Abstract
Rhbg is a membrane glycoprotein that is involved in NH3/NH4+ transport. Several models have been proposed to describe Rhbg, including an electroneutral NH4+/H+ exchanger, a uniporter, an NH4+ channel, or even a gas channel. In this study, we characterized the pH sensitivity of Rhbg expressed in Xenopus oocytes. We used two-electrode voltage clamp and ion-selective microelectrodes to measure NH4+-induced [and methyl ammonium (MA+)] currents and changes in intracellular pH (pHi), respectively. In oocytes expressing Rhbg, 5 mM NH4Cl (NH3/NH4+) at extracellular pH (pHo) of 7.5 induced an inward current, decreased pHi, and depolarized the cell. Raising pHo to 8.2 significantly enhanced the NH4+-induced current and pHi changes, whereas decreasing bath pH to 6.5 inhibited these changes. Lowering pHi (decreased by butyrate) also inhibited the NH4+-induced current and pHi decrease. In oocytes expressing Rhbg, 5 mM methyl amine hydrochloride (MA/MA+), often used as an NH4Cl substitute, induced an inward current, a pHi increase (not a decrease), and depolarization of the cell. Exposing the oocyte to MA/MA+ at alkaline bath pH (8.2) enhanced the MA+-induced current, whereas lowering bath pH to 6.5 inhibited the MA+ current completely. Exposing the oocyte to MA/MA+ at low pHi abolished the MA+-induced current and depolarization; however, pHi still increased. These data indicate that 1) transport of NH4+ and MA/MA+ by Rhbg is pH sensitive; 2) electrogenic NH4+ and MA+ transport are stimulated by alkaline pHo but inhibited by acidic pHi or pHo; and 3) electroneutral transport of MA by Rhbg is likely but is less sensitive to pH changes.
Keywords: NH4+ transport, Rh B glycoprotein, methyl ammonium, ammonium/hydrogen exchange, ammonium channel, gas channel
several studies have shown that Rhbg and Rhcg are involved in transport of NH3/NH4+ (1, 11, 12, 14–16, 20, 24, 27, 28). In our previous studies (19, 22), we demonstrated that electrogenic NH4+ transport is enhanced in oocytes expressing Rhbg. We have also shown that methyl amine (MA) and methyl ammonium (MA+) are transported by Rhbg, yet their mode of transport differs from that of NH3/NH4+ (22). Whereas transport of NH3/NH4+ by Rhbg is predominantly electrogenic, transport of MA/MA+ has an apparent electroneutral as well as an electrogenic component. We have also demonstrated that amiloride is a partial inhibitor of NH3/NH4+ and MA/MA+ transport by Rhbg. Other functional characteristics of Rhbg, such as the effect of pH, are yet to be determined.
The physiological roles of Rhbg and Rhcg in acid-base transport and in renal NH3/NH4+ handling are becoming more evident. Rhcg is highly expressed in the epididymis, where it may be involved in the mechanisms of acidifying the epididymal fluid needed for maturation of sperm (3). Interestingly, Rhcg knockout mice were found to have lower pH of the epididymal fluid compared with wild type. In the kidney, chronic metabolic acidosis increased Rhcg protein expression in cells of the medullary collecting duct (CD) (25). The same study indicated an increase in apical and basolateral Rhcg expression. Metabolic studies demonstrated that mice lacking Rhcg had impaired urinary NH4+ excretion in response to acid loads (3). A recent study on mice with renal CD-specific Rhcg deletion (CD-KO) (9) showed that urinary NH4+ excretion was less in KO mice than in control mice and that after acid loading CD-KO mice developed more severe metabolic acidosis than control mice. Studies on Rhbg showed that genetic deletion of pendrin, an apical Cl−/HCO3− exchanger in intercalated cells, decreased Rhbg expression. Recently, Bishop et al. (2) generated intercalated cell-specific Rhbg KO mice and demonstrated that when acid loaded urinary ammonium excretion was significantly less in KO mice versus control mice.
The pH sensitivity of Rhbg is of significant importance for several reasons. First, a change in pH will shift the equilibrium of the ammonia-to-ammonium balance with acidic pH favoring increased [NH4+]/[NH3] and thus may affect transport of NH4+ by Rhbg. Second, it has been proposed that Rhbg may function as an NH4+/H+ exchanger that is driven by the H+ gradient (12, 14, 27). Therefore, a pH change will also affect transport of NH4+ by Rhbg. Third, a transport model based on the crystal structure of AmtB (a bacterial homolog of Rh proteins) suggests a mechanism of recruitment of extracellular NH4+ and eventual transport and release of neutral NH3 intracellularly. Both processes are likely pH sensitive. Finally, pH sensitivity of this transporter is probably highly significant in acid-base disturbances where renal Rhbg, for example, may play an important role in the adaptive response to acidosis. For these reasons we investigated the sensitivity of Rhbg to pH. To do so, we examined the effects of pH changes on NH3/NH4+ and MA/MA+ transport by Rhbg.
METHODS
All experiments were conducted on oocytes expressing Rhbg or injected with H2O as a control. NH3/NH4+ and MA/MA+ transport were assessed from measurements of changes in intracellular pH (pHi), membrane potential (Vm), and whole cell currents induced by exposing the oocytes to bath solutions containing 5 mM NH4Cl or 5 mM methyl ammonium chloride. Bath solutions were HCO3− free and buffered with HEPES. The standard bathing solution was amphibian ND96 medium and had the following composition in mM: 100 NaCl, 2 KCl, 1.2 CaCl2, and 5 HEPES buffered to pH 7.5. The NH3/NH4+ solutions had 5 mM NH4Cl replacing NaCl. Similarly, the MA/MA+ solution had 5 mM methyl ammonium chloride replacing NaCl. The standard bath solution had a pH of 7.5. Other bath solutions used had a pH of either 8.2 or 6.5. These solutions were prepared by titrating the specified solution with NaOH or HCl, respectively. The osmolality of every solution was ∼200 mosmol/kgH2O.
Isolation of Oocytes
All experiments were conducted on oocytes in stage 5 or 6. Isolation of oocytes from Xenopus laevis frogs (Xenopus Express) was done according to standard operating procedures as described previously (19, 21). Briefly, the frog was anesthetized in water containing 0.2% tricaine adjusted to pH 7.2 with NaDH and buffered with 5 mM HEPES. The oocytes were extracted from a 1-cm incision in the abdominal wall where a lobe of the ovary was externalized and its distal portion cut. The oocytes were isolated by treating the excised piece of ovary with sterile-filtered Ca2+-free solution containing collagenase type I-A (2–3 mg/ml) for 30–40 min, followed by several washes in Ca2+-free solution. Free oocytes were rinsed several times with sterile OR3 medium, sorted, and then stored at 18°C. The protocols describing animal handling procedures and isolation of oocytes were approved by the Institutional Animal Care and Use Committee of Tulane University Medical School.
Capped RNA Preparation and Injection of Oocytes
Capped RNA (cRNA) for Rhbg was prepared from transcribed Rhbg cDNA with the mMessage mMachine T7 transcription kit from Ambion (Foster City, CA) as described previously (22). The concentration of cRNA was determined by UV absorbance, and its quality was assessed by formaldehyde/MOPS/1% agarose gel electrophoresis. Oocytes in OR3 medium were visualized with a dissecting microscope and injected with 50 nl of cRNA for Rhbg (0.2 μg/μl, for a total of 10 ng of RNA). Control oocytes were injected with 50 nl of sterile H2O. The sterile pipettes had tip diameters of 20–30 μm. They were backfilled with paraffin oil and were connected to a Nanoject motor-driven pipette (Drummond Scientific). Injected oocytes were used on the third day after injection with cRNA and for the following 4–5 days.
pH and Electrophysiological Measurements in Frog Oocytes
pHi measurements and voltage-clamp experiments were conducted as previously reported (19, 22) and are briefly described here. pHi was measured by H+-selective microelectrodes of the liquid ion exchanger type (H+ ionophore 1, cocktail B) obtained from Fluka (Buchs, Switzerland). The microelectrodes were manufactured as described by Siebens and Boron (26). The electrodes were fitted with a holder with an Ag-AgCl wire attached to a high-impedance probe of a WPI FD-223 electrometer and calibrated. The pH electrodes were calibrated in standard solutions of pH 6 and 8. All the electrodes used in this study had a slope of >56 mV/pH. The pH electrodes were not selective to MA+ over a concentration range from 1 to 20 mM.
Initial rates of change of pHi (dpHi/dt) were determined by fitting pHi vs. time to a linear regression line. Statistical significance was judged from Student's t-tests. Whenever possible, measurements were determined under control and test conditions in the same oocyte.
Two-Electrode Voltage Clamp
Two-electrode voltage clamp (OC-725, Warner Instruments, Hamden, CT) was used to measure whole cell currents. Electrodes were filled with 3 M KCl solution and had resistances of 1–4 MΩ. Bath electrodes were also filled with 3 M KCl and were directly immersed in the chamber. Oocytes were clamped at −60 mV, and long-term readings of current were sampled at a rate of once per second. Inward flow of cations is defined by convention as inward current (negative current).
Abbreviations
NH3/NH4+ refers to an equilibrated mix of ammonia gas (NH3) and ammonium ions (NH4+) obtained by dissolving NH4Cl in solution. MA/MA+ refers to methyl amine (MA) in equilibrium with methyl ammonium (MA+) obtained by dissolving methyl amine hydrochloride salt in solution. pHi refers to intracellular pH; pHo is extracellular or bath pH.
RESULTS
Transport of NH3/NH4+ by Rhbg
Basic effect.
Several studies have shown that Rhbg and Rhcg are involved in transport of NH3/NH4+. In our previous studies, we demonstrated that electrogenic NH4+ transport is enhanced in oocytes expressing Rhbg. Thus in oocytes expressing Rhbg, exposure to NH3/NH4+ caused a fast and significant decrease in pHi, a depolarization of the cell, and an inward current (19, 22). Characteristically absent was any pHi increase, suggesting minimal activation of NH3 transport (relative to NH4+) that would normally cause alkalinization. These changes were significantly more pronounced than NH3/NH4+-induced changes in H2O-injected oocytes. However, because H2O-injected oocytes exhibited similar, albeit much smaller, changes induced by NH3/NH4+, it was suggested that there is endogenous background NH4+ transport in native oocytes (4–6, 19, 21).
On the other hand, we showed that MA/MA+, often used to substitute for NH3/NH4+, is also a substrate of Rhbg. However, exposure to MA/MA+ caused an increase in pHi (not a decrease), a depolarization of the cell, and an inward current (19, 22). In H2O-injected oocytes there was no significant MA/MA+ transport that can be detected by similar measurements. This suggests that transport of MA/MA+ in H2O-injected oocytes is much less than in oocytes expressing Rhbg. In this study, we relied on measurements of pHi, Vm, and voltage-clamped current in oocytes expressing Rhbg to characterize the pH sensitivity of this transporter.
pH Dependence of NH4+ Transport by Rhbg
Sensitivity to extracellular pH.
EFFECT OF EXTRACELLULAR ALKALINIZATION ON NH4+-INDUCED CURRENT.
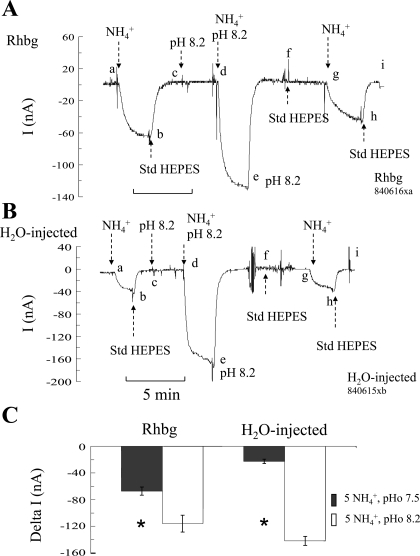
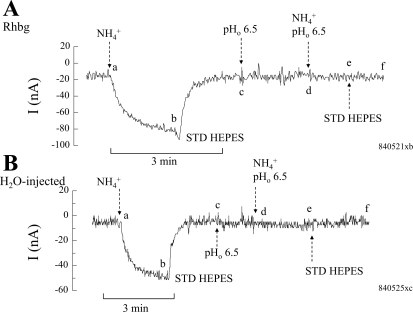
To assess the role of pHo, we measured the NH4+-induced current (voltage clamped at −60 mV) at normal bath pH of 7.5 and compared it to that at alkaline pH of 8.2. In oocytes expressing Rhbg (Fig. 1A), exposing the cell to 5 mM NH4Cl at bath pH of 7.5 caused an inward current (segment ab) that was readily reversed upon removal of bath NH4+ (segment bc). At point c, bath pH was raised to 8.2 with no discernable effect on current (segment cd). Exposing the same oocyte to 5 mM NH4Cl at a bath pH of 8.2 caused a larger inward current (segment de) compared with that at pH 7.5. The NH4+-induced current was reversed upon removal of bath NH4+ (segment ef). At point f, bath pH was returned to normal (7.5). Another NH4+ pulse at pH 7.5 was repeated, causing an inward current (segment gh) that was smaller than that at pH 8.2. In nine paired experiments, the NH4+-induced current averaged −67 ± 6.2 nA at pHo 7.5 compared with −116 ± 12.8 nA at pHo 8.2 (P < 0.001). These experiments suggested that raising pHo in oocytes expressing Rhbg enhanced electrogenic NH4+ transport.
Fig. 1.
Effect of alkaline extracellular pH (pHo) on NH4+-induced current (I). A: in oocytes expressing Rhbg, exposure to NH3/NH4+ (5 mM) at pH 7.5 induced an inward current (clamped at −60 mV) of −67 ± 6.2 nA (segment ab). Exposing the oocyte to 5 mM NH3/NH4+ at pHo of 8.2 induced a significantly larger inward current of −116 ± 12.8 nA (segment de). A change in bath pH from 7.5 to 8.2 (or back to 7.5) by itself did not cause any change in current (segments cd and fg, respectively). Segment ghi is a repeat of the 1st control pulse (NH3/NH4+ at pHo of 7.5), causing similar results. B: in H2O-injected oocytes, exposure to NH3/NH4++ at bath pH of 7.5 induced an inward current (segment ab) that was significantly enhanced when the oocyte was exposed to NH3/NH4+ at pH 8.2 (segment de). Segment ghi is a repeat of the initial NH3/NH4+ pulse at pH 7.5, with comparable results. C: summary graph indicating inward currents induced by 5 mM NH3/NH4+ at bath pH of 7.5 and 8.2 in oocytes expressing Rhbg and those injected with H2O. NH4+-induced current was enhanced by alkaline pH in Rhbg and control oocytes. *Statistical significance between pH 7.5 and 8.2. Std HEPES, standard HEPES.
We then investigated whether extracellular alkalinization affected NH4+ transport in H2O-injected oocytes. As shown in Fig. 1B, exposing H2O-injected (control) oocytes to 5 mM NH4Cl at pHo 7.5 caused an inward current (segment ab), which was readily reversed upon removal of bath NH4Cl (segment bc). By comparison to Rhbg, the NH4+-induced current in H2O-injected oocyte was significantly smaller than in Rhbg oocytes (compare with Fig. 1A). At point c, the pH in the bath was raised to 8.2, causing no change in current (segment cd). Addition of 5 mM NH4Cl at pHo 8.2 caused a significantly bigger inward current (segment de), which readily reversed upon removal of bath NH4+ (segment ef). The rest of the experiment was a repeat of the first NH4+ pulse. Bath pH was returned to normal 7.5 with no change in current (segment fg). The oocyte was then reexposed to 5 mM NH4Cl at pH 7.5, causing an inward current (segment gh) that readily recovered upon removal of bath NH4+ (segment hi). This second pulse at pHo 7.5 (segment ghi) was comparable to the first NH4+ pulse (segment abc) and much smaller than the NH4+ pulse at higher bath pH (segment cde). In six paired experiments on H2O-injected oocytes, the NH4+-induced current averaged −22 ± 3.1 nA at pHo 7.5 but was increased to −142 ± 6.8 nA (P < 0.001) at pHo 8.2.
The experiments of Fig. 1 clearly indicate that electrogenic NH4+ transport is enhanced at alkaline pH. This effect is evident in both H2O-injected oocytes and those expressing Rhbg. These results are summarized in Fig. 1C. It is important to note that whereas raising pHo activated NH4+ transport in oocytes in general, this does not exclude the possibility that alkaline pH may have activated transport by Rhbg also.
EFFECT OF EXTRACELLULAR ALKALINIZATION ON NH4+-INDUCED PHI CHANGES.
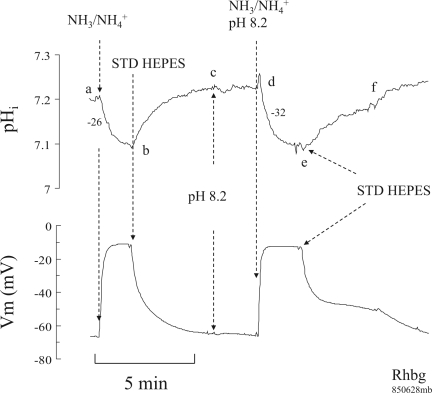
In the next set of experiments, we investigated the effect of raising pHo on the NH4+-induced pHi changes. In oocytes expressing Rhbg (Fig. 2), 5 mM NH4Cl in the bath caused a pHi decrease and depolarization of the cell (segment ab) as demonstrated in earlier studies. Removal of bath NH4Cl reversed these changes, and pHi and Vm fully recovered (segment bc). Switching bath pH to 8.2 did not cause significant pHi or Vm changes (segment cd). Adding 5 mM NH4Cl to the bath at pH 8.2 caused a faster decrease in pHi (segment de) and depolarized the cell. These changes were reversed upon removal of bath NH4+ (segment ef).
Fig. 2.
Effect of alkaline bath pH on NH3/NH4+-induced intracellular pH (pHi) and membrane potential (Vm) changes in oocytes expressing Rhbg. Exposing oocytes to 5 mM NH3/NH4+ at pH 7.5 caused a decrease in pHi and depolarization of the cell (segment ab). Removal of NH3/NH4+ reversed these changes (segment bc). Raising bath pH from 7.5 to 8.2 did not cause any significant pHi or Vm changes (segment cd). Exposing the oocyte to NH3/NH4+ at bath pH of 8.2 caused a decrease in pHi and depolarization (segment de) that readily reversed when NH3/NH4+ was removed from the bath (segment ef). The rate of NH3/NH4+-induced pHi decrease at pHo 7.5 was significantly slower than at pHo of 8.2. Numbers next to the pHi tracing indicate the rate of pHi decrease in 10−4 pH units/s.
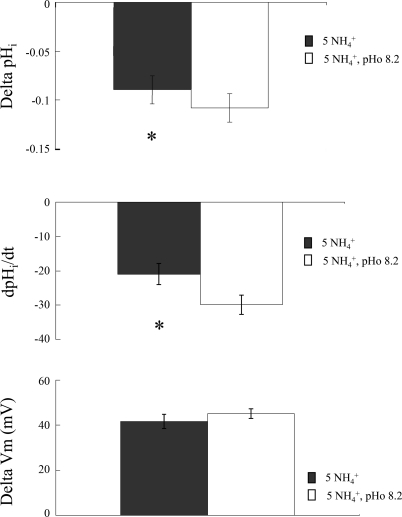
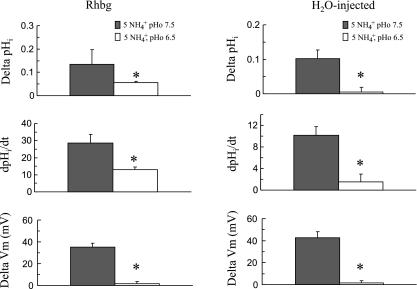
Figure 3 summarizes these results on 13 paired experiments on oocytes expressing Rhbg. As indicated, bath NH3/NH4+ at pH 7.5 caused pHi to decrease by 0.09 ± 0.01 pH units at a rate of −21 ± 3.1 × 10−4 pH units/s and depolarized the cell by 42 ± 3.1 mV. On the other hand, at pHo 8.2 NH4+ caused pHi to decrease by 0.11 ± 0.01 pH units (P < 0.01) at a rate of −30 ± 2.8 × 10−4 pH units/s (P < 0.01). However, the Vm change of 45 ± 2.1 mV at pH 8.2 was not significantly different from the NH4+-induced depolarization of 42 ± 3.1 mV at pH 7.5 (P > 0.05).
Fig. 3.
Summary of effect of extracellular alkalinization on NH3/NH4+-induced changes in oocytes expressing Rhbg. *Statistical significance (P < 0.05). dpHi/dt, rate of change in pHi.
Similar experiments were repeated on H2O-injected oocytes. As shown in Fig. 4, exposing control oocytes to 5 mM NH4Cl caused a pHi decrease of 0.02 pH units at a rate of −4.0 × 10−4 pH units/s (segment ab). Vm depolarized by 45 mV. These effects were fully reversed upon removal of bath NH4Cl (segment bc). At point c, bath pH was raised to 8.2, with no significant effects on pHi or Vm (segment cd). Exposing the cell to 5 mM NH4Cl at pH 8.2 caused a pHi decrease of 0.06 pH units at a rate of −9.3 × 10−4 pH units/s and a depolarization of 54 mV (segment de). These changes fully recovered when NH4+ was removed from the bath (segment ef). In six paired experiments, bath NH4Cl at pH 7.5 induced an average decrease in pHi of 0.04 ± 0.01 pH units at a rate of −6 ± 3.0 × 10−4 pH units/s and Vm depolarized by 35 ± 2.6 mV. Bath NH4Cl at pHo 8.2 caused pHi to decrease by 0.07 ± 0.02 pH units at a rate of −12 ± 2.2 × 10−4 pH units/s, and Vm depolarized by 52 ± 3.2 mV. The rates of pHi decrease and depolarization were significantly different from those induced by NH4+ at pH 7.5 (P < 0.01). In contrast to the NH4+-induced current, the pHi and voltage changes were clearly larger in Rhbg-expressing oocytes than in H2O-injected oocytes.
Fig. 4.
Effect of extracellular alkalinization on NH3/NH4+-induced pHi and Vm changes in H2O-injected oocytes. Exposing oocytes to 5 mM NH3/NH4+ at bath pH of 7.5 caused a slow decrease in pHi (segment ab) and significant depolarization of the cell. Removal of bath NH3/NH4+ reversed these changes (segment bc). Raising bath pH to 8.2 did not cause any effect on pHi or Vm (segment cd). Exposing the oocyte to 5 mM NH3/NH4+ at bath pH of 8.2 slightly but significantly enhanced the rate of NH4+-induced pHi decrease (segment de). pHi and Vm readily recovered when NH3/NH4+ was removed from the bath.
EFFECT OF EXTRACELLULAR ACIDIFICATION ON NH4+-INDUCED CURRENT.
In the next set of experiments, we examined the effect of lowering bath pH on NH4+-induced current in Rhbg and H2O-injected oocytes. In oocytes expressing Rhbg (Fig. 5A), 5 mM NH4Cl in the bath at pH 7.5 induced an inward current (segment ab) as previously observed. Removal of NH4Cl reversed this effect fully (segment bc). At point c, bath pHo was decreased to 6.5 with no effect on current (segment cd). However, switching the bath solution to a pH of 6.5 completely inhibited the NH4+-induced current usually observed at pH 7.5 (segment de). At point e, the bath solution was returned to standard HEPES (pH 7.5) with no effect in current.
Fig. 5.
Effect of extracellular acidification on NH4+-induced current. A: in oocytes expressing Rhbg, exposure to 5 mM NH3/NH4+ at bath pH of 7.5 caused an inward current (segment ab) that readily reversed on removal of bath NH3/NH4+. Lowering bath pH to 6.5 did not cause any change in current (segment cd). Exposing the oocyte to 5 mM NH3/NH4+ at bath pH of 6.5 completely inhibited the NH4+- induced change in current (segment de). B: in H2O-injected oocytes, similar effects of bath pH were observed: 5 mM NH3/NH4+ at pHo 7.5 caused an inward current (segment ab); 5 mM NH3/NH4+ in the bath at pHo 6.5 did not induce any change in current (segment de).
Similar experiments were conducted on H2O-injected oocytes. As shown in Fig. 5B, exposing control oocytes to 5 mM NH4Cl at pH 7.5 caused an inward current (segment ab), which readily recovered upon removal of bath NH4+ (segment bc). As previously reported, the NH4+-induced current in H2O-injected oocytes was significantly less than that caused by NH4+ in oocytes expressing Rhbg (compare segment abc in Fig. 5, A and B). At point c, bath pH was decreased to 6.5, causing no change in the whole cell current (segment cd). Exposing the oocyte to 5 mM NH4Cl at pH 6.5 did not cause any significant change in current as well (segment de). At point e, the bath solution was switched back to standard HEPES with no effect on current (segment ef).
In 11 experiments on oocytes expressing Rhbg, NH4+-induced inward current at pH 7.5 averaged −56 ± 4.7 nA and was completely blocked at pH 6.5. In seven experiments on H2O-injected oocytes, NH4+-induced inward current at pH 7.5 averaged −31 ± 6.4 nA and was also completely blocked at pH 6.5. As stated above, the NH4+ current at pH 7.5 in oocytes expressing Rhbg was significantly larger than that in H2O-injected oocytes (P < 0.01). The results of these experiments indicate that electrogenic NH4+ transport in control oocytes and those expressing Rhbg are inhibited at low pHo.
EFFECT OF EXTRACELLULAR ACIDIFICATION ON NH4+-INDUCED PHI CHANGES.
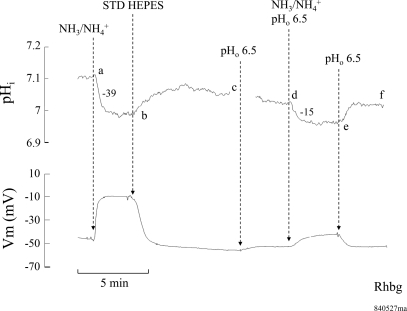
To further investigate the role of pHo, we measured the NH3/NH4+-induced pHi changes at a low bath pH of 6.5. As shown in Fig. 6, exposing an Rhbg-expressing oocyte to 5 mM NH4Cl in the bath caused the usual pHi decrease (segment ab) and depolarization of the cell. Removal of bath NH4Cl reversed these changes completely (segment bc). Switching bath pH to 6.5 caused a very small decrease in pHi and Vm (segment cd). Exposing the oocyte again to 5 mM NH4Cl but at pHo of 6.5 caused a smaller decrease in pHi (segment de) at a slower rate of acidification (−15 vs. −39 × 10−4 pH units/s). Moreover, the NH4+-induced depolarization was significantly blunted. Removal of bath NH4Cl fully reversed these effects, and pHi and Vm recovered (segment ef). In six paired experiments, 5 mM NH3/NH4+ at bath pH of 7.5 decreased pHi by 0.14 ± 0.001 pH units at a rate of −29 ± 4.9 × 10−4 pH units/s and Vm depolarized by 35 ± 3.6 mV. At bath pH of 6.5, 5 mM NH3/NH4+ decreased pHi by 0.06 ± 0.03 pH units at a rate of −13 ± 1.4 × 10−4 pH units/s and Vm depolarized by only 2.0 ± 1.8 mV.
Fig. 6.
Effect of extracellular acidification on NH3/NH4+-induced pHi and Vm changes in oocytes expressing Rhbg. Exposing the oocyte to 5 mM NH4Cl at pH 7.5 caused a decrease in pHi and depolarization (segment ab) that readily reversed upon removal of NH4Cl from the bath (segment bc). Lowering bath pH to 6.5 did not cause any significant change in pHi but depolarized the cell slightly (segment cd). Exposing the oocyte to 5 mM NH4Cl at pH 6.5 caused a slower and smaller decrease in pHi, and the depolarization of the cell was significantly inhibited (segment de). The rate of pHi decrease induced by NH4Cl was −39 × 10−4 pH units/s at pHo 7.5 but only −15 × 10−4 pH units/s at pHo 6.5.
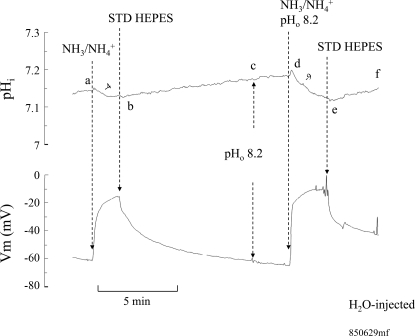
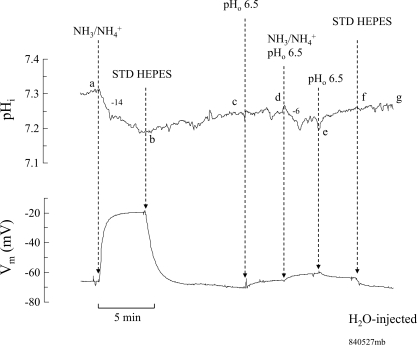
Similar experiments were also conducted on H2O-injected oocytes. As shown in Fig. 7, 5 mM NH4Cl in the bath at pH 7.5 caused a small change in pHi and Vm as shown previously (segment ab). Removal of NH3/NH4+ from the bath caused pHi and Vm to recover (segment bc). Switching pHo to 6.5 did not significantly alter pHi but slightly depolarized the cell (segment cd). Exposing the oocyte again to 5 mM NH4Cl but at pH 6.5 caused a smaller change of pHi, at a slower rate, and significantly blocked the depolarization of the cell (segment de). Removal of bath NH3/NH4+ fully reversed these changes (segment ef). In five paired experiments, 5 mM NH3/NH4+ at bath pH of 7.5 decreased pHi by 0.1 ± 0.02 pH units at a rate of −10 ± 1.6 × 10−4 pH units/s and Vm depolarized by 43 ± 5.6 mV. On the other hand, at bath pH of 6.5, 5 mM NH3/NH4+ hardly decreased pHi (0.005 ± 0.0008 pH units at a rate of −2 ± 1.5 × 10−4 pH units/s) or caused any depolarization of Vm (ΔVm = 2 ± 1.6 mV). These data confirm the results based on current measurements that NH4+ transport in oocytes is inhibited by acidic pHo. These data are summarized in Fig. 8.
Fig. 7.
Effect of extracellular acidification on NH4+-induced pHi and Vm changes in H2O-injected oocytes. Bath NH4Cl (5 mM) at pH 7.5 caused a decrease in pHi at a rate of −14 × 10−4 pH units/s and depolarized the cell (segment ab). These changes were reversed by removal of NH4Cl from the bath (segment bc). Switching bath pH to 6.5 did not affect pHi and only slightly depolarized the oocyte (segment cd). Exposing the oocyte to 5 mM NH4Cl at pH 6.5 decreased pHi at a rate of −6 × 10−4 pH units/s and only slightly depolarized the cell (segment de). These changes were reversed when NH4Cl was washed out from the bath (segment ef).
Fig. 8.
Summary graph comparing the effects of extracellular acidification on NH4+-induced pHi and Vm changes in Rhbg-expressing and H2O-injected oocytes. Lowering bath pH significantly inhibited the NH3/NH4+-induced changes in oocytes expressing Rhbg (left) and H2O-injected oocytes (right). *Statistical significance between pH 7.5 and pH 6.5.
The above data indicate that pHo clearly affects NH4+ transport in oocytes in general and by Rhbg as well. In the next set of experiments, we investigated the role of pHi in affecting NH4+ transport.
Sensitivity to intracellular pH: effects of intracellular acidification on NH4+-induced current.
The data presented so far demonstrated that, in control oocytes as well as those expressing Rhbg, NH4+ transport is very sensitive to changes in pHo. In the next set of experiments, we investigated whether NH4+ transport was affected by changes in pHi. To do so, we acidified the intracellular medium by exposing the oocyte to 20 mM butyrate. In previous studies (20, 22) and as demonstrated below, bath butyrate decreased pHi by ∼0.2–0.3 pH units. Accordingly, we measured the NH4+-induced current at steady-state pHi and at acidic pHi (in the presence of butyrate).
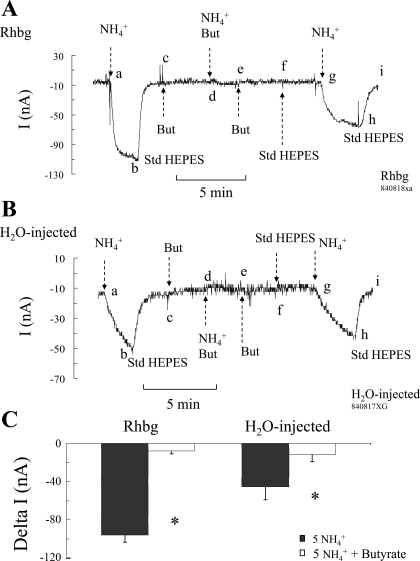
In oocytes expressing Rhbg (Fig. 9A), exposing the oocyte to NH4Cl (5 mM) caused the usual inward current (segment ab), which readily recovered upon removal of NH4Cl from the bath (segment bc). At point c, the bath solution was switched to a solution containing 20 mM butyrate at pH 7.5. This is expected to lower pHi as described above; however, there was no effect on the current, with the voltage clamped at −60 mV (segment cd). In the presence of butyrate, the oocyte was exposed again to 5 mM NH4Cl, but this time the NH4+-induced current was completely blocked (segment de). These steps were subsequently reversed: NH4Cl was first removed from the bath with no effect on current (segment ef), and then butyrate was removed and current did not change as well (segment fg). At point g, presumably pHi went back to steady state; NH4Cl was applied again to the bath, resulting in an inward current (segment gh) as observed in the first pulse (compare with segment ab). The NH4+-induced current averaged −84 ± 6.1 nA (n = 11) at steady-state pHi conditions but was only −8 ± 3.1 nA (n = 6; P < 0.05) at low pHi (in the presence of butyrate). These results indicate that intracellular acidification inhibited NH4+-induced current in oocytes expressing Rhbg.
Fig. 9.
Effect of intracellular acidification on NH4+-induced current. A: in oocytes expressing Rhbg, NH4Cl (5 mM) caused an inward current (segment ab) that readily reversed upon removal of NH4Cl from the bath (segment bc). Butyrate (But; 20 mM) in the bath did not cause any change in whole cell current (segment cd). Butyrate decreased pHi by ∼0.3 units (see Fig. 12). Subsequent additions of NH4Cl (5 mM) to the bath in the continued presence of butyrate did not induce any significant inward current (segment de). At point e, NH4Cl was removed from the bath; then at point f butyrate was also removed, with no effects on current. Readdition of NH4Cl to the bath in the absence of butyrate caused an inward current (segment gh) similar to the 1st pulse (segment ab). B: in H20-injected oocytes, similar effects of butyrate on NH4Cl-induced current were observed. NH4Cl (5 mM) in the absence of butyrate caused an inward current (segments ab and gh). NH4Cl (5 mM) in the presence of butyrate did not induce any inward current (segment bc). C: summary of the effects of intracellular acidification (induced by 20 mM butyrate) on NH4+-induced current in Rhbg-expressing and H2O-injected oocytes. In both cases intracellular acidification significantly inhibited NH4+-induced current. *Statistical significance (P < 0.05).
Similar experiments were also conducted on H2O-injected oocytes. As shown in Fig. 9B, 5 mM NH4Cl in the bath caused the usual inward current (segment ab), which recovered upon return of bath solution to standard HEPES (segment bc). At point c, butyrate (20 mM) was added to the bath, causing no change in whole cell current (segment cd). Bath NH4Cl in the presence of butyrate (and presumably at lower pHi) caused no change in current (segment de). These steps were then sequentially reversed: NH4Cl was first removed (segment ef) and then butyrate (segment fg), causing no change in current in both cases. The last step was a repeat of the initial NH4+ control pulse: NH4Cl added to the bath in the absence of butyrate caused an inward current (segment gh) as observed initially (compare with segment ab). The NH4+-induced change in current averaged −43 ± 8.4 nA (n = 7) in the absence of butyrate but was only −12 ± 7.6 nA (n = 5) at low pHi (in the presence of butyrate).
This experiment yielded results that are qualitatively similar to those obtained in oocytes expressing Rhbg. These results demonstrate that intracellular acidification inhibits NH4+-induced current in control oocytes also. The data on Rhbg and H2O-injected oocytes are summarized in Fig. 9C.
pH Dependence of MA/MA+ Transport by Rhbg
We have thus far demonstrated that NH4+ transport in oocytes expressing Rhbg as well as in H2O-injected oocytes is pH dependent. In principle, NH4+ transport seems to be inhibited by low pH (extracellular as well as intracellular) and activated by alkaline pHo. It is evident that Rhbg transport of NH4+ is clearly affected by pH changes, yet the background transport of NH4+ in H2O-injected (control) oocytes was also pH sensitive, although with some quantitative differences. Therefore the next series of experiments were conducted to isolate the pH sensitivity of Rhbg from that of native transport in the oocyte preparation. To do so, we measured MA/MA+-induced current and pHi changes while varying bath pH (pHo) or pHi.
Sensitivity of MA/MA+ current to extracellular pH.
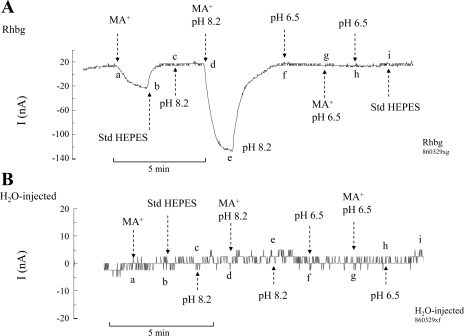
In the first set of experiments, we measured the MA+-induced inward current (clamp voltage at −60 mV) at normal bath pH (pHo) of 7.5, at alkaline pHo of 8.2, and at acidic pHo of 6.5. In oocytes expressing Rhbg (Fig. 10A), exposure of the cell to MA/MA+ (5 mM) at bath pH of 7.5 caused an inward current of −35 ± 5.9 nA (n = 4; segment ab), which was readily reversed upon removal of MA/MA+ (segment bc). At point c, bath pH was raised to 8.2, which did not cause any change in the whole cell current (segment cd). Exposing the oocytes again to MA/MA+ (5 mM) at bath pH of 8.2 caused an inward current (segment de) that was substantially larger than that at bath pH of 7.5. In four experiments on oocytes expressing Rhbg, MA+-induced current at pHo 8.2 averaged −147 ± 4.4 nA. The MA+-induced current was reversed upon removal of bath MA/MA+ (segment ef). Switching bath pH to 6.5 did not cause any change in the clamped current (segment fg). Exposing the oocytes to MA/MA+ (5 mM) at pHo 6.5 did not induce any current (segment gh). This is in contrast to the usual inward current induced by MA+ at normal pH or the enhanced MA+-induced current at alkaline pH.
Fig. 10.
Effect of pHo on methyl ammonium (MA+)-induced current. A: in oocytes expressing Rhbg, methyl amine (MA)/MA+ (5 mM) at pHo 7.5 caused an inward current (segment ab) that readily reversed upon removal of MA/MA+ from the bath (segment bc). Raising bath pH to 8.2 did not cause any change in current (segment cd). MA/MA+ at pHo 8.2 caused a significantly larger inward current (segment de) that recovered upon removal of MA/MA+ (segment ef). Lowering bath pH to 6.5 did not cause any change in current (segment fg). MA/MA+ at pHo 6.5 did not cause any inward current (segment gh). No changes in current were detected in subsequent removal of MA/MA+ at pHo 6.5 (segment hi) or upon restoring bath pH to 7.5 (at point i). B: in H2O-injected oocytes, MA/MA+ (5 mM) did not induce any current at pH 7.5 (segment ab), or at pH 8.2 (segment de) or pH 6.5 (segment gh). Changing bath pH from 7.5 to 8.2 or to 6.5 also did not cause any changes in current.
In contrast, in H2O-injected oocytes (Fig. 10B) exposure to MA/MA+ (5 mM) in the bath did not cause any inward current at pH 7.5 (segment ab) as observed above. Similarly, exposure to MA/MA+ did not induce any current at pHo 8.2 (segment de) or at pHo 6.5 (segment gh). Six similar experiments were conducted.
These data indicate that electrogenic MA+ transport by Rhbg is sensitive to changes in external pH. MA+-induced currents are enhanced by alkaline external pH and inhibited by acidic bath pH. These effects occur in oocytes expressing Rhbg and are completely absent in H2O-injected oocytes.
Sensitivity of MA/MA+ current to intracellular pH.
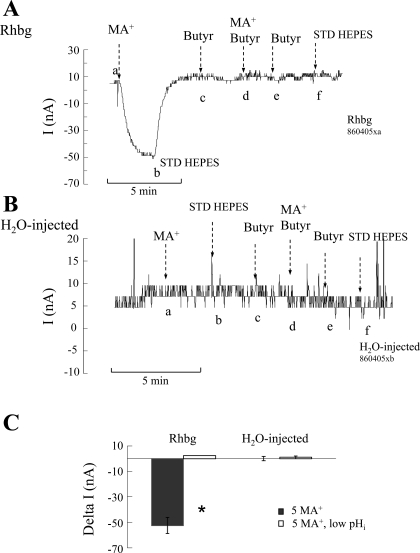
To investigate the effect of pHi on Rhbg transport of MA/MA+, we lowered pHi by exposing the oocyte to a bath solution containing 20 mM butyrate. As described above, in earlier studies we showed that 20 mM butyrate decreased pHi by ∼0.3 pH units. In this study on oocytes expressing Rhbg (Fig. 11A), we measured the MA+-induced current at normal bath pH (7.5), which averaged −52 ± 6.3 nA (n = 3) (segment ab). Removal of MA/MA+ from the bath reversed this effect (segment bc). The same oocytes were then superfused by a solution containing 20 mM butyrate, with no effect on current (segment cd). After a period of ∼3 min (during which pHi usually decreases to a stable, more acidic value), the oocytes were exposed to 5 mM MA/MA+ in the continued presence of butyrate. In oocytes expressing Rhbg, exposure to MA/MA+ in the presence of butyrate did not induce any inward current (segment de).
Fig. 11.
Effect of intracellular acidification [induced by 20 mM butyrate (Butyr)] on MA+-induced current. A: in oocytes expressing Rhbg, MA/MA+ (5 mM) caused an inward current (segment ab) that readily reversed upon washout of MA/MA+ from the bath (segment bc). pHi was lowered by exposing the oocyte to butyrate (20 mM), which did not cause any change in whole cell current (segment cd). In the presence of butyrate, MA/MA+ in the bath did not cause any inward current (segment de). MA/MA+ was then removed from the bath (segment ef), followed by butyrate (at point f) with no effects on current. B: in H2O-injected oocytes, MA/MA+ (5 mM) in the bath did not induce any inward current in the absence of butyrate (segment ab) or in its presence (segment de). C: summary graph indicating the effect of intracellular acidification (lowered by butyrate) on MA+-induced current in Rhbg-expressing and H2O-injected oocytes. Decreasing pHi completely blocked the MA+-induced current in oocytes expressing Rhbg. In H2O-injected oocytes MA+ did not induce any current at steady-state pHi or when pHi was lowered by butyrate. *Statistical significance (P < 0.01).
Similar experiments were also conducted on H2O-injected oocytes. In those experiments (Fig. 11B), MA/MA+ did not elicit any significant inward current in the absence (segment ab) or presence (segment de) of butyrate. The effects of pHi acidification on MA+-induced current in Rhbg and H2O-injected oocytes are summarized in Fig. 11C.
Effect of intracellular acidification on MA-induced pHi changes.
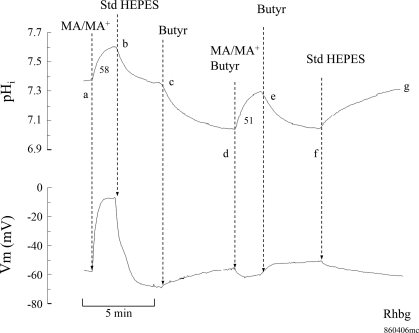
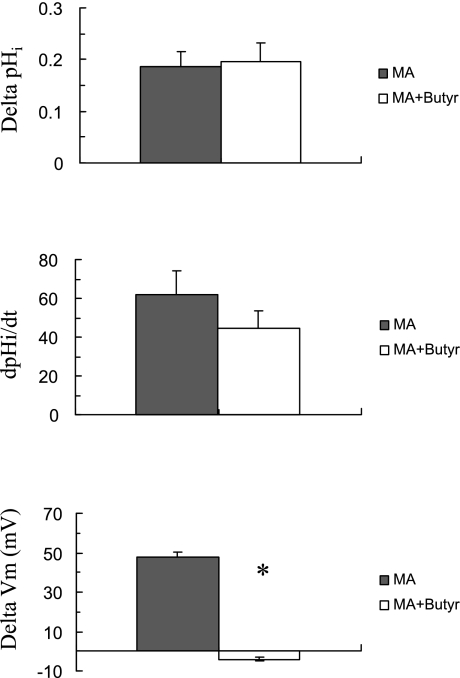
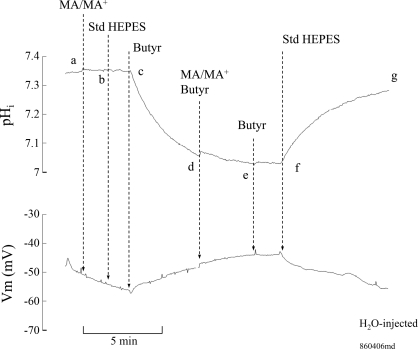
The possible inhibition of MA/MA+ transport by acidic shifts in pHi was further investigated. To do so, we measured MA/MA+-induced changes in Vm and pHi at steady state as well as acidic pHi. As shown in Fig. 12, exposing an oocyte expressing Rhbg to MA/MA+ (5 mM) caused the usual rapid pHi increase and significant depolarization of the cell (segment ab). In four experiments, MA/MA+ caused pHi to increase by 0.19 ± 0.03 pH units at a rate of 62 ± 12 × 10−4 pH units/s and Vm depolarized by 48 ± 2.4 mV. pHi and Vm fully recovered upon removal of MA/MA+ from the bath (segment bc). At point c, exposing the oocytes to 20 mM butyrate in the bath caused a decrease in pHi by 0.33 ± 0.01 (4), and Vm depolarized by 9 ± 3.2 mV (segment cd). In the continued presence of bath butyrate, exposing the oocytes to MA/MA+ (5 mM) caused pHi to increase by 0.20 ± 0.04 pH units at a rate of 45 ± 8.8 × 10−4 pH units/s (segment de). However, it is important to note that in the presence of butyrate MA/MA+ did not cause any depolarization but rather a small hyperpolarization of −4 ± 1.1 mV (segment de). The MA/MA+-induced changes in Vm and pHi were completely reversed upon removal of MA/MA+ from the bath (segment ef). Superfusing the oocytes with control standard HEPES solution (removal of butyrate) resulted in full recovery of pHi and Vm to their initial steady-state values (segment fg). It is noteworthy that while MA/MA+ still induced a pHi increase at low pHi, the Vm changes were completely blocked. This suggests that in oocytes expressing Rhbg the electrogenic component of MA/MA+ transport was blocked at low pHi. This is consistent with the current measurements shown above (see Fig. 11). The results of this and similar experiments on oocytes expressing Rhbg are summarized in Fig. 13.
Fig. 12.
Effect of intracellular acidification on MA/MA+-induced changes in pHi and Vm in oocytes expressing Rhbg. Exposing the oocyte to MA/MA+ (5 mM) caused an increase in pHi at a rate of 58 × 10−4 pH units/s and depolarized the cell (segment ab). These changes were reversed upon washout of MA/MA+ from the bath (segment bc). Exposing the oocyte to 20 mM butyrate caused significant intracellular acidification of 0.3 pH units and slightly depolarized the oocyte (segment cd). At low pHi subsequent addition of MA/MA+ to the bath still caused an increase in pHi at a rate of 51 × 10−4 pH units/s, but there was a small hyperpolarization of the cell (segment de). These changes were fully reversed upon removal of MA/MA+ (segment ef) and then butyrate (segment fg). Bath pH was maintained at 7.5 throughout.
Fig. 13.
Summary graph indicating the effect of low pHi on MA/MA+-induced pHi and Vm changes in oocytes expressing Rhbg. Lowering pHi did not inhibit the MA/MA+-induced increase in pHi but completely blocked the change in Vm.
For comparison, similar experiments were conducted on H2O-injected oocytes. As shown in Fig. 14, exposure of control oocytes to 5 mM MA/MA+ did not cause any significant changes in pHi and Vm (segment ab), after which MA/MA+ was removed from the bath (segment bc). At point c, 20 mM butyrate in the bath caused a substantial decrease in pHi of 0.33 ± 0.03 pH units (3) and a small depolarization of the cell of 8 ± 0.5 mV (segment cd). MA/MA+ (5 mM) in the presence of butyrate did not elicit any pHi increase, and Vm continued to depolarize slowly (segment de). At point e, MA/MA+ was removed from the bath, with no significant changes. pHi and Vm fully recovered when butyrate was removed from the bath (segment fg). These results confirm that MA/MA+ effects on pHi and Vm are Rhbg specific.
Fig. 14.
Effect of intracellular acidification on MA/MA+-induced changes in H2O-injected oocytes. Exposing control oocytes to 5 mM MA/MA+ did not induce any significant changes in pHi or Vm at steady-state pHi (segment ab) or when pHi was lowered by 20 mM butyrate (segment de). Tracing shows that butyrate caused substantial decrease in pHi (segment cd) and small depolarization of the cell. All changes were reversible.
DISCUSSION
This study examined the sensitivity of Rhbg to pH. To do so, we investigated whether NH3/NH4+ and MA/MA+ transport by Rhbg were affected by changes in pH. Voltage-clamp experiments and pHi monitoring were conducted to assess transport in Xenopus oocytes expressing Rhbg. The sensitivity of MA/MA+ and NH3/NH4+ transport to pH was examined when pHo was increased or decreased and when pHi was acidified. Our results indicate a significant sensitivity of Rhbg to changes in pH. In general, transport by Rhbg was stimulated when pHo was alkaline. However, transport by Rhbg was inhibited when pHo or pHi was acidified.
Few studies have addressed the role of pH in regulating transport by members of the Rh family. Using micromolar concentrations of MA/MA+ in oocytes expressing Rhbg, Ludewig (12) reported that MA/MA+ did not induce any inward current and that uptake of labeled MA/MA+ was enhanced with increased pHo. Even though there was a pHo stimulation of labeled MA/MA+ uptake in H2O-injected oocytes (yet smaller than in Rhbg), it was concluded that Rhbg transport of MA/MA+ was electroneutral and that Rhbg acted as an NH4+/H+ (or MA+/H+) exchanger. Mak et al. (14) also used uptake studies of labeled MA/MA+ and demonstrated dependence of uptake on pHo. That study demonstrated that the pH-enhanced MA/MA+ uptake could not be fully explained by an effect on electroneutral MA uptake. In a more thorough examination of the pH effect, the study maintained a constant electroneutral MA concentration while pHo and total MA/MA+ concentrations were varied. This study also reported that uptake of labeled MA/MA+ was not affected by manipulating Vm in voltage-clamp experiments. Both studies propose that Rhbg acts as an electroneutral MA+/H+ (or NH4+/H+)exchanger. Our data do not support that Rhbg is behaving as an NH4+/H+ exchanger.
Electrogenic NH4+ and MA+ Transport by Rhbg
We have previously demonstrated that NH4+ transport by Rhbg is electrogenic because there is a direct and proportional relationship between NH4+-induced inward current and the NH4+ gradient. This relationship is saturable. It is also clear that there is an endogenous electrogenic NH4+ transport in oocytes, as shown by other studies as well (4, 6, 21, 24). However, the magnitude of this transport is enhanced severalfold by expression of Rhbg. Whereas it is difficult to disprove that expression of Rhbg might activate endogenous transport, it is more likely that a de novo transport by Rhbg is expressed even though it may resemble endogenous NH4+ transport. It is interesting to note that Xenopus oocytes do express Rh-related proteins (7, 23).
Our data also indicate that a component of MA/MA+ transport by Rhbg is electrogenic. In our experiments, MA/MA+ caused an inward current and depolarization of the cell, much like NH4+. This effect was completely absent in H2O-injected oocytes, suggesting that 1) there is no significant endogenous electrogenic MA+ transport in oocytes and 2) MA and/or MA+ are good substrates for Rhbg. We used a concentration of 5 mM MA/MA+ in most experiments; however, a MA+-induced current and depolarization were clearly evident in response to 1 mM MA/MA+ (18). In contrast, Ludewig (12) reported no MA+-induced current. However, in his experiments, micromolar concentrations of MA+ were used. This could be explained by a subthreshold effect of MA+ rather than the absence of electrogenic transport.
pH Dependence of NH4+ Transport by Rhbg
In addressing the role of pHo in affecting transport of NH4+ by Rhbg, our data indicate that alkaline pHo causes 1) an increase in NH4+-induced current, 2) a faster rate of NH4+-induced changes in pHi (dpHi/dt), 3) a bigger NH4+-induced decrease in pHi (ΔpHi), and 4) no apparent change in NH4+-induced depolarization. These results indicate that in oocytes expressing Rhbg extracellular alkalinization activates electrogenic NH4+ influx. However, extracellular alkalinization promoted similar changes in NH4+ transport in H2O-injected oocytes. It should be noted, however, that the voltage and pH changes were not as large. In other words, endogenous NH4+ transport was also enhanced by extracellular alkalinization.
An unexpected observation was that the percent increase in NH4+-induced current at pH 8.2 seemed to be more pronounced in H2O-injected oocytes compared with oocytes expressing Rhbg. The reasons behind the difference in response are unclear, especially since the nature of the endogenous NH4+ current in H2O-injected oocytes is unknown. However, in oocytes expressing Rhbg the NH3/NH4+-induced current and pHi changes are determined by how the relative flux of NH3 to NH4+ (JNH3/JNH4+) changes (17). For example, if JNH4+ and JNH3 change significantly but proportionally, then the change in current (and ΔpHi) would be less than if JNH4+ changed, even moderately, but without a matching change in JNH3. It is therefore possible that at alkaline bath pH, the relative change in JNH3/JNH4+ is different for Rhbg than for H2O-injected oocytes. Our results on MA/MA+, where there is no significant endogenous transport, are consistent with this possibility.
The effect of extracellular acidification on NH4+ transport in oocytes expressing Rhbg was in the opposite direction. In oocytes expressing Rhbg, acidic pHo caused 1) complete inhibition of NH4+-induced current, 2) inhibition of NH4+-induced rate of pHi decrease, 3) smaller NH4+-induced pHi decrease (ΔpHi), and 4) inhibition of NH4+-induced change in Vm. These results indicate that extracellular acidification inhibits electrogenic NH4+ transport by Rhbg. However, acidic pHo caused similar but smaller effects on NH4+ transport in H2O-injected oocytes.
Because the effect of pHo on NH4+ transport was qualitatively similar in H2O-injected and Rhbg oocytes, two possibilities could be proposed. First, expression of Rhbg upregulates endogenous NH4+ transport with no distinct NH4+ pathway through Rhbg. Alternatively, NH4+ transport by Rhbg is distinct from that of endogenous NH4+ in control oocytes even though they share several similar characteristics, yet both are activated by extracellular alkalinization.
We propose the latter hypothesis as the working model for NH4+ transport by Rhbg. This model is supported by the following observations: 1) If endogenous NH4+ transport was inhibited by a decrease in pHo (or activated by an increase in pHo), this does not exclude the possibility that transport by Rhbg may be affected similarly. Given the fact that Xenopus oocytes possess membrane proteins belonging to the Rh family, endogenous NH4+ transport may resemble that of Rhbg. 2) Amiloride was able to inhibit electrogenic NH4+ transport in oocytes expressing Rhbg but not in H2O-injected oocytes (18). This observation does not favor an upregulation of endogenous NH4+ transport by Rhbg expression. 3) MA/MA+ acts as specific substrate for Rhbg, causing intracellular changes that are different from those in H2O-injected oocytes.
The dependence of NH4+ transport on changes in pHo raises two possibilities. The first is that NH4+ transport could be driven by the H+ gradient. This possibility is favored by the studies proposing that Rhbg functions as NH4+/H+ exchanger (12–14, 27). Alternatively, Rhbg may be directly affected by pH, possibly through a charge or allosteric effect resulting in inhibition by low pH and activation by an increase in pH.
To distinguish between these two possibilities, we investigated the dependence of NH4+ transport by Rhbg on pHi. If Rhbg is an NH4+/H+ exchanger driven by the H+ gradient, then raising intracellular [H+] should also lead to activation of transport. In our experiments, lowering pHi by exposing oocytes to 20 mM butyrate caused 1) inhibition of NH4+ current, 2) inhibition of NH4+-induced ΔpHi, 3) inhibition of NH4+-induced rate of pHi decrease, and 4) inhibition of NH4+-induced depolarization. These observations do not favor NH4+/H+ exchange but rather indicate that Rhbg is pH sensitive. It is inhibited by low pH, intracellular or extracellular, and activated by extracellular alkalinization.
The implications of inhibiting transport by Rhbg at low pH are difficult to interpret. NH3/NH4+ transport in the collecting duct increases during acidosis. Therefore, if Rhbg (and Rhcg for that matter) is a major route of NH3/NH4+ excretion, it is expected that transport would be enhanced rather than inhibited at low pH. To resolve this apparent disparity, at least two issues need to be addressed. First, a profile of Rhbg dependence over a range of pH values would determine how activity of Rhbg changes at different pH values. Second, the effects of low pH on NH4+ versus NH3 transport need to be determined. Our data clearly indicate that electrogenic NH4+ transport was inhibited at low pH. It is not clear, however, whether NH3 transport (and consequently net NH3/NH4+ transport) was similarly affected. In fact, the experiments with MA/MA+ showed that electroneutral MA transport was not significantly altered. A role of Rhbg in acidosis is demonstrated in studies where tissue-specific deletion of Rhbg in mouse CD caused more severe acidosis and impaired NH4+ excretion in response to acid loading (2).
pH Dependence of MA/MA+ Transport by Rhbg
Transport of MA/MA+ by Rhbg provides additional insight into the properties of this mechanism. Our experiments indicate that expressing Rhbg in oocytes promotes transport of electrogenic MA+ and electroneutral MA. The former (MA+) appears to be transported by Rhbg much like NH4+. However, there was no evidence of electrogenic MA+ transport in H2O-injected oocytes where MA+ did not induce any current or depolarization of the cell. Thus in electrical measurements MA+ is very useful as a good substrate for Rhbg. The fact that charged MA+ induces electrical changes similar to NH4+ in Rhbg oocytes but not in H2O-injected oocytes argues against the possibility that enhanced NH4+ conductance and current by Rhbg is caused by activating endogenous NH4+ transport.
On the other hand, MA/MA+ transport by Rhbg induced an increase in pHi consistent with electroneutral transport of MA by Rhbg. This is unique in the oocyte, especially that NH3 hardly causes any detectible pHi alkalinization. Moreover, MA-induced alkalinization in H2O-injected oocytes is minimal compared with that in oocytes expressing Rhbg. One likely possibility is that Rhbg may promote MA transport in addition to the charged MA+. The present study cannot distinguish between direct transport of MA and an enhanced membrane permeability of the neutral molecule by the oocytes upon expression of Rhbg. One observation in favor of direct transport of MA/MA+ by Rhbg is that amiloride inhibited MA-induced alkalinization as well as MA+-induced current and depolarization. However, inhibition of MA-induced alkalinization by low pHi was less pronounced.
A recent study (10) resolved the molecular structure of AmtB, a bacterial homolog of Rh glycoproteins from Escherichia coli, by X-ray crystallography at 1.35-Å resolution. On the basis of molecular analysis of this structure, a model was proposed in which a 20-Å-long hydrophobic pore serves as a channel that has four NH3 or NH4+ binding sites. The first site facing the extracellular medium serves as a vestibule that recruits NH3/NH4+ binding predominantly as an charged cationic NH4+. Once NH4+ is recruited, H+ is released extracellularly and NH3 is conducted through the hydrophobic pore favored by molecular interactions that lower pKa in the pore. On the intracellular side, NH3 recruits an intracellular H+ and is released as NH4+. Of the four NH3/NH4+ binding sites, only the first at the entry site accepts a fully protonated NH4+. The other three sites are occupied by fully deprotonated NH3 (8). According to this model, transport is essentially pH sensitive. As such, decreasing pHo is thought to lower the rate of transport by opposing the proton release from NH4+. The opposite effect is expected by raising pHo. These observations are consistent with our results about the effect of bath pH on NH4+ and MA+ transport. On the other hand, decreasing pHi should not lead to inhibition of transport. A decrease in pHi is expected to favor reprotonation of NH3 and therefore acceleration of transport. In our experiments this was not the case, but rather a decrease in pHi caused an inhibition of NH4+ transport by Rhbg. Whether these differences are unique to Rhbg compared with AmtB will be addressed in future studies.
The model suggesting Rhbg to be an electroneutral NH4+/H+ exchanger was largely based on uptake studies of labeled MA/MA+ and its sensitivity to changes of pHo. It is important to note that in those studies it was not possible to distinguish between electroneutral MA and electrogenic MA+ transport, both of which seem to be promoted by Rhbg expression. Moreover, in those studies only pHo could be manipulated, and therefore inhibition by low pHi (which does not favor the exchange model) could not be examined.
In summary, our study demonstrated that transport of NH4+ and MA/MA+ by Rhbg is pH sensitive. Electrogenic NH4+ and MA+ transport are stimulated by alkaline pHo but inhibited by acidic pHi or pHo. Electroneutral MA transport by Rhbg is likely but seems to be less sensitive to pH changes. An H+ gradient driving transport by Rhbg (as suggested by an exchange model) cannot be fully explained by our data. On the other hand, direct effects of pH on Rhbg are very likely.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-DK-62295 and American Heart Association Southern Affiliate Grant 0255258B.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Mrs. Karen Brown for excellent technical assistance.
REFERENCES
- 1.Avent ND. A new chapter in Rh research: Rh proteins are ammonium transporters. Trends Mol Med 7: 94–96, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Bishop JM, Verlander JW, Lee HW, Nelson RD, Weiner AJ, Handlogten ME, Weiner ID. Role of the Rhesus glycoprotein, Rh B glycoprotein, in renal ammonia excretion. Am J Physiol Renal Physiol (August18, 2010). doi:10.1152/ajprenal.00277.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, Devuyst O, Marini AM. A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456: 339–343, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Burckhardt BC, Fromter E. Pathways of NH3/NH4+ permeation across Xenopus laevis oocyte cell membrane. Pflügers Arch 420: 83–86, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Burckhardt BC, Thelen P. Effect of primary, secondary and tertiary amines on membrane potential and intracellular pH in Xenopus laevis oocytes. Pflügers Arch 429: 306–312, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Cougnon M, Bouyer P, Hulin P, Anagnostopoulos T, Planelles G. Further investigation of ionic diffusive properties and of NH4+ pathways in Xenopus laevis oocyte cell membrane. Pflügers Arch 431: 658–667, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Huang CH, Liu PZ. New insights into the Rh superfamily of genes and proteins in erythroid cells and nonerythroid tissues. Blood Cells Mol Dis 27: 90–101, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Ishikita H. Modulation of the protein environment in the hydrophilic pore of the ammonia transporter protein AmtB upon GlnK protein binding. FEBS Lett 581: 4293–4297, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Lee HW, Verlander JW, Bishop JM, Igarashi P, Handlogten ME, Weiner ID. Collecting duct-specific Rh C glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 296: F1364–F1375, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JK, Khademi S, Harries W, Savage D, Miercke L, Stroud RM. Water and glycerol permeation through the glycerol channel GlpF and the aquaporin family. J Synchrotron Radiat 11: 86–88, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Peng J, Mo R, Hui C, Huang CH. Rh type B glycoprotein is a new member of the Rh superfamily and a putative ammonia transporter in mammals. J Biol Chem 276: 1424–1433, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Ludewig U. Electroneutral ammonium transport by basolateral rhesus B glycoprotein. J Physiol 559: 751–759, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludewig U. Ion transport versus gas conduction: function of AMT/Rh-type proteins. Transfus Clin Biol 13: 111–116, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Mak DO, Dang B, Weiner ID, Foskett JK, Westhoff CM. Characterization of ammonia transport by the kidney Rh glycoproteins RhBG and RhCG. Am J Physiol Renal Physiol 290: F297–F305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marini AM, Matassi G, Raynal V, Andre B, Cartron JP, Cherif-Zahar B. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat Genet 26: 341–344, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Mayer M, Schaaf G, Mouro I, Lopez C, Colin Y, Neumann P, Cartron JP, Ludewig U. Different transport mechanisms in plant and human AMT/Rh-type ammonium transporters. J Gen Physiol 127: 133–144, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musa-Aziz R, Jiang L, Chen LM, Behar KL, Boron WF. Concentration-dependent effects on intracellular and surface pH of exposing Xenopus oocytes to solutions containing NH3/NH4+. J Membr Biol 228: 15–31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakhoul NL, Abdulnour-Nakhoul SM, Boulpaep EL, Rabon E, Schmidt E, Hamm LL. Substrate specificity of Rhbg: ammonium and methyl ammonium transport. Am J Physiol Cell Physiol 299: C695–C705, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakhoul NL, Dejong H, Abdulnour-Nakhoul SM, Boulpaep EL, Hering-Smith K, Hamm LL. Characteristics of renal Rhbg as an NH4+ transporter. Am J Physiol Renal Physiol 288: F170–F181, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Nakhoul NL, Hamm LL. Non-erythroid Rh glycoproteins: a putative new family of mammalian ammonium transporters. Pflügers Arch 447: 807–812, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Nakhoul NL, Hering-Smith KS, Abdulnour-Nakhoul SM, Hamm LL. Transport of NH3/NH4+ in oocytes expressing aquaporin-1. Am J Physiol Renal Physiol 281: F255–F263, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Nakhoul NL, Schmidt E, Abdulnour-Nakhoul SM, Hamm LL. Electrogenic ammonium transport by renal Rhbg. Transfus Clin Biol 13: 147–153, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Peng J, Huang CH. Rh proteins vs. Amt proteins: an organismal and phylogenetic perspective on CO2 and NH3 gas channels. Transfus Clin Biol 13: 85–94, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Planelles G. Ammonium homeostasis and human Rhesus glycoproteins. Nephron Physiol 105: p11––p17., 2007 [DOI] [PubMed] [Google Scholar]
- 25.Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Han KH, Handlogten ME, Verlander JW, Weiner ID. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F397–F408, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Siebens AW, Boron WF. Effect of electroneutral luminal and basolateral lactate transport on intracellular pH in salamander proximal tubules. J Gen Physiol 90: 799–831, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westhoff CM, Wylie DE. Transport characteristics of mammalian Rh and Rh glycoproteins expressed in heterologous systems. Transfus Clin Biol 13: 132–138, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Zidi-Yahiaoui N, Mouro-Chanteloup I, D'Ambrosio AM, Lopez C, Gane P, Le van Kim C, Cartron JP, Colin Y, Ripoche P. Human Rhesus B and Rhesus C glycoproteins: properties of facilitated ammonium transport in recombinant kidney cells. Biochem J 391: 33–40, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]