Abstract
The evolution of specialized excretory cells is a cornerstone of the metazoan radiation, and the basic tasks performed by Drosophila and human renal systems are similar. The development of the Drosophila renal (Malpighian) tubule is a classic example of branched tubular morphogenesis, allowing study of mesenchymal-to-epithelial transitions, stem cell-mediated regeneration, and the evolution of a glomerular kidney. Tubule function employs conserved transport proteins, such as the Na+, K+-ATPase and V-ATPase, aquaporins, inward rectifier K+ channels, and organic solute transporters, regulated by cAMP, cGMP, nitric oxide, and calcium. In addition to generation and selective reabsorption of primary urine, the tubule plays roles in metabolism and excretion of xenobiotics, and in innate immunity. The gene expression resource FlyAtlas.org shows that the tubule is an ideal tissue for the modeling of renal diseases, such as nephrolithiasis and Bartter syndrome, or for inborn errors of metabolism. Studies are assisted by uniquely powerful genetic and transgenic resources, the widespread availability of mutant stocks, and low-cost, rapid deployment of new transgenics to allow manipulation of renal function in an organotypic context.
Keywords: nephrolithiasis, renal acidosis, xanthinuria, Bartter syndrome, stem cells
The Need for Models of Renal Function
the renal system occupies a critical and privileged role in the body. As it filters the entire blood volume every few minutes, it is positioned not just for osmoregulation and elimination of waste, but receives early indications of immune, toxic, and other insults to the body. To aid our understanding at all levels, from medical to biophysical, it is necessary to identify models that are informative for the question posed. Historically, ion and solute transport has been modeled with simpler preparations, from frog skin to toad bladder; at present, transgenic mouse models present potent, although cumbersome, systems to assess gene function in an organismal context. This review will outline the emerging and exciting benefits (and limitations) of the simple genetic model, the fruit fly Drosophila melanogaster, as a quick, inexpensive and surprisingly relevant system for study of development, transport, defense, and renal disease.
Speed and Cost
There is a trade-off between genetic power and biomedical relevance in any experimental system (22). At one extreme, Escherichia coli divide every 20 min and so allow genetic mutagenesis and selection to be performed overnight on populations of hundreds of millions. At the other, human renal physiology is essentially an observational science; the range of experimental interventions is ethically circumscribed, and mutational analysis is based on serendipitous (rare) occurrences. In this context, the mouse, with its combination of mammalian body plan and established transgenic technology, provides an excellent balance of relevance and power (55, 65, 66). Nonetheless, it takes several years and tens of thousands of dollars to produce a new experimental mouse, and so investigation obligatorily occurs against a background of major, and reliably renewed, grant funding. By contrast, stock Drosophila transgenics can be obtained by mail for a few dollars and custom transgenics for a few hundred dollars, in 3 mo from drawing board to laboratory bench. Strategic use of the fruit fly thus confers significant nimbleness to a research program, and it is even possible to use Drosophila as a test-bed for subsequent mouse transgenic work, thus obtaining both cost, speed, and ethical (3Rs: “replacement, reduction, refinement”) (40) advantages.
However, given the intellectual investment required to embrace a new model, two issues must be addressed first: relevance of the model to biomedical research and benefits that potentially accrue.
Relevance to Humans
At first sight, the insect body-plan might seem too far from human to be useful. Insects have a hemocoel (blood-filled body cavity) rather than a vascular blood system, and the renal tubules float freely in the hemocoel. Furthermore, the insect renal system is aglomerular, and urine formation is based on active transport rather than selective reabsorption of an ultrafiltrate (5). However, these are not necessarily problems; Drosophila tubules are easily dissected intact, without painstaking microdissection (24). As discussed below, the insect nephrocyte/tubule system represents an intermediate step in the evolution of the glomerular kidney. Additionally, the basic tasks performed by the two systems are fundamentally similar: transport, excretion, and osmoregulation. In addition, the machinery by which these tasks are accomplished is also instantly recognizable. Of the simple models, Drosophila is comfortably the best in terms of homology; despite 400 million years of divergent evolution, 70% of Drosophila genes have clear human homologs (9). For the kidney, the case is even better, as the strict steric constraints on transport proteins allow even closer parallels do be drawn at the transcriptome level (64).
Benefits of Drosophila
Not only do the two renal systems have more in common than might be suspected, but the advantages conferred by Drosophila genetics are immense.
Sequenced, compact genome. The genome is 180 Mb, of which ∼130 Mb is euchromatin, making it about the size of a single human chromosome. The number of genes is ∼13,500, broadly comparable with humans, and ∼70% of Drosophila genes have human homologs (9).
Mutants for one-half of genes. The far-sighted pioneers of Drosophila biology placed their precious mutants into public-domain stock centers, which ship by mail. So, for example, it is possible today to work with the direct descendants of white1, the first mutation ever discovered in Drosophila (in 1910, by Thomas Hunt Morgan), incidentally an ABC transporter with massive renal expression (26). The tradition of freely sharing reagents continues to this day.
RNAi stocks for nearly all genes. In addition to classic mutants and P-element insertional alleles, systematic mutagenesis programs have produced RNAi lines for nearly all Drosophila genes (20). Again, these are available by mail at nominal cost.
Tissue- and cell type-specific drivers. One of the key advantages for organotypic study of gene function is the ability to drive transgenic constructs (reporter fusions, overexpressors, dominant negatives or RNAi) in cell types or at times of the experimenter's choosing. This is made possible by the binary GAL4/UAS system of adapted P-elements (8), composed of generic GAL4 “driver” flies (in which the innocuous yeast GAL4 transcription factor is expressed in a specific pattern) and UAS lines (in which cargoes of interest are placed downstream of the UAS element bound by GAL4). With an appropriate panel of renal-specific GAL4 drivers (e.g., see Ref. 60), experiments of great physiological precision are possible; for example, Drosophila was the first animal in which genetically encoded calcium reporters were expressed, to study real-time renal calcium signaling (51).
Ready-to-go vectors for promoter analysis, transgene expression, GFP and epitope fusions (1, 7), and for generation of dsRNA (41).
Although P-element-mediated transformation is very potent, it has also proved possible to mutate flies sequence specifically with both ends-out and ends-in homologous recombination (50).
Speed and cost. The Drosophila life-cycle lasts ∼2 wk, so multigenerational breeding experiments are performed easily. Each line of flies costs ∼$20 a year to keep alive, so it is possible to maintain a panel of informative “reagents” for long-term use.
The Drosophila Tubule
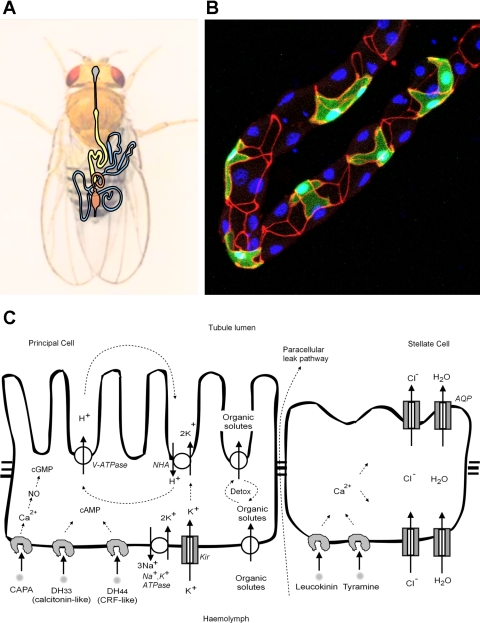
A brief diagrammatic overview of the tubule is provided in Fig. 1. The four tubules are packed as two pairs in a tightly determined, asymmetric pattern within the thorax and abdomen, and their main segments are composed of two main cell types; a metabolically active principal cell, and the smaller, intercalated stellate cell.
Fig. 1.
Structure and function of the adult Malpighian (renal) tubule. A: disposition of the tubules (blue) in the body cavity. The foregut (grey), midgut (yellow), and hindgut (orange) are also shown. B: photomicrograph of a tubule, showing stellate cells (marked green with transgenic green fluorescent protein), tight (“septate”) junctions labeled red with a-Discs-large, and nuclei labeled blue with 4,6-diamidino-2-phenylindole. C: overview of function of the 2 major cell types in the main segment of the tubule.
Development
There are uncanny similarities between development of flies and humans; indeed, homeotic genes were famously discovered in flies (52). Accordingly, the development of the Drosophila tubule has been studied in immense detail (6) as a model for tubulogenesis (57). The similarities in renal development between the two organisms have been reviewed elsewhere (18, 70); however, critically, both involve cells from more than one embryonic lineage, one of which undergoes a mesenchymal-to-epithelial transition (MET). The sequence is as follows.
1) Cell specification. A group of cells at the front of the hindgut are specified to a tubule fate by Kruppel and Cut; ectopic expression of these cells elsewhere in the gut leads to excretory competence, but not ectopic tubules (31).
2) Eversion of tubule primordia. Kruppel expression is refined to four clusters of cells, which acquire distinct dorsoventral identities under control of Decapentaplegic and Brinker (31).
3) Cell division. Initially all, then later a subset of cells under control of Wingless and Spitz (61), undergo two to five rounds of division.
4) Elongation by rearrangement. The short, fat tubules are elongated by rearrangements at cell-cell junctions. Simultaneously, the two pairs of tubules ramify into precisely defined areas of the body cavity, with the right-hand pair lying next to the gut anteriorly, and the left pair wrapping around the hindgut. Such lateral asymmetry is reminiscent of the human body plan. Mutations in punt or schnurri cause abnormal localization of tubules within the body cavity (6).
5) Mesenchymal recruitment. As the tubules elongate, a population of caudal mesoderm cells, expressing Tiptop and Teashirt, intercalate in specific regions of the tubules; they undergo MET and become the secondary or stellate cells (18). This is reminiscent of MET in development of the human nephron (57). Disruption of Hibris signaling compromises this insertion and is eventually fatal (18).
6) Functional differentiation. As embryonic development completes, the tubule develops functional competence, as is visible from the formation of uric acid crystals (43). As will be discussed later, urate nephrolithiasis is constitutive and symptomless in insect renal tubules (46).
Stem Cells and the Potential for Regeneration
A good model for stem cell regeneration in the kidney is important, because 1) some renal carcinomas can be ascribed to stem cell populations that escape normal controls, perhaps as a result of chronic inflammation (3); and 2) stem cell cloning (48) may itself provide a path to therapeutic replacement of kidneys damaged by polycystic kidney disease or malignancy (48, 58). It is thus interesting to explore the relevance of stem cell models in the genetically potent model Drosophila to human medicine.
In flies, the cell numbers established in embryogenesis are normally fixed throughout the life of the fly, and remarkably consistent between individuals (60). However, cells with properties resembling stem cells have been identified in the lower tubule. Under appropriate conditions, they divide and differentiate into both the two major cell types (principal and stellate), thus recapitulating cells with both ectodermal and mesenchymal origins (58). Furthermore, manipulation of the JAK/STAT pathway in just tubule stem cells is sufficient to produce tumorous overgrowth (58).
Nephrocytes and Evolution of the glomerular Kidney
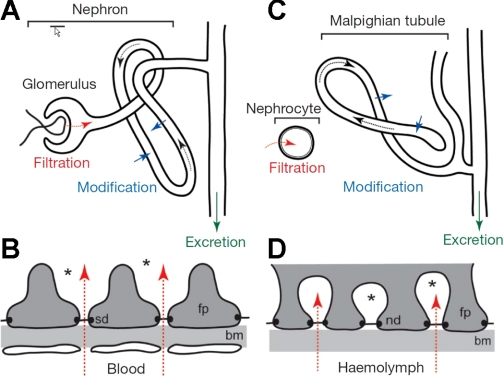
A conceptual barrier to the adoption of Drosophila as a kidney model is that invertebrate renal systems are aglomerular and so lack the structural complexity of the glomerular kidney. However, recently, it has been proposed that insects represent a window into the evolution of the glomerular kidney, specifically that the podocytes and the tubule have recognizable homologs in insects, but which are physically separate within the body cavity (70). The insect tubule is, of course, recognizable as an analog of the vertebrate renal tubule. It generates a primary urine, selectively reabsorbs some solutes, and like the vertebrate kidney has several distinct morphological and physiological regions. There is a second class of excretory cell in insects, i.e., nephrocytes. These cells sit singly or in small clusters (“garland cells”) within the body cavity and are known for roles in detoxification by filtration and endocytosis, followed by metabolism or sequestration. Furthermore, the nephrocyte plasma membrane is thrown into deep infoldings of ∼30-nm diameter, guarded by nephrin-containing barriers, strongly reminiscent of the glomerular podocyte slit diaphragm. The proposal is thus that in insects both filtration and transport roles exist in different tissues, but that it has not been evolutionarily necessary to bring them together (Fig. 2).
Fig. 2.
Similarities between glomerular and insect renal systems. Overviews of the glomerular (A) and insect renal systems (C). The podocyte slit diaphragm (B; sd) shows a striking resemblance to the basal labyrinth of insect nephrocytes (D). bm, Basement membrane; fp, foot process; nd, nephrocyte diaphragm. From Ref. 70.
Why has this not proved a necessary adaptation? The key may lie in the relative size of the organisms, and thus of the tasks faced by their renal systems. In insects, a combination of diffusion and stirring from mechanical movements of the body, combined with the action of a dorsal heart, suffices to maintain hemocoel mixing. Added to this, the tubules are capable of transporting their own volume of fluid every 10 s (the fastest per-cell rate known) (44). The size and high transport rates apparently render a pumped ultrafiltration system unnecessary. By contrast, the much larger body plan of most vertebrates demands a pumped vascular circulation. That is, the structural specializations required to provide excretory oversight of rapidly circulating blood are more extreme. Insects may thus provide a unique opportunity to dissect the differing contributions of these two cell types (nephrocyte and tubule epithelia) to our sophisticated kidney structure (glomerular capillaries/podocytes and transporting epithelia/nephron).
Function
As insect tubules can move their own cell volumes of fluid every 10 s, they offer an excellent model for the study of ion transport (6, 22). The middle region of the tubule (the “main segment”) (60) generates the primary urine, and two specialized cell types are involved (Fig. 1C). Four classes of transporters dominate the metabolically active principal cell: a basolateral Na+-K+-ATPase (63) and basolateral inward-rectifier K+ channels (25), and apically, a plasma membrane V-ATPase (15), and an alkali-metal/proton exchanger of the NHA, rather than the more familiar NHE, family (17). Together, these transporters and channels effect the active transport of potassium from the basolateral (hemocoel) to apical (tubule lumen) surface. The stellate cells provide the anion shunt conductance and water conductance via chloride channels (47) and aquaporins, respectively. Chloride is also thought to move paracellularly through the tight (in insects, septate) junctions, although the relative contributions of transcellular and paracellular routes may vary between insects. The Na+ driven Cl−/HCO3− exchanger (NDAE1) (54) and Na+ -K+-2Cl− cotransporter (NKCC) (34) are also present in tubules and may contribute to transepithelial chloride flux.
Disease Models
Drosophilists have moved with great speed to apply their favorite organism to human disease. Many of the analogies drawn are rather labored. Nevertheless, both renal function, and the underlying genes responsible, are rather well conserved; so some of the best Drosophila models of human disease occur in the renal context. Table 1 illustrates this point, showing that many of the classic human renal disease loci have Drosophila cognates that are both very close in sequence similarity (assessed by BLASTP) and with enriched expression in the Drosophila tubule (assessed by microarray).
Table 1.
Human renal disease loci conserved between fly and human
| Gene | Affymetrix Signal | Enrichment (Tubule:Fly) | BLAST Score | Human Disease | Notes |
|---|---|---|---|---|---|
| CG10226 | 1055 | 26 | 0 | Colchicine resistance | MDR1 homolog |
| CG1315 | 1934 | 23 | 1.00E-123 | Citrullinemia | Argininosuccinate synthase |
| ry | 770 | 19 | 0 | Xanthinuria, type I | Xanthine oxidase |
| CG31999 | 2097 | 16 | 2.00E-011 | Hyperuricemic nephropathy | Uromodulin/fibulin-like |
| CG31116 | 1162 | 11 | 1.00E-111 | Bartter syndrome types 3 and 4 | CLCNKA/B homolog |
| Spat | 1682 | 9.7 | 3.00E-091 | Hyperoxaluria, primary type 1 | Serine-pyruvate aminotransferase |
| CG1140 | 1729 | 9.5 | 2.00E-174 | Ketoacidosis: SCOT deficiency | Citric acid synthase |
| CG9547 | 1458 | 7.9 | 4.00E-171 | Glutaricacidemia type I | Glutaryl-CoA deydrogenase |
| CG6126 | 2316 | 7.5 | 4.00E-056 | Hypouricemia, 1 | SLC22A12 urate transporter |
| CG17119 | 1294 | 7 | 7.00E-073 | Cystinosis, nephropathic | CTNS cystine transporter |
| ir | 1099 | 5.5 | 2.00E-078 | Bartter syndrome type 2 | Inward-rectifier K+ channel |
| Drip | 589 | 5.1 | 4.00E-037 | Diabetes insipidus, nephrogenic, autosomal recessive | AQP2 water channel |
| CG5284 | 1335 | 4 | 0 | Dent disease | CLCK2 chloride channel |
| vha55 | 4016 | 3.7 | 0 | Renal tubular acidosis with deafness | V-ATPase subunit |
| wal | 3073 | 3.5 | 3.00E-112 | Glutaricaciduria type IIA | Electron transfer flavoprotein, α-polypeptide |
| CG7834 | 3035 | 2.1 | 1.00E-082 | Glutaricaciduria type IIB | Electron transfer flavoprotein, β-polypeptide |
The Homophila database (9) of Drosophila cognates of human genetic disease loci was mined for terms such as “nephro,” “renal,” “emia,” or “acidosis,” and the expression patterns of Drosophilacognates were assessed using the FlyAtlas.org online resource of tissue-specific Affymetrix microarray data (10). Genes shown here are ranked by their enriched expression in tubules compared with the whole fly, taking a BLAST threshold of 1E-10 as interesting.
MDR1, multidrug resistance 1; AQP2, aquaporin-2.
Transport genes.
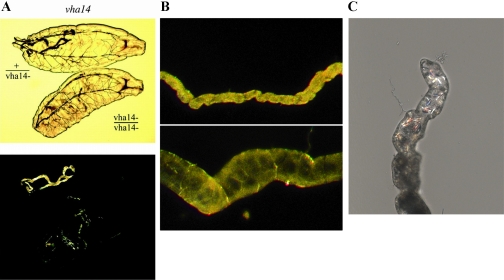
The first animal knockout of a V-ATPase subunit was demonstrated in Drosophila (15). The vha55 gene, encoding the B subunit, was shown to be essential, and dying animals had a renal acidification deficit, shared with mutations in any of the plasma membrane V-ATPase genes (2) (Fig. 3A). Drosophila work thus presaged the subsequent discovery that sublethal human V-ATPase mutations demonstrated renal tubular acidosis (38). Several classes of Bartter syndrome, salt-wasting diseases of the newborn, also have close homologs in Drosophila (Table 1). Of note, ATP-sensitive inward rectifier potassium channels (Kir) have been shown to be important in Drosophila renal function, as fluid secretion can be blocked with a spectrum of sulfonylurea antidiabetics (25). Drosophila tubules also express four aquaporins (Drip, CG17664, CG7777, and CG4019) at high levels (64) and are sensitive to organomercurials (23); one of the genes, Drip, has been shown to encode a functional water channel specifically in the secondary, stellate cell (39).
Fig. 3.
Examples of renal disease models in Drosophila. A: first instar larvae heterozygous or homozygous for a null mutation of a V-ATPase subunit, viewed by transmitted light (top) or birefringence (lower). The healthy heterozygotes have tubules packed with birefringent uric acid crystals; the dying homozygous sibs are unable to precipitate urate as uric acid. This phenotype is observed in null alleles of all 13 genes that encode the renal V-ATPase holoenzyme (2). B: tubules from rosy mutants (bottom) are bloated and deformed compared with wild-type (top), as a result of blockage by xanthine concretions (out of plane of photo). This recapitulates the symptoms of the analogous human disease, xanthinuria type I (64). C: birefringent calcium oxalate crystals forming in the initial segment of isolated tubules incubated in oxalate-containing medium (Cabrero P, personal communication). The relatively transparent tubules thus allow nucleation of oxalate stones to be followed in real time.
Inborn errors of metabolism and nephrolithiasis.
Interestingly, many human genetic diseases result from mutations of metabolic enzymes. Typically, the result of such a mutation is the depletion of downstream metabolites and the accumulation of upstream metabolites. While the former can often have serious sequelae, the renal system is often left to cope with the latter (accumulation). This is why nephrolithiasis frequently accompanies other presentations of inborn errors of metabolism (IEMs) (12, 69). Xanthinuria results from a breakdown of purine metabolism. Xanthinuria type I results from mutations in xanthine oxidase (19), and xanthinuria type II from defects in molybdopterin cofactor sulfurase, the enzyme which inserts the molybdenum-containing cofactor into molybdoenzymes such as xanthine oxidase (35, 67). Intriguingly, the Drosophila homologs (rosy and maroon-like) have long been known to recapitulate this form of nephrolithiasis, showing reduced viability, sensitivity to dietary purines, and bloated, malformed renal tubules (Fig. 3B), blocked by orange concretions (29). Laborious biochemical analysis (27, 45) and the cloning of candidate loci in Drosophila resulted in a clear understanding of the nature of the disease even as it was being described for the first time in humans. This must form one of the better Drosophila models of a human disease, and the metabolic perturbations have recently been followed further by metabolomics; perturbations in metabolite levels of rosy and maroon-like are broadly similar, and are detectable up to four metabolites away from the molecular lesion (37), allowing experimental testing of mathematical models for purine metabolism (14).
Drosophila tubules are relatively benign test-beds for stone disease. The simple tubule architecture means that stones are rarely lethal. Indeed, insects produce two classes of stone constitutively without ill effect. Calcium phosphate is stored in the tubule lumen as concentric “spherites,” perhaps to provide a calcium reserve (59), and the tubule lumen can be packed with uric acid crystals (Fig. 3A), providing a natural model for urate nephrolithiasis (28).
Of course, xanthinuria is a relatively rare cause of kidney stones, but the overall problem is massive, with 250,000 emergency room admissions annually in the US alone. The dominant form of stone in humans (∼70%) is formed of calcium oxalate. Several genes associated with hyperoxaluria are enriched in the Drosophila tubule (Table 1), suggesting that it might prove a useful model. Indeed, recently it has been shown that oxalate lithiasis can be modeled in Drosophila in response to dietary oxalate loading both in vivo and in vitro (21, 32, 33). Drosophila Prestin (Slc26a5) has been shown to act as a chloride/oxalate exchanger, analogous to the role of the apical SLC26A6 Cl−/ox2− exchanger in the human kidney (21). As the tubule is relatively transparent, it has proved possible to watch the nucleation and growth of oxalate crystals in the lumen in real time (Fig. 3C), offering exciting opportunities not possible in a structured glomerular kidney.
Metabolic disorders.
As accumulation of metabolites can contribute to nephrolithiasis, the kidney can be impacted by a range of IEMs (12). Given the close sequence conservation for most enzymes and transporters, it is perhaps not so surprising to see several such diseases represented with close Drosophila cognates in Table 1. Generally, these models exist only in silico and have yet to be investigated experimentally. However, given the extraordinary prevalence of some of these IEMs, particularly in certain ethnic and cultural groups (53), the potential availability of simple models may be attractive. For example, classic citrullinemia type I, a urea-cycle defect in argininosuccinate synthase (4), has an incidence of 1:17,000 live births in Saudi Arabia, but is almost unknown in the West. Interestingly, the single Drosophila gene encoding argininosuccinate synthase is exclusively expressed in the tubule, implying that, in contrast to NCBI GEO-documented widespread expression in humans, citrulline handling in Drosophila is a uniquely renal task, providing a compact, single-tissue model (Table 1).
Things You Can't Do with Drosophila Tubules
There are certain areas for which it would not be sensible to adopt the Drosophila tubule as a model, even though a clear gene homolog exists. For example, although insects show both lateral asymmetry of their viscera (Fig. 1A) (13) and planar cell polarity (56), their cells lack primary cilia, except in sperm and sensory dendrites (30, 42). Thus ciliary dysfunction, the major cause of polycystic kidney disease, cannot be modeled in tubules (71). Nonetheless, the TRPP channel gene Pkd2 is expressed in sperm (which have flagella) (68), and TRPN to the tips of mechanosensory cilia (42), and so fertility or sensory transduction could be used as a surrogate to screen for primary ciliary function in flies.
Endocrine signaling differs too. Although there are orthologs of enzymes such as angiotensin-converting enzyme (ACE) (36), there is no recognizable ortholog of the renin-angiotensin system. Insect ACE (a dipeptidase with broad specificity) must thus have other targets. Nor is there a clear insect homolog of Klotho, the fashionable anti-aging protein (62). Neuroendocrine control is via very distant homologs of corticotropin-releasing factor (CRF), calcitonin, tyramine, and some short invertebrate-specific peptides such as CAPA and the insect kinins (Fig. 1C). These have been reviewed elsewhere (11, 16, 49). Calcitonin-like and CRF-like peptides signal through cAMP in the principal cell to activate cation conductance. CAPA acts through calcium in the principal cell; however, because this cell also contains a calcium-activated nitric oxide synthase and high levels of cGMP-dependent protein kinase, CAPA also induces downstream elevation of nitric oxide and cGMP to stimulate cation transport. Tyramine and leucokinin both act to stimulate chloride shunt conductance through calcium in the stellate cell. A full range of second messenger pathways are thus amenable to genetic perturbation and phenotypic analysis in this tissue.
Conclusion: A Model for All Reasons
Despite the obvious issues with size and phylogeny, the Drosophila renal system provides a broad phylogenetic fix on conserved aspects of renal function. Importantly, Drosophila brings exquisite transgenic technologies to bear on renal physiology that would be inconceivable in a mammalian model or time prohibitive. The ability to work in an organotypic, rather than cell culture, context provides a valuable bridge from cell biology to organismal function. The high speed and low cost of deployment, combined with readily available mutant stocks, allows nimbleness in progressing appropriate research hypotheses. We have striven to show here that, most importantly, the Drosophila model is relevant to a broad range of fundamental questions and emerging areas in renal development and function.
GRANTS
This work was supported by the UK Biotechnology and Biological Science Research Council (BBSRC) and by National Institutes of Health Grants EY017732 and DK083007.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We are grateful to Prof. S. A. Davies for a critical reading of the manuscript.
REFERENCES
- 1.Akbari OS, Oliver D, Eyer K, Pai CY. An Entry/Gateway cloning system for general expression of genes with molecular tags in Drosophila melanogaster. BMC Cell Biol 10: 8, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan AK, Du J, Davies SA, Dow JAT. Genome-wide survey of V-ATPase genes in Drosophila reveals a conserved renal phenotype for lethal alleles. Physiol Genomics 22: 128–138, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature 432: 324–331, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Beaudet AL, O'Brien WE, Bock HG, Freytag SO, Su TS. The human argininosuccinate synthetase locus and citrullinemia. Adv Hum Genet 15: 161–196, 1986 [DOI] [PubMed] [Google Scholar]
- 5.Beyenbach KW, Liu PL. Mechanism of fluid secretion common to aglomerular and glomerular kidneys. Kidney Int 49: 1543–1548, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Beyenbach KW, Skaer H, Dow JAT. The developmental, molecular, and transport biology of Malpighian tubules. Annu Rev Entomol 55: 351–374, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Boy AL, Zhai Z, Habring-Muller A, Kussler-Schneider Y, Kaspar P, Lohmann I. Vectors for efficient and high-throughput construction of fluorescent Drosophila reporters using the PhiC31 site-specific integration system. Genesis 48: 452–456, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Brand AH, Perrimon N. Targetted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Chien S, Reiter LT, Bier E, Gribskov M. Homophila: human disease gene cognates in Drosophila. Nucleic Acids Res 30: 149–151, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila models of human disease. Nat Genet 39: 715–720, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Coast G. The endocrine control of salt balance in insects. Gen Comp Endocrinol 152: 332–338, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Cochat P, Pichault V, Bacchetta J, Dubourg L, Sabot JF, Saban C, Daudon M, Liutkus A. Nephrolithiasis related to inborn metabolic diseases. Pediatr Nephrol 25: 415–424, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coutelis JB, Petzoldt AG, Speder P, Suzanne M, Noselli S. Left-right asymmetry in Drosophila. Semin Cell Dev Biol 19: 252–262, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Curto R, Voit EO, Sorribas A, Cascante M. Validation and steady-state analysis of a power-law model of purine metabolism in man. Biochem J 324: 761–775, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies SA, Goodwin SF, Kelly DC, Wang Z, Sozen MA, Kaiser K, Dow JAT. Analysis and inactivation of vha55, the gene encoding the vacuolar ATPase B-subunit in Drosophila melanogaster reveals a larval lethal phenotype. J Biol Chem 271: 30677–30684, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Davies SA, Terhzaz S. Organellar calcium signalling mechanisms in Drosophila epithelial function. J Exp Biol 212: 387–400, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Day JP, Wan S, Allan AK, Kean L, Davies SA, Gray JV, Dow JAT. Identification of two partners from the bacterial Kef exchanger family for the apical plasma membrane V-ATPase of Metazoa. J Cell Sci 121: 2612–2619, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Denholm B, Sudarsan V, Pasalodos-Sanchez S, Artero R, Lawrence P, Maddrell S, Baylies M, Skaer H. Dual origin of the renal tubules in Drosophila: mesodermal cells integrate and polarize to establish secretory function. Curr Biol 13: 1052–1057, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Dent CE, Philpot GR. Xanthinuria: an inborn error of metabolism. Lancet I: 182–185, 1954 [DOI] [PubMed] [Google Scholar]
- 20.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Dow JA, Cabrero P, Hirata T, Chang M, Romero MF. A simple model for nephrolithiasis: Drosophila Prestin (Slc26a5) functions as a Cl−/Oxalate exchanger in the gut with kinetics similar to mammalian Slc26a6. Proc Physiol Soc 16: C15, 2009 [Google Scholar]
- 22.Dow JA, Davies SA. Integrative physiology and functional genomics of epithelial function in a genetic model organism. Physiol Rev 83: 687–729, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Dow JA, Kelly DC, Davies SA, Maddrell SH, Brown D. A novel member of the major intrinsic protein family in Drosophila—are aquaporins involved in insect Malpighian (renal) tubule fluid secretion? J Physiol 489: P110–P111, 1995 [Google Scholar]
- 24.Dow JA, Maddrell SH, Görtz A, Skaer NV, Brogan S, Kaiser K. The Malpighian tubules of Drosophila melanogaster: a novel phenotype for studies of fluid secretion and its control. J Exp Biol 197: 421–428, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Evans JM, Allan AK, Davies SA, Dow JA. Sulphonylurea sensitivity and enriched expression implicate inward rectifier K+ channels in Drosophila melanogaster renal function. J Exp Biol 208: 3771–3783, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Evans JM, Day JP, Cabrero P, Dow JA, Davies SA. A new role for a classical gene: white transports cyclic GMP. J Exp Biol 211: 890–899, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Glassman E, Mitchell HK. Mutants of Drosophila melanogaster deficient in xanthine dehydrogenase. Genetics 44: 153–162, 1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutman AB, Yu TF. Uric acid nephrolithiasis. Am J Med 45: 756–779, 1968 [DOI] [PubMed] [Google Scholar]
- 29.Hadorn E, Schwinck I. A mutant of Drosophila without isoxanthopterine which is non-autonomous for the red eye pigments. Nature 177: 940–941, 1956 [PubMed] [Google Scholar]
- 30.Han YG, Kwok BH, Kernan MJ. Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr Biol 13: 1679–1686, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Hatton-Ellis E, Ainsworth C, Sushama Y, Wan S, VijayRaghavan K, Skaer H. Genetic regulation of patterned tubular branching in Drosophila. Proc Natl Acad Sci USA 104: 169–174, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirata T, Cabrero P, Dow JAT, Romero MF. Drosophila Prestin provides an in vivo model for oxalate kidney stone formation. J Am Soc Nephrol 21: 486A, 2010 [Google Scholar]
- 33.Hirata T, Kato A, Cabrero P, Chang MH, Dow JA, Romero MF. Drosophila Slc26a5 functions as a Cl−/oxalate exchanger similar to mammalian Slc26a6. J Am Soc Nephrol 20: 385A, 2009 [Google Scholar]
- 34.Ianowski JP, O'Donnell MJ. Basolateral ion transport mechanisms during fluid secretion by Drosophila Malpighian tubules: Na+ recycling, Na+:K+:2Cl− cotransport and Cl− conductance. J Exp Biol 207: 2599–2609, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Ichida K, Matsumura T, Sakuma R, Hosoya T, Nishino T. Mutation of human molybdenum cofactor sulfurase gene is responsible for classical xanthinuria type II. Biochem Biophys Res Commun 282: 1194–1200, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Isaac RE, Bland ND, Shirras AD. Neuropeptidases and the metabolic inactivation of insect neuropeptides. Gen Comp Endocrinol 162: 8–17, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Kamleh MA, Hobani Y, Dow JAT, Watson DG. Metabolomic profiling of Drosophila using liquid chromatography Fourier transform mass spectrometry. FEBS Lett 582: 2916–2922, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Karet FE, Finberg KE, Nelson RD, Nayir A, Mocan H, Sanjad SA, Rodriguez-Soriano J, Santos F, Cremers CW, Di Pietro A, Hoffbrand BI, Winiarski J, Bakkaloglu A, Ozen S, Dusunsel R, Goodyer P, Hulton SA, Wu DK, Skvorak AB, Morton CC, Cunningham MJ, Jha V, Lifton RP. Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet 21: 84–90, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Kaufmann N, Mathai JC, Hill WG, Dow JA, Zeidel ML, Brodsky JL. Developmental expression and biophysical characterization of a Drosophila melanogaster aquaporin. Am J Physiol Cell Physiol 289: C397–C407, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi K, Bang Lu Y, Xian Li M, Nishi I, Hsiao KJ, Choeh K, Yang Y, Hwu WL, Reichardt JK, Palmieri F, Okano Y, Saheki T. Screening of nine SLC25A13 mutations: their frequency in patients with citrin deficiency and high carrier rates in Asian populations. Mol Genet Metab 80: 356–359, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Kondo T, Inagaki S, Yasuda K, Kageyama Y. Rapid construction of Drosophila RNAi transgenes using pRISE, a P-element-mediated transformation vector exploiting an in vitro recombination system. Genes Genet Syst 81: 129–134, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Lee J, Moon S, Cha Y, Chung YD. Drosophila TRPN(=NOMPC) channel localizes to the distal end of mechanosensory cilia. PLoS One 5: e11012, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X, Kiss I, Lengyel JA. Identification of genes controlling Malpighian tubule and other epithelial morphogenesis in Drosophila melanogaster. Genetics 151: 685–695, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maddrell SH. The fastest fluid-secreting cell known: the upper Malpighian tubule cell of Rhodnius. BioEssays 13: 357–362, 1991 [Google Scholar]
- 45.Mitchell HK, Glassman E. Hypoxanthine in rosy and maroon-like mutants of Drosophila melanogaster. Science 129: 268, 1959 [DOI] [PubMed] [Google Scholar]
- 46.Munn CA. Note on a method of obtaining uric acid crystals from the Malpighian tubes of insects and from the nephridium of pulmonate Mollusca. J Physiol 7: 128–129, 1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Donnell MJ, Rheault MR, Davies SA, Rosay P, Harvey BJ, Maddrell SH, Kaiser K, Dow JA. Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am J Physiol Regul Integr Comp Physiol 274: R1039–R1049, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Perin L, Giuliani S, Sedrakyan S, Da Sacco S, De Filippo RE. Stem cell and regenerative science applications in the development of bioengineering of renal tissue. Pediatr Res 63: 467–471, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Predel R, Wegener C. Biology of the CAPA peptides in insects. Cell Mol Life Sci 63: 2477–2490, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rong YS, Golic KG. Gene targeting by homologous recombination in Drosophila. Science 288: 2013–2018, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Rosay P, Davies SA, Yu Y, Sozen MA, Kaiser K, Dow JAT. Cell-type specific calcium signalling in a Drosophila epithelium. J Cell Sci 110: 1683–1692, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Rubin GM, Lewis EB. A brief history of Drosophila's contributions to genome research. Science 287: 2216–2218, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Saadallah AA, Rashed MS. Newborn screening: experiences in the Middle East and North Africa. J Inherit Metab Dis 30: 482–489, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Sciortino CM, Shrode LD, Fletcher BR, Harte PJ, Romero MF. Localization of endogenous and recombinant Na+-driven anion exchanger protein NDAE1 from Drosophila melanogaster. Am J Physiol Cell Physiol 281: C449–C463, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Shao H, Sinasac DS, Burrage LC, Hodges CA, Supelak PJ, Palmert MR, Moreno C, Cowley AW, Jr, Jacob HJ, Nadeau JH. Analyzing complex traits with congenic strains. Mamm Genome 21: 276–286, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simons M, Gault WJ, Gotthardt D, Rohatgi R, Klein TJ, Shao Y, Lee HJ, Wu AL, Fang Y, Satlin LM, Dow JT, Chen J, Zheng J, Boutros M, Mlodzik M. Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization. Nat Cell Biol 11: 286–294, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh SR, Hou SX. Multipotent stem cells in the Malpighian tubules of adult Drosophila melanogaster. J Exp Biol 212: 413–423, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh SR, Liu W, Hou SX. The adult Drosophila Malpighian tubules are maintained by multipotent stem cells. Cell Stem Cell 1: 191–203, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Southall TD, Terhzaz S, Cabrero P, Chintapalli VR, Evans JM, Dow JAT, Davies SA. Novel subcellular locations and functions for secretory pathway Ca2+/Mn2+-ATPases. Physiol Genomics 26: 35–45, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Sözen MA, Armstrong JD, Yang MY, Kaiser K, Dow JAT. Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proc Natl Acad Sci USA 94: 5207–5212, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sudarsan V, Pasalodos-Sanchez S, Wan S, Gampel A, Skaer H. A genetic hierarchy establishes mitogenic signalling and mitotic competence in the renal tubules of Drosophila. Development 129: 935–944, 2002 [DOI] [PubMed] [Google Scholar]
- 62.Torres PU, Prie D, Molina-Bletry V, Beck L, Silve C, Friedlander G. Klotho: an antiaging protein involved in mineral and vitamin D metabolism. Kidney Int 71: 730–737, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Torrie LS, Radford JC, Southall TD, Kean L, Dinsmore AJ, Davies SA, Dow JA. Resolution of the insect ouabain paradox. Proc Natl Acad Sci USA 101: 13689–13693, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J, Kean L, Yang J, Allan AK, Davies SA, Herzyk P, Dow JA. Function-informed transcriptome analysis of Drosophila renal tubule. Genome Biol 5: R69, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang T, Giebisch G. Tubule function in transgenic mice. Exp Nephrol 6: 447–453, 1998 [DOI] [PubMed] [Google Scholar]
- 66.Wang T, Giebisch G, Aronson PS. Use of transgenic animals to study renal acid-base transport. J Nephrol 155: S151–S160, 2002 [PubMed] [Google Scholar]
- 67.Watanabe T, Ihara N, Itoh T, Fujita T, Sugimoto Y. Deletion mutation in Drosophila ma-l homologous, putative molybdopterin cofactor sulfurase gene is associated with bovine xanthinuria type II. J Biol Chem 275: 21789–21792, 2000 [DOI] [PubMed] [Google Scholar]
- 68.Watnick TJ, Jin Y, Matunis E, Kernan MJ, Montell C. A flagellar polycystin-2 homolog required for male fertility in Drosophila. Curr Biol 13: 2179–2184, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Watts RW. Metabolic causes of renal stone formation. Postgrad Med J 53, Suppl 2: 7–24, 1977 [PubMed] [Google Scholar]
- 70.Weavers H, Prieto-Sanchez S, Grawe F, Garcia-Lopez A, Artero R, Wilsch-Brauninger M, Ruiz-Gomez M, Skaer H, Denholm B. The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 457: 322–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Q, Taulman PD, Yoder BK. Cystic kidney diseases: all roads lead to the cilium. Physiology (Bethesda) 19: 225–230, 2004 [DOI] [PubMed] [Google Scholar]