Abstract
The INK4a proteins p16INK4a and p19ARF regulate cell cycle arrest and senescence. However, the role of these proteins in controlling these processes in the normal kidney and following injury is unknown. We performed unilateral ureteral obstruction (UUO) to induce fibrosis in 2- to 3-mo-old wild-type (WT) C57/B6 and INK4a knockout mice. By quantitative RT-PCR, p16INK4a levels were increased sixfold in WT mice 7 days after UUO and p19ARF remained undetectable. Kidney sections were examined to determine levels and localization of p16INK4a, apoptosis, fibrosis, and senescent cells. INK4a knockout mice displayed mesangial cell proliferation, increased matrix deposition, and myofibroblast differentiation under normal conditions. Following UUO, INK4a knockout mice displayed 10-fold increased tubular and interstitial cell proliferation, 75% decreased collecting duct apoptosis, 2-fold greater collagen and fibronectin deposition, and no cell senescence by senescence-associated β-galactosidase staining compared with WT mice. Both INK4a knockout mesangial cells and kidney lysates from knockout mice following injury showed elevated levels of IL-6 by ELISA compared with WT samples. INK4a knockout epithelial cell cultures displayed increased mesenchymal cell markers when exposed to transforming growth factor-β. These results confirm that p16INK4a controls cell proliferation and matrix production and mitigates fibrosis following injury and suggest that the mechanism involves a role in limiting inflammation and cell proliferation.
Keywords: mesangial cell, UUO
under normal conditions, less than 1% of kidney cells are proliferating. By maintaining a quiescent state, cells are able to quickly respond to injury by reentering the cell cycle and proliferating (21, 22). This change in cellular state is a highly regulated process that occurs by means of a number of proteins including the cyclin-dependent kinases (CDK) that induce proliferation. To prevent unimpeded proliferation, cellular “checks and balances” have evolved including the cyclin-dependent kinase inhibitors, or CKIs. The Cip/Kip family (p21 and p27) and the INK4a family (p16 and p19) of CKIs have been extensively studied and have been shown to be major factors in tumor suppression, aging, and the response to cell injury (8).
The INK4a gene locus encodes transcripts for the p16INK4a and p19ARF proteins under distinct promoters. The proteins have different first exons while the rest of the proteins are made from alternative reading frames of shared second and third exons (23). By selectively binding CDKs 4 and 6, p16INK4a effectively prevents association with its cyclin-D counterpart. This suppresses the phosphorylation of the retinoblastoma (Rb) family proteins and prevents the cell from entering the S phase of the cell cycle (7).
p19ARF is able to arrest the cell cycle at both the G1 and G2 phases. However, it does so without connection to the CDK system. p19ARF selectively binds and promotes the degradation of MDM2, a protein that promotes ubiquination and degradation of p53. The in vivo functions of these proteins have been studied using knockout mice (24). INK4a-deficient mice contain an altered transcript that replaces exons two and three with a pgk-neo cassette, eliminating p16 and p19 expression. The phenotype of these mice appears mostly normal at 2 mo of age; however, increased proliferation of hematopoietic tissues such as the spleen occurred, and these mice eventually develop tumors at an average age of 29 wk and have increased susceptibility to carcinogen-induced sarcomas and lymphomas, while their heterozygous and homozygous WT counterparts remain normal (23). These results clearly demonstrate the widely accepted tumor-suppressor role of the INK4a genes.
Increased p16 and p19 expression with resulting effects on the pRb and p53 pathways has also been shown to be associated with cell senescence, a condition characterized by irreversible growth arrest, in which cells remain metabolically viable and develop a flattened appearance. In cell culture, both telomere erosion as a result of excess replication and DNA damage from various types of cell stress have been shown to trigger senescence and increased p16 and p19 expression (8). In vitro, decreased p16 and p19 expression results in an extended cell lifespan and decreased senescence in response to stress.
While much is now known about the role of the INK4a gene in tumor suppression and age-related cell senescence, its role in regulating cell growth and differentiation in response to injury is unknown. Under normal conditions, p16 and p19 expression in the mouse kidney is low to undetectable (4) but has been shown to increase with age (9, 14). In addition, p16 expression in tubular epithelial and interstitial cells has been shown to have a relative correlation with sclerotic glomeruli, interstitial fibrosis, and tubular atrophy (15). Whether INK4a gene expression increases with injury to function as an “emergency brake” to halt excessive cellular proliferation under promitotic conditions as described in other tissues (4) or as a normal process to keep proliferation in check is another topic under debate (3).
In a mouse model of liver injury, it has been shown that fibrosis is significantly more pronounced in INK4a/p53 double knockout compared with wild-type (WT) mice due to increased proliferation and decreased senescence of activated matrix-producing hepatic stellate cells (10). However, to date, nothing is known about the role of INK4a proteins in controlling cell proliferation in the normal kidney and in the response to kidney injury including the development of cell senescence and fibrosis. Accordingly, in this study, we have examined the effect of INKa deletion on normal kidney function and the response to unilateral ureteral obstruction (UUO), a fibrosis-inducing injury.
MATERIALS AND METHODS
Mice strains.
Homozygous INK4a knockout mice strain B6.129-Cdkn2atm1Rdp maintained on a B6;129 background were obtained from the NCI mouse repository (Frederick, MD). B6.129F2/J mice were used as the WT strain for all experiments. All procedures involving laboratory animals were evaluated and approved by The New York Medical College Institutional Animal Care and Use Committee and followed the guidelines for laboratory animal welfare.
Quantitative real-time PCR.
Kidneys were removed aseptically, and RNA was isolated using an RNeasy Mini Kit (Qiagen, Valencia, CA) and quantified using a spectrophotometer (Eppendorf, Hauppauge, NY). cDNA was prepared by reverse transcription of 1 ug total RNA using a qScript cDNA synthesis kit (Quanta, Surrey, UK) according to the manufacturer's instructions. Quantitative real-time PCR was performed using PerfeCTa Sybr Green Fastmix Low Rox (Quanta) and the Mx3000 real-time PCR system (Stratagene, La Jolla, CA). Specific primers were designed based on published sequences. The p16 sense sequence was 5′-AACTCTTTCGGTCGTACCCC-3′, and antisense was 5′-GCGTGCTTGAGCTGAAGCTA-3′. The 18S sense sequence was 5′-TGTCTCAAAGATTAAGCCATGCAT-3′, and anti-sense, 5′-AACCATAACTGATTTAA-TGAGCCATTC-3′. RNA expression levels were based on the cycle threshold values and were reported compared with the reference gene 18S.
Senescence-associated β-galactosidase staining.
Cells and tissues were fixed with 2% paraformaldehyde and 0.5% glutaraldehyde in PBS. Following washes in PBS, cells and tissues were incubated with 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 1 mM MgSO4 and 1 mg/ml X-gal in 40 mM citric acid buffer (pH 6.0) overnight or for 6 h, respectively at 37°C.
Proximal tubular and mesangial cell culture.
Kidneys were isolated under sterile conditions. Proximal tubule cells were prepared using the method of Terryn et al. (26). This method was shown to yield pure populations of proximal tubule cells with minimal fibroblast contamination (26). The medulla was dissected from bisected kidneys, and 1-mm2 fragments were cut from the remaining cortex. Tissue fragments were digested in 0.1% type-2 collagenase solution for 30 min at 37°C. Digested tissue was filtered through 100-μm nylon sieves (Fisher). The initial filtrate containing glomeruli was washed with HBSS, resuspended in MEM with 10% FBS, and seeded on 10-cm dishes. Adherent glomeruli were cultured for outgrowth of mesangial cells. Retained proximal tubule fragments were washed off the inverted sieve using HBSS and seeded on 0.4-μ collagen-coated Transwell filters (Corning) and cultured in DMEM with 1% FBS containing nonessential amino acids, 0.55 mmol/l sodium pyruvate, insulin, transferrin, and selenium, and 50 nmol/l hydrocortisone.
Small interference RNA transfections.
Mesangial cells (passage 2) from B6;129 mice were transfected with 50 or 200 nM p16 small interference (si) RNA (Dharmacon ON-TARGET plus SMARTpool, Lafayette, CO) using Nucleofector electroporation. Control cells were transfected with nontargeting siRNA (Dharmacon).
Antibodies and reagents.
For immunohistochemistry and Western blotting, primary antibodies used include 5′-nucleotidase (5′-NT; BD Pharmingen, San Jose, CA), p16 (F-12, Santa Cruz Biotechnology, Santa Cruz, CA), α-smooth muscle actin (SMA; clone 1A4, Sigma, St. Louis, MO), fibronectin (ab2413, Abcam, Cambridge, MA), Ki67 (Ab15580, Abcam), β-tubulin (clone TUB2.1, Sigma), S100A4 (DakoCytomation, Carpinteria, CA), FITC-conjugated peanut lectin agglutinin (PNA; Vector Labs, Burlingame, CA), and vimentin (C-20, Santa Cruz Biotechnology). Donkey anti-rabbit, anti-goat, and anti-mouse secondary antibodies used in Western blots were from Amersham Biosciences (UK). FITC donkey anti-goat and goat anti-mouse and anti-rabbit secondary antibodies were from Jackson ImmunoResearch (West Grove, PA). IL-6 ELISAs were performed with a commercially available kit (Peprotech, Rocky Hill, NJ) according to the manufacturer's instructions. Tissue lysates were diluted 1:10, and cell culture supernatants were diluted 1:2 in sample buffer. Results are reported as means ± SE for three samples measured in duplicate.
UUO.
Eight- to ten-week-old male mice were anesthetized with ketamine and xylazine. Mice were placed on a heating pad at 37°C. The left ureter was identified and ligated with 3-0 sutures at two sites near the renal hilum through a midline incision.
Immunohistochemistry.
Following deparaffinization, slides were incubated in antigen retrieval solution (Dako) at 90–95°C and then allowed to cool to room temperature. Slides were blocked with peroxidase, incubated with avidin and biotin blocking solution (Dako), and blocked with 10% swine serum in PBS for 1 h at room temperature. Primary antibody was applied overnight at 4°C. Slides were stained using a Dako LSAB+ system-HRP kit and developed with DAB and counterstained with hematoxylin.
Immunofluorescence.
Kidneys were fixed in 4% paraformaldehyde and frozen in O.C.T. Five-micrometer cryostat sections were cut and applied to Probe-on plus slides (Fisher Scientific, Pittsburgh, PA). Slides were treated with 0.2% Triton X-100 in PBS and blocked with 10% goat serum for 1 h. Samples were incubated with a primary antibody overnight at 4°C. A secondary antibody was applied the following day. Following washes in PBS, slides were incubated with Hoechst and examined by immunofluorescence using a Nikon Eclipse 1000 microscope equipped with a CCD camera. Quantitation of interstitial cell and matrix area was performed using Photoshop and National Institutes of Health Image J software.
Western blotting.
Cells cultures were washed with PBS and lysed in RIPA buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 0.5% Triton X-100 in PBS) containing Complete Mini protease inhibitor (Roche, Indianapolis, IN) and 2 mM PMSF. Protein concentrations were measured using a Bradford assay (Bio-Rad, Hercules, CA). The protein samples were denatured by adding SDS sample buffer and heating at 90°C for 5 min. Ten to forty micrograms of sample containing β-mercaptoethanol were resolved on 4–20% Tris glycine polyacrylamide gels. Gels were transferred to polyvinylidene difluoride membranes (Immobilon-P, Millipore, Bedford, MA) and blocked with 5% nonfat dry milk in PBS-T (0.1% Tween 20 in PBS). The membranes were then incubated overnight at 4°C with primary antibody in blocking buffer. This was followed by washing and 1-h incubation with an appropriate horseradish peroxidase-conjugated secondary antibody. Protein bands were detected by chemiluminiscence using a commercial kit (Pierce) according to the manufacturer's instructions. For quantification of protein levels, autoradiographs were scanned with the Scion Image densitometry program, and results were corrected for variations in the amount of protein loaded on each lane using corresponding β-tubulin levels.
Terminal transferase-mediated dUTP nick-end labeling staining.
Terminal transferase-mediated dUTP nick-end labeling (TUNEL) staining was performed with a commercially available kit (In Situ Cell Death Detection Kit, TMR Red, Roche). Cryostat tissue sections were incubated with fixation solution followed by permeabilization solution according to the manufacturer's instructions. Tissue sections were labeled with TUNEL reaction mixture for 1 h at 37°C. Cells were labeled with Hoechst and examined by immunofluorescence microscopy. Results are reported as means ± SD TUNEL-positive cells/mm2 (n = 5 mice/group).
Transforming growth factor-β ELISA.
A transforming growth factor (TGF)-β1 Emax ImmunoAssay kit (Promega) with a sensitivity of 20–25 pg/ml was used for quantification of TGF-β levels. Protein lysates from WT and INK4a knockout control and UUO kidneys (n = 5/group) were diluted in sample buffer to a concentration of 1 μg/μl and assayed in duplicate according to the manufacturer's instructions.
Statistical analysis.
Data were collected from three to five independent experiments and presented as means ± SD. Student's t-test was used to compare two series of data. A P value of <0.05 was considered significant.
RESULTS
Localization of p16INK4a in normal mouse kidney.
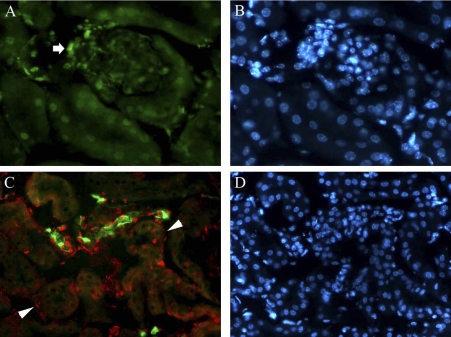
To determine the cellular localization of p16INK4a in normal kidneys, we examined 3-mo-old mice by immunofluorescence (Fig. 1). p16INK4a was detected in glomerular and extraglomerular mesangial cells (Fig. 1A, arrow). Double labeling with PNA lectin demonstrated that p16INK4a is expressed predominately in collecting ducts in the cortex (Fig. 1C) and medulla and in peritubular interstitial cells (arrowheads).
Fig. 1.
Localization of p16INK4a in normal mouse kidney cortex. A: p16INK4a expression in glomerular and extraglomerular mesangial cells (arrow) and a group of surrounding tubular nuclei. B: Hoechst nuclear staining of tissue shown in A. C: peanut lectin agglutinin (PNA; green) and p16INK4a (red) double labeling demonstrating p16 expression in cortical collecting duct epithelial cells and surrounding interstitial cells (arrowheads). D: Hoechst nuclear stain of the tissue shown in C. Original magnification ×400.
INK4a deletion causes mesangial and interstitial cell activation.
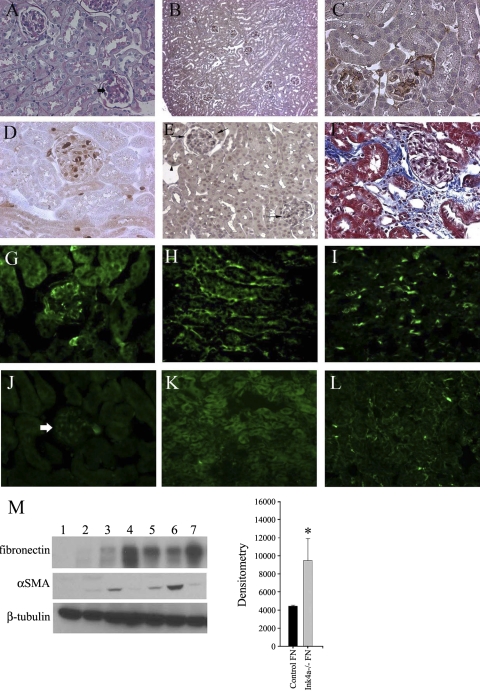
The role of p16INK4a and p19ARF expression in normal kidney homeostasis in young mice was examined by comparison of 2- to 3-mo-old WT and INK4a knockout mice with deletion of the p16INKa and p19ARF genes. As shown in Fig. 2A, periodic acid-Schiff staining demonstrated mesangial proliferation and matrix deposition in knockout mice. Immunohistochemical staining showed glomerular fibronectin deposition in all glomeruli in knockout mice (Fig. 2B, low power and Fig. 2C, high power). To determine whether this glomerular pathology was associated with inflammatory cell infiltration, kidneys were labeled with S100A4, a marker for kidney monocytes, macrophages (11, 12), and fibroblasts from epithelial-mesenchymal transition. As shown in Fig. 2D, S100A4-positive cells were detected primarily in knockout glomeruli and in smaller numbers in the medullary interstitium. Labeling with F4/80, an additional macrophage marker, demonstrated a similar pattern of cell infiltration in both control and UUO kidneys (data not shown). The increased glomerular cell number correlated with glomerular Ki67 expression, a marker for cell proliferation (Fig. 2E). A 10-fold increase in medullary tubular epithelial cell Ki67 was also detected in knockout kidneys. A comparison of S100A4 and Ki67 expression in WT and INK4a knockout kidneys (n = 5) is shown in Table 1. To determine whether increased glomerular inflammation is associated with mesangial and interstitial fibroblast activation, α-SMA expression, a marker for myofibroblasts, was examined. α-SMA was detected in glomeruli (Fig. 2G), medullary (Fig. 2H), and papillary (Fig. 2I) interstitial cells. In contrast, α-SMA was only detected in vascular smooth muscle cells in corresponding areas in WT kidneys (Fig. 2, J–L). The proliferative and inflammatory changes in INK4a knockout mice resulted in patchy areas of perivascular fibrosis detected by Masson-Trichrome staining (Fig. 2F). Comparisons of fibronectin and α-SMA levels in WT and knockout mice were also made by Western blot analysis (Fig. 2M), demonstrating a nearly threefold increased expression of fibronectin in knockout mice (lanes 3–8).
Fig. 2.
Glomerular proliferation and matrix expansion and increased α-smooth muscle actin (SMA) expression in INK4a −/− mouse kidneys. A: periodic acid-Schiff (PAS) staining demonstrating glomerular matrix expansion (arrows). B and C: low- and high-power magnification fibronectin immunhistochemistry, respectively, showing increased deposition in glomeruli of knockout mice. D: S100A4 immunohistochemistry demonstrating macrophage infiltration of glomeruli. E: Ki67-positive proliferating glomerular cells (arrows) with less intense staining in surrounding tubular epithelial cells. F: Masson trichrome stain showing an area of perivascular fibrosis (blue). G–I: α-SMA immunofluorescence demonstrating expression in glomeruli (G), medullary fibroblasts (H), and peritubular cells in the papilla (I) with no signal in corresponding wild-type (WT) sections (J–L). M: Western blot analysis of fibronectin, α-SMA, and β-tubulin levels in WT (lanes 1 and 2) and INK4a knockout kidneys (lanes 3–7) with corresponding densitometry for fibronectin. *P = 0.002.
Table 1.
Comparison of proliferating and infiltrating cells in wild-type and INK4a knockout kidneys
| WT Kidney | Ink4a −/− | WT UUO | Ink4a −/− UUO | |
|---|---|---|---|---|
| % Tubular Ki67 | 0.5 ± 0.2 | 5.3 ± 3.6* | 1.21 ± 0.4 | 12.6 ± 0.08† |
| % Interstitial Ki67 | 0.8 ± 0.7 | 5.9 ± 1.4* | 2.74 ± 0.7 | 23.3 ± 0.06† |
| Glomerular Ki67 | 0.9 ± 1.5 | 5.4 ± 1.9* | 0.7 ± 1.0 | 4.5 ± 0.7† |
| S100A4 cells/high-power field | ||||
| Interstitium | 0.25 ± 0.5 | 10.6 ± 3.9* | 19.2 ± 8.4 | 14.8 ± 6.4‡ |
| Glomeruli | 0.14 ± 0.3 | 3.5 ± 2.8* | 1.8 ± 0.8 | 1.0 ± 1.8‡ |
Values are means ± SE WT, wild-type; UUO, unilateral ureteral obstuction.
P < 0.0001 compared with WT.
P < 0.001 compared with WT.
P = not significant.
Increased p16INK4a expression after kidney injury.
Results presented above indicate that the INK4a gene may have an important role in controlling cell proliferation and matrix production in normal young mouse kidneys. This suggested a possible role in controlling the response to kidney injury. To determine whether p16INK4a is increased following fibrosis inducing acute kidney injury, 2- to 3-mo-old B6.129 mice (n = 3 for each time point) underwent UUO and tissue was collected at 1, 3 and 7 days following obstruction for preparation of RNA for quantitative PCR (Fig. 3). UUO day 1 mice had similar low levels of p16 mRNA as control mice. 3 days following UUO p16 levels were increased two-fold, and by day 7, more than six-fold. Attempts at amplification of p19ARF using 3 different sets of primers were unsuccessful, suggesting very low mRNA expression for this protein.
Fig. 3.
Expression levels of p16INK4a in mice before and after unilateral ureteral obstruction (UUO) at 1, 3, and 7 days. Real-time PCR analysis is reported as relative expression of p16 mRNA. Values are means ± SE from 3 mice at each time point. *P < 0.05, **P < 0.001.
p16INK4a localization in interstitial and tubular epithelial cells following UUO.
The effect of fibrosis inducing acute kidney injury on p16INK4a expression and its cellular localization in 2- to 3-mo-old mice was examined by immunofluorescence in mice following UUO (Fig. 4). As shown in Fig. 4A, p16INK4a is detected in 5′-NT-positive interstitial cells in normal WT mice, demonstrating p16 expression in cortical fibroblasts (20). Seven days following UUO (Fig. 4B), much of the 5′-NT is absent suggesting fibroblast transdifferentiation. This is corroborated by Fig. 4D demonstrating interstitial infiltration with α-SMA-positive myofibroblasts that coexpress p16INK4a. Myofibroblasts are not detected in control mice, and p16INK4a-positive interstitial cells are α-SMA negative (Fig. 4C, arrowheads). Finally, Fig. 4, E and F, show that p16 is increased in medullary collecting duct cells following UUO.
Fig. 4.
p16INK4a expression and interstitial cell differentiation in control mice and in response to 7 days of UUO. A and B: double labeling for p16INK4a and 5′-nucleotidase (NT) demonstrating p16INK4a expression in cortical fibroblasts. 5′-NT expression is markedly diminished following UUO. C and D: double labeling for p16INK4a and α-SMA demonstrating p16INK4a expression in α-SMA-positive myofibroblasts following UUO. α-SMA is only expressed in vascular smooth muscle cells, and p16INK4a-positive interstitial cells in control kidneys are α-SMA negative (arrowheads). E and F: double labeling for p16INK4a and PNA showing increased p16INK4a expression in outer medullary collecting duct cells following UUO. Original magnification ×400.
INK4a knockout mice develop increased fibrosis compared with WT mice.
p16INK4a has been hypothesized to act as an emergency brake on cell proliferation following stress, and increased levels have been shown to induce cell senescence and decrease extracellular matrix formation (1, 3). Because INK4a knockout mice demonstrate increased cell proliferation and matrix formation and p16INK4a levels are increased at 7 days following UUO in both epithelial cells and myofibroblasts, we next examined the effect of INK4a deletion on the development of kidney fibrosis following UUO. As shown in Fig. 5, kidneys from INK4a knockout mice developed increased areas of fibrosis detected by Masson-trichrome stain for matrix deposition (Fig. 5, A and B, low-power, C and D, high-power magnification) and periodic acid-Schiff stain demonstrating thickened, irregular tubular basement membranes (Fig. 5, E and F, arrows). Areas of fibronectin deposition, a major component of matrix formation in fibrosis, were examined by immunohistochemistry (Fig. 5G). Quantification of tissue deposition showed a 1.5-fold increase in collagen and a nearly 2-fold increase in fibronectin deposition in INK4a knockout mice compared with WT mice (Fig. 5H).
Fig. 5.
Increased kidney fibrosis in INK4a knockout mice following UUO. Low-power (A and B) and high-power (C and D) images of Masson trichrome-stained kidneys 10 days following UUO demonstrating increased collagen deposition (blue stain) in knockout kidneys are shown. E and F: PAS staining demonstrating increased tubular basement membrane thickening and irregularity in knockout kidneys (arrows). G and H: fibronectin immunohistochemistry showing increased matrix deposition in knockout kidneys. I and J: quantification of Masson trichrome- and fibronectin-stained areas, respectively, in sections from 3 different WT and knockout kidneys. Original magnification ×40 (A and B) and ×400 (C–H). *P = 0.05, **P = 0.002.
Decreased cell senescence and apoptosis in INK4a knockout mice following UUO.
Increased p16INK4a expression has been correlated with tubular atrophy and senescence in kidneys of both aging and injured human and rodent kidney (13, 14). To determine whether UUO results in increased cell senescence and examine the effect of INK4a deletion, tissue was stained for senescence-associated (SA) β-galactosidase, a marker for in vivo cell senescence (6) (Fig. 6). Ten days following UUO, staining was detected in cortical tubular epithelial cells, glomeruli, and interstitial cells (Fig. 6, A–C). Labeling of serial sections with PNA lectin, a marker for collecting ducts, demonstrated SA-β-galactosidase staining in both proximal tubules and cortical collecting ducts. (data not shown). SA-β-galactosidase staining was nearly undetectable in INK4a knockout kidneys following UUO (Fig. 6D). Previous studies have shown that tubular cell apoptosis in dilated collecting ducts and interstitial cells occurs following UUO and may have an important role in the loss of kidney function following injury (2). p16INK4a and p19ARF have been shown to promote apoptosis through p53-dependent and -independent mechanisms. Apoptosis was examined by TUNEL staining in WT and INK4a knockout kidneys at 10 days following UUO. As shown in Fig. 6, E and G, TUNEL-positive cells were detected in dilated medullary tubules and the papilla in WT kidneys and were increased over threefold compared with knockout kidneys. TUNEL staining was detected in scattered tubular epithelial cells in knockout kidneys (Fig. 6F) and was similar to normal WT kidneys. To determine whether decreased cell senescence and apoptosis in INK4a knockout kidneys correlated with increased cell proliferation and decreased inflammatory cell infiltration, Ki67 and S100A4 expression was examined by immunofluorescence in WT and knockout kidneys at 3 days following UUO. As shown in Table 1, knockout kidneys displayed a 10-fold increase in tubular and interstitial cell proliferation compared with WT kidneys. S100A4 staining demonstrated that knockout kidneys had a 18-fold lower increase in interstitial and a 50% decrease in glomerular macrophages compared with WT kidneys.
Fig. 6.
Decreased senescence-associated (SA) β-galactosidase staining and decreased tubular cell apoptosis in INK4a knockout mice following UUO. A: WT normal kidney demonstrating low levels of tubular SA-β-galactosidase staining (blue). B: increased staining in cortical tubules and glomeruli (arrows) in WT kidneys 10 days following UUO. C: high-power image of WT kidney following UUO demonstrating staining of interstitial cells (arrows). D: INK4a knockout kidney 10 days following UUO. E: terminal transferase-mediated dUTP nick-end labeling (TUNEL) staining detecting apoptotic cells (red, arrow) in dilated tubule of WT mouse 10 days following UUO. F: corresponding area in INK4a knockout mouse with decreased TUNEL-positive cells. Low-power images of kidney medulla and papilla demonstrating TUNEL-positive cells in WT kidney following UUO (G) with corresponding area in knockout kidney showing reduced number of positive cells (H) are shown. I: quantification of TUNEL-positive cells in WT and INK4a knockout kidneys 10 days following UUO. Values are means ± SD for 5 mice/group. *P < 0.01.
IL-6 and TGF-β1 levels are increased in INK4a knockout mice.
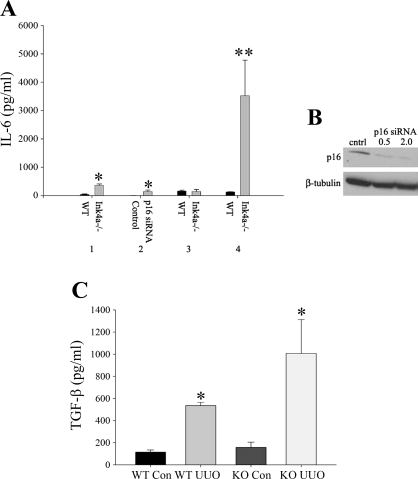
The glomerular proliferation and influx of inflammatory cells in control conditions and increased fibrosis following UUO in INK4a knockout mice suggested that INK4a proteins may have a role in controlling inflammatory cytokine production. To examine this possibility, IL-6 levels were measured by ELISA in cultured mesangial cells and IL-6 (Fig. 7A) and TGF-β levels (Fig. 7C) were measured in lysates from control and obstructed kidneys from WT and knockout mice. IL-6 levels in conditioned medium from knockout cells was significantly elevated compared with WT cells (145 ± 47 vs. 3.6 ± 0.27 pg/ml, P < 0.05). To determine whether changes in IL-6 expression were due to p16 deletion alone, mesangial cells were transfected with p16 siRNA and conditioned medium was collected 72 h posttransfection. siRNA transfection resulted in a 70% reduction in p16 levels by Western blot analysis (Fig. 7B). Consistent with knockout cells, IL-6 levels were significantly elevated in p16 siRNA-transfected cells compared with control siRNA-transfected cells (359 ± 49.5 vs. 35.8 ± 19.5 pg/ml, P = 0.05). Measurement of IL-6 levels in kidney lysates showed no difference in control WT and knockout mice (154 ± 31 vs. 143 ± 73 pg/ml). One week following UUO, levels were significantly elevated in knockout compared with WT mice (3,528 ± 1,250 vs. 124 ± 12 pg/ml, P < 0.001). TGF-β1 levels were significantly increased in control knockout compared with WT mice (157 ± 50 vs. 114 ± 17 pg/ml, P < 0.05) and in knockout mice following UUO (1,006 ± 343 vs. 536 ± 25 pg/ml, P < 0.05).
Fig. 7.
A: increased IL-6 and transforming growth factor (TGF)-β1 levels in INK4a knockout mice following injury. IL-6 was measured by ELISA in conditioned culture medium, and tissue lysates and reported as means ± SE. 1, WT and INK4a knockout mesangial cells; 2, control and p16 small interference (si) RNA-transfected WT mesangial cells; 3, WT and INK4a knockout control kidney lysates; 4, WT and INK4a knockout kidney lysates 3 days following UUO. *P < 0.05, **P < 0.0001. B: Western blot of p16 levels in mesangial cells transfected with indicated amounts (μl) of p16 siRNA. C: TGF-β1 levels in kidney lysates from control WT and knockout (KO) kidneys and WT and knockout kidneys 7 days following UUO. *P < 0.05.
Effect of oxidative stress and TGF-β on primary cultures of kidney cells from WT and INK4a knockout mice.
To confirm the observed in vivo profibrotic effects of INK4a deletion, primary cultures of mesangial and proximal tubule cells from INK4a knockout mice were compared with WT cells following oxidative stress using H2O2 and treatment with TGF-β. Under control conditions, no differences in cell cycle stages were detected between WT (Fig. 8A) and knockout mesangial cells (Fig. 8B) using flow cytometry. Analysis of cell cycle stages revealed no reduction in the S phase population of knockout mesangial cells following H2O2 treatment (Fig. 8D), with a 40% reduction detected in WT cells (Fig. 8C). Analysis of cell senescence using SA-β-galactosidase staining demonstrated an 85% reduction in the number of senescent mesangial cells 48 h following H2O2 treatment (Fig. 8E). It was also of interest to determine whether INK4a deletion resulted in phenotypic changes in epithelial cells in response to profibrotic stimuli. Proximal tubule epithelial cells were isolated from WT and INK4a knockout mice and grown to confluence on collagen-coated permeable supports. Treatment with H2O2 resulted in increased SA-β-galactosidase and p16 staining in WT cells and no staining in knockout cells, demonstrating that INK4a knockout epithelial cells are resistant to oxidative stress-induced senescence (Fig. 9, A–F). To examine phenotypic changes to profibrotic stimuli, confluent cells were treated with TGF-β for 48 h and examined for expression of markers characteristic of epithelial-mesenchymal transdifferentiation. As shown in Fig. 9, increased α-SMA, vimentin, and fibronectin expression was detected by immunofluorescence in knockout epithelial cells compared with WT cells in both control conditions and following treatment with TGF-β.
Fig. 8.
Mesangial cells from INK4a knockout mice are resistant to stress-induced premature senescence. Shown is a flow cytometry cell cycle stage analysis using 4,6-diamidino-2-phenylindole-stained mesangial cells. A: WT. B: INK4a knockout. C: WT 24 h following treatment with 400 μM H2O2. D: INK4a knockout following H2O2 treatment. E: SA-β-galactosidase staining of WT and INK4a knockout mesangial cells 24 h following H2O2 treatment, demonstrating an 85% reduction in senescent cells from knockout mice. *P < 0.0001.
Fig. 9.
Proximal tubule epithelial cells from INK4a knockout mice express increased mesenchymal markers under control conditions and in response to TGF-β. Cells were grown to confluence on collagen-coated Transwell inserts and treated with 400 μm H2O2 for 3 h to induce senescence or 5 ng/ml TGF-β for 48 h. A–D: SA-β-galactosidase staining in WT and INK4a knockout proximal tubule cells as indicated demonstrating decreased staining in knockout cells following treatment with 400 μm H2O2. E–H: p16INK4a immunofluorescence demonstrating increased p16INK4a expression following H2O2 treatment. Immunofluorescence demonstrates increased α-SMA (I–L), fibronectin (M–P), and vimentin (Q–T) expression in INK4a knockout cells compared with WT cells. Original magnification ×100.
DISCUSSION
Fibrosis following kidney injury is associated with tubular cell apoptosis, activation of pericytes with transdifferentiation into proliferating myofibroblasts that are responsible for increased matrix production, and invasion of inflammatory cells. In this study, we provide evidence that in normal young mice, mesangial cell and fibroblast p16INK4a expression contribute to decreased cell proliferation and excess matrix formation and increased p16INK4a expression following injury acts to limit excess cell proliferation, inflammation, and resulting kidney fibrosis. Increased p16INK4a expression and cell senescence were detected in both tubular epithelial and interstitial cells following UUO. INK4a knockout mice display increased cell proliferation and decreased cell senescence and increased fibrosis in response to injury, a result confirmed by examination of primary cultures of mesangial and proximal tubule epithelial cells from these mice. UUO is an established model to study the effect of gene knockouts on the development of kidney fibrosis (2). This study provides the first evidence for the importance of the INK4a gene in controlling mesenchymal cell proliferation and matrix formation in the normal mouse and cell senescence and the development of fibrosis following kidney injury. Normal human kidney biopsies have been shown to express p16INK4a in glomerular mesangial cells, distal tubule epithelium, and interstitial cells (25), a pattern similar to results from normal mouse kidneys, suggesting that p16INK4a may have a role in regulating these cell types in humans. More definitive studies using conditional or cell-specific INK4a knockout mice will be necessary to determine the specific mechanisms underlying the observed effects of INK4a deletion.
The senescent phenotype has been most extensively studied in fibroblasts. Although senescent cells are unable to divide, they are intact and metabolically viable. Senescent fibroblasts increase expression of inflammatory mediators and growth factors and matrix-degrading enzymes and decrease production of extracellular matrix proteins (1). The changes in gene expression in senescent fibroblasts differ from senescent epithelial cells and endothelial cells, suggesting that the inflammatory response is cell type specific. Because of their resistance to apoptosis, senescent cells may accumulate over time. In vitro studies have shown that senescent cells secrete a variety of proteins that stimulate immune surveillance. These changes may induce the immune clearance of both senescent cells and neighboring activated cells. Our finding of decreased macrophage infiltration in INK4a knockout mice following UUO may be explained by decreased cell senescence in these mice. Senescence may therefore act as a homeostatic mechanism that may limit tissue fibrosis, as recently demonstrated in a mouse model of reversible liver fibrosis (10). These findings also raise the possibility that the decreased apoptosis observed in INK4a knockout mice following UUO may contribute to early fibrosis by reducing the removal of profibrotic cell types, including senescent or injured epithelial cells and fibroblasts. Studies of the effect of INK4a deletion in a reversible model of kidney injury will be necessary to examine this possibility.
In human kidneys, p16INK4a increases in cortical tubular epithelial cells and medullary epithelial and interstitial cells with age and is correlated with tubular atrophy and interstitial fibrosis. These studies suggest that induction of p16INK4a may be a primary cause of aging in the kidney. Examination of human kidney biopsies with glomerular disease and proteinuria for p16INK4a expression demonstrated increased glomerular, interstitial, and tubular p16INK4a expression compared with normal adult kidney and kidneys with tubulointerstitial disease (15). The level of p16 and SA-β-galactosidase expression correlated with levels of tubular atrophy and interstitial fibrosis.
Why premature senescence occurs in these diseases and following UUO remains to be determined. In human kidneys, replicative senescence due to telomere shortening may occur but has not been demonstrated in vivo. As hypothesized in liver fibrosis, signals that activate cell hyperproliferation such as the Akt and p38 MAPK signaling may also activate p16 and other factors (12).
Results from this study differ considerably from studies of the effect of knockout of other CDK inhibitors on kidney injury, including p27 and p21. Following UUO, p21 knockout mice showed increased myofibroblast proliferation and no effect on apoptosis or fibrosis compared with WT mice (5). p27 knockout mice displayed increased tubular epithelial cell proliferation and apoptosis following UUO (19). The increased fibrosis observed in INK4a knockout mice can be attributed to increased interstitial cell proliferation and matrix formation followed by decreased macrophage infiltration. Increased p16INK4a expression in myofibroblasts in response to mitogenic signals may provide a brake on proliferation and matrix production and thereby limit fibrosis.
As demonstrated in a mouse model of liver fibrosis, it will be of interest to determine whether decreased myofibroblast senescence also contributes to increased fibrosis. More detailed studies of myofibroblast senescence and interstitial cell-specific knockouts of senescence-inducing proteins such as p16 and p53 will be needed to examine this possibility.
Studies of the effects of CDK inhibitors on inflammation following injury are contradictory. In contrast to the increased inflammatory phenotype of INK4a deletion, p21 knockout macrophages were shown to express lower inflammatory markers, resulting in decreased inflammation in atherosclerotic lesions in p21 −/− ApoE knockout mice (16). In a study of mechanical arterial injury, increased immune cell infiltration due to increased SDF-1 activation was shown in p21 −/− mice (18). Our finding that INK4a deletion results in increased kidney inflammation in control mice may appear contradictory to studies demonstrating that cell senescence results in a proinflammatory phenotype. In younger animals and following injury, increased p16INK4a levels that are not high enough to cause senescence may help to reduce inflammation. In addition, our study examined only early time points in fibrosis. The initial inflammatory cell infiltrate occurs almost immediately following UUO. Increased inflammatory cell infiltration in response to increased p16INK4a expression and cell senescence may occur later after injury. The mechanism by which INK4a deletion results in increased inflammation is unknown. p16INK4a has been shown to decrease NF-κB activity by binding to the RelA subunit and interfering with transactivation (27). Overexpression of p16INK4a in activated synovial fibroblasts decreases MCP-1 production and reduces inflammation in mouse models of rheumatoid arthritis (17). While increased TGF-β levels in control knockout kidneys can be explained by production from increased tissue macrophages, IL-6 levels were increased only after UUO and in cell culture conditions. Additional studies are necessary to determine the mechanism responsible for increased kidney macrophage infiltration in normal knockout mice.
In conclusion, examination of INK4a knockout mice indicates that p16INK4a plays an important role in regulating cell proliferation, inflammation, and fibrosis in both the normal mouse kidney and following injury. Activation of p16INK4a and other factors in response to cell stress may limit excess inflammation and induce cell senescence, thereby reducing the development of fibrosis.
GRANTS
This work was funded by National Institute of Diabetes and Digestive and Kidney Diseases grant DK067881 (M. D. Plotkin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8: 729–740, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 75: 1145–1152, 2009 [DOI] [PubMed] [Google Scholar]
- 3.del Arroyo AG, Peters G. The Ink4a/Arf network—cell cycle checkpoint or emergency brake? Adv Exp Med Biol 570: 227–247, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Furth EE, Gustafson KS, Dai CY, Gibson SL, Menard-Katcher P, Chen T, Koh J, Enders GH. Induction of the tumor-suppressor p16(INK4a) within regenerative epithelial crypts in ulcerative colitis. Neoplasia 8: 429–436, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes J, Brown P, Shankland SJ. Cyclin kinase inhibitor p21CIP1/WAF1 limits interstitial cell proliferation following ureteric obstruction. Am J Physiol Renal Physiol 277: F948–F956, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Itahana K, Campisi J, Dimri GP. Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol Biol 371: 21–31, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Ivanchuk SM, Mondal S, Dirks PB, Rutka JT. The INK4A/ARF locus: role in cell cycle control and apoptosis and implications for glioma growth. J Neurooncol 51: 219–229, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell 127: 265–275, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114: 1299–1307, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell 134: 657–667, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Hir M, Hegyi I, Cueni-Loffing D, Loffing J, Kaissling B. Characterization of renal interstitial fibroblast-specific protein 1/S100A4-positive cells in healthy and inflamed rodent kidneys. Histochem Cell Biol 123: 335–346, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173: 1617–1627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melk A, Kittikowit W, Sandhu I, Halloran KM, Grimm P, Schmidt BM, Halloran PF. Cell senescence in rat kidneys in vivo increases with growth and age despite lack of telomere shortening. Kidney Int 63: 2134–2143, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Melk A, Schmidt BM, Takeuchi O, Sawitzki B, Rayner DC, Halloran PF. Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int 65: 510–520, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Melk A, Schmidt BM, Vongwiwatana A, Rayner DC, Halloran PF. Increased expression of senescence-associated cell cycle inhibitor p16INK4a in deteriorating renal transplants and diseased native kidney. Am J Transplant 5: 1375–1382, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Merched AJ, Chan L. Absence of p21Waf1/Cip1/Sdi1 modulates macrophage differentiation and inflammatory response and protects against atherosclerosis. Circulation 110: 3830–3841, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Nonomura Y, Nagasaka K, Hagiyama H, Sekine C, Nanki T, Tamamori-Adachi M, Miyasaka N, Kohsaka H. Direct modulation of rheumatoid inflammatory mediator expression in retinoblastoma protein-dependent and -independent pathways by cyclin-dependent kinase 4/6. Arthritis Rheum 54: 2074–2083, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Olive M, Mellad JA, Beltran LE, Ma M, Cimato T, Noguchi AC, San H, Childs R, Kovacic JC, Boehm M. p21Cip1 modulates arterial wound repair through the stromal cell-derived factor-1/CXCR4 axis in mice. J Clin Invest 118: 2050–2061, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ophascharoensuk V, Fero ML, Hughes J, Roberts JM, Shankland SJ. The cyclin-dependent kinase inhibitor p27Kip1 safeguards against inflammatory injury. Nat Med 4: 575–580, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Picard N, Baum O, Vogetseder A, Kaissling B, Le Hir M. Origin of renal myofibroblasts in the model of unilateral ureter obstruction in the rat. Histochem Cell Biol 130: 141–155, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price PM, Safirstein RL, Megyesi J. The cell cycle and acute kidney injury. Kidney Int 76: 604–613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sang L, Coller HA. Fear of commitment: Hes1 protects quiescent fibroblasts from irreversible cellular fates. Cell Cycle 8: 2161–2167, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho RA. Role of the INK4a locus in tumor suppression and cell mortality. Cell 85: 27–37, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Sharpless NE, Ramsey MR, Balasubramanian P, Castrillon DH, DePinho RA. The differential impact of p16(INK4a) or p19(ARF) deficiency on cell growth and tumorigenesis. Oncogene 23: 379–385, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Sis B, Tasanarong A, Khoshjou F, Dadras F, Solez K, Halloran PF. Accelerated expression of senescence associated cell cycle inhibitor p16INK4A in kidneys with glomerular disease. Kidney Int 71: 218–226, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Terryn S, Jouret F, Vandenabeele F, Smolders I, Moreels M, Devuyst O, Steels P, Van Kerkhove E. A primary culture of mouse proximal tubular cells, established on collagen-coated membranes. Am J Physiol Renal Physiol 293: F476–F485, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Wolff B, Naumann M. INK4 cell cycle inhibitors direct transcriptional inactivation of NF-kappaB. Oncogene 18: 2663–2666, 1999 [DOI] [PubMed] [Google Scholar]