Abstract
Recently, the use of overexpression of telomerase reverse transcriptase (TERT) has led to the generation of immortalized human cell lines. However, this cell immortalization approach has not been reported in well-differentiated mouse cells, such as renal epithelial cells. We sought to establish and then characterize a mouse collecting duct cell line, using ectopic expression of mTERT. Isolated primary cortical collecting duct (CCD) cell lines were transduced with mouse (m)TERT, using a lentiviral vector. mTERT-negative cells did not survive blasticidin selection, whereas mTERT-immortalized cells proliferated in selection media for over 40 subpassages. mTERT messenger RNA and telomerase activity was elevated in these cells, compared with an SV40-immortalized cell line. Flow cytometry with Dolichos biflorus agglutinin was used to select the CCD principal cells, and we designated this cell line mTERT-CCD. Cells were well differentiated and exhibited morphological characteristics typically found in renal epithelial cells, such as tight junction formation, microvilli, and primary cilia. Further characterization using standard immunofluorescence revealed abundant expression of aquaporin-2 and the vasopressin type 2 receptor. mTERT-CCD cells exhibited cAMP-stimulated/benzamil-inhibited whole cell currents. Whole cell patch-clamp currents were also enhanced after a 6-day treatment with aldosterone. In conclusion, we have successfully used mTERT to immortalize mouse collecting duct cells that retain the basic in vivo phenotypic characteristics of collecting duct cells. This technique should be valuable in generating cell lines from genetically engineered mouse models.
Keywords: epithelial, polycystic
a recent development in cell immortalization uses overexpression of telomerase reverse transcriptase (TERT), the catalytic subunit of the telomerase enzyme (28), to immortalize primary cell lines. This enzyme provides for the replacement of short segments of DNA that are lost during cell replication (10). Loss of telomerase activity is thought to be involved in cell senescence in culture and in the process of aging (16, 32). Telomerase is abundantly expressed in cells that routinely undergo cell division, whereas it is expressed at low levels in most well-differentiated somatic cells (10, 14, 16, 18). In addition, mouse (m)TERT levels also appear to diminish over time in primary cell cultures, in part, leading to cell senescence and cell death (1, 6, 10, 14, 18). Work with human primary cell lines has shown that ectopic expression of human TERT (hTERT) can lead to continued cell replication and hence, immortalization (21, 31, 37, 39). More importantly, recent reports have shown immortalized hTERT cells remain differentiated, thus preserving the phenotypic properties that closely resemble in vivo characteristics (37, 39).
A current literature search revealed no successful mTERT immortalization of differentiated cultured mouse cells, whereas mTERT has been used to immortalize mouse embryonic fibroblasts, embryonic stem cells (2, 13), and spermatocytes (11). The lack of mTERT-mediated immortalization in well-differentiated mouse cells might be due to the intrinsically higher level of TERT expression in mouse compared with human cells, the delivery method of the TERT gene, or cell line variability (7, 19, 29).
A common immortalization strategy is to culture cells derived from excised tissue of the Immortomouse. In addition, cell lines such as renal epithelial cells have been established by transfecting the cells with the SV-40 temperature-sensitive (tsA58) large T antigen (38). Although these cells lines have provided valuable information, studies have shown this approach could result in a loss of phenotypic features as these lines become genomically unstable (32, 36, 37, 39). Another often-used strategy is to breed the Immortomouse with genetically altered mice and to subsequently generate immortalized cell lines from the resulting offspring. However, genetic background issues can complicate this approach. Alleles of interest that produce a specific phenotype in one mouse model may be modified by background alleles that are unique to other mouse strains, such as those carrying the SV40 T antigen (3, 4, 15, 22, 34). Creating a transgenic mouse from mice with different genetic backgrounds may produce confounding effects due to background alleles interfering with the modified genes (9, 20, 25, 30, 35, 41).
The purpose of this study was to evaluate the feasibility of establishing immortalized mouse renal epithelial cells using ectopic expression of mTERT. If successful, this would be of substantial importance to the field, as this technology would allow cell lines to be generated directly from genetically engineered or wild-type mouse models.
METHODS
Cell immortalization.
Wild-type mice from a mixed genetic background (129 and C57B6/J) were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Institutional Animal Care and Use Committee approved the protocol. Mice were euthanized using CO2 asphyxiation, the kidneys were removed, and then transverse sections were immediately placed in 1% collagenase type IVS (Sigma) in DMEM/F-12 media (Media Tech). Tissue sections were incubated in collagenase, on a rotator, for 30 min at room temperature. Next, slices of renal cortex were placed in a glass petri dish containing media, and then collecting ducts were hand dissected using fine forceps under a stereomicroscope. It should be noted that although the majority of dissected tubules were collecting ducts, it was impossible to visually verify that this was a pure population of collecting duct cells/tubules. Renal epithelial cells and tubules were then transferred to a conical tube containing media and centrifuged at 750 g for 10 min. The supernatant was removed, and the tissue was resuspended in fresh media, containing 0.2 μg/ml dexamethasone, 10 nM triiodothrionine, 1× insulin-transferrin-sodium selenite (ITS), and 5% FBS (Fisher) and placed onto Snapwell permeable membranes, in six-well plates (12-mm diameter, 0.4-mm pore, polyester; Costar). All media additives were purchased from Sigma except FBS. No antibiotics were added to the media, in preparation for the transduction with the mTERT lentiviral expression construct. Cells were maintained in a 37°C humidified incubator with 5% CO2 for 2 days to allow cell colony formation.
The mTERT expression plasmid was generated using the ViraPower Lentiviral Expression System (Invitrogen, Carlsbad, CA). A full-length cDNA clone of mTERT was obtained from inner medullary collecting duct (IMCD) cells by RT-PCR, using sequence-specific primers. BP Clonase was used to clone the mTERT gene into pDONR221, generating the entry vector. Recombination of the mTERT entry vector with the pLenti6/UBC/V5-DEST Gateway Vector, containing a blasticidin selection marker, resulted in the destination vector. Lentivirus was generated in 293FT cells by cotransfection with the mTERT expression plasmid and the ViraPower packaging mix, using Lipofectamine 2000 (Invitrogen) to increase transfection efficiency. The supernatant, containing the lentivirus with the mTERT gene and blasticidin resistance gene, was then harvested 72 h posttransfection and filtered through a 0.45-μm nylon membrane. To assay the viral titer, 2 × 105 IMCD cells were plated in each well of a six-well plate. Then, 10-fold serial dilutions of the viral supernatant (from 10−2 to 10−6) were prepared, and IMCD cells were transduced with the lentivirus containing mTERT and the blasticidin resistance marker in five of the six wells. IMCD cells in the remaining well received an empty vector. Cells containing the blasticidin resistance gene also contained the mTERT gene, so complete media with 5 μg/ml blasticidin was used to select cells containing the mTERT gene 48 h posttransduction. Surviving colonies were visualized with crystal violet staining (Sigma). IMCD cells transduced with the empty vector did not survive.
The highest viral titer, 1.36 × 106 transducing units (TU)/ml, was used to introduce mTERT into the dissected primary collecting duct cells. Cells immortalized with the mTERT gene were selected 48 h posttransduction, using 5 μg/ml blasticidin in complete media. Blasticidin was included in the media for 40 subpassages to maintain selection pressure and reduce the possibility of culturing cells that did not contain the mTERT gene. For patch-clamp studies, cells were maintained only in culture media without antibiotics. There were no apparent differences in cell morphology or in cell responsiveness between cells cultured with or without antibiotics. One week posttransduction, cells were trypsinized with 0.25% trypsin-EDTA (Invitrogen) and transferred to a 24-well culture plate (Fisher) to visualize cell morphology. A glass column, affixed to the plate with vacuum grease, was used to select cells with visual epithelial characteristics. Cells within the glass column were removed using 0.25% trypsin-EDTA and replated into T-75 flasks until confluent.
Control cell line.
The BAP2 cell line was used as a control because it was derived from the collecting duct using microdissection in a similar manner as the mTERT-immortalized cells. BAP2 is a collecting duct cell line expressing normal Tg737 (ift88) derived from an orpk mouse crossed with the Immortomouse (40). BAP2 cells were grown to confluence at 33°C in DMEM/F-12 with 0.2 μg/ml dexamethasone, 10 nM triiodothrionine, 1× ITS, 5% FBS, and 12 U/ml IFN-γ. When cells were confluent, they were placed at 37°C in complete media without IFN-γ for 5 days until differentiated.
Flow cytometry.
Visually selected mTERT-immortalized cells were incubated in PBS with 0.1% FBS containing 20 μg/ml of the biotinylated lectin, Dolichos biflorus agglutinin (DBA; Vector), at 37°C for 30 min. The cells were washed one time in PBS to remove unbound lectin and then incubated at 37°C for 30 min in PBS containing 10 μg/ml cascade blue-avidin-neutravidin conjugate (Invitrogen). Cells were then washed two times in PBS to remove unbound fluorophore and were immediately sorted on a Becton Dickinson FACSAria. The selected cells were plated in a T-25 flask and then expanded and used for subsequent experiments.
Magnetic selection of epithelial cells.
In an effort to further ensure the purity of this cell line, epithelial cells were selected using an EasySep Mouse Epithelial Cell Enrichment Kit from Stemcell Technologies (Vancouver, BC). Cortical collecting duct epithelial cells previously selected by flow cytometry were enriched by depletion of hematopoietic and endothelial cells. Briefly, nonepithelial cells were specifically labeled with biotinylated antibodies and subsequently labeled with dextran-coated magnetic nanoparticles. The cell suspension was placed in the EasySep magnet so that the magnetically labeled cells adhered to the sides of the tube and the epithelial cells were decanted into a fresh tube. Enriched epithelial cells were resuspended in complete media without antibiotics and used in electrophysiology experiments.
Immunofluorescence.
mTERT-CCD and BAP2 cells were plated onto eight-well chamber slides (BD Bioscience) or onto Snapwell permeable membranes and grown to confluence. To visualize apical DBA and the tight junction marker zonula occludens (ZO)-1, cells grown on Snapwell permeable membranes were fixed with 4% paraformaldehyde (PFA) at room temperature for 10 min, washed three times with PBS, and then 20 μg/ml of FITC-conjugated DBA lectin (Vector) was incubated in the apical compartment of the permeable support for 30 min at room temperature. Cells were washed five times with PBS to remove unbound lectin and then were permeabilized using 0.5% saponin (Sigma) for 5 min at room temperature to allow ZO-1 access to its binding sites. All other cells, on eight-well chamber slides, were fixed and permeabilized as described above. All cells were blocked with 5% IgG-free BSA (Jackson ImmunoResearch) and 5% normal serum from the secondary antibody's host animal for 1 h at room temperature. Primary antibodies were added for overnight incubation at 4°C. Secondary antibodies were incubated 1 h at room temperature. Subsequently, cells were washed two times with PBS-T containing 1% BSA and 1% normal serum and then three times with PBS. A 1:500 dilution of Hoechst (Invitrogen) was added to the final PBS wash and incubated for 15 min. Slides were mounted with Vectashield (Vector), sealed with nail polish, and then viewed on a Leica confocal (TCS SP5) inverted microscope (DMI5000); images were acquired using LAS software. Antibodies used were 1:50 vasopressin 2 receptor (V2R; Abcam), 1:100 aquaporin-2 (AQP2; Abcam), 1:500 ZO-1 (Santa Cruz Biotechnology). Secondary antibodies, 1:1,000 dilution, were conjugated with Dylight fluorophores (488 or 549 nm, Jackson ImmunoResearch). To confirm antibody specificity, secondary antibodies were added to cells without primary antibodies. No fluorescence was observed in these cells.
Real-time quantitative PCR.
Cells were grown to 80–90% confluence on six-well plates (Corning). Total RNA was isolated from TERT-immortalized and BAP2 (control) cells using TRIzol reagent (Invitrogen, Carlsbad, CA) and reverse-transcribed using SuperScript One-Step RT-PCR System (Invitrogen). BAP2 and mTERT mRNA were quantified by RT-PCR using an iCycler iQ (Bio-Rad) with RT2 SYBR Green qPCR Master Mix (SABiosciences) and mTERT-specific primer mix (PPM03702A; SABiosciences). Cellular GAPDH mRNA was used as the normalization control.
Telomerase activity.
Telomerase activity was measured using real-time quantitative telomeric repeat amplification protocol (RTQ-TRAP) as described by Karystinou et al. (17). Cells (106) were lysed in 200 μl of 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate hydrate (CHAPS) buffer (10 mM Tris, pH 8.0, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, 0.5% CHAPS and 200 U of RNAsin), and incubated at 4°C for 30 min. Cell lysate was centrifuged at 12,000 g for 30 min at 4°C, and telomerase activity of the supernatant was measured. Each RTQ-TRAP assay reaction (25 μl total) contained 1× SYBR Green RT-PCR Master mix (SABiosciences), 0.2 μg of T4 gene protein, 0.4 μM of primers TS (5′-GCGCGG[CTTACC]3CTAACC-3′) and ACX (5′-AATCCGTCGAGCAGAGTT-3′) and cell lysate containing 1 μg of total protein. Samples were incubated at 25°C for 20 min to allow TS primer elongation by the telomerase in the cell extracts. Extended primer was amplified (95°C for 10 min, followed by 40 cycles at 95°C for 20 s, 50°C for 30 s, and then 72°C for 90 s), and threshold cycles (Ct) of the lysates were compared as a measure of the telomerase activity of the mTERT and BAP2 cells. Cell lysates from three separate samples were run in triplicate, and lysis buffer was used as the negative control.
Flow-dependent changes in cytosolic calcium concentration.
mTERT-CCD and control cells were grown on 35-mm round glass no. 1 coverslips (Glycotech) until 100% confluent and then incubated in phenol red-free DMEM/F-12 containing 5 μg/ml fura 2-AM, resuspended in DMSO/20% pluronic acid (Teflabs), for 1 h at 37°C. The cells were then rinsed with PBS and attached to a parallel plate chamber (Glycotech) using a vacuum seal. An IPC-8 pump (Ismatec) was used to perfuse cells with a modified, low-sodium Ringer solution. Modified Ringer contained (in mM) 10 NaCl, 4.5 KCl, 1 CaCl2, 2 MgCl2, 10 d-glucose, 10 HEPES, 130 NMDG, and 1 probenecid (Invitrogen). All chemicals were purchased from Sigma except probenecid. The perfusion chamber was mounted on a Nikon TE 200U inverted microscope, and cells were visualized with a QuantEM 512SC camera (Photometrics). Microscope stage and objectives were contained within an environmental chamber (Okolabs), which maintained a consistent temperature of 37°C and was covered with black cloth to block out ambient light. Excitation wavelengths were set at 340 and 380 nm, and the emission wavelength was 515 nm. Cells were equilibrated for 10 min and then subjected to a perfusion rate of 5 ml/min. Changes in the fura 2 ratio were monitored with Easy Ratio Pro software (Photon Technology International).
Transmission electron microscopy.
mTERT-CCD and BAP2 cells were cultured on Snapwell filters in normal media. Cells were treated under four different conditions: control, 100 nM aldosterone, 10 μM amiloride, or 100 nM aldosterone in the presence of 10 μM amiloride. Aldosterone was added for 6 days before fixation. Samples were blinded to prevent bias. Filters were fixed in 2.5% each formaldehyde/glutaraldehyde, in 0.1 M sodium cacodylate buffer, pH 7.4 (Electron Microscopy Sciences) overnight at room temperature. The filters were then washed in maleate buffer, pH 5.2, and postfixed in 1% osmium tetroxide in phosphate buffer for 1.5 h at 4°C. After washing in maleate buffer, filters were stained with a solution of 1% uranyl acetate in maleate buffer, pH 6.0. Samples were dehydrated in a graded series of ethanol, placed in molds, and embedded in EM Bed 812 in a 60°C oven overnight. Semithin sections were stained with toluidine blue, while ultrathin sections were stained with uranyl acetate and lead citrate and then examined by light microscopy and transmission electron microscopy (TEM), respectively. Ultrathin sections (90 nm thick) were obtained using a Leica EM UC6 ultramicrotome (Leica Microsystems) mounted on copper grids and observed in an FEI Tecnai T12 Spirit electron microscope, operated at 80 kV (FEI). Images were recorded with an AMT 2K CCD camera at various magnifications.
Electrophysiological characterization.
Patch micropipettes with a tip resistance of 3–6 MΩ were fabricated using a Sutter P-97 micropipette puller (Sutter Instruments, Novato, CA). Micropipettes were filled with an electrolyte solution containing (in mM) 100 potassium gluconate, 30 KCl, 10 NaCl, 20 HEPES, and 0.5 EDTA. The bath contained DMEM/F-12 50/50 cell culture medium. All experiments were performed at room temperature. The cells were initially sealed in the pipettes with seal resistance of 1–3 GΩ. Whole cell clamp conditions were obtained by applying suction to the pipette until an abrupt decrease in resistance and increase in capacitance were observed. The holding potential was set at −60 mV. Subsequently, the membrane potential was clamped to a series of voltages ranging from −130 to +20 mV in 15-mV increments for 800 ms each, returning to the holding potential of −60 mV for 1 s between each test voltage. This protocol was implemented using an Axopatch 200B patch-clamp amplifier, Digidata 1440A digitizer, and pCLAMP 10 software (Axon Instruments, Sunnyvale, CA). Channel activation by forskolin (1 μM) and subsequent blockade by benzamil (10 μM) were accomplished by perfusion of these agents into the bath solution. Upregulation of the epithelial sodium channel (ENaC) was achieved with the addition of 100 nM aldosterone to cultured cells for 6 days, before whole cell patch clamp. Statistical significance was calculated using GraphPad Prism (La Jolla, CA).
RESULTS
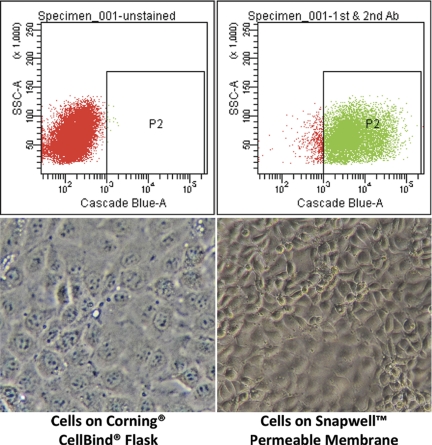
mTERT-negative cells did not survive blasticidin selection, whereas mTERT-immortalized cells continued to proliferate in selection media for >40 subpassages. In the absence of blasticidin, mTERT cells remained differentiated and retained CCD phenotypic characteristics. These renal epithelial cells were then subjected to flow cytometry (Fig. 1), and DBA-positive cells were selected to ensure the cell population originated from principal cells of the collecting duct. In addition, sorted mTERT-CCD cells were labeled with DBA and visualized by fluorescence microscopy (see Fig. 3).
Fig. 1.
Isolation of cortical collecting duct (CCD) cells by flow cytometry. Top left: unstained cell fluorescence dot plot. Top right: Dolichos biflorus agglutinin (DBA)-stained cells showed a shift in fluorescence that is separate from unstained cells, and FACSAria software was gated to select the DBA-positive cells. Bottom left: brightfield image of mouse telomerase reverse transcriptase (mTERT)-CCD cells grown on glass at passage 25 (×20 objective with ×10 camera zoom). Bottom right: brightfield image of mTERT cells grown on Snapwell membranes at passage 30 (×20 objective with ×10 camera zoom) showing typical epithelial cell morphology.
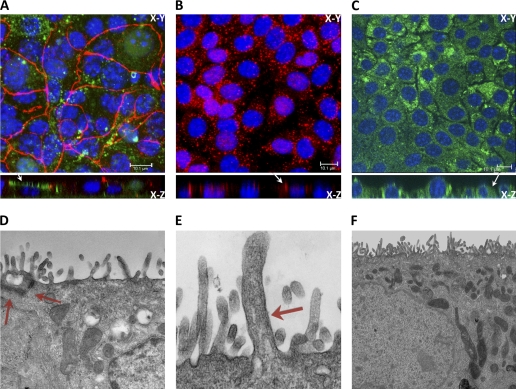
Fig. 3.
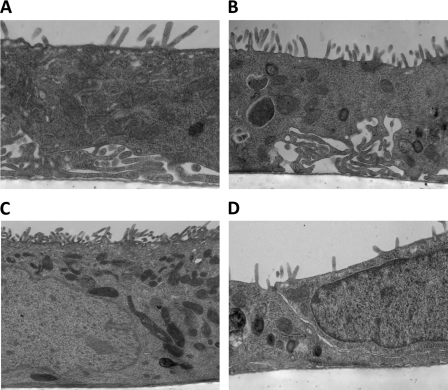
Cell characterization by confocal and electron microscopy. A–C: confocal images of fluorescently labeled DBA (green), located at the apical membrane (see arrow) and ZO-1 (red; A), aquaporin-2 (AQP2; B), located at the apical compartment (arrow), and V2R, located at the basolateral compartment (arrow; C). D–F: electron micrographs of mTERT-CCD cells showing tight junction (×2,700 magnification; D), primary cilium (×4,400 magnification; E), and extensive microvilli (×11,000 magnification; F). Note in E the large membrane protrusion and standard ciliary dimensions were used as criteria for our reference to this structure as a primary cilium. We suggest the basal body and centriole to be merely out of this thin optical plane presented in above micrograph, as primary cilia were routinely seen in these cells by DIC and immunofluorescence.
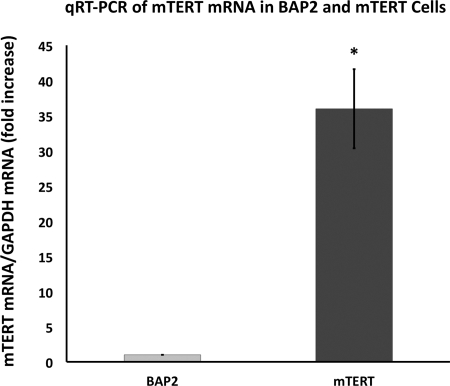
Quantitative RT-PCR was used to determine whether these cells had a greater mTERT expression level than BAP2 cells. As shown in Fig. 2, mTERT-CCD cells expressed a 32-fold higher level of mTERT mRNA compared with BAP2 control cells. Telomerase activity was also measured, using RTQ-TRAP. As shown in Fig. 2, mTERT-immortalized cells had 8.5-fold higher telomerase activity than cells immortalized using SV40. We have designated this cell line as mTERT-CCD.
Fig. 2.
Quantatative (q)RT-PCR of TERT mRNA from mTERT-CCD cells and a collecting duct cell line expressing normal Tg737 (BAP2) shows a 32-fold higher expression of TERT mRNA in the mTERT-immortalized cells. Error bars represent SD; n = 6. Real-time quantitative telomeric repeat amplification protocol (RTQ-TRAP) results confirm there is 8.5-fold higher telomerase activity in mTERT-CCD cells compared with BAP2 cells. Results are calculated using differences in cycle threshold between the 2 cell lines. *P = 0.002.
Brightfield, confocal microscopy, and EM were used to visualize the morphological characteristics of mTERT-CCD cells grown on permeable membranes.As shown in Figs. 1 and 3, mTERT-CCD cells exhibited an “epithelial-like” appearance at both the EM and light levels. The EM micrographs revealed an abundance of microvilli, the presence of a primary cilium on the apical surface, and well-developed cell-to-cell junction complexes. Collecting duct cells in vivo uniquely express AQP2 at the apical membrane, and V2R at the basolateral membrane. Therefore, we assessed the expression and localization of these two proteins in mTERT-CCD cells. As shown in Fig. 3, mTERT-CCD cells expressed these two key proteins in specific locations unique to CCD cells in vivo. Differentiated CCD cells have well-defined tight junctions and express apical DBA. Figure 3 demonstrated tight junctions in the mTERT-CCD cells with ZO-1 immunofluorescence (A) and an electron micrograph of a tight junction complex (D). A z-stack projection was used to show apical expression of DBA (A).
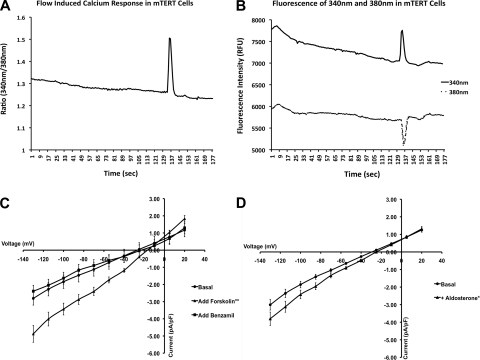
Patch clamp was used to measure the whole cell currents in the mTERT-CCD cells (8). Renal collecting duct epithelial cells express an amiloride/benzamil-sensitive sodium channel that is upregulated by cAMP and by the mineralocorticoid aldosterone. Whole cell currents were increased from basal levels with the addition of 1 μM forskolin and were subsequently blocked by 10 μM benzamil (Fig. 4). Prolonged administration of aldosterone increases basolateral surface area and the transepithelial transport of Na+ in collecting duct cells (24). Cells treated with 100 nM aldosterone for 6 days before patch clamp had significantly elevated whole cell currents compared with cells treated only with vehicle (Fig. 4).
Fig. 4.
Functional characterization of mTERT-CCD cells. A: spike in the 340/380-nm fura 2 ratio in response to an increase in laminar flow from 0.5 to 5 ml/min at the apical membrane. B: decrease in 380-nm and increase in 340-nm fluorescence intensities indicating a calcium response. C: comparison of whole cell currents in mTERT-CCD cells with no treatment, after stimulation with forskolin, and subsequent blockade by benzamil. Each current was normalized by its cell capacitance. Average cell capacitance was 26.05 ± 3.28 pF; n = 4. **P < 0.0001 (error bars represent SE). D: aldosterone stimulation of mTERT-CCD cells. Whole cell currents of mTERT-CCD cells after administration of aldosterone for 6 days compared with cells without aldosterone treatment. Each current was normalized by its cell capacitance. Average cell capacitance was 26.79 ± 2.74 pF; n = 4. *P < 0.0001 (error bars represent SE).
mTERT-CCD cells grown on filter supports and treated for 6 days with 100 nM aldosterone were fixed for TEM, either in the presence or absence of 10 μM amiloride added apically. The EM images in Fig. 5 showed that aldosterone-treated mTERT-CCD cells demonstrated an abundance of basolateral infoldings and space expansion. However, cells treated with 10 μM apical amiloride had decreased or absent basolateral spaces.
Fig. 5.
Characterization of transepithelial Na+ transport by transmission electron microscopy. A: control (no treatment). Magnification ×6,500. B: 6 days of aldosterone treatment. Magnification ×4,400. C: 6 days of aldosterone treatment in the presence of amiloride. Magnification ×2,700. D: amiloride treatment alone. Magnification ×4,400.
DISCUSSION
Telomerase is a ribonucleoprotein complex, consisting of the catalytic subunit TERT and an RNA component that adds TTAGGG repeats to the chromosome ends, thereby maintaining the length of the telomeres (23). It has also been suggested that telomerase has other functions, such as regulating glycolysis, altering telomere interactions with the nuclear membrane, and protecting the cell against apoptosis (5). Telomeres protect against chromosome fusion and destruction. Cell senescence has been linked to shortening and loss of telomeres, while constitutive maintenance of telomeres may lead to cell proliferation and immortalization (5, 33). Although TERT is present in proliferating human cells, such as stem cells, progenitor cells, and immune cells, it is largely undetectable in terminally differentiated cells. This was the rationale for the initial use of TERT overexpression as a potential strategy for cell immortalization. The major advantage of this approach is the absence of viral oncogene expression, which increases the likelihood of generating cell lines that more closely mimic cells in vivo. This has been substantiated in reports where hTERT-immortalized cell lines have been shown to retain many of the in vivo characteristics of native cells. As stated by Voglauer et al. (37), hTERT immortalization is a “major improvement over the use of viral oncogenes for the establishment of human benign tumor cell lines.” Toouli et al. (36) indicated that TERT-immortalized cells had “fewer phenotypic and karyotypic changes compared to SV 40 Tag immortalized cells, providing a useful source of relatively normal cells for in vitro studies.”
Although there have been several reports of TERT immortalization in mouse cells (embryonic fibroblasts, embryonic stem cells, and spermatocytes), there are no reports of successful mTERT immortalization in fully differentiated, slowly proliferating cells, such as renal epithelial cells. It has been proposed that mouse cells have more telomerase activity and longer telomeres compared with their human counterparts, suggesting that TERT addition might be relatively ineffective in immortalizing differentiated cells in this species. The availability of a large number of genetically engineered mouse models of human disease and the development of conditional floxed alleles, in which the activation of Cre can be used to knock out specific genes/proteins, provided a compelling rationale to assess whether TERT could be used to immortalize mouse renal epithelial cells. The findings of our study demonstrate that it is possible to establish a renal epithelial cell line that corresponds to the primary cells. Quantitative RT-PCR (Fig. 2) indicates that mTERT mRNA levels are substantially greater compared with an immortalized mouse collecting duct cell line that was created using the SV-40tsA58 large T antigen (BAP2). Several lines of evidence suggest that mTERT overexpression is responsible for cell immortalization and proliferation. The increased mTERT mRNA suggests that the higher mTERT expression level is driving cell proliferation and that the mTERT-CCD cell line is not the result of a spontaneous transformation. In addition, the telomerase activity is 8.5-fold higher in mTERT-CCD cells than in BAP2 cells. We have also successfully established mTERT-immortalized collecting duct cell lines from a congenital polycystic kidney (cpk) mutation mouse model and the corresponding control mouse (Steele SL, Guay-Woodford L, and Bell PD, unpublished observations). Our ability to immortalize cells from two other mouse strains, after only one attempt, reduces the likelihood that the immortalization of the mTERT-CCD cells is due to a spontaneous immortalization or to some concomitant mutation occurring at or near the time that the cells were subjected to mTERT transduction. The cpk mTERT cells were maintained in selection media for 4 subpassages and continue to proliferate and remain differentiated for 10 subpassages without blasticidin selection. In addition, primary collecting duct cells that were transduced with an empty vector died when subjected to antibiotic selection. Had these cells survived, we would suspect they were spontaneously immortalized. Although it has been reported that collecting duct cells do spontaneously immortalize (12), the frequency of this event is so small we do not feel that it is a practical method for creating cell lines.
One possible reason we were successful in generating this cell line could be related to the method of TERT immortalization. Renal epithelial cells are notoriously difficult to transduce or transfect using conventional techniques, which has prompted us to use lentiviral vectors to insert genes into these cells. This efficient system of transducing genes into cells may be an important reason we were successful in generating the mTERT-CCD cell line.
Dedifferentiation and increased tumorigenesis have been suggested as a possible effect of TERT overexpression in cells (13, 37). Thus it was important to determine whether we could isolate a pure population of principal cells of the collecting duct and to determine whether these cells retained phenotypic characteristics of collecting duct cells found in vivo. mTERT-immortalized cells were subjected to flow cytometry, using DBA selection, to isolate principal cells of the collecting duct. The mTERT-CCD cell morphology was analyzed using brightfield, confocal microscopy, and EM. mTERT-CCD cells appear to have morphological characteristics that are very similar to the control cell line, as well as to collecting duct cells in vivo. This includes well-developed junction complexes between cells, abundant microvilli, and primary cilia. In addition, these cells lack the vacuoles and lysosomal compartments that are often found in oncogene-transformed cells (26, 27). The mTERT-CCD cells also exhibit Na+ transport via ENaC, as assessed using patch clamp and whole cell conductance. Specifically, inward currents are increased by elevations in cAMP and inhibited by benzamil. In addition, prolonged treatment with aldosterone enhances inward currents, which is consistent with upregulation of ENaC activity.
In addition to collecting duct cell morphology, we found that mTERT-CCD cells express AQP2 water channels and V2R. We also show that increasing cAMP with forskolin and aldosterone administration both lead to increases in amiloride/benzamil-sensitive ENaC channel activity, as reflected by changes in the whole cell currents. These data indicate that mTERT-CCD cells have the key morphological, biochemical, and functional properties of the renal collecting duct.
In conclusion, we report that mTERT can be used to successfully immortalize mouse renal collecting duct epithelial cells. mTERT-CCD cells retain the features found in principal cells of the collecting duct in vivo. This immortalization technique should be very important to a number of researchers, given the large number of genetically altered mouse models.
GRANTS
This project was supported by a Veterans Affairs Merit Grant (P. D. Bell), National Institutes of Health Grants P30DK074038 and R01 DK55534 (L. Guay-Woodford), DK32032 (P. D. Bell), and KO1 DK075652 (R. J. Kolb), and National Science Foundation RII Grant EPS-00903795 (H. Yao).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Drs. Bradley Yoder (University of Alabama, UAB) and Robert Fenton (University of Aarhus, Aarhus, Denmark) for generously providing the BAP2 cell line and antibody reagents, respectively; Rick Peppler at the MUSC Hollings Cancer Center Flow Cytometry Core Facility for help with flow cytometric sorting and analysis; Melissa Chimento and Edward Phillips, at the UAB EM facility, for their technical assistance; Yujing Dang for technical assistance with the TRAP assay; and Barbara J. Harris for administrative assistance.
mTERT-CCD cells are available upon request from P. D. Bell (bellpd@musc.edu) or visit the UAB Recessive Polycystic Kidney Disease Core Center website (http://www.rpkdcc.uab.edu/) for more information.
REFERENCES
- 1.Allsopp RC, Weissman IL. Replicative senescence of hematopoietic stem cells during serial transplantation: does telomere shortening play a role? Oncogene 21: 3270–3273, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Armstrong L, Saretzki G, Peters H, Wappler I, Evans J, Hole N, von Zglinicki T, Lako M. Overexpression of telomerase confers growth advantage, stress resistance, and enhanced differentiation of ESCs toward the hematopoietic lineage. Stem Cells 23: 516–529, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Asamoto M, Hokaiwado N, Cho YM, Shirai T. Effects of genetic background on prostate and taste bud carcinogenesis due to SV40 T antigen expression under probasin gene promoter control. Carcinogenesis 23: 463–467, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Asamoto M, Hokaiwado N, Cho YM, Takahashi S, Ikeda Y, Imaida K, Shirai T. Prostate carcinomas developing in transgenic rats with SV40 T antigen expression under probasin promoter control are strictly androgen dependent. Cancer Res 61: 4693–4700, 2001 [PubMed] [Google Scholar]
- 5.Belgiovine C, Chiodi I, Mondello C. Telomerase: cellular immortalization and neoplastic transformation. Multiple functions of a multifaceted complex. Cytogenet Genome Rese 122: 255–262, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science 279: 349–352, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Boehm JS, Hahn WC. Immortalized cells as experimental models to study cancer. Cytotechnology 45: 47–59, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bubien JK. Whole cell sodium conductance of principal cells freshly isolated from rat cortical collecting duct. Am J Physiol Cell Physiol 269: C791–C796, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Carlson GA, Borchelt DR, Dake A, Turner S, Danielson V, Coffin JD, Eckman C, Meiners J, Nilsen SP, Younkin SG, Hsiao KK. Genetic modification of the phenotypes produced by amyloid precursor protein overexpression in transgenic mice. Hum Mol Genet 6: 1951–1959, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Dahse R, Fiedler W, Ernst G. Telomeres and telomerase: biological and clinical importance. Clin Chem 43: 708–714, 1997 [PubMed] [Google Scholar]
- 11.Feng LX, Chen Y, Dettin L, Pera RAR, Herr JC, Goldberg E, Dym M. Generation and in vitro differentiation of a spermatogonial cell line. Science 297: 392–395, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Gaeggeler HP, Gonzalez-Rodriguez E, Jaeger NF, Loffing-Cueni D, Norregaard R, Loffing J, Horisberger JD, Rossier BC. Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J Am Soc Nephrol 16: 878–891, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Geserick C, Tejera A, Gonzalez-Suarez E, Klatt P, Blasco MA. Expression of mTert in primary murine cells links the growth-promoting effects of telomerase to transforming growth factor-beta signaling. Oncogene 25: 4310–4319, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Greenberg RA, Allsopp RC, Chin L, Morin GB, DePinho RA. Expression of mouse telomerase reverse transcriptase during development, differentiation and proliferation. Oncogene 16: 1723–1730, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Hager JH, Hodgson JG, Fridlyand J, Hariono S, Gray JW, Hanahan D. Oncogene expression and genetic background influence the frequency of DNA copy number abnormalities in mouse pancreatic islet cell carcinomas. Cancer Res 64: 2406–2410, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature 345: 458–460, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Karystinou A, Dell'Accio F, Kurth TB, Wackerhage H, Khan IM, Archer CW, Jones EA, Mitsiadis TA, De Bari C. Distinct mesenchymal progenitor cell subsets in the adult human synovium. Rheumatology (Oxford) 48: 1057–1064, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science 266: 2011–2015, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Kipling D, Cooke HJ. Hypervariable ultra-long telomeres in mice. Nature 347: 400–402, 1990 [DOI] [PubMed] [Google Scholar]
- 20.Lambert R. Genetic background: understanding its importance in mouse-based biomedical research. In: Resource Manual. Bar Harbor, ME: Jackson Laboratory, 2007 [Google Scholar]
- 21.Lee KM, Choi KH, Ouellette MM. Use of exogenous hTERT to immortalize primary human cells. Cytotechnology 45: 33–38, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacKay K, Striker LJ, Pinkert CA, Brinster RL, Striker GE. Glomerulosclerosis and renal cysts in mice transgenic for the early region of SV40. Kidney Int 32: 827–837, 1987 [DOI] [PubMed] [Google Scholar]
- 23.McEachern MJ, Krauskopf A, Blackburn EH. Telomeres and their control. Annu Rev Genet 34: 331–358, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Minuth WW, Gross P, Gilbert P, Kashgarian M. Expression of the alpha-subunit of Na/K-ATPase in renal collecting duct epithelium during development. Kidney Int 31: 1104–1112, 1987 [DOI] [PubMed] [Google Scholar]
- 25.Montagutelli X. Effect of the genetic background on the phenotype of mouse mutations. J Am Soc Nephrol 11: S101–S105, 2000 [PubMed] [Google Scholar]
- 26.Morelli C, Barbisan F, Iaccheri L, Tognon M. SV40-immortalized human fibroblasts as a source of SV40 infectious virions. Mol Med 10: 112–116, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimura T, Kawai N, Kawai M, Notake K, Ichihara I. Fusion of SV40-induced endocytotic vacuoles with the nuclear membrane. Cell Struct Funct 11: 135–141, 1986 [DOI] [PubMed] [Google Scholar]
- 28.Nugent CI, Lundblad V. The telomerase reverse transcriptase: components and regulation. Genes Dev 12: 1073–1085, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Prowse KR, Greider CW. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc Natl Acad Sci USA 92: 4818–4822, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schauwecker PE. Complications associated with genetic background effects in models of experimental epilepsy. Prog Brain Res 135: 139–148, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Sealey DCF, Zheng L, Taboski MAS, Cruickshank J, Ikura M, Harrington LA. The N-terminus of hTERT contains a DNA-binding domain and is required for telomerase activity and cellular immortalization. Nucl Acids Res 38: 2019–2035, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shay JW, Wright WE. Telomeres and telomerase: implications for cancer and aging. Radiat Res 155: 188–193, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Sherr CJ, DePinho RA. Cellular senescence: mitotic clock or culture shock? Cell 102: 407–410, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Sola C, Tronik D, Dreyfus M, Babinet C, Rougeon F. Renin-promoter SV40 large T-antigen transgenes induce tumors irrespective of normal cellular expression of renin genes. Oncogene Res 5: 149–153, 1989 [PubMed] [Google Scholar]
- 35.Sommardahl C, Cottrell M, Wilkinson JE, Woychik RP, Johnson DK. Phenotypic variations of orpk mutation and chromosomal localization of modifiers influencing kidney phenotype. Physiol Genomics 7: 127–134, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Toouli CD, Huschtscha LI, Neumann AA, Noble JR, Colgin LM, Hukku B, Reddel RR. Comparison of human mammary epithelial cells immortalized by simian virus 40 T-antigen or by the telomerase catalytic subunit. Oncogene 21: 128–139, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Voglauer R, Grillari J, Fortschegger K, Wieser M, Sterovsky T, Gunsberg P, Katinger H, Pfragner R. Establishment of human fibroma cell lines from a MEN1 patient by introduction of either hTERT or SV40 early region. Int J Oncol 26: 961–970, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Whitehead RH, Robinson PS. Establishment of conditionally immortalized epithelial cell lines from the intestinal tissue of adult normal and transgenic mice. Am J Physiol Gastrointest Liver Physiol 296: G455–G460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wieser M, Stadler G, Jennings P, Streubel B, Pfaller W, Ambros P, Riedl C, Katinger H, Grillari J, Grillari-Voglauer R. hTERT alone immortalizes epithelial cells of renal proximal tubules without changing their functional characteristics. Am J Physiol Renal Physiol 295: F1365–F1375, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE, Jr, Schafer JA, Balkovetz DF. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol 282: F541–F552, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Yoshiki A, Moriwaki K. Mouse phenome research: implications of genetic background. ILAR J 47: 94–102, 2006 [DOI] [PubMed] [Google Scholar]