Abstract
Dynamin is a large GTPase involved in several distinct modes of cell endocytosis. In this study, we examined the possible role of dynamin in UT-A1 internalization. The direct relationship of UT-A1 and dynamin was identified by coimmunoprecipitation. UT-A1 has cytosolic NH2 and COOH termini and a large intracellular loop. Dynamin specifically binds to the intracellular loop of UT-A1, but not the NH2 and COOH termini. In cell surface biotinylation experiments, coexpression of dynamin and UT-A1 in HEK293 cells resulted in a decrease of UT-A1 cell surface expression. Conversely, cells expressing dynamin mutant K44A, which is deficient in GTP binding, showed an increased accumulation of UT-A1 protein on the cell surface. Cell plasma membrane lipid raft fractionation experiments revealed that blocking endocytosis with dynamin K44A causes UT-A1 protein accumulation in both the lipid raft and nonlipid raft pools, suggesting that both caveolae- and clathrin-mediated mechanisms may be involved in the internalization of UT-A1. This was further supported by 1) small interfering RNA to knock down either caveolin-1 or μ2 reduced UT-A1 internalization in HEK293 cells and 2) inhibition of either the caveolae pathway by methyl-β-cyclodextrin or the clathrin pathway by concanavalin A caused UT-A1 cell membrane accumulation. Functionally, overexpression of dynamin, caveolin, or μ2 decreased UT-A1 urea transport activity and decreased UT-A1 cell surface expression. We conclude that UT-A1 endocytosis is dynamin-dependent and mediated by both caveolae- and clathrin-coated pit pathways.
Keywords: membrane protein, urea transport, endocytosis
urea transport in the kidney is important for the production of concentrated urine. The major mechanism for delivering urea to the inner medullary interstitium is urea transport mediated by the facilitated UT-A urea transporters. Four mammalian UT-A isoforms (UT-A1, A2, A3, and A4; UT-A4 is only found at the mRNA level) have been identified in the kidney medulla (21, 33). UT-A1 is the largest form, whereas the others are structurally truncated forms of UT-A1. UT-A1 is expressed in the apical plasma membrane of epithelial cells in the terminal inner medullary collecting duct and plays an important role in urea reabsorption and the generation of the corticomedullary osmolarity gradient (30). UT-A1 is physiologically significant in the kidney since the UT-A1/A3 knockout mouse has reduced urine concentration ability (15).
The regulation of UT-A1 is complicated. It occurs in multiple steps at different levels, including gene transcription, protein expression, and posttranslational modification [such as phosphorylation (3), glycosylation (5), and ubiquitination (6, 37)]. As a membrane protein, UT-A1 must be present in the cell membrane to facilitate urea transport. Therefore, regulating the retention of UT-A1 in the apical plasma membrane may be an important mechanism for controlling urea transport activity. The accumulation of UT-A1 protein in the apical plasma membrane is determined by how many UT-A1 molecules are delivered to the membrane and how many are internalized back to the cytoplasm. Any processes that cause increased insertion into, or decreased removal from, the apical plasma membrane will result in increased UT-A1 expression at the cell surface. UT-A1 trafficking to the cell surface has been studied (3, 5, 6, 20, 24). However, the regulatory mechanisms for UT-A1 internalization, which may be an important mechanism for downregulation of the protein, are largely unknown.
Endocytosis is involved in a number of important physiological and pathophysiological processes, such as receptor downregulation, receptor signaling, neurotransmission, nutrient uptake, and pathogen entry (10, 44). Membrane proteins can be endocytosed by a variety of mechanisms, including caveolae-mediated endocytosis, clathrin-dependent endocytosis, macropinocytosis, and lipid raft-dependent/caveolae-independent endocytosis (10, 42). The caveolae- and clathrin-mediated endocytotic pathways are the two major routes for endocytosis in eukaryotic cells. Dynamin is essential for these two distinct endocytic processes (18). Dynamin is a large molecular weight GTP-binding protein with high intrinsic GTPase activity (28, 40). Three distinct dynamin genes have been identified in mammals: dynamin-1 is expressed exclusively in neurons; dynamin-2 is found in all tissues; and dynamin-3 is restricted to the testis, brain, lung, and heart (18, 40, 44). Dynamin has been identified in both caveolae- and clathrin-coated pits (23, 25). Dynamin directly associates with caveolin, the major component of caveolae (23, 45), and with the clathrin-coated pit accessory protein, amphiphysin II (44). Dynamin functions as a membrane scission protein in both caveolae- and clathrin-endocytic pathways. A dynamin mutation (K44A) that interferes with GTP binding and hydrolysis severely inhibits endocytosis (4, 19, 22).
Clathrin-mediated endocytosis is the best-studied endocytic pathway. This process is carried out through its association with the AP-2 clathrin adaptor complex at the plasma membrane (11). AP-2 is a heterotetramer and is composed of four subunits: two large subunits (α and β2); a medium subunit (μ2); and a small subunit (σ2). The μ2 subunit interacts with the cytoplasmic domain of membrane-bound receptors containing tyrosine-based internalization motifs (8, 28a). More than 30 accessory proteins are believed to control the clathrin-coated pit internalization pathway. Clathrin and the adaptor protein complex (AP-2) constitute the major coat constituents (8). Dynamin facilitates the clathrin endocytic process by promoting membrane invagination and fission of cargo bearing clathrin-coated vesicles from the plasma membrane (39).
Another major endocytic process is the caveolae pathway, which is distinct from clathrin-mediated endocytosis (18). Caveolae are small invaginated microdomains of plasma membranes that are enriched in cholesterol and sphingolipids. The protein caveolin is the major component of caveolae and is necessary for the generation of caveolae. Compared with the clathrin-mediated pathway, the caveolae endocytic pathway is less studied. The caveolae pathway is responsible for internalization of glycosylphosphatidylinositol-anchored proteins, cholera toxin B (18), glucose transporter 4 (32), and insulin receptor (12).
We reported that UT-A1 is predominately localized in lipid rafts in the cell plasma membrane and internalized through the caveolae-mediated pathway (14). In the current study, we investigated the role of dynamin in the UT-A1 cellular internalization and found that UT-A1 internalization is dynamin-dependent and utilizes both caveolae- and clathrin-mediated endocytic pathways.
MATERIALS AND METHODS
Plasmid construction.
The dynamin and dynamin-K44A constructs in pEGFP vector were kindly provided by Dr. McNiven (Mayo Clinic College of Medicine). Human caveolin-1-specific siRNA expression vector was kindly provided by Dr. Xie (Medical College of Ohio). Human dynamin-specific siRNA expression vector and control scrambled sequence vector were described before (14). To construct human μ2 siRNA expression vector, a specific sequence (GAGGGTATCAAGTATCGTC) was selected and cloned into pSuppressor vector (IMGENEX, San Diego, CA) which contains the U6 promoter. The encoding full-length of dynamin from pEGFP-dynamin, caveolin from ATCC (MGC-13070), and μ2 from GST-μ2 (29) were polymerase chain reaction (PCR) amplified and subcloned into oocyte expression vector pGH19.
Rat UT-A1 cDNA in oocyte expression vector pGH19 (pGH19-UT-A1) was made as described before (14). A fragment of 378 nt corresponding to the 126 aa NH2 terminus, 124 nt corresponding to the 48 aa COOH terminus, and a fragment of 537 nt corresponding to 179 aa intracellular loop of urea transporter UT-A1 (Fig. 1B) were obtained by PCR by using pcDNA3-UT-A1 as the template. The amplified fragments (N-A1, C-A1, and Lp-A1) were cloned in-frame with the GAL4 DNA-binding domain into the pGBKT7 vector (Clontech). The pGBKT7 vector contains a T7 promoter and a NH2-terminal c-myc tag. These three constructs were originally generated as baits for yeast two-hybrid assay. All constructs were verified by DNA sequencing.
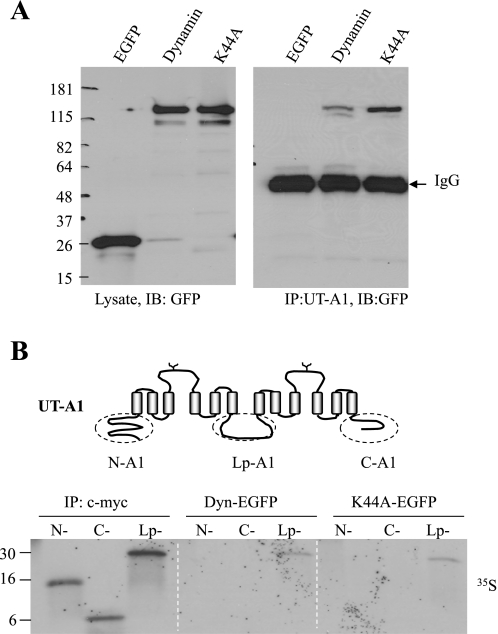
Fig. 1.
UT-A1 urea transporter associates with dynamin. A: UT-A1-HEK293 cells were transfected with pEGFP, pEGFP-dynamin, or pEGFP-dynamin K44A for 48 h. Cells were lysed with RIPA buffer and the supernatants were used for immunoprecipitation with UT-A1 antibody. Total cell lysates or UT-A1-precipitated samples were processed for Western blot with GFP antibody. B: dynamin binds to UT-A1 intracellular loop. EGFP-Dynamin and EGFP-K44A were prepared by transfection of pEGFP-Dyn or pEGFP-K44A into HEK293 cells. The proteins were precipitated with GFP antibody and conjugated to the protein A beads. An equal amount of [35S]-labeled N-, C-, and Lp-A1 synthesized in reticulocyte lysates was added to GFP-Dyn or GFP-K44A beads for pulldown assay. The three fragments of N-, C-, and Lp-A1 tagged with c-myc were precipitated with c-myc antibody as control. The [35S] signal was detected by autoradiography.
Cell culture and transfection.
HEK293 cells and permanent cell lines of 293 cells expressing UT-A1 were cultured in DMEM supplemented with 10% fetal calf serum at 37°C in 5% CO2. Cells were grown in six-well plates or 100-mm dishes to 80% confluency and transfected with indicated constructs (pcDNA3-UT-A1, pEGFP-dynamin, or pSupressor siRNA expression plasmids). Transfection was carried out using Lipofectamine (Invitrogen) according to the manufacturer's instructions. After 48 h, cells were processed for lipid raft fractionation, cell surface biotinylation, immunoprecipitation, or Western blot.
Cell surface biotinylation.
To investigate UT-A1 expression in the cell membrane, cell surface biotinylation was performed (14). Forty-eight hours after transfection or treatment with chemicals, the cells were placed on ice and rinsed with cold PBS. Then, the cells were incubated twice with ice-cold borate buffer (85 mM NaCl, 4 mM KCl, 15 mM Na2B4O7, pH 8.0) containing 1.0 mg/ml sulfo-NHS-SS-biotin (Pierce) for 20 min at 4°C. The excess biotin was quenched by 0.1 M lysine. After being washed with PBS, the cells were scraped in RIPA buffer (150 mM NaCl, 10 mM Tris·HCl, pH 7.5, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS and protease inhibitors). Equal amounts of proteins were added to 20 μl of streptavidin beads overnight. The biotin-conjugated proteins were eluted and processed for Western blot with UT-A1 antibody.
Immunoprecipitation and Western blot.
The cells were lysed with RIPA buffer. The cell suspension was centrifuged at 14,000 rpm at 4°C for 10 min. The protein concentration of each cellular extract was determined with the BCA protein assay reagent (Pierce). For immunoprecipitations, the postnuclear supernatants were incubated with UT-A1 antibody overnight at 4°C, and then with protein A beads (Amersham Biosciences) for 1 h at 4°C with rotation. Beads were collected by washing and centrifuging with the lysis buffer. Proteins were eluted by Laemmli sample buffer and subjected to SDS-PAGE and Western blot analysis. The membranes were incubated with primary antibody, followed by anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibodies (Amersham) and developed by ECL (Amersham). The following primary antibodies were used: anti-UT-A1 (29), anti-caveolin-1 (BD Bioscience), anti-dynamin-2 (Santa Cruz Biotechnology), anti-transferrin receptor (Invitrogen), anti-Na+-K+-ATPase α-subunit (Sigma), and anti-μ2 (kindly provided by Dr. Bonifacino, National Institute of Child Health and Human Development/National Institutes of Health).
GFP-dynamin protein purification and in vitro binding assay.
The GFP-dynamin and K44A dynamin were prepared by transfection of the relevant constructs into HEK293 cells. Forty-eight hours after transfection, the cells were lysed in RIPA buffer. The supernatant of cell lysates (∼2 mg) were incubated with 10 μl of anti-GFP antibody (Invitrogen) for 2 h at 4°C, and then 10 μl of protein A beads (Amersham) for another 2 h. After being washed with RIPA buffer, GFP-dynamin, conjugated to beads, was used for the subsequent 35S-labeled N-A1, C-A1, and Lp-A1 binding assay.
The three intracellular fragments of UT-A1 (N-A1, C-A1, and Lp-A1) were translated in vitro using the TNT T7-coupled rabbit reticulocyte translation system (Promega) in the presence of [35S]methionine. The translation reaction in a final volume of 50 μl included 25 μl of reticulocyte lysate, 2 μl of 10× reaction buffer, 1 μl of 1 mM amino acid mixture (minus methionine), 2 μl of 10 mCi/ml [35S]methionine (Amersham), 1 μg of DNA template (pGBKT7-N-A1, -C-A1, and -Lp-A1), and 1 μl of T7 RNA polymerase. The mixtures were incubated for 45 min at 30°C. The reaction was terminated by storing on ice. Twenty microliters of reaction mixture were incubated with dynamin or K44A dynamin-conjugated beads, and another 10 μl of mixture were used as control to monitor the protein synthesis by immunoprecipitation with c-myc antibody. After the incubation, the beads were washed with RIPA and pelleted by centrifugation. The proteins were eluted by boiling in Laemmli sample buffer, run on SDS-PAGE, and detected by autoradiography.
Lipid raft isolation.
UT-A1 HEK293 cells were grown on 100-mm plates until 80% confluency and transfected with pEGFP-dynamin or K44A dynamin or vector for 48 h. Cells were then processed for cell surface biotinylation as above. Lipid raft fractionation was performed with a 5–40% sucrose discontinuous gradient as described (14). Here, instead of Triton X-100, Brij96 (7) was used as the nonionic detergent in the lysis buffer. The cells from two 100-mm plates for each group were lysed in 900 μl of ice-cold 0.5% Brij96/TNEV buffer (10 mM Tris·HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 2 mM Na vanadate, and protease inhibitor cocktail) for 30 min on ice. Lysates were centrifuged at 10,000 rpm for 3 min at 4°C. The protein concentration was determined by BCA protein assay and equal amounts of supernatant in 500 μl were mixed with 500 μl of 80% sucrose in TNEV and transferred to 13 × 51-mm Beckman centrifuge tubes. Three milliliters of 35% sucrose in TNEV were layered carefully on top of the mixture followed by another 1 ml layer of 5% sucrose. The sucrose gradient was then centrifuged in a SW 50.1 rotor (Beckman) at 34,000 rpm (∼110,000 g) for 20 h at 4°C. Fractions (∼400 μl) were collected from the top to bottom of the tube. Fifty microliters of each fraction were saved for Western blot. The remaining volume (∼350 μl) was added to 800 μl of RIPA and mixed in a cold room for 2 h, followed by addition of 20 μl of streptavidin beads and continued incubation for another 2 h. After being washed, the pulled-down samples were eluted and analyzed by Western blot with relevant antibodies. NIH ImageJ software was used to quantify the band density.
Oocyte urea flux and biotinylation experiments.
Xenopus laevis oocytes were prepared and maintained as described in a previous report (14). All capped cRNAs were transcribed in vitro from linearized cDNAs with T7 polymerase using the mMESSAGE mMACHINE T7 Ultra Kit (Ambion). Two nanograms of UT-A1 and 5 ng of different combinations of dynamin, caveolin, or μ2 in a total volume of 23 nl water were injected into each oocyte. Three days later, healthy oocytes were selected for functional study and protein expression. The urea uptake assay and oocyte biotinylation were performed as before (14).
Statistical analysis.
Urea flux data are expressed as means ± SD. Statistical analysis of the data was performed by one-way ANOVA followed by Tukey HSD tests. Differences were considered as significant at *P < 0.05 or **P < 0.01.
RESULTS
UT-A1 urea transporter directly associates with dynamin.
Dynamin directly interacts with many cargo proteins (16, 39). We first examined whether UT-A1 associated with dynamin. UT-A1 HEK293 cells were transiently transfected with enhanced green fluorescent protein (EGFP)-tagged dynamin or mutant (EGFP-K44A) constructs for 48 h. pEGFP vector was used as a control. Cells were processed for coimmunoprecipitation. As shown in Fig. 1A, no GFP pulldown was detectable with the EGFP vector. Dynamin, either wild-type or K44A mutant, was precipitated by the UT-A1 antibody.
Dynamin binds to the intracellular loop of UT-A1.
UT-A1 has both cytosolic NH2 and COOH termini and a large intracellular loop (Fig. 1B). To investigate the dynamin-binding site in UT-A1, the three cytoplasmic fragments of UT-A1 were prepared and labeled with [35S]methionine by in vitro translation with rabbit reticulocyte lysate and used for GFP-dynamin and GFP-K44A pulldown experiments. Figure 1B shows that the loop segment of UT-A1 contains the binding site for dynamin.
Dynamin K44A increases UT-A1 expression in the plasma membrane.
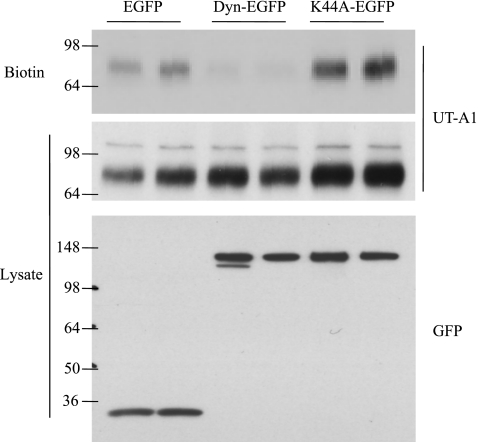
To evaluate the effect of dynamin on UT-A1 membrane expression, UT-A1 HEK293 cells were transfected with dynamin or its mutant K44A and UT-A1 cell surface expression was examined using a biotinylation assay. UT-A1 membrane expression was markedly decreased when the cells were transfected with dynamin. In contrast, the dominant-negative dynamin GTPase (K44A), which blocks endocytosis, leads to UT-A1 accumulation at the cell surface (Fig. 2).
Fig. 2.
Overexpression of dynamin decreases UT-A1 cell surface accumulation. UT-A1-HEK293 cells were transfected with pEGFP, pEGFP-Dynamin, or pEGFP-K44A for 48 h. Cells were processed for biotinylation. Total cell lysate and biotinylated samples were immunoblotted with UT-A1 or GFP antibodies.
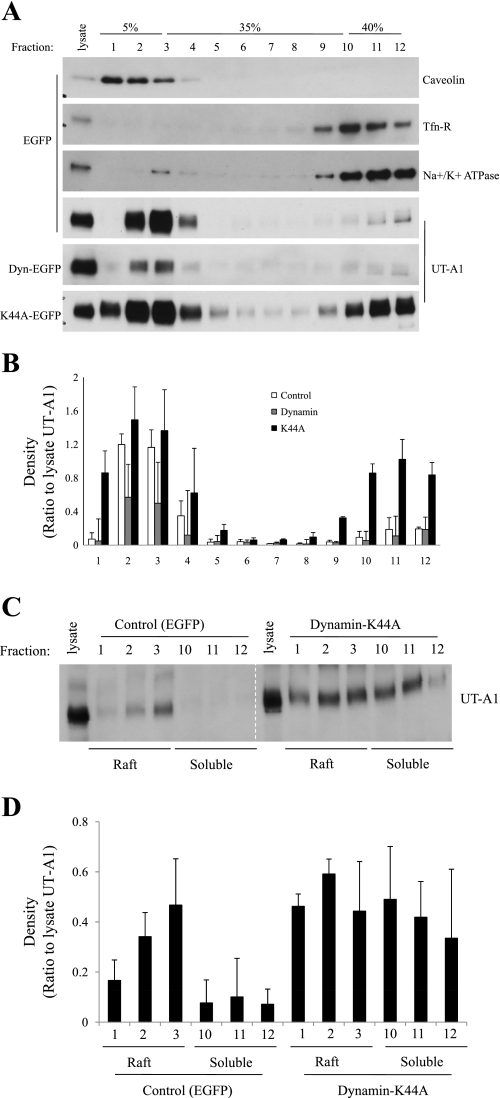
Inhibition of endocytosis by dynamin K44A causes UT-A1 accumulation in both lipid raft and nonlipid raft fractions.
Cell membrane UT-A1 is concentrated in lipid raft microdomains (14). To investigate whether inhibition of endocytosis by K44A dynamin would affect UT-A1 distribution at the cell membrane, wild-type and K44A dynamin were transfected into UT-A1 HEK293 cells. Plasma membrane lipid raft and nonraft membrane were fractionated on a sucrose density gradient by ultracentrifugation (13, 14). Since Brij96 was shown to better preserve membrane lipid rafts (7), we used Brij96 instead of Triton X-100. Caveolin-1 was used as a lipid raft-positive control marker. Transferrin receptor and Na+-K+-ATPase α-subunit (17) were applied as markers for nonraft fractions. When using Brij 96, the lipid raft appears in the first three fractions (1–3) as indicated by caveolin. As shown in Fig. 3A, the cell membrane UT-A1 was mainly found in lipid raft fractions in the control group, consistent with our previous findings (14). Overexpression of dynamin did not change the UT-A1 distribution in the membrane subfraction. However, blocking endocytosis by coexpression of a dominant-negative K44A dynamin resulted in UT-A1 accumulation in both the lipid raft and nonlipid raft subdomains. Figure 3B shows the densitometry analysis of UT-A1 distribution in the membrane fractions of Fig. 3A from three separate experiments. The relative intensities of each band were normalized to the UT-A1 band from total lysate. Interestingly, there was very little UT-A1 present in the first fraction in control and dynamin groups, while transfection of K44A dynamin significantly increased UT-A1 population in fraction 1 as well as fractions 10–12.
Fig. 3.
Dynamin K44A changes UT-A1 distribution in the cell membrane. A: lipid raft isolation. UT-A1-HEK293 cells were grown in 100-mm dishes and transfected with pEGFP, pEGFP-Dynamin, or pEGFP-K44A for 48 h. Cells were lysed in ice-cold 0.5% Brij96/TNEV buffer. After centrifugation at 10,000 rpm for 3 min, supernatants were collected and protein concentration was determined. The same amount of proteins in supernatant was loaded on the 5–40% sucrose gradient and centrifuged at 34,000 rpm for 20 h at 4°C. Equal fraction volumes were collected from the top to bottom of the gradient and analyzed by immunoblotting with relevant antibodies. B: quantification of UT-A1 distribution in the membrane fractions from 3 separate experiments. The relative intensities of each band were normalized to the UT-A1 band from total lysate. C: analysis of lipid rafts after biotinylation. The cells were grown and transfected with indicated constructs as above. Before the cells were processed for lipid raft isolation, cell surface proteins were labeled by sulfo-NHS-SS-biotin in borate buffer. Fractions 1–3 (raft) and fractions 10–12 (nonraft) collected from the density gradients were mixed with streptavidin beads for at least 2 h. After being washed, the bound proteins on the beads were analyzed by Western blot with UT-A1 antibody. D: densitometry analysis of UT-A1 distribution in the membrane fractions of C from 3 separate experiments.
The lipid component of plasma membrane and cytosolic organelle membrane is significantly different. The ratio of cholesterol to phospholipid is 1.0 in plasma membrane, 0.15 in endoplasmic reticulum, 0.2 in Golgi, 0.5 in late endosomes, and 0.1 in mitochondria (43). The lipid raft isolation study (13, 14) performed here represents mostly the plasma membrane subfractions. To exclude the possible cytosolic organelle membranes, particularly the early endosome, we combined cell surface biotinylation with lipid raft fractionation. The cells were first biotinylated and then processed for sucrose gradient flotation. Plasma membrane UT-A1 (pulled down by streptavidin) distribution was analyzed by immunoblotting with UT-A1 antibody. Fractions 1–3 were selected as lipid raft and fractions 10–12 as nonraft. Figure 3C is representative of three individual experiments of biotin-lipid raft isolation study, showing that K44A dynamin causes plasma membrane UT-A1 accumulation in both raft and nonraft subdomains.
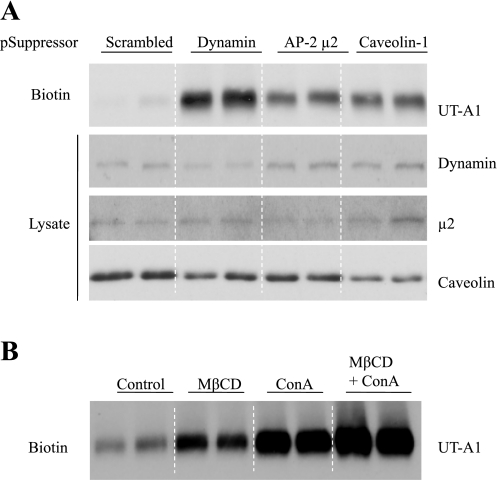
Knocking down caveolin or μ2 increases UT-A1 cell membrane expression.
To confirm that both endocytic pathways are involved in UT-A1 internalization, we utilized RNAi technology to inhibit synthesis of the key components of each process. Transfection with caveolin or μ2-specific siRNA significantly reduced their respective protein expression levels (Fig. 4A). Cell surface biotinylation assay showed that RNAi-mediated depletion of either caveolin or μ2 leads to UT-A1 accumulation at the plasma membrane. Knocking down dynamin by siRNA also reduced UT-A1 internalization.
Fig. 4.
Both caveolae and clathrin pathways are involved in UT-A1 endocytosis. A: knockdown of dynamin, μ2, or caveolin-1 by RNA interference increases UT-A1 membrane expression. UT-A1-HEK293 cells were transfected with pSuppressor/scramble, dynamin, AP-2 μ2, or caveolin-1 for 48 h. Cells were processed for biotinylation. Total cell lysate and biotinylated samples were blotted with the corresponding antibodies. B: inhibition of the caveolae or clathrin pathway causes UT-A1 cell membrane accumulation. UT-A1-HEK293 cells were treated with 5 mM methyl-β-cyclodextrin (MβCD) or 250 μg/ml concanavalin A (ConA) or MβCD + ConA for 1 h; cells were then processed for biotinylation assay. The biotinylated samples were blotted for UT-A1.
Inhibition of the caveolae- or the clathrin-mediated pathway increases UT-A1 cell membrane expression.
To further clarify the preceding findings, UT-A1 endocytosis was examined by two endocytic pathway inhibitors. UT-A1 HEK293 cells were treated with methyl-β-cyclodextrin (MβCD) which depletes cholesterol and inhibits caveolin-dependent endocytosis, or concanavalin A (ConA), which inhibits clathrin-coated pit-mediated endocytosis. Figure 4B shows that both inhibition of the caveolin-dependent pathway by MβCD and of the clathrin-dependent pathway by ConA significantly increased UT-A1 cell membrane accumulation.
Overexpression of caveolin and μ2 decreases UT-A1 cell membrane expression and urea transport activity.
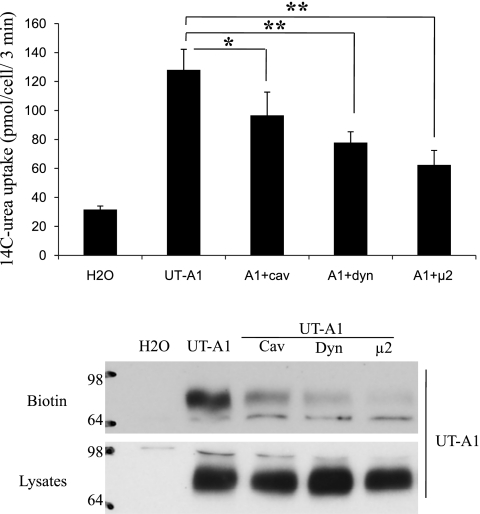
Finally, we examined the effect of coinjection of dynamin, caveolin, and μ2 on UT-A1 urea transport activity. In our previous publication (14), we showed that overexpression of caveolin decreases UT-A1 urea transport activity. Here, we examined the change in UT-A1 functional activity when overexpressing μ2 in oocytes. As shown in Fig. 5, coinjection of dynamin or μ2 decreases UT-A1 urea transporter activity. This effect is consistent with decreased UT-A1 expression in the surface membrane.
Fig. 5.
Overexpression of dynamin, caveolin, and μ2 reduces UT-A1 activity. A: oocytes were injected with cRNA encoding UT-A1 (2 ng/cell) alone or together with dynamin, caveolin, or μ2 (5 ng/cell) for 3 days. Urea transport activity was measured by [14C] urea flux (n = 6 oocytes/time point). B: UT-A1 expression in total cell lysates or biotinylated samples was analyzed by immunoblotting with UT-A1 antibody. The results are representative of 3 separate experiments. *P < 0.05, **P < 0.01.
DISCUSSION
The process of dynamin-mediated endocytosis involves numerous protein-protein and protein-lipid interactions (31). The role of dynamin in the clathrin-coated pit endocytic pathway is well-studied. Dynamin is targeted to coated pits through interactions with amphiphysin II, which also binds AP-2 and clathrin (42, 44). In addition, accumulating evidence shows that dynamin can also directly bind to the cargo proteins, as seen with voltage-gated calcium channels (16) and AT1A receptors (39). The interaction of dynamin and cargo proteins promotes endocytosis of the cargo protein. Dynamin interaction can also prevent dissociation of captured cargo protein (41). In the current study, we found that dynamin directly associates with UT-A1 at the intracellular loop region (Fig. 1B). Dynamin directly interacts with caveolin (45). We previously reported that UT-A1 interacts with caveolin (14). However, we could not demonstrate UT-A1 association with μ2 by GST pulldown (data not shown). Therefore, the entry of UT-A1 into caveolae could be mediated by caveolin and/or dynamin. On the other hand, how UT-A1 is recruited into clathrin-coated pits is less clear. Dynamin regulates the late steps of clathrin-mediated endocytosis (19, 40). It is unlikely that it acts as a UT-A1 recruiting protein.
Cell surface-specific protein internalization through different pathways is highly selective. Aquaporin-2 (AQP2) is concentrated in clathrin-coated pits on the apical surface of collecting duct principal cells and is internalized through a clathrin-dependent pathway (38). Shimkets et al. (35) reported that the activity of the epithelial sodium channel (ENaC) is regulated by clathrin-mediated endocytosis. The glucose transporter GLUT8 (34), ROMK (9, 26), and AT1 receptor (39) were reported to be internalized via the clathrin pathway. In contrast, other proteins favor the caveolae-dependent endocytosis route. These include FcRI complexes (13), glucose transporter 4 (32), and insulin receptors (12). The endocytic mechanism of UT-A1 may differ significantly from that of AQP2 (38) and ENaC (35). Interestingly, unlike UT-A1, which is mainly localized in lipid rafts, a large amount of AQP2 resides outside of lipid rafts (14). This could explain why AQP2 internalization was reported to occur through the clathrin pathway (38). We previously reported that UT-A1 endocytosis is through the caveolae endocytic pathway (14). Here, we found that UT-A1 is also internalized via the clathrin-coated pit pathway. Originally, we made this finding when we examined isolated membrane lipid rafts and discovered that when endocytosis was blocked by dynamin K44A, significant amounts of UT-A1 accumulated in nonraft fractions (Fig. 3). By knocking down μ2, a component of the clathrin-mediated endocytic machinery, we determined that internalization of UT-A1 is also clathrin-mediated. Strikingly, inhibition of UT-A1 endocytosis by ConA is more pronounced than by MβCD. This suggests that the clathrin-coated pit pathway could be the predominant mode of UT-A1 internalization.
In fact, internalization by both caveolin- and clathrin-dependent pathways has been observed for several receptors (27, 36). When in the presence of a low concentration of ligand, epidermal growth factor (EGF) receptor is exclusively internalized by the clathrin pathway, while at a high concentration of ligand, EGF receptor is ubiquitinated and internalized through both caveolae and clathrin pathways (36). The majority of previous work on endocytosis of membrane proteins was concentrated on the clathrin-coated pit pathway, with the caveolae pathway relatively less studied. Increasing evidence shows that some membrane proteins, such as ROMK (27), are internalized through the caveolae pathway in addition to the clathrin pathway.
Why do some membrane proteins utilize two distinct endocytic pathways? These two routes have different properties and are regulated by different mechanisms (36). Caveolae-mediated endocytosis is much slower than clathrin-mediated endocytosis (18). As Aguilar and Wendland (1) stated that “two pathways are better than one”, the two pathways could be regulated in concert in response to different physiological demands. It is not clear from the current study how UT-A1 enters the two different pathways. The status of protein phosphorylation and/or ubiquitination might determine the choice of endocytic pathway. Further study is required to address how the UT-A1 endocytic process is triggered, how UT-A1 is selectively incorporated into caveolae- or clathrin-coated vesicles, and the significance of these two distinct pathways in the regulation of UT-A1 urea transport activity.
GRANTS
This work was supported by American Heart Association Beginning Grant-In-Aid 0765202B and National Institutes of Health (NIH) Grant R21-DK-080431 to G. Chen, and NIH Grants P01-DK-61521 and R01-DK-41707 to J. M. Sands.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. M. McNieven for kindly providing the dynamin constructs, Dr. Haucke for GST-μ2 construct, Dr. Z.-J. Xie for caveolin siRNA plasmid, and Dr. J. Bonifacino for μ2 antibody.
REFERENCES
- 1.Aguilar RC, Wendland B. Endocytosis of membrane receptors: two pathways are better than one. Proc Natl Acad Sci USA 102: 2679–2680, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blount MA, Mistry AC, Fröhlich O, Price SR, Chen G, Sands JM, Klein JD. Phosphorylation of UT-A1 urea transporter at serines 486 and 499 is important for vasopressin-regulated activity and membrane accumulation. Am J Physiol Renal Physiol 295: F295–F299, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao H, Chen J, Awoniyi M, Henley JR, McNiven MA. Dynamin 2 mediates fluid-phase micropinocytosis in epithelial cells. J Cell Sci 120: 4167–4177, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Fröhlich O, Yang Y, Klein JD, Sands JM. Loss of N-linked glycosylation reduces urea transporter UT-A1 response to vasopressin. J Biol Chem 281: 27436–27442, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Chen G, Huang H, Fröhlich O, Yang Y, Klein JD, Price SR, Sands JM. MDM2 E3 ubiquitin ligase mediates UT-A1 urea transporter ubiquitination and degradation. Am J Physiol Renal Physiol 295: F1528–F1534, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Jen A, Warley A, Lawrence MJ, Quinn PJ, Morris RJ. Isolation at physiological temperature of detergent-resistant membranes with properties expected of lipid rafts: the influence of buffer composition. Biochem J 417: 525–533, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Conner SD, Schmid SL. Differential requirements for AP-2 in clathrin-mediated endocytosis. J Cell Biol 162: 773–779, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cope G, Murthy M, Golbang AP, Hamad A, Liu CH, Cuthbert AW, O'Shaughnessy KM. WNK1 affects surface expression of the ROMK potassium channel independent of WNK4. J Am Soc Nephrol 17: 1867–1874, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Donaldson JG, Porat-Shliom N, Cohen LA. Clathrin-independent endocytosis: a unique platform for cell signaling and PM remodeling. Cell Signal 21: 1–6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorsey FC, Muthusamy T, Whitt MA, Cox JV. A novel role for a YXXPhi motif in directing the caveolin-dependent sorting of membrane-spanning proteins. J Cell Sci 120: 2544–2554, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Fagerholm S, Ortegren U, Karlsson M, Ruishalme I, Strålfors P. Rapid insulin-dependent endocytosis of the insulin receptor by caveolae in primary adipocytes. PLoS One 4: e5985, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fattakhova G, Masilamani M, Borrego F, Gilfillan AM, Metcalfe DD, Coligan JE. The high-affinity immunoglobulin-E receptor (FcepsilonRI) is endocytosed by an AP-2/clathrin-independent, dynamin-dependent mechanism. Traffic 7: 673–685, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Feng X, Huang H, Yang Y, Fröhlich O, Klein JD, Sands JM, Chen G. Caveolin-1 directly interacts with UT-A1 urea transporter: the role of caveolae/lipid rafts in UT-A1 regulation at the cell membrane. Am J Physiol Renal Physiol 296: F1514–F1520, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenton RA, Chou CL, Stewart GS, Smith CP, Knepper MA. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci USA 101: 7469–7474, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Gutierrez G, Miranda-Laferte E, Neely A, Hidalgo P. The Src homology 3 domain of the beta-subunit of voltage-gated calcium channels promotes endocytosis via dynamin interaction. J Biol Chem 282: 2156–2162, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Hartmann AM, Blaesse P, Kranz T, Wenz M, Schindler J, Kaila K, Friauf E, Nothwang HG. Opposite effect of membrane raft perturbation on transport activity of KCC2 and NKCC1. J Neurochem 111: 321–331, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Henley JR, Krueger EWA, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol 41: 85–99, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heymann JA, Hinshaw JE. Dynamins at a glance. J Cell Sci 122: 3427–3431, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue T, Terris J, Ecelbarger CA, Chou CL, Nielsen S, Knepper MA. Vasopressin regulates apical targeting of aquaporin-2 but not of UT1 urea transporter in renal collecting duct. Am J Physiol Renal Physiol 276: F559–F566, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Karakashian A, Timmer RT, Klein JD, Gunn RB, Sands JM, Bagnasco SM. Cloning and characterization of two new isoforms of the rat kidney urea transporter: UT-A3 and UT-A4. J Am Soc Nephrol 10: 230–237, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Karim Z, Vepachedu R, Gorska M, Alam R. UNC119 inhibits dynamin and dynamin-dependent endocytosis processes. Cell Signal 22: 128–137, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Kim YN, Bertics PJ. The endocytosis-linked protein dynamin associates with caveolin-1 and is tyrosine phosphorylated in response to the activation of a noninternalizing epidermal growth factor receptor mutant. Endocrinology 143: 1726–1731, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Klein JD, Fröhlich O, Blount MA, Martin CF, Smith TD, Sands JM. Vasopressin increases plasma membrane accumulation of urea transporter UT-A1 in rat inner medullary collecting ducts. J Am Soc Nephrol 17: 2680–2686, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Krauss M, Kukhtina V, Pechstein A, Haucke V. Stimulation of phosphatidylinositol kinase type I-mediated phosphatidylinositol (4,5)-bisphosphate synthesis by AP-2mu-cargo complexes. Proc Natl Acad Sci USA 103: 11934–11939, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin DH, Sterling H, Wang Z, Babilonia E, Yang B, Dong K, Hebert SC, Giebisch G, Wang WH. ROMK1 channel activity is regulated by monoubiquitination. Proc Natl Acad Sci USA 102: 4306–4311, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin DH, Yue P, Pan CY, Sun P, Zhang X, Han Z, Roos M, Caplan MJ, Giebisch G, Wang WH. POSH stimulates the ubiquitination and the clathrin-independent endocytosis of ROMK1 channels. J Biol Chem 284: 29614–29624, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNiven MA. Dynamin: a molecular motor with pinchase action. Cell 94: 151–154, 1998 [DOI] [PubMed] [Google Scholar]
- 28a.Motley AM, Berg N, Taylor MJ, Sahlender DA, Hirst J, Owen DJ, Robinson MS. Functional analysis of AP-2 α and μ2 subunits. Mol Biol Cell 17: 5298–5308, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naruse M, Klein JD, Ashkar ZM, Jacobs JD, Sands JM. Glucocorticoids downregulate the rat vasopressin-regulated urea transporter in rat terminal inner medullary collecting ducts. J Am Soc Nephrol 8: 517–523, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Nielsen S, Terris J, Smith CP, Hediger MA, Ecelbarger CA, Knepper MA. Cellular and subcellular localization of the vasopressin-regulated urea transporter in rat kidney. Proc Natl Acad Sci USA 93: 5495–5500, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishi K, Saigo K. Cellular internalization of green fluorescent protein fused with herpes simplex virus protein VP22 via a lipid raft-mediated endocytic pathway independent of caveolae and Rho family GTPases but dependent on dynamin and Arf6. J Biol Chem 282: 27503–27517, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Ros-Baro A, Lopez-Iglesias C, Peiro S, Bellido D, Palacin M, Zorzano A, Camps M. Lipid rafts are required for GLUT4 internalization in adipose cells. Proc Natl Acad Sci USA 98: 12050–12055, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sands JM. Molecular mechanisms of urea transport. J Membr Biol 191: 149–163, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Schmidt U, Briese S, Leicht K, Schürmann A, Joost HG, Al-Hasani H. Endocytosis of the glucose transporter GLUT8 is mediated by interaction of a dileucine motif with the beta2-adaptin subunit of the AP-2 adaptor complex. J Cell Sci 119: 2321–2331, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Shimkets RA, Lifton RP, Canessa CM. The activity of the epithelial sodium channel is regulated by clathrin-mediated endocytosis. J Biol Chem 272: 25537–25541, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci USA 102: 2760–2765, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart GS, O'Brien JH, Smith CP. Ubiquitination regulates the plasma membrane expression of renal UT-A urea transporters. Am J Physiol Cell Physiol 295: C121–C129, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Sun TX, Van Hoek A, Huang Y, Bouley R, McLaughlin M, Brown D. Aquaporin-2 localization in clathrin-coated pits: inhibition of endocytosis by dominant-negative dynamin. Am J Physiol Renal Physiol 282: F998–F1011, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Szaszák M, Gáborik Z, Turu G, McPherson PS, Clark AJ, Catt KJ, Hunyady L. Role of the proline-rich domain of dynamin-2 and its interactions with Src homology 3 domains during endocytosis of the AT1 angiotensin receptor. J Biol Chem 277: 21650–21656, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Takei K, Yoshida Y, Yamada H. Regulatory mechanisms of dynamin-dependent endocytosis. J Biochem (Tokyo) 137: 243–247, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol 10: 583–596, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Ungewickell EJ, Hinrichsen L. Endocytosis: clathrin-mediated membrane budding. Curr Opin Cell Biol 19: 417–425, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9: 112–124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, Haas M, Liang M, Cai T, Tian J, Li S, Xie Z. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J Biol Chem 279: 17250–17259, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Yao Q, Chen J, Cao H, Orth JD, McCaffery JM, Stan RV, McNiven MA. Caveolin-1 interacts directly with dynamin-2. J Mol Biol 348: 491–501, 2005 [DOI] [PubMed] [Google Scholar]