Abstract
The role of mechanical forces in the regulation of glomerulotubular balance in the proximal tubule (PT) and Ca2+ signaling in the distal nephron was first recognized a decade ago, when it was proposed that the microvilli in the PT and the primary cilium in the cortical collecting duct (CCD) acted as sensors of local tubular flow. In this review, we present a summary of the theoretical models and experiments that have been conducted to elucidate the structure and function of these unique apical structures in the modulation of Na+, HCO3−, and water reabsorption in the PT and Ca2+ signaling in the CCD. We also contrast the mechanotransduction mechanisms in renal epithelium with those in other cells in which fluid shear stresses have been recognized to play a key role in initiating intracellular signaling, most notably endothelial cells, hair cells in the inner ear, and bone cells. In each case, small hydrodynamic forces need to be greatly amplified before they can be sensed by the cell's intracellular cytoskeleton to enable the cell to regulate its membrane transporters or stretch-activated ion channels in maintaining homeostasis in response to changing flow conditions.
Keywords: proximal tubule, cortical collecting duct, microvilli, primary cilia, polycystic kidney disease, fluid flow in renal tubule
mechanical forces due to fluid flow and hydrodynamic pressure are not only sensed by renal epithelial cells but provide the key signals that regulate glomerulotubular balance in the proximal tubule (PT) and ion transport in the distal nephron, including the cortical collecting duct (CCD). In key papers at the beginning of this decade, Guo et al. (25) and Praetorius and Spring (57) proposed that the flow-induced bending moments on the microvilli of the brush-border epithelium and the primary cilia of Madin-Darby canine kidney (MDCK) cells, a cell line derived from the collecting duct of the canine kidney, respectively, provided the afferent signal for the regulation of ion transport at the proximal and distal portions of the nephron. What was not previously appreciated is that renal epithelial cells, like hair cells in the inner ear (33) and osteocytes in calcified bone tissue (21), have special sensing machinery that enables them to sense variations in luminal fluid shear stress (FSS) and amplify these mechanical signals so that they can be transmitted to the intracellular cytoskeleton for the purpose of regulating key membrane transport proteins. Such structures are needed, since the FSS experienced by renal epithelial cells is an order of magnitude smaller than that prevailing in the vasculature, where a surface glycocalyx on endothelial cells (ECs) can serve this important function, as reviewed previously (84).
The brush border is a remarkable mechanosensing apparatus that involves ∼4,000 membrane-bound actin filament bundles (microvilli) that are equally spaced and nearly identical in height on each PT epithelial cell. Prior to 2000, the primary function of these fingerlike projections was thought to be the amplification of membrane surface area for transport (90). Little thought was given to mechanotransduction, since the flow velocities in the brush border were known to be negligibly small (3). All epithelial cells in the renal tubule, perhaps with the exception of intercalated cells in the fully differentiated distal nephron [although this remains controversial (67)], have a single primary cilium, a nonmotile 9 + 0 microtubular structure projecting from the cell's centriole. Long ignored, the role of the cilium as a mechanosensor was initially identified by Praetorius and Spring (57), who performed bending experiments on MDCK cell primary cilia. With the identification of polycystin-1 and -2, the products of the two genes implicated in autosomal-dominant polycystic kidney disease (PKD) localized to the renal cilium, this organelle has been thrust into the spotlight as a key player in renal epithelial biology (52, 98). In this review, we present a focused summary of the theoretical models and various experiments that have been conducted over the past decade to elucidate the structure and function of these unique apical structures in the modulation of Na+, HCO3−, and water reabsorption in the PT, and, in the distal nephron, ion transport via increases in intracellular Ca2+ concentration ([Ca2+]i).
Basic Models for Transmission of Hydrodynamic Forces in the PT
Although it was demonstrated four decades ago (64) that the fraction of filtered fluid reabsorbed by the rat PT was nearly constant over the entire physiological range of flow (glomerulotubular balance), little attention had been paid to the mechanosensing of hydrodynamic forces by the brush border in the PT cells. Similarly, Gertz and Boylan (24) and Wilcox and Baylis (93) had written extensive reviews on glomerulotubular balance and the related perfusion-absorption balance of the key epithelial transporters at the apical and basolateral membranes, but the afferent mechanism that enabled the PT cells to sense the flow rate remained a mystery. Prior studies had considered changes in the brush-border geometry with flow (46) or solute gradients in the brush border (37), but no convincing quantitative mechanism could be developed.
While the ultrastructure and mechanosensory function of the stereocilia in the inner ear were well recognized in the 1980s (30, 71) and the fluid shear hypothesis had been proposed for the fluid flow excitation of bone cells (82), at least initially there seemed to be a much greater similarity between brush-border epithelia and ECs. In each case, the apical surface was covered by a dense porous surface layer: microvilli in the case of the PT and a glycocalyx composed of various glycoproteins in the case of ECs. For ECs, hundreds of papers had been written since the landmark paper by Dewey et al. (13) in which ECs were first grown in culture and exposed to FSS. These papers assumed that the FSS due to the flowing blood, typically 10–20 dyn/cm2, acted directly on the apical membrane, whereas in vivo experiments (75) clearly showed that G proteins and other receptor molecules were buried in a 0.4-μm-thick glycocalyx layer. Michel (49) and Weinbaum (81) proposed that it was this layer that functioned as the molecular sieve for plasma proteins, as opposed to the junctional strands, and that the classical Starling force balance should be applied locally across the glycocalyx layer, rather than between lumen and tissue, as had been widely assumed. This new conceptual model was subsequently confirmed in a series of experiments reviewed by Weinbaum et al. (84).
Early hydrodynamic models, for the brush border (3) and for the endothelial glycocalyx layer (EGL) (32, 66), clearly predicted that fluid velocities were greatly attenuated by the surface layer and nearly negligible at the level of the apical membrane. This raised a fundamental paradox: How could FSS be communicated to the cytoskeleton of either cell when the FSS on the apical membrane surface was vanishingly small? This basic paradox was first answered for PT epithelia by Guo et al. (25) and then applied to ECs by Weinbaum et al. (85) using key insights obtained from the basic model for PT epithelia. Although the basic mechanism for the transmission of FSS into the actin cytoskeleton is very similar, we shall show that the cytoskeletal response of tall columnar brush-border cells is nearly diametrically opposite that of cultured ECs subject to FSS (see Cell Culture Studies to Examine the Effect of FSS on Cytoskeletal Organization and Transporter Trafficking in PT).
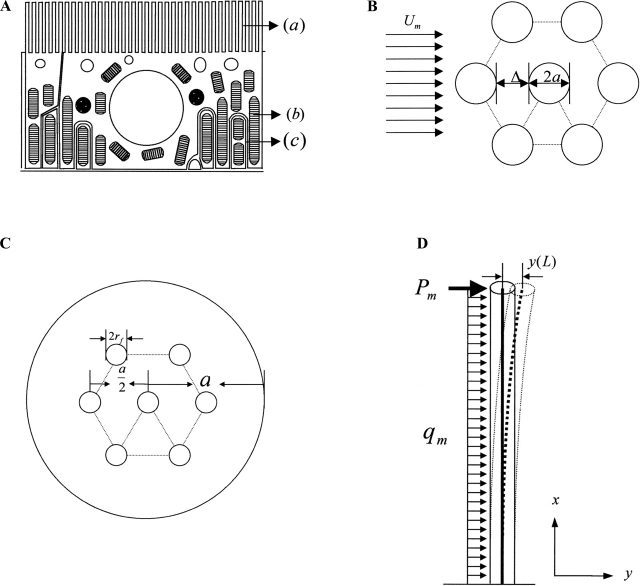
A schematic diagram of the basic ultrastructural and elastohydrodynamic model of Guo et al. (25) is shown in Fig. 1. The ultrastructural model is based on the detailed electron-microscopic observations of Maunsbach et al. (46), which show that the microvilli are remarkably similar in height (Fig. 1A) and are arranged in a highly organized hexagonal array (Fig. 1B), in which there is an organized central actin filament bundle (28, 60) involving, on average, seven central actin filaments (Fig. 1C) (45). Guo et al. (25) observed that if the sole purpose of the microvilli were to increase transport area, there would be no need for such local uniformity in microvilli height and spacing and theoretically predicted that the central actin filament bundle is sufficiently stiff to undergo only small lateral deflections when subjected to hydrodynamic loading (Fig. 1D). To support this hypothesis, Guo et al. developed a mathematical model to predict the detailed velocity profile within the brush border and an elastohydrodynamic model to predict the bending deformation of the microvilli for physiological loading conditions based on the measured flexural rigidity EI (where E is Young's modulus and I is moment of inertia) of individual actin filaments (35).
Fig. 1.
A: schematic diagram of an epithelial cell in proximal tubule (PT) S2 segment. Cells are characterized by densely spaced microvilli (a), numerous mitochondria (b), and extensive interdigitation of the lateral membrane near the basal surface (c). B: idealized model of brush border in transverse section showing hexagonal microvillus array. C: idealized structural model for the arrangement of axial F-actin filaments in microvillus cross section. The 7 F-actin filaments form a hexagonal array, in which the 6 off-axis filaments are equally spaced on a circle that is the half-radius of the cross section; a is the radius of the microvillus membrane, and rf is the radius of the actin filament. D: geometry of the deformed microvillus. Note axial deflection of its central filament. Mechanical loading is a combination of a concentrated force (Pm) acting at the tip, accounting for approximately three-fourths of the total force, and a uniform loading (qm), accounting for approximately one-fourth of the total force. [From Guo et al. (25).]
The key predictions of the foregoing model were as follows. 1) The velocity at the microvilli tips at the edge of the brush border is 1/400th the velocity at the tubule centerline. This velocity is further attenuated by another factor of 400 over a narrow transition distance of <0.2 μm at the brush-border edge and, thus, is negligible over >90% of the layer thickness. 2) The drag force (Pm) in the narrow transition region is 74% of the total drag on the microvilli, and this force contributes 86% of the total bending moment [torque (T)] on the microvillus. 3) The deflection of the microvilli tips is <4 nm for a 2.5-mm-long microvillus or <5% of the open gap of 74 nm between microvilli (46). Thus the microvilli act as stiff bristles that are exquisitely designed to transmit torque to the actin cortical web beneath the apical membrane. 4) Although the predicted force on each microvillus is very small (<0.01 pN), using a moment balance, Weinbaum et al. (83) showed a ∼40-fold amplification of the force arising from the resisting moment of the central actin filament bundle at the base of the microvilli. This amplification is sufficient to deform the anchoring filaments in the actin cortical web and initiate signaling through linker molecules such as ezrin (38). While standard transmission electron micrographs typically give microvilli the appearance of soft flexible projections roughly uniform in height (45), rapid freeze scanning electron micrographs clearly show the rigidity of these projections and their remarkable uniformity (Fig. 2) (54).
Fig. 2.
Freeze-fracture appearance of the apical pole of a PT epithelial cell with its brush border. [Adapted from Orci et al. (54); reprinted with permission from Elsevier.]
Maunsbach et al. (46) were the first to report that increases in luminal fluid flow in PTs perfused in situ were accompanied by changes in microvillus geometry associated with increases in tubule diameter. The open gap in Fig. 1C increased from 62.1 to 90.4 nm when the tubule flow was increased from 5 to 45 nl/min. More recently, Du et al. (14) found that when perfusion rate (Q) in isolated mouse PT was increased from 5 to 25 nl/min, there was a nonlinear response with a 50% increase in diameter between the lowest and the highest perfusion rate. These increases in microvilli spacing and tubule diameter change the FSS at the edge of the brush border and the forces Pm and qm in Fig. 1D. Using the insights of Guo et al. (25), Du et al. (14) developed a simplified local control volume analysis in which one could relate torque at any perfusion rate and tubule radius (R) to a low flow reference state (Qr) as follows
| (1) |
where the subscript r denotes the low-flow reference state, L is the length of the microvilli, and δ is the thickness of the narrow transition layer at the brush-border edge, where the force Pm is applied. Typical values are as follows: R = 15 μm, L = 2.5 μm, and δ = 150 nm. For these values, one can show that the simpler approximation to Eq. 1, T/Tr = (Rr/R)2(Q/Qr), is accurate to 6% for diameter changes with increased flow of up to 50%. Since volume reabsorption (Jv) at any perfusion rate can be related to its measured value at the low-flow reference state r, Eq. 1 or its simpler approximation can be used to predict the change in T as Q and R increase with flow.
The experiments of Du et al. (14) described below (see In Vitro Experimental Verification of Flow-Induced Bending Moment Hypotheses in PT: Na+, HCO3−, and Water Reabsorption in the PT) show that Jv (equivalently Na+ reabsorption) and HCO3− reabsorption (JHCO3) in the PT scale in direct proportion to the bending moment on the microvilli in response to axial flow and that this direct proportionality is obeyed only if the changes in diameter with axial flow described by Eq. 1 are taken into account. Modulation of PT apical Na+/H+ exchanger (NHE3) and H+-ATPase activities is thus directly related to changing microvillus bending moments, and the stiff microvilli serve as the afferent sensor, at least for NHE3. Recent experiments on cultured PT cells suggest that H+-ATPase is associated with microtubules, as opposed to the actin cytoskeleton (Y. Duan, unpublished observations).
Weinbaum et al. (83) also presented a comparative analysis of the ultrastructural model for PT microvilli and other cells with central actin filament bundles, where mechanotransduction of fluid flow is widely recognized to initiate intracellular signaling. Stereocilia on hair cells (26, 71) and the dendritic processes of osteocytes (27, 69, 99) have a central actin filament bundle with the same type of cross-linking that is proposed for the PT brush-border microvilli in Fig. 1C. In stereocilia and osteocytes, the central actin filaments are linked by 12-nm fimbrin cross-filaments that rotate 60° counterclockwise and advance 12.5 nm axially between cross-link attachments (12). This central bundle is encircled by a double helix of brush-border myosin I similar to the microvilli in the small intestine (22). In the case of stereocilia, Tilney and Tilney (71) showed that the individual actin filaments are able to slide relative to one another, enabling the stereocilia to undergo bending rotation about a tapered ankle at its point of insertion into the cuticular matrix at its base, creating tension in the tip links at the apex of the stereocilia. It is not known whether there are lateral links between the microvilli in the PT. In the case of osteocyte cell processes, there are recently discovered integrin attachments to the canalicular wall (47, 80), wherein the tethered cell processes are able to slide relative to the fixed integrin attachments, creating cell-level membrane strains that are two orders of magnitude larger than the very tiny whole tissue strains in bone due to locomotion. In both cases, strain in the membrane created by fluid flow is able to open stretch-activated ion channels, initiating intracellular biochemical responses, a behavior that appears to be similar to the initial entry of extracellular Ca2+ resulting from the bending of primary cilia in the distal nephron and the activation of apical Ca2+-dependent K+ channels (see Mechanoinduced [Ca2+]i Responses of the CCD).
In Vitro Experimental Verification of Flow-Induced Bending Moment Hypotheses in PT: Na+, HCO3−, and Water Reabsorption in the PT
There has been a long-standing debate as to why the glomerulotubular balance convincingly demonstrated by Schnermann et al. (64) in vivo could not also be demonstrated in vitro in single perfused tubules. For four decades, the classical experiments of Burg and Orloff (8) on isolated rabbit tubules had been used as a counterexample to show that flow-activated Na+ and fluid absorption appeared to be insignificant in vitro, at least in rabbit tubules.
The development of an in vitro mouse model (14, 16) and the torque-dependent axial flow hypothesis (25) have allowed for a major reassessment of the experiments of Burg and Orloff (8). Du et al. (14, 16) measured Na+ (fluid) and HCO3− reabsorption in isolated mouse PTs in response to a fivefold increase in perfusion rates. Experimental data show a doubling of Na+ and HCO3− reabsorption in PTs isolated from mouse kidney, in contrast to the earlier experiments with rabbit PTs (8). Since the PTs were isolated, this was clear evidence that flow-activated Na+ and HCO3− absorption can occur in the absence of peritubular Starling forces, neuronal control, and systemic hormonal regulation, factors that can be altered by increased glomerular filtration rate in vivo. Du et al. (16) also showed that the doubling of Na+ and HCO3− reabsorption occurs without a change in HCO3− permeability and that the flow-induced changes in HCO3− concentration in the PT fluid and increase in the albumin concentration on the basolateral side of the tubule cannot account for the increment of Na+ and HCO3− absorption by axial flow. Experimental data support the view that axial flow directly stimulates NHE3- and H+-ATPase-mediated Na+ and HCO3− absorption in PTs, since flow-stimulated fluid (i.e., Na+) and HCO3− reabsorption were significantly diminished in NHE3 knockout mice and were significantly inhibited by blockers for NHE3 and H+-ATPase (16).
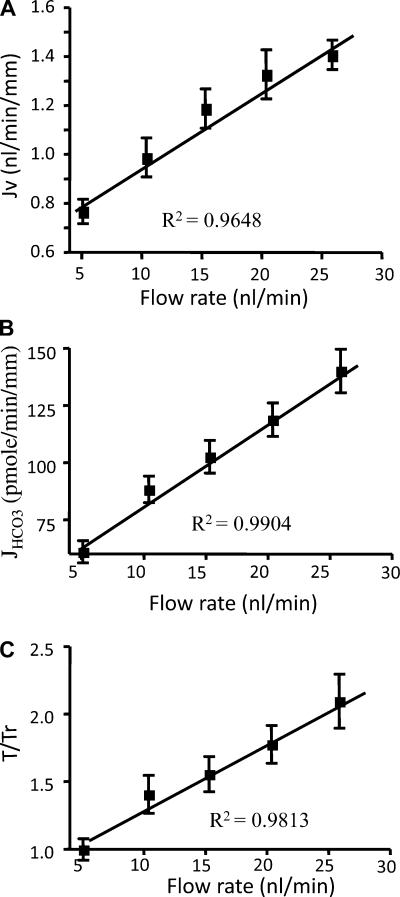
The motivation for the foregoing experiments was to test the hypothesis of Guo et al. (25) that the brush-border microvilli serve as the mechanosensors of axial flow along the PT. According to this hypothesis (see Basic Models for Transmission of Hydrodynamic Forces in the PT), the drag force on each microvillus produces a bending moment on an actin filament bundle (Fig. 1) that is transmitted to the underlying cortical cytoskeleton. In this regard, an important observation in Fig. 3, A and B, is that a fivefold increase in axial flow leads to only a twofold increase in fluid and HCO3− reabsorption. This striking nonlinear behavior is accurately predicted by the torque (bending moment) formula given by Eq. 1 or its simplified form [T/Tr = (Rr/R)2(Q/Qr)]. This formula, derived by Du et al. (14), shows that the bending moment depends inversely on the square of the tubule diameter or radius and that, for a compliant tubule, this could produce a significant blunting of the axial flow effect on reabsorption. In fact, careful measurements of tubule diameter with flow in Fig. 4A show a ∼50% increase in diameter when the perfusion rate in the mouse tubule is increased fivefold. When the diameter changes with Q are applied in Eq. 1, there is a nearly exact twofold increase in bending moment for a fivefold increase in Q and, thus, Na+ and HCO3− reabsorption are directly proportional to the bending moment on the microvilli. In the classical in vivo experiment of Schnermann et al. (64), the fractional Na+ absorption was constant when flow rate increased by a factor of 5, but the transport activity only increased by a factor of 2 in vitro. In vivo, it is less likely that an increase in glomerular filtration rate would lead to significant increases in tubule diameter. Additional data supporting the notion that brush-border microvilli function as a flow sensor are as follows: increased luminal fluid viscosity increased Na+ reabsorption at a constant perfusion rate, and disruption of the cytoskeleton with cytochalasin D eliminated the perfusion-dependent increase in Na+ and HCO3− reabsorption (16).
Fig. 3.
A and B: torque-dependent fluid (Na+) and HCO3− absorption (Jv and JHCO3−). C: torque ratio. Note that JV and JHCO3− scale linearly with T/Tr as flow rate increases. [From Du et al. (14, 16).]
Fig. 4.
Flow-induced changes in PT transport. A: inner diameter change with increase in axial flow for mouse and rabbit. B and C: torque ratio and reabsorption in mouse and rabbit PTs for 3-fold change in axial flow. [Data from Du et al. (14) and Burg and Orloff (8).]
The bending moment hypothesis of Guo et al. (25) can also be used to reexamine the widely cited in vitro experiments of Burg and Orloff (8) on rabbit PTs. The absent effect of flow on proximal Na+ reabsorption was reconsidered taking into account the changes in tubule diameter with flow shown in Fig. 4A. Note that the rabbit tubule is more compliant than the mouse tubule and that, for the experiment of Burg and Orloff, where flow is increased threefold from 6 to 18 nl/min, the increase in tubule inner diameter was 41% compared with only 30% for the mouse. If these increases in tubule diameter are inserted into our torque formula (Eq. 1), there is a 38% increase in microvillus bending moment for the rabbit but a 61% increase for the mouse for this threefold increase in flow (Fig. 4B). Even more remarkably, the measured change in Na+ reabsorption over this range was 37% for the rabbit and 60% for the mouse, in nearly perfect agreement with the predicted change in microvillus torque (Fig. 4C). It appears that rabbit PTs were more distensible than mouse PTs (Fig. 4A), so that perfusion-dependent changes in luminal diameter precluded large deviations in microvillus torque (14, 77) and, hence, Na+ reabsorption.
The renal primary cilium is a sensory organelle that can sense fluid flow and initiate Ca2+-based signaling for the maintenance of normal epithelial function and developmental processes. The importance of the renal cilium is highlighted by the fact that mutations of cilia-associated proteins lead to epithelial dedifferentiation and overproliferation in PKD (23, 74). It is still an open question whether primary cilia can serve as mechanosensors in flow-activated Na+ and HCO3− reabsorption in PTs. However, it has been shown that increased [Ca2+]i by exposure to the Ca2+ ionophore A23187 reduced Na+ and HCO3− absorption in the PT, and a calmodulin inhibitor (W-7) stimulated PT transport (79). Given the fact that flow stimulates the primary cilia and increases Ca2+ influx and mobilization in PT cells (57), increased flow would lead to reduced Na+ and HCO3− reabsorption, in contradiction to the observed flow-activated PT transport. Therefore, it is unlikely that primary cilia play a primary role in flow-activated PT transport, even though these cilia can protrude above the brush-border microvilli (14, 77).
It is important to investigate the downstream mechanism and the messenger of the flow signal. Signaling molecules, such as ANG II and nitric oxide, are important regulators of PT Na+ and HCO3− transport (15, 78). Fluid movement induces angiotensin (AT1) receptor externalization from condensed apical recycling endosomes in cultured mouse PT cells, indicating that fluid flow is important for directing AT1 receptors to the apical surface and allowing them to interact with ANG II to produce cellular signals (36). Whether ANG II plays a role in flow-activated PT transport has not been reported. Bobulescu et al. (6) and Hu et al. (31) demonstrated that dopamine stimulates NHE3 endocytosis via a PKC-mediated mechanism in opossum kidney cells. They reported that dopamine acutely decreases surface NHE3 expression in a dose- and time-dependent fashion without altering total cellular NHE3. This effect, which is dependent on dopamine (DA1 and DA2) receptors and DA receptor-stimulated NHE3 endocytosis, can be blocked by PKA inhibition or by mutation of two PKA target serines (Ser560 and Ser613) on NHE3 (6, 31). Dopamine decreased Na+ reabsorption only in PTs in which transport had been stimulated by norepinephrine (4). Our preliminary data show that dopamine inhibits flow-stimulated Na+ and HCO3− absorption in mouse PTs, suggesting that recruitment of NHE3 to the apical membrane is critical for flow-stimulated Na+ and HCO3− absorption (17).
Cell Culture Studies to Examine the Effect of FSS on Cytoskeletal Organization and Transporter Trafficking in PT
There are numerous studies on the regulation of NHE3-mediated Na+ and HCO3− transport in cultured PT cells. These studies are less technically challenging than those performed in perfused tubules typically isolated from rodent kidneys and, in addition, allow for careful control of FSS. The effect of FSS on cytoskeletal organization and junctional protein regulation has been studied extensively in cultured ECs (10). Thi et al. (70) showed that an intact EGL is essential for the transmission of FSS to the intracellular cytoskeleton and that if the EGL is compromised, actin stress fibers will not form, nor will there be a disruption of junctional protein complexes. This is explained in terms of a “bumper-car” model for the dense peripheral actin band (DPAB) that is associated with the adherens junction (AJ) under static conditions. Exposure of the cells to FSS for 5 h resulted in dramatic redistribution of F-actin and various junction-associated proteins, including vinculin, ZO-1, and connexin 43, in ECs, provided the EGL was intact, but little effect on paxillin, a basal focal adhesion protein, strongly suggesting that the EGL served as the apical flow sensor in ECs.
Mouse PT cells provided a useful tool for studying flow-induced changes on cytoskeletal organization and transporter trafficking for several reasons. The immortalized mouse PT cell line BUMPT-306 expresses the Na+-glucose transporter megalin and has structural and functional features of a homogenous population of differentiated PT cells (68). To elucidate the mechanism by which luminal flow modulates ion transport in renal tubules, cell culture studies have been performed using murine PT cells. These epithelial cells, when cultured under static conditions, demonstrated numerous pronounced cytosolic actin stress fibers on their basal membrane, which may relate to the dedifferentiation of the cells (7). Subsequently, Essig et al. (19) reported that, after the onset of laminar FSS, PT cells underwent phenotypic changes, including a disappearance of stress fibers from the basal surface and a reinforcement of the lateral actin network. More recently, Duan et al. (18) demonstrated that a FSS of only 1 dyn/cm2 led to formation of tight junctions and AJs and an accumulation of focal adhesion proteins (vinculin and paxillin) in the basement membrane. These findings are surprisingly opposite to that observed for confluent EC monolayers, where the major event is creation of stress fibers, disruption of junctions, and dispersion of focal adhesion proteins (70), as noted above. In addition, ECs require a 10-fold higher FSS than that applied on epithelial cells to induce cytoskeletal changes.
To explain the foregoing discrepancies, Duan et al. (18) proposed a “junction-buttressing” model for the PT cells shown in Fig. 5. In static control cells, strong expression of stress fibers on the basement membrane leads to a firm adhesion of the cells to their substrate. This creates a tension on the cell lateral membrane accompanied by a compressive resistance of the internal cytoskeleton. This results in a rounded canopy at the apical membrane and the pulling away of the cell from its neighbors. Cell junctions cannot form until the stress fibers are disrupted at the basal surface and this membrane tension is released. A small FSS applied to the apical surface was able to create enough rotational moment at the cell base to cause these tall cuboidal cells to tilt, their basolateral surfaces to come into contact, and tight junctions, AJs, and a DPAB form (Fig. 5B). In comparison, for endothelial cells, Thi et al. (70) proposed that the DPAB, with its vascular endothelial-cadherin bonds, functions like a “rubber bumper” in the static control condition. Unless the rotational moment applied on the EGL at the apical surface of the ECs exceeds the strength of the opposing moment provided by the DPAB with its AJ, cytoskeletal reorganization will not take place. These results indicate that the brush-border microvilli on the apical surface of the epithelial cells play the same role as the EGL, in that the fluid drag on these apical structures produces a rotational moment on the cell in response to FSS. The large difference in effective lever arm provided by the tall cuboidal epithelial cells compared with the flat ECs is the main reason that a FSS of only 1/10th of that applied on the ECs is necessary for epithelial cells to undergo cytoskeletal reorganization.
Fig. 5.
Conceptual “junctional buttressing” model for cytoskeleton reorganization and junctional formation of mouse PT cells in response to fluid shear stress (FSS). A: under static condition, there are neither adherens junctions (AJs) nor tight junctions (TJs). Cells express numerous cytosolic stress fibers at their base. B: after exposure to FSS, stress fibers disappear from cell base, and AJs, TJs, and dense peripheral actin bands (DPAB) form. [From Duan et al. (18); copyright (2008) National Academy of Sciences.]
The three potential mechanosensors of FSS in PT cells are as follows: microvilli, primary cilia, and glycocalyx. Scanning electron microscopy and confocal microscopy have been used to examine whether these structures are present on the surfaces of cultured mouse PT cells. Scanning electron microscopy reveals a denser array of microvilli on the apical cell surface in mouse than in human primary cultured PT cells (1). Immunofluorescence with anti-tubulin staining showed that primary cilia are completely absent in murine cells at 5 days of culture but appear when cells are cultured for >8 days. Since experiments were done in PT cells that were cultured for only 5 days, the possibility that primary cilia could contribute to FSS-induced cytoskeletal reorganization can be ruled out (18). Whether the glycocalyx plays a sensor role in regulating mechanotransduction in PT cells was examined after the addition of heparinase III to digest the glycocalyx. Experimental data show that heparinase III had no effect on FSS-induced changes in cytoskeletal reorganization and junctional formation, supporting the view that microvilli are the flow sensor in PT cells (18).
It would be important to know if FSS induces ion transporters, NHE3, H+-ATPase, and Na+-K+-ATPase trafficking, and expression that accompanies changes of Na+ and HCO3− absorption. It is our hope that these ongoing studies in our laboratory will soon provide new information about flow-induced PT transport and regulation.
Transport Models for the PT That Incorporate Flow-Induced Torque
Glomerulotubular balance or perfusion-absorption balance is a characteristic of the intact PT. It is natural, therefore, to try to define the cellular machinery required to achieve this overall transport response. Guo et al. (25) (see Basic Models for Transmission of Hydrodynamic Forces in the PT) proposed that the brush-border microvilli are the flow sensors and that they transmit a signal to the actin cytoskeleton. The effectors of the response comprise the ensemble of membrane transporters, the activity of which is modulated with flow. At the time they proposed their hypothesis, Guo et al. cited observations of Maddox et al. (43) and Preisig (59), who reported increases in luminal membrane NHE3 transporter density in response to increases in luminal flow rate; Preisig also presented evidence that activity of the peritubular Na+-3HCO3− cotransporter also increased. Additional background to this issue includes the observation that when proximal Na+ reabsorption was driven by coupled transport of organics, there was a coordinate increase in peritubular K+ permeability (5, 48). It remains unknown whether modulation of peritubular K+ permeability is also a feature of perfusion-absorption balance, although the ATP dependence of peritubular K+ channels, identified by Tsuchiya et al. (73), would suggest that it is. To identify a plausible transporter ensemble underlying flow- and torque-dependent transport, Weinstein et al. (89) employed a mathematical model of the PT. Their first observation was that an isolated increase in luminal membrane NHE3 activity or increases in all the luminal membrane transporters would not be particularly effective in modulating epithelial Na+ reabsorption (Fig. 6A). Furthermore, luminal-only increases in transporter density would produce large (and likely lethal) increases in cell volume. In contrast, coordinated modulation of luminal and peritubular transporters produced coordinate increases in Na+ reabsorption, with negligible changes in cell volume and composition (Fig. 6B). Weinstein et al. then examined model performance when model transporter density (Par) was linearly proportional to microvillous torque, according to the formula
| (2) |
where TM and TM0 are the computed microvillous torque and reference torque and TS is the uniform torque sensitivity for modulation of luminal and peritubular membrane transporter density. With this formulation, in a compliant PT, TS = 1.6 yielded nearly perfect perfusion-absorption balance over a fivefold range of luminal perfusion rates, from 15 to 75 nl/min (Fig. 7).
Fig. 6.
A and B: impact of scaling PT transporter density on transepithelial Na+ flux and cell volume. Baseline bath conditions and the open-circuited epithelial model were used to calculate variation of transporter density: luminal membrane Na+/H+ exchanger (NHE3) alone, all luminal membrane transporters, or coordinate scaling of luminal plus peritubular transporters. [From Weinstein et al. (89).]
Fig. 7.
Impact of torque-modulated transport on fractional proximal reabsorption. Inlet conditions vary from 15 to 75 nl/min. PT model is solved iteratively, so that outlet pressure is exactly 9 mmHg for all perfusion rates. For the compliant tubule (3%/mmHg), there is torque-dependent modulation of luminal plus peritubular transporter density, with a scaling coefficient of 2.2, 1.6, or 1.0. For the noncompliant tubule, axial pressure drop (early proximal to late proximal) ranges from 3 to 24 mmHg; in the compliant case (torque scale = 2.2), axial pressure drops 5–15 mmHg. [From Weinstein et al. (89).]
These considerations underscore the connection between modulating transepithelial Na+ reabsorption and preserving cellular volume and composition during flux variation (cellular homeostasis). Weinstein et al. (89) utilized gross one-to-one scaling of all luminal and peritubular transporters. Weinstein (86) addressed the issue of identifying critical transporters for cell volume homeostasis in the PT. At the core of that study was the observation that, for steady-state calculations, over a physiological range of transporter perturbations, the performance of the model and its linearization were nearly identical. This allowed for an efficient and systematic exploration of parameter dependence on cell variables to determine effective parameter control. The key finding was that, for a number of perturbations of cell volume (increased glucose entry, increased Na+/H+ exchange, peritubular K+ or HCO3− increase, or hypotonic shock), volume-dependent activation of peritubular K+-Cl− or Na+-HCO3− cotransport would protect cell volume and promote epithelial Na+ reabsorption. A subsequent study documented the essential linearity of the time-dependent PT cell model, and this allowed application of linear control theory to identify an optimal ensemble of transporters along with their parameter dependence (87). This approach allowed for specification of a cost function, such as rapidity of cell volume recovery. For this cost, cell volume and peritubular potential difference figured prominently as control variables, but not all the findings were intuitive. For example, cell volume acted to increase peritubular K+-Cl− cotransport and to blunt luminal entry steps but to decrease peritubular Na+-HCO3− activity. On reflection, the selected peritubular configuration allowed cell volume recovery with slower cytosolic acidification to avoid acid-driven increase of luminal NHE3.
Ultimately, linearization of the PT model as a dynamical system allowed for formulation of a tracking problem, which captured the essentials of flow-dependent reabsorption (88). In this approach, PT linearization yields a singular system of the form
| (3) |
where u is the vector of physiological model variables and u′ is its time derivative and p is the vector of model parameters (transporter densities), all considered deviations from a reference condition; S, R, and Q are matrices. Because the matrix S is singular, with more than half of the rows zero, corresponding to the algebraic equations of the model, this problem has a degree of difficulty in applying linear control theory. In the problem considered, the cost function was embodied in a matrix M, for which the product Mu identified cell volume, cell HCO3−, and luminal membrane Na+ flux. Given this cost function, an optimal feedback control matrix F is first identified by solution of an algebraic Riccati equation, so that p(t) = Fu(t) provides the feedback component of model parameters. Then a tracking problem is formulated in which r(t) = [0,0,v(t)] is the vector that states that we seek a function in which cell volume and pH have zero deflection, while luminal membrane Na+ flux follows a prescribed function of time v(t). Specifically, we seek to minimize the error e(t)
| (4) |
The solution to the problem provides a second term to the parameter function, p(t), which is an autonomous (feedforward) term driven by the variation in luminal fluid flow rate. In the problem that was formulated, luminal flow was prescribed as a ramp pattern, doubling linearly over 50 s and then declining back to baseline. Once this problem was solved for the linear model, the parameter function (feedback terms and autonomous terms) could be incorporated into the full nonlinear PT model to see how effective they would be where it counts. Those calculations are shown in Fig. 8, in which the model achieves more than twofold changes in Na+ flux, with only trivial changes in cell volume or HCO3− concentration. Figure 8 shows the fractional changes of nine key model parameters over time for midproximal luminal fluid. These calculations demonstrate that the model makes substantial use of feedforward increases in luminal glucose entry and NHE3 activity and activation of peritubular transporters K+-Cl− and Na+-HCO3− and the Na+-K+-ATPase. There is little use of luminal membrane anion transport or peritubular K+ permeability in the optimal solution. Translating these model results into biological terms, the model predicts that the luminal membrane signal (from microvilli) will be transmitted to peritubular transporters (perhaps via the actin cytoskeleton) and that transporter densities will change in a manner that may not be predicted from feedback-dependent considerations.
Fig. 8.
Parameter deviations during tracking in the full proximal tubule model. Using mid-proximal fluid (glucose- and HCO3−-depleted), the fractional changes (deviations relative to baseline) in 9 of the transporter densities are shown. [HIE(K+) is peritubular K+ permeability.] The total deviation (T) is the sum of an autonomous term (A), imported directly from the dynamical system, plus a feedback term (F), computed from the reduced optimal feedback matrix and the trajectory point. [From Weinstein and Sontag (88); reprinted with permission from Springer.]
Basic Model for Transmission of Hydrodynamic Forces in the CCD
Primary cilia are single, immotile organelles with a 9 + 0 microtubule structure derived from a centrosome and are present in nearly all vertebrate cells (91), a notable exception being the intercalated cells of the distal nephron, albeit this is debatable (41). These cilia are missing a central microtubule pair and its associated dynein motors and regulatory proteins. The growth of the cilia requires intraciliary or intraflagellar transport from the cell's mother centriole. This construction and the various functions that have been ascribed to primary cilia, such as left-right asymmetry in nodal flow (53), are summarized by Praetorius and Spring (57). Primary cilia have also been studied in bone (44, 92, 96) and cartilage (34). However, by far the greatest interest in primary cilia has focused on their role in renal epithelial cells in health and disease (PKD), a picture that emerged from the study of cells in culture and in isolated tubules perfused in their native geometry. This review focuses on the Ca2+ response of principal and adjacent intercalated cells comprising the distal nephron to mechanical forces induced by variations in luminal flow rate and stretch in normal tubules and tubules from models of PKD.
The role of the primary cilium as a mechanotransducer was first suggested in 1997 by Schwartz et al. (65), who noticed that the primary cilia in cultured rat kangaroo kidney epithelial (PtKt) cells would undergo large bending deformations with exposure to increasing superfusate FSS. This bending deformation was described by “heavy elastica” theory for a cantilever beam using an empirical relation for flow past a circular cylinder. The first demonstration that cilia played a key role in Ca2+ signaling and extracellular Ca2+-dependent intracellular Ca2+ release was the pioneering experiment of Praetorius and Spring (57). They demonstrated, by application of negative pressure locally and direct mechanical manipulation, that bending of the MDCK cilium, rather than FSS acting on the apical membrane of cultured epithelial cells, as is the case for ECs, leads to an increase in [Ca2+]i due to Ca2+ influx through mechanosensitive channels residing in the cilium or its base. This was further confirmed by additional experiments in which removal of the primary cilia in MDCK cells by pretreatment with chloral hydrate abrogated the Ca2+ response (58). A more sophisticated theoretical model was then developed by Liu et al. (41); in their model, hydrodynamic drag forces on a hexagonal array of cilia were quantitatively evaluated and used to interpret experiments performed in isolated microperfused New Zealand White rabbit CCDs subjected to flow and stretch. These models (see below) made it possible to compare the response of cultured cells and native perfused tubules to FSS.
The primary purpose of the heavy elastica model (65) was to determine the bending rigidity (EI) of the primary cilium and its change in shape with flow. A basic question in comparing the effects of flow superfusing cultured cells in a flow chamber with the effects of flow within the lumen of a microperfused CCD is as follows: Is the average velocity of the flow in the chamber matched with the average velocity of the flow in the CCD or with the FSS acting on the cilia tips? In the studies of Praetorius and Spring (57) and Liu et al. (41), the former was matched; even at the highest flow rate, Liu et al. reported that the FSS at the cilia tips was only about one-fifth of that in the perfused CCD. A second question is as follows: How are the effects of FSS on the cilia separated from the effects of FSS on the apical membrane and, in the case of the tubule, the effect of stretch caused by flow? Woda et al. (94) observed that an increase in flow in the CCD microperfused in vitro produced a 20% increase in diameter. Liu et al. tried to isolate the effects of flow and stretch and the Ca2+ response in three experiments: 1) flow in microperfused intact CCD in vitro, 2) flow in a split-open CCD affixed to the bottom of an open flow chamber, and 3) flow in an occluded microperfused tubule. To help dissect this behavior, idealized models for the split-open and intact CCD flow geometries were considered (Fig. 9, A and B). A more detailed model of the primary cilia ultrastructure was also constructed to enable predictions of the actual forces and moments on the microtubule pairs in the ciliary axoneme (Fig. 9C). The models for the flow and deformation of the cilia (Fig. 9, A and B) considered the flow interaction between the individual cilia but neglected the presence of intercalated cells, given that they account for <25% of the cell population in this segment in this species (62). The theoretical model could separately estimate the fluid shear force acting on the apical surface and the drag and bending moments on the cilia, including the forces on the individual microtubule pairs in Fig. 9C, where a simplified symmetrical eight-microtubule-pair approximation was used.
Fig. 9.
Theoretical models for split-open cortical collecting duct (CCD) showing, in an en face view, hexagonal cell geometry and cell dimensions (A) and a perfused tubule showing arrangement of primary cilia at the tubule wall (B). Solid and dotted lines represent cilia in different planes. C: simplified mathematical model of microtubule distribution inside a primary cilium. Microtubules, which terminate in the transition zone at the top of the basal body, transfer the torque on the axoneme to the centriole in the basal body. Because of the stiffness of the microtubules in the primary cilium and its supporting structure, a 2.5-μm cilium bends only slightly because of the drag forces exerted by the fluid flow. U, average velocity. B: cross section of the cilium. The “9 + 0” microtubule structure of primary cilium is reduced to 8 symmetrically arranged microtubule doublets. The model is used to predict tensile and compressive forces at the base of each microtubule pair when a torque is applied about an axis passing through microtubules pairs 1 and 5. [From Liu et al. (41).]
The key results of this analysis are summarized in Table 1. The height of the cilia in the native CCD was 2.5 μm, but predictions are also shown for a confluent monolayer of MDCK cells with 8-μm cilia placed in the same flow chamber for comparison with the experiments of Praetorius and Spring (57) and Rydholm et al. (61). Two flow rates [high (25 μl/s) and low (3.2 μl/s)] were applied to split-open CCD cells in the study of Liu et al. (41). 1) Cilia tip velocities at the high flow rate were only one-sixth of those predicted for the perfused tubule, and the FSS was only 0.056 dyn/cm2, a value 10-fold lower than that experienced by the perfused tubule. For ECs, such low FSS would not be expected to produce intracellular signaling (2), suggesting that, in the intact and split-open tubule, the [Ca2+]i response is due primarily to bending deformation of the cilia, although the drag on the cilium is only about one-eighth of the total FSS drag on the apical membrane of the epithelial cell in the split-open and perfused tubules. 2) The tip deflection of a 2.5-μm cilium is extremely small, 1.9 nm for the split-open tubule and 13 nm for the perfused CCD, but increased 100-fold for the long (8-μm) cilia found on MDCK cells. These predictions, like those described by Schwartz et al. (65), assume a rigid cantilever boundary condition at the insertion of the cilium into the basal body in Fig. 1C and no rotation of the basal body inside the cell. This assumption is confirmed by the side-view images of bent cilia in MDCK cells (61). These images show that the FSS on a long cilium causes the ciliary axoneme to bend near its insertion into its basal body but that the basal body is firmly attached to the intracellular cytoskeleton and does not swivel inside the cell at physiological values of FSS up to 0.59 dyn/cm2, values comparable to a perfused tubule in Table 1. Thus the deflections in Table 1 reflect the bending of the ciliary axoneme, not the rotation of its basal body.
Table 1.
Calculated results of theoretical models for split-open and perfused CCDs
| Chamber With Split-Open Tubule | Perfused Tubule (25 μm ID) | |||
|---|---|---|---|---|
| Cilium length, μm | 2.5 (short) | 8.0 (long) | 2.5 | |
| Flow rate, μl/s | 3.2 (slow) | 25 (fast) | 25 (fast) | 5* |
| Cilium diameter, μm | 0.2 | 0.2 | 0.2 | 0.2 |
| Tip velocity, μm/s | 2.27 | 17.9 | 37.4 | 119 |
| Wall shear stress, dyn/cm2 | 0.0070 | 0.056 | 0.021 | 0.52 |
| Shear drag/cell, pN | 0.078 | 0.62 | 0.23 | 5.8 |
| Drag on cilium, pN | 0.010 | 0.078 | 0.43 | 0.56 |
| Torque on cilium, pN·μm | 0.017 | 0.13 | 2.44 | 0.91 |
| Tip deflection, nm | 0.23 | 1.9 | 227 | 13 |
| Force on microtubule pair 3, pN | 0.114 | 0.86 | 16.3 | 6.06 |
CCDs, cortical collecting ducts. Results for 8-μm cilia are predictions for a confluent monolayer of Madin-Darby canine kidney cells if they were exposed to shear in the same flow chamber as that used in Liu et al. (41). Surface area of the cell is 111.3 μm2.
Units are nl/min. [Adapted from Liu et al. (41).]
Rydholm et al. (61) also developed a finite-element model (FEM) in which the ciliary axoneme is covered by a 10-nm membrane and inserted into an apical membrane with the same viscoelastic properties, but the central axoneme is simplified: it is treated as a uniform axial beam, in contrast to the detailed microtubule structure shown in Fig. 9C. This viscoelastic model is used to predict the distribution of membrane stresses in the apical and cilium membranes and also to explain the delay of the [Ca2+]i response observed by Liu et al (41) and Praetorius and Spring (57) (see Mechanoinduced [Ca2+]i Responses of the CCD). The model shows that the maximum membrane stresses are developed in the vicinity of the insertion of the axoneme into the basal body in the apical and cilium membranes, suggesting that Ca2+ entry occurs in this region. This behavior can be explained using the microtubule model shown in Fig. 9C. As shown in Table 1, the drag and torque on the cilium are very small, 0.43 pN and 2.5 pN·μm, respectively, even for the long (8-μm) cilium at the high flow rate in vitro (41). However, the drag force at the base of the cilium is greatly amplified because of the long moment arm, and, in microtubule pair 3, the pair farthest from the cilium axis, force on the basal body is 16 pN. Even in the much shorter (2.5-μm) cilium in the perfused tubule, force on the outer microtubule pair is 6 pN (Table 1), which is large enough to deform the local actin network supporting the membrane. These results suggest that the concentrated forces acting at the point of insertion of the microtubules into the basal body elicit the Ca2+ response, and not the bending per se, of the ciliary axoneme.
Mechanoinduced [Ca2+]i Responses of the CCD
The native CCD consists of two morphologically and functionally distinct cell types. Principal cells reabsorb Na+ and water (in the presence of vasopressin) and secrete K+, whereas intercalated cells function in acid-base homeostasis to transport H+ and HCO3− and can, under certain conditions, absorb K+. As described above, principal cells possess a central apical cilium, whereas intercalated cells, generally considered to be devoid of cilia in their fully differentiated state, are decorated with abundant apical microvilli and microplicae (39, 41, 94). There is no evidence of gap junctional communication between these two cell types (41).
An acute increase in luminal flow rate in the CCD microdissected from the kidney of the New Zealand White rabbit and microperfused in vitro in its cylindrical native geometry, which is sufficient to lead to a 5–20% increase in tubular diameter within 1 s, leads to a biphasic increase in global [Ca2+]i in principal and intercalated cells (39, 41, 94). Specifically, in the fully differentiated CCD, [Ca2+]i rapidly increases from ∼100 to 350 nM within 11 s of an increase in luminal flow rate and is followed by a gradual decay to a plateau value, which remains elevated above baseline for ≥20 min during a period of sustained high flow (39, 41, 94). This mechanoinduced response is developmentally regulated: the peak [Ca2+]i elicited by high flow is significantly less in principal and intercalated cells in CCDs isolated from 1-wk-old newborn than older animals (40, 95). The modest response of the neonatal principal cell to flow cannot be explained by the absence of an apical central cilium, as cilia are longer at birth than in adulthood, and their length decreases with advancing age (20, 40). Alternative explanations are as follows: expression of conducting Ca2+ channels and inositol trisphosphate (IP3) receptors are developmentally regulated, and/or IP3 receptors and Ca2+ channels are not fully assembled to allow IP3-assisted Ca2+-induced Ca2+ release in neonatal CCDs, as has been reported in developing neurons (72, 97).
To determine whether flow across the apical membrane and/or circumferential stretch mediates the flow-induced [Ca2+]i transient in the CCD, fura 2-loaded tubules were studied in the three configurations by Liu et al. (41) (see Basic Model for Transmission of Hydrodynamic Forces in the CCD): 1) microperfused in vitro in their native geometry (and, thus, subject to flow and stretch), 2) split open to expose the luminal surface to superfusate flow in the absence of stretch (to isolate the effect of flow across the cilium), or 3) occluded (to examine the effects of circumferential stretch and the duration/magnitude of the flow impulse). Analysis of the responses of individual cells in each of the three preparations to flow/stretch revealed significant differences in the temporal delay to and amplitude of the [Ca2+]i transient, suggesting that distinct mechanical triggers and/or signaling pathways underlie the response. The temporal delay can, in part, be explained by the viscoelastic FEM in Rydholm et al. (61) (see below). We confirmed that, in perfused tubules subject to a rapid increase in tubular fluid flow rate sufficient to increase tubular diameter by 20%, [Ca2+]i increased threefold in principal and intercalated cells (Fig. 10A). This [Ca2+]i transient was completely inhibited by thapsigargin, an irreversible inhibitor of the endoplasmic reticulum Ca2+-ATPase that prevents refilling of intracellular Ca2+ pools and leads to depletion of internal stores, or 2-aminoethoxydiphenyl borate, a cell-permeant inhibitor of the IP3 receptor (42, 56). These observations imply that IP3-mediated release of endoplasmic reticulum Ca2+ stores is required for the flow/stretch response.
Fig. 10.
Representative traces of intracellular Ca2+ concentration ([Ca2+]i) responses of principal (PC) and intercalated cells (IC) to acute increase in luminal flow rate in a microperfused CCD (A), increase in superfusate flow rate in a split-open CCD (B), and circumferential stretch in an occluded CCD (C). Horizontal bars identify onset and termination of high flow. [From Liu et al. (41).]
On the basis of the work of Praetorius and Spring (57) in cultured cells, we sought to test whether the flow/stretch-induced Ca2+ influx in the mammalian CCD was localized to the apical membrane. CCDs subject to an acute increase in luminal flow rate in the nominal absence of luminal Ca2+ failed to exhibit a sustained plateau elevation of [Ca2+]i, suggesting that the flow-induced plateau elevation in [Ca2+]i in perfused CCDs is mediated by luminal Ca2+ entry into the epithelial cells. The complete inhibition of the flow/stretch-induced increase in [Ca2+]i in tubules perfused and briefly bathed in the absence of Ca2+ implies that basolateral Ca2+ entry pathways coupled with internal Ca2+ release are necessary for the flow-induced [Ca2+]i response and suggests that the cilium may transmit a mechanical force to the cytoskeleton, which “talks” to the basolateral membrane.
In split-open CCDs, we first determined a threshold flow rate of 3.2 μl/s, below which a response was not detected. However, an increase in superfusate flow from 3.2 to 25 μl/s led to a twofold increase in [Ca2+]i in principal and intercalated cells (Fig. 10B); even at this higher flow rate, the cilium is subject to forces and torques that are nearly sevenfold lower than those predicted in the microperfused CCD (Table 1). The time to peak [Ca2+]i was delayed (∼30 s) compared with the 11-s delay following an increase in flow in perfused CCDs. At 20 min of sustained high flow, [Ca2+]i remained elevated. However, a reduction in superfusate flow from 25 to 3.2 μl/s led to a fall in [Ca2+]i to baseline values measured at the initial slow flow rate. As the FSS on the apical membrane of the renal epithelial cells in the split-open tubules superfused at the fast flow rate is only 0.056 dyn/cm2, which is well below the threshold for a Ca2+ response in ECs, our data suggest that the triggering stimulus for the Ca2+ response in split-open CCDs is not the drag force due FSS acting on the apical membrane but, more likely, the torque on the basal body at the base of the cilium, where the ciliary axoneme is anchored to the cytoskeleton. It is unlikely that bending deformation of the cilium per se leads to activation of stretch-activated Ca2+ channels, since Rydholm et al. (61) showed that that the stresses in the membrane of the cilium are small, except at its base. Furthermore, the tip deflection for a 2.5-μm cilium at even the higher flow rate of 25 μl/s was <2 nm (Table 1).
In the occluded CCD, a rapid increase in luminal volume, sufficient to increase tubular diameter by 20% within 0.6 s, led to a fourfold increase in [Ca2+]i in principal and intercalated cells (Fig. 10C), an increase double that measured in split-open tubules. Peak [Ca2+]i was reached within 10 s in both cell types, a time course very similar to that in the perfused tubule. The magnitude of the increase in [Ca2+]i in the occluded tubule depended on the degree and speed of circumferential stretch. Thus a rapid (<1 s) increase in luminal volume, sufficient to increase tubular diameter by only 5%, led to a twofold increase in [Ca2+]i in principal and intercalated cells, whereas a slow (>3 min) expansion of tubular diameter by 20% failed to elicit a [Ca2+]i transient in either cell type. These observations suggest that the trigger for the flow-induced Ca2+ response is not likely circumferential stretch but that the important signals are the magnitude of the FSS generated by the time rate of expansion of the inner tubule volume and the resulting torque on the cilium. The difference in the time scale of the [Ca2+]i response for the perfused and occluded CCDs compared with the split-open CCD could be due to the difference in the viscoelastic behavior of the CCD in their circular, as opposed to their planar, geometry. In the circular geometry, the cytoskeleton of the epithelial cell is in a state of residual stress, which is released when the CCD is split open. The time scale for the relaxation of this residual stress is a cellular viscoelastic response sometimes referred to as Rayleigh stiffness damping (63). This possibility is explored by Rydholm et al. (61): their FEM predicts a [Ca2+]i delay time of ∼30 s for MDCK cells subject to FSS.
The following question remains: What model described above best represents the physiological condition for the distal nephron in vivo? Increases in tubular diameter on the order of 15% in response to high flows, as detected in isolated microperfused CCDs, would predict significant changes in distal tubular volume in the kidney with an intact capsule, necessitating compression of adjacent renal tissue. As there is no evidence that this occurs, it is more likely that 1) tubules in vivo experience only minimal circumferential stretch, 2) the [Ca2+]i response in the split-open tubule is the most relevant, and 3) the forces and torques on primary cilia of principal cells and, possibly, the microvilli/microplicae of intercalated cells leading to deformation of the cytoskeleton are key.
Autosomal recessive PKD, now ascribed to mutations in polyductin/fibrocystin (52, 76), is characterized by the presence of ectatic collecting ducts, which remain contiguous with the filtering nephron. The Oak Ridge polycystic kidney (orpk) mouse, which bears an insertional mutation in the Tg737 gene encoding the intraflagellar transport protein product polaris (50, 51), exhibits a renal disease phenotypically resembling autosomal recessive PKD. The primary cilia in the kidney (Fig. 11) of orpk mice are short (40, 55). To further examine whether it is the structural and/or functional integrity of the cilium that determines the fully differentiated hydrodynamic response to flow, we sought to examine the effect of changes in luminal flow rate on the [Ca2+]i response in microperfused CCDs with stunted cilia isolated from orpk mice (40).
Fig. 11.
Scanning electron micrographs of collecting ducts on postnatal day 7 (P7, A–C) and 14 (P14, D–E) wild-type (WT), Oak Ridge polycystic kidney (orpk) heterozygous, and orpk homozygous mutant mice. WT collecting ducts at P7 (A) and P14 (D) possess principal cells with a single central cilium and intercalated cells distinguished by apical microvilli and/or microplicae. Collecting ducts in orpk heterozygous kidneys at P7 (B) and P14 (E) appear phenotypically similar to those in WT animals. Cilia on principal cells from orpk homozygous mutants (C and F) are shorter than those in age-matched WT and orpk heterozygous animals. Scale bar, 1 μm. [From Liu et al. (40).]
Flow-induced increases in [Ca2+]i were not detected in cells within dilated, ectatic portions of cystic collecting ducts (CDs). This finding was not unexpected, given that the shear and drag forces on the cilia and apical membranes of cells in these dilated sections of the nephron were expected to be less than those experienced by cells in more proximal portions of normal diameter. The magnitude of the flow-induced [Ca2+]i transients in segments of normal diameter, just proximal to cystic dilations of ectatic CDs from 2-wk-old, but not 1-wk-old, mutant orpk animals was blunted compared with the responses of cells in segments isolated from age-matched wild-type (WT) controls. Modeling of the hydrodynamic forces prevailing in these segments revealed that the drag and torque on the WT cilia are 7 and 14 times greater than the drag and torque on the short mutant cilia at the slow and fast flow rates, respectively. The observation that the peak [Ca2+]i response elicited by flow in the 2-wk-old mutant tubule was half of that measured in the age-matched control indicates that the response does not scale linearly with drag and bending moments on the stunted cilia. This could be due to the differing viscoelastic properties of epithelial cells in cystic CDs and WT controls, much like red blood cells in sickle cell disease (11). In 1-wk-old animals, the flow-induced [Ca2+]i response in mutant CDs was similar to that detected in controls, a result unexpected on the basis of the shorter cilia length in the former, predicted to be associated with blunted hydrodynamic forces. The similar mechanoinduced responses at this developmental age may reflect functional immaturity of an as yet unidentified signal transduction pathway early in postnatal life or that the Ca2+ response in immature CDs is not due to fluid flow but, rather, another stimulus (e.g., circumferential stretch).
In every experiment described above, the flow-induced [Ca2+]i response of intercalated cells, devoid of cilia but decorated with apical microvilli and microplicae (Fig. 11), was similar to that in principal cells. A logical explanation for the intercalated cell response is that the afferent sensors are the microvilli and/or microplicae, which undergo flow-induced deformations similar to those described above for the PT, a possibility that must await experimental investigation. An alternative explanation is that the [Ca2+]i response of the intercalated cell is due to autocrine/paracrine signaling secondary to flow- or stretch-induced release of an extracellular factor. Indeed, there is considerable evidence for mechanoinduced release of extracellular nucleotides, including ATP and UTP, both of which, via binding to purinergic receptors, trigger increases in [Ca2+]i (9, 29).
Summary
Table 2 has been prepared to compare the important flow-dependent mechanisms and responses of PT cells, CCD cells, and ECs to fluid flow. It emphasizes flow-dependent activation and the relationship between cellular structure and function indicating key references.
Table 2.
Summary comparison of flow-dependent mechanisms in PT, CCD, and endothelial cells (ECs)
| Flow-Dependent Response/Mechanism | PT Cells | CCD Principal Cells | ECs |
|---|---|---|---|
| Mechanosensing structure | Microvilli | Primary cilia | EGL |
| Basic mechanism | FSS on microvilli tips creates bending moment (torque) on microvilli at insertion into actin cytoskeleton | Fluid drag on primary cilia produces bending moment on insertion into basal body where membrane stresses are greatest | FSS on core protein tips produces rotational moment on transmembrane proteins linked to actin cortical web |
| Typical FSS | 1.0 dyn/cm2, 0.1 Pa | <0.5 dyn/cm2 | 10 dyn/cm2 |
| Downstream target | Activation of Na+ and HCO3− reabsorption through stimulation of NHE3 and H+-ATPase | Opening of stretch-activated Ca2+ channels at base of primary cilia leads to release of Ca2+ from intracellular stores | FSS leads to release of Ca2+ from intracellular stores and production of eNOS |
| Reorganization of actin and junctional proteins in response to FSS in culture | Basal cytosolic actin stress fibers, present under static control, disappear, AJs and TJs form, and focal adhesion proteins accumulate in basement membrane | Cytoskeletal reorganization in response to FSS has not been studied | FSS leads to formation of stress fibers, disruption of DPAB, and redistribution of junction-associated proteins vinculin, ZO-1, and Cx43 |
| Relation of cell geometry to mechanosensitive response | PT cells are tall and columnar but are exposed to FSS 1/10th that of ECs. This is compensated for by microvilli with a large aspect ratio, typically >25. Long lever arm leads to a ∼40-fold increase in stress at the base of the central actin filament bundle at its insertion into the apical membrane | Principal cells have a central cilium with a 9 + 0 microtubule structure; primary cilia are exposed to higher velocities over their entire length and, thus, large bending moments | ECs are flat compared with PT cells and, thus, need larger FSS to produce the same rotational moment on cadherin bonds in their AJs; also thickness of the EGL is typically <1/5th the height of the microvilli |
| Physiological significance | Glomerulotubular balance and its related perfusion absorption balance | Principal and intercalated cells of the CCD respond to FSS with a biphasic increase in global [Ca2+]i; impact on transport is under investigation but includes BK channel-mediated flow-stimulated K+ secretion (39) | FSS plays a key role in release of Ca2+ and NO in the vasodilatory response of blood vessels |
PT, proximal tubule; EC, endothelial cell; EGL, endothelial glycocalyx; FSS, fluid shear stress; NHE3, Na+/H+ exchanger; NO, nitric oxide; eNOS, endothelial NO synthase; AJ, adherens junction; TJ, tight junction; Cx43, connexin 43; BK channel, large-conductance K+ channel; [Ca2+]i, intracellular Ca2+ concentration; DPAB, dense peripheral actin band.
Conclusion
This review has articulated the importance of transmitting luminal flow rate to the lining epithelial cells of the proximal and distal nephron. A special emphasis has been placed on the importance of mathematical models in developing quantitative hypotheses for the design and interpretation of experiments to elucidate the structure and function of epithelial cells in the PT and distal tubule. In the case of the PT, a long-standing mystery as to why the glomerulotubular balance demonstrated in vivo more than four decades ago did not appear to be present in single perfused PTs has been resolved by examination of the biomechanics of the brush-border microvilli as an afferent sensor of luminal flow. Two important unresolved issues in the nephron involve 1) the cross talk and coupling between membrane transport proteins at the apical and basolateral aspects of the brush-border cells and 2) the cytoskeletal signaling associated with transporter trafficking and expression. In the case of the CCD, primary cilia have been proposed as the key structural element in the [Ca2+]i response to FSS. Important unresolved issues for the distal nephron are to more precisely determine the FSS prevailing in vivo, unravel the components of the signaling pathways activated by flow and their developmental expression and regulation, and clarify the molecular basis for the intercalated cell response to FSS.
GRANTS
Our research on the PT and the CCD was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-62289, DK-038470, DK-051391, DK-29857, and P30 DK-079307.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Baer PC, Bereiter-Hahn J, Schubert R, Geiger H. Differentiation status of human renal proximal and distal tubular epithelial cells in vitro: differential expression of characteristic markers. Cells Tissues Organs 184: 16–22, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Barbee KA, Mundel T, Lal R, Davies PF. Subcellular distribution of shear stress at the surface of flow-aligned and nonaligned endothelial monolayers. Am J Physiol Heart Circ Physiol 268: H1765–H1772, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Basmadjian D, Dykes DS, Baines AD. Flow through brush borders and similar protuberant wall structures. J Membr Biol 56: 183–190, 1980 [DOI] [PubMed] [Google Scholar]
- 4.Baum M, Quigley R. Inhibition of proximal convoluted tubule transport by dopamine. Kidney Int 54: 1593–1600, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck JS, Potts DJ. Cell swelling, co-transport activation and potassium conductance in isolated perfused rabbit kidney proximal tubules. J Physiol 425: 369–378, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bobulescu IA, Quinones H, Gisler SM, DiSole F, Hu MC, Shi M, Zhang J, Fuster DG, Wright N, Mumby M, Moe OW. Acute regulation of renal Na+/H+ exchanger NHE3 by dopamine: role of protein phosphatase 2A. Am J Physiol Renal Physiol 298: F1205–F1213, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown D, Stow JL. Protein trafficking and polarity in kidney epithelium: from cell biology to physiology. Physiol Rev 76: 245–297, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Burg MB, Orloff J. Control of fluid absorption in the renal proximal tubule. J Clin Invest 47: 2016–2024, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat 194: 335–342, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu JJ, Usami S, Chien S. Vascular endothelial responses to altered shear stress: pathologic implications for atherosclerosis. Ann Med 41: 19–28, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Dao M, Lim CT, Suresh S. Mechanics of the human red blood cell deformed by optical tweezers. J Mech Phys Solids 51: 2259–2280, 2003 [Google Scholar]
- 12.DeRosier DJ, Tilney LG, Egelman E. Actin in the inner ear: the remarkable structure of the stereocilium. Nature 287: 291–296, 1980 [DOI] [PubMed] [Google Scholar]
- 13.Dewey CF, Jr, Bussolari SR, Gimbrone MA, Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng 103: 177–185, 1981 [DOI] [PubMed] [Google Scholar]
- 14.Du Z, Duan Y, Yan Q, Weinstein AM, Weinbaum S, Wang T. Mechanosensory function of microvilli of the kidney proximal tubule. Proc Natl Acad Sci USA 101: 13068–13073, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du Z, Ferguson W, Wang T. Role of PKC and calcium in modulation of effects of angiotensin II on sodium transport in proximal tubule. Am J Physiol Renal Physiol 284: F688–F692, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Du Z, Yan Q, Duan Y, Weinbaum S, Weinstein AM, Wang T. Axial flow modulates proximal tubule NHE3 and H-ATPase activities by changing microvillus bending moments. Am J Physiol Renal Physiol 290: F289–F296, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Du Z, Yan Q, Weinbaum S, Weinstein A, Wang T. Role of dopamine in modulating flow-dependent sodium and bicarbonate transport in mouse proximal tubule (Abstract). FASEB J 24: 1024.–1022., 2010 [Google Scholar]
- 18.Duan Y, Gotoh N, Yan Q, Du Z, Weinstein AM, Wang T, Weinbaum S. Shear-induced reorganization of renal proximal tubule cell actin cytoskeleton and apical junctional complexes. Proc Natl Acad Sci USA 105: 11418–11423, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Essig M, Terzi F, Burtin M, Friedlander G. Mechanical strains induced by tubular flow affect the phenotype of proximal tubular cells. Am J Physiol Renal Physiol 281: F751–F762, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Evan AP, Satlin LM, Gattone VH, 2nd, Connors B, Schwartz GJ. Postnatal maturation of rabbit renal collecting duct. II. Morphological observations. Am J Physiol Renal Fluid Electrolyte Physiol 261: F91–F107, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Fritton SP, Weinbaum S. Fluid and solute transport in bone: flow-induced mechanotransduction. Annu Rev Fluid Mech 41: 347–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furukawa R, Fechheimer M. The structure, function, and assembly of actin filament bundles. Int Rev Cytol 175: 29–90, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Gallagher AR, Germino GG, Somlo S. Molecular advances in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 17: 118–130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gertz KH, Boylan JW. Glomerular-tubular balance. In: Handbook of Physiology. Renal Physiology. Washington, DC: Am. Physiol. Soc., 1973, p. 763–790 [Google Scholar]
- 25.Guo P, Weinstein AM, Weinbaum S. A hydrodynamic mechanosensory hypothesis for brush border microvilli. Am J Physiol Renal Physiol 279: F698–F712, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Hackney CM, Furness DN. Mechanotransduction in vertebrate hair cells: structure and function of the stereociliary bundle. Am J Physiol Cell Physiol 268: C1–C13, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Han Y, Cowin SC, Schaffler MB, Weinbaum S. Mechanotransduction and strain amplification in osteocyte cell processes. Proc Natl Acad Sci USA 101: 16689–16694, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassen OE, Hermann L. The presence of an axial structure in the microvillus of the mouse convoluted proximal tubule cell. Lab Invest 11: 610–616, 1962 [PubMed] [Google Scholar]
- 29.Homolya L, Steinberg TH, Boucher RC. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J Cell Biol 150: 1349–1360, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howard J, Hudspeth AJ. Mechanical relaxation of the hair bundle mediates adaptation in mechanoelectrical transduction by the bullfrog's saccular hair cell. Proc Natl Acad Sci USA 84: 3064–3068, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu MC, Fan L, Crowder LA, Karim-Jimenez Z, Murer H, Moe OW. Dopamine acutely stimulates Na+/H+ exchanger (NHE3) endocytosis via clathrin-coated vesicles: dependence on protein kinase A-mediated NHE3 phosphorylation. J Biol Chem 276: 26906–26915, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Hu X, Weinbaum S. A new view of Starling's hypothesis at the microstructural level. Microvasc Res 58: 281–304, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Hudspeth AJ. Making an effort to listen: mechanical amplification in the ear. Neuron 59: 530–545, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen CG, Poole CA, McGlashan SR, Marko M, Issa ZI, Vujcich KV, Bowser SS. Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol Int 28: 101–110, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Kishino A, Yanagida T. Force measurements by micromanipulation of a single actin filament by glass needles. Nature 334: 74–76, 1988 [DOI] [PubMed] [Google Scholar]
- 36.Kolb RJ, Woost PG, Hopfer U. Membrane trafficking of angiotensin receptor type-1 and mechanochemical signal transduction in proximal tubule cells. Hypertension 44: 352–359, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Krahn TA, Weinstein AM. Acid/base transport in a model of the proximal tubule brush border: impact of carbonic anhydrase. Am J Physiol Renal Fluid Electrolyte Physiol 270: F344–F355, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Lamprecht G, Weinman EJ, Yun CH. The role of NHERF and E3KARP in the cAMP-mediated inhibition of NHE3. J Biol Chem 273: 29972–29978, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Liu W, Morimoto T, Woda C, Kleyman TR, Satlin LM. Ca2+ dependence of flow-stimulated K secretion in the mammalian cortical collecting duct. Am J Physiol Renal Physiol 293: F227–F235, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, Satlin LM. Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol 289: F978–F988, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285: F998–F1012, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Ma HT, Patterson RL, van Rossum DB, Birnbaumer L, Mikoshiba K, Gill DL. Requirement of the inositol trisphosphate receptor for activation of store-operated Ca2+ channels. Science 287: 1647–1651, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Maddox DA, Fortin SM, Tartini A, Barnes WD, Gennari FJ. Effect of acute changes in glomerular filtration rate on Na+/H+ exchange in rat renal cortex. J Clin Invest 89: 1296–1303, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci USA 104: 13325–13330, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maunsbach AB, Christensen EI. Functional ultrastructure of the proximal tubule. In: Handbook of Physiology. Renal Physiology. Bethesda, MD: Am. Physiol. Soc., 1992, sect. 8, p. 41–107 [Google Scholar]
- 46.Maunsbach AB, Giebisch GH, Stanton BA. Effects of flow rate on proximal tubule ultrastructure. Am J Physiol Renal Fluid Electrolyte Physiol 253: F582–F587, 1987 [DOI] [PubMed] [Google Scholar]
- 47.McNamara LM, Majeska RJ, Weinbaum S, Friedrich V, Schaffler MB. Attachment of osteocyte cell processes to the bone matrix. Anat Rec (Hoboken) 292: 355–363, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Messner G, Koller A, Lang F. The effect of phenylalanine on intracellular pH and sodium activity in proximal convoluted tubule cells of the frog kidney. Pflügers Arch 404: 145–149, 1985 [DOI] [PubMed] [Google Scholar]
- 49.Michel CC. Starling: the formulation of his hypothesis of microvascular fluid exchange and its significance after 100 years. Exp Physiol 82: 1–30, 1997 [DOI] [PubMed] [Google Scholar]
- 50.Moyer JH, Lee-Tischler MJ, Kwon HY, Schrick JJ, Avner ED, Sweeney WE, Godfrey VL, Cacheiro NL, Wilkinson JE, Woychik RP. Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science 264: 1329–1333, 1994 [DOI] [PubMed] [Google Scholar]
- 51.Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. The Oak Ridge polycystic kidney (orpk) disease gene is required for left-right axis determination. Development 127: 2347–2355, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Nonaka S, Yoshiba S, Watanabe D, Ikeuchi S, Goto T, Marshall WF, Hamada H. De novo formation of left-right asymmetry by posterior tilt of nodal cilia. PLoS Biol 3: e268, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orci L, Humbert F, Brown D, Perrelet A. Membrane ultrastructure in urinary tubules. Int Rev Cytol 73: 183–242, 1981 [DOI] [PubMed] [Google Scholar]
- 55.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol 151: 709–718, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peppiatt CM, Collins TJ, Mackenzie L, Conway SJ, Holmes AB, Bootman MD, Berridge MJ, Seo JT, Roderick HL. 2-Aminoethoxydiphenyl borate (2-APB) antagonises inositol 1,4,5-trisphosphate-induced calcium release, inhibits calcium pumps and has a use-dependent and slowly reversible action on store-operated calcium entry channels. Cell Calcium 34: 97–108, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 184: 71–79, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol 191: 69–76, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Preisig PA. Luminal flow rate regulates proximal tubule H-HCO3 transporters. Am J Physiol Renal Fluid Electrolyte Physiol 262: F47–F54, 1992 [DOI] [PubMed] [Google Scholar]
- 60.Rostgaard J, Thuneberg L. Electron microscopical observations on the brush border of proximal tubule cells of mammalian kidney. Z Zellforsch Mikrosk Anat 132: 473–496, 1972 [DOI] [PubMed] [Google Scholar]
- 61.Rydholm S, Zwartz G, Kowalewski JM, Kamali-Zare P, Frisk T, Brismar H. Mechanical properties of primary cilia regulate the response to fluid flow. Am J Physiol Renal Physiol 298: F1096–F1102, 2010 [DOI] [PubMed] [Google Scholar]
- 62.Satlin LM, Schwartz GJ. Postnatal maturation of rabbit renal collecting duct: intercalated cell function. Am J Physiol Renal Fluid Electrolyte Physiol 253: F622–F635, 1987 [DOI] [PubMed] [Google Scholar]
- 63.Sato M, Ohshima N, Nerem RM. Viscoelastic properties of cultured porcine aortic endothelial cells exposed to shear stress. J Biomech 29: 461–467, 1996 [DOI] [PubMed] [Google Scholar]
- 64.Schnermann J, Wahl M, Liebau G, Fischbach H. Balance between tubular flow rate and net fluid reabsorption in the proximal convolution of the rat kidney. I. Dependency of reabsorptive net fluid flux upon proximal tubular surface area at spontaneous variations of filtration rate. Pflügers Arch 304: 90–103, 1968 [DOI] [PubMed] [Google Scholar]
- 65.Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol Renal Physiol 272: F132–F138, 1997 [DOI] [PubMed] [Google Scholar]
- 66.Secomb TW, Hsu R, Pries AR. Motion of red blood cells in a capillary with an endothelial surface layer: effect of flow velocity. Am J Physiol Heart Circ Physiol 281: H629–H636, 2001 [DOI] [PubMed] [Google Scholar]
- 67.Shibazaki S, Yu Z, Nishio S, Tian X, Thomson RB, Mitobe M, Louvi A, Velazquez H, Ishibe S, Cantley LG, Igarashi P, Somlo S. Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum Mol Genet 17: 1505–1516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sinha D, Wang Z, Price VR, Schwartz JH, Lieberthal W. Chemical anoxia of tubular cells induces activation of c-Src and its translocation to the zonula adherens. Am J Physiol Renal Physiol 284: F488–F497, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Tanaka-Kamioka K, Kamioka H, Ris H, Lim SS. Osteocyte shape is dependent on actin filaments and osteocyte processes are unique actin-rich projections. J Bone Miner Res 13: 1555–1568, 1998 [DOI] [PubMed] [Google Scholar]
- 70.Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a “bumper-car” model. Proc Natl Acad Sci USA 101: 16483–16488, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tilney LG, Tilney MS. Functional organization of the cytoskeleton. Hear Res 22: 55–77, 1986 [DOI] [PubMed] [Google Scholar]
- 72.Tschop J, Braun GS, Borscheid R, Horster MF, Huber SM. Ontogeny of purinergic receptor-regulated Ca2+ signaling in mouse cortical collecting duct epithelium. Cell Physiol Biochem 12: 75–82, 2002 [DOI] [PubMed] [Google Scholar]
- 73.Tsuchiya K, Wang W, Giebisch G, Welling PA. ATP is a coupling modulator of parallel Na,K-ATPase-K-channel activity in the renal proximal tubule. Proc Natl Acad Sci USA 89: 6418–6422, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Verghese E, Weidenfeld R, Bertram JF, Ricardo SD, Deane JA. Renal cilia display length alterations following tubular injury and are present early in epithelial repair. Nephrol Dial Transplant 23: 834–841, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res 79: 581–589, 1996 [DOI] [PubMed] [Google Scholar]
- 76.Wang S, Luo Y, Wilson PD, Witman GB, Zhou J. The autosomal recessive polycystic kidney disease protein is localized to primary cilia, with concentration in the basal body area. J Am Soc Nephrol 15: 592–602, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Wang T. Flow-activated transport events along the nephron. Curr Opin Nephrol Hypertens 15: 530–536, 2006 [DOI] [PubMed] [Google Scholar]
- 78.Wang T. Nitric oxide regulates HCO3− and Na+ transport by a cGMP-mediated mechanism in the kidney proximal tubule. Am J Physiol Renal Physiol 272: F242–F248, 1997 [DOI] [PubMed] [Google Scholar]
- 79.Wang T, Chan YL. Cholinergic effect on rat proximal convoluted tubule. Pflügers Arch 415: 533–539, 1990 [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, McNamara LM, Schaffler MB, Weinbaum S. A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc Natl Acad Sci USA 104: 15941–15946, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weinbaum S. Models to solve mysteries in biomechanics at the cellular level: a new view of fiber matrix layers. Ann Biomed Eng 26: 627–643, 1998 [DOI] [PubMed] [Google Scholar]
- 82.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech 27: 339–360, 1994 [DOI] [PubMed] [Google Scholar]
- 83.Weinbaum S, Guo P, You L. A new view of mechanotransduction and strain amplification in cells with microvilli and cell processes. Biorheology 38: 119–142, 2001 [PubMed] [Google Scholar]
- 84.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 9: 121–167, 2007 [DOI] [PubMed] [Google Scholar]
- 85.Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci USA 100: 7988–7995, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weinstein AM. Modeling epithelial cell homeostasis: steady-state analysis. Bull Math Biol 61: 1065–1091, 1999 [DOI] [PubMed] [Google Scholar]
- 87.Weinstein AM. Modeling epithelial cell homeostasis: assessing recovery and control mechanisms. Bull Math Biol 66: 1201–1240, 2004 [DOI] [PubMed] [Google Scholar]
- 88.Weinstein AM, Sontag ED. Modeling proximal tubule cell homeostasis: tracking changes in luminal flow. Bull Math Biol 71: 1285–1322, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weinstein AM, Weinbaum S, Duan Y, Du Z, Yan Q, Wang T. Flow-dependent transport in a mathematical model of rat proximal tubule. Am J Physiol Renal Physiol 292: F1164–F1181, 2007 [DOI] [PubMed] [Google Scholar]
- 90.Welling LW, Welling DJ. Surface areas of brush border and lateral cell walls in the rabbit proximal nephron. Kidney Int 8: 343–348, 1975 [DOI] [PubMed] [Google Scholar]
- 91.Wheatley DN, Wang AM, Strugnell GE. Expression of primary cilia in mammalian cells. Cell Biol Int 20: 73–81, 1996 [DOI] [PubMed] [Google Scholar]
- 92.Whitfield JF. Primary cilium—is it an osteocyte's strain-sensing flowmeter? J Cell Biochem 89: 233–237, 2003 [DOI] [PubMed] [Google Scholar]
- 93.Wilcox CS, Baylis C. Glomerular-tubular balance and proximal regulation in the kidney. In: Physiology and Pathophysiology, edited by Seldin DW, Giebisch G. New York: Raven, 1985 [Google Scholar]
- 94.Woda CB, Leite M, Jr, Rohatgi R, Satlin LM. Effects of luminal flow and nucleotides on [Ca2+]i in rabbit cortical collecting duct. Am J Physiol Renal Physiol 283: F437–F446, 2002 [DOI] [PubMed] [Google Scholar]
- 95.Woda CB, Miyawaki N, Ramalakshmi S, Ramkumar M, Rojas R, Zavilowitz B, Kleyman TR, Satlin LM. Ontogeny of flow-stimulated potassium secretion in rabbit cortical collecting duct: functional and molecular aspects. Am J Physiol Renal Physiol 285: F629–F639, 2003 [DOI] [PubMed] [Google Scholar]
- 96.Xiao Z, Zhang S, Mahlios J, Zhou G, Magenheimer BS, Guo D, Dallas SL, Maser R, Calvet JP, Bonewald L, Quarles LD. Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. J Biol Chem 281: 30884–30895, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamamoto K, Nakano M, Hashimoto K, Shimohama S, Kato N. Emergence of a functional coupling between inositol-1,4,5-trisphosphate receptors and calcium channels in developing neocortical neurons. Neuroscience 109: 677–685, 2002 [DOI] [PubMed] [Google Scholar]
- 98.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13: 2508–2516, 2002 [DOI] [PubMed] [Google Scholar]
- 99.You LD, Weinbaum S, Cowin SC, Schaffler MB. Ultrastructure of the osteocyte process and its pericellular matrix. Anat Rec A Discov Mol Cell Evol Biol 278: 505–513, 2004 [DOI] [PubMed] [Google Scholar]