Abstract
Follistatin-like 1 (FSTL1) is a secreted protein with homology to both Follistatin and the SPARC/BM40 family of matricellular proteins. In this study, we sought to determine the expression patterns of Fstl1 and its cognate receptor Dip2a in the adult, and to assess the consequences of Fstl1 inactivation on development and homeostasis of the kidney. We find that FSTL1 circulates at high levels in both the human and the mouse and that it is also locally expressed in the loop of Henle in the kidney. To begin to understand the in vivo functions of Fstl1, we generated a mouse mutant using a genetrap approach. The hypomorphic Fstl1 genetrap strain displays a strong reduction in FSTL1 expression at the protein level, but it does not show overt developmental defects. FSTL1 has previously been implicated in diverse disease processes as a regulator of inflammatory cytokine expression, and we therefore evaluated the response of the genetrap strain to cisplatin-mediated acute kidney injury, a disease model with highly cytokine-dependent pathology. We find that although TNF-α and Il6 levels are unchanged relative to wild-type, renal Il-1β expression is increased in genetrap mice following cisplatin treatment. Furthermore, histopatological analysis, expression of the tissue injury marker Havcr1, and measurement of serum creatinine demonstrate that reduction of Fstl1 expression sensitizes the kidney to acute cisplatin nephrotoxicity, suggesting a role for FSTL1-mediated Il-1β suppression in protection of the kidney from acute nephrotoxic injury.
Keywords: Tsc36, acute kidney injury
follistatin-like 1 (FSTL1) was originally isolated as a TGF-β-responsive gene in osteoblast cells (18). On the basis of protein sequence, it has been classified both as a Follistatin homolog and as a member of the SPARC/BM40 family of matricellular proteins (5). However, the functions of FSTL1 at the molecular level remain largely unknown. Knock down of the zebrafish Fstl1 ortholog shows that it acts redundantly with chordin and noggin in dorso-ventral axis formation, suggesting that it functions as a BMP antagonist (3). FSTL1 also regulates a number of distinct cellular and inflammatory processes suggesting functions other than BMP antagonism. In synovial cells, FSTL1 treatment reduces expression of matrix metalloproteases 1 and 3 and prostaglandin E2 (20). In macrophages, overexpression of FSTL1 leads to upregulation of the proinflammatory cytokines IL-1β, TNF-α, and IL-6 (12). Tissue levels of IFN-γ also increase on viral overexpression of FSTL1 (2). In rat ventricular myocytes, FSTL1 overexpression increases activating phosphorylation of Akt (13).
FSTL1 has been implicated in several distinct pathologies. Expression of FSTL1 is reduced in numerous human cancer cell lines (5, 11, 19), as well as experimentally transformed cells (8). Interestingly, its reintroduction to cancer cells reduces proliferation and invasiveness (8, 19). In rheumatoid arthritis, FSTL1 is a strong autoantigen and displays potent proinflammatory properties as well as ameliorative effects on tissue proteases and prostaglandin E2 secretion from synovial cells (2, 12, 20, 21). In experimental cardiac ischemia, systemic administration of FSTL1 is protective, and experiments in cultured cardiomyocytes suggest that this effect originates from direct anti-apoptotic effects of FSTL1. Similarly, administration of FSTL1 accelerates revascularization in the hindlimb ischemia model, and cell-based studies demonstrate an anti-apoptotic effect on primary endothelial cells (15).
We previously showed that Fstl1 is robustly expressed in the developing kidney (1), and it has recently been shown that FSTL1 is represented in the urinary proteome postnatally (10). We set out to enquire whether expression of this novel and intriguing protein is maintained in the adult organ, and what consequences loss of Fstl1 might have for organ development and homeostasis. We find that FSTL1 circulates at high levels in both the human and mouse and that it is also locally expressed in a segment of the loop of Henle. A hypomorphic Fstl1 mouse strain displaying a strong reduction in FSTL1 expression at the protein level does not show overt effects on embryonic kidney development, but is sensitized to cisplatin nephrotoxicity, possibly as a result of increased renal Il-1ß expression following injury.
MATERIALS AND METHODS
RNA purification and qPCR analysis.
Tissue slices were added to 1 ml TRIzol (Invitrogen 15596026) on ice, homogenized immediately, and snap-frozen. Crude total RNA was purified from 500 μl of lysate according to the manufacturer's instructions, and further purified using the RNeasy Mini kit (Qiagen 74106) with DNase treatment. One microliter of Ribolock (Fermentas EO0381) was added, and cDNA was generated from 1 μg of RNA using the qScript cDNA kit (Quanta Biosciences 95048–100). For QPCR, 1 μl of cDNA was used as template in a 25-μl reaction using iQ SYBR Green SuperMix (BioRad 170–8880) on a MyiQ real-time detection system (Bio-Rad). Cycling parameters were 95°C for 15 s, 55°C for 45 s. Primer sequences can be found in Supplemental Table 1 (the online version of this article contains supplemental data). All primers were 95% efficient or better. β-Actin was used as a housekeeping gene in all analyses. In analyses of tissue arrays (Origene Technologies) for normal human tissue (HMRT102) and normal mouse tissue (MNRT101), specific gene expression was expressed relative to β-actin abundance assuming equivalent efficiency between primer sets. For Fstl1, Havcr1, and cytokine analyses in mouse tissues, five biological samples isolated from distinct individuals were assayed, and each biological sample was assayed in technical triplicate. Technical replicates were averaged and 1/ΔCT was calculated for each biological sample. Standard error was determined within each group of biological replicates and P values were calculated using Student's t-test.
Human sample acquisition.
Our protocol for collection of blood from healthy volunteers was reviewed and approved by the Institutional Review Board at Maine Medical Center. Normal plasma was collected from healthy volunteers and coded numerically. Gender of the study participant was collected and coded with the samples. Blood samples were immediately placed on ice. Samples were processed within 4 h by centrifugation and plasma was aliquoted and stored at −80°C. Tissue samples were acquired from Zyagen Laboratories following review and approval by the Institutional Review Board at Maine Medical Center.
Immunoblot analysis.
Mouse serum (2 μl/lane) or human plasma (0.4 μl per lane) proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. Human FSTL1 was detected with a goat anti-FSTL1 antibody (Abcam AB11805) and mouse FSTL1 was detected with a goat antibody from R&D Systems (BAF1694). FSTL1 protein was visualized using a secondary donkey anti-goat horseradish peroxidase-conjugated antibody (Jackson Immuno-Research 705–035-147) and ECL chemiluminescent substrate (GE Life Sciences RPN2109) for signal detection. Specificity of the anti-human FSTL1 antibody was determined by incubating 0.5 μg of the antibody with 1.0 μg of blocking peptide (Abcam AB23213) in 4% BSA/TBST overnight at 4°C.
In situ hybridization.
In situ hybridization (ISH) of paraffin-embedded adult kidney tissue was performed as previously described (1).
Immunofluorescence.
Kidneys from adult mice and embryonic day 17.5 (E17.5) embryos were fixed in 4% paraformaldehyde overnight, dehydrated in methanol, and paraffin-embedded for sectioning. Deparaffinized sections were then subjected to antigen retrieval (DAKO Target Retrieval Solution S2369) and blocked in 5% donkey serum in PBS. Endogenous biotin was blocked using a kit from Vector Laboratories (SP-2001). Slides were incubated with FITC-conjugated lectins Lotus Tetragonolobus or Dolichos Biflorus Agglutinin (Vector Laboratories FL1321 and FL1031) to visualize the proximal tubule brush border and collecting ducts, respectively. FSTL1 was detected by incubating slides with a goat antibody from R&D Systems (AF1738) and a biotinylated donkey anti-goat antibody (Jackson ImmunoResearch 705–065-147) followed by incubation with streptavidin-Alexa Fluor 568 (Invitrogen S-11226). Nuclei were visualized by staining with DAPI (Invitrogen D1306). Negative controls were processed in the same manner, except for the omission of the primary antibody. For detection of FSTL1 protein in HEK293, cells transfected with a mouse Fstl1 expression construct were fixed in formalin 48 h after transfection, blocked in 0.3% Triton X-100/5% donkey serum/PBS, incubated with goat anti-FSTL1, followed by donkey anti-goat Alexa Fluor 568 (Invitrogen A11057). Nuclei were counterstained with DAPI.
Cell culture and transfection.
Human embryonic kidney cells (HEK293) were cultured at 37°C with 5% CO2 in DMEM supplemented with 10% bovine serum, glutamine, penicillin/streptomycin, and Amphotericin B. Transfection of HEK293 cells with an expression construct for mouse fstl1 was performed in a 24-well plate during a 6-h period with Lipofectamine 2000 (Invitrogen 52887) according to the manufacturer's instructions. Controls were transfected in a similar fashion with an irrelevant plasmid. Primary human renal proximal tubule epithelial cells (RPTECs; Lonza CC-2553) were cultured at 37°C with 5% CO2 in REGM basal medium with supplements (Lonza CC-3190), penicillin/streptomycin, and Amphotericin B.
Cisplatin toxicity assay in RPTECs.
RPTECs were plated in a 96-well plate and cultured for 2 days. Two hours before the addition of cisplatin (Sigma P4394), the cells were incubated with varying concentrations of 48-h conditioned medium from HEK293 cells transfected either with a mouse Fstl1 expression construct or an empty control vector. Cisplatin was added to the experimental wells at a concentration of 75 μM. After 48 h, the conditioned medium was assayed in triplicate wells for the release of lactate dehydrogenase (LDH) at a wavelength of 490 nm using the CytoTox-96 kit (Promega) according to the manufacturer's directions. The LDH release of cells treated with cisplatin was first adjusted by correcting for a background amount of LDH release in matching RPTEC cultures not treated with cisplatin. The percentage of adjusted LDH release into the conditioned medium by cells exposed to cisplatin and FSTL1 conditioned medium was normalized to the same data obtained for cells treated with cisplatin and a matching percentage of control conditioned medium. The dose of cisplatin to be used in this experiment was determined from a 72-h dose-response study: triplicate wells of RPTECs were treated with varying doses of cisplatin and the percentage of LDH release at 24, 48, and 72 h was measured. Degree of cell death for a particular dose and time point was expressed as the percentage LDH released into medium compared with the total LDH content of the cell culture. The latter was determined by adding the LDH value measured following lysis of the cell culture at harvest to the LDH value for the culture medium.
ES cell culture, blastocyst injection, and genotyping.
ES cells obtained from the German Genetrap Consortium (GGTC) were expanded using ES cell culture conditions recommended by GGTC. Blastocyst injection was performed according to standard procedures (6). Resulting mice were bred onto the ICR background for five generations before experiments were conducted. The position of the genetrap within the second intron of the Fstl1 locus of the P086B03 ES cell line was initially determined by rapid amplification of cDNA ends by GGTC. The exact location was determined by walking through the intron using a reverse PCR primer located within the genetrap (5′-GCA TCC GAC TTG TGG TCT CGC TGT T-3′) and a selection of forward primers complementary to exon 2, spaced at 500 nucleotide intervals upstream of the genetrap. A genotyping assay that distinguishes between wild-type, heterozygous, and homozygous animals was developed based on oligonucleotides that generate an amplicon in this assay, with the addition of a third primer located downstream of the genetrap. Forward primer: 5′-ACT GGC TTA AAT TTC ACC CCT CAG G-3′, reverse primer 1: 5′-GCA TCC GAC TTG TGG TCT CGC TGT T-3′, reverse primer 2: 5′-GTT TCT GGG GTA GCC TGG CCC CGC C-3′.
Cisplatin treatment of mice.
Animal care in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals was approved by the Institutional Animal Care and Use Committee of Maine Medical Center. Groups of 5 genetrap homozygous mice and age- and strain-matched controls were injected with 15 mg/kg cisplatin (Sigma P4394) in PBS intraperitoneally and killed after 90 h. Blood was collected by cardiac puncture at the time of death, and serum was prepared immediately to avoid hemolysis. Harvested kidneys were cut into ∼3-mm transverse slices that were either fixed in 10% neutral buffered formalin for sectioning and histological staining or immediately processed for RNA extraction.
Measurements of kidney injury.
Periodic acidic Schiff's stained kidney sections were scored for tubule occlusion by counting the proportion of transversely sectioned tubules that were occluded by protein casts in three high-power (×400) fields per kidney. In all, ∼60 transversely sectioned tubules were scored per section, giving a total of ∼180 tubules counted per individual kidney. Serum creatinine was measured by the Biochemical Analysis Service at the Jackson Laboratory. P values for both of these datasets were calculated using Student's t-test.
RESULTS
Fstl1 is expressed in a partially overlapping pattern with its cognate receptor Dip2a in human and mouse tissues.
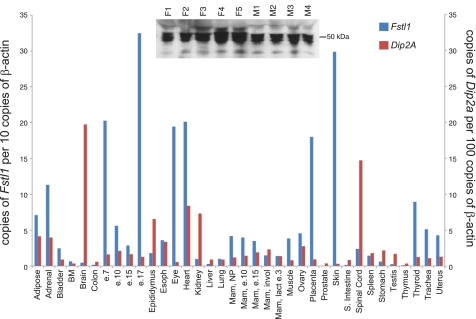
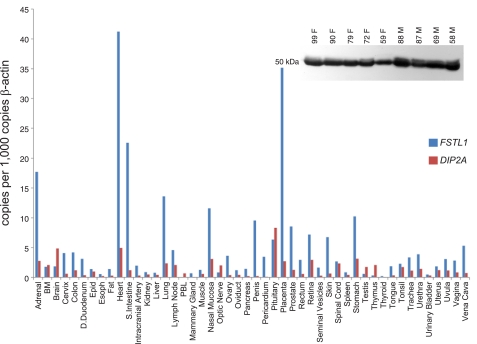
In a previous study, we characterized Fstl1 in the mouse embryo where expression is specifically localized to the central nervous system, heart, vasculature, lung, kidney, and skin (1). To ascertain whether this expression pattern is maintained in the adult, we performed rt-qPCR analysis of Fstl1 on tissue panels derived from both mouse and human (Figs. 1 and 2). In the mouse, we find lower expression in most adult organs relative to the E17.5 embryo, and relatively low level expression in the adult brain, spinal cord, lung, and kidney. However, the adult heart and skin maintain strong Fstl1 expression. Interestingly, immunoblot analysis of mouse serum reveals high levels of circulating FSTL1 protein (Fig. 1, inset), indicating the possibility that the protein functions through endocrine signaling. To evaluate the possibility for FSTL1 activity at distant sites, we assessed the expression of the transcript for the cognate FSTL1 receptor Dip2a (14) using the same tissue panel. We find strong expression of Dip2a transcript in brain, spinal cord, and kidney, suggesting that circulating FSTL1 may activate signaling at these sites. Comparison with human tissue reveals interesting likenesses such as strong FSTL1 expression in heart, weak expression in kidney, and circulation of protein in plasma, but also some key differences such as weak expression in skin. Similarly, interesting species differences are seen with DIP2a, such as weak expression in the human kidney. From our expression analysis, we conclude that adult Fstl1 expression differs significantly from embryonic expression, in that several strongly positive organs in the embryo such as central nervous system, lung, and kidney display only weak expression in the adult. However, other sites such as the heart express Fstl1 abundantly, possibly serving as sources of circulating protein. In the mouse, the Dip2a receptor is expressed in the kidney, potentially facilitating signaling by circulating FSTL1 or by small amounts of locally produced protein. To ensure the specificities of antibodies used to detect FSTL1 in mouse and human blood samples, we performed specificity controls by immunodetection of FSTL1 production by transfected cells (Supplemental Fig. 1) and blocking with the immunizing peptide (Supplemental Fig. 2A), respectively. To address the possibility that FSTL1 is represented differently in plasma and serum, we compared equivalent volumes of serum and plasma from the same animals by immunoblot (Supplemental Fig. 2B). Although there may be slightly more protein in plasma, the difference is minimal.
Fig. 1.
Expression of Fstl1 and its cognate receptor Dip2A in mouse tissues. Tissue arrays were screened by rt-qPCR for expression of Fstl1 and Dip2A transcripts. Inset: immunoblot analysis of Follistatin-like 1 (FSTL1) in serum from individual female (F) and male (M) mice.
Fig. 2.
Expression of FSTL1 and its cognate receptor DIP2A in human tissues. Tissue arrays were screened by rt-qPCR for expression of FSTL1 and DIP2A transcripts. Inset: immunoblot analysis of FSTL1 in plasma from 5 healthy female (F) and 4 healthy male (M) donors.
FSTL1 expression is limited to the distal tubule in the mouse kidney during embryonic development and to the loop of Henle during adult life.
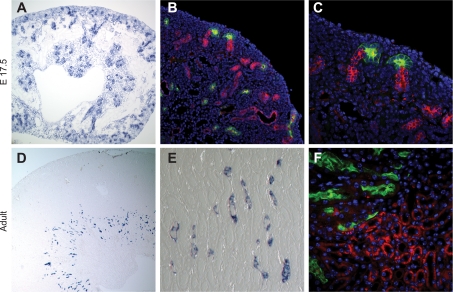
To localize gene and protein expression in the kidney, we performed a series of ISH and immunostaining experiments (Fig. 3). ISH reveals expression in cortical tubules of the embryonic kidney (Fig. 3A). Immunostaining together with the collecting duct marker dolichos bifloris agglutinin (DBA) clearly shows that positive tubule segments in the embryonic kidney are distal, as they can be seen to fuse with collecting ducts within the nephrogenic zone (Fig. 3, B and C). Limited protein expression can be seen in DBA-positive collecting ducts located in the medullary region of the kidney (Fig. 3B). As expected from the rt-qPCR study, RNA expression in the adult organ is more limited, with ISH revealing a sparse population of thin Fstl1-positive tubules in the outer medulla (Fig. 3, D and E). These do not colocalize with DBA (data not shown) or the proximal tubule marker lotus tetragonolobus lectin (LTL; Fig. 3F). By morphological criteria and exclusion of marker colocalization, we propose that FSTL1 is expressed in a segment of the loop of Henle.
Fig. 3.
Expression of FSTL1 in the kidney. Mouse kidney tissue was subjected to in situ hybridization using a riboprobe specific for Fstl1 (A, D, E) or immunostaining with a FSTL1-specific antibody (B, C, F). A: in embryonic tissue, RNA expression can be seen in nephric tubules as well as in the cortical nephrogenic zone. B, C: protein expression (red) is seen in distal tubules connecting to dolichos bifloris agglutinin (DBA) lectin-stained collecting ducts (green), and limited colocalization with DBA can also be seen. D, E: in the adult kidney, RNA is expressed exclusively in frequent narrow tubules within the outer medulla. F: protein expression (red) does not overlap with proximal tubule-specific lotus tetragonolobus lectin (LTL) staining (green) or with collecting duct-specific DBA lectin staining (data not shown), and we therefore conclude on the basis of tissue localization that protein expression is specific to the loop of Henle.
Hypomorphic mouse strain carrying a gene trap in the second intron of Fstl1.
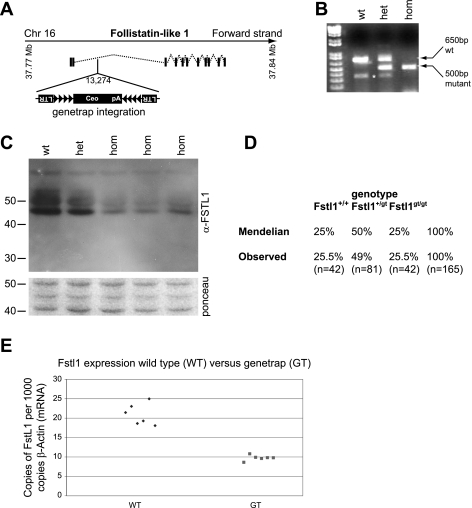
To understand the functional significance of FSTL1 for development and homeostasis of the murine kidney, we generated a Fstl1 mutant mouse strain. Mice were derived from the P086B03 embryonic stem cell line obtained from the German Genetrap Consortium. The genetrap is integrated 13,274 bases from the first ATG within the coding sequence in the expected orientation (Fig. 4A). We developed a 3-primer genotyping assay to distinguish wild-type (Fstl1+/+), heterozygous (Fstl1gt/+), and homozygous (Fstl1gt/gt) animals (Fig. 4B). We intercrossed heterozygous animals (maintained on an ICR background) and harvested litters at E12.5 to assess the degree of FSTL1 inactivation by Western blot of whole embryo lysates (Fig. 4C). There is a profound reduction in protein expression in homozygous animals compared with wild-type, and significant attenuation of expression in heterozygotes. We performed ∼15 intercross matings, genotyped the animals at 2 wk of age, and monitored for at least 2 mo. Ratios of genotypes retrieved at postnatal day 14 (P14) were Mendelian, indicating that embryonic lethality is not associated with this mutation (Fig. 4D). Furthermore, we detected no overt phenotype in heterozygous or homozygous animals at 2 mo of age. Subsequent monitoring of a smaller cohort of animals to 6 mo of age did not reveal any obvious phenotype. At 2 mo of age, there is an ∼50% reduction in Fstl1 expression in the kidney of the homozygous genetrap mouse compared with wild-type (Fig. 4E). We thus conclude that the homozygous Fstl1 mutant is a strong hypomorph and that this mutation is not associated with any overt phenotype. Subsequent to generation of the genetrap mouse mutant, an alternate Fstl1 transcript was reported at Ensembl that is not predicted to be trapped in ES clone P086B03 (ENSMUST00000023511), providing a possible explanation for the hypomorphic nature of the mouse strain. The coding sequences of the two Fstl1 transcripts are identical with the exception that the internally initiated transcript lacks the first methionine and part of the signal sequence, indicating that it represents a partial transcript.
Fig. 4.
Development of a Fstl1-specific genetrap mouse strain. A: gene-trap is located in intron 2 of the Fstl1 locus. The genetrap contains multiple recombination sites intended for reversal of the trap to a conditional conformation in addition to a cDNA-encoding human CD2 for cell surface labeling of trapped cells. Despite attempts with validated anti-CD2 antibodies, we were unable to immunodetect this tag in tissue by either immunochemistry or immunoblotting. B: PCR-based genotyping distinguishes wild-type, heterozygous, and homozygous animals. C: immunoblotting for FSTL1 on lysates from whole embyronic day 12.5 (E12.5) embryos demonstrates strongly reduced protein abundance in homozygous (hom) genetrap animals compared with wild-type (wt). The heterozygote (het) displays an intermediate protein level. Ponceau S staining of the appropriate region of the blotted membrane demonstrates approximately equivalent loading of samples. D: genotyping data from offspring of heterozygous intercrosses. Close to perfect Mendelian ratios of the 3 possible genotypes were retrieved in a sample of 165 pups. E: rt-qPCR analysis of kidneys from adult animals demonstrates an ∼50% reduction in the level of Fstl1 transcript in homozygous genetrap tissue compared with wild-type.
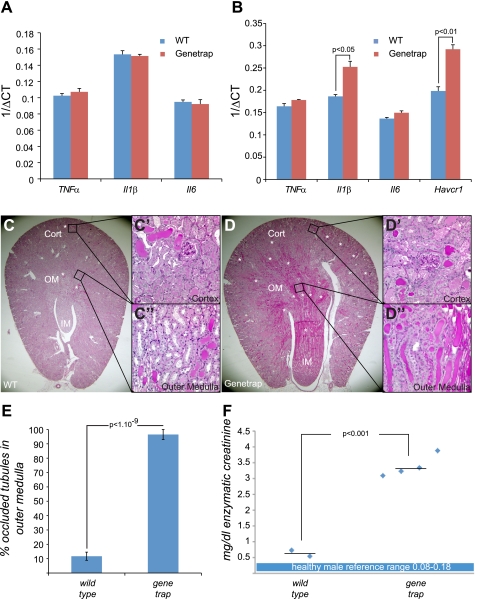
Fstl1 hypomorph displays higher levels of renal Il-1β expression than wild-type following injury and is sensitized to cisplatin nephrotoxicity.
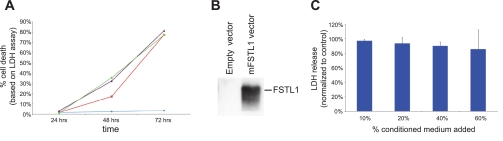
FSTL1 displays strong effects on the expression of inflammatory cytokines in adenoviral transduction studies. Specifically, FSTL1 appears to regulate Il-1β, Il-6, and TNF-α (2). To test the possibility that FSTL1 regulates renal expression of these cytokines in vivo, we compared their expression in wild-type and Fstl1gt/gt kidney tissue by rt-qPCR (Fig. 5A). In the uninjured kidney, we find no significant differences in expression of any of these cytokines between wild-type and mutant. Furthermore, we detected no expression differences between wild-type and mutant males vs. females, or young (6 wk) vs. old (8 mo) animals (data not shown). To probe whether FSTL1 may regulate expression of any of these cytokines in the injured kidney, we analyzed tissue from animals treated with cisplatin (15 mg/kg). Cisplatin nephrotoxicity is associated with extensive increase in expression of a variety of cytokines, including Il-1β, Il-6, and TNF-α (4, 17). We find no noteworthy differences in levels of TNF-α or Il6 in kidney tissue from wild-type and genetrap animals, but we do find a significant (2.6-fold) increase in Il-1β in genetrap tissue compared with wild-type (Fig. 5B). From this analysis, we can conclude that FSTL1 suppresses Il-1β expression in kidney tissue following cisplatin injury. Interestingly, histopathological analysis of tissue from cisplatin-treated animals reveals a consistent exacerbation of injury in kidneys from genetrap animals (Fig. 5, C and D; Supplemental Fig. 3). The percentage of tubules occluded by protein casts per high-power field within the outer medulla of wild-type kidneys (Fig. 5C′, C′′, E) is ∼10%, whereas genetrap kidneys display ∼90% occluded tubules within this region (Fig. 5D′, D′′, E), demonstrating that tissue injury in kidneys of genetrap animals is more severe than wild-type following cisplatin treatment. To obtain quantitative measurements of acute kidney injury, we measured expression of the murine kidney injury marker 1 (KIM1) ortholog Havcr1 locally in kidney tissue as well as levels of the functional marker creatinine in serum. Corroborating the histological analysis, we find significant increases in both Havcr1 and creatinine in genetrap vs. wild-type. We therefore conclude that reduced Fstl1 expression exacerbates acute nephrotoxic injury. To ascertain whether the exacerbation of tissue injury in the genetrap following cisplatin treatment might be explained by a direct cytoprotective effect of FSTL1 on proximal tubule cells, we conducted a cisplatin cytotoxicity assay in the presence or absence of recombinant FSTL1 protein. RPTECs were treated with a variety of doses of cisplatin (Fig. 6A), and 75 μM was selected as a dose that resulted in significant (30%) cell death after 48 h. Conditioned medium from HEK293 cells transfected either with an empty vector or with a mouse Fstl1 expression construct (Fig. 6B) was mixed with RPTEC medium at concentrations from 10 to 60%. Cell death 48 h after cisplatin treatment was unaffected by the addition of FSTL1 at any concentration (Fig. 6C), strongly suggesting that the protein does not have a direct cytoprotective effect on proximal tubule epithelial cells.
Fig. 5.
Cytokine expression and tissue injury in kidneys from cisplatin-treated mice. rt-qPCR was used to measure expression of cytokines Il-1β, Il-6, and TNF-α and the kidney injury marker Havcr1 in tissue (A, B), and positive acidic Schiff's histological stain was used to demonstrate tissue injury (C, D). A: cytokine expression was compared in uninjured kidney tissue from groups of 5 wild-type and 5 genetrap homozygous animals. B: cytokine expression was compared in kidney tissue from mice treated with 15 mg/kg cisplatin. Tissue injury was also compared in these animals using the Havcr1 ortholog of human kidney injury marker 1 (KIM1). C: morphology of cisplatin-injured kidney tissue displays differences between wild-type and genetrap homozygote: in both, the most severe tubular damage can be seen in the outer medulla (C, D). Higher magnification reveals a moderate degree of tubular cast occlusion in the wild-type kidney (C′, C′′), with a majority of tubules in the section being patent. Severe tubular damage can be seen in comparable regions of the genetrap homozygous kidney (D′, D′′), where few tubules remain patent. These tissue sections are representative of a total sample size of 5 animals in each group. For additional sections, see Supplemental Fig. 3. E: scoring of occluded tubules within the outer medulla of wild-type and genetrap kidneys. The proportion of transversely sectioned tubules occluded by casts in 3 high-power fields from each kidney was compared. F: creatinine was measured in serum from wild-type and genetrap animals treated with cisplatin. Relative to the upper reference value for healthy males (0.18), creatinine in cisplatin-treated wild-type animals (average 0.64) is elevated ∼3-fold, whereas creatinine in cisplatin-treated genetrap animals (average 3.38) is elevated ∼16-fold. Diamonds represent individual values and horizontal lines represent averages for each group. Healthy male reference values are shown as a blue box.
Fig. 6.
Assay for cytoprotective effect of FSTL1 on proximal tubule epithelial cells. A: human renal proximal tubule epithelial cells (RPTECs) were treated with vehicle (blue), 50 μM (red), 75 μM (purple), or 100 μM (green) cisplatin over 3 days, and cell death measured by lactate dehydrogenase (LDH) release into the culture medium was calculated as a percentage of the total LDH in the culture. B: control and FSTL1 containing conditioned media were produced by transfecting HEK293 cells either with an empty vector or a mouse Fstl1 expression vector, and harvesting medium 48 h after transfection. Immunoblot of 5 μl of conditioned medium with anti-FSTL1 antibody demonstrates FSTL1 content in medium from cells transfected with the expression construct. C: RPTEC cultures were supplemented either with freshly harvested control conditioned medium or conditioned medium containing FSTL1 at concentrations varying from 10 to 60% and treated with 75 μM cisplatin for 48 h. LDH release in FSTL1-treated cultures is compared with release in control cultures treated with the same concentration of conditioned medium. At all concentrations of conditioned medium, LDH release in FSTL1-treated cultures is ∼90–100% of that in control cultures.
DISCUSSION
In this study, we set out to understand the expression patterns of Fstl1 and its cognate receptor Dip2a in the adult and to assess the consequences of Fstl1 inactivation on development and homeostasis of the kidney. We find that FSTL1 circulates at high levels in both the human and mouse and that it is also locally expressed in the loop of Henle in the adult mouse. A hypomorphic Fstl1 mouse strain displaying a strong reduction in FSTL1 expression at the protein level does not show overt effects on embryonic kidney development, but it does display increased renal Il-1ß expression following cisplatin treatment and is sensitized to cisplatin nephrotoxicity.
Fstl1 is expressed in numerous organs in the adult and circulates at high levels. In both the mouse and human, transcript levels are modest in the kidney compared with other organs such as the heart. ISH reveals a restricted domain of Fstl1 expression in the outer medulla of the kidney, identified as the loop of Henle by morphological criteria. Tubular staining for FSTL1 protein can also be seen in this region, indicating that the protein may act locally within the outer medulla, a kidney region that is highly susceptible to acute kidney injury. Furthermore, readily detectable levels of FSTL1 can be found in the circulation. Tubules of the nephron are intimately associated with interstitial blood vessels, and it is probable that circulating FSTL1 reaches most cells of the kidney. To ascertain the basis for biological FSTL1 function, we determined the expression pattern of the cognate receptor Dip2a, which was recently identified in a protein interaction screen (14). DIP2a is required for the anti-apoptotic and promigratory effects of FSTL1 on endothelial cells and mediates FSTL1 activation of Akt. We find that Dip2a is expressed in the kidney of the mouse, indicating that locally and remotely produced FSTL1 may signal through this receptor. Interestingly, relatively weak expression of Dip2a is seen in the human kidney, possibly indicating a species difference in receptor expression.
To evaluate the role of Fstl1 in the kidney, a hypomorphic mouse strain was generated from a genetrapped embryonic stem cell provided by the GGTC. Loss-of-function experiments in zebrafish and chick demonstrated important roles for Fstl1 orthologs in dorsoventral axis formation (3), early mesoderm development, somitogenesis, and myogenesis (22), and we placed particular emphasis on analyzing hypomorphic Fstl1 mouse embryos for similar phenotypes. Despite a profound reduction in FSTL1 protein, no overt developmental defects could be detected. This could, of course, simply reflect incomplete gene inactivation to reduce protein expression below the level required for normal development. However, in the antisense gene inactivation experiments performed by Towers et al. (22) in chick embryos, Fstl1 inactivation was also partial, yet profound phenotypes were observed. This raises the possibility that redundant expression of homologous genes in the mouse may mask developmental effects of inactivating Fstl1. Indeed, in zebrafish, morpholino inactivation of the Fstl1 ortholog alone is not sufficient to provoke a developmental phenotype, supporting this interpretation (3).
Several studies showed that FSTL1 regulates inflammatory cytokine expression. The cisplatin nephrotoxicity model was selected for this study because its pathology is closely associated with the inflammatory response (16). Numerous cytokines such as Il-1β, Il-6, and TNF-α are upregulated in the kidney following cisplatin injury (4, 17), and in the case of Il-1β and TNF-α, this regulation occurs at both transcriptional and protein levels. For IL6, only protein levels have been reported (4). In the uninjured kidney, loss of Fstl1 does not affect expression of Il-1β, Il-6, or TNF-α, indicating that Fstl1 is not required to maintain steady-state expression of these cytokines. However, following injury significant differences can be seen between wild-type and Fstl1 hypomorphic mice in renal cytokine expression, where Il-1β is elevated. The finding that cytokine expression is increased in the hypomorph could simply indicate that Fstl1 has a renoprotective effect, its absence causing generally elevated expression of inflammatory cytokines. However, the lack of any protective effect of FSTL1 protein on primary epithelial cells suggests that this may not be the case, and the disproportionate elevation of Il-1β expression argues that the inflammatory response is specifically modulated. Several gain-of-function studies demonstrated effects of FSTL1 on cytokine expression. Perhaps due to the complex interrelationships between various cytokines, reports are conflicting. In a lipopolysaccharide-induced model of joint inflammation, local FSTL1 treatment suppresses Il6 (9). However, systemic adenoviral Fstl1 expression promotes TNF-α, IL-6, and IL-1β (12), and antibody neutralization of FSTL1 suppresses IL6 and IL-1β in collagen-induced arthritis (2). Phenotypically, results using distinct disease models are also contradictory. In collagen-induced arthritis, adenoviral Fstl1 expression strongly exacerbates tissue injury (2), whereas adenoviral Fstl1 expression ameliorates ischemic heart injury (13). One possible reason for the difference in outcomes is the differing rates of disease onset in these models. In collagen-induced arthritis, tissue destruction occurs over the course of several weeks (12), whereas the acute cardiac ischemia model spans only 24 h (13). Thus, perceived contradictions in the action of FSTL1 may partly be explained by the kinetics of the disease models used, with FSTL1 filling distinct functions in the acute and chronic inflammatory responses. In an acute nephrotoxic injury model, we find evidence for an ameliorative role of FSTL1. Although no specific causal role has been reported for Il-1β in acute kidney injury, it is a possibility that the renoprotective action of FSTL1 is mediated through modulation of this cytokine. Future studies exploring molecular targets of FSTL1 in the kidney will shed light on the specific pathways in which this novel protein interacts.
GRANTS
This work was supported by National Institutes of Health/National Center for Research Resources 2P20RR18798 (project 1, LO).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
Additional technical core support was provided by Maine Medical Center Research Institute's Center of Excellence in Stem and Progenitor Cell Biology core facilities in histology, bioinformatics, and ES cell culture and FACS (2P20RR18798) as well as Center of Excellence in Vascular Biology core facilities in mouse transgenics and MRI (2P20RR15555). The P086B03 ES cell line was kindly provided by the German Genetrap Consortium (http://genetrap.gsf.de/ggtc/aboutus/consortium.php).
REFERENCES
- 1.Adams D, Larman B, Oxburgh L. Developmental expression of mouse Follistatin-like 1 (Fstl1): dynamic regulation during organogenesis of the kidney and lung. Gene Expr Patterns 7: 491– 500, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clutter SD, Wilson DC, Marinov AD, Hirsch R. Follistatin-like protein 1 promotes arthritis by upregulating IFN-gamma. J Immunol 182: 234– 239, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dal-Pra S, Furthauer M, Van-Celst J, Thisse B, Thisse C. Noggin1 and Follistatin-like2 function redundantly to Chordin to antagonize BMP activity. Dev Biol 298: 514– 526, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, Oh DJ, Lu L, Klein CL, Dinarello CA, Edelstein CL. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther 322: 8– 15, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Hambrock H, O, Kaufmann B, Müller S, Hanisch F, G, Nose K, Paulsson M, Maurer P, Hartmann U. Structural characterization of TSC-36/Flik: analysis of two charge isoforms. J Biol Chem 279: 11727– 11735, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Woodbury, New York: Cold Spring Harbor Laboratory Press, 1994 [Google Scholar]
- 7.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol 286: F552– F563, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Johnston IM, Spence HJ, Winnie JN, McGarry L, Vass JK, Meagher L, Stapleton G, Ozanne BW. Regulation of a multigenic invasion programme by the transcription factor, AP-1: reexpression of a down-regulated gene, TSC-36, inhibits invasion. Oncogene 19: 5348– 5358, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Kawabata D, Tanaka M, Fujii T, Umehara H, Fujita Y, Yoshifuji H, Mimori T, Ozaki S. Ameliorative effects of follistatin-related protein/TSC-36/FSTL1 on joint inflammation in a mouse model of arthritis. Arthritis Rheum 50: 660– 668, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Lee RS, Monigatti F, Lutchman M, Patterson T, Budnik B, Steen JA, Freeman MR, Steen H. Temporal variations of the postnatal rat urinary proteome as a reflection of systemic maturation. Proteomics 8: 1097– 1112, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Mashimo J, Maniwa R, Sugino H, Nose K. Decrease in the expression of a novel TGF beta1-inducible and ras-recision gene, TSC-36, in human cancer cells. Cancer Lett 113: 213– 219, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Miyamae T, Marinov AD, Sowders D, Wilson DC, Devlin J, Boudreau R, Robbins P, Hirsch R. Follistatin-like protein-1 is a novel proinflammatory molecule. J Immunol 177: 4758– 4762, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel DR, Walsh K. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation 117: 3099– 3108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouchi N, Asaumi Y, Ohashi K, Higuchi A, Sono-Romanelli S, Oshima Y, Walsh K. Disco-interacting protein 2 homolog A functions as a follistatin-like 1 receptor. J Biol Chem 285: 7127– 7134, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, Walsh K. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric oxide synthesis-dependent mechanism. J Biol Chem 283: 32802– 32811, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73: 994– 1007, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110: 835– 842, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibanuma M, Mashimo J, Mita A, Kuroki T, Nose K. Cloning from a mouse osteoblastic cell line of a set of transforming-growth-factor-beta 1-regulated genes, one of which seems to encode a follistatin-related polypeptide. Eur J Biochem 217: 13– 19, 1993 [DOI] [PubMed] [Google Scholar]
- 19.Sumitomo K, Kurisaki A, Yamakawa N, Tsuchida K, Shimizu E, Sone S, Sugino H. Expression of a TGF-beta1 inducible gene, TSC-36, causes growth inhibition in human lung cancer cell lines. Cancer Lett 155: 37– 46, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Tanaka M, Ozaki S, Kawabata D, Kishimura M, Osakada F, Okubo M, Murakami M, Nakao K, Mimori T. Potential preventive effects of follistatin-related protein/TSC-36 on joint destruction and antagonistic modulation of its autoantibodies in rheumatoid arthritis. Int Immunol 15: 71– 77, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M, Ozaki S, Osakada F, Mori K, Okubo M, Nakao K. Cloning of follistatin-related protein as a novel autoantigen in systemic rheumatic diseases. Int Immunol 10: 1305– 1314, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Towers P, Patel K, Withington S, Isaac A, Cooke J. Flik, a chick follistatin-related gene, functions in gastrular dorsalisation/neural induction and in subsequent maintenance of midline Sonic hedgehog signalling. Dev Biol 214: 298– 317, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.