Abstract
The mammalian myocardium expresses four adenosine receptor (AR) subtypes: A1AR, A2aAR, A2bAR, and A3AR. The A1AR is well known for its profound antiadrenergic effects, but the roles of other AR subtypes in modulating contractility remain inconclusive. Thus, the objective of this study was to determine the direct and indirect effects of A2aAR and A2bAR on cardiac contractility. Experiments were conducted in paced, constant pressure-perfused isolated hearts from wild-type (WT), A2aAR knockout (KO), and A2bAR KO mice. The A2aAR agonist CGS-21680 did not alter basal contractility or β-adrenergic receptor agonist isoproterenol (Iso)-mediated positive inotropic responses, and Iso-induced effects were unaltered in A2aAR KO hearts. However, A2aAR gene ablation resulted in a potentiation of the antiadrenergic effects mediated by the A1AR agonist 2-chloro-N-cyclopentyladenosine. The nonselective AR agonist 5′-N-ethylcarboxamido adenosine and the selective A2bAR agonist BAY 60-6583 induced coronary flow-independent increases in contractility, but BAY 60-6583 did not alter Iso-induced contractile responses. The A1AR antiadrenergic effect was not potentiated in A2bAR KO hearts. The expression of all four AR subtypes in the heart and ventricular myocytes was confirmed using real-time quantitative PCR. Taken together, these results indicate that A2aAR does not increase cardiac contractility directly but indirectly alters contractility by modulating the A1AR antiadrenergic effect, whereas A2bAR exerts direct contractile effects but does not alter β-adrenergic or A1AR antiadrenergic effects. These results indicate that multiple ARs differentially modulate cardiac function.
Keywords: A2a adenosine receptor, A2b adenosine receptor, knockout mice, cardiac contractility
adenosine is an autocoid that exerts several well-known receptor-mediated effects in the heart via four different adenosine receptors (ARs): A1AR, A2aAR, A2bAR, and A3AR. A1AR exerts negative chronotropic and dromotropic effects by modulating sinoatrial and atrioventricular nodal conduction, A2aAR and A2bAR activation induces coronary vasodilation, and there are no known direct effects of A3AR stimulation (2, 9, 22). All four ARs are also known to exert cardioprotective effects against myocardial ischemia-reperfusion injury (26). Although all four AR subtypes are expressed in the heart, adenosine exerts little, if any, direct effect on ventricular function in the normal myocardium.
The most well-characterized AR subtype in the ventricular myocardium is A1AR, which remains the only AR subtype identified in ventricular myocytes by radioligand binding (18). Evidence to date has indicated that A1AR exerts little, if any, direct effects on contractility, but its activation does attenuate the positive inotropic effects of β-adrenergic stimulation at the whole heart and myocyte levels (7, 12). There is evidence for the presence of A2aAR on ventricular myocytes, but there are contradictory reports on the ability of this receptor to alter cardiac contractility. Previous work from our laboratory (13) has provided immunological evidence for the expression of A2aAR in rat ventricular myocytes, although the A2aAR-selective agonist CGS-21680 exerted no effects on myocyte shortening or cAMP. Additional studies from our laboratory (14, 16) have indicated that A2aAR stimulation has no effects on contractility in isolated rat hearts and intact porcine myocardium. Our findings are consistent with those of a study by Headrick et al. (31) in which it was also shown that A2aAR activation has no contractile effects in isolated mouse hearts. In contrast, other reports (6, 8, 20, 30, 33) have indicated that A2aAR stimulation increases contractility in isolated rat and mouse hearts and in isolated rat cardiomyocytes.
In addition to inconsistencies in the observed direct effects of A2aAR on contractility, there are also discrepancies regarding the indirect effects of A2aAR activation. It has been reported that the A2aAR agonist CGS-21680 potentiates the positive inotropic effects of β-adrenergic receptor stimulation in the mouse heart (30) but not in rat ventricular myocytes (8). In addition, it has been observed that the AR antagonist ZM-241385, which exhibits some selectivity toward A2aAR, potentiates A1AR antiadrenergic effects in isolated perfused rat hearts and myocytes (8, 23). However, when the hypothesis that A2aAR antagonizes the A1AR antiadrenergic effect was tested using AR knockout (KO) mice, it was observed that the A1AR antiadrenergic effect was reduced in A2aAR KO mice (30). The basis for such inconsistencies regarding A2aAR-mediated direct and indirect contractile effects remains unclear.
A2bAR, which exhibits a low affinity for adenosine, is the least well-characterized AR in the mammalian heart. Although it has been shown to be expressed in several cell types in the myocardium (4, 11, 25, 27, 28), its expression in mammalian ventricular myocytes has yet to be confirmed. The contractile effects of A2bAR have not been studied in detail, due to the lack of readily available selective agonists and antagonists. One study (31) using wild-type (WT) mice reported that A2bAR may be responsible for a small, but sustained, positive inotropic effect after adenosine administration in isolated perfused mouse hearts. In the only study (28) conducted thus far with a selective A2bAR-selective agonist, BAY 60-6583 exerted dose-dependent increases in ventricular function in parallel with coronary vasodilation. Thus, similar to A2aAR, the effects of A2bAR stimulation on cardiac contractility remain inconclusive.
Given these inconsistencies, the objectives of this study were to examine the direct effects of A2aAR and A2bAR on cardiac contractility as well as A2aAR and A2bAR modulation of β-adrenergic contractile responses. Furthermore, real-time quantitative PCR was used to assess the expression of all four AR subtypes in whole ventricles and isolated ventricular myocytes.
MATERIALS AND METHODS
Materials.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. The A2bAR agonist BAY 60-6583 was a kind gift from Dr. Thomas Krahn at Bayer Healthcare (Wuppertal, Germany). 2-chloro-N-cyclopentyladenosine (CCPA), CGS-21680, 5′-N-ethylcarboxamido adenosine (NECA), and BAY 60-6583 were prepared as 10 mM stock in DMSO and diluted as necessary. Isoproterenol (Iso) was prepared as a 10 mM stock solution in PBS with 0.1% sodium metabisulfite.
Animals.
The animals in this study were maintained and used in accordance with guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996) and were evaluated and approved by the Institutional Animal Care and Use Committee of Wayne State University. Ten- to twelve-week-old male C57BL/6 (WT) mice were purchased from Jackson Laboratories (Bar Harbor, ME). The progenitors for A2aAR and A2bAR constitutive global homozygous KO (−/−) mice were generous gifts from Dr. Joel Linden (La Jolla Institute for Allergy and Immunology, La Jolla, CA) and Dr. Stephen Tilley (University of North Carolina, Chapel Hill, NC), respectively. The genotype of the offspring was validated by PCR (using tail genomic DNA) or RT-PCR (using mRNA isolated from hearts after some experiments). In a subset of isolated hearts, the respective genotypes were also verified by lack of responsiveness to the A2aAR agonist CGS-21680 and the A2bAR agonist BAY 60-6583.
Isolated heart preparation (general protocol).
Hearts were excised from anesthetized (60 mg/kg pentobarbital sodium) and heparinized (500 units) mice, placed in cold (4°C) saline, and mounted on the perfusion apparatus. Hearts were perfused at constant coronary perfusion pressure (70 mmHg) with standard Krebs-Henseleit buffer (KHB). Heart temperature was maintained at 37°C with constant-temperature reservoirs and by partially submerging the heart into a water-jacketed chamber filled with KHB. Hearts were paced at 420 beats/min via pacing wires inserted into the right ventricle, and a fluid-filled balloon was inserted into the left ventricle (LV) across the mitral valve and connected to a pressure transducer, permitting continuous measurements of LV pressure and heart rate (HR). Ventricular function [LV systolic pressure (LVSP), +dP/dt, and −dP/dt], HR, coronary flow (CF), and perfusion pressure were continuously monitored and recorded throughout the experiments. All data collected were analyzed with PowerLab and Chart Software for Windows (AD Instruments, Colorado Springs, CO).
Experimental protocols.
Hearts were allowed to equilibrate for ∼20 min before any experimental protocols. Dose-response curves were generated by exposing the hearts to increasing concentrations of agents (100, 500, and 1,000 nM), and steady-state responses were recorded. To determine the effects of AR agonists on β-adrenergic responses, hearts were pretreated with CCPA, CGS-21680, or BAY 60-6583 (all at 200 nM) for 5 min before and during Iso exposure. Steady-state responses to Iso (2 and 10 nM) were assessed after 2 min. Only a single experimental protocol was conducted in each heart.
CCPA and CGS-21680 exhibit selectivity for human and rat A1AR and A2aAR, respectively, whereas NECA is nonselective and activates A2bAR at high nanomolar concentrations (34). BAY 60-6583 is a new agonist that has been reported to have a Ki of 0.33 μM for mouse A2bAR (1). It has also been reported that this agonist exerts no effects on cAMP in Chinese hamster ovary cells transfected with human A1AR and A2aAR (10).
Myocyte isolation protocol.
Adult mouse ventricular myocytes were isolated from hearts of WT and A2aAR KO hearts using standard retrograde perfusion/enzymatic dissociation methods, as previously described (24). After isolation and Ca2+ restoration to 1 μM, myocytes were preplated on 10-cm tissue culture-treated dishes for 2 h at 37°C to remove fibroblasts and other nonmyocytes, which adhere to plastic. The unattached cell supernatant was then gravity settled through a 5% BSA solution to further enrich the pellet in cardiomyocytes. The supernatant was discarded, and the resulting pellet was used for RNA isolation.
RNA isolation and real-time quantitative PCR.
Total RNA was isolated from WT and A2aAR KO mouse ventricular tissue and isolated ventricular myocytes using TRIzol reagent according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). RNA was digested with DNase I and purified using a RNeasy Mini Kit (QIAGEN, Valencia, CA), and RNA quality and quantity were validated by spectrophotometry and agarose gel electrophoresis. All reagents necessary for real-time PCR were purchased from Applied Biosystems (Carlsbad, CA). Reverse transcription was performed on 1 μg total RNA using the High-Capacity cDNA reverse transcription kit. Gene expression was assayed with TaqMan Universal PCR Master Mix and FAM-labeled TaqMan inventoried gene expression assays according to the manufacturer's instructions. Real-time PCR was conducted in a StepOnePlus Real-Time PCR System, and data were analyzed by the comparative 2−ΔCt method (where Ct is threshold cycle) and expressed as a percentage of the internal endogenous control gene GAPDH.
Data and statistical analysis.
Data were analyzed with GraphPad Prism software and are presented as means ± SE. Functional data were analyzed using one- or two-way ANOVA where appropriate. Statistically significant differences between gene expression in the heart and myocyte preparations were calculated by an unpaired t-test. Statistically significant differences in the values were taken at P < 0.05.
RESULTS
Baseline cardiac contractile parameters and CF from WT, A2aAR KO, and A2bAR KO mice are shown in Table 1. There were no differences in baseline hemodynamics among the groups. There were also no differences in body weights or heart weight-to-body weight ratios.
Table 1.
Baseline hemodynamics in WT and AR KO mouse hearts
| LVSP, mmHg | LV +dP/dt, mmHg/s | LV −dP/dt, mmHg/s | CF, ml/min | |
|---|---|---|---|---|
| WT mice | 93 ± 4 | 3,650 ± 304 | −2,157 ± 156 | 2.46 ± 0.17 |
| A2aAR KO mice | 86 ± 3 | 3,399 ± 132 | −1,889 ± 131 | 2.50 ± 0.19 |
| A2bAR KO mice | 88 ± 4 | 3,720 ± 224 | −2,081 ± 150 | 2.47 ± 0.14 |
Values are means ± SE.
Baseline values were taken before treatment with the adenosine receptor (AR) agonists or isoproterenol (Iso) in wild-type (WT) and AR knockout (KO) mouse hearts. LVSP, left ventricular systolic pressure; CF, coronary flow.
A2aAR effects on cardiac contractility.
The contractile effects of A2aAR activation were evaluated in WT mouse hearts using the A2aAR-selective agonist CGS-21680. As shown in Table 2 the A2aAR-selective agonist CGS-21680 increased CF by 50%, but this was associated with no significant effects on contractility even at the highest concentration tested (1 μM). As shown in Table 2, administration of Iso resulted in dose-dependent increases in all contractile parameters and CF. Pretreatment with CGS-21680 (200 nM) before and during Iso stimulation did not alter β-adrenergic contractile responses at either Iso concentration.
Table 2.
Hemodynamic effects of CGS-21680, Iso, and CGS-21680 + Iso
| LVSP, mmHg | +dP/dt, mmHg/s | −dP/dt, mmHg/s | CF, ml/min | |
|---|---|---|---|---|
| CGS-21680 dose response | ||||
| Baseline | 96 ± 6 | 3,667 ± 424 | −2,123 ± 281 | 2.2 ± 0.1 |
| 100 nM | 96 ± 8 | 3,768 ± 590 | −2,216 ± 444 | 3.3 ± 0.3* |
| 500 nM | 97 ± 9 | 3,868 ± 604 | −2,307 ± 471 | 3.3 ± 0.2* |
| 1,000 nM | 98 ± 8 | 3,911 ± 619 | −2,384 ± 485 | 3.5 ± 0.2* |
| Iso dose response | ||||
| Baseline | 91 ± 5 | 3,509 ± 202 | −2,010 ± 118 | 2.3 ± 0.1 |
| 2 nM | 162 ± 7* | 7,691 ± 567* | −4,798 ± 392* | 3.0 ± 0.1* |
| 10 nM | 181 ± 8* | 9,845 ± 813* | −6,036 ± 458* | 3.2 ± 0.1* |
| CGS-21680 + Iso dose response | ||||
| Baseline | 91 ± 5 | 3,305 ± 354 | −1956 ± 178 | 2.5 ± 0.2 |
| 200 nM CGS-21680 | 88 ± 4 | 3,264 ± 352 | −1,902 ± 153 | 3.5 ± 0.2* |
| 2 nM Iso | 152 ± 6* | 6,626 ± 703* | −4,581 ± 444* | 3.4 ± 0.2* |
| 10 nM Iso | 179 ± 8* | 9,237 ± 1271* | −5,886 ± 524* | 3.4 ± 0.2* |
Values are means ± SE. Baseline values were taken before treatment with the A2aAR agonist CGS21680 or Iso in WT mouse hearts.
P < 0.05.
The ability of A2aAR to indirectly alter myocardial contractility (i.e., by modulating the A1AR-mediated antiadrenergic response) was investigated using WT and A2aAR KO mice. As shown in Fig. 1, the β-adrenergic agonist Iso increased LVSP, +dP/dt, and −dP/dt by 76%, 109%, and 133%, respectively, at a concentration of 2 nM Iso and by 107%, 190%, and 204%, respectively, at 10 nM Iso. Pretreatment of additional hearts with the A1AR agonist CCPA exerted a potent antiadrenergic effect where contractility was attenuated significantly (at 2 nM Iso, LVSP, +dP/dt, and −dP/dt were reduced by 54%, 56%, and 58%, respectively). The A1AR agonist also blunted the effects of 10 nM Iso, although the extent of inhibition was lower (27%, 29%, and 32% reductions of LVSP, +dP/dt, and −dP/dt, respectively).
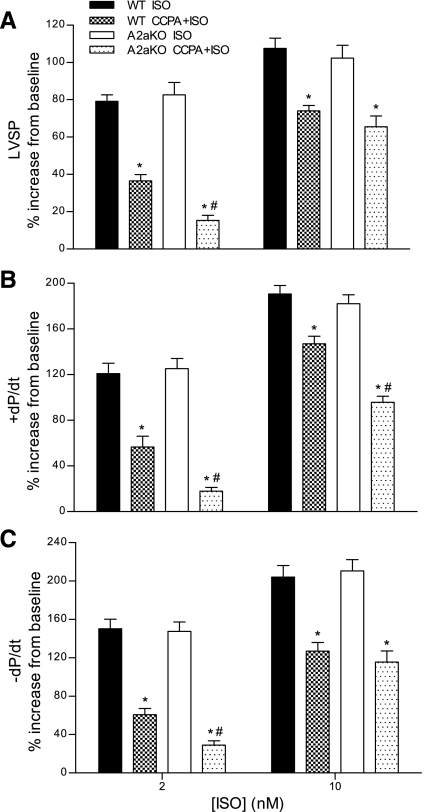
Fig. 1.
A2a adenosine receptor (AR) modulation of the A1AR antiadrenergic effect. The A1AR-mediated antiadrenergic response was evaluated using wild-type (WT) and A2aAR knockout (A2aKO) mouse hearts. A: left ventricular systolic pressure (LVSP). B: +dP/dt. C: −dP/dt. Data are expressed as percent increases above baseline values after isoproterenol (Iso) stimulation. For A1AR activation, hearts were preincubated with 200 nM of the A1AR agonist 2-chloro-N-cyclopentyladenosine (CCPA) for 5 min before Iso stimulation. Data are means ± SE; n ≥ 5 hearts/group. *P < 0.05 vs. Iso; #P < 0.05, A2aKO mice with CCPA + Iso vs. WT mice with CCPA + Iso.
Deletion of A2aAR had no effect on Iso-mediated contractile responses (Fig. 1); however, the A1AR antiadrenergic effect was significantly potentiated in A2aAR KO hearts compared with their WT counterparts. In A2aAR KO hearts, CCPA reduced the effects of 2 nM Iso by 81%, 86%, and 80% for LVSP, +dP/dt, and −dP/dt, respectively. Compared with WT controls, these values represent a further reduction in contractility by 58%, 68%, and 52% for LVSP, +dP/dt, and −dP/dt, respectively (Fig. 1). The potentiation of the A1AR antiadrenergic effect in A2aAR KO hearts was more pronounced at the lower Iso concentration, as the only significant difference between WT and A2aAR KO hearts at 10 nM Iso was on +dP/dt (with a further reduction of 35% in A2aAR KO hearts; Fig. 1B).
A2bAR effects on cardiac contractility.
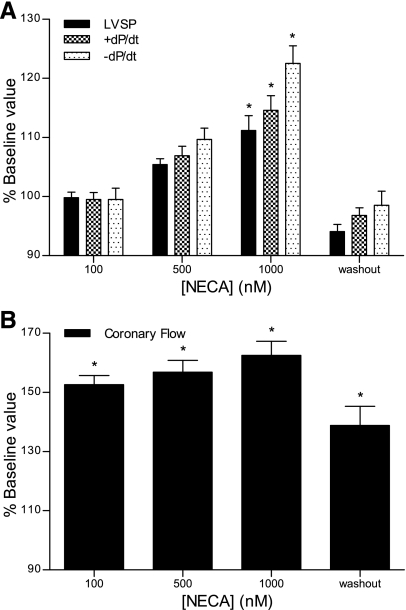
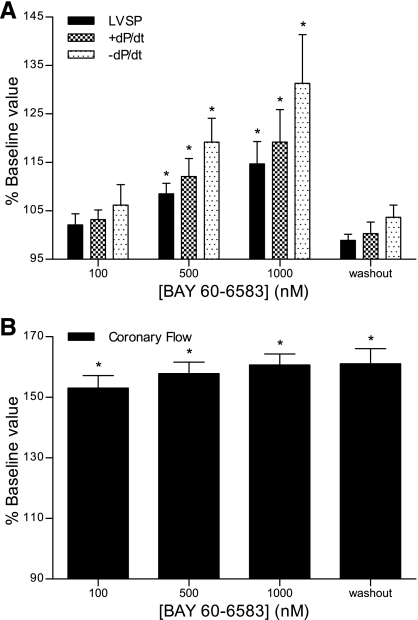
The effects of A2bAR activation were evaluated in WT hearts using the nonselective agonist NECA and the A2bAR-selective agonist BAY 60-6583. In contrast to the A2aAR agonist, both NECA (Fig. 2A) and BAY 60-6583 (Fig. 3A) increased contractility. NECA exerted statistically significant effects on all three contractile parameters at a concentration of 1 μM, with LVSP, +dP/dt, and −dP/dt increasing by 11%, 15%, and 22%. Although NECA increased CF by ∼60% (Fig. 2B), there was a dissociation of the contractile and vasodilatory effects. CF was nearly maximal at 100 nM NECA, at a 10-fold lower dose than needed to increase contractility. In addition, the NECA-induced increase in contractile parameters abated within 10 min after agonist treatment was discontinued, but CF remained elevated. Similar effects were observed with the selective A2bAR agonist BAY 60-6583. At concentrations of 500 nM and 1 μM, BAY 60-6583 significantly increased contractility, with LVSP, +dP/dt, and −dP/dt increasing by 13%, 18%, and 29% at the higher concentration (Fig. 3A). The A2bAR agonist increased CF by >50% at 100 nM, with little additional increase as the concentration was increased (Fig. 3B). As seen with NECA, CF remained elevated after the discontinuation of BAY 60-6583 treatment, but contractility decreased to baseline levels. The results shown in Table 3 indicate that the hemodynamic effects of BAY 60-6583 were absent in A2bAR KO hearts.
Fig. 2.
Effects of the nonselective agonist 5′-N-ethylcarboxamido adenosine (NECA) on cardiac contractility (A) and coronary flow (B). Constant pressure-perfused and paced hearts were treated with increasing concentrations (100, 500, and 1,000 nM) of NECA. Baseline contractility was measured before drug infusion, and all data are expressed as percent baseline values. Washout measurements were taken 10 min after the discontinuation of treatment with the highest concentration of NECA. Data are means ± SE; n ≥ 5 experiments/group. *P < 0.05 vs. baseline values.
Fig. 3.
Effects of the A2bAR agonist BAY 60-6583 on cardiac contractility (A) and coronary flow (B). Constant pressure-perfused and paced hearts were treated with increasing concentrations (100, 500, and 1,000 nM) of BAY 60-6583. Baseline contractility was measured before drug infusion, and all data are expressed as percent baseline values. Washout measurements were taken 10 min after responses to the 1,000 nM concentration were determined. Data are means ± SE; n ≥ 5 experiments/group. *P < 0.05 vs. baseline values.
Table 3.
Hemodynamic effects of BAY 60-6583 in A2b AR KO hearts
| LVSP, mmHg | +dP/dt, mmHg/s | −dP/dt, mmHg/s | CF, ml/min | |
|---|---|---|---|---|
| Baseline | 89 ± 3 | 3,140 ± 286 | −1,983 ± 117 | 2.5 ± 0.2 |
| 100 nM BAY 60-6583 | 88 ± 3 | 3,177 ± 294 | −1,996 ± 126 | 2.5 ± 0.2 |
| 500 nM BAY 60-6583 | 88 ± 3 | 3,184 ± 309 | −2,007 ± 117 | 2.5 ± 0.2 |
| 1000 nM BAY 60-6583 | 90 ± 3 | 3,321 ± 376 | −2,116 ± 163 | 2.6 ± 0.3 |
Values are means ± SE. Baseline values were taken before treatment with the A2bAR agonist BAY 60-6583.
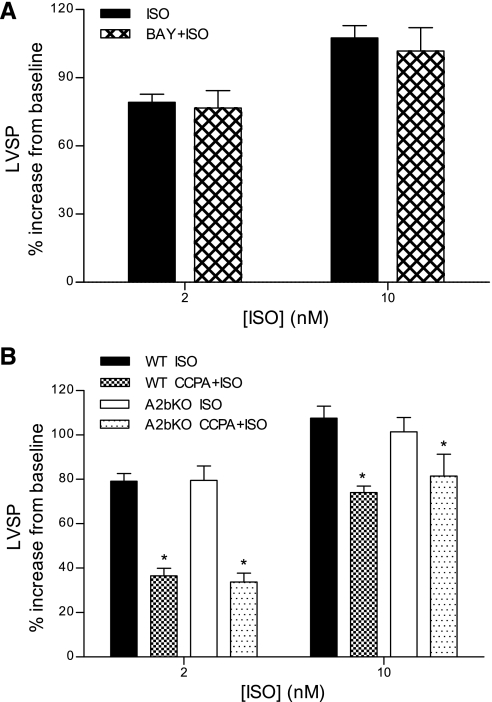
Additional experiments determined whether A2bAR modulated the contractile effects of β-adrenergic stimulation or the A1AR antiadrenergic effect. As shown in Fig. 4A, the A2bAR agonist (200 nM) did not alter the positive inotropic effects of Iso on LVSP. A similar trend was observed for +dP/dt and −dP/dt, where, in the presence of BAY 60-6583, contractility remained indistinguishable from that with Iso alone (data not shown). The results shown in Fig. 4B indicate that deletion of A2bAR had no effect on Iso-induced increases in LVSP, nor did it alter the A1AR antiadrenergic effect. Similar patterns were observed for +dP/dt and −dP/dt (data not shown). These findings indicate that, unlike in A2aAR KO hearts, there was no potentiation of the A1AR antiadrenergic effect in A2bAR KO hearts.
Fig. 4.
Effects of A2bAR stimulation (A) and A2bAR deletion (B) on β-adrenergic receptor-induced increases in LVSP. A: Iso protocols were conducted in WT hearts in the absence or presence of the A2bAR agonist BAY 60-6583 (200 nM). Data are expressed as percent increases above baseline values after Iso stimulation. B: contractile effects of Iso in the absence or presence of the A1AR agonist CCPA (200 nM) in WT and A2bAR knockout (A2bKO) mouse hearts. Data are means ± SE; n ≥ 4 experiments/group. Statistically significant differences (*P < 0.05) are shown for CCPA + ISO vs. ISO.
Gene expression of ARs.
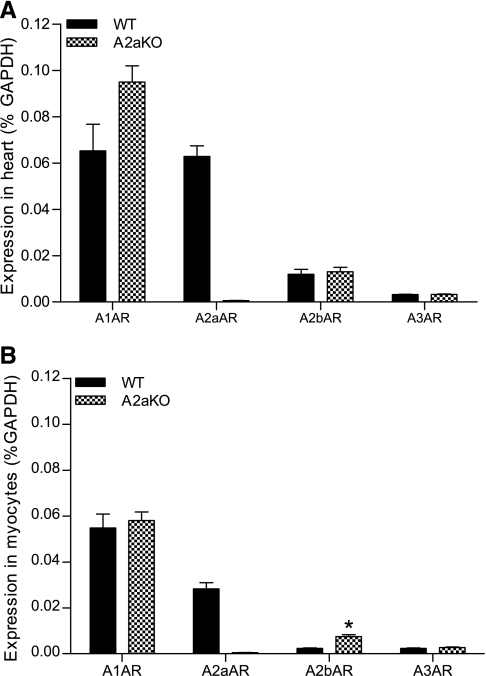
Real-time quantitative PCR was used to assess the expression levels of the genes encoding all four AR subtypes in adult murine ventricular myocardium and isolated ventricular myocytes in WT and A2aAR KO hearts. As shown in Fig. 5A, all four AR subtypes were expressed in the mouse ventricle, with A1AR and A2aAR subtypes expressed to a much greater extent than A2bAR and A3AR subtypes. Deletion of A2aAR did not result in any significant changes in the expression of the other receptor subtypes. Figure 5B shows that the mRNA for all four subtypes was expressed in enriched myocyte preparations, albeit at lower levels than in intact hearts (with the exception of A1AR). The enrichment of the myocyte sample (∼80% cardiomyocytes) was based on the mRNA levels of troponin T compared with mRNA levels of the fibroblast marker discoidin domain receptor, type 2, vascular endothelial cadherin, and smooth muscle markers calponin and SM22. Myocytes isolated from A2aAR KO hearts exhibited similar levels of receptor mRNA expression, although the A2bAR message was greater than in WT myocytes.
Fig. 5.
AR gene expression in the murine myocardium (A) and ventricular cardiomyocytes (B) of WT and A2aKO hearts. Relative AR gene expression was assessed by real-time PCR in mouse ventricles and isolated, enriched ventricular myocyte preparations. All data were normalized to GAPDH and are expressed as a percentage of GAPDH. Data are means ± SE; n ≥ 3. *Statistically significant difference from the heart at P < 0.05.
DISCUSSION
A2aAR and A2bAR subtypes are expressed in coronary vascular tissue, where their potent vasodilatory effects have been well characterized. However, the effects of A2aAR on cardiac function are controversial, at best, and the effects of A2bAR stimulation on cardiac contractility have not been fully examined. The results of the present study indicate that the A2aAR agonist CGS-21680 exerted potent coronary vasodilation but did not elicit any direct effects on contractility, nor indirectly alter the β-adrenergic positive inotropic effect. However, deletion of A2aAR potentiated the A1AR-mediated antiadrenergic effect. High doses (≥500 nM) of the nonselective agonist NECA and the A2bAR-selective agonist BAY 60-6583 increased contractility independent of their coronary vasodilatory effects. Stimulation of A2bAR with BAY 60-6583 did not alter the effects of β-adrenergic receptor stimulation, and the A1AR antiadrenergic effect was not altered in A2bAR KO hearts. Real-time quantitative PCR results indicated the presence of both A2aAR and A2bAR in ventricular myocytes, supporting distinct roles for these receptors in modulating cardiac contractility.
A2aAR modulation of cardiac contractility.
The present findings indicate that A2aAR stimulation exerts potent coronary vasodilatory effects but no direct effects on cardiac contractility. CF increased by 50%, but cardiac function did not change with increasing concentrations of the A2aAR agonist (up to 1 μM). These results are consistent with our previous reports as well as those of others (13, 16, 19, 31) showing that A2aAR stimulation does not alter cardiac function in isolated perfused rat hearts or isolated cardiac myocytes. In contrast, it has been reported that the A2aAR agonist CGS-21680 (100 nM) increased LV developed pressure in isolated mouse hearts by ∼80% (20). However, this same concentration also increased CF by 500%, and the A2aAR dose-response curves for LV function and coronary vasodilation exhibited similar EC50 values. Willems and Headrick (31) concluded that this significant increase in contractility was due to the Gregg phenomenon or the garden hose effect (flow-induced increases in ventricular function). In the only other study to date in mouse myocardium, Tikh et al. (30) concluded that A2aAR stimulation with CGS-21680 increased contractility by ∼5–10%, but these authors did not present specific data to support this conclusion. There have been few in vivo studies in large or small animals due to the fact that the majority of A2aAR agonists exert significant reductions in afterload, thus invalidating the use of standard measurements of cardiac function. We (14) have previously reported, however, that using load-insensitive measurements of cardiac contractility [preload recruitable stroke work (PRSW) and PRSW area] in an intact porcine preparation that an intracoronary infusion of CGS-21680 increased regional coronary blood flow by 150% but exerted no effects on regional cardiac contractility.
The present findings also indicate that A2aAR does not alter β-adrenergic receptor-mediated increases in cardiac contractility. In WT hearts, the A2aAR agonist CGS-21680 (200 nM) did not alter Iso-mediated increases in LVSP, +dP/dt, or −dP/dt. In addition, contractile responses to Iso were not altered in A2aAR KO hearts, indicating that gene ablation did not alter the normal β-adrenergic response. Results from a previous study (30) in isolated perfused mouse hearts indicated that CGS-21680 (100 nM) potentiated the effects of Iso on +dP/dt by ∼60%. It is not clear why our findings are different from this study, but our observations are consistent with previous reports (19, 23) showing that A2aAR stimulation did not potentiate β-adrenergic effects in rat isolated hearts and cardiomyocytes.
Our observations do indicate, however, that A2aAR activation does modulate the A1AR antiadrenergic effect. Both WT and A2aAR KO hearts displayed A1AR antiadrenergic responses, but this response was potentiated in A2aAR KO hearts. At 2 nM Iso, A1AR antiadrenergic effects in A2aAR KO hearts were increased by ∼40% compared with WT hearts. With 10 nM Iso, only the blunting of +dP/dt was potentiated in A2aAR KO hearts. These results suggest that the absence of A2aAR increases the sensitivity of the A1AR antiadrenergic effect but may not alter the effects of higher levels of β-adrenergic receptor stimulation. These data are consistent with a previous observation showing that ZM-241385, an A2aAR antagonist, potentiated the A1AR antiadrenergic response in isolated perfused rat hearts (19). However, our present observations contradict those of Tikh et al. (30), who reported that the A1AR antiadrenergic effect was significantly reduced in the A2aAR KO mouse. Their conclusion was based on only a single concentration of Iso (10 nM), whereas we observed much greater modulation of the A1AR antiadrenergic effect at 2 versus 10 nM Iso. Although we used a slightly higher concentration of CCPA (200 vs. 100 nM) than those authors, we observed a similar A1AR antiadrenergic effect during 10 nM Iso stimulation. Our higher concentration of CCPA was associated with an ∼20% increase in CF, indicating the activation of vascular A2aAR, an effect that was absent in A2aAR KO hearts.
Our observation that deletion of A2aAR modulates cardiac A1AR is consistent with other reports suggesting that A2aAR can modulate A1AR activity. It has been reported that A1AR and A2aAR form functional heterodimers (in a heterologous expression system and native rat striatum and human brain caudate nucleus) in which A2aAR has the ability to decrease the affinity of A1AR for agonists (3, 5). There is also evidence that A2aAR can modulate A1AR effects in the heart. We (15) reported that the AR antagonist ZM-241385, which exhibits some selectivity for A2aAR, blocked the infarct size-reducing effects of CCPA in intact rats. It has also been reported that A2aAR activation antagonizes the A1AR stimulatory effects of protein phosphatase 2A activity in the mouse myocardium, an effect that is absent in A2aAR KO mice (29). Thus, it is possible that, in the absence of A2aAR, inhibitory effects on A1AR are relieved, and A1AR can exert a stronger antiadrenergic effect, as observed in this study.
A2bAR modulation of cardiac contractility.
A2bAR is the least well-characterized AR in the heart, although its role in the modulation of CF is well recognized (22). In contrast to A1AR and A2aAR, A2bAR displays a lower affinity for adenosine, and it has been reported that the expression of myocardial A2bAR is much lower than that of A1AR and A2aAR (20). A2bAR is coupled to a Gs pathway and is also coupled to Gq in select cell types, but A2bAR G protein coupling in the heart has not been determined. In addition, there is a general lack of knowledge on the potential regulation of cardiac function via this receptor subtype.
Both the nonselective AR agonist NECA and the A2bAR-selective agonist BAY 60-6583 increased CF and contractility. Both agonists increased CF by ∼50% at 100 nM, and this was associated with no effect on LV contractile function. Increasing the agonist concentrations resulted in little additional increases in CF but did significantly increase contractility (∼15–20%) assessed by all three parameters. In addition, after completion of the dose-response curves, the contractile effects of both agonists disappeared after a 10-min washout period, whereas CF remained elevated. These observations indicate that the contractile effects of both agonists were independent of the increases in CF. The increase in contractility with NECA was not apparent until a concentration of 1 μM, consistent with the lower affinity of this agonist for A2bAR. The effect of NECA on contractility was absent in A2bAR KO hearts, although coronary vasodilation persisted due to its effects on A2aAR (data not shown; n = 2). The A2bAR selectivity of BAY 60-6583 was confirmed by the lack of effect of this agonist on both CF and contractility in A2bAR KO hearts. Despite the moderate increases in contractility with A2bAR stimulation, β-adrenergic contractile responses were not altered by BAY 60-6583, and the A1AR antiadrenergic effect was not affected in the absence of A2bAR.
Our observations that A2bAR activation exerted a modest positive inotropic effect are consistent with a limited number of previous studies. It has been reported that adenosine and NECA (at high concentrations) produced small increases in contractility in isolated rat hearts, consistent with a possible A2bAR effect (19). Willems and Headrick (31) also reported a similar small, but sustained, flow-independent effect of adenosine in isolated perfused mouse hearts and concluded that this was likely due to A2bAR stimulation. In contrast, it has been shown that NECA increased contractility in isolated perfused mouse hearts by ∼140% at a concentration of 100 nM in association with a 300% increase in CF (20). However, it must be noted that these hearts were unpaced, and, given that NECA decreases HR via A1AR activation, interpretation of cardiac function with large changes in HR is very difficult. In a subsequent study by the same laboratory (28), BAY 60-6583 exerted a significant increase in LV pressure in isolated mouse hearts in parallel with increases in CF.
The indirect effects of A2aAR and direct effects of A2bAR on cardiac contractility are consistent with the expression of both AR subtypes in ventricular myocytes as assessed by real-time quantitative PCR. The pattern of AR subtype mRNA expression that we observed in mouse heart ventricular tissue is essentially identical to that reported previously (21). However, A2aAR and A2bAR mRNA expression are significantly reduced in isolated ventricular myocytes, and even with our efforts to reduce contamination by preplating the myocyte suspension for 2 h, the presence of nonmyocytes cannot be completely excluded. Our observations that there was no change in A1AR expression in A2aAR KO hearts and myocytes indicates that the potentiation of the A1AR antiadrenergic effect in these hearts is not likely to be due to an upregulation of A1AR. It has been published that KO of A2bAR also does not alter the myocardial expression of other AR subtypes (10).
Our observations that NECA and BAY 60-6583 exerted flow-independent increases in cardiac contractility are consistent with the expression of A2bAR in cardiac myocytes, as supported by our RT-PCR data. A previous study (32) provided evidence for A2bAR mRNA in isolated rat ventricular myocytes, but these authors did not describe procedures to reduce contamination by nonmyocytes. Furthermore, there is evidence for low-affinity A2bAR in cultured fetal chick cardiomyocytes based on differential responses to several AR agonists (including NECA) and antagonists (17). Although there is no direct evidence to date of A2bAR protein expression in adult mammalian ventricular cardiomyocytes, the present functional data are consistent with this hypothesis. Given the increases in adenosine that occur during conditions of myocardial O2 supply/demand imbalance, such as myocardial ischemia and hypoxia, it is possible that cardiac function may be modulated by low-affinity A2bAR.
Conclusions.
In summary, these findings indicate that A2aAR and A2bAR modulate cardiac contractility via indirect and direct effects, respectively. A2aAR activation exerted no direct effects on cardiac contractility or the β-adrenergic contractile response. A2bAR stimulation induced a modest increase in basal cardiac contractility independent of coronary vasodilation but had no effect on the β-adrenergic contractile response. Contractility experiments conducted using A2aAR and A2bAR KO mice further indicated that A2aAR indirectly modulates cardiac contractility by attenuating the A1AR-mediated antiadrenergic response but that A2bAR does not exhibit such indirect effects. These modulatory effects of A2aAR and A2bAR on ventricular contractility are consistent with our quantitative real-time PCR data, thus supporting the expression of these two AR subtypes on ventricular cardiomyocytes. Taken together, it appears that the two stimulatory ARs, A2aAR and A2bAR, play distinct roles in modulating cardiac contractility.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-066132 (to R. D. Lasley).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Dr. Joel Linden (La Jolla Institute for Allergy and Immunology, La Jolla, CA) and Dr. Stephen Tilley (University of North Carolina, Chapel Hill, NC) for the generous gifts of A2aAR and A2bAR KO breeders. The authors also acknowledge Dr. Thomas Krahn (Bayer Healthcare, Wuppertal, Germany) for providing BAY 60-6583.
REFERENCES
- 1.Auchampach JA, Kreckler LM, Wan TC, Maas JE, van der Hoeven D, Gizewski E, Narayanan J, Maas GE. Characterization of the A2B adenosine receptor from mouse, rabbit, and dog. J Pharmacol Exp Ther 329: 2–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belardinelli L, Shryock JC, Snowdy S, Zhang Y, Monopoli A, Lozza G, Ongini E, Olsson RA, Dennis DM. The A2A adenosine receptor mediates coronary vasodilation. J Pharmacol Exp Ther 284: 1066–1073, 1998 [PubMed] [Google Scholar]
- 3.Casado V, Barrondo S, Spasic M, Callado LF, Mallol J, Canela E, Lluis C, Meana J, Cortes A, Salles J, Franco R. Gi protein coupling to adenosine A1-A2A receptor heteromers in human brain caudate nucleus. J Neurochem 114: 972–980, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Epperson S, Makhsudova L, Ito B, Suarez J, Dillmann W, Villarreal F. Functional effects of enhancing or silencing adenosine A2b receptors in cardiac fibroblasts. Am J Physiol Heart Circ Physiol 287: H2478–H2486, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Ciruela F, Ferre S, Casado V, Cortes A, Cunha RA, Lluis C, Franco R. Heterodimeric adenosine receptors: a device to regulate neurotransmitter release. Cell Mol Life Sci 63: 2427–2431, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobson JG, Jr, Fenton RA. Adenosine A2 receptor function in rat ventricular myocytes. Cardiovasc Res 34: 337–347, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Dobson JG, Jr, Ordway RW, Fenton RA. Endogenous adenosine inhibits catecholamine contractile responses in normoxic hearts. Am J Physiol Heart Circ Physiol 251: H455–H462, 1986 [DOI] [PubMed] [Google Scholar]
- 8.Dobson JG, Jr, Shea LG, Fenton RA. Adenosine A2A and β-adrenergic calcium transient and contractile responses in rat ventricular myocytes. Am J Physiol Heart Circ Physiol 295: H2364–H2372, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drury AN, Szent-Gyorgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol 68: 213–237, 1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation 115: 1581–1590, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Feng W, Song Y, Chen C, Lu ZZ, Zhang Y. Stimulation of adenosine A2B receptors induces interleukin-6 secretion in cardiac fibroblasts via the PKC-δ-P38 signalling pathway. Br J Pharmacol 159: 1598–1607, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenton RA, Moore ED, Fay FS, Dobson JG., Jr Adenosine reduces the Ca2+ transients of isoproterenol-stimulated rat ventricular myocytes. Am J Physiol Cell Physiol 261: C1107–C1114, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Kilpatrick EL, Narayan P, Mentzer RM, Jr, Lasley RD. Cardiac myocyte adenosine A2a receptor activation fails to alter cAMP or contractility: role of receptor localization. Am J Physiol Heart Circ Physiol 282: H1035–H1040, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Lasley RD, Jahania MS, Mentzer RM., Jr Beneficial effects of adenosine A2a agonist CGS-21680 in infarcted and stunned porcine myocardium. Am J Physiol Heart Circ Physiol 280: H1660–H1666, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Lasley RD, Kristo G, Keith BJ, Mentzer RM., Jr The A2a/A2b receptor antagonist ZM-241385 blocks the cardioprotective effect of adenosine agonist pretreatment in in vivo rat myocardium. Am J Physiol Heart Circ Physiol 292: H426–H431, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Lasley RD, Mentzer RM., Jr Adenosine improves recovery of postischemic myocardial function via an adenosine A1 receptor mechanism. Am J Physiol Heart Circ Physiol 263: H1460–H1465, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Liang BT, Haltiwanger B. Adenosine A2a and A2b receptors in cultured fetal chick heart cells. High- and low-affinity coupling to stimulation of myocyte contractility and cAMP accumulation. Circ Res 76: 242–251, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Martens D, Lohse MJ, Schwabe U. [3H]-8-cyclopentyl-1,3-dipropylxanthine binding to A1 adenosine receptors of intact rat ventricular myocytes. Circ Res 63: 613–620, 1988 [DOI] [PubMed] [Google Scholar]
- 19.Monahan TS, Sawmiller DR, Fenton RA, Dobson JG., Jr Adenosine A2a-receptor activation increases contractility in isolated perfused hearts. Am J Physiol Heart Circ Physiol 279: H1472–H1481, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Morrison RR, Talukder MA, Ledent C, Mustafa SJ. Cardiac effects of adenosine in A2A receptor knockout hearts: uncovering A2B receptors. Am J Physiol Heart Circ Physiol 282: H437–H444, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Morrison RR, Teng B, Oldenburg PJ, Katwa LC, Schnermann JB, Mustafa SJ. Effects of targeted deletion of A1 adenosine receptors on postischemic cardiac function and expression of adenosine receptor subtypes. Am J Physiol Heart Circ Physiol 291: H1875–H1882, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Mustafa SJ, Morrison RR, Teng B, Pelleg A. Adenosine receptors and the heart: role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol 161–188, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norton GR, Woodiwiss AJ, McGinn RJ, Lorbar M, Chung ES, Honeyman TW, Fenton RA, Dobson JG, Jr, Meyer TE. Adenosine A1 receptor-mediated antiadrenergic effects are modulated by A2a receptor activation in rat heart. Am J Physiol Heart Circ Physiol 276: H341–H349, 1999 [DOI] [PubMed] [Google Scholar]
- 24.O'Connell TD, Ni YG, Lin K, Han H, Yan Z. Isolation and culture of adult mouse cardiac myocytes for signaling studies. Alliance Cell Signal 1: 1–9, 2003 [Google Scholar]
- 25.Olanrewaju HA, Qin W, Feoktistov I, Scemama JL, Mustafa SJ. Adenosine A2A and A2B receptors in cultured human and porcine coronary artery endothelial cells. Am J Physiol Heart Circ Physiol 279: H650–H656, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Peart JN, Headrick JP. Adenosinergic cardioprotection: multiple receptors, multiple pathways. Pharmacol Ther 114: 208–221, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Shen J, Halenda SP, Sturek M, Wilden PA. Novel mitogenic effect of adenosine on coronary artery smooth muscle cells: role for the A1 adenosine receptor. Circ Res 96: 982–990, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Teng B, Ledent C, Mustafa SJ. Up-regulation of A2B adenosine receptor in A2A adenosine receptor knockout mouse coronary artery. J Mol Cell Cardiol 44: 905–914, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tikh EI, Fenton RA, Chen JF, Schwarzschild MA, Dobson JG., Jr Adenosine A1 and A2A receptor regulation of protein phosphatase 2A in the murine heart. J Cell Physiol 216: 83–90, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Tikh EI, Fenton RA, Dobson JG., Jr Contractile effects of adenosine A1 and A2A receptors in isolated murine hearts. Am J Physiol Heart Circ Physiol 290: H348–H356, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Willems L, Headrick JP. Contractile effects of adenosine, coronary flow and perfusion pressure in murine myocardium. Pflügers Arch 453: 433–441, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Xi J, McIntosh R, Shen X, Lee S, Chanoit G, Criswell H, Zvara DA, Xu Z. Adenosine A2A and A2B receptors work in concert to induce a strong protection against reperfusion injury in rat hearts. J Mol Cell Cardiol 47: 684–690, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu H, Stein B, Liang B. Characterization of a stimulatory adenosine A2a receptor in adult rat ventricular myocyte. Am J Physiol Heart Circ Physiol 270: H1655–H1661, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Yan L, Burbiel JC, Maass A, Müller CE. Adenosine receptor agonists: from basic medicinal chemistry to clinical development. Expert Opin Emerg Drugs 8: 537–76, 2003 [DOI] [PubMed] [Google Scholar]