Abstract
The invitation to present the 2010 Hans Ussing lecture for the Epithelial Transport Group of the American Physiological Society offered me a unique, special, and very surprising opportunity to join in saluting a man whom I met only once, but whose work was the basis, not only for my career, but also for finding the molecular defect in the inherited disease cystic fibrosis (CF). In this context, I will venture to make the tribute with a new explanation of why a mutation in a single gene that codes for an anion channel can cause devastation of multiple epithelial systems with pathogenic mucus. In so doing, I hope to raise awareness of a new role for that peculiar anion around which so much physiology revolves, HCO3−. I begin by introducing CF pathology as I question the name of the disease as well as the prevalent view of the basis of its pathology by considering: 1) mucus, 2) salt, and 3) HCO3−. I then present recent data showing that HCO3− is required for normal mucus discharge, and I will close with conjecture as to how HCO3− may support mucus discharge and why the failure to transport this electrolyte is pathogenic in CF.
Keywords: mucus expansion, mucus obstruction, mucovisicidosis, exocrine gland
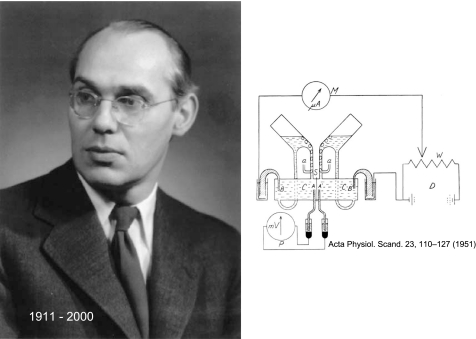
if any disease owed its pathophysiological definition to one man, it is cystic fibrosis (CF) to Hans Ussing (Fig. 1). The concepts and principles he put forward for coupling electrical current to movements of charged solutes preceded their application in studies of CF by nearly 30 years. Perhaps, ironically the single electrolyte disturbance first noted in CF sweat (13) was described at almost the same time that Ussing described his classic and now-famous treatment of active ion transport, entitled “Active transport of sodium as the source of electric current in the short-circuited isolated frog skin” (57). No doubt, his most famous creation was the “Ussing chamber” (as it is now called) for measurements of spontaneous transepithelial voltage, short-circuit current, and conductance, but he was responsible for many, if not most, of the concepts underlying epithelial ion transport, among them: exchange diffusion, unidirectional fluxes and the flux-ratio equation, two-membrane hypothesis, and solvent drag, to mention a few, but to be fair this list is short and interested readers are directed to the splendid biographical essay by Hvid Larsen on Ussing's career accomplishments (33).
Fig. 1.
Hans H. Ussing (30 December 1911–22 December 2000). The portrait is from 1955 at the time Ussing became a Fellow of the Royal Danish Academy of Sciences and Letters. Reprinted with permission from the Royal Danish Academy of Sciences and Letters. The drawing depicts what is now known as the “Ussing chamber.” Reprinted from Ussing HH and Zerahn K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand 23: 110–127, 1951, with permission from Wiley.
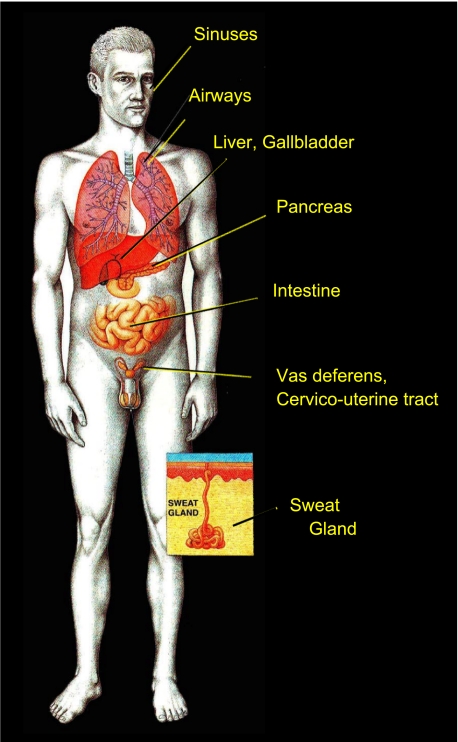
But really, what is CF? Even now after more than 60 years, CF is still best known as a disease of thick, sticky mucus that clogs the lungs and kills children. Nonetheless, CF is basically a disease of epithelial electrolyte transport (44). It affects the epithelia of hollow organs and glands: the sinuses, salivary glands, respiratory airways, liver bile ducts, pancreatic ducts, small intestine, colon, vas deferens, the cervico-uterine tract, and, of course, sweat glands (Fig. 2). Because of the number of different exocrine glands involved, it is sometimes referred to as an exocrinopathy (51). All of these complications arise from the mutation in a single gene that encodes a protein best known for its ability to conduct chloride (21), i.e., Cystic Fibrosis Transmembrane Regulator (CFTR, see Ref. 48). Defects in this protein lead to Cl− impermeability in tissues where it is expressed (45), but still, what links a defect in an electrolyte transport protein to the deadly mucus of CF?
Fig. 2.
A disease of epithelial electrolyte transport. Since much of the pathology is expressed in exocrine systems, Cystic fibrosis could be classified as an exocrinopathy. Inset illustrates an eccrine sweat gland in the skin. Drawing is reprinted with permission from Robert Osti.
It may be audaciously presumptive, but I would like to attempt an answer to this question by focusing on only three substances that are abnormal in CF: 1) mucus, 2) salt, and 3) HCO3−. Beginning with mucus, I simply ask the reader to consider the histopathology and appearance of mucoid substances found in affected organs.
Abnormal Mucus
Lungs.
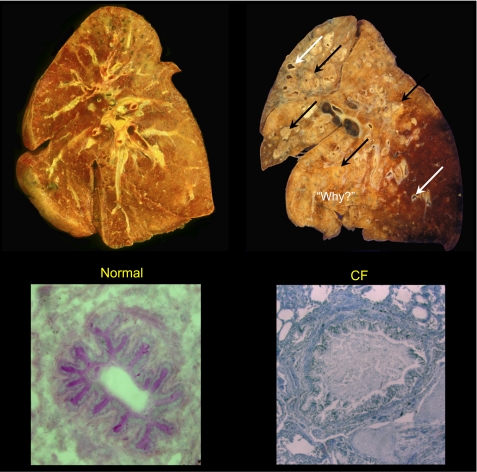
Most perilous are the changes that occur in the CF lung. Figure 3 compares a coronal section through a normal (left) with a section through a severely diseased CF (right) lung. The immediate question is “why?” Why does a lung that begins life normally (as the lung on the left) with time become so devastated, destroyed, or, as we more politely say, “remodeled,” as is the lung on the right? Clearly, most of the airways of the affected lung are filled with purulent pus, and what was described early on as concretions of mucus or mucoid substance that filled the small airways, where lung disease was presumed to begin, even before purulent secretions appear (Fig. 3) (8, 60). These lungs are characterized by bronchiectatic lesions that fill with purulent mucus that block air passage until so much of the lung is involved that respiration becomes impossible.
Fig. 3.
Coronal sections of whole lungs and cross sections of small airways. Note the uniform parenchyma of normal lung (top left) in contrast to the lung from a cystic fibrosis (CF) patient (top right). The latter shows an enormous number of ectatic lesions (arrows) with most airways completely filled and obstructed with purulent mucoid accumulations (black arrows). Examined microscopically, the lumens of normal small airways are virtually free of secretions (bottom left); in contrast, bronchioles from a CF patient can be obstructed and distended by mucus secretions even without significant evidence of inflammation (bottom right). The destruction demands “Why?” a defect in electrolyte transport is so destructive. The coronal sections are courtesies of Walter Finkbeiner, University of California, San Francisco, and the CF cross section is a courtesy of Carl Doershuk (Case-Western Reserve University).
Intestine.
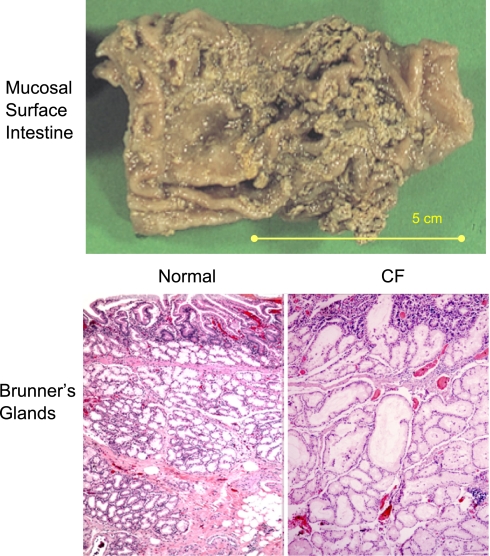
As another example (Fig. 4), we can see mucus partially covering the surface of a resected specimen of the small intestine from a CF patient. The image may not appear so remarkable, yet the pathologist who examined the specimen commented that he could not remove the mucus coat without removing the underlying epithelial cells. This comment is consistent with the general notion that CF mucus is not only thick and viscid but also adhesive.
Fig. 4.
Mucus adherence and accumulation in CF. Top: a small intestine from a CF patient with mucus accumulated and “stuck” to luminal epithelial surface. Image was kindly provided by Peter Durie and Ernest Cutz, Universty of Toronto, Canada. Bottom: histology of submucosal Brunner's glands of small intestine illustrating normal microanatomy of acini and ducts below the intestinal crypts (bottom left) in contrast to the deranged acini and ducts that have become massively distended by accumulated mucoid secretions (bottom right). Micrographs were provided by Walt Finkbeiner, University of California, San Francisco.
Glands.
Unfortunately, opportunities to assess the properties of mucus in CF (as shown in Fig. 4) are few, and most conclusions are based on the histologic and microanatomic appearance of tissues such as in Brunner's glands (Fig. 4), where duct blockage appears to result in continued accumulation of mucoid material that distends the duct and acinar lumens, ultimately forming lacunae lined by transformed squamous epithelial cells. In general, the material appears mucoid and stains with periodic acid Schiff (PAS) reagent (12, 15). Because other tissues (e.g., pancreas, bile ducts, gall bladder, submucosal glands, salivary glands, and uterine cervix) can all show very similar abnormalities that arise from mucus, “cystic fibrosis of the pancreas” is truly “mucoviscidosis” (a general state of thick mucus) of many organs. Thus it seems this disease was misnamed. The fact that advances in understanding CF over the past three decades have occurred mainly in understanding fluid and electrolyte transport probably contributed to the puzzling fact that the name “mucoviscidosis,” introduced by Farber 65 years ago (16), has been all but forsaken. Like Farber, I see “cystic fibrosis” more clearly as “mucoviscidosis.”
Abnormal Salt
Airways.
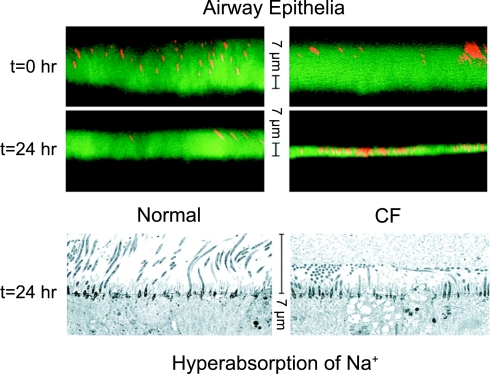
The first application of Ussing's chamber to CF tissue appeared in 1983 when Knowles et al. (29) showed that excised nasal tissues from CF subjects spontaneously displayed significantly more lumen-negative transepithelial potential differences (TEP) and lower transepithelial conductances (Gt) than did tissues from control subjects (Table 1). The results corroborated their groundbreaking observations of luminal TEPs in vivo in airways (28) and in the sweat duct(43). Even though these studies of native tissue in vitro (29) did not reveal significantly increased short-circuit current or net flux of Na+ or Cl−, the larger effect of the Na+ channel inhibitor amiloride on the spontaneous TEP in CF tissue supported the interpretation that active Na+ absorption was enhanced in CF airways (28), which in turn supported the idea that CF mucus was thick and viscid due to dehydration caused by excessive absorption of salt and water. The advent of confocal microscopy made measurements of specimen thickness possible and provided further support for this concept in air-liquid interface cultures of primary airway epithelial cells. Cultures from CF subjects developed and maintained an airway surface fluid depth that was only about one-third normal and had significantly enhanced rates of fluid removal (active absorption) from the surface layer (Fig. 5) (34). While this evidence supports the now commonly accepted idea that “mucoviscidosis” is caused by excessive absorption of Na+ (and therefore fluids) (7), it is important to recognize that these studies were carried out on cell cultures not native tissues, although the cultured cells were clearly well differentiated. However, two observations, one in sweat glands and the second in other organs affected in CF, leave me less certain of this cause of “mucoviscidosis.”
Table 1.
Bioelectric properties and solute fluxes across excised CF nasal polyps and normal nasal turbinates
| Normal | CF | |
|---|---|---|
| TEP, mV | −4.7 ± 0.8 | −11.7 ± 3.1* |
| Gt, mS/cm2 | 15.6 ± 1.2 | 7.6 ± 3.0* |
| Isc, mEq·cm−2·h−1 | 2.6 ± 0.9 | 2.7 ± 1.0 |
| Na+ net flux, mEq·cm−2·h−1 | 2.5 ± 0.8 | 3.5 ± 1.2 |
Data are means of average values (± SE) for turbinates from 6 normal and 3 cystic fibrosis (CF) subjects. TEP, transepithelial potential; Gt, Lower transepithelial conductance; short-circuit current.
Significantly different from normal values. From Knowles MR, Stutts MJ, Spock A, Fischer N, Gatzy JT, and Boucher RC. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science 221: 1067–1070, 1983
Fig. 5.
Prevalent view of mucoviscidosis. Mucus in airway surface fluid is considered to be thick and dessicated in CF due to excessive absorption of Na+ (fluid). Confocal microscopic images in the top show thicknesses of fluid surface layers at time 0 and 24 h later covering cultured normal (left) and CF (right) bronchial epithelial cells. The images below show electron micrographs of tissues 24 h after applying the same fluid load above each culture of cells. The thin CF layer above and the apparently compressed cilia in the image below indicate excessive fluid absorption. Reprinted and adapted from Matsui et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 95: 1005–1015, 1998, with permission from Elsevier.
Sweat glands.
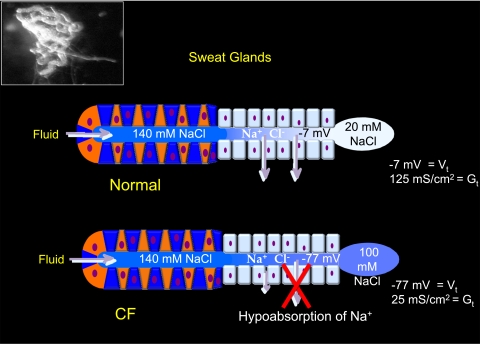
The sweat glands in CF reflect two fundamental failures in electrolyte transport in this disease: to secrete fluids (50) and to absorb salt (43). Intuitively, the failure to secrete fluids might compound the problem of dehydrated mucus just as would an enhanced absorption of Na+. However, the failure to absorb electrolytes places this defect diametrically opposed to the enhanced salt absorption in the lung. Figure 6 illustrates the functions of a normal and a CF sweat gland, but I should note that thermoregulatory (cholinergically mediated) secretion of sweat is not affected, whereas β-adrenergically mediated secretion (here and in other organs) is defective in CF. Thus, during sweating in CF or unaffected individuals, normal volumes of fluid are secreted into the water-impermeable sweat duct where normally NaCl is absorbed in great excess relative to water, thus producing a hypotonic sweat on the skin that contains ∼20 mM NaCl (Fig. 6, top). However, in CF, the sweat duct fails to efficiently absorb salt and thus sweat emerges that contains four to five times more NaCl or ∼100 mM (Fig. 6, bottom). Note that in this tissue, as in the airway, the lumen is much more electronegative relative to the interstitium in the CF duct than in the normal duct; i.e., −77 mV vs. −7 mV (46) even though the rate of salt absorption is depressed by three- to fourfold compared with normal absorption (Fig. 4) (6). Also, like the airway, despite the fact that salt absorption is clearly much less in the CF sweat duct, the effect of amiloride on its spontaneous TEP is much larger than on that of the normal duct, depolarizing the normal duct by only ∼10 mV and the CF duct by ∼100 mV (5). It must be noted that neither the magnitude of the TEP nor the impact of amiloride on the TEP can be taken as a measure of the underlying net salt transport activity.
Fig. 6.
Functions of normal and CF sweat glands. Inset: an isolated sweat gland as a single coiled tubule with the secretory coil in the left half of the image connected to, but distinguishable from, the reabsorptive duct in the right half of the image, which is illustrated at the bottom. Thermoregulated sweating is normal in CF and secretes the same load of isotonic precursor sweat from the secretory coil (blue and red cells on the left) to the more distal reabsorptive ducts (pale blue cells on the right). As the secreted fluid passes through the normal duct (top), Na+ and Cl− are absorbed in excess of water, yielding a quite hypotonic sweat secretion on the skin surface. In contrast, as the fluid passes through the CF sweat gland, Cl− and consequently Na+ cannot be efficiently absorbed, yielding a much higher concentration of excreted salt in the sweat (bottom). Note that even though the rate of salt absorption in the normal duct is fivefold greater than in the CF duct, the transepithelial voltage associated with normal absorption is only about 1/10 of that of the CF duct. Thus the transepithelial potential (Vt) indicates relative separation of charge due to the absorptive process and not the rate of the Na+ absorption.
This discrepancy in salt absorption between the airways and sweat ducts requires that we look to other affected organs and begs for an explanation since absorption in both tissues requires the same anion channel for the uptake of Cl− and the same cation channel for the uptake of Na+. The cation channel is Epithelial Na Channel (ENaC) known for its sensitivity to amiloride, and the anion channel is Cystic Fibrosis Transmembrane Regulator (CFTR), cloned in 1989 as the defective gene in CF (26, 48, 49). Numerous functions have been attributed to the CFTR protein (32), but the best known, most commonly accepted, and the one that pertains here is that of a Cl−-selective anion channel that together with ENaC mediates the concurrent absorption of Na+ and Cl− across the apical membrane of some absorptive epithelia. Thus salt absorption is mediated by ENaC and CFTR in the ducts of sweat, salivary, and bronchial submucosal glands, as well as in the colon, uterine cervix, and airways but not in the small intestine, pancreas, liver, or gall bladder. All of these organs are affected in CF, and all, except the sweat glands (which secretes almost no mucus), are compromised by mucus adherence and blockage. These results are tabulated in Table 2, which dramatizes the conundrum of how a defect in CFTR can cause the same pathological mucoviscidosis by altering a mechanism that is not common to all the affected tissues.
Table 2.
Overview of systems affected in CF
| Affected Tissues | Mucus Secretion | Na+ (ENaC) Absorption | HCO3− Secretion |
|---|---|---|---|
| Pancreas | + | 0 | + |
| Liver | + | 0 | + |
| Gall bladder | + | 0 | + |
| Small intestine | + | 0 | + |
| Colon | + | + | + |
| Salivary glands | + | + | + |
| Airways | + | + | + |
| Submucosal glands | + | + | + |
| Vas deferens | + | ? | + |
| Uterine cervix | + | + | + |
| Sweat glands | 0 | + | + |
Organ systems known to be disturbed in CF are listed on the left and the presence of mucus secretion, epithelial Na channel (ENaC) Na+ Absorption (ENaC/CFTR-dependent Na+ absorption), and HCO3− secretion are indicated as present (+), absent (0), or uncertain (?) in the column under the respective headings.
The following conclusions are based on what I have reviewed: 1) The same defective anion channel (CFTR) paradoxically results in enhanced salt absorption in CF airways and inhibited salt absorption in CF sweat ducts. 2) Not all tissues affected by mucoviscidosis express ENaC-CFTR-dependent salt/fluid absorption. 3) Enhanced fluid absorption does not appear to explain mucoviscidosis in all affected organs.
Abnormal HCO3−
Since it seems unlikely that the thick mucus in CF is due to aberrant mucus biosynthesis, and since hyperabsorption does not seem to explain mucoviscidosis in all the affected mucus-producing organs, I would like to consider whether there is a common denominator. Looking back, the name “cystic fibrosis of the pancreas” (the organ first associated with the disease) suggests a starting point (4). Of all the organs of the body, the pancreas is the organ of HCO3− secretion, which almost immediately implicates it in the disease process, but this anion has been neglected in favor of studies of Cl−, Na+, and fluid, possibly because physiologists do not like HCO3− and for good reason: HCO3− is a salt, a gas, a base, and a chelator; it is a pain to study and thus frequently is omitted or ignored even though it is the most important extracellular buffer.
After elevated NaCl was found in the sweat (14), HCO3− was the next ion found to be abnormal in CF. Hadorn et al. (22), Gaskin et al. (19), and Kopelman et al. (30) all found that even in CF patients with sufficient pancreatic enzymes to support digestion, the concentration and secreted amounts of HCO3− were significantly depressed in pancreatic juices collected in vivo compared with control subjects.
The defect in HCO3− secretion extends beyond the pancreas and is present in other tissues as well. In the duodenum, Pratha et al. (40) found that cAMP-mediated HCO3− secretion was almost fivefold greater in normal than in CF tissue. Since access to human tissue is limited, the creation of the CF mouse model lacking the expression of CFTR protein has been extremely instructive for gastrointestinal abnormalities; similar results were observed in the duodenum (9), jejunum, and ileum (52) as well as in the colon (23) and gall bladder (11). I should note that cAMP-mediated HCO3− secretion (i.e., CFTR dependent) is not the only source of HCO3− in the gut (40, 53).
In the airways, Smith and Welsh (54) found a cAMP-mediated HCO3−-dependent short-circuit current (Isc) in primary cultures of human bronchial epithelial (HBE) that was not present in CF-HBE cells, and Coakley et al. (10) observed that compared with HBEs from normals, CF-HBEs were significantly slower to correct for an acid load in the airway surface liquid, apparently secondary to an inability of CF cells to secrete HCO3− normally. Song et al. (55) measured the pH of secretions from tracheobronchial submucosal glands and found the pH of CF secretions to be 0.6 pH units lower than normal secretions, which would translate into more than a threefold difference in HCO3− concentration, assuming a physiological level of CO2 at 5%.
Even in the sweat gland, the pH of CF sweat tends to be more acid, but intriguingly, whereas sweat ducts from patients with severe CFTR mutations (such as ΔF508) show little if any HCO3− conductance, patients with the “milder” mutation R117H retain substantial HCO3− conductance (47) and generally do not have pancreatic insufficiency. In the male reproductive tract, the seminal fluid is normally highly alkaline (pH 8.2; calculated HCO3− ∼150 mM), whereas in CF, this fluid is acidic (pH ∼6.6; calculated HCO3− <5 mM) (17, 25).
A review of the literature shows that there is either direct evidence or good, indirect evidence that all of the glands that are affected in CF also secrete HCO3− (Table 2). There is a close correspondence between the ability to secrete HCO3− in all organs pathologically affected in CF and, the ability to secrete gel-forming mucins, excepting the sweat gland, which secretes almost no mucus and is not structurally affected.
The single point I make here is that CFTR-dependent HCO3− secretion is defective in CF epithelia.
Is There Evidence That HCO3− is Linked to Mucoviscidosis?
The evidence I have reviewed above leads to a new hypothesis that HCO3− is required for the normal discharge of gel-forming mucins. To validate that hypothesis, we designed experiments to test the impact of manipulating conditions affecting HCO3− secretion on mucus discharge. We fabricated a simple temperature-controlled apparatus from syringe barrels, rubber stoppers, O-rings, and glass capillaries (18) and vertically mounted mouse ileums (5–6 cm) for bottom-to-top perfusion (∼1 ml/min) of the lumen with defined solutions. The luminal perfusate was collected in aliquots at intervals of 3–5 min during the experiment. The solutions bathing the serosal surface (external) were also defined and changed as needed. The collected perfusates were assayed for mucus content by WGA-lectin binding or by PAS staining of the filtrand retained on imobilon-P film (an adaptation of the dot blot technique) (2, 18, 35).
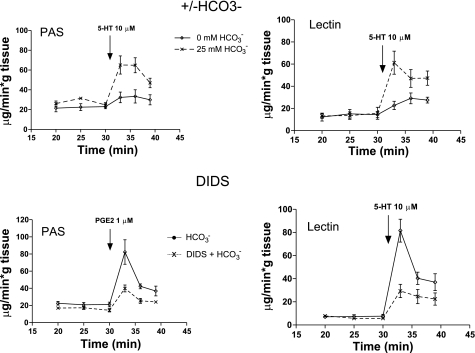
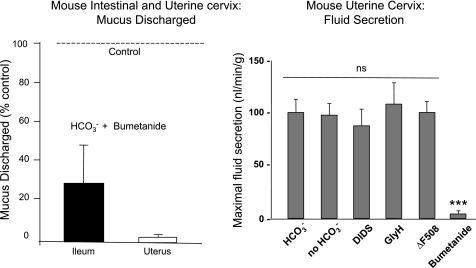
We monitored mucus discharged into the lumen before and after stimulation [by PGE2 or serotonin (5-HT)] in the presence and absence of various inhibitors. We found that, independent of the method of stimulation or the assay technique, when HCO3− was absent from the serosal compartment bathing the tissue, the amount of mucus appearing in the perfusate was significantly depressed (Fig. 7). Adding DIDS to the bath in the presence of HCO3− [to block the Na+ bicarbonate cotransporter (NBC)], also markedly reduced the amount of mucus discharged into the luminal perfusate (Fig. 7). These results show rather convincingly that the presence of HCO3−, and presumably its concurrent secretion, are needed for normal mucus discharge, but to be relevant to the disease, the effect should also depend on CFTR function.
Fig. 7.
HCO3−-dependent mucus discharge. Top: lumens of isolated, perfused ileal segments were incubated with 25 mM HCO3− (dashed lines) and without HCO3− (solid lines) in the serosal bathing media. Bottom: luminally perfused segments were incubated in 25 mM HCO3− with (dashed lines) or without DIDS (solid lines) in the serosal media to block HCO3− secretion. Aliquots of perfusates were assayed for mucus content at the times indicated. In either case, interfering with HCO3− secretion reduces mucus discharge. Discharged mucus was assayed by periodic acid Schiff reagent (PAS, left) and confirmed by conjugated WGA-Lectin binding assays (right). Both assays render similar results. Reprinted and adapted with permission from the American Society for Clinical Investigation from Garcia MA, Yang N, and Quinton PM. 2009. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest 119: 2613–2622.
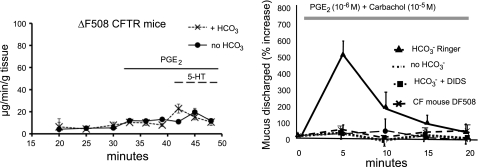
When we used ileums from mice homozygous for the severe ΔF508 mutation, which disrupts processing of the CFTR protein to the membrane, we observed that the amount of mucus released did not discernibly increase with stimulation and was not affected by the presence or absence of HCO3− (Fig. 8). We also found that applying naphthalen-2-ylamino-acetic acid (3,5-dibromo-2,4,-dihydroxy-benzylidene)-hydrazide (GlyH-101; courtesy A. Verkman) to block CFTR substantially reduced mucus discharge (not shown). These results strongly implicate CFTR as being critical to mucus discharge, presumably via a role in supporting HCO3− secretion.
Fig. 8.
Mucus discharge in intestine and uterine tracts. Left: intestine from mice homozygous for the ΔF508 mutation show severely blunted response to stimulation by PGE2 (10−6 M) and 5-HT (10−5 M). The amount of mucus release does not differ between segments in the presence or absence of HCO3− when stimulated with PGE2 (n = 5, P = 0.2) or serotonin (5-HT, P = 0.475) assayed by lectin-WGA-DAB. Right: the uterus responds to manipulations of HCO3−and HCO3− transport qualitatively in the same manner as does the ileum, but in contrast to the ileum, virtually no mucins appear in the perfusate in the absence of HCO3− or in the presence of the HCO3− transport inhibitor DIDS. As in the intestine, CF tissue does not respond to stimulation even in the presence of HCO3−. Reprinted and adapted with permission from the American Society for Clinical Investigation from Garcia MA, Yang N, and Quinton PM. 2009. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest 119: 2613–2622 and adapted from Muchekehu RW and Quinton PM. A new role for bicarbonate secretion in cervico-uterine mucus release. J Physiol 588: 2329–2342, 2010.
Are Other Organs Affected Similarly?
I have already emphasized that CF is a multiorgan disease of mucus pathology. Thus, if the lack of HCO3− is a unifying defect, one would predict that the effects of HCO3− should be present in other tissues in addition to the ileum. We thus tested the mouse reproductive tract since the cervical mucus of CF women is more viscous and undergoes little if any cyclical thinning (31). We adapted the perfusion system described above to the mouse uterus (a smaller tissue) by cannulating the vaginal os and one of the uterine horns while ligating the other so that the organ could be mounted and vertically perfused akin to the preparation of the ileum (35). We first observed that stimulating mucus discharge reproducibly from this organ required both carbachol (a muscarinic cholinergic agonist) and PGE2 simultaneously and further that in terms of mucus discharged, this tissue seemed to be more sensitive than the ileum to disturbing HCO3− transport. In bath solutions replete with HCO3−, we found a five- to sixfold increase in the amount of mucus in the perfusate within the first 5 min after stimulation (Fig. 8); however, if HCO3− was removed from the bath, or DIDS or GlyH-101 was added, or if the reproductive tract was from a CF mouse, virtually no mucus was discharged into the perfusate (Fig. 8) (35). Of note, the two organs predominantly secrete different mucins: the ileum secretes MUC2 (3) and the uterus secretes MUC5B (20). Thus the effect of HCO3− on mucus discharge is not mucin or tissue specific, and HCO3− participates in the formation of mucus gels in this tissue as well.
But is The Effect on Mucus Discharge Just Because of Decreased Fluid Secretion?
I must address the fact that CFTR is central to cAMP-mediated fluid (Cl−) secretion (50) and that concurrent fluid secretion with mucus secretion may be requisite to transport mucus by a lavage effect from the tissue into the perfusate. If fluid secretion in the gut or uterus is dependent on HCO3−, the depressed discharge of mucus into the luminal perfusate could be due to decreased fluid secretion and not directly to the lack of HCO3− per se. Indeed, if we inhibited the driver for fluid secretion, the Na+/K+/2Cl− cotransporter (NKCC) with bumetanide, stimulated mucus discharge was strongly depressed in both the intestine and the uterus (Fig. 9). These results demonstrated that the transport of gel-forming mucins from the crypts, intervillar space, and uterine glands depends on concurrent fluid secretion and demands an evaluation of the effect of removing HCO3− on fluid secretory activity. We measured fluid secretion gravimetrically in closed loops of ileums (18), but since the uterus is much smaller, we devised a volumetric measure of fluid secretion by ligating the vaginal os and one uterine horn while then cannulating the other horn with a small glass capillary (280 μM i.d. × 3 cm) (35). After equilibration, increases in weight of the loops and advances of the fluid-air interface meniscus in the connected capillary were converted to rates of fluid secretion. In both preparations, removing HCO3−, inhibiting transport by adding DIDS or GlyH-101, or using CF mouse tissues did not affect stimulated fluid secretion compared with controls in HCO3−-replete medium; only addition to the bath of the secretory inhibitor bumetanide blocked fluid secretion (Fig. 9). These results show that the fluid stimulated concurrently with mucus discharge was not affected by the presence or absence of HCO3− and that this fluid secretion almost certainly does not depend on CFTR. Thus 1) mucus discharge requires HCO3−, CFTR, and fluid secretion; 2) fluid secretion does not require HCO3−; and 3) HCO3− effects on mucus discharge are not caused by depressed fluid secretion.
Fig. 9.
Effects of inhibiting fluid secretion. Adding bumetanide to block Na+/K+/2Cl cotransporter and fluid secretion (right) decreases the amount of mucus discharged (left) into the perfusate of the ileum to <30% and of the uterus to less than a few percent compared with controls without bumetanide (dashed line, 100%) stimulated with PGE2 (ileum) or PGE2 and carbachol (uterus). Fluid secretion measured in the uterus is only blocked by bumetanide (***P < 0.001) and not by the absence of HCO3− or the presence of DIDS, GlyH-101, or the defective gene (ΔF508) expressed in the CF mouse (right). Reprinted and adapted with permission from the American Society for Clinical Investigation from Garcia MA, Yang N, and Quinton PM. 2009. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest 119: 2613–2622 and adapted from Muchekehu RW and Quinton PM. A new role for bicarbonate secretion in cervico-uterine mucus release. J Physiol 588: 2329–2342, 2010.
So What Can HCO3− Do to Mucins?
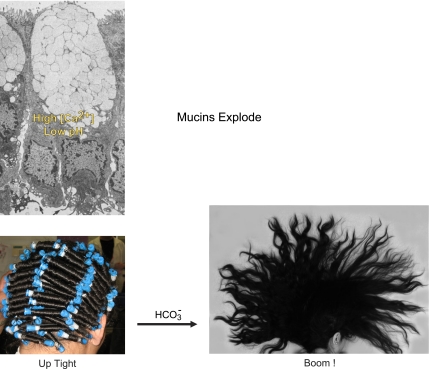
Here the issue becomes a bit “explosive” if we recall that intracellularly mucins are stored highly condensed and compacted in granules (58) (Fig. 10). Furthermore, the gel-forming mucins are massive glycoprotein molecules that can be millions of Daltons in weight and microns in length (27). The core protein is a long polymer highly populated with polysaccharide side chains that carry an extremely large number of fixed negative charges. The electrostatic forces from the negative charges are critical to keeping the deployed mucin molecule extended, making it almost “frictionless” and creating its gel-forming ability (39, 56). Upon quick reflection, it is easy to see that the cell encounters a huge problem in overcoming the repulsive electrostatic forces of the anionic charges as it packs these enormous molecules in intracellular granules for storage until secretion. Its solution is simple: the forces of the negative charges are neutralized and shielded behind the positive charges on small cations, namely, Ca2+ and protons, i.e., a pH < 6 and Ca2+ concentrations possibly >200 mM (37, 38, 58). Protons associate with individual anionic sites to neutralize charge, and Ca2+ not only neutralizes charge but also forms divalent electrostatic bridges between anionic sites that stabilize the molecule. Upon secretion of the granule, the mucins virtually explode and expand within a couple of seconds to a volume that is up to three orders of magnitude larger than the condensed mucins. Rapid expansion of the gel is likely important for forming the gel from these reptating molecular strings, but the shielding cations must be removed before this phenomenon can occur. The negative charges on the fixed anionic sites must be exposed so that their repulsive electrostatic forces can “explode” the condensed mucin molecule into the massive expansion that will form the mucin gel as dramatized in Fig. 10.
Fig. 10.
Explosion of condensed and compacted mucin. Mucins are stored in granules of goblet cells under conditions of very high Ca2+ concentration and very low pH (top left) to prevent condensed compacted mucin molecules from expanding under the electrostatic forces of their fixed negative charges. Bottom images dramatize mucin expansion as an exploding coiffure that is compacted in “curlers” that explode within seconds into a massive free-flowing mane when exposed to HCO3− as it is discharged; cf. Fig. 11. Top left is from Neutra MR, O'Malley LJ, and Specian RD. Regulation of intestinal goblet cell secretion. II. A survey of potential secretagogues. Am J Physiol Gastrointest Liver Physiol 242: G380–G387, 1982. Bottom left courtesy of Chelsea Johnson-Root; bottom right courtesy of Bayda Bahur.
The displacement of Ca2+ is presumed to be due to simple cation exchange of Ca2+ and H+ for monovalent Na+ and K+ in the mucin matrix upon exocytotic release of the mucin granule and that the mucin would then swell by a Donnan effect as these ions contribute to the osmotic pressure in the mucin matrix (1). However, the efficiency of this exchange should depend on the relative attraction of the cations for the fixed anionic sites of the mucin. If Ca2+ is more strongly attracted to the mucin sites, for example, than Na+, the process should be slower and may not go to completion. However, if the competition is between anions for Ca2+, and the competing anion is mobile and binds Ca2+ more strongly than the fixed anions, the displacement of Ca2+ within the mucin molecule is virtually assured and the cation exchange is all but guaranteed. Now, enter HCO3− as the competing mobile anion. HCO3− exists in equilibrium with its salt CO3−2, which can form CaCO3, while HCO3− itself can also form CaHCO3+ to tie up Ca2+ (36).
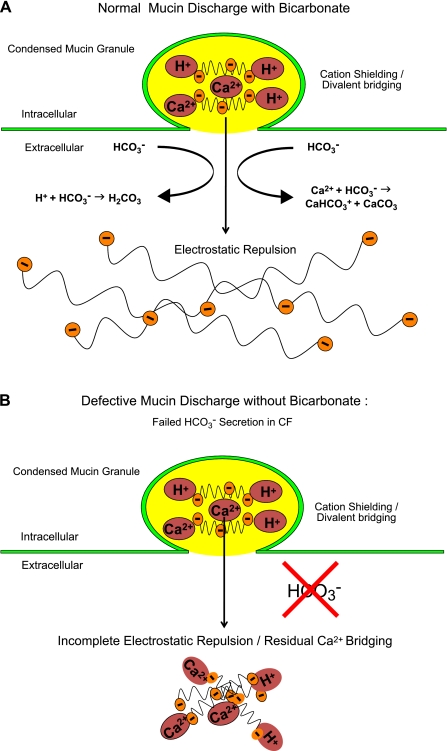
By illustrative analogy to coiffures, Fig. 10 illustrates the predicted effect of HCO3− on condensed mucins as they are excreted. More specifically, as illustrated less poetically in Fig. 11A, as the granules with mucins bound with Ca2+ and H+ enter the extracellular milieu, HCO3− immediately combines with Ca2+ and neutralizes H+ to form CaHCO3+ and CaCO3 as well as H2CO2 (which yields H2O and CO2). Without the shielding cations, the repulsive electrostatic forces of the fixed negative ions on the mucin core are exposed and can force open the compacted mucins into lengthy strings. The strings become entangled by reptating into a network to form a soluble, transportable mucus gel. In contrast, in CF mucin granules (Fig. 11B), if HCO3− is not present or secreted, more Ca2+ and H+ would remain with the mucins impeding their expansion and ability to reptate into normal gels. The predicted result is akin to what we witness in CF with aggregating mucus accumulating on the surfaces and in the ducts of mucus-secreting epithelial organs (see Figs. 3–4 above).
Fig. 11.
Models of effects of HCO3−. A: prediction of effects of HCO3− during normal mucin discharge. Repulsive electrostatic forces on the mucin polysaccharides are unshielded as HCO3− competes with the fixed negative sites for Ca2+ and H+, disrupting divalent bridges and forcing the mucin molecules to rapidly expand into an extended network of charged polymers. B: prediction of the effect of poor HCO3− secretion during mucin discharge as might occur in cystic fibrosis. Without competition from HCO3−, the repulsive electrostatic forces of the fixed negative sites on the mucin polysaccharides remain shielded and bound by Ca2+ and H+, leaving molecules that adhere to each other and remain relatively condensed and viscous Reprinted and adapted with permission from the American Society for Clinical Investigation from Garcia MA, Yang N, and Quinton PM. 2009. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest 119: 2613–2622.
Can We Cause Mucoviscidosis?
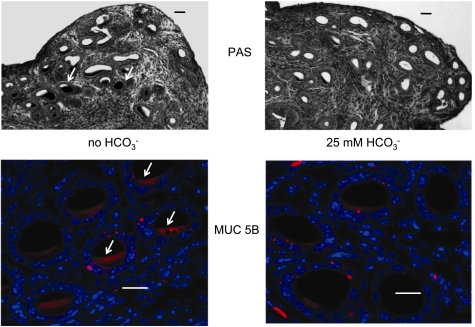
Based on these observations and notions, one might predict that removing HCO3− would reduce the amount of mucus released from the epithelia, as we demonstrated above (Figs. 7 and 8) and “cause cystic fibrosis” in the subject tissue. In fact, when we looked for mucus in mouse uterine glands with either PAS or MUC5B antibody, we observed that mucus appeared to be accumulated, if not “stuck,” within the ducts of the glands incubated without HCO3− compared with those incubated with HCO3− (Fig. 12). I describe the results as “mucoviscidosis.”
Fig. 12.
Mucoviscidosis in the absence of HCO3−. Uterine tissue was incubated in HCO3−-free (left) and HCO3−-containing (right) Ringer solutions for 20 min and then stimulated with PGE2 + carbachol for 20 min. Significantly more PAS-positive material remains in the lumens of tissue stimulated without HCO3− (arrows, top left) compared with tissue stimulated with HCO3− (top right). Tissue stained for MUC5B mucin (red) and counterstained with DAPI (blue nuclei, bottom) correlates with the PAS staining in the corresponding micrographs above (top: bar 20 μm. bottom: bar 50 μm). Adapted from Muchekehu RW and Quinton PM. A new role for bicarbonate secretion in cervico-uterine mucus release. J Physiol 588: 2329–2342, 2010.
These findings and conjectures tempt me to remark on what may happen in CF and why mucus appears to be excreted excessively, even if less mucins are produced (24). I surmise that inflammatory responses must appear, but first, I should note that the loss of organ function in CF is generally a gradual process. The lungs, pancreas, and liver are usually destroyed over years, if not decades, apparently one or a few subunits, acini, or lobules at a time. Thus mucus obstruction is not sudden and universal even in the same organ; some threshold or trigger must be crossed or activated to initiate the pathogenic event. The gradual process also informs that, in relative terms, the localized pathogenic event is infrequent. Hence, I can imagine that when mucus stagnates, if not cleared quickly, it may start a viscous cycle that leads to the demise of the affected unit. That is, if stagnated mucus is not removed (e.g., by lavage), the tissue must recognize it as debris requiring removal by innate inflammatory processes, which would including more mucin secretion. Without HCO3− to support mucus discharge, the added mucins will aggregate and accumulate along with previously accumulated debris, which they are intended to, but cannot, remove. Hence, mucus accumulates until eventually the tissue is damaged, remodeled, and destroyed by normal inflammatory responses. These events impede the clearance of pathogens in the lungs and of prematurely activated proteolytic enzymes in the pancreas, rendering these organs particularly vulnerable. I do not intend to ignore the role of fluid secretion in this process. Indeed, the data presented here show that it is crucial, and it remains prudent to appreciate that the pathology in CF is likely multifactorial, involving Na+ absorption, Cl− secretion, HCO3− secretion, and other confounding phenomena.
Before ending what may be viewed as an enormous amount of speculation, I would be remiss were I not to offer at least two caveats. First, as Einstein warned, “make it simple, just not too simple.” It is entirely possible, and very likely in fact, that I have oversimplified the role HCO3− in unraveling mucins. For example, mucins are likely packaged in very structured arrays maintained not only by cationic bridges and shielding but also by intramolecular disulfide and hydrophobic bonds. Thus unraveling the nascent mucin must involve efficient cleavage and breaking of these bonds as well. Moreover, there may be a substantial, perhaps greater, impact of HCO3− on this process, especially if higher pH is requisite or if Ca2+ must be removed from binding sites in nodes that organize the protein cores into loops of mucin molecules (27, 42).
Second, I have presumed that HCO3− existing in the luminal medium facilitates the mucin expansion but perhaps HCO3− is concurrently secreted with mucin. Based on my training, I assume that HCO3− is secreted primarily, if not exclusively, by the neighboring transport epithelial cells that surround mucin-secreting goblet cells and are the source of the luminal HCO3−. Intuitively, based on characteristics of electrolyte-transporting cells that seem to be missing in goblet cells, I expect that goblet cells cannot participate in significant HCO3− secretion. However, the evidence that Bestrophin-2 is a HCO3−-permeable channel(41) in the basolateral membrane of goblet cells (59) raises this question to an intriguingly new level.
Understanding of the process and, in particular, how and where HCO3− influences this new-found role, requires much more intense and intricate investigation, but perhaps what we have seen here will provide a new view of CF animated by bringing the disease process to basic principles of chemistry and physics. In closing, I leave you with three final points: 1)CFTR-dependent HCO3− secretion is defective in CF. 2) HCO3− is required for normal mucus formation. 3) Defective HCO3− transport leads to “mucoviscidosis.”
GRANTS
Funding was provided by the National Institutes of Health Grant HL-084042, the Cystic Fibrosis Foundation, Cystic Fibrosis Research, Inc., the Olmsted Trust, and kind anonymous gifts.
DISCLOSURES
The author has no conflicts of interest in publishing this work.
ACKNOWLEDGEMENTS
I sincerely thank the organizers of the Ussing Lecture for their efforts and their kindness in offering me this truly unique honor of trying to give tribute to a man whom I and so many others owe so much: Professor Hans Ussing. Drs. Jessie Yang, Ruth Muchekehu, and Abi Garcia should be clearly recognized for their contributions to the design, data analysis, and execution of experimental protocols presented here. Drs. J. Kaunitz, Y. Akiba, and G. Flores were critical in establishing improved mucus assays, and Kirk Taylor contributed crucial technical support to the projects.
REFERENCES
- 1.Aitken ML, Verdugo P. Donnan mechanism of mucin release and conditioning in goblet cells: the role of polyions. Symp Soc Exp Biol 43: 73–80, 1989 [PubMed] [Google Scholar]
- 2.Akiba Y, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Dynamic regulation of mucus gel thickness in rat duodenum. Am J Physiol Gastrointest Liver Physiol 279: G437–G447, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Allen A, Hutton DA, Pearson JP. The MUC2 gene product: a human intestinal mucin. Int J Biochem Cell Biol 30: 797–801, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease. Am J Dis Children 56: 344–399, 1938 [Google Scholar]
- 5.Bijman J, Quinton P. Permeability properties of cell membranes and tight junctions of normal and cystic fibrosis sweat ducts. Pflugers Arch 408: 505–510, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Bijman J, Quinton PM. Influence of abnormal Cl- impermeability on sweating in cystic fibrosis. Am J Physiol Cell Physiol 247: C3–C9, 1984 [DOI] [PubMed] [Google Scholar]
- 7.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med 261: 5–16, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Burgel PR, Montani D, Danel C, Dusser DJ, Nadel JA. A morphometric study of mucins and small airway plugging in cystic fibrosis. Thorax 62: 153–161, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke LL, Harline MC. Dual role of CFTR in cAMP-stimulated HCO3− secretion across murine duodenum. Am J Physiol Gastrointest Liver Physiol 274: G718–G726, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Coakley RD, Grubb BR, Paradiso AM, Gatzy JT, Johnson LG, Kreda SM, O'Neal WK, Boucher RC. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci USA 100: 16083–16088, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuthbert AW. Bicarbonate secretion in the murine gallbladder–lessons for the treatment of cystic fibrosis. Jop 2: 257–262, 2001 [PubMed] [Google Scholar]
- 12.Damjanovich L, Szeifert GT, Szabo M, Papp Z. Pathological confirmation of foetal cystic fibrosis following prenatal diagnosis. Acta Morphol Hung 38: 141–148, 1990 [PubMed] [Google Scholar]
- 13.diSant'Agnese PA, Darling RC, Perera GA, Shea E. Abnormal electrolyte composition of sweat in cystic fibrosis of the pancreas; clinical significance and relationship to the disease. Pediatrics 12: 549–563, 1953 [PubMed] [Google Scholar]
- 14.Di Sant'Agnese PA, Darling RC, Perera GA, Shea E. Sweat electrolyte disturbances associated with childhood pancreatic disease. Am J Med 15: 777–784, 1953 [DOI] [PubMed] [Google Scholar]
- 15.Doggett RG, Bentinck B, Harrison GM. Structure and ultrastructure of the labial salivary glands in patients with cystic fibrosis. J Clin Pathol 24: 270–282, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farber S. Some organic digestive disturbances in early life. J Michigan State Med Soc 44: 587–594, 1945 [Google Scholar]
- 17.Flume PA, Yankaskas JR. Reproductive issues. In: Cystic Fibrosis in Adults, edited by Yankaskas JR, Knowles MR. Philadelphia, PA: Lipencott-Raven, 1999, p. 449–464 [Google Scholar]
- 18.Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest 119: 2613–2622, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaskin KJ, Durie PR, Corey M, Wei P, Forstner GG. Evidence for a primary defect of pancreatic HCO3−secretion in cystic fibrosis. Pediatr Res 16: 554–557, 1982 [DOI] [PubMed] [Google Scholar]
- 20.Gipson IK. Mucins of the human endocervix. Front Biosci 6: D1245–1255, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Gray MA, Plant S, Argent BE. cAMP-regulated whole cell chloride currents in pancreatic duct cells. Am J Physiol Cell Physiol 264: C591–C602, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Hadorn B, Johansen PG, Anderson CM. Pancreozymin secretin test of exocrine pancreatic funtion in cystic fribrosis and the significance of the result for the pathogenesis of the disease. Can Med Assoc J 98: 377–385, 1968. [PMC free article] [PubMed] [Google Scholar]
- 23.Harmon GS, Dumlao DS, Ng DT, Barrett KE, Dennis EA, Dong H, Glass CK. Pharmacological correction of a defect in PPAR-gamma signaling ameliorates disease severity in Cftr-deficient mice. Nature medicine 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henke MO, Renner A, Huber RM, Seeds MC, Rubin BK. MUC5AC and MUC5B mucins are decreased in cystic fibrosis airway secretions. Am J Respir Cell Mol Biol 31: 86–91, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Kaplan E, Shwachman H, Perlmutter AD, Rule A, Khaw KT, Holsclaw DS. Reproductive failure in males with cystic fibrosis. N Engl J Med 279: 65–69, 1968 [DOI] [PubMed] [Google Scholar]
- 26.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. Identification of the cystic fibrosis gene: genetic analysis. Science 245: 1073–1080, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Kesimer M, Makhov AM, Griffith JD, Verdugo P, Sheehan JK. Unpacking a gel-forming mucin: a view of MUC5B organization after granular release. Am J Physiol Lung Cell Mol Physiol 298: L15–L22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knowles M, Gatzy J, Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med 305: 1489–1495, 1981 [DOI] [PubMed] [Google Scholar]
- 29.Knowles MR, Stutts MJ, Spock A, Fischer N, Gatzy JT, Boucher RC. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science 221: 1067–1070, 1983 [DOI] [PubMed] [Google Scholar]
- 30.Kopelman H, Corey M, Gaskin K, Durie P, Weizman Z, Forstner G. Impaired chloride secretion, as well as bicarbonate secretion, underlies the fluid secretory defect in the cystic fibrosis pancreas. Gastroenterology 95: 349–355, 1988 [DOI] [PubMed] [Google Scholar]
- 31.Kopito LE, Kosasky HJ, Shwachman H. Water and electrolytes in cervical mucus from patients with cystic fibrosis. Fertil Steril 24: 512–516, 1973 [PubMed] [Google Scholar]
- 32.Kunzelmann K. CFTR: interacting with everything? News Physiol Sci 16: 167–170, 2001 [DOI] [PubMed] [Google Scholar]
- 33.LarsenHans HEH. Ussing–scientific work: contemporary significance and perspectives. Biochim Biophys Acta 1566: 2–15, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 95: 1005–1015, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Muchekehu RW, Quinton PM. A new role for bicarbonate secretion in cervico-uterine mucus release. J Physiol 588: 2329–2342, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nayakama FS. Calcium activity, complex and ion-pair in saturated CaCO3. Soil Science 106: 429–434, 1968 [Google Scholar]
- 37.Nicaise G, Maggio K, Thirion S, Horoyan M, Keicher E. The calcium loading of secretory granules. A possible key event in stimulus-secretion coupling. Biol Cell 75: 89–99, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Perez-Vilar J, Olsen JC, Chua M, Boucher RC. pH-dependent intraluminal organization of mucin granules in live human mucous/goblet cells. J Biol Chem 280: 16868–16881, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Pettersson T, Dedinaite A. Normal and friction forces between mucin and mucin-chitosan layers in absence and presence of SDS. J Coll Int Sci 324: 246–256, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Pratha V, Hogan D, Martensson B, Conrad D, Light M, Isenberg JI. Duodenal Bicarbonate Transport is impaired in Cystic Fibrosis Patients. Pediatr Pulmonol Supplement 17: 225, 1999 [Google Scholar]
- 41.Qu Z, Hartzell HC. Bestrophin Cl− channels are highly permeable to HCO3−. Am J Physiol Cell Physiol 294: C1371–C1377, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quinton PM. Birth of mucus. Am J Physiol Lung Cell Mol Physiol 298: L13–L14, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinton PM. Chloride impermeability in cystic fibrosis. Nature 301: 421–422, 1983 [DOI] [PubMed] [Google Scholar]
- 44.Quinton PM. Cystic fibrosis: a disease in electrolyte transport. FASEB J 4: 2709–2717, 1990 [DOI] [PubMed] [Google Scholar]
- 45.Quinton PM. Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev 79: S3–S22, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Quinton PM, Bijman J. Higher bioelectric potentials due to decreased chloride absorption in the sweat glands of patients with cystic fibrosis. N Engl J Med 308: 1185–1189, 1983. [DOI] [PubMed] [Google Scholar]
- 47.Reddy MM, Quinton PM. Control of dynamic CFTR selectivity by glutamate and ATP in epithelial cells. Nature 423: 756–760, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary. DNA Sci 245: 1066–1073, 1989 [DOI] [PubMed] [Google Scholar]
- 49.Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N, Collins FS, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science 245: 1059–1065, 1989 [DOI] [PubMed] [Google Scholar]
- 50.Sato K, Sato F. Defective beta adrenergic response of cystic fibrosis sweat glands in vivo and in vitro. J Clin Invest 73: 1763–1771, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwiebert EM, Benos DJ, Fuller CM. Cystic fibrosis: a multiple exocrinopathy caused by dysfunctions in a multifunctional transport protein. Am J Med 104: 576–590, 1998 [DOI] [PubMed] [Google Scholar]
- 52.Seidler U, Blumenstein I, Kretz A, Viellard-Baron D, Rossmann H, Colledge WH, Evans M, Ratcliff R, Gregor M. A functional CFTR protein is required for mouse intestinal cAMP-, cGMP- and Ca(2+)-dependent HCO3− secretion. J Physiol 505: 411–423, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sellers ZM, Childs D, Chow JY, Smith AJ, Hogan DL, Isenberg JI, Dong H, Barrett KE, Pratha VS. Heat-stable enterotoxin of Escherichia coli stimulates a non-CFTR-mediated duodenal bicarbonate secretory pathway. Am J Physiol Gastrointest Liver Physiol 288: G654–G663, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Smith JJ, Welsh MJ. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest 89: 1148–1153, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song Y, Salinas D, Nielson DW, Verkman AS. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am J Physiol Cell Physiol 290: C741–C749, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Tanaka T. Gels. Sci Am 244: 124–136, 138, 1981 [DOI] [PubMed] [Google Scholar]
- 57.Ussing HH, Zerahn K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand 23: 110–127, 1951 [DOI] [PubMed] [Google Scholar]
- 58.Verdugo P, Deyrup-Olsen I, Aitken M, Villalon M, Johnson D. Molecular mechanism of mucin secretion. I. The role of intragranular charge shielding. J Dent Res 66: 506–508, 1987 [DOI] [PubMed] [Google Scholar]
- 59.Yu K, Lujan R, Marmorstein A, Gabriel S, Hartzell HC. Bestrophin-2 mediates bicarbonate transport by goblet cells in mouse colon. J Clin Invest 120: 1722–1735, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zuelzer WW, Newton WA. The pathogenesis of fibrocystic disease of the pancreas. A study of 36 cases with special reference to the pulmonary lesions. Pediatrics 4: 53–69, 1949 [PubMed] [Google Scholar]