Abstract
Previous studies have suggested that inhibition of the mammalian target of rapamycin (mTOR) by rapamycin suppresses myocardial hypertrophy. However, the role of mTOR in the progression of cardiac dysfunction in pathological hypertrophy has not been fully defined. Interestingly, recent reports indicate that the inflammatory response, which plays an important role in the development of heart failure, is enhanced by rapamycin under certain conditions. Our aim in this study was to determine the influence of mTOR on pathological hypertrophy and to assess whether cardiac mTOR regulates the inflammatory response. We generated transgenic mice with cardiac-specific overexpression of wild-type mTOR (mTOR-Tg). mTOR-Tg mice were protected against cardiac dysfunction following left ventricular pressure overload induced by transverse aortic constriction (TAC) (P < 0.01) and had significantly less interstitial fibrosis compared with littermate controls (WT) at 4 wk post-TAC (P < 0.01). In contrast, TAC caused cardiac dysfunction in WT. At 1 wk post-TAC, the proinflammatory cytokines interleukin (IL)-1β and IL-6 were significantly increased in WT mice but not in mTOR-Tg mice. To further characterize the effects of mTOR activation, we exposed HL-1 cardiomyocytes transfected with mTOR to lipopolysaccharide (LPS). mTOR overexpression suppressed LPS-induced secretion of IL-6 (P < 0.001), and the mTOR inhibitors rapamycin and PP242 abolished this inhibitory effect of mTOR. In addition, mTOR overexpression reduced NF-κB-regulated transcription in HL-1 cells. These data suggest that mTOR mitigates adverse outcomes of pressure overload and that this cardioprotective effect of mTOR is mediated by regulation of the inflammatory reaction.
Keywords: cardiac hypertrophy, heart failure, inflammation, transgenic mice, mammalian target of rapamycin
cardiac hypertrophy occurs in a wide variety of clinically important conditions, such as hypertension and valvular heart disease, and increases the risk of heart failure (14). Whereas left ventricular (LV) pressure overload initially induces adaptive hypertrophy (17), sustained pressure overload eventually results in pathological hypertrophy (4). Pathological hypertrophy is characterized by multiple pathophysiological features, including fibrosis and cardiac dysfunction (4). Studies using rapamycin indicate that inhibition of the mammalian target of rapamycin (mTOR) suppresses myocardial hypertrophy induced by mechanical stresses in animal models (36, 48, 50). However, the role of mTOR in the progression of cardiac dysfunction that accompanies pathological hypertrophy has not been fully defined.
Fibrosis is commonly observed in patients with cardiac hypertrophy and correlates with the severity of LV remodeling (42). Levels of proinflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β, increase during heart failure (8); these cytokines are known to accelerate the accumulation of collagen following the inflammatory response (38). Interestingly, recent reports showed that rapamycin enhances the inflammatory response in immune cells (43). Since cytokines are generated in cardiomyocytes as well as immune cells (21), it is likely that cardiac mTOR suppresses cytokine production during the progression of cardiac dysfunction in pathological hypertrophy.
Rapamycin binds to FKBP12 (FK506-binding protein) to form a complex that in turn binds mTOR, thereby inhibiting signaling to p70S6 kinase (p70S6K, S6K1) and eukaryotic initiation factor 4E-binding protein 1 (4EBP1) (3). mTOR forms two functional complexes, a rapamycin-sensitive mTOR complex (mTORC1), which includes the scaffolding protein raptor, and a rapamycin-insensitive complex (mTORC2) that contains rictor (56). mTORC1 activates p70S6K, which promotes translation by phosphorylating the ribosomal protein S6. mTORC1 also inhibits 4EBP1 binding to eIF4E (eukaryotic translation initiation factor 4E), further promoting translation (18). mTORC2 activates Akt through phosphorylation at Ser473 (22). Several new ATP-competitive inhibitors of mTOR, such as PP242, which inhibits both mTORC1 and mTORC2, have been developed and characterized (10, 23). By blocking both mTOR complexes, these new inhibitors allow us to assess the role of mTOR more completely.
To explore the role of mTOR expression in cardiac hypertrophy and dysfunction, we examined mTOR levels in hearts from cardiac patients and controls and generated transgenic mice with cardiac-specific overexpression of wild-type mTOR. Intriguingly, our studies demonstrate that overexpression of wild-type mTOR mitigates cardiac dysfunction in response to pressure overload-induced hypertrophy. In addition, the inflammatory response was reduced in the hearts of mTOR-Tg mice compared with controls. We assessed the role of cardiac mTOR in the inflammatory response in a cardiomyocyte cell line by manipulating mTOR activity by overexpression or with mTOR inhibitors.
MATERIALS AND METHODS
Human ventricular myocardial tissue.
All human tissue was acquired with approval from the appropriate research ethics board at Massachusetts General Hospital (Boston MA). The investigation conforms with the principles outlined in the Declaration of Helsinki. All personal information related to each heart was de-identified before receipt in the laboratory, in accordance with the Privacy Rule standards of the Health Insurance Portability and Accountability Act. Myocardial tissue was collected from explanted hearts of transplant patients with heart failure [average fractional shortening (%FS), 14.9 ± 2.3%] in nonischemic dilated cardiomyopathy or from donor control hearts, as previously described (30). Donor control hearts were not transplanted for a variety of reasons, including lack of a suitable recipient, blood transfusion while in the emergency room, and age of donor. Donor hearts were used only if there were no macroscopic, laboratory, or instrumental signs of cardiac disease.
Generation of transgenic mice with cardiac overexpression of wild-type mTOR.
Animal experiments in this study were approved by the Institution Animal Care and Use Committee at Beth Israel Deaconess Medical Center, Harvard Medical School (Boston MA). The cDNA encoding hemagglutinin (HA)-tagged wild-type rat mTOR was subcloned downstream of the murine α-myosin heavy chain (α-MHC) promoter (generous gift of Dr. Jeffrey Robbins, Cincinnati Children's Hospital Research Foundation) and used to generate transgenic mice through oocyte injection as performed previously (32). To genotype, we used the real-time quantitative PCR system. Genomic DNA was reacted with a TaqMan probe using the QuantiFast SYBR green PCR kit (Qiagen, Valencia, CA). Three viable lines (line 4, line 52, and line 53) were generated from independent founders. Line 4 (mTOR-Tg) was backcrossed to C57BL/6 for more than eight generations, and the other lines were backcrossed for three generations. All data for baseline characterization of mTOR-Tg mice were collected from 12- to 14-wk-old male mice. Male wild-type (WT) littermates were used as controls.
Pressure overload inducing cardiac hypertrophy.
Mice were subjected to transverse aortic constriction (TAC) as previously described (55). Male mTOR-Tg mice (12–14 wk old) were anesthetized by intraperitoneal delivery of a mixture of ketamine (80–100 mg/kg) and xylazine (12 mg/kg). After a thoracotomy was performed, the transverse aortic arch was ligated. On the basis of our previous echocardiographic study results indicating that wild-type mice develop cardiac hypertrophy and dysfunction at 4 wk post-TAC, we examined mTOR-Tg and WT mice at 1 and 4 wk post-TAC. Cardiac function and signaling molecules examined in sham-operated mice were not different from those in nonoperated mice in the baseline study with wild-type male C57BL/6 mice. To confirm whether TAC treatment induces similar levels of pressure overload in both mTOR-Tg and WT mice, we simultaneously measured the pressure gradient between right and left carotid arteries using a Millar catheter as previously described (55). Nonoperated mTOR-Tg or WT mice were used as controls in the TAC study.
Echocardiography.
Echocardiography was performed on nonanesthetized mice using a high-frequency (10 MHz) linear transducer (13 L, VingMed 5; GE Medical Services, Milwaukee, WI). M-mode images used for measurements were taken at the papillary muscle level (32). We measured LV diastolic dimension, LV systolic dimension, and %FS.
Quantitative RT-PCR.
Accumulation of PCR product was monitored in real time, and the crossing threshold (Ct) was determined with 7300ABI (Applied Biosystems, Foster City, CA). Relative change in gene expression was determined using the ΔΔCt method with normalization to GAPDH. Quantitative RT-PCR (QRT-PCR) were performed with the following sets of primers: forward 5′-TGTTCCGACGAATCTCAAAGC and reverse 5′-TCATATGTTCCTGGCACAGCC for human mTOR, forward 5′-GCAAATTCCATGGCACCGT and reverse 5′- TCGCCCCACTTGATTTTGG for human GAPDH, forward 5′-GTGAAAAGTGGACTCTGGTTAATGAC and reverse 5′-CATCGTGAGTATCCCGAGGAAT for rat mTOR, forward 5′-AGAAGGAGTGGCTAAGGACCAA and reverse 5′-GCATAACGCACTAGGTTTGCC for mouse IL-6, forward 5′-CCTTCCAGGATGAGGACATGAG and reverse 5′-CGTCACACACCAGCAGGTTATC for mouse IL-1β, and forward 5′-TGGTGAAGCAGGCATCTGAG and reverse 5′-TGCTGTTGAAGTCGCAGGAG for mouse GAPDH. TaqMan probes for mouse atrial natriuretic factor (ANF) and mouse connective tissue growth factor (CTGF) were purchased from Applied Biosystems.
Histological assays of cardiac tissue from TAC-treated transgenic mice.
Midventricle short-axis heart sections (5 μm) from male WT and mTOR-Tg mice were fixed in 4% paraformaldehyde. To identify macrophages, we immunostained sections with anti-Mac-2 monoclonal antibody (Cedarlane Lab, Hornby, ON, Canada). Signals were enhanced with the ABC kit (Vector Laboratories, Burlingame, CA). To visualize fibrotic tissue, we stained the sections with Masson's trichrome. To objectively quantify the amount of tissue fibrosis, we developed a prespecified, genotype-blinded image selection method. Images selected for analysis from each section at the midpapillary muscle level contained the largest amount of fibrosis. Percent fibrosis was determined using ImageJ to quantify blue (fibrotic) vs. non-blue (nonfibrotic) pixels. The results are presented as percent change in fibrosis per image area (not whole heart) from WT sham.
Cardiomyocyte isolation.
LV cardiomyocytes were isolated using a perfused-heart method, as described previously (32). Images were captured digitally using the Leica Application Suite. ImageJ was used to trace individual cells and calculate their surface areas.
Terminal deoxynucleotidyl transferase dUTP nick-end label staining.
Terminal deoxynucleotidyl transferase dUTP nick-end label (TUNEL) staining was performed with Apoptag (Millipore) according to the manufacturer's instructions, as described previously (34). More than 2,000 nuclei were counted in each heart from each group (n = 4 for each week 1 group; n = 3 for each week 4 group). In total, over 6,000 nuclei were evaluated in each group.
Cell culture and transfection.
The HL-1 cardiomyocyte cell line was a generous gift from Dr William Claycomb (Louisiana State University Medical Center, New Orleans, LA). The cells were cultured and transfected as described previously (6). HL-1 cells were transfected with either wild-type mTOR vector or backbone pcDNA1.0 vector (mock) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. One day before transfection, 1.0 × 106 HL-1 cells were transferred into each well of a six-well plate containing 2 ml of supplemented Claycomb medium. The next day, HL-1 cells were transfected with 10 μg/well mTOR or control DNA vector using Lipofectamine2000. Two days after the transfection, the HL-1 cells were treated with 50 nM rapamycin (Cell Signaling, Danvers, MA) or 0.4 μM PP242 (Sigma) 30 min before stimulation with 1 μg/ml Escherichia coli LPS (Sigma). On the basis of our preliminary data, treated cells were harvested 5 h after LPS stimulation for luciferase assay. In separate experiments, the supernatants were collected 24 h after LPS stimulation for measurement of cytokine production. In the experiment for mTOR signaling pathway, cells were starved overnight and then pretreated with rapamycin (50 nM) or PP242 (0.4 μM) for 1 h before LPS stimulation.
Western blotting.
After animals were deeply anesthetized, hearts and other tissues were removed, snap frozen, and crushed in liquid nitrogen. The tissue was homogenized in cold lysis buffer [20 mM Tris·HCl (pH 7.6), 150 mM NaCl, 1% Triton X-100, 2 mM EGTA, 1 mM PMSF, 1 mM DTT, 1 mM sodium orthovanadate, 1 μg/ml leupeptin, and 1 μg/ml aprotinin]. For whole cell lysates of the HL-1 cells, cultured cells were collected with the cold lysis buffer. Western blotting was performed as described previously (33). Primary antibodies to HA (12CA5; Roche, Indianapolis, IN), GAPDH (Cell Signaling), S6 (Cell Signaling), phospho-S6 (Ser235/236; Cell Signaling), 4EBP1 (Cell Signaling), phospho-4EBP1 (Thr37/46; Cell Signaling), Akt (Cell Signaling), phospho-Akt (Ser473; Cell Signaling), and mTOR (Cell Signaling) were used for immunoblotting.
Determination of secreted cytokines in HL-1 cells.
Levels of IL-6, TNF-α, and IL-1β were measured simultaneously using multiplexed cytokine assays (Meso Scale Discovery, Gaithersburg, MD) according to the manufacturer's protocol. The standard curves established from curve-fitting models exhibited low variability and a dynamic range between 3 and 5 logs (44).
Luciferase assay.
HL-1 cells were cotransfected with a pGL4.32[luc2P/NF-κB-RE/Hygro] vector (Promega, Madison, WI), a Renilla pGL4.75[hRluc/CMV] vector (Promega), and a wild-type mTOR plasmid (or mock vector). Two days after the transfection, cells were pretreated with either control medium or an mTOR inhibitor for 30 min before 5-h incubation with LPS. NF-κB luciferase activity was measured using the Dual-Glo luciferase assay system (Promega) (27).
Statistical analysis.
Data are means ± SE. Group differences were analyzed using the two-tailed Student's t-test. For multiple comparisons, a one- or two-way ANOVA post hoc test was used. For all analyses, P values <0.05 were considered significant.
RESULTS
Changes in cardiac mTOR expression in human heart failure.
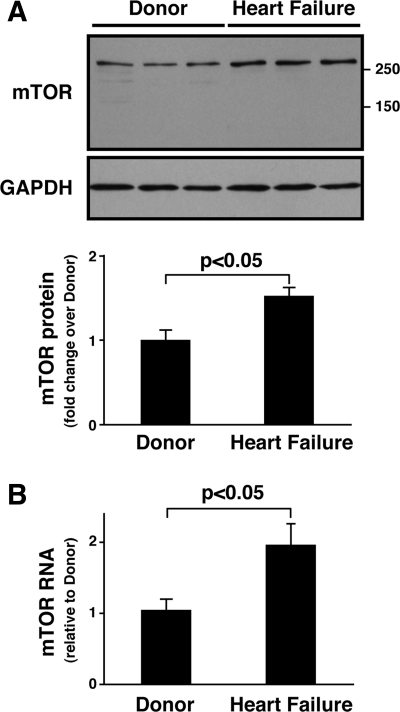
Tissue derived from the hearts of five heart failure patients and five donors was subjected to Western blotting (Fig. 1A). The two groups were matched for sex (1 male and 4 females in each group) and age (54.4 ± 6.0 vs. 54.2 ± 7.4 yr, heart failure vs. donor). Immunoblotting demonstrated that the mTOR protein level in hearts from heart failure patients was about 60% higher than in hearts from donor controls (P < 0.05), whereas levels of GAPDH protein were comparable (Fig. 1A). Quantification by QRT-PCR revealed that cardiac mTOR mRNA levels were also elevated: they were about 90% higher in heart failure patients than donor controls (P < 0.05, Fig. 1B). These results indicate that cardiac mTOR expression increases at protein and mRNA levels during heart failure.
Fig. 1.
Mammalian target of rapamycin (mTOR) expression is elevated in patients with heart failure. A: mTOR protein levels. Top, representative Western blots showing mTOR protein levels in extracts of human heart tissue from donor control and heart failure patients. GAPDH was used as a loading control. Bottom, densitometric quantification of mTOR protein expression. Values are means ± SE. Data are expressed as the degree of change (fold change) over the mean value in donor hearts. B: mTOR mRNA levels. mTOR mRNA was measured in extracts of donor and patient hearts by quantitative RT-PCR (QRT-PCR). Values are means = SE; n = 5 for each group.
Generating transgenic mice with cardiac overexpression of wild-type mTOR.
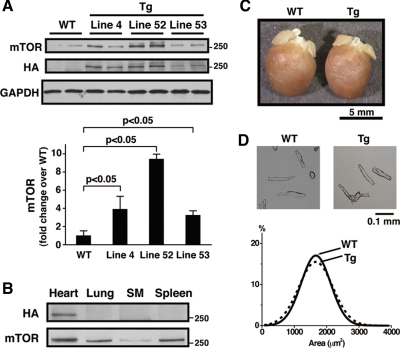
To examine the functional consequences of increased mTOR expression in the heart, we generated transgenic mice with cardiac-specific overexpression of wild-type mTOR driven by the murine α-MHC promoter, as described previously (32). Three lines were produced, and all demonstrated stable Mendelian inheritance and normal life span (over 12 mo). Immunoblotting with an antibody against the incorporated HA epitope demonstrated the presence of the transgene mTOR-HA in all three lines (Fig. 2A). The mTOR expression level in hearts from each line of transgenic mice was over threefold higher than that in WT hearts (P < 0.05, Fig. 2A). Line 4 (mTOR-Tg), which showed moderate transgene expression, was used for detailed baseline characterization and for the TAC experiments. Anti-HA blotting demonstrated cardiac-specific transgene expression in mTOR-Tg (Fig. 2B). At baseline, heart weight and body weight were not different in mTOR-Tg mice compared with WT mice (Table 1 and Fig. 2C). Morphological analysis of isolated cardiomyocytes showed no difference in cardiomyocyte surface area between mTOR-Tg and WT (Fig. 2D). Echocardiography found no difference in systolic function between mTOR-Tg and WT mice (Table 1). Thus chronic elevated expression of wild-type mTOR does not modify heart or cardiomyocyte size in mTOR-Tg mice and does not affect cardiac function at baseline.
Fig. 2.
Cardiac-specific overexpression of hemagglutinin (HA)-tagged mTOR in mice. A: cardiac mTOR protein expression in transgenic mice overexpressing wild-type mTOR. Top, representative Western blots of cardiac mTOR protein and HA-tagged mTOR expression. Immunoblotting was performed on extracts from hearts of 12- to 14-wk-old male mice from 3 viable lines of transgenic mice overexpressing wild-type mTOR: line 4 (mTOR-Tg), line 52, and line 53, as well as littermate negative mice (WT). GAPDH was used as a loading control. Bottom, densitometric quantification of cardiac mTOR. Values are means ± SE; n = 3 in each group. Data were normalized to the mean value of mTOR protein expression in WT. B: cardiac-specific expression of HA-tagged mTOR in mTOR-Tg mice. Samples (40 μg) of whole cell lysates from various organs in 13-wk-old male mTOR-Tg mice were separated by SDS-PAGE and immunoblotted with antibodies to HA and mTOR. SM, skeletal muscle. This immunoblot is representative of 3 independent experiments. C: gross view of representative hearts from 13-wk-old male WT and mTOR-Tg mice. D: isolated cardiomyocytes. Ventricular cardiomyocytes were isolated from 12- to 14-wk-old male mTOR-Tg and WT mouse hearts. Top, representative cardiomyocytes from each group; bottom, quantification of cell surface area. The surface areas of over 300 cells from 3 individual mice in each group were measured using ImageJ, and the size distributions were plotted, showing no significant difference in size.
Table 1.
General and cardiac echo measurements in WT and mTOR-Tg mice
| WT | mTOR-Tg | |
|---|---|---|
| Age, wk | 14.0 ± 0.2 | 13.9 ± 0.2 |
| Body weight, g | 23.9 ± 0.5 | 22.2 ± 0.6 |
| Tibia length, mm | 23.4 ± 0.3 | 23.4 ± 0.2 |
| Heart weight, mg | 120.7 ± 3.5 | 119.5 ± 3.2 |
| Heart weight/body weight, mg/g | 5.1 ± 0.2 | 5.4 ± 0.2 |
| Heart weight/tibia length, mg/mm | 5.2 ± 0.2 | 5.1 ± 0.1 |
| Left ventricular diameter in diastole, mm | 2.9 ± 0.1 | 3.0 ± 0.1 |
| Left ventricular diameter in systole, mm | 1.3 ± 0.1 | 1.2 ± 0.1 |
| Fractional shortening, % | 56 ± 2 | 61 ± 2 |
| Heart rate, beats/min | 702 ± 10 | 695 ± 17 |
Values are means ± SE; n = 8 wild-type littermates (WT) and n = 11 transgenic mice with cardiac-specific overexpression of wild-type mTOR (mTOR-Tg).
mTOR overexpression in transgenic mice protects against the development of pathological cardiac hypertrophy.
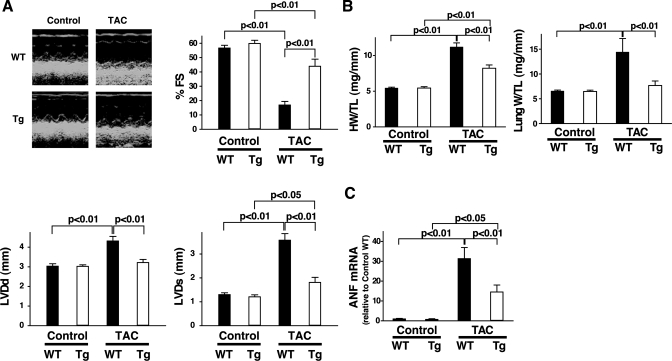
To investigate the effect of elevated cardiac mTOR expression on the development of pathological hypertrophy, we subjected mTOR-Tg and WT mice to TAC. The TAC-induced pressure gradient between right and left carotid arteries was not significantly different between WT and mTOR-Tg mice (57.8 ± 11.3 vs. 53.1 ± 6.6 mmHg, respectively). Four weeks after TAC, echocardiography demonstrated substantially reduced cardiac function in WT mice compared with age-matched nonoperated WT mice (Fig. 3A). In WT mice, TAC treatment also increased heart weight and lung weight (Fig. 3B). Ventricular ANF expression increased dramatically in TAC-operated WT mice compared with age-matched nonoperated WT (Fig. 3C). These measures demonstrate that the WT mice subjected to TAC exhibited pathological hypertrophy and cardiac dysfunction. In contrast, mTOR-Tg mice exposed to TAC exhibited little indication of cardiac dysfunction, less LV dilation (Fig. 3A), less cardiac hypertrophy, and less lung congestion (Fig. 3B) than TAC-operated WT. In addition, TAC induced a significantly smaller increase in ANF expression in mTOR-Tg than in WT mice (Fig. 3C). Together, these data indicate that chronic overexpression of cardiac mTOR can mitigate pathological hypertrophy and prevent cardiac dysfunction induced by TAC.
Fig. 3.
Pressure overload increased cardiac hypertrophy in mTOR-Tg mice. A: echocardiographic analyses. Left, representative M-mode images of nonoperated (control) and transverse aortic constriction (TAC)-operated mTOR-Tg and WT mice. Right, mean scores for %fractional shortening (%FS), left ventricular (LV) diameter in systole (LVDs), and left ventricular diameter in diastole (LVDd). Values are means ± SE; n = 6 in each group. B: ratios of heart weight to tibia length (HW/TL; left) and lung weight to tibia length (LW/TL; right). Hearts and lungs from each group were harvested after echocardiography was performed. Values are means ± SE; n = 6 in each group. C: fold changes in atrial natriuretic factor (ANF) mRNA levels. Ventricular ANF mRNA levels were quantified with QRT-PCR. Values are means ± SE; n = 6 in each group.
Expression of mTOR protein and mTOR signaling pathway in pathological hypertrophy.
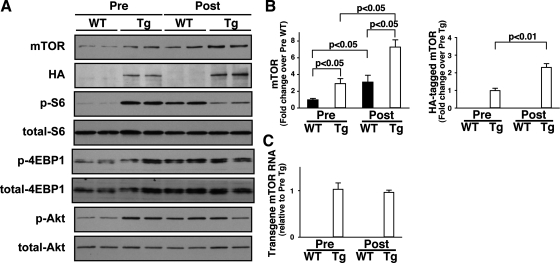
In addition to elevated mTOR expression, phosphorylation levels of S6 and Akt were greater in nonoperated mTOR-Tg mice than in nonoperated WT mice, suggesting that overexpression of wild-type mTOR induces functional activation of both mTORC1 and mTORC2 at baseline (Fig. 4A). As predicted based on our results in cardiac tissue from patients with heart failure (Fig. 1A), TAC increased mTOR protein expression in WT mice. mTOR protein levels in WT mice following TAC were similar to the baseline mTOR protein level in mTOR-Tg mice (Fig. 4, A and B). Intriguingly, mTOR protein levels in the mTOR-Tg mice also increased significantly above baseline following TAC (Fig. 4, A and B). Western blotting with anti-HA showed that expression of the transgenic mTOR after TAC increased similarly to total mTOR (2.3 ± 0.2-fold change over pre-TAC level, P < 0.01, Fig. 4, A and B). Since the transgene in the mTOR-Tg mice is of rat mTOR origin, we used primers specific to the rat mTOR sequence to distinguish the transgene mRNA from endogenous mouse mTOR mRNA. As expected, QRT-PCR using the rat-specific primers did not detect endogenous mTOR mRNA in WT mice. In mTOR-Tg mice, we detected rat mTOR at baseline and saw no change in its expression following TAC (Fig. 4C). These data imply that in heart failure and cardiac hypertrophy, mTOR protein levels may be controlled by posttranslational modification as well as changes in mTOR mRNA level.
Fig. 4.
Activation of mTOR signaling pathways in TAC-operated mTOR-Tg mice. A: representative immunoblots of mTOR signaling molecules. Pre-TAC hearts were harvested from 12- to 14-wk-old nonoperated male mice. Post-TAC hearts were harvested 4 wk after TAC. Immunoblotting was performed with indicated antibodies. Blots are representative of 5–6 independent samples in each group. 4EBP1, eukaryotic initiation factor 4E-binding protein. B: densitometric quantification of mTOR and HA-mTOR protein levels. Data were normalized to the mean protein level in pre-TAC WT. Values are means ± SE; n = 5–6 in each group. C: transgene mTOR mRNA levels. Transgene mTOR mRNA level before and after TAC was quantified with QRT-PCR. Values are means ± SE; n = 5–6 in each group.
Interestingly, although there was a trend toward increased phosphorylation of the mTORC1 substrates S6 and 4EBP1 and the mTORC2 substrates Akt in WT mice, TAC did not affect the already elevated phosphorylation levels of 4EBP1 and Akt in mTOR-Tg mice at 4 wk after TAC (Fig. 4A). Phosphorylation of S6 in mTOR-Tg was decreased at 4 wk post-TAC (Fig. 4A).
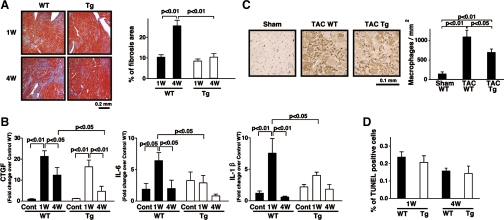
Interstitial fibrosis and inflammatory response in mTOR-Tg mice after pressure overload.
Apoptosis is an important pathophysiological feature of heart failure during cardiac hypertrophy. Despite that connection, we did not observe any significant difference between WT and mTOR-Tg mice in the percentage of apoptotic cells at either 1 or 4 wk post-TAC (Fig. 5D). Fibrosis is another pathophysiological feature frequently seen in pathological hypertrophy and is critical to the pathogenesis of heart failure. We investigated the extent of interstitial fibrosis in mTOR-Tg vs. WT mice after TAC. Masson's trichrome staining demonstrated that interstitial fibrosis was about 60% lower in mTOR-Tg mice compared with WT mice at 4 wk post-TAC, although whereas no significant difference between the two groups was observed at 1 wk post-TAC (Fig. 5A). CTGF, a key mediator in deposition of extracellular matrix, is upregulated by several proteins, including transforming growth factor-β1 and angiotensin II, in cardiac hypertrophy (35). TAC treatment increased CTGF mRNA levels to a similar level in WT and mTOR-Tg mice at 1 wk post-TAC. However, at 4 wk post-TAC, the level of CTGF expression in WT mice was more than threefold greater than that in mTOR-Tg mice (Fig. 5B). These findings suggest that a trigger of fibrosis such as inflammation might be more persistent or stronger in WT mice than in mTOR-Tg mice after 1 wk post-TAC. The proinflammatory cytokine IL-6 is known as a strong prognostic predictor in patients with heart failure (24). IL-1β plays an important role in the extension of the fibrotic scar during the progression to heart failure in cardiac hypertrophy (47). Expression of IL-6 and IL-1β in WT mice at 1 wk post-TAC was significantly higher than in nonoperated controls and WT mice at 4 wk post-TAC. In contrast, the expression levels of IL-6 and IL-1β in mTOR-Tg mice were not significantly different between control, 1 wk post-TAC, and 4 wk post-TAC (Fig. 5B). In addition, the level of IL-6 and IL-1β expression in WT mice at 1 wk post-TAC was significantly higher than that in mTOR-Tg mice at 1 wk post-TAC (Fig. 5B). These cytokine data indicated a significant inflammatory reaction at 1 wk post-TAC; therefore, we investigated macrophage accumulation at that time. Histological assay demonstrated that the number of accumulated macrophages in post-TAC WT mice was significantly greater than that in post-TAC mTOR-Tg mice (Fig. 5C). These data suggest that mTOR overexpression suppresses inflammation, a potential trigger of cardiac fibrosis.
Fig. 5.
Interstitial fibrosis and inflammatory response in mTOR-Tg mice after LV pressure overload. A: pathological analysis. Left, representative photos of Masson's trichrome staining in cardiac sections from TAC-operated WT and Tg mice at 1 and 4 wk post-TAC; right, quantitative analysis of interstitial fibrosis examined by Masson's trichrome staining (n = 3 in each group). B: expression of mouse connective tissue growth factor (CTGF), IL-6, and IL-1β mRNAs. mRNA was measured by QRT-PCR. Values are means ± SE; n = 4–6 in each group. C: macrophage infiltration. Left, representative photos of anti-Mac-2 immunostained hearts from sham control, TAC-operated WT, and TAC-operated mTOR-Tg mice. Macrophages are stained brown. Right, quantitative analysis of macrophage infiltration. Macrophages were counted in hearts from several animals in each group (sham, n = 3; 1 wk post-TAC, n = 4; 4 wk post-TAC, n = 4). D: terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay. Apoptotic cells have TUNEL-positive nuclei. Values are means ± SE; the percentage of TUNEL-positive nuclei was calculated from over 6,000 nuclei in each group.
mTOR signaling in mTOR-overexpressed HL-1 cells.
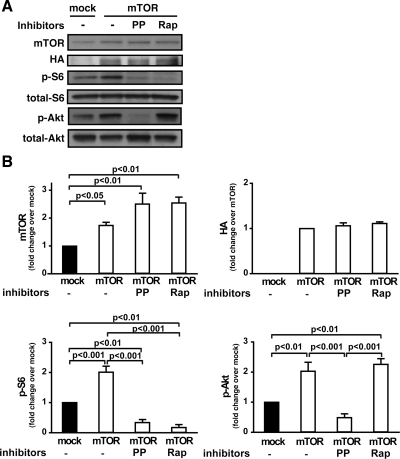
To further characterize the role of cardiac mTOR in the inflammatory response, we used cardiomyocytes of the HL-1 cell line. Immunoblotting confirmed that HL-1 cells transfected with mTOR overexpress mTOR (P < 0.05, Fig. 6, A and B). Overexpression of mTOR significantly enhanced phosphorylation of both S6 and Akt (Fig. 6, A and B), suggesting that increased mTOR expression activates both mTORC1 and mTORC2. The selective ATP-competitive mTORC inhibitor PP242 has been shown to suppress both mTORC1 and mTORC2 (10). In mTOR-transfected HL-1 cells, treatment with PP242 substantially inhibited both mTORC1 and mTORC2, as demonstrated by suppression of S6 and Akt phosphorylation (phospho-S6, −83.5%, P < 0.001; phospho-Akt, −76%, P < 0.001) (Fig. 6, A and B). In contrast, rapamycin suppressed S6 phosphorylation but had no effect on Akt phosphorylation in mTOR-transfected HL-1 cells (Fig. 6, A and B), confirming that rapamycin is an mTORC1-specific inhibitor under these conditions.
Fig. 6.
mTOR overexpression in HL-1 cells. A: representative immunoblots of HA, mTOR, phospho-Akt, phospho-S6, total Akt, and total S6 in HL-1 cells transfected with mTOR. HL-1 cells were transfected with mock vector or mTOR using Lipofectamine 2000 as described in materials and methods. Forty-eight hours after transfection, the cells were treated in the presence or absence of PP242 (PP; 0.4 μM) or rapamycin (Rap; 50 nM) for 1 h. B: quantitative analysis of mTOR, HA, phospho-S6, and phospho-Akt. Data were normalized to levels in mock-transfected controls in each experiment. Values are means ± SE; results are from 3–4 independent experiments.
mTOR overexpression suppresses cytokine release and inhibits NF-κB activity in cardiomyocytes in vitro.
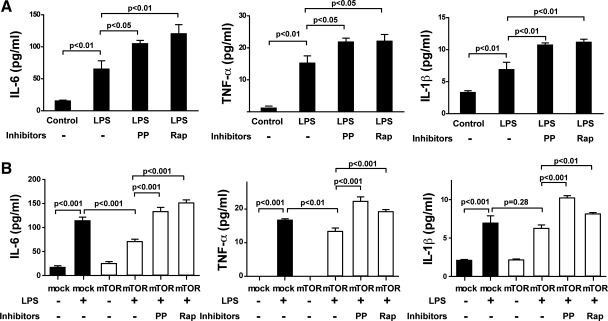
Toll-like receptor 4 (TLR4) regulates secretion of TNF-α, IL-6, and IL-1β in the heart (52). Treatment with LPS, which activates TLR4, significantly enhanced secretion of proinflammatory cytokines IL-6 (4.2-fold, P < 0.01), TNF-α (12.8-fold, P < 0.01), and IL-1β (2.1-fold, P < 0.01) from cardiomyocytes of the HL-1 cell line (Fig. 7, A and B). mTOR overexpression substantially suppressed IL-6 (P < 0.001) and TNF-α (P < 0.01) secretion in LPS-stimulated cells (Fig. 7B). PP242 and rapamycin abolished the ability of mTOR overexpression to inhibit LPS-induced cytokine secretion of IL-6 and TNF-α (Fig. 7B). LPS induction of IL-1β secretion was not inhibited by mTOR overexpression; however, PP242 and rapamycin significantly increased IL-1β secretion in mTOR-transfected cells following LPS stimulation (Fig. 7, A and B). The data strongly suggest that mTOR inhibits the inflammatory response triggered by LPS in cardiomyocytes, particularly with respect to IL-6.
Fig. 7.
mTOR modulates secretion of proinflammatory cytokines in HL-1 cardiomyocytes. A: HL-1 cells were pretreated with medium or mTOR inhibitor (PP or Rap) 30 min before 24-h treatment with 1 μg/ml LPS. Cell supernatants were analyzed for IL-6, tumor necrosis factor (TNF)-α, and IL-1β. B: HL-1 cells transiently transfected with mTOR or mock vector were treated with or without the mTOR inhibitor PP or Rap 30 min before 24-h treatment with or without 1 μg/ml LPS. Cell supernatants were analyzed for IL-6, TNF-α, and IL-1β. Values are means ± SE; representative data from 1 of 3 independent experiments performed in triplicate are shown.
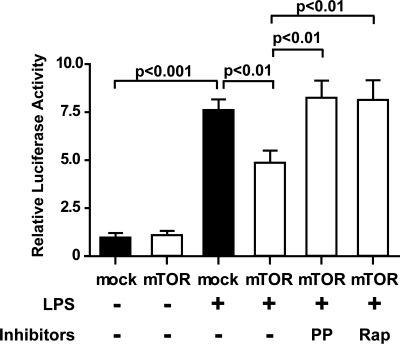
To explore the mechanism by which mTOR regulates cytokines secretion, we assayed the activity of NF-κB, a major regulator of IL-6, TNF-α and IL-1β in the proinflammatory response (Fig. 8). Using a luciferase assay on cells cotransfected with NF-κB-regulated luciferase expression vector, we found that LPS stimulation significantly increased NF-κB-mediated luciferase activity in cardiomyocytes (7.6 ± 0.5-fold change over control, P < 0.001, Fig. 8). mTOR overexpression reduced the NF-κB-mediated luciferase activity in LPS-stimulated cells by 36% compared with mock transfection (P < 0.01, Fig. 8) . Both PP242 and rapamycin abolished this effect of mTOR overexpression (P < 0.01, Fig. 8).
Fig. 8.
Activation of mTOR signaling reduces NF-κB activation following LPS stimulation in HL-1 cells. HL-1 cells were transfected with mTOR or mock vector as indicated. An NF-κB assay was performed as described in materials and methods. After transfection, the cells were treated with or without mTOR inhibitor (PP or Rap) 30 min before 5-h treatment with 1 μg/ml LPS. Values are means ± SE; representative data from 1 of 3 independent experiments performed in triplicate are shown.
DISCUSSION
Our data show that cardiac mTOR expression is increased in human heart failure and in a mouse model of pathological hypertrophy generated by LV pressure overload (TAC). We also have demonstrated that cardiac-specific mTOR overexpression reduces the adverse effects of TAC on cardiac morphology and function, including cardiac hypertrophy, LV dilatation, cardiac dysfunction, interstitial fibrosis, and inflammation. Our in vitro findings using HL-1 cardiomyocytes show that mTOR can reduce cytokine secretion and suggest that cardiac mTOR suppresses the inflammatory response induced by LPS, at least in part, by modulating NF-κB activity.
Previous studies have suggested that cardiac hypertrophy is inhibited by rapamycin (36, 48, 50), suggesting a role for mTOR. A recent study showing that deletion of cardiac mTOR accelerates progression of heart failure in TAC-induced cardiac hypertrophy (58) implies that cardiac mTOR plays an important role in cardioprotection against heart failure during pathological hypertrophy. However, there has been little investigation of mTOR's role in fibrosis and inflammation, important pathogenetic phenomena associated with pathological hypertrophy and cardiac dysfunction. Our observation that expression of cardiac mTOR was elevated in explanted human hearts from transplant patients with heart failure and in mice with pathological hypertrophy suggested that it plays a role in either the pathogenesis of heart failure or the adaptive response to heart failure. To address whether increased mTOR expression is an adaptive or maladaptive response, we generated transgenic mice with cardiac-specific overexpression of wild-type mTOR (mTOR-Tg) and subjected them to TAC-induced pressure overload. Intriguingly, mTOR overexpression protected the heart against pressure overload-induced cardiac dysfunction and reduced the degree of interstitial fibrosis at 4 wk post-TAC in mTOR-Tg mice compared with WT mice. In addition, CTGF mRNA levels were significantly lower in mTOR-Tg mice than in WT mice at the 4 wk post-TAC, consistent with previous reports that CTGF expression in cardiac fibroblasts and cardiomyocytes promotes expression of collagen and other extracellular matrix proteins involved in fibrosis (5, 26).
Inflammation followed by fibrosis is a key feature in the pathogenesis of pathological hypertrophy (54). Previous reports using animal models demonstrated that increased proinflammatory cytokine expression plays an important role in the pathogenesis of heart failure with pathological hypertrophy (47). The mechanisms that trigger inflammation in human heart failure and animal models of heart failure, including TAC, are multifactorial and have not been clearly established (2). However, reactive oxygen species (ROS) are generated at high levels in TAC-induced cardiac hypertrophy (51, 59), and ROS level seem to be tightly associated with the inflammatory response during the progression of heart failure (25). Although the effects of proinflammatory cytokines, including IL-6, TNF-α, and IL-1β, are complex (20), prolonged secretion and excessive levels of cytokines are known to be key features in the pathogenesis of heart failure (9). The significant increase in IL-6 and IL-1β we observed in WT mice after pressure overload was limited in mTOR-Tg mice, resulting in the infiltration of fewer macrophages in mTOR-Tg mice compared with WT at 1 wk post-TAC. This suggests that cardiac mTOR exerts an inhibitory pressure on cytokine production or secretion, consistent with recent reports showing that rapamycin enhances the inflammatory response in immune cells (43). Inhibition of mTOR by rapamycin has been shown to promote production of proinflammatory cytokines via the transcription factor NF-κB in immune cells (53). In mice systemically injected with LPS, rapamycin treatment increases mortality and is accompanied by an increase in serum IL-1β levels (45). Since cytokines are generated in cardiomyocytes as well as immune cells (21), it is likely that activated mTOR signaling in mTOR-Tg mouse heart suppresses the increase in IL-6 and IL-1β observed at 1 wk post-TAC in WT mice. In our in vitro experiments using HL-1 cardiomyocytes, LPS increased secretion of IL-6, IL-1β, and TNF-α, which was partially blocked by overexpression of mTOR. Experiments with rapamycin and PP242 showed that mTORC1 was predominantly responsible for this decrease in LPS-induced cytokine secretion. Rapamycin and PP242 were also equally effective in reversing the effect of mTOR overexpression on LPS-induced NF-κB activation in HL-1 cardiomyocytes, again implicating mTORC1. These findings are consistent with a previous report that mTOR activation due to mTOR overexpression reduces NF-κB activation in endothelial cells (40). NF-κB is an important transcription factor in the TLR4-mediated signaling pathway in immune cells (39) and cardiomyocytes (12). ROS, which are generated in the TAC model of heart failure (51, 59), mediate TLR4-dependent activation of NF-κB (1). Our findings suggest that mTOR plays a key regulatory role in NF-κB signaling in TLR-induced cytokine release in cardiomyocytes. Other transcriptional regulators important in TLR4 signaling, such as AP-1 (16), might also contribute to LPS-induced cytokine regulation in cardiomyocytes; however, we did not examine these in the current study.
IL-6 generation was suppressed by mTOR activation in our in vivo and in vitro studies. In contrast, LPS-induced IL-1β secretion in HL-1 cardiomyocytes was not inhibited by mTOR activation, although it was upregulated by mTOR inhibitors. Thus mTOR activation in cardiomyocytes predominantly affects IL-6 generation rather than IL-1β generation. A previous report demonstrated that most of the IL-1β immunoreactivity during cardiac hypertrophy is localized to endothelial cells and interstitial macrophages rather than to the myocardium (47). IL-6 is produced in response to IL-1 (41) and promotes inflammation in the heart by recruiting leukocytes to cardiomyocytes (57). Thus the inhibitory effect of cardiac mTOR on IL-6 generation might be sufficient to suppress the subsequent inflammatory response, including that mediated by IL-1β generation from other cells in the heart. A critical role for IL-6 in hypertrophy and heart failure is supported by previous reports showing that IL-6 contributes to cardiomyocyte hypertrophy and fibroblast proliferation in animal models (7, 13) and that IL-6 is a strong prognostic predictor in patients with heart failure (11). Together, these data suggest that controlling IL-6 generation might be a key factor in the cardioprotective effect of mTOR.
In the current study, mTOR-Tg mice did not show cardiac hypertrophy at baseline despite significant activation of downstream molecules in both the mTORC1 and mTORC2 pathways. In previous studies, transgenic mice with cardiac-specific overexpression of constitutively active mTOR also displayed normal heart size (46). Rapamycin treatment suppressed myocardial hypertrophy induced by LV pressure overload, accompanied by inhibition of S6K1 activity (48). However, rapamycin treatment did not affect cardiomyocyte size at baseline (48). Knockout mouse models demonstrated that S6 kinases alone are not essential for the development of cardiac hypertrophy (37). These findings suggest that mTORC1 is not sufficient to induce cardiac hypertrophy. In our mTOR-Tg mice, Akt phosphorylation was also increased at baseline, suggesting that mTORC2 was activated. Using transgenic mouse models, we and others have reported that Akt activation induces cardiac hypertrophy (32, 49). Unlike the animals in these previous studies, our mTOR-Tg mice showed activation of both mTORC1 and mTORC2. mTORC1 pathways generate feedback on upstream molecules, such as insulin receptor substrate (IRS)-1 (19). These feedback loops might contribute to maintaining normal heart size in the mTOR-Tg mice. Further work is necessary to define the mechanisms underlying these signaling pathways in cardiac hypertrophy and understand how they interact.
mTOR mRNA and protein levels were elevated in patients with advanced heart failure. Accelerated transcription of cardiac mTOR or enhanced mTOR mRNA stability could account in part for the increase in mTOR expression in heart failure. At present, we have no data to provide insight into the mechanism underlying this increase in mTOR expression gene. One possible contributor is thyroid hormone, which activates cardiac mTOR accompanied with an increase in mTOR expression (28, 29). Thyroid hormone has been shown to prevent cardiac dysfunction in animal models of heart failure and has some protective effects in clinical trials (15). However, based on the literature, heart failure appears to be associated with decreased thyroid hormone activity, so it seems unlikely that thyroid hormone is responsible for the elevated mTOR expression we observed in clinical samples and in our animal model for heart failure. Posttranslational modification of mTOR may also be involved in the control of mTOR expression in the heart. In the current study, we observed an increase in mTOR-HA transgene level in post-TAC mTOR-Tg mice. The increase in transgene protein was not accompanied by any significant change in the mRNA level, suggesting that stability of the mTOR protein plays an important role in regulation of mTOR function in the heart. In other tissues, the protein FBXW7 (F-box and WD repeat domain-containing 7) has been shown to regulate mTOR protein stability (31). A similar mechanism might increase mTOR in the heart. Further experiments are required to explore the mechanism underlying increased mTOR expression in the heart.
In summary, we found that endogenous mTOR expression was elevated significantly in patients with advanced heart failure and in mice with pathological hypertrophy and cardiac dysfunction. Although chronic mTOR activation did not modify heart size or affect cardiac function at baseline, it inhibited the progression of cardiac dysfunction in pathological hypertrophy, in addition to reducing interstitial fibrosis. Our data suggest that the anti-inflammatory effect of mTOR activation may preserve cardiac function by controlling collagen generation and attenuating fibrosis. Improved understanding of mTOR's cardioprotective mechanism may have important implications not only in cardiac hypertrophy but also in other heart diseases such as myocardial infarction.
GRANTS
This work was supported in part by National Institutes of Health (NIH) Grants HL-098423 (to T. Matsui) and HL-077543 (to A. Rosenzweig), a China Scholarship Council grant (to X. Song), a Leducq Network for Research Excellence grant (to A. Rosenzweig), and an NIH Training Grant (to M. A. Rosenberg).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Drs. Federica del Monte, Thomas E. MacGillivray, and Judith K Gwathmey for providing human hearts.
REFERENCES
- 1.Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol 172: 2522–2529, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Aukrust P, Gullestad L, Ueland T, Damas JK, Yndestad A. Inflammatory and anti-inflammatory cytokines in chronic heart failure: potential therapeutic implications. Ann Med 37: 74–85, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Avruch J, Hara K, Lin Y, Liu M, Long X, Ortiz-Vega S, Yonezawa K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene 25: 6361–6372, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Berenji K, Drazner MH, Rothermel BA, Hill JA. Does load-induced ventricular hypertrophy progress to systolic heart failure? Am J Physiol Heart Circ Physiol 289: H8–H16, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. CTGF expression is induced by TGF- beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol 32: 1805–1819, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA 95: 2979–2984, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz JA, Booth AJ, Lu G, Wood SC, Pinsky DJ, Bishop DK. Critical role for IL-6 in hypertrophy and fibrosis in chronic cardiac allograft rejection. Am J Transplant 9: 1773–1783, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diwan A, Tran T, Misra A, Mann DL. Inflammatory mediators and the failing heart: a translational approach. Curr Mol Med 3: 161–182, 2003 [DOI] [PubMed] [Google Scholar]
- 9.El-Menyar AA. Cytokines and myocardial dysfunction: state of the art. J Card Fail 14: 61–74, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol 7: e38, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari R. Interleukin-6: a neurohumoral predictor of prognosis in patients with heart failure: light and shadow. Eur Heart J 23: 9–10, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, Kelly RA. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest 104: 271–280, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredj S, Bescond J, Louault C, Delwail A, Lecron JC, Potreau D. Role of interleukin-6 in cardiomyocyte/cardiac fibroblast interactions during myocyte hypertrophy and fibroblast proliferation. J Cell Physiol 204: 428–436, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation 109: 1580–1589, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Gerdes AM, Iervasi G. Thyroid replacement therapy and heart failure. Circulation 122: 385–393, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Gu JQ, Ikuyama S, Wei P, Fan B, Oyama J, Inoguchi T, Nishimura J. Pycnogenol, an extract from French maritime pine, suppresses Toll-like receptor 4-mediated expression of adipose differentiation-related protein in macrophages. Am J Physiol Endocrinol Metab 295: E1390–E1400, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Gunther S, Grossman W. Determinants of ventricular function in pressure-overload hypertrophy in man. Circulation 59: 679–688, 1979 [DOI] [PubMed] [Google Scholar]
- 18.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110: 177–189, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Harrington LS, Findlay GM, Lamb RF. Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem Sci 30: 35–42, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Hedayat M, Mahmoudi MJ, Rose NR, Rezaei N. Proinflammatory cytokines in heart failure: double-edged swords. Heart Fail Rev 15: 543–562, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Herskowitz A, Choi S, Ansari AA, Wesselingh S. Cytokine mRNA expression in postischemic/reperfused myocardium. Am J Pathol 146: 419–428, 1995 [PMC free article] [PubMed] [Google Scholar]
- 22.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127: 125–137, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Janes MR, Limon JJ, So L, Chen J, Lim RJ, Chavez MA, Vu C, Lilly MB, Mallya S, Ong ST, Konopleva M, Martin MB, Ren P, Liu Y, Rommel C, Fruman DA. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med 16: 205–213, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jug B, Salobir BG, Vene N, Sebestjen M, Sabovic M, Keber I. Interleukin-6 is a stronger prognostic predictor than high-sensitive C-reactive protein in patients with chronic stable heart failure. Heart Vessels 24: 271–276, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Khaper N, Bryan S, Dhingra S, Singal R, Bajaj A, Pathak CM, Singal PK. Targeting the vicious inflammation-oxidative stress cycle for the management of heart failure. Antioxid Redox Signal 13: 1033–1049, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Koitabashi N, Arai M, Kogure S, Niwano K, Watanabe A, Aoki Y, Maeno T, Nishida T, Kubota S, Takigawa M, Kurabayashi M. Increased connective tissue growth factor relative to brain natriuretic peptide as a determinant of myocardial fibrosis. Hypertension 49: 1120–1127, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci USA 101: 1315–1320, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuzman JA, O'Connell TD, Gerdes AM. Rapamycin prevents thyroid hormone-induced cardiac hypertrophy. Endocrinology 148: 3477–3484, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Kuzman JA, Vogelsang KA, Thomas TA, Gerdes AM. l-Thyroxine activates Akt signaling in the heart. J Mol Cell Cardiol 39: 251–258, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Makarenko I, Opitz CA, Leake MC, Neagoe C, Kulke M, Gwathmey JK, del Monte F, Hajjar RJ, Linke WA. Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ Res 95: 708–716, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, Balmain A. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science 321: 1499–1502, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem 277: 22896–22901, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Matsui T, Nagoshi T, Hong EG, Luptak I, Hartil K, Li L, Gorovits N, Charron MJ, Kim JK, Tian R, Rosenzweig A. Effects of chronic Akt activation on glucose uptake in the heart. Am J Physiol Endocrinol Metab 290: E789–E797, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 104: 330–335, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Matsui Y, Sadoshima J. Rapid upregulation of CTGF in cardiac myocytes by hypertrophic stimuli: implication for cardiac fibrosis and hypertrophy. J Mol Cell Cardiol 37: 477–481, 2004 [DOI] [PubMed] [Google Scholar]
- 36.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation 109: 3050–3055, 2004 [DOI] [PubMed] [Google Scholar]
- 37.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Dorfman AL, Longnus S, Pende M, Martin KA, Blenis J, Thomas G, Izumo S. Deletion of ribosomal S6 kinases does not attenuate pathological, physiological, or insulin-like growth factor 1 receptor-phosphoinositide 3-kinase-induced cardiac hypertrophy. Mol Cell Biol 24: 6231–6240, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medzhitov R. Origin and physiological roles of inflammation. Nature 454: 428–435, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388: 394–397, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Minhajuddin M, Bijli KM, Fazal F, Sassano A, Nakayama KI, Hay N, Platanias LC, Rahman A. Protein kinase C-delta and phosphatidylinositol 3-kinase/Akt activate mammalian target of rapamycin to modulate NF-kappaB activation and intercellular adhesion molecule-1 (ICAM-1) expression in endothelial cells. J Biol Chem 284: 4052–4061, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng SB, Tan YH, Guy GR. Differential induction of the interleukin-6 gene by tumor necrosis factor and interleukin-1. J Biol Chem 269: 19021–19027, 1994 [PubMed] [Google Scholar]
- 42.Rudolph A, Abdel-Aty H, Bohl S, Boye P, Zagrosek A, Dietz R, Schulz-Menger J. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol 53: 284–291, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Saemann MD, Haidinger M, Hecking M, Horl WH, Weichhart T. The multifunctional role of mTOR in innate immunity: implications for transplant immunity. Am J Transplant 9: 2655–2661, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Saric J, Li JV, Swann JR, Utzinger J, Calvert G, Nicholson JK, Dirnhofer S, Dallman MJ, Bictash M, Holmes E. Integrated cytokine and metabolic analysis of pathological responses to parasite exposure in rodents. J Proteome Res 9: 2255–2264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmitz F, Heit A, Dreher S, Eisenacher K, Mages J, Haas T, Krug A, Janssen KP, Kirschning CJ, Wagner H. Mammalian target of rapamycin (mTOR) orchestrates the defense program of innate immune cells. Eur J Immunol 38: 2981–2992, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Shen WH, Chen Z, Shi S, Chen H, Zhu W, Penner A, Bu G, Li W, Boyle DW, Rubart M, Field LJ, Abraham R, Liechty EA, Shou W. Cardiac restricted overexpression of kinase-dead mammalian target of rapamycin (mTOR) mutant impairs the mTOR-mediated signaling and cardiac function. J Biol Chem 283: 13842–13849, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shioi T, Matsumori A, Kihara Y, Inoko M, Ono K, Iwanaga Y, Yamada T, Iwasaki A, Matsushima K, Sasayama S. Increased expression of interleukin-1 beta and monocyte chemotactic and activating factor/monocyte chemoattractant protein-1 in the hypertrophied and failing heart with pressure overload. Circ Res 81: 664–671, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, Izumo S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation 107: 1664–1670, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest 115: 2108–2118, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soesanto W, Lin HY, Hu E, Lefler S, Litwin SE, Sena S, Abel ED, Symons JD, Jalili T. Mammalian target of rapamycin is a critical regulator of cardiac hypertrophy in spontaneously hypertensive rats. Hypertension 54: 1321–1327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest 115: 1221–1231, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Timmers L, Sluijter JP, van Keulen JK, Hoefer IE, Nederhoff MG, Goumans MJ, Doevendans PA, van Echteld CJ, Joles JA, Quax PH, Piek JJ, Pasterkamp G, de Kleijn DP. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res 102: 257–264, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, Kolbe T, Stulnig TM, Horl WH, Hengstschlager M, Muller M, Saemann MD. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 29: 565–577, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol 131: 471–481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao CY, Chen M, Zsengeller Z, Li H, Kiss L, Kollai M, Szabo C. Poly(ADP-ribose) polymerase promotes cardiac remodeling, contractile failure, and translocation of apoptosis-inducing factor in a murine experimental model of aortic banding and heart failure. J Pharmacol Exp Ther 312: 891–898, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Yang Q, Guan KL. Expanding mTOR signaling. Cell Res 17: 666–681, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Youker K, Smith CW, Anderson DC, Miller D, Michael LH, Rossen RD, Entman ML. Neutrophil adherence to isolated adult cardiac myocytes. Induction by cardiac lymph collected during ischemia and reperfusion. J Clin Invest 89: 602–609, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang D, Contu R, Latronico MV, Zhang JL, Rizzi R, Catalucci D, Miyamoto S, Huang K, Ceci M, Gu Y, Dalton ND, Peterson KL, Guan KL, Brown JH, Chen J, Sonenberg N, Condorelli G. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest 120: 2805–2816, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang P, Xu X, Hu X, van Deel ED, Zhu G, Chen Y. Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure. Circ Res 100: 1089–1098, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]