJ. Electrotonic interaction during canine ventricular repolarization. Circ Res 43: 348–356, 1978 [DOI] [PubMed] [Google Scholar]
- 44.Vetter FJ, McCulloch AD. Three-dimensional analysis of regional cardiac function: a model of rabbit ventricular anatomy. Prog Biophys Mol Biol 69: 157–183, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Voss F, Opthof T, Marker J, Bauer A, Katus HA, Becker R. There is no transmural heterogeneity in an index of action potential duration in the canine left ventricle. Heart Rhythm 6: 1028–1034, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Walker NL, Burton FL, Kettlewell S, Smith GL, Cobbe SM. Mapping of epicardial activation in a rabbit model of chronic myocardial infarction. J Cardiovasc Electrophysiol 18: 862–868, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Wiegerinck RF, van Veen TA, Belterman CN, Schumacher CA, Noorman M, de Bakker JM, Coronel R. Transmural dispersion of refractoriness and conduction velocity is associated with heterogeneously reduced connexin43 in a rabbit model of heart failure. Heart Rhythm 5: 1178–1185, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Yan GX, Shimizu W, Antzelevitch C. Characteristics and distribution of M-cells in arterially perfused canine left ventricular wedge preparations. Circulation 98: 1921–1927, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Yan GX, Antzelevitch C. Cellular basis for the normal T-wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation 98: 1928–1936, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Yan GX, Rials SJ, Wu Y, Liu T, Xu X, Marinchak RA, Kowey PR. Ventricular hypertrophy amplifies transmural repolarization dispersion and induces early afterdepolarization. Am J Physiol Heart Circ Physiol 281: H1968–H1975, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Zaritsky R, Pertsov A. Simulation of 2-D spiral wave interactions on a Pentium-based cluster. In: Proceedings of Neural, Parallel, and Scientific Computations, edited by Bekakos MP, Ladde GS, Medhin NG, Sambandham M. Atlanta, GA: Dynamic, 2002 [Google Scholar]
- 52.Zygmunt AC, Eddlestone GT, Thomas GP, Nesterenko VV, Antzelevitch C. Larger late sodium conductance in M-cells contributes to electrical heterogeneity in canine ventricle. Am J Physiol Heart Circ Physiol 281: H689–H697, 2001 [DOI] [PubMed] [Google Scholar]