Abstract
The KCNQ1 K+ channel plays a key role in the regulation of several physiological functions, including cardiac excitability, cardiovascular tone, and body electrolyte homeostasis. The metabolic sensor AMP-activated protein kinase (AMPK) has been shown to regulate a growing number of ion transport proteins. To determine whether AMPK regulates KCNQ1, we studied the effects of AMPK activation on KCNQ1 currents in Xenopus laevis oocytes and collecting duct epithelial cells. AMPK activation decreased KCNQ1 currents and channel surface expression in X. laevis oocytes, but AMPK did not phosphorylate KCNQ1 in vitro, suggesting an indirect regulatory mechanism. As it has been recently shown that the ubiquitin-protein ligase Nedd4-2 inhibits KCNQ1 plasma membrane expression and that AMPK regulates epithelial Na+ channels via Nedd4-2, we examined the role of Nedd4-2 in the AMPK-dependent regulation of KCNQ1. Channel inhibition by AMPK was blocked in oocytes coexpressing either a dominant-negative or constitutively active Nedd4-2 mutant, or a Nedd4-2 interaction-deficient KCNQ1 mutant, suggesting that Nedd4-2 participates in the regulation of KCNQ1 by AMPK. KCNQ1 is expressed at the basolateral membrane in mouse polarized kidney cortical collecting duct (mpkCCDc14) cells and in rat kidney. Treatment with the AMPK activators AICAR (2 mM) or metformin (1 mM) reduced basolateral KCNQ1 currents in apically permeabilized polarized mpkCCDc14 cells. Moreover, AICAR treatment of rat kidney slices ex vivo induced AMPK activation and intracellular redistribution of KCNQ1 from the basolateral membrane in collecting duct principal cells. AICAR treatment also induced increased ubiquitination of KCNQ1 immunoprecipitated from kidney slice homogenates. These results indicate that AMPK inhibits KCNQ1 activity by promoting Nedd4-2-dependent channel ubiquitination and retrieval from the plasma membrane.
Keywords: KCNE1, ion channels, potassium transport
metabolic stress inhibits the expression and activities of a variety of membrane transport proteins, which may act to limit the dissipation of transmembrane ionic gradients and thus conserve the energy required to maintain them in tissues under conditions of ischemia or hypoxia (28). However, the molecular mechanisms linking membrane transport activity to cellular metabolism are unclear. The metabolic sensor AMP-activated protein kinase (AMPK) has emerged as a regulator of a growing number of epithelial transport proteins, including the cystic fibrosis transmembrane conductance regulator (CFTR), the epithelial Na+ channel (ENaC), the vacuolar H+-ATPase (V-ATPase), and the creatine transporter SLC6A8 (15, 26, 29, 34, 46). The regulation of membrane transport proteins by AMPK may afford the sensitive coupling of ion transport to underlying cellular metabolic status in epithelia and other tissues with high metabolic demands (28).
AMPK is a ubiquitous, heterotrimeric Ser/Thr kinase composed of a catalytic α- and regulatory β- and γ-subunits. AMPK activity is exquisitely sensitive to metabolic perturbations, with allosteric activation occurring in response to elevated intracellular AMP:ATP ratios through preferential binding of AMP over ATP to the γ-subunit. Activation of AMPK also requires phosphorylation of Thr-172 in the activation loop of the α-subunit by upstream kinases, which include the LKB1 protein complex and the Ca2+/calmodulin-dependent kinase kinase-β (35). Many studies have established that a key function of AMPK is to regulate energy balance within the cell. Once activated, AMPK phosphorylates a variety of substrates, the overall effect of which is to switch off ATP-consuming processes and to switch on ATP-generating pathways in cells (16, 28).
Several studies have reported inhibition of membrane transport proteins, most notably ENaC, via the E3 ubiquitin-protein ligase Nedd4-2, which ubiquitinates target membrane proteins and enhances their internalization and degradation (1, 21, 25). We have recently shown that ENaC inhibition by AMPK is mediated by Nedd4-2 (8, 15). Therefore, we considered that AMPK may regulate other ion channels and transporters through its ability to activate Nedd4-2. Recently, the KCNQ1 K+ channel has shown to be regulated by Nedd4-2 via internalization from the plasma membrane and subsequent degradation when both proteins were expressed in HEK-293 cells (42).
KCNQ1 is a low-conductance and voltage-dependent potassium channel (41). KCNQ1 can associate with regulatory KCNE β-subunits, resulting in channel complexes with different electrical and pharmacological properties (50, 51). KCNQ1 channels are expressed in several different tissues, where they regulate important physiological functions. In cardiomyocytes, KCNQ1 currents are partly responsible for terminating the cardiac action potential (41, 53, 56). Mutations in KCNQ1 causing channel dysfunction may result in the cardiac long QT syndrome, a disorder characterized by serious, life-threatening arrhythmias (52, 53). In epithelial tissues, KCNQ1 is a regulator of significant energy-consuming ion transport processes, such as acid and chloride secretion in gastric and colonic epithelia, respectively, where it has been proposed to play an important role by compensating for membrane depolarization induced by these secretory processes (37, 44, 63). In the kidney, KCNQ1 is expressed in several segments of the nephron (66), although its function has not been well characterized (36). It has been suggested that activation of these channels in the proximal tubule minimizes the depolarization of the luminal membrane associated with electrogenic Na+-dependent glucose and amino acid transport (59, 60). Also, KCNQ1 knockout mice were found to have hypokalemia, urinary and fecal salt wasting, and volume depletion, thereby indicating an important role for this channel in total-body salt and fluid homeostasis (59, 60). The potential role of KCNQ1 in the distal nephron of the kidney has not been previously studied in detail.
In this study, we tested whether KCNQ1 is a target for regulation by AMPK and found that AMPK inhibits KCNQ1 channel activity both in the Xenopus laevis oocyte expression system and in mouse polarized kidney cortical collecting duct (mpkCCDc14) cells. As with ENaC, this regulation appears to be mediated through the ubiquitin ligase Nedd4-2 and involves a downregulation of KCNQ1 expression at the basolateral membrane by AMPK. We propose that KCNQ1 inhibition by AMPK may limit cellular K+ recycling under conditions of metabolic stress, thus reducing cellular energy expenditure for epithelial ion transport.
MATERIALS AND METHODS
Reagents and chemicals.
All chemicals used were obtained from Sigma (St. Louis, MO) unless otherwise noted. 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranosyl 5′-monophosphate (ZMP) was obtained from Toronto Research Chemicals (North York, ON). Chromanol 293B and L-364,373 were obtained from Tocris Bioscience (Ellisville, MO). PKA catalytic subunit (cPKA) was obtained from Promega (Madison, WI). Anti-V5 and horseradish peroxidase (HRP)-conjugated anti-V5 antibodies and TO-PRO-3 nuclear stain were obtained from Invitrogen. Anti-AMPK-α-pThr172 antibody was obtained from Cell Signaling Technology. Goat polyclonal anti-KCNQ1 antibody (clone C-20) was obtained from Santa Cruz Biotechnology, and rabbit polyclonal anti-KCNQ1 antibody was obtained from Alomone Laboratories, Rabbit anti-ubiquitin antibody was obtained from Sigma-Aldrich. Rat polyclonal antibody against zona occludens-1 (ZO)-1 was a gift from Dr. Gerald Apodaca (2). Secondary HRP-conjugated anti-mouse and anti-rabbit IgG antibodies were obtained from Amersham Biosciences. Plasmids for mammalian or oocyte expression of wild-type (WT) and mutant N-terminal FLAG-tagged X. laevis Nedd4-2 (FLAG-xNedd4-2) and c-Myc-tagged human KCNQ1 and KCNE1 subunits (gifts of Dr. Dan Roden) were generated and used as described previously (8, 43). Purified recombinant active human AMPK holoenzyme (α1-T172D, β1, γ1) was kindly provided by Dr. Dietbert Neumann and synthesized as described (54). Paraformaldehyde was obtained from Electron Microscopy Sciences (Hatfield, PA).
Oocyte expression.
Maintenance of X. laevis frogs, surgical extraction of ovaries, and collagenase treatment of oocytes were carried out as described (15). Complementary RNAs (cRNAs) for all proteins expressed in oocytes were synthesized using the mMessage mMachine kit (Ambion, Austin, TX) following the manufacturer's instructions. The purity and quantity of cRNA were assessed by agarose gel electrophoresis. Stage V-VI oocytes were injected within 1 day of collagenase treatment with 3.5 ng KCNQ1 cRNA alone or plus 3.5 ng KCNE1 cRNA. To test for Nedd4-2 effects, 1.5 ng WT, 0.7 ng constitutively active (CA), or 5 ng dominant negative (DN) Nedd4-2 cRNAs were also injected. Oocytes were then maintained in ND96 solution containing 96 mM NaCl, 4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM HEPES, pH 7.4 supplemented with 2.5 mM sodium pyruvate and 50 μg/ml gentamycin at 18°C for up to 3 days before experimentation.
Two-electrode voltage clamp.
Two-electrode voltage-clamp (TEV) measurements were performed using oocytes continuously perfused with ND96 solution, as described previously (15). Current-voltage relationships were measured by holding at −80 mV for 400 ms and stepping to a series of test potentials from −100 to +100 mV in 20-mV increments for 4,000 ms, followed by a tail pulse at −80 mV. KCNQ1-mediated K+ currents were defined as the current difference measured in the absence vs. the presence of 50 μM chromanol 293B in the bath solution. To activate cellular AMPK for experiments, 40 mM potassium-ZMP (K-ZMP) or potassium-gluconate (K-gluconate; control) was microinjected into oocytes (32 nl/oocyte). TEV measurements were performed 1–4 h postinjection (15).
Surface labeling of myc-KCNQ1 channels expressed in X. laevis oocytes.
Experiments were performed as previously described with minor modifications (57). Oocytes expressing c-Myc-tagged KCNQ1 (43) were washed three times for 5 min at 4°C with ND96 with 1% BSA and then incubated for 30 min in 1% BSA/ND96 at 4°C to prevent nonspecific antibody binding. Oocytes were then incubated for 60 min at 4°C with monoclonal anti-c-Myc antibody clone 9E10 (Roche, 400 μg/ml, 1:400 in 1% BSA/ND96), washed five times for 10 min at 4°C with 1% BSA/ND96, and incubated with 2 μg/ml HRP-conjugated affinity-purified sheep anti-mouse IgG antibody in 1% BSA/ND96 for 60 min. Oocytes were washed thoroughly 10 times, initially in 1% BSA/ND96 (5 min at 4°C), and then 5 times in ND96 without BSA (5 min at 4°C). Individual oocytes were placed in 100 μl SuperSignal ELISA Pico Chemiluminescent Substrate (Pierce) and incubated at room temperature for 1 min. Chemiluminescence was quantitated with a TD-20/20 Luminometer (Turner Designs). The results from 15–20 oocytes were averaged per batch, and results from two batches of oocytes are presented in relative light units (RLU).
In vitro phosphorylation assays.
HEK-293T cells were maintained in Dulbecco's modified Eagle's medium (BioWhittaker), 10% fetal bovine serum, and penicillin/streptomycin, passaged twice weekly, seeded at subconfluent density into 60-mm dishes 1 day before transient transfection with 3 μg human KCNQ1 or CFTR plasmid DNA, and lysed 2 days after transfection. KCNQ1 or CFTR was immunoprecipitated from cell lysates using the anti-c-Myc monoclonal antibody or the m24–1 anti-CFTR monoclonal antibody coupled to protein A/G beads (Pierce), as described (34, 43). In vitro phosphorylation was performed using 1 μg/μl purified active AMPK holoenzyme with γ-[32P]ATP labeling, as previously described (15). As a positive control for KCNQ1 phosphorylation, parallel in vitro phosphorylation was performed using purified cPKA. After SDS-PAGE and transfer to nitrocellulose membranes, immunoblotting for expression of c-Myc-KCNQ1 or CFTR was first performed and quantitated using a Versa-Doc Imager with Quantity One software (Bio-Rad). After the chemiluminescent signal had decayed, phosphorylated bands on the membrane were imaged by exposure to a phosphoscreen, and the bands were quantitated using a Bio-Rad Phosphorimager.
Protein preparation and western blotting from cells.
Cells were lysed on ice for 15 min by adding ice-cold lysis buffer containing 20 mM Tris, pH 7.4, 0.5% Nonident P-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 1 μg/μl leupeptin, 500 μM DTT, 5 mM PMSF, and complete protease inhibitor cocktail tablets (Roche). Following incubation, the samples were centrifuged at 16,000 g for 10 min. The cleared supernatant was aliquoted to a new tube, and the protein was quantified by a Bradford assay. Western blot analysis was performed using 25 μg cellular protein/sample. Following SDS-PAGE on a 4–12% gradient gel (NuPage, Invitrogen), proteins were transferred to a nitrocellulose membrane, blocked in 1× Tris-buffered saline with Tween (0.03%) (1× TBST) and 5% nonfat dry milk for 1 h. Membranes were incubated with rabbit anti-KCNQ1 or other appropriate primary antibody overnight at 4°C and, after washing in TBST, were incubated for 1 h at room temperature with secondary antibody. After three additional 15-min washes in 1× TBST, signals were detected using the ECL-Plus reagent (Amersham Biosciences).
Transepithelial short-circuit current measurements.
mpkCCDc14 cells were cultured as described (15), grown on Snapwell filters (1.2-cm2 surface area; Corning Costar) for 7 days, and then mounted in Ussing chambers (Physiologic Instruments) for short-circuit current (Isc) measurements. Transepithelial potential difference was clamped to zero, and Isc was recorded using Ag-AgCl electrodes in 3 M KCl agar bridges as previously described (31, 33). Transepithelial resistance was monitored by inducing 1-s transepithelial voltage pulses of ±2 mV each minute throughout the recordings. Transepithelial resistances were >1,000 Ω·cm2 for all filters throughout the experiments. All preparations were allowed to equilibrate in Ussing chambers for 15 min before the experiments were performed. Isc was defined as positive for cation flow from the apical to the basolateral chamber. For basolateral K+ current measurements, amiloride (20 μM) was added to the apical bath to block electrogenic Na+ absorption. To investigate the activity of basolateral K+ channels in isolation, the apical membrane was permeabilized by addition of 20 μM amphotericin B in an apical-to-basolateral K+ gradient (140:5 mM), while the Na+ concentration was maintained at 25 mM on both sides of the monolayers as previously described (13). The resulting Isc is due to the movement of K+ through channels through the basolateral membrane (IK). For measurements of amiloride-sensitive transepithelial Isc, various inhibitors were added as described in results and Supplemental Fig. S1, legend (supplementary material for this article is available online at the journal web site).
Immunofluorescence labeling and confocal microscopy in CCD cells.
mpkCCDc14 cell monolayers were permeabilized and fixed in 4% (wt/vol) paraformaldehyde using a previously described pH-shift protocol (3, 4). All incubations were performed at room temperature. Cells were incubated with primary antibodies against KCNQ1 (1:200) and ZO-1 (1:200) for 1 h, washed three times with block buffer for 5 min, and then incubated with fluorescent-labeled secondary antibodies (donkey anti-goat conjugated to FITC at 1:1,000, pseudocolored red, and goat anti-rat conjugated to CY3 at 1:2,000, pseudocolored blue; Jackson Immunologicals) for 1 h. After three additional 5-min washes with block buffer, the cells were rinsed with PBS, treated with TO-PRO-3 (1:400 in PBS; pseudocolored green) for 5 min, and then mounted as described previously (3). Imaging was performed using a TCS-SL confocal microscope (Leica) equipped with argon, green helium-neon, and red helium-neon lasers. Images were acquired using a ×100 1.4-numerical aperture oil objective. Photomultipliers were set to 600–900 V and zoom at ×4. Images were collected every 0.25 μm and averaged three times. The images were saved in a TIFF format and compiled using Volocity software (Improvision, Lexington, MA).
Kidney tissue slice preparation.
All animal experiments were approved by the Institutional Animal Care and Utilization Committee at the University of Pittsburgh, in accordance with the NIH Guide for Care and Use of Laboratory Animals. Adult male Sprague-Dawley rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (65 mg/kg). The rats were perfused via the left ventricle with Ringer solution (pH 7.4) at 37°C as previously described (12, 13, 61). Both kidneys were removed from the rat at the time of the perfusion, then cut into 4- to 5-mm-thick sections and quickly placed in Ringer solution (pH 7.4) at 37°C equilibrated with 5% CO2-95% air, and further sectioned into 0.5-mm slices as described (13, 26). The slices were first incubated at 37°C for 10 min in equilibrated Ringer buffer. These slices were then simultaneously transferred to fresh vials containing Ringer buffer or to Ringer buffer with AICAR (2 mM) for 75 min at 37°C. At the end of the incubation period, several slices were quickly frozen in liquid nitrogen for Western blot preparation, and other slices were fixed by immersion in periodate-lysine containing 4% paraformaldehyde, followed by washes, quenching, and cryoprotection in 30% sucrose as described (29). These experiments were repeated in at least three different kidney tissue slices preparations.
Immunofluorescence labeling and Western blot of kidney tissue slices.
Kidney slices were embedded in Tissue-Tek and sectioned using a cryostat (4-μm thick) as described (11, 29). The cryosections were picked up on polylysine-coated slides, rehydrated in PBS for 15 min, and treated with 1% SDS for antigen retrieval (14). Blocking and immunostaining of the sections was performed as described (11, 29), using the primary rabbit polyclonal KCNQ1 antibody (1:50 dilution). The secondary antibody used was a goat anti-rabbit IgG conjugated to FITC (1:60 dilution, Jackson Immunologicals). Tissues were also colabeled with an antibody raised in chicken against the E subunit of the V-ATPase, followed by a secondary antibody goat anti-chicken CY3 (1:800, Jackson Immunologicals) as previously described (26). Alternatively, tissues were colabeled with an antibody raised in goat against aquaporin-2 (AQP2; 1:400, Santa Cruz Biotechnology), a principal cell marker, followed by a secondary donkey anti-goat antibody coupled to CY3 (1:800, Jackson Immunologicals). Sections were mounted in Vectashield (Vector Labs), then examined using a Leica laser-scanning microscope, and imported into Adobe Photoshop, as previously described (26).
Frozen kidney slices were briefly thawed on ice and placed in lysis buffer [150 mM NaCl, 10 mM Tris (pH 7.4), 1% Triton X-100, 0.5% Igepal, 1 mM EDTA, 1 mM EGTA (pH 7.4), 200 μM PMSF, and one-quarter tablet of Complete protease inhibitor cocktail per 10 ml of buffer solution, 50 mM NaF, 5 mM Na pyrophosphate, and 0.1 mM Na orthovanadate]. The samples were homogenized using a glass/Teflon potter (Fisher) on ice, and the homogenate was centrifuged at 13,000 rpm in a Fisher accuSpin microcentrifuge for 15 min at 4°C. The supernatant was used for protein quantification using the Bio-Rad protein assay reagent, and 50 μg of protein/sample were subjected to SDS-PAGE, as described above. Immunoblotting was performed as above, and membranes were incubated with anti-AMPK-α-pThr172 antibody (1:2,500) in 5% milk in TBS-Tween followed by HRP-conjugated secondary anti-rabbit antibody (1:10,000).
Ubiquitination of KCNQ1 channels in rat kidney slices.
Kidney slices were treated with Ringer buffer or with Ringer buffer containing AICAR (2 mM) for 75 min, then frozen, and homogenized in lysis buffer as described above. Equal amounts of whole kidney lysate proteins (1,000 μg/sample) were precleared with 50 μl of protein A/G Sepharose and incubated for 1 h at 4°C. Lysates were then rotated for 1 h at 4°C in the presence or absence of 2 μg/ml goat anti-KCNQ1 antibody. After the addition of 50 μl of protein A/G-Sepharose, the protein solutions were incubated overnight at 4°C. After extensive washing, bound proteins were eluted by heating samples at 65°C for 15 min in 25 μl of 2× Laemmli sample buffer containing 100 mM DTT. Immunoprecipitated KCNQ1 was analyzed by Western blotting using a rabbit anti-ubiquitin antibody (1:200) to detect ubiquitinated KCNQ1, or rabbit anti-KCNQ1 (1:1,000) to control for total KCNQ1 protein in whole lysates and the immunoprecipitated fraction. Protein signals were detected by chemiluminescence and quantitated using Versa-Doc with Quantity One software (Bio-Rad), and the results were normalized to the control values.
Statistical analysis.
TEV data generated from different oocyte batches were pooled, and statistical analyses were performed using an ANOVA factorial model to account for batch-to-batch variability in chromanol 293B-sensitive KCNQ1 currents. In the figure legends, n indicates the number of oocytes measured, and N indicates the number of oocyte batches used. For other biochemical experiments, statistics were performed using unpaired or paired Student's t-tests, as described for each figure. StatView (SAS Institute, Cary, NC) was used for statistical analyses. In all cases, P values <0.05 were considered significant.
RESULTS
AMPK activation inhibits KCNQ1 and KCNQ1/KCNE1 currents expressed in oocytes.
To determine whether the metabolic-sensing kinase AMPK regulates KCNQ1 K+ channel activity, chromanol 293B-sensitive currents were measured by TEV in X. laevis oocytes that had been injected with KCNQ1 cRNA alone or together with KCNE1 cRNA using the TEV technique (Fig. 1). Under basal conditions, oocytes expressing KCNQ1 channels evoked relatively slowly activating K+ currents at potentials more positive than −40 mV (Fig. 1A, top left). Coexpression of KCNE1 increased whole-cell currents and drastically altered the kinetics of the KCNQ1 channel, resulting in extremely slowly activating and deactivating potassium currents at potentials more positive than −20 mV (Fig. 1A, bottom left). Typical current-voltage curves with KNCQ1 alone or with coexpression of KCNE1 are shown in Fig. 1B. Activation of AMPK by microinjection of oocytes with the potassium salt of the AMP analog ZMP, which is the active intracellular metabolite of the AMPK activator AICAR (17), significantly reduced KCNQ1 and KCNQ1/KCNE1 currents compared with oocytes that were injected with a K+-gluconate control solution (Fig. 1A, right, and C). These results suggest that the activity of KCNQ1 K+ channels is inhibited by AMPK in this system.
Fig. 1.
AMP-activated protein kinase (AMPK) inhibits KCNQ1 and KCNQ1/E1 channels expressed in Xenopus laevis oocytes. cRNAs encoding KCNQ1 alone or KCNQ1 along with KCNE1 were injected into X. laevis oocytes 3 days before the performance of two-electrode voltage clamp (TEV) experiments, as described in materials and methods. A: representative current-time sweeps with voltage-clamp steps between −100 and +100 mV are shown in oocytes expressing KCNQ1 alone (top) or KCNQ1+KCNE1 (bottom) after microinjection with either K+-gluconate (left) or K+-5-aminoimidazole-4-carboxamide-1-β-d-ribofuranosyl 5′-monophosphate (ZMP; right) 1–4 h before the measurements. B: representative chromanol 293B-sensitive current-voltage (I-V) curves are shown for oocytes expressing KCNQ1 alone (●) or KCNQ1+KCNE1 (○) that were previously microinjected with K+-gluconate control solution. C: summary of mean ± SE chromanol 293B-sensitive currents measured at +100-mV potential in oocytes expressing KCNQ1 alone (left) or KCNQ1 + KCNE1 (right) following microinjection with either K+-gluconate control (gray bars) or the AMPK activator K+-ZMP (black bars). *P < 0.01, by ANOVA for the indicated comparisons; n = 20–26 oocytes for each condition from 3 separate batches.
AMPK does not directly phosphorylate KCNQ1 in vitro.
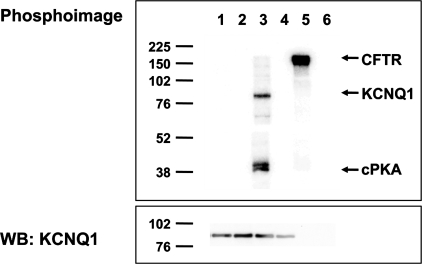
Previous studies have shown that AMPK can regulate ion channel activity by direct phosphorylation, so we tested whether AMPK directly phosphorylates KCNQ1 in vitro. KCNQ1 was transiently expressed in HEK-293T cells, immunoprecipitated from cell lysates, and then incubated with γ-[32P]ATP in the presence or absence of purified recombinant AMPK (Fig. 2). The samples were then resolved by SDS-PAGE and transferred to nitrocellulose membranes. No detectable 32P incorporation into KCNQ1 was observed in the samples treated with AMPK. CFTR was used as a positive control to confirm AMPK activity (34), and PKA was used as a positive control to demonstrate phosphorylation of KCNQ1 by another kinase (49). The inability of AMPK to phosphorylate KCNQ1 in vitro suggests that its regulation of KCNQ1 occurs via an indirect mechanism that does not depend on direct channel phosphorylation.
Fig. 2.
AMPK does not directly phosphorylate KCNQ1 in vitro. HEK-293T cells transfected to express either KCNQ1 (lanes 1–4) or CFTR (lanes 5 and 6) were lysed, and then the channels were immunoprecipitated from cell lysates for in vitro phosphorylation assays using either no added kinase (lanes 2, 4, and 6), purified AMPK holoenzyme (lanes 1 and 5), or purified PKA catalytic subunit (lane 3), as described in materials and methods. Representative phospho-screen (top) and Western blot (WB; bottom) images of the same nitrocellulose membrane are shown from 1 of 3 repeat experiments. Positive controls confirmed that AMPK was able to phoshorylate CFTR (lane 5), and PKA was able to phosphorylate KCNQ1 (lane 3), as previously described (34, 49). However, there was no detectable KCNQ1 phosphorylation by AMPK in vitro (lane 1). Western blot analysis confirmed comparable KCNQ1 expression in lanes 1–4 (bottom).
Nedd4-2 catalytic activity is required for AMPK-dependent inhibition.
Recent studies in ventricular cardiac myocytes have shown that KCNQ1 channels are regulated by members of the E3 ubiquitin-protein ligase family by direct interaction with a highly conserved atypical PY-motif (LPTY) located at the COOH-terminal tail of KCNQ1 (42). Previous studies from our laboratory have shown that AMPK downregulates ENaC via functional regulation of the E3 ubiquitin ligase Nedd4-2, presumably inducing ubiquitination, internalization from the plasma membrane, and eventual degradation of ENaC (8, 15). Therefore, we hypothesized that the AMPK-dependent regulation of KCNQ1 is also mediated via functional regulation of Nedd4-2. Expression of a KCNQ1 PY-motif Tyr-to-Ala mutant (Y662A) significantly enhanced chromanol 293B-sensitive currents compared with WT KCNQ1-associated currents and prevented the current inhibition by the AMPK activator ZMP (Fig. 3A). These findings suggest that KCNQ1 inhibition by AMPK requires the binding of Nedd4-2 to KCNQ1 and that tonic inhibition of the channel by endogenous Nedd4-2 in the oocyte may be relieved by expression of this Nedd4-2 binding-deficient KCNQ1 mutant. To determine the functional role of Nedd4-2 in the regulation of KCNQ1 by AMPK, we measured chromanol 293B-sensitive whole cell currents in oocytes coexpressing KCNQ1/KCNE1 channels, along with either WT or mutant xNedd4-2 constructs. Coexpression of WT Nedd4-2 with KCNQ1/KCNE1 decreased currents by >50% compared with those of oocytes expressing KCNQ1/KCNE1 alone under control conditions (Fig. 3B). Coexpression of a ubiquitin ligase-deficient, dominant-negative (DN) xNedd4-2 mutant (C938S) greatly increased basal KCNQ1/KCNE1 currents (Fig. 3C), presumably by preventing endogenous xNedd4-2-dependent KCNQ1 ubiquitination and degradation. Under this condition, the AMPK activator ZMP failed to downregulate KCNQ1 (Fig. 3C), suggesting that Nedd4-2-mediated KCNQ1 ubiquitination is involved in the AMPK-dependent inhibition of KCNQ1. Conversely, coexpression of a constitutively active (CA) xNedd4-2 mutant (S338A, S444A), which lacks two dominant SGK1/PKA phosphorylation sites known to promote cellular sequestration of Nedd4-2 through enhanced binding to 14-3-3 proteins (7, 39), substantially decreased KCNQ1/KCNE1 currents. Again, ZMP failed to inhibit KCNQ1/KCNE1 current with coexpression of this xNedd4-2 mutant (Fig. 3D). Taken together, these findings indicate that modulation of Nedd4-2 activity is necessary for the AMPK-dependent inhibition of KCNQ1/KCNE1.
Fig. 3.
AMPK-dependent regulation of KCNQ1 channels is mediated via functional regulation of Nedd4-2. Two-electrode voltage clamp (TEV) experiments were performed on oocytes expressing KCNQ1 (wild-type or mutant) and KCNE1 along with or without xNedd4-2 (wild-type or mutant). The oocytes were then microinjected with either the AMPK activator ZMP (black bars) or K+-gluconate (KG) control (gray bars) 1–4 h before experimentation, and chromanol 293B-sensitive KCNQ1/E1 currents were measured by TEV at a voltage of +100 mV. A, left: ZMP significantly reduced KCNQ1/E1 currents compared with KG-treated oocytes. Right: expression of KCNQ1 with a PY-motif mutation (Y662A) significantly increased KCNQ1/E1 currents and prevented ZMP-dependent current inhibition. NS, not significant. B: coexpression of wild-type xNedd4-2 (1.5 ng cRNA/oocyte) inhibited KCNQ1/E1 currents, and ZMP treatment inhibited KCNQ1/E1 currents in both the presence and absence of exogenous Nedd4-2 coexpression. C: coexpression of ubiquitin-ligase activity-deficient xNedd4-2 mutant (C938S; 5 ng/oocyte) enhanced KCNQ1/E1 currents and prevented inhibition of these currents by ZMP. DN, dominant negative. D: coexpression of a constitutively active (CA) Nedd4-2 mutant (S338A/S444A; 0.7 ng) dramatically reduced KCNQ1/E1 currents and prevented inhibition of these currents by ZMP. For each condition 22–30 oocytes were recorded. *P < 0.05 by ANOVA for indicated comparison.
AMPK activation inhibits KCNQ1 surface membrane expression in oocytes.
As AMPK-dependent regulation of KCNQ1 activity appears to be mediated by Nedd4-2, we hypothesized that the inhibitory mechanism involves an inhibition of channel expression at the plasma membrane. To test this hypothesis, we performed antibody surface labeling experiments in X. laevis oocytes expressing external c-Myc epitope-tagged KCNQ1 along with KCNE1 with or without prior injection of the AMPK activator ZMP (Fig. 4). As measured by luminometry of peroxidase-conjugated antibody-labeled oocytes, surface expression of KCNQ1 channels decreased by 40–45% with ZMP treatment (Fig. 4A). Nonspecific labeling of H2O-injected oocytes not expressing KCNQ1/KCNE1 was minimal (∼10% of control signal; Fig. 4A). Parallel measurements of KCNQ1-dependent current revealed a comparable ∼50% reduction in current with ZMP treatment (Fig. 4B). These findings suggest that the inhibition of KCNQ1 by AMPK results from decreased KCNQ1 plasma membrane expression. Over the time course of ZMP treatment (3 h), there was no significant difference in total KCNQ1 expression in the oocytes, as detected by Western blotting (Fig. 4C), suggesting that there was a net redistribution of channels from the plasma membrane to internal cellular compartments.
Fig. 4.
AMPK activation reduces plasma membrane expression of KCNQ1/KCNE1 channels. A: KCNQ1 tagged with an extracellular c-Myc epitope was coexpressed with KCNE1 subunits in X. laevis oocytes. The oocytes were then microinjected with either ZMP (black bars) or K+-gluconate control (gray bars) and incubated for 3 h. The c-myc epitope inserted in the S1-S2 extracellular loop of KCNQ1 enabled the detection of channel surface expression by a chemiluminescence assay. Activation of AMPK with ZMP significantly reduced the surface expression of KCNQ1/KCNE1 channels by ∼40–45% compared with oocytes injected with K+-gluconate. Oocytes injected with water instead of cRNA (white bar) were tested to determine background signal, which accounted for <10% of control values. To pool data from different batches of oocytes, surface expression values were normalized to the corresponding mean values of wild-type control oocytes. Values are means ± SE of 34–38 oocytes for each condition from 2 separate batches of oocytes, *P < 0.01 by unpaired t-test. B: chromanol 293B-sensitive currents were determined in parallel in oocytes from the same experimental groups. Values are means ± SE of 10–12 oocytes for each condition from 2 separate batches of oocytes, *P < 0.01 by unpaired t-test. C: representative KCNQ1 Western blot analysis showing no significant difference in whole cell expression of KCNQ1 protein between oocytes treated with ZMP or K+-gluconate.
Functional KCNQ1 channels are expressed at the basolateral membrane of mpkCCDc14 cells.
KCNQ1 channels are expressed in most segments of the nephron, particularly in the S3 segment of the proximal tubule, the distal convoluted tubule, and the cortical collecting duct (66). We have previously characterized the effects of AMPK on Na+ transport in the mouse polarized kidney cortical collecting duct mpkCCDc14 cell line. We first confirmed that mouse kidneys and mpkCCDc14 cells, which have a principal cell phenotype and current activation response to the hormones aldosterone and vasopressin (6), express functional KCNQ1/KCNE1 channels. Immunoblotting for KCNQ1 and KCNE1 revealed characteristic bands at an apparent molecular mass of 75 and 25 kDa, respectively (Fig. 5A, left). Both bands were virtually eliminated when the immunoblots were probed using anti-KCNQ1 and anti-KCNE1 antibodies that had been preadsorbed with their respective immunizing peptides (Fig. 5A, right). Moreover, immunofluorescence localization studies using confocal microscopy revealed that KCNQ1 has a distinct distribution at or near the basolateral membrane in these cells (Fig. 5B).
Fig. 5.
KCNQ1 and KCNE1 expression in mouse kidney homogenates and mpkCCDc14 cells (A) and KCNQ1 cellular localization in polarized mpkCCDc14 cells (B). A, left: 30 μg of mouse total kidney homogenate (lane 1), or 50 μg of nonpolarized (lane 2) or polarized (lane 3) mpkCCDc14 cell lysates were immunoblotted with antibodies against either KCNQ1 (top), KCNE1 (middle), or β-actin (bottom), as described in materials and methods. Right: parallel samples run on the same membrane were incubated with the KCNQ1 or KCNE1 antibodies along with 10-fold excess of immunizing peptide. B: polarized mpkCCDc14 cells grown on Transwell filters were immunostained with TOPRO nuclear stain (green), KCNQ1 (red), and the tight junction marker zonula occludens (ZO)-1 (blue) as described in materials and methods. Pseudocoloring was performed for each fluorophore to enhance clarity of the images. Representative x-y sections are shown at the level of the tight junction (row A) and at a midsection of the monolayer (row B). Merged images are shown on the right. Bottom: x-z reconstruction of merged images demonstrates KCNQ1 in a basolateral membrane distribution. Scale bar = 10 μm.
AMPK inhibits endogenous KCNQ1 activity in mpkCCDc14 cells.
To test whether AMPK activation inhibits KCNQ1 currents in polarized mpkCCDc14 cells, we performed Isc measurements on these cells polarized on filters and mounted in Ussing chambers in the presence and absence of the AMPK activators AICAR (2 mM) and metformin (1 mM). A time course study demonstrated that both of these drugs significantly activated AMPK, as measured by phospho-Thr172 AMPK immunoblots, as early as 2–4 h after treatment (Fig. 6A). This AMPK activation persisted through 24 h after treatment with either drug (Fig. 6B). To specifically measure KCNQ1-mediated currents at the basolateral membrane of the cells, amiloride was added to the apical bath to inhibit ENaC first, and then amphotericin B was added to permeabilize the apical membrane and thereby isolate the basolateral membrane for Isc measurements. The specific benzodiazepine KCNQ1 activator (L-364,373; 50 μM) was then added to induce KCNQ1 activity at the basolateral membrane, and finally the specific KCNQ1 channel blocker chromanol 293B (50 μM) was added to inhibit the channel (Fig. 6C). KCNQ1-dependent current was defined as the difference between peak current after L-364,373 treatment and the current after chromanol 293B treatment. In cells that were pretreated overnight (16 h) with 1 mM metformin, the KCNQ1-dependent current was significantly reduced by ∼50% relative to control (Fig. 6D). Similar inhibition of KCNQ1 current was found following overnight 2 mM AICAR treatment (Fig. 6E). Together, these data indicate that AMPK activation inhibits KCNQ1 activity in kidney collecting duct cultured collecting duct principal-like cells.
Fig. 6.
AMPK activation inhibits basolateral KCNQ1 currents in polarized mpkCCDc14 cells. A: representative Western blot of cell lysates showing 24-h time course of AMPK activity (pThr172-α1-AMPK; top) with β-actin as loading control (bottom) following treatment of polarized mpkCCDc14 cells with 1 mM metformin. B: summary of means ± SE relative AMPK activities (normalized to control at time 0) following treatment for up to 24 h with 2 mM AICAR (○), 1 mM metformin (●), or vehicle control (◊). *P < 0.05, unpaired t-test compared with control at time 0; n = 3 replicate experiments for each drug treatment data point; n = 6 for controls. C: representative short-circuit current (Isc) tracings of mpkCCDc14 cells pretreated for 16 h with 1 mM metformin (dark gray trace) or vehicle (light gray trace) in the presence of an apical-to-basolateral K+ gradient, as described in materials and methods. Cell monolayers were mounted in Ussing chambers and treated first with 20 μM amiloride apically to inhibit ENaC, then 20 μM amphotericin B apically to isolate conductance at the basolateral membrane, then 50 μM of the KCNQ1 activator L-364,373 basolaterally, then 50 μM chromanol 293B basolaterally, and finally 2 mM BaCl2 basolaterally at the indicated times. One-second transepithelial voltage pulses of ±2 mV were performed each minute throughout the recordings but were removed from the displayed current trace for clarity. D: summary of means ± SE basolateral KCNQ1-mediated Isc (defined as difference between peak current following L-364,373 treatment and the current measured after chromanol 293B treatment) with or without 1 mM metformin pretreatment for 16 h. E: summary of means ± SE basolateral KCNQ1-mediated Isc with or without 2 mM AICAR pretreatment for 16 h. *P < 0.05, unpaired t-test, compared with control; n = 8 filters analyzed in 4 separate experiments for all conditions. Inset: representative immunoblots of cell lysates for pThr172-α1-AMPK and β-actin following the AICAR treatment experiment.
The AMPK activator AICAR reduces KCNQ1 expression at the basolateral pole of rat principal cells.
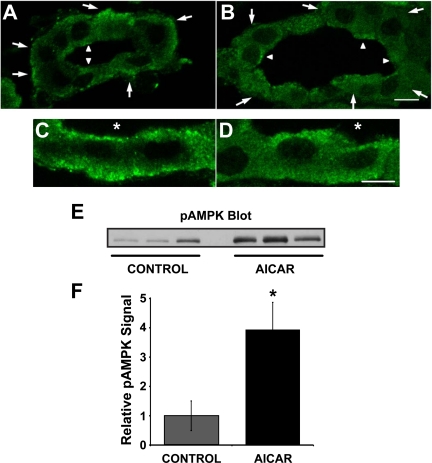
By immunofluorescence labeling, we observed that KCNQ1-associated fluorescence was present at the basolateral pole of collecting duct principal cells in kidney slice tissue treated under control conditions in Ringer buffer for 75 min (Fig. 7A). Principal cells (larger arrows) were identified by their lack of immunofluorescence labeling for an intercalated cell marker, the V-ATPase, or by costaining with the principal cell marker AQP2 (not shown). KCNQ1 immunolabeling in principal cells of kidney slices treated with the AMPK activator AICAR (2 mM) for 75 min showed qualitatively a more diffuse basolateral labeling (Fig. 7B). Higher-magnification images of principal cells treated with either Ringer buffer alone (Fig. 7C) or with AICAR for 75 min (Fig. 7D) confirmed more diffuse cytoplasmic labeling in AICAR-treated kidney slices.
Fig. 7.
AICAR induces a redistribution of KCNQ1 away from the basolateral pole in collecting duct principal cells of rat kidney slices. Rat kidney slices were treated for 75 min in either control Ringer buffer alone (A and C) or Ringer buffer+2 mM AICAR (B and D) before immunofluorescence staining and laser-scanning confocal microscopy. Principal cells were identified by costaining with the principal cell marker aquaporin-2 (AQP2) or by the absence of the intercalated cell marker V-ATPase (not shown). In A and B, large arrows outside tubules indicate principal cells, and small arrowheads within tubular lumens indicate intercalated cells (scale bar = 20 μm). C and D are higher magnification images of principal cells (scale bar = 10 μm). Asterisk (*) denotes lumen. These images are representative of at least 3 separate experiments using 3 different kidney slice preparations. Three separate kidney slice lysates were immunoblotted for the active AMPK using an antibody raised against AMPK-α-pTh172 (E). Values are relative means ± SE of AMPK-α-pThr172 signal for 3 separate slices normalized to β-actin (F). *P < 0.05, unpaired t-test.
To confirm AMPK activation in kidney slices treated with AICAR, we performed Western blotting of kidney slice lysates using an anti-AMPK-α-pThr172 antibody, which recognizes the active form of the kinase (Fig. 7E). Quantification of the anti-AMPK-α-pThr172 signal normalized to β-actin revealed that AICAR treatment for 75 min induced a significant approximately threefold activation of AMPK in kidney slices compared with slices treated with Ringer buffer alone (Fig. 7F).
AMPK activation by AICAR enhances ubiquitination of KCNQ1 in kidney slices.
As Nedd4-2 activity is required for the regulation of KCNQ1 by AMPK (Fig. 3), we hypothesized that enhanced ubiquitination of the channel would be observed with AICAR treatment of kidney slices. KCNQ1 was immunoprecipitated from kidney slice homogenates after treatment for 75 min with AICAR (vs. Ringer buffer alone), and immunoblotting was performed on the tissue lysates and immunoprecipitates (Fig. 8). Comparable amounts of total KCNQ1 were present in the lysates (Fig. 8A, top left) and immunoprecipitates (Fig. 8A, bottom right), but there was an increase in ubiquitinated KCNQ1 in the AICAR-treated kidney slices (Fig. 8A, top right). Densitometric quantification of the relative KCNQ1 ubiquitination from four replicate experiments confirmed that AICAR treatment increased ubiquitinated KCNQ1 to ∼2–2.5 times that of control-treated kidney slices (Fig. 8B).
Fig. 8.
AMPK activation stimulates ubiquitination of KCNQ1 channels in rat kidney slices. Rat kidney slices were treated for 75 min in either control Ringer buffer alone of Ringer buffer containing 2 mM AICAR before lysis, homogenization, immunoprecipitation (IP), and immunoblotting, as described in materials and methods. A: representative immunoblot showing increased ubiquitination of KCNQ1 in rat kidney slices treated with AICAR compared with kidney slices treated with control buffer (top right). Ubiquitination of KCNQ1 produced a shift in the observed molecular weight of KCNQ1, consistent with the conjugation of ubiquitin chains. The amount of KCNQ1 protein in the immunoprecipitate was determined by reprobing with an anti-KCNQ1 antibody (bottom right). No ubiquitin or KCNQ1 signals were detected in parallel samples incubated without anti-KCNQ1 antibody during the immunoprecipitation. Kidney slice lysate samples were probed for KCNQ1 and β-actin (left) to assess basal expression. B: densitometric analysis of ubiquitinated KCNQ1 normalized to the corresponding amount of KCNQ1 immunoprecipitated from lysates of kidney slices treated with AICAR or control buffer. Values are means ± SE relative to control values of 4 independent kidney slice preparations. *P < 0.01 by paired t-test.
Inhibition of basolateral K+ channels dampen amiloride-sensitive Isc after stimulation with aldosterone and vasopressin in polarized mpkCCDc14 cells.
To test the potential physiological significance of conductance through basolateral KCNQ1 and other K+ channels in the transepithelial reabsorption of Na+ in kidney collecting duct principal cells, we measured amiloride-sensitive Isc across polarized mpkCCDc14 cell monolayers under control conditions or following stimulation with either 1 μM aldosterone for 4 h and/or 100 nM desmopressin (dDAVP) for 15–30 min before and after treatment with the KCNQ1 channel blocker chromanol 293B (50 μM; see Supplemental Fig. S1). The nonspecific K+ channel blocker barium (2 mM BaCl2) was added to the apical bath to block any K+ secretion at the apical membrane and thus more specifically measure transepithelial Na+ reabsorption. In cells cultured in basic medium overnight, inhibition of basolateral KCNQ1 channels by chromanol 293B caused a very slight but consistent inhibition of amiloride-sensitive transepithelial Isc, by 2.2 ± 0.3% (Supplemental Fig. S1B). This very minor effect was not appreciably altered by either prior aldosterone or dDAVP stimulation of amiloride-sensitive Isc. However, basolateral treatment with 2 mM BaCl2 caused a more substantial 35.3 ± 3.4% reduction in amiloride-sensitive current (Supplemental Fig. S1B). These results confirm that basolateral membrane K+ recycling plays a significant physiological role in enhancing transepithelial Na+ reabsorption in CCD cells (27). However, under the conditions of this study the KCNQ1 channel appears to play only a very minor role in supporting transepithelial Na+ reabsorption in these CCD cells, with other K+ channel(s) likely largely responsible for this process.
DISCUSSION
In this study, we identified the KCNQ1 K+ channel as a new ion channel target for regulation by the metabolic sensor AMPK in the heterologous X. laevis oocyte expression system, in mpkCCDc14 polarized kidney collecting duct cells, and in native rat kidney slices (Figs. 1, 4, 6, and 7). Similar to ENaC (8, 15), AMPK-dependent inhibition of KCNQ1 does not appear to occur via direct phosphorylation of the channel by AMPK (Fig. 2), but rather via the ubiquitin-protein ligase Nedd4-2 (Fig. 3). This AMPK-dependent regulation is blocked in a KCNQ1 mutant that lacks a functional Nedd4-2 interaction motif and in dominant-negative and constitutively active Nedd4-2 mutants.
There are differing reports in the literature regarding the distribution and subcellular localization of KCNQ1 in the kidney. Wingo and colleagues (66) described basolateral expression of KCNQ1 in the distal convoluted tubule, connecting tubule, and inner medullary collecting duct but more diffuse and apical staining in the cortical collecting duct of mice. Vallon and colleagues (59) did not detect a clear-cut signal for KCNQ1 downstream of the proximal tubule in mice. Differences in apparent KCNQ1 distribution along the nephron between these studies may reflect the different antibodies, fixation, and immunolocalization staining techniques used. Our studies revealed a basolateral distribution of the KCNQ1 channel in mpkCCDc14 cells (Fig. 5) and in principal cells from rat kidney slices (Fig. 7). In addition, the subcellular localization in rat kidney slices appears to be more apical in the neighboring intercalated cells (Fig. 7, A and B), as previously suggested by Wingo and colleagues (66).
We also found that AMPK activation by treatment with either AICAR or metformin, two mechanistically distinct AMPK activators, inhibited basolateral conductance mediated by KCNQ1 in these cells (Fig. 6). Moreover, the KCNQ1 channel redistributed away from the basolateral pole of principal cells to a more diffuse cytoplasmic distribution and became more heavily ubiquitinated when rat kidney ex vivo slices were treated with AICAR (Figs. 7 and 8). Taken together, these findings suggest that AMPK inhibits KCNQ1 by promoting its Nedd4-2-dependent ubiquitination, thereby decreasing functional channel expression at the basolateral plasma membrane, presumably through enhanced endocytic retrieval.
It has been previously demonstrated that Nedd4-2 reduces KCNQ1 protein levels when expressed in HEK-293 cells through enhanced ubiquitination of the channel (42). This model is very similar to that proposed for ENaC regulation by Nedd4-2 (21). Furthermore, both channels appear to be upregulated by SGK1 through inhibition of the association of Nedd4-2 with the channels (18, 24). Unlike ENaC, however, KCNQ1 is expressed largely at the basolateral membrane in collecting duct principal cells. Although Nedd4-2 expression is largely present in an apical and subapical cytosolic distribution, a previous report has found that Nedd4-2 is distributed throughout the plasma membrane and not specifically localized to the apical membrane in kidney epithelial cells (40). In HEK-293 cells and cardiomyocytes, Jespersen and colleagues (42) found that KCNQ1 and KCNQ1/KCNE1 currents were reduced by coexpression of Nedd4-2 but not by a catalytically inactive Nedd4-2 mutant. Additional experiments showed that Nedd4-2-mediated inhibition of KCNQ1 is strictly dependent on the PY-motif located in the COOH-terminal domain of the channel (42). These results suggest that KCNQ1 internalization and stability are physiologically regulated by its Nedd4-2-dependent ubiquitination. This mechanism may thereby be important in regulating the surface density of the KCNQ1 channels in cardiomyocytes and other cell types. As KCNQ1 activity is a key regulator of ion transport, regulation of this channel may allow for concerted regulation of ion transport in several tissues.
Our findings that AMPK inhibits the basolateral expression and activity of KCNQ1 in kidney collecting duct principal cells can likely be extrapolated to other tissues where KCNQ1 plays an important role. Specifically, KCNQ1 appears to facilitate cAMP-mediated CFTR Cl− secretion in the colon and lung by providing a basolateral recycling pathway for K+ entry mediated by the basolateral Na+-K+-2Cl− cotransporter, whose activity is required to maintain a favorable gradient for apical Cl− secretion (48, 65). Of note, Walker et al. (64) found that AMPK activation, either acutely or in the setting of chronic inflammation, inhibits CFTR activity in mouse colon in vivo. We have similarly reported that AMPK inhibits CFTR in polarized human colonic T84 and bronchial Calu-3 cells (32, 33). Based on this study, it is reasonable to propose that concomitant AMPK-dependent inhibition of basolateral KCNQ1 activity in colonic and lung epithelial cells may help to potentiate the inhibition of CFTR-mediated Cl− secretion by AMPK at the apical membrane. The inhibition of various ion transport pathways by AMPK under conditions of metabolic and other cellular stresses would appear to play an adaptive role. Specifically, limiting the diffusive fluxes of K+ and other ions under conditions of tissue ischemia via AMPK would limit the ongoing need for Na+-K+-ATPase activity to maintain transmembrane ionic gradients and is thus consistent with the role of AMPK in inhibiting cellular ATP-consuming processes.
Based on our results, it seems plausible that other transport proteins that are regulated by Nedd4-2 are also be regulated by AMPK (e.g., other voltage-gated K+ and Na+ channels, the Na+-glucose cotransporter SGLT1, glutamate transporters, the Na+-phosphate cotransporter NaPi IIb, and ClC-Ka/barttin) (9, 10, 19, 23, 38, 55, 62). Moreover, recent studies suggest that Nedd4-2 represents a final common mediator for the regulation of transport proteins by various kinases in addition to AMPK, including SGK1, PKA, IκB kinase-β, Akt/PKB, and JNK1 (18, 22, 30, 45, 58). Thus Nedd4-2 appears to be a locus for the integration and coordination of various cellular signaling pathways and kinases in the regulation of transport protein activity.
While KCNQ1 activity appears to play an important role in electrogenic Na+-coupled solute reabsorption in the kidney proximal tubule (60), the physiological role of KCNQ1 in the distal nephron is unclear at present. Our preliminary results in mpkCCDc14 cells do not suggest that basolateral KCNQ1 K+ recycling plays an important role in collecting duct Na+ reabsorption under basal conditions or following hormonal stimulation with aldosterone or vasopressin (Supplemental Fig. S1). However, KCNQ1 has been described to play an important role in the cell swelling-induced regulatory volume decrease response in both proximal and distal primary renal epithelial cells (5). Moreover, a recent report implicates KCNQ1 channels in lipopolysaccharide-induced apoptosis of distal kidney cells, which is mediated by KCNQ1-dependent KCl efflux (apoptotic volume decrease) (20). Interestingly, AMPK has been shown to have both anti-inflammatory and antiapoptotic effects (31, 47), so it is conceivable that KCNQ1 inhibition by AMPK contributes to these effects. Further studies are warranted to address the physiological relevance of the regulation of KCNQ1 by AMPK in the distal nephron of the kidney.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK075048 (to K. R. Hallows) and R01 DK084184 (to N. M. Pastor Soler) and P30 DK079307, the Pittsburgh Center for Kidney Research. C. Rondanino was supported by the Urinary Tract Epithelial Imaging Core of the Pittsburgh Center for Kidney Research. This work was also supported by American Heart Association Grant 09GRNT2060539 (to N. M. Pastor-Soler) and a Postdoctoral Fellowship Award 0825540D (to R. Alzamora).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dan Roden for the gift of KCNQ1 and KCNE1 plasmids, Gerry Apodaca for providing anti-ZO1 antibody, Alain Vandewalle for providing mpkCCDc14 cells, and Dietbert Neumann for providing purified active human AMPK holoenzyme. We also thank Marcelo Carattino for technical advice with the oocyte antibody surface labeling experiments.
REFERENCES
- 1.Abriel H, Staub O. Ubiquitylation of ion channels. Physiology (Bethesda) 20: 398–407, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Acharya P, Beckel J, Ruiz WG, Wang E, Rojas R, Birder L, Apodaca G. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am J Physiol Renal Physiol 287: F305–F318, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J Cell Biol 125: 67–86, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacallao R, Stelzer EH. Preservation of biological specimens for observation in a confocal fluorescence microscope and operational principles of confocal fluorescence microscopy. Methods Cell Biol 31: 437–452, 1989 [DOI] [PubMed] [Google Scholar]
- 5.Belfodil R, Barriere H, Rubera I, Tauc M, Poujeol C, Bidet M, Poujeol P. CFTR-dependent and -independent swelling-activated K+ currents in primary cultures of mouse nephron. Am J Physiol Renal Physiol 284: F812–F828, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol 10: 923–934, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Bhalla V, Daidie D, Li H, Pao AC, LaGrange LP, Wang J, Vandewalle A, Stockand JD, Staub O, Pearce D. Serum- and glucocorticoid-regulated kinase 1 regulates ubiquitin ligase neural precursor cell-expressed, developmentally down-regulated protein 4–2 by inducing interaction with 14–3-3. Mol Endocrinol 19: 3073–3084, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, Pearce D, Hallows KR. AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4-2. J Biol Chem 281: 26159–26169, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Boehmer C, Henke G, Schniepp R, Palmada M, Rothstein JD, Broer S, Lang F. Regulation of the glutamate transporter EAAT1 by the ubiquitin ligase Nedd4-2 and the serum and glucocorticoid-inducible kinase isoforms SGK1/3 and protein kinase B. J Neurochem 86: 1181–1188, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Boehmer C, Laufer J, Jeyaraj S, Klaus F, Lindner R, Lang F, Palmada M. Modulation of the voltage-gated potassium channel Kv1.5 by the SGK1 protein kinase involves inhibition of channel ubiquitination. Cell Physiol Biochem 22: 591–600, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Bouley R, Breton S, Sun T, McLaughlin M, Nsumu NN, Lin HY, Ausiello DA, Brown D. Nitric oxide and atrial natriuretic factor stimulate cGMP-dependent membrane insertion of aquaporin 2 in renal epithelial cells. J Clin Invest 106: 1115–1126, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouley R, Pastor-Soler N, Cohen O, McLaughlin M, Breton S, Brown D. Stimulation of AQP2 membrane insertion in renal epithelial cells in vitro and in vivo by the cGMP phosphodiesterase inhibitor sildenafil citrate (Viagra). Am J Physiol Renal Physiol 288: F1103–F1112, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Breton S, Brown D. Cold-induced microtubule disruption and relocalization of membrane proteins in kidney epithelial cells. J Am Soc Nephrol 9: 155–166, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Brown D, Lydon J, McLaughlin M, Stuart-Tilley A, Tyszkowski R, Alper S. Antigen retrieval in cryostat tissue sections and cultured cells by treatment with sodium dodecyl sulfate (SDS). Histochem Cell Biol 105: 261–267, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Carattino MD, Edinger RS, Grieser HJ, Wise R, Neumann D, Schlattner U, Johnson JP, Kleyman TR, Hallows KR. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J Biol Chem 280: 17608–17616, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Carling D. The AMP-activated protein kinase cascade–a unifying system for energy control. Trends Biochem Sci 29: 18–24, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229: 558–565, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J 20: 7052–7059, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dieter M, Palmada M, Rajamanickam J, Aydin A, Busjahn A, Boehmer C, Luft FC, Lang F. Regulation of glucose transporter SGLT1 by ubiquitin ligase Nedd4-2 and kinases SGK1, SGK3, and PKB. Obes Res 12: 862–870, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Duranton C, Rubera I, L'Hoste S, Cougnon M, Poujeol P, Barhanin J, Tauc M. KCNQ1 K+ channels are involved in lipopolysaccharide-induced apoptosis of distal kidney cells. Cell Physiol Biochem 25: 367–378, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Eaton DC, Malik B, Bao HF, Yu L, Jain L. Regulation of epithelial sodium channel trafficking by ubiquitination. Proc Am Thorac Soc 7: 54–64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edinger RS, Lebowitz J, Li H, Alzamora R, Wang H, Johnson JP, Hallows KR. Functional regulation of the epithelial Na+ channel by IkappaB kinase-beta occurs via phosphorylation of the ubiquitin ligase Nedd4-2. J Biol Chem 284: 150–157, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Embark HM, Bohmer C, Palmada M, Rajamanickam J, Wyatt AW, Wallisch S, Capasso G, Waldegger P, Seyberth HW, Waldegger S, Lang F. Regulation of CLC-Ka/barttin by the ubiquitin ligase Nedd4-2 and the serum- and glucocorticoid-dependent kinases. Kidney Int 66: 1918–1925, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Embark HM, Bohmer C, Vallon V, Luft F, Lang F. Regulation of KCNE1-dependent K+ current by the serum and glucocorticoid-inducible kinase (SGK) isoforms. Pflügers Arch 445: 601–606, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Flores SY, Debonneville C, Staub O. The role of Nedd4/Nedd4-like dependant ubiquitylation in epithelial transport processes. Pflügers Arch 446: 334–338, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Gong F, Alzamora R, Smolak C, Li H, Naveed S, Neumann D, Hallows KR, Pastor-Soler NM. Vacuolar H+-ATPase apical accumulation in kidney intercalated cells is regulated by PKA and AMP-activated protein kinase. Am J Physiol Renal Physiol 298: F1162–F1169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray DA, Frindt G, Zhang YY, Palmer LG. Basolateral K+ conductance in principal cells of rat CCD. Am J Physiol Renal Physiol 288: F493–F504, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Hallows KR. Emerging role of AMP-activated protein kinase in coupling membrane transport to cellular metabolism. Curr Opin Nephrol Hypertens 14: 464–471, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Hallows KR, Alzamora R, Li H, Gong F, Smolak C, Neumann D, Psstor-Soler NM. AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells. Am J Physiol Cell Physiol 296: C672–C681, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallows KR, Bhalla V, Oyster NM, Wijngaarden MA, Lee JK, Li H, Chandran S, Xia X, Huang Z, Chalkley RJ, Burlingame AL, Pearce D. Phosphopeptide screen uncovers novel phosphorylation sites of Nedd4-2 that potentiate its inhibition of the epithelial Na+ channel. J Biol Chem 285: 21671–21678, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hallows KR, Fitch AC, Richardson CA, Reynolds PR, Clancy JP, Dagher PC, Witters LA, Kolls JK, Pilewski JM. Up-regulation of AMP-activated kinase by dysfunctional cystic fibrosis transmembrane conductance regulator in cystic fibrosis airway epithelial cells mitigates excessive inflammation. J Biol Chem 281: 4231–4241, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Hallows KR, Kobinger GP, Wilson JM, Witters LA, Foskett JK. Physiological modulation of CFTR activity by AMP-activated protein kinase in polarized T84 cells. Am J Physiol Cell Physiol 284: C1297–C1308, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Hallows KR, McCane JE, Kemp BE, Witters LA, Foskett JK. Regulation of channel gating by AMP-activated protein kinase modulates cystic fibrosis transmembrane conductance regulator activity in lung submucosal cells. J Biol Chem 278: 998–1004, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Hallows KR, Raghuram V, Kemp BE, Witters LA, Foskett JK. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest 105: 1711–1721, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8: 774–785, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev 85: 319–371, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heitzmann D, Warth R. No potassium, no acid: K+ channels and gastric acid secretion. Physiology (Bethesda) 22: 335–341, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Henke G, Maier G, Wallisch S, Boehmer C, Lang F. Regulation of the voltage gated K+ channel Kv1.3 by the ubiquitin ligase Nedd4-2 and the serum and glucocorticoid inducible kinase SGK1. J Cell Physiol 199: 194–199, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Ichimura T, Yamamura H, Sasamoto K, Tominaga Y, Taoka M, Kakiuchi K, Shinkawa T, Takahashi N, Shimada S, Isobe T. 14–3-3 proteins modulate the expression of epithelial Na+ channels by phosphorylation-dependent interaction with Nedd4-2 ubiquitin ligase. J Biol Chem 280: 13187–13194, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Itani OA, Stokes JB, Thomas CP. Nedd4-2 isoforms differentially associate with ENaC and regulate its activity. Am J Physiol Renal Physiol 289: F334–F346, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Jespersen T, Grunnet M, Olesen SP. The KCNQ1 potassium channel: from gene to physiological function. Physiology (Bethesda) 20: 408–416, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Jespersen T, Membrez M, Nicolas CS, Pitard B, Staub O, Olesen SP, Baro I, Abriel H. The KCNQ1 potassium channel is down-regulated by ubiquitylating enzymes of the Nedd4/Nedd4-like family. Cardiovasc Res 74: 64–74, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Kanki H, Kupershmidt S, Yang T, Wells S, Roden DM. A structural requirement for processing the cardiac K+ channel KCNQ1. J Biol Chem 279: 33976–33983, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev 82: 245–289, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Lee IH, Dinudom A, Sanchez-Perez A, Kumar S, Cook DI. Akt mediates the effect of insulin on epithelial sodium channels by inhibiting Nedd4-2. J Biol Chem 282: 29866–29873, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Li H, Thali RF, Smolak C, Gong F, Alzamora R, Wallimann T, Scholz R, Pastor-Soler NM, Neumann D, Hallows KR. Regulation of the creatine transporter by AMP-activated protein kinase in kidney epithelial cells. Am J Physiol Renal Physiol 299: F167–F177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu C, Liang B, Wang Q, Wu J, Zou MH. Activation of AMP-activated protein kinase alpha1 alleviates endothelial cell apoptosis by increasing the expression of anti-apoptotic proteins Bcl-2 and survivin. J Biol Chem 285: 15346–15355, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Mall M, Wissner A, Schreiber R, Kuehr J, Seydewitz HH, Brandis M, Greger R, Kunzelmann K. Role of KVLQT1 in cyclic adenosine monophosphate-mediated Cl− secretion in human airway epithelia. Am J Respir Cell Mol Biol 23: 283–289, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Marx SO, Kurokawa J, Reiken S, Motoike H, D'Armiento J, Marks AR, Kass RS. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science 295: 496–499, 2002 [DOI] [PubMed] [Google Scholar]
- 50.McCrossan ZA, Abbott GW. The MinK-related peptides. Neuropharmacology 47: 787–821, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Melman YF, Krummerman A, McDonald TV. KCNE regulation of KvLQT1 channels: structure-function correlates. Trends Cardiovasc Med 12: 182–187, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Moss AJ, Kass RS. Long QT syndrome: from channels to cardiac arrhythmias. J Clin Invest 115: 2018–2024, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev 85: 1205–1253, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Neumann D, Woods A, Carling D, Wallimann T, Schlattner U. Mammalian AMP-activated protein kinase: functional, heterotrimeric complexes by co-expression of subunits in Escherichia coli. Protein Expr Purif 30: 230–237, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Palmada M, Dieter M, Speil A, Bohmer C, Mack AF, Wagner HJ, Klingel K, Kandolf R, Murer H, Biber J, Closs EI, Lang F. Regulation of intestinal phosphate cotransporter NaPi IIb by ubiquitin ligase Nedd4-2 and by serum- and glucocorticoid-dependent kinase 1. Am J Physiol Gastrointest Liver Physiol 287: G143–G150, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Peroz D, Rodriguez N, Choveau F, Baro I, Merot J, Loussouarn G. Kv7.1 (KCNQ1) properties and channelopathies. J Physiol 586: 1785–1789, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seebohm G, Strutz-Seebohm N, Birkin R, Dell G, Bucci C, Spinosa MR, Baltaev R, Mack AF, Korniychuk G, Choudhury A, Marks D, Pagano RE, Attali B, Pfeufer A, Kass RS, Sanguinetti MC, Tavare JM, Lang F. Regulation of endocytic recycling of KCNQ1/KCNE1 potassium channels. Circ Res 100: 686–692, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Snyder PM, Olson DR, Kabra R, Zhou R, Steines JC. cAMP and serum and glucocorticoid-inducible kinase (SGK) regulate the epithelial Na+ channel through convergent phosphorylation of Nedd4-2. J Biol Chem 279: 45753–45758, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Vallon V, Grahammer F, Richter K, Bleich M, Lang F, Barhanin J, Volkl H, Warth R. Role of KCNE1-dependent K+ fluxes in mouse proximal tubule. J Am Soc Nephrol 12: 2003–2011, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Vallon V, Grahammer F, Volkl H, Sandu CD, Richter K, Rexhepaj R, Gerlach U, Rong Q, Pfeifer K, Lang F. KCNQ1-dependent transport in renal and gastrointestinal epithelia. Proc Natl Acad Sci USA 102: 17864–17869, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Balkom BW, van Raak M, Breton S, Pastor-Soler N, Bouley R, van der Sluijs P, Brown D, Deen PM. Hypertonicity is involved in redirecting the aquaporin-2 water channel into the basolateral, instead of the apical, plasma membrane of renal epithelial cells. J Biol Chem 278: 1101–1107, 2003 [DOI] [PubMed] [Google Scholar]
- 62.van Bemmelen MX, Rougier JS, Gavillet B, Apotheloz F, Daidie D, Tateyama M, Rivolta I, Thomas MA, Kass RS, Staub O, Abriel H. Cardiac voltage-gated sodium channel Nav1.5 is regulated by Nedd4-2 mediated ubiquitination. Circ Res 95: 284–291, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Waldegger S. Heartburn: cardiac potassium channels involved in parietal cell acid secretion. Pflügers Arch 446: 143–147, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Walker J, Jijon HB, Churchill T, Kulka M, Madsen KL. Activation of AMP-activated protein kinase reduces cAMP-mediated epithelial chloride secretion. Am J Physiol Gastrointest Liver Physiol 285: G850–G860, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Warth R, Riedemann N, Bleich M, Van Driessche W, Busch AE, Greger R. The cAMP-regulated and 293B-inhibited K+ conductance of rat colonic crypt base cells. Pflügers Arch 432: 81–88, 1996 [DOI] [PubMed] [Google Scholar]
- 66.Zheng W, Verlander JW, Lynch IJ, Cash M, Shao J, Stow LR, Cain BD, Weiner ID, Wall SM, Wingo CS. Cellular distribution of the potassium channel KCNQ1 in normal mouse kidney. Am J Physiol Renal Physiol 292: F456–F466, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.