Abstract
In vitro experiments showed that the connecting tubule (CNT) sends a signal that dilates the afferent arteriole (Af-Art) when Na+ reabsorption in the CNT lumen increases. We call this process CNT glomerular feedback (CTGF) to differentiate it from tubuloglomerular feedback (TGF), which is a cross talk between the macula densa (MD) and the Af-Art. In TGF, the MD signals the Af-Art to constrict when NaCl transport by the MD is enhanced by increased luminal NaCl. CTGF is mediated by CNT Na+ transport via epithelial Na+ channels (ENaC). However, we do not know whether CTGF occurs in vivo or whether it opposes the increase in Af-Art resistance caused by TGF. We hypothesized that CTGF occurs in vivo and opposes TGF. To test our hypothesis, we conducted in vivo micropuncture of individual rat nephrons, measuring stop-flow pressure (PSF) as an index of glomerular filtration pressure. To test whether activation of CTGF opposes TGF, we used benzamil to block CNT Na+ transport and thus CTGF. CTGF inhibition with the ENaC blocker benzamil (1 μM) potentiated the decrease in PSF at 40 and 80 nl/min. Next, we tested whether we could augment CTGF by inhibiting NaCl reabsorption in the distal convoluted tubule with hydrochlorothiazide (HCTZ, 1 mM) to enhance NaCl delivery to the CNT. In the presence of HCTZ, benzamil potentiated the decrease in PSF at 20, 40, and 80 nl/min. We concluded that in vivo CTGF occurs and opposes the vasoconstrictor effect of TGF.
Keywords: afferent arteriole, macula densa, connecting tubule, glomerular filtration rate, sodium ion transport, benzamil, stop-flow pressure
we and others have shown that, in the renal cortex, the connecting tubule (CNT), a segment of the nephron located between the distal convoluted tubule and collecting duct, returns to the vascular pole of the glomerulus and accompanies the afferent arteriole (Af-Art) for varying distances (2, 6, 7, 28). This morphology is compatible with cross talk between the CNT and the Af-Art. In vitro experiments showed that the CNT sends a signal that dilates the Af-Art when Na+ reabsorption in the CNT increases. We called this process CNT glomerular feedback (CTGF) to differentiate it from tubuloglomerular feedback (TGF), which is cross talk between the macula densa (MD) and the Af-Art (23, 24). From a homeostatic point of view, TGF is a negative feedback loop that senses increases in NaCl at the MD and increases Af-Art resistance while at the same time decreasing glomerular filtration rate (GFR), thus favoring Na+ retention. On the other hand, CTGF is a positive feedback loop that decreases Af-Art resistance in response to increases in NaCl in the CNT. A decrease in Af-Art resistance increases both renal blood flow and GFR and thus favoring Na+ excretion. NaCl reabsorption is mediated by the thiazide-sensitive Na+-Cl− cotransporter (NCC) in the distal convoluted tubule and the amiloride/benzamil-sensitive epithelial Na+ channel (ENaC) in the CNT and cortical collecting duct (17, 18, 21). Thus benzamil blocks CTGF (23) while hydrochlorothiazide (HCTZ), by blocking Na+ reabsorption in the distal convoluted tubule, enhances downstream delivery of Na+ to the CNT and theoretically may enhance CTGF.
Although there is some evidence that the distal nephron regulates Af-Art tone (19), direct demonstration of CTGF has only occurred in vitro to our knowledge (23) and could have been an artifact of the in vitro preparation. Thus we know of no direct in vivo evidence demonstrating the existence of CTGF and its physiological significance. Here we tested the hypothesis that CTGF occurs in vivo and opposes the vasoconstrictor effect of TGF. To test this hypothesis, we conducted micropuncture of individual rat nephrons, measuring stop-flow pressure (PSF) as an index of glomerular filtration pressure while the late proximal perfusion rate was increased from 0 to 10, 20, 40, and 80 nl/min.
METHODS
All experiments were approved by the Henry Ford Health System Institutional Animal Care and Use Committee and were conducted in accord with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats weighing 200–300 g were anesthetized with thiobutabarbital (125 mg/kg body wt ip). Body temperature was maintained at 37.5°C using a feedback-controlled, heated surgical table. A tracheotomy was performed with PE-240 tubing to allow spontaneous breathing of room air. The right jugular vein was catheterized with PE-10 tubing for maintenance infusion of 0.9% NaCl at a rate of 1.5 ml/h. The left femoral artery was catheterized with PE-10 tubing attached to a pressure transducer and recorder that monitored blood pressure continuously. The left kidney was approached through a flank incision, freed of adherent fat and connective tissue, and placed in a Lucite cup, eliminating the effects of body movement. It was then bathed in saline and immobilized by surrounding it loosely with saline-soaked cotton. The bladder was cannulated to allow free urine flow. After a 30- to 45-min equilibration period, proximal tubules were identified by injecting phosphate buffer (pH 7.4) containing 7% of a green dye made from FD&C yellow #5, FD&C yellow #6, and FD&C blue #1 in a random proximal segment using a glass pipette (OD 6–8 μm at the tip). A nephron was used when at least three downstream proximal segments were identified, indicating an early proximal puncture site. Early proximal portions were blocked with a very tenacious grease (Type T Medium Temperature Vacuum Grease; Apiezon). We injected grease blocks of ∼420 μm in length, because we previously established that, by using this particular type of grease and block length, we consistently obtain a seal that is both impermeable and immobile. A perfusion pipette (OD 8–10 μm) attached to a nanoliter infusion pump was inserted in the last superficial proximal segment to perfuse the loop of Henle and distal nephron with a solution containing (in mmol/l): 140 NaCl, 10 HEPES, 1 CaCO3, 0.5 K2HPO4, 4 KHCO3, 1.2 MgSO4, 5.5 glucose, 0.5 sodium acetate, 0.5 sodium lactate, and 0.5% of the above-mentioned green dye (pH 7.4). To measure PSF, a pressure pipette (OD 3–4 μm) attached to a micropressure system (model 900A; World Precision Instruments, Sarasota, FL) was inserted in an early proximal segment. To generate a PSF curve, the late proximal perfusion rate was increased from 0 to 10, 20, 40, and 80 nl/min while measuring PSF. Each perfusion rate was maintained for 1–5 min as required to observe a stable PSF. Next, the perfusate was replaced with one containing the compounds to be tested, and a second PSF curve was generated.
The ENaC inhibitor benzamil and the NCC inhibitor HCTZ were purchased from Sigma.
Statistics.
Data are expressed as means ± SE. Repeated-measures ANOVA with two repeated factors (time and perfusion rate) was performed, followed by Student's paired t-tests, which were used to compare PSF for each flow rate between the control and experimental flow-response curves. Hochberg's step-up procedure was used to adjust the P values for multiple comparisons so that the family wise type I error rate, predefined as 0.05, was controlled.
RESULTS
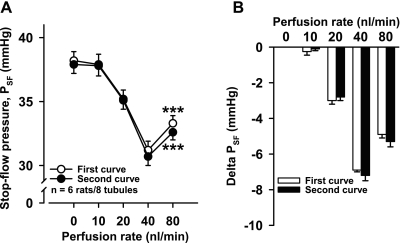
We first performed a time-control experiment consisting of two consecutive PSF dose-response curves (DRC). In the first, PSF was measured while increasing perfusion rate from 0 to 10, 20, 40, and 80 nl/min. PSF decreased from 38.2 ± 0.7 to 37.9 ± 0.8, 35.2 ± 0.7, and 31.2 ± 0.7 mmHg at 10, 20, and 40 nl/min, respectively; however, at 80 nl/min, PSF increased to 33.3 ± 0.6 mmHg. When we returned the flow rate to 0 nl/min and repeated the protocol, PSF was not significantly different from the first DRC (n = 6 rats/8 tubules; Fig. 1), confirming that the PSF response to increased tubular perfusion rate is reversible and reproducible.
Fig. 1.
A: two consecutive stop-flow pressure (PSF) curves were induced by increasing the late proximal tubule perfusion rate (0, 10, 20, 40, 80 nl/min), which lowered PSF. However, at 80 nl/min, PSF increased (***P < 0.001, 80 vs. 40 nl/min), indicating afferent arteriole (Af-Art) dilatation. There was no difference between the first (○) and second (●) curve. B: delta PSF (calculated by taking each PSF at 0, 10, 20, 40, and 80 nl/min and subtracting PSF at 0 nl/min).
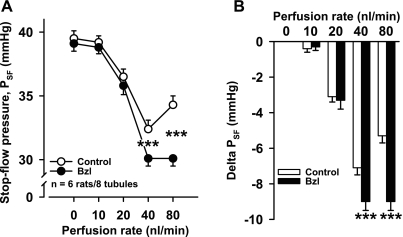
To test whether CTGF opposes TGF, we studied the effect of the CTGF blocker benzamil. In the absence of benzamil, increasing the perfusion rate from 0 to 40 nl/min caused PSF to decrease as expected. In the presence of benzamil, the decrease in PSF at 40 nl/min was potentiated. Furthermore, increasing the perfusion rate to 80 nl/min in the absence of benzamil caused PSF to increase compared with 40 nl/min in the presence of benzamil (n = 6 rats/8 tubules; Fig. 2).
Fig. 2.
Effect of benzamil-induced connecting tubule glomerular feedback (CTGF) inhibition on PSF. A: benzamil (1 μM) potentiated the decrease in PSF at 40 and 80 nl/min [***P < 0.001, control (○) vs. benzamil (●)], suggesting that 1) CTGF was activated at 40 and 80 nl/min and 2) CTGF antagonized tubuloglomerular feedback (TGF). B: delta PSF (calculated by taking each PSF at 0, 10, 20, 40, and 80 nl/min and subtracting PSF at 0 nl/min). Bzl, benzamil.
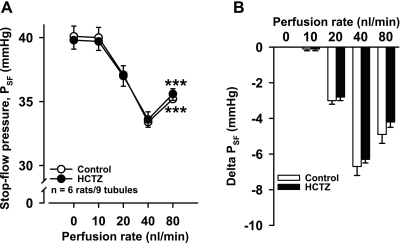
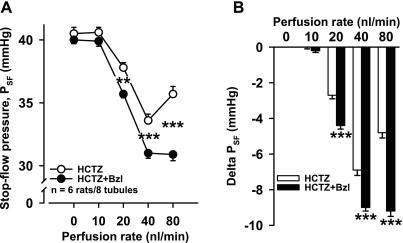
To see if we could enhance CTGF by increasing NaCl delivery to the CNT, we used HCTZ to inhibit distal convoluted tubule NaCl absorption. Adding 1 mM HCTZ to the tubular perfusate induced the same PSF response as control (n = 6 rats/9 tubules; Fig. 3). However, addition of benzamil to the perfusate along with HCTZ significantly potentiated the decrease in PSF at perfusion rates of 20, 40, and 80 nl/min (n = 6 rats/8 tubules; Fig. 4).
Fig. 3.
Effect of hydrochlorothiazide (HCTZ)-induced increases in connecting tubule Na+ delivery on PSF. A: increasing the late proximal tubule perfusion rate lowered PSF. However, at 80 nl/min, PSF increased (***P < 0.001, 80 vs. 40 nl/min), indicating Af-Art dilatation. There was no difference between control (○) and 1 mM HCTZ (●). B: delta PSF (calculated by taking each PSF at 0, 10, 20, 40, and 80 nl/min and subtracting PSF at 0 nl/min).
Fig. 4.
Effect of benzamil-induced CTGF inhibition plus HCTZ on PSF. A: in the presence of HCTZ, benzamil potentiated the decrease in PSF at 20, 40, and 80 nl/min [**P < 0.01 and ***P < 0.001, HCTZ (○) vs. HCTZ + benzamil (●)], suggesting that, in the presence of HCTZ, 1) CTGF was activated at 20, 40, and 80 nl/min, 2) CTGF antagonized TGF, and 3) when CTGF was absent, TGF was potentiated. B: delta PSF (calculated by taking each PSF at 0, 10, 20, 40, and 80 nl/min and subtracting PSF at 0 nl/min).
Mean blood pressure was continuously monitored via the left femoral artery while perfusing the late proximal tubule. It remained stable in all animals (Table 1).
Table 1.
Mean blood pressure during two consecutive PSF dose-response curves in rats
| Perfusion rate, nl/min |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First PSF dose-response curve |
Second PSF dose-response curve |
|||||||||
| 0 | 10 | 20 | 40 | 80 | 0 | 10 | 20 | 40 | 80 | |
| Time control | 110 ± 2 | 110 ± 2 | 110 ± 2 | 110 ± 2 | 110 ± 2 | 110 ± 2 | 110 ± 2 | 110 ± 2 | 110 ± 2 | 110 ± 2 |
| Control vs. benzamil (1 μM) | 110 ± 2 | 110 ± 2 | 110 ± 2 | 110 ± 2 | 110 ± 2 | 110 ± 2 | 110 ± 2 | 110 ± 2 | 110 ± 2 | 110 ± 2 |
| Control vs. HCTZ (1 mM) | 117 ± 4 | 117 ± 4 | 117 ± 4 | 117 ± 4 | 117 ± 4 | 117 ± 4 | 117 ± 4 | 117 ± 4 | 117 ± 4 | 117 ± 4 |
| HCTZ vs. HCTZ + benzamil | 115 ± 2 | 115 ± 2 | 115 ± 2 | 115 ± 2 | 115 ± 2 | 115 ± 2 | 115 ± 2 | 115 ± 2 | 115 ± 2 | 115 ± 2 |
Values are means ± SE; n = 6 rats in each group. PSF, stop-flow pressure; HCTZ, hydrochlorothiazide.
DISCUSSION
Our data support the hypothesis that CTGF occurs in vivo and counters TGF. Increasing late proximal tubule perfusion rate from 0 to 10, 20, and 40 nl/min lowered PSF; however, when perfusion rate was raised to 80 nl/min, PSF increased significantly compared with 40 nl/min, indicating Af-Art dilatation. We reasoned that, if TGF and CTGF are simultaneously activated by perfusion of the distal tubule but have opposing effects, then CTGF inhibition should potentiate the TGF response, that is, the decrease in PSF. We used benzamil to block CNT Na+ transport (and thus CTGF) and found that CTGF inhibition with benzamil potentiated a decrease in PSF at 40 and 80 nl/min, suggesting that CTGF was activated at 40 and 80 nl/min and antagonized TGF. Next, we examined whether increasing NaCl delivery to the CNT without altering NaCl delivery to the MD would enhance CTGF and antagonize TGF. For this, we used HCTZ, which blocks NCC in the distal convoluted tubule. We found that adding HCTZ to the tubular fluid did not alter the PSF response; however, it did have an effect on the benzamil, which now potentiated the PSF response at 20, 40, and 80 nl/min, suggesting that, in the presence of HCTZ, benzamil potentiates TGF, probably by blocking CTGF. This occurred even at low flow rates (20 nl/min), whereas, in the absence of HCTZ, benzamil stimulated TGF at 40 nl/min. In theory, HCTZ should have increased delivery of NaCl to the CNT and activated CTGF to the point that it inhibited TGF. Thus, even though theoretically PSF should have been less in the presence of HCTZ, we found no difference. One likely explanation is that HCTZ inhibits NaCl absorption in the loop of Henle and thus increases delivery to both the MD and CNT. Increased delivery of NaCl to the MD would enhance TGF, whereas increased delivery to the CNT should enhance CTGF, so that both would cancel out. HCTZ has been shown to inhibit Na+ reabsorption in the loop of Henle, possibly by blocking carbonic anhydrase (27). Nevertheless, inhibition of ENaC with benzamil clearly shows that CTGF antagonizes TGF and that HCTZ enhances this effect. The fact that benzamil augmented the decrease in PSF at 20, 40, and 80 nl/min in the presence of HCTZ is likely a result of both inhibition of enhanced CTGF and stimulated TGF at all flow rates as explained above.
Two mechanisms contribute to renal blood flow autoregulation: the myogenic response and TGF. Recent studies have pointed to the existence of another mechanism of renal blood flow autoregulation simply dubbed the third mechanism, which has been identified in vivo in mice (13), rats (11, 12, 25, 26, 29), and dogs (14, 15) by analyzing the renal vascular resistance curve immediately after changing renal perfusion pressure. Apparently this third mechanism is slower than the myogenic response and TGF, and is present even when TGF is abolished, for example, in A1 adenosine receptor knockout mice (10). Seeliger et al. (25) studied renal blood flow autoregulation and determined that this mechanism counterbalances TGF and may be related to CTGF. Our data showed that, in the presence of HCTZ, blocking CTGF with benzamil potentiated the decrease in PSF at 20, 40, and 80 nl/min, suggesting that, at these rates, 1) CTGF was activated and 2) CTGF antagonized TGF, since when CTGF was absent TGF was potentiated. CTGF might be related to the so-called third mechanism and also agrees with Mörsing et al. (19) who showed that the distal nephron regulates Af-Art tone in vivo.
The present study corroborates previous in vitro evidence that CTGF is mediated by Na+ transport via ENaC in the CNT (23). We have also shown that in vitro CTGF is mediated by prostaglandins and epoxyeicosatrienoic acids (22). A limitation of in vivo studies such as the present one is that they are less suited to dissect signaling pathways. Compounds that affect CTGF will also likely affect TGF.
Because some amiloride analogs inhibit Na+-H+ exchangers (NHEs), they could potentially affect TGF. Eleven NHE isoforms (NHE1–11 or SLC9A1–11) have been described to date in the human genome (9), five of which are expressed at the plasma membrane of renal epithelial cells (3), NHE3 being the main isoform expressed in adults at the apical (luminal) membrane of the proximal tubule (4). We chose to use the benzyl analog of amiloride (benzamil) because of its high specificity for ENaC compared with NHEs. The inhibitory constant (Ki) of benzamil for ENaC is 10−8 M, whereas its Ki for NHE3 is 10−4 M (20). Other NHE isoforms present in the kidney have similarly low affinities for benzamil (1, 5, 16, 30). Thus the concentration of benzamil we used is at least 100 times lower than the known Ki values for NHE isoforms, making it highly unlikely that our results with benzamil where affected by inhibition of NHE.
It could be argued that using a flow of 80 nl/min to perfuse the late proximal tubule is too high, since the maximum TGF response is achieved with flows between 30 and 40 nl/min. By using a higher flow, namely 80 nl/min, we were able to demonstrate the existence of additional mechanisms of cross talk between the nephron and the Af-Art in the absence of further activation of TGF. We found that, after maximum TGF response, further raising perfusion rate caused an increase in PSF, reflecting Af-Art dilation. We also considered that such high flow may damage the tubular epithelium and as a consequence TGF response would be decreased. However, the fact that inhibition of CTGF with benzamil restored and potentiated the maximum TGF response suggests that this was not the case. The normal flow in the distal nephron is variable under physiological conditions. Good and Wright (8) reported the range of distal flow rate may change from 4 nl/min (nondiuretic animals) to 38 nl/min (volume-expanded animals). Thus we found that blocking CTGF with benzamil potentiated the vasoconstrictor response to TGF at flows starting at 20 nl/min, which are within the physiological range.
In summary, our studies provide direct evidence of CTGF existence in vivo and further confirm our in vitro finding of cross talk between the CNT and Af-Art. A high flow rate stimulates Na+ transport in the CNT via ENaC, activates CTGF, and dilates the Af-Art. Inhibiting CTGF with benzamil potentiated the TGF response. We concluded that CTGF occurs in vivo and counters the vasoconstrictor effect of TGF. In the absence of CTGF, TGF is potentiated.
CTGF is a novel regulatory mechanism of the renal microcirculation that may explain the Af-Art dilatation and increased GFR observed during high-salt intake, perhaps by antagonizing or resetting TGF. During high-salt intake, O2 consumption by the nephron is higher because of increased Na+ reabsorption; thus, CTGF could help protect the kidney from ischemia by increasing renal blood flow. On the other hand, CTGF may be detrimental in certain situations, such as in diabetes with osmotic diuresis, where Af-Art dilation might increase intraglomerular pressure and perhaps glomerular damage.
GRANTS
This study was supported by National Institutes of Health Program Project Grants HL-028982 and HL-088036.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Part of this research was published in abstract form in Experimental Biology 2010 in Anaheim, CA, April 24–28, 2010.
REFERENCES
- 1.Anderie I, Blum R, Haase W, Grinstein S, Thevenod F. Expression of NHE1 and NHE4 in rat pancreatic zymogen granule membranes. Biochem Biophys Res Commun 246: 330– 336, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Barajas L, Powers K, Carretero OA, Scicli AG, Inagami T. Immunocytochemical localization of renin and kallikrein in the rat renal cortex. Kidney Int 29: 965– 970, 1986 [DOI] [PubMed] [Google Scholar]
- 3.Bobulescu IA, Moe OW. Na+/H+ exchangers in renal regulation of acid-base balance. Semin Nephrol 26: 334– 344, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobulescu IA, Moe OW. Luminal Na(+)/H (+) exchange in the proximal tubule. Pflugers Arch 458: 5– 21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuthbert AW, Fanelli GM. Effects of some pyrazinecarboxamides on sodium transport in frog skin. Br J Pharmacol 63: 139– 149, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dørup J, Mörsing P, Rasch R. Tubule-tubule and tubule-arteriole contacts in rat kidney distal nephrons. A morphologic study based on computer-assisted three-dimensional reconstructions. Lab Invest 67: 761– 769, 1992 [PubMed] [Google Scholar]
- 7.Faarup P. On the morphology of the juxtaglomerular apparatus. Acta Anat 60: 20– 38, 1965 [DOI] [PubMed] [Google Scholar]
- 8.Good DW, Wright FS. Luminal influences on potassium secretion: sodium concentration and fluid flow rate. Am J Physiol Renal Fluid Electrolyte Physiol 236: F192– F205, 1979 [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev 89: 193– 277, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Just A. Mechanisms of renal blood flow autoregulation: dynamics and contributions. Am J Physiol Regul Integr Comp Physiol 292: R1– R17, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Just A, Arendshorst WJ. Dynamics and contribution of mechanisms mediating renal blood flow autoregulation. Am J Physiol Regul Integr Comp Physiol 285: R619– R631, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Just A, Arendshorst WJ. Nitric oxide blunts myogenic autoregulation in rat renal but not skeletal muscle circulation via tubuloglomerular feedback. J Physiol 569: 959– 974, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Just A, Arendshorst WJ. A novel mechanism of renal blood flow autoregulation and the autoregulatory role of A1 adenosine receptors in mice. Am J Physiol Renal Physiol 293: F1489– F1500, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Just A, Ehmke H, Toktomambetova L, Kirchheim HR. Dynamic characteristics and underlying mechanisms of renal blood flow autoregulation in the conscious dog. Am J Physiol Renal Physiol 280: F1062– F1071, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Just A, Ehmke H, Wittmann U, Kirchheim HR. Role of angiotensin II in dynamic renal blood flow autoregulation of the conscious dog. J Physiol 538: 167– 177, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang'ethe W, Aimanova KG, Pullikuth AK, Gill SS. NHE8 mediates amiloride-sensitive Na+/H+ exchange across mosquito Malpighian tubules and catalyzes Na+ and K+ transport in reconstituted proteoliposomes. Am J Physiol Renal Physiol 292: F1501– F1512, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Madsen KM, Tisher CC. Structural-functional relationships along the distal nephron. Am J Physiol Renal Fluid Electrolyte Physiol 250: F1– F15, 1986 [DOI] [PubMed] [Google Scholar]
- 18.Meneton P, Loffing J, Warnock DG. Sodium and potassium handling by the aldosterone-sensitive distal nephron: the pivotal role of the distal and connecting tubule. Am J Physiol Renal Physiol 287: F593– F601, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Mörsing P, Velazquez H, Ellison D, Wright FS. Resetting of tubuloglomerular feedback by interrupting early distal flow. Acta Physiol Scand 148: 63– 68, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Orlowski J. Heterologous expression and functional properties of amiloride high affinity (NHE-1) and low affinity (NHE-3) isoforms of the rat Na/H exchanger. J Biol Chem 268: 16369– 16377, 1993 [PubMed] [Google Scholar]
- 21.Reilly RF, Ellison DH. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol Rev 80: 277– 313, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Ren Y, D'Ambrosio M, Garvin JL, Wang H, Carretero OA. Possible mediators of connecting tubule glomerular feedback (CTGF). Hypertension 53: 319– 323, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren Y, Garvin JL, Liu R, Carretero OA. Crosstalk between the connecting tubule and the afferent arteriole regulates renal microcirculation. Kidney Int 71: 1116– 1121, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Ren Y, Garvin JL, Liu R, Carretero OA. Cross-talk between arterioles and tubules in the kidney. Pediatr Nephrol 24: 31– 35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seeliger E, Wronski T, Ladwig M, Dobrowolski L, Vogel T, Godes M, Persson PB, Flemming B. The renin-angiotensin system and the third mechanism of renal blood flow autoregulation. Am J Physiol Renal Physiol 296: F1334– F1345, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Siu KL, Sung B, Moore LC, Birzgalis A, Chon KH. Very low frequency modulation in renal autoregulation. Conf Proc IEEE Eng Med Biol Soc 1: 771– 774, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Unwin RJ, Walter SJ, Giebisch G, Capasso G, Shirley DG. Localization of diuretic effects along the loop of Henle: an in vivo microperfusion study in rats. Clin Sci (Lond) 98: 481– 488, 2000 [PubMed] [Google Scholar]
- 28.Vio CP, Figueroa CD, Caorsi I. Anatomical relationship between kallikrein-containing tubules and the juxtaglomerular apparatus in the human kidney. Am J Hypertens 1: 269– 271, 1988 [DOI] [PubMed] [Google Scholar]
- 29.Wronski T, Seeliger E, Persson PB, Forner C, Fichtner C, Scheller J, Flemming B. The step response: a method to characterize mechanisms of renal blood flow autoregulation. Am J Physiol Renal Physiol 285: F758– F764, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Yu FH, Shull GE, Orlowski J. Functional properties of the rat Na/H exchanger NHE-2 isoform expressed in Na/H exchanger-deficient Chinese hamster ovary cells. J Biol Chem 268: 25536– 25541, 1993 [PubMed] [Google Scholar]