Abstract
Stem cells transplanted to the ischemic myocardium usually encounter massive cell death within a few days of therapy. Hypoxic preconditioning (HPC) is currently employed as a strategy to prepare stem cells for increased survival and engraftment in the heart. However, HPC of stem cells has provided varying results, supposedly due to the differences in the oxygen concentration, duration of exposure, and passage conditions. In the present study, we determined the effect of HPC on rat mesenchymal stem cells (MSCs) exposed to 0.5% oxygen concentration for 24, 48, or 72 h. We evaluated the expression of prosurvival, proangiogenic, and functional markers such as hypoxia-inducible factor-1α, VEGF, phosphorylated Akt, survivin, p21, cytochrome c, caspase-3, caspase-7, CXCR4, and c-Met. MSCs exposed to 24-h hypoxia showed reduced apoptosis on being subjected to severe hypoxic conditions. They also had significantly higher levels of prosurvival, proangiogenic, and prodifferentiation proteins when compared with longer exposure (72 h). Cells taken directly from the cryopreserved state did not respond effectively to the 24-h HPC as those that were cultured under normoxia before HPC. Cells cultured under normoxia before HPC showed decreased apoptosis, enhanced expression of connexin-43, cardiac myosin heavy chain, and CD31. The preconditioned cells were able to differentiate into the cardiovascular lineage. The results suggest that MSCs cultured under normoxia before 24-h HPC are in a state of optimal expression of prosurvival, proangiogenic, and functional proteins that may increase the survival and engraftment in the infarct heart. These results could provide further insights into optimal preparation of MSCs which would greatly influence the effectiveness of cell therapy in vivo.
Keywords: myocardial infarction, cell therapy
the oxygen concentration in the microenvironment of stem cells plays an important role in controlling stem cell potency, proliferation, and differentiation ability (1, 26, 28). Stem cells that are normally cultured at ambient air in an in vitro environment differ in their exposure to the concentration of oxygen, compared with their natural physiological niches where they reside and function (7, 9). During cell transplantation procedures to treat conditions such as myocardial infarction (MI), the cultured stem cells encounter a sudden shortage in oxygen availability when transplanted into an ischemic heart tissue. A growing body of evidence attributes failure of stem cell therapy to the extensive loss of transplanted stem cells upon introducing them to such a harsh ischemic environment, which is high in inflammation factors and free radicals generated by oxidative stress (14, 38). Varying the oxygen exposure level while culturing the stem cells may play a major role in determining the survival of the cell as it is an essential adaptive factor for sustaining many of the energy-driven cellular processes along with playing an important role in cell signaling mechanisms that determine the course of a cell's fate.
Myocardial infarction, resulting in congestive heart failure, is still a leading cause of death (29), due to the severe loss of heart tissue which the body endogenously is incompetent to repair and restore completely, resulting eventually in failure of the organ (40). Our previous study showed that transplanted mesenchymal stem cells (MSCs) improved cardiac function and infarct site oxygenation in a rat model at 4 wk (4). However, there has been increasing concern over the low rate of engraftment of transplanted stem cells as a majority of them are lost within a few days of transplantation (2, 31, 39, 43). To counter such loss upon transplantation, MSCs have been preconditioned in several ways, including hypoxic preconditioning (HPC), to enhance the retention and survival of these cells in the MI heart (12, 16, 18, 41, 42). In our earlier studies we observed that the ischemic region in the infarct heart of murine models could reach an oxygen level as low as 0.2% (4, 25), which would imply that the survival and proliferation of transplanted stem cells in the infarct tissue would require the cells to adapt to very low oxygen tension in the infarct heart. Currently, in clinics as well as in research laboratories, freshly isolated (30) and cryopreserved MSCs (5, 8, 17) are being used for cell therapy. In this study using cryogenic rat MSCs, we have attempted to further examine hypoxic preconditioning at 0.5% oxygen concentration at different time periods of hypoxia and passage of MSCs in culture. We also examined whether cryopreservation could affect the response of these cells to preconditioning.
We observed that rat MSCs exposed to a hypoxic culture environment at 0.5% O2 were in a state of prosurvival and adaptation even after 24 and 48 h. However, HPC for 72 h using 0.5% oxygen concentration showed no significant levels of prosurvival or angiogenic protein expressions in these cells. Furthermore, we also show that cryopreserved MSCs that were in normoxic culture for at least a passage after being thawed respond positively to HPC and are more stable than MSCs that are freshly thawed and preconditioned directly in hypoxia. MSCs that have already been in normoxic culture before the 24-h HPC showed more effective differentiation into cardiomyocyte-like and endothelial cells than freshly thawed MSC cultures.
MATERIALS AND METHODS
In Vitro Culture of MSCs
Cryopreserved primary rat mesenchymal stem cells (MSCs), isolated from the bone marrow of adult Fisher 344 rats, were procured from Chemicon (Billerica, MA). The cells were characterized by the supplier to be positive for CD29 (integrin-β1) and CD54 (ICAM-1). The cells were thawed and cultured using Dulbecco's modified Eagle's medium (1× DMEM, low glucose + GlutaMAX-1) containing 10% heat-inactivated fetal bovine serum (FBS) and penicillin-streptomycin (Gibco). Accutase (Chemicon), a cell-detachment solution containing proteolytic and collagenolytic enzymes, was used for separation of adherent cells. Aerobic culture was maintained at 37°C in a humidified environment at 5% CO2 in air. Hypoxic cultures were grown in a humidified atmosphere of 0.5% O2 and 5% CO2 with a balance of N2 using in a specialized environmental chamber (C-Chamber and ProOx Model C21, BioSpherix) housed within a standard cell culture incubator. To study the optimal time period for HPC, frozen MSCs of passage 2 were thawed and allowed to grow a passage under normoxic (aerobic) conditions before they were split again and grown in 0.5% hypoxia for 24, 48, and 72 h. Control cells of the same passage were grown for a typical period of 48 h under normoxic culture environment. To investigate the response of different serial passages to HPC, MSCs from passages 2–6 were also cultured for 24 h under hypoxia. Passage 1 MSCs were thawed and maintained in normoxia for HPC of subsequent passages (until passage 6).
Measurement of Apoptosis
Phosphatidylserine exposure on the outer leaflet of the plasma membrane of MSCs exposed to normoxia and severe hypoxia (0.1% O2) for 48 h was measured using Invitrogen Annexin-V FITC Apoptosis Assay Kit according to the manufacturer's instructions. HPC-MSCs (24 h) and non-HPC MSCs were exposed to severe hypoxia for 48 h along with serum deprivation by using 2% heat-inactivated FBS. Briefly, cells were rinsed with PBS twice, and 1 × 106 cells/ml were resuspended in 100 μl of binding buffer. Five microliters of annexin V and 5 μl propidium iodide (PI) were added to the cells, which were incubated for 15 min in the dark. Annexin V binding buffer (400 μl) was then added, after which cells were analyzed immediately using a FACSCalibur (BD) and analyzed using CellQuest software (BD). Approximately 1 × 104 cells were analyzed in each sample.
Immunoblot Analysis
Cell lysates of MSCs were prepared using radio-immunoprecipitation assay (RIPA) buffer. The lysates were centrifuged at 12,500 g for 20 min at 4°C, and the supernatant was separated. The protein concentration in the lysates was determined using a Pierce detergent-compatible protein assay kit. For Western blot analysis, 75 to 100 μg of protein lysate per sample were denatured in 2× SDS-PAGE sample buffer and subjected to SDS-PAGE on a 10% to 12% Tris-glycine gel. The separated proteins were transferred to a polyvinylidene difluoride (PVDF) membrane, and then the membrane was blocked with 5% (wt/vol) nonfat milk powder in TBST solution (10 mM Tris, 100 mM NaCl, and 0.1% Tween 20) for 45 min at room temperature. The membranes were incubated with the primary antibodies overnight at 4°C. Antibodies against Akt, phosphorylated (p)Akt (Ser473), ERK1/2, pERK1/2, cytochrome c, and procaspases-3 and -7 were purchased from Cell Signaling Technology (Beverly, MA). α-Tubulin, p21, survivin, Bcl-2, VEGF, fusin (CXCR4) and c-Met antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Hypoxia-inducible factor-1α (HIF-1α) antibody was procured from Chemicon (Billerica, MA). The bound antibodies were detected with horseradish peroxidase (HRP)-labeled sheep anti-mouse IgG or HRP-labeled donkey anti-rabbit IgG (Amersham Biosciences) using an enhanced chemiluminescence detection system (ECL Advanced Kit). Proteins on the autoradiograph films were quantified by densitometry analysis using Image Gauge software (version 3.45; UN-SCANIT software, Silk Scientific, Orem, UT). Samples were collected from three independent experiments and analyzed using Western blots.
Enzyme-Linked Immunosorbent Assay
To quantify VEGF levels, cell lysates of MSCs grown in normoxia (48 h) and hypoxia (24, 48, and 72 h) were collected for enzyme-linked immunosorbent assay (ELISA). ELISA was carried out according to the manufacturer's recommendations (RayBio Rat VEGF ELISA kit). Briefly, standards and culture media were incubated at room temperature with sample buffer in 96-well plates for 2.5 h and then with biotin-labeled anti-Rat VEGF detection antibody for 1 h. Next, a streptavidin-HRP conjugate was added at room temperature for 45 min. Bound VEGF was detected by adding tetramethylbenzidine substrate solution for 30 min. The reaction was then stopped using sulfuric acid, and the plates were read immediately at 450 nm.
In Vitro Differentiation Assay for MSCs
Cardiomyogenesis.
MSCs were grown under normoxic (passage 3) and hypoxic (passages 2 and 3) culture for 24 h. MSCs were induced to differentiate in their respective oxygen environments to cardiomyocyte-like cells using 5-azacytidine. The cells were seeded in 60-mm petri dishes at a density of 7.5 × 104 cells/ml and cultured for 1 wk using DMEM containing 5% heat-inactivated FBS and penicillin-streptomycin. After 1 wk, cells were treated with 10 μM 5-azacytidine (Sigma-Aldrich) for 24 h. The cells were then washed with PBS (1×), and fresh DMEM containing 5% FBS was added. Expression of cardiomyocyte markers was examined by immunocytochemistry between 2 and 3 wk after treatment.
Endothelial differentiation.
MSCs were grown under normoxic (passage 3) and hypoxic (passages 2 and 3) culture for 24 h. MSCs were induced to differentiate in their respective oxygen environments into an endothelial lineage. The cells were seeded in 60-mm petri dishes at 5.5 × 104 cells/ml and grown in complete Clonetics Cambrex EGM-2 medium (Lonza, NJ). Expression of endothelial cell marker was examined by immunocytochemistry after 2 wk of culture.
Immunocytochemistry
Cells grown in both normoxic and hypoxic cultures were fixed with 2% paraformaldehyde for 5 min. The cell membrane was permeabilized with 0.5% Triton X-100 (1× PBS) for 15 min followed by 30 min of incubation with normal goat serum (Jackson ImmunoResearch) to block nonspecific binding. After being washed with PBS (1×), the cells were incubated overnight with the primary antibodies. Cardiomyocyte-specific antibodies used were monoclonal anti-mouse heavy chain cardiac myosin antibody (1:50; Abcam) and monoclonal anti-mouse connexin-43 (1:250; Chemicon). The antibody specific to the endothelial lineage was monoclonal anti-mouse platelet/endothelial cell adhesion molecule (PECAM)-1 (CD31) (1:50; Chemicon). The secondary antibodies used to visualize the attachment of the primary antibodies were goat anti-mouse conjugated with Alexa Fluor 488 or Alexa Fluor 594, and goat anti-rabbit conjugated with Alexa Fluor 488 (Invitrogen). The nuclei of the cells were stained with hard set mounting medium containing 4,6-diamidino-2-phenylindole (DAPI; Vector Laboratories).
Data Analysis
The statistical significance of the results was evaluated using one-way ANOVA and Bonferroni's multiple-comparison tests. The values are expressed as means ± SD. An overall P value of < 0.05 was considered significant.
RESULTS
Effect of Duration of HPC on the Prosurvival and Functional Characteristics of MSCs
Time-dependent expression of survival factors on HPC of MSCs.
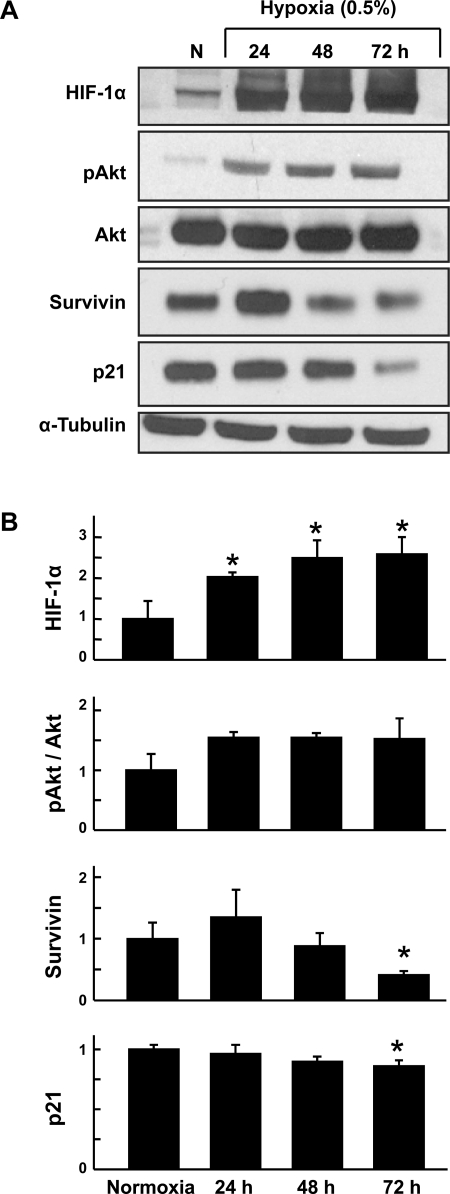
Rat MSCs were grown under hypoxia (0.5% O2) for 24, 48, and 72 h. Prosurvival proteins expressed by the cells under hypoxic culture were compared with those expressed in cells grown under normoxic culture (20% O2). Expression of the hypoxia marker HIF-1α was increased significantly at all three time points of hypoxic culture compared with normoxic culture (control) cells (Fig. 1). To determine the optimal duration of HPC of MSCs, the levels of prosurvival proteins such as pAkt, p-ERK1/2, Bcl-2, and survivin were determined using Western blot analysis. Phosphorylated Akt (pAkt), a critical regulator of phosphatidylinositol 3 (PI3)-kinase-mediated cell survival, showed increased level in HPC cells. No significant changes in pERK1/2 and ERK1/2 were observed between the control and the HPC cells (data not shown). In addition, the expression of the cell survival protein survivin was significantly decreased after 72-h exposure to hypoxia. p21, a cell cycle regulatory protein, showed a significant decrease in expression level after 72 h of HPC. The results showed that at 24 h of hypoxic preconditioning, expression of proteins involved in survival and differentiation was upregulated when compared with cells exposed to prolonged hypoxia.
Fig. 1.
Time-dependent expression of survival factors on hypoxic preconditioning (HPC) of mesenchymal stem cells (MSCs). Rat MSCs were grown under hypoxia (0.5% O2) for 24, 48, and 72 h. Proteins expressed by the cells under hypoxic culture were compared with those expressed in cells grown under normoxic culture (N, 20% O2). A: representative Western blots. B: quantitation of Western blot data. Data were obtained from 3 independent experiments and are expressed as means ± SD, relative to normoxic levels. *P < 0.05, hypoxic groups vs. normoxia (1-way ANOVA and Bonferroni's multiple-comparison test). The results show that HPC upregulated the expression of the hypoxic marker, hypoxia-inducible factor 1α (HIF-1α), and prosurvival proteins in MSCs compared with cells grown in normoxia.
Induction of apoptosis in MSCs by HPC.
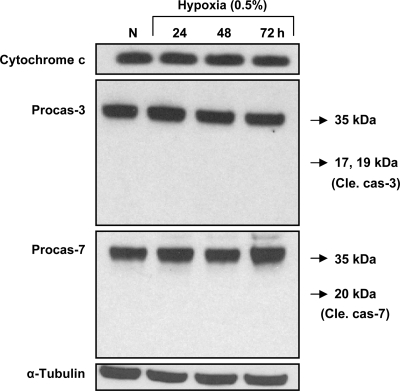
No significant induction of apoptosis was observed in MSCs upon exposure to hypoxia for as long as 72 h (Fig. 2). Cytochrome c, pro-caspase-3, and pro-caspase-7 showed no significant change in expression in either control or HPC cells. Cleaved caspase-3 and cleaved caspase-7 were not detected in the HPC cells, indicating that 0.5% O2, sublethal hypoxia, by itself did not induce cell death by apoptosis.
Fig. 2.
Induction of apoptosis in MSCs during HPC. Rat MSCs were grown under hypoxia (0.5% O2) for 24, 48, and 72 h. Apoptotic marker proteins expressed by the cells under hypoxic culture were compared with those expressed in cells grown under normoxic culture (20% O2). Pro-caspase (Procas)-7 showed no significant change in expression in control and cells subjected to HPC for 24, 48, and 72 h. The cleaved forms of caspase-3 (Cle cas-3) and caspase-7 (Cle cas-7) were not detected in the preconditioned cells, indicating that sublethal hypoxia at 0.5% oxygen did not trigger cell death.
Enhanced levels of angiogenic factors in MSCs upon HPC.
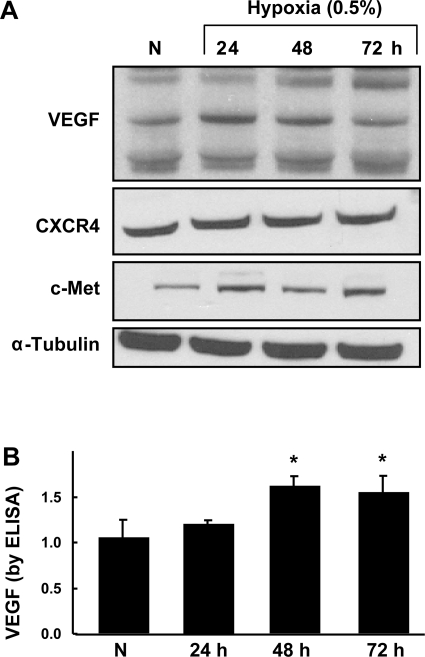
VEGF, a proangiogenic growth factor, showed a significant increase after 48 h of HPC (Fig. 3). We used both Western blotting and ELISA to obtain the result. CXCR4 and c-Met, the receptors of motogenic factors SDF-1 and hepatocyte growth factor (HGF), respectively, were expressed in all conditions but showed no significant changes upon preconditioning.
Fig. 3.
Enhanced levels of angiogenic factors in MSCs upon HPC. Rat MSCs were grown under hypoxia (0.5% O2) for 24, 48, and 72 h. A: representative Western blots of VEGF, CXCR4, and c-Met. B: quantification of VEGF using ELISA. Data were obtained from 3 independent experiments and are expressed as means ± SD, relative to normoxic levels. *P < 0.05, hypoxic groups vs. normoxia (1-way ANOVA and Bonferroni's multiple-comparison test). VEGF showed a significant increase after 48 h of HPC.
Apoptosis of MSCs on exposure to severe hypoxia reduced on preconditioning.
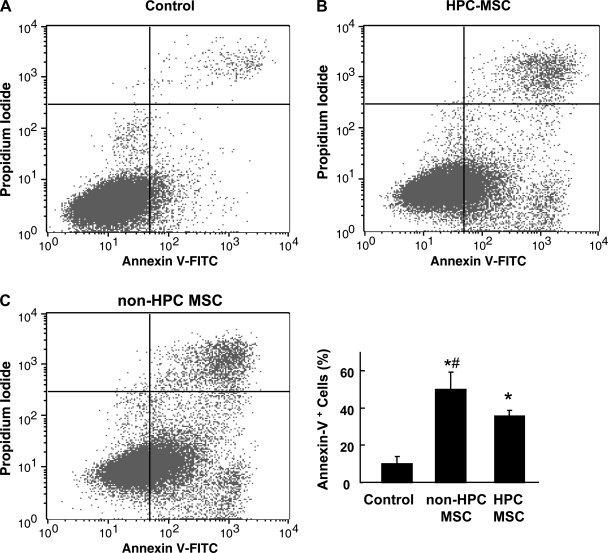
Apoptosis of MSCs on exposure to 0.1% oxygen showed significant reduction on preconditioning at 0.5% hypoxia for 24 h compared with MSCs that were not preconditioned before exposure to severe hypoxia (Fig. 4). The control MSCs grown in normoxia showed the least amount of apoptosis.
Fig. 4.
Quantitation of apoptotic cells by flow cytometric analysis of and non-HPC MSCs labeled with annexin V and propidium iodide on exposure to severe hypoxia. Rat MSCs preconditioned for 24 h in (0.5% O2) hypoxia (HPC-MSCs) along with those cultured in normoxia (non-HPC MSCs) were exposed to severe hypoxia. Apoptosis evaluated by annexin V staining was compared between control cells grown in normoxia (A), HPC-MSCs exposed to severe hypoxia (B), and non-HPC MSCs exposed to severe hypoxia (C). Data were obtained from 3 independent experiments and are expressed as means ± SD, relative to normoxic levels. *P < 0.05, hypoxic groups vs. normoxia, #P < 0.05 HPC-MSCs vs. non-HPC MSCs (1-way ANOVA and Bonferroni's multiple-comparison test). A significant decrease in apoptosis was observed in HPC-MSCs compared with non-HPC MSCs.
Effect of Cell Passage on the Prosurvival, Proangiogenic, and Functional Characteristics of MSCs Upon HPC
Effect of cell passage on the expression level of survival factors.
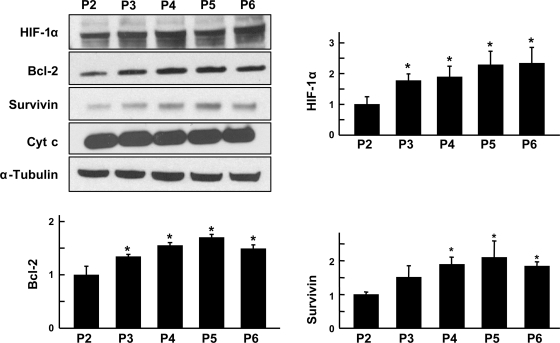
Given that 24 h of HPC was found to enhance prosurvival and profunctional proteins in MSCs, expression levels of prosurvival, angiogenic, and differentiation-inducing proteins were analyzed in different passages of MSCs using Western blotting. The lowest passage used for HPC was passage 2, which was freshly thawed, while the subsequent higher passages were cultured and maintained in normoxia before HPC. HIF-1α expression was observed to increase with passage and was significant in all passages of MSCs compared with passage 2 (Fig. 5). However, neither pAkt nor pERK1/2 showed any change in the passages studied (data not shown). Bcl-2 and survivin activity was higher upon HPC in the higher passages compared with passage 2. No significant changes were observed in the expression of p21 in any of the five passages (data not shown). Release of cytochrome c, an intermediate protein in apoptosis, did not significantly change in any of the passages. The results showed that prosurvival factors were predominantly expressed in passages 3–6.
Fig. 5.
Effect of cell passage on the expression level of survival factors. MSCs of passage 2 to passage 6 (P2–P6) were grown under hypoxic culture for 24 h, and protein levels of prosurvival factors were analyzed using Western blotting. Representative blots and quantitation are shown. Data were obtained from 3 independent experiments and are expressed as means ± SD, relative to P2 levels. *P < 0.05, higher passages vs. passage 2 (1-way ANOVA and Bonferroni's multiple-comparison test). The results show that the expression of prosurvival factors is predominantly increased in passages 3 to 5. Cyt c, cytochrome c.
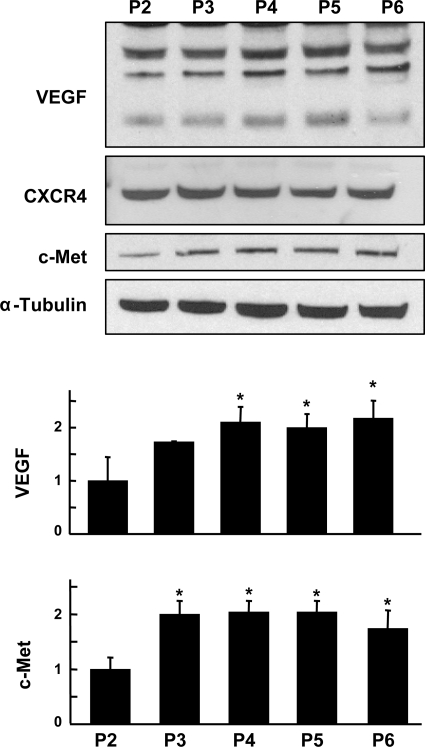
Evaluation of VEGF, CXCR4, and c-Met expression in MSC passages 2 to 6 after 24 h of HPC.
VEGF showed a significant increase in passages 4–6 compared with passage 2 after 24 h of HPC (Fig. 6). CXCR4, the receptor of motogenic factor SDF-1, showed no significant change in any of the passages. c-Met, the receptor of motogenic factor HGF, showed a significant increase in all higher passages examined compared with passage 2.
Fig. 6.
Evaluation of VEGF, CXCR4, and c-Met expression in MSC passages 2 to 6 after 24 h of HPC. Representative blots and quantitation of VEGF and c-Met are shown. Data were obtained from 3 independent experiments and are expressed as means ± SD, relative to P2 levels. *P < 0.05, higher passages vs. passage 2 (1-way ANOVA and Bonferroni's multiple-comparison test). VEGF and c-Met showed an increased expression in higher passages compared with lower passages after 24 h of HPC.
Effect of Hypoxia on the Differentiation of MSCs Into Cardiac and Endothelial Lineages
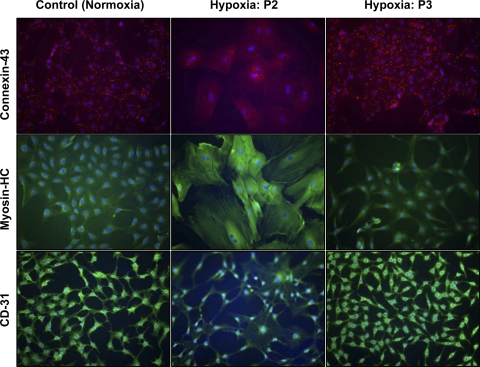
To determine the effect of sublethal hypoxia on the differentiation capacity of MSCs into cardiovascular lineages, the cells were induced to differentiate after 24 h of HPC into cardiomyocyte and endothelial lineages in the same hypoxic environment (passages 2 and 3). These experimental groups were compared with the differentiation of MSCs grown in normoxia (control, passage 3). Figure 7 shows the differentiation of MSCs into cardiomyocytes (positive staining for myosin heavy chain and connexin-43 especially along the cell membrane) and endothelial lineage (positive staining for CD31). The results showed that the differentiation potential of HPC-MSCs in a hypoxic environment was unaltered in passage 3 cells compared with MSCs grown under 20% O2. The passage 3 cells were in normoxic culture before HPC. However, hypoxic preconditioning of passage 2 MSCs that were freshly thawed and not in normoxic culture before HPC failed to show positive differentiation staining along the cell membrane as observed in the control.
Fig. 7.
Effect of hypoxia on the differentiation of MSCs into cardiac and endothelial lineages. Control MSCs were grown under normoxic (20% O2) conditions and HPC-MSCs of passages 2 and 3 at 0.5% O2 for 24 h. MSCs were induced to differentiate in their respective oxygen environment into cardiomyocyte-like cells using 5-azacytidine. Immunofluorescence staining is shown for cardiac markers heavy chain (HC) cardiac myosin (green) and connexin-43 (red along the membrane), superimposed on 4,6-diamidino-2-phenylindole (DAPI) staining for the nucleus (blue). Also shown are expressions of endothelial markers CD31 (green) along with DAPI staining for the nucleus (blue) for both normoxic and hypoxic MSCs after 2 wk of culture in complete endothelial medium. Magnification, ×200.
DISCUSSION
In this study we have analyzed the in vitro response of cryopreserved mesenchymal stem cells to hypoxic preconditioning at 0.5% oxygen concentration. The results showed that early passages of MSCs that are cultured under normoxic (20% oxygen) conditions before hypoxic preconditioning are in a state of prosurvival and profunction and retained their potential to differentiate into the cardiovascular lineage. The preconditioned cells were observed to undergo apoptosis to a lesser extent on exposure to severe hypoxia than MSCs that had not been preconditioned. Despite the many potential stem cell contenders for the repair and regeneration of damaged cardiac tissue (27), MSCs remain one of the preferred choices of adult stem cells for treating MI. This is mainly attributed to the potential of MSCs to differentiate into cells of the cardiovascular lineage and ability to repair damaged tissue without eliciting an immune response in the host (6). Both freshly isolated (30) and cryopreserved MSCs (5, 8, 17) are currently being used in clinics as well as in research laboratories. The present study was performed on cryopreserved MSCs derived from rat bone marrow.
The concept of HPC of stem cells is based on the knowledge that cells grown under a normoxic (20% O2) culture environment undergo DNA damage induced by oxidative stress, which is one of the major extrinsic factors responsible for cell senescence (22). In the physiological state, the circulating bone marrow stem cells have been described to experience ∼12% oxygen concentration in the blood, while the stem cells in the bone marrow can experience an oxygen environment as low as 1–2% (7, 9). Many groups have used hypoxic preconditioning of MSCs under different oxygen concentrations and periods of time (16, 21, 34). The MSCs used have been obtained in different states, such as freshly isolated or cryopreserved (5, 17). The studies have reported dissimilar results (10).
We opted to study HPC of rat MSCs at 0.5% O2 for several reasons. Our previous study determined the concentration of oxygen to be as low as 0.2–1% in the ischemic heart (4, 25). To meet the goal of repairing damaged myocardial tissue, a concentration of oxygen that promotes MSC differentiation toward a cardiovascular lineage is required, while avoiding oxygen concentrations at which MSCs are known to favor differentiation into other lineages such as osteogenic and adipogenic. A study done with human MSCs isolated from bone marrow showed that differentiation of MSCs into the osteogenic and adipogenic lineage was impaired at 1% oxygen but was restored when the oxygen levels were raised to 3% (19). Furthermore, a study by Hu et al. (20) has showed that HPC of rat MSCs at 0.5% O2 resulted in a significant increase in angiogenesis and cardiac function upon transplantation compared with normoxic-cultured MSCs.
We observed that the optimal duration for hypoxic preconditioning of MSCs was 24 h. The key target of hypoxic preconditioning strategy is to stimulate the survival mechanism in the cell as a response to a drastic reduction in oxygen availability. HIF-1α, which is a key mediator of the hypoxic responses in cells, is prone to oxygen-dependent degradation and is stable at lower concentrations of oxygen. MSCs cultured in a hypoxic environment for 24, 48, and 72 h showed an upregulation of HIF-1α activity. HIF-1α-mediated transcriptional responses can directly influence the activation of angiogenic and vasodilatory factors, such as VEGF and inducible nitric oxide synthase (37). The present results show significantly higher levels of VEGF in hypoxic-preconditioned cells. Since VEGF has also been shown to decrease apoptosis, delay senescence, and increase the expression of prosurvival factors, apart from its contributing role in angiogenesis (13, 33), an increase in its expression would be crucial for the survival of the cells in the infarct cardiac tissue.
The PI3 kinase pathway, similar to HIF-1α, is activated in response to hypoxia and cytokines (11). Upregulation of pAkt in response to PI3 kinase activation promotes cell survival by either directly targeting proteins involved in apoptosis or by modulating cell metabolism when under stress (11). We observed increased levels of pAkt levels upon hypoxic preconditioning at 24–48 h (significant at Student's t-test: *P < 0.05, compared with control). A comparable effect was not observed at 72 h of hypoxic preconditioning, which could indicate that either the cells are adapting to hypoxia on exposure to longer periods or most likely these cells under prolonged hypoxia have a lower probability of survival. Studies on treating MI hearts using MSCs overexpressing pAkt have shown an increased release of paracrine factors and enhanced cytoprotection compared with normoxic-cultured MSCs (15). We did not observe a significant change in ERK, while proteins that are known to play a role in cell cycle progression and proliferation, survivin (3) and p21 (24), were decreased significantly upon 72-h exposure to hypoxia. Low expression of ERK has been observed in MSCs exposed to <1% oxygen, suggesting that MSCs escape senescence under hypoxic conditions by downregulation of p16, a tumor suppressor gene (22). Although a moderate increase in survivin is seen at 24 h, the measurements were not reproducible. However, at 72 h of hypoxic preconditioning, a significant decrease was observed in survivin levels, indicating that apoptosis could occur in MSCs exposed to prolonged hypoxia. Similar effects were observed on the expression of p21, because a significant decrease was observed at 72 h of culture under hypoxia. This protein has been reported to play a dual role in stem cells: preserving “stemness” at basal levels by inhibiting cell cycle progression while increasing expression of p21 (as observed here at normoxic and 24-h hypoxic preconditioning) could limit self-renewal and promote differentiation (23).
Expression of CXCR4 and c-Met receptors to motogenic factors SDF-1 and HGF, respectively, play a significant role in the migration of these cells to the ischemic tissue (36). These receptors were expressed in the hypoxic-preconditioned MSCs at all conditions. CXCR4 showed a trend to increase; however, the increase was not significant as was observed in another study using human MSCs preconditioned at 1% hypoxia (21). Again, the difference in results might be due to the different species studied. In the rat MSCs even in the normoxic-cultured cells, CXCR4 is expressed at relatively high levels and shows only a marginal increase on hypoxic preconditioning. We did not observe any significant induction of apoptosis in the 72-h exposure MSCs to 0.5% O2. Neither a difference in the cytochrome c release nor a cleavage of caspase-3 or caspase-7 was observed in the hypoxic-preconditioned MSCs, indicating that sublethal hypoxia by itself did not induce cell death by apoptosis in MSCs. Although these cells showed no substantial apoptosis at the 0.5% O2, on decreasing the oxygen levels further down to 0.1%, the percentage of cell death increased to 50% in MSCs that have not been preconditioned, around 35% in preconditioned and only 10% in cells grown in normoxic conditions. The conditions in vivo might have a different effect on the transplanted MSCs, but these results obtained indicate that hypoxic preconditioning does reduce apoptosis under severe hypoxic and reduced serum conditions than cells exposed to such environment directly from the normoxic culture.
Cell therapy requires the use of stem cells at their earliest passages to retain their self-renewal and “stemness” characteristics. In clinical practice, cryopreserved stem cells are thawed just before implanting or infusing into the body of the patient (5, 8). This procedure might be required when multiple doses of cells have to be delivered at different times. A study on freezing and storage observed that freshly isolated human MSCs from the bone marrow showed similar cell proliferation, differentiation, and characteristic markers compared with cryopreserved MSCs grown in normoxic culture at passages 1 and 2 (17). However, although the frozen MSCs expressed the characteristic surface markers, there was a significant reduction in the expression levels of surface markers in the cryopreserved MSCs that were thawed as opposed to cells that were cultured from fresh isolates (17).
In our study we determined whether all passages would respond similarly to hypoxic preconditioning in terms of the expression of prosurvival factors. We treated MSCs starting with the lowest cryopreserved passage for 24 h under hypoxia. The MSCs that were freshly thawed and preconditioned in hypoxia showed the least response to the 24-h hypoxic preconditioning compared with passages 3–6, which were kept in normoxic culture for at least 24 h before hypoxic exposure. The results showed a significant difference in protein expression in all passages of hypoxic-preconditioned MSCs in comparison to passage 2 cells. Although cell cycle and prosurvival proteins pAkt, ERK, and p21 expression showed no significant changes among the different passages, other proteins were observed to show a difference in their levels compared with passage 2. Proteins involved with prosurvival and angiogenesis—Bcl2, survivin, HIF-1α, and VEGF—showed significant increased levels on exposure to hypoxic preconditioning in higher passages that were in normoxic culture before HPC functions compared with lower passages that had a short recovery from being thawed from the cryopreserved state. In addition, an increased level of c-Met receptors was expressed at all higher passages, which could indicate that cells taken out of cryopreservation might not possess effective migratory function as those cells that have been cultured in normoxia before HPC. However, there was no significant change in the expression of apoptotic protein cytochrome c in the passages observed.
Since the goal of hypoxic preconditioning of MSCs is to prepare the cells to adapt for the ischemic environment in the infarct hearts after transplantation, we examined passage 2 MSCs (directly obtained from cryopreservation) and passage 3 MSCs (obtained from normoxic culture) after 24 h HPC for their ability to differentiate into the cardiomyocyte and endothelial lineage in the same hypoxic environment. They were compared with MSCs grown in normoxic culture which have been differentiated previously into the cardiovascular lineage in vitro (32, 35). Cells of passage 3 showed similar differentiation morphology of cardiomyocyte-like cells and endothelial cells to the differentiated MSCs grown under normoxic culture. These cells were observed to adapt better and differentiate after the 24-h hypoxic conditioning than the passage 2 MSCs, which showed a different morphology and expression of stress fibers. This could be due to the poor survival probability (lower expression of Bcl-2, survivin) of cryopreserved MSCs in a hypoxic environment than the loss of differentiation capacity (p21, involved in differentiation, shows no change in all passages studied). Cryopreserved rat MSCs show no detrimental effect of hypoxia on their differentiation capacity at 0.5% O2 if they are kept in normoxic culture before HPC. Thus, the results of the present study emphasize an important consideration in the application of hypoxic preconditioning of MSCs for cell therapy.
GRANTS
This work was supported by American Heart Association Predoctoral Fellowship Grant GRT00007883 (to S. M. Chacko) and National Institutes of Health Grant EB-006153 (to P. Kuppusamy).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Abdollahi H, Harris LJ, Zhang P, McIlhenny S, Srinivas V, Tulenko T, Dimuzio PJ. The role of hypoxia in stem cell differentiation and therapeutics. J Surg Res. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham MR, Gerstenblith G. Preconditioning stem cells for cardiovascular disease: an important step forward. Circ Res 100: 447–449, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Ambrosini G, Adida C, Sirugo G, Altieri DC. Induction of apoptosis and inhibition of cell proliferation by survivin gene targeting. J Biol Chem 273: 11177–11182, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Chacko SM, Khan M, Kuppusamy ML, Pandian RP, Varadharaj S, Selvendiran K, Bratasz A, Rivera BK, Kuppusamy P. Myocardial oxygenation and functional recovery in infarct rat hearts transplanted with mesenchymal stem cells. Am J Physiol Heart Circ Physiol 296: H1263–H1273, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chin SP, Poey AC, Wong CY, Chang SK, Teh W, Mohr TJ, Cheong SK. Cryopreserved mesenchymal stromal cell treatment is safe and feasible for severe dilated ischemic cardiomyopathy. Cytotherapy 12: 31–37, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Chugh AR, Zuba-Surma EK, Dawn B. Bone marrow-derived mesenchymal stems cells and cardiac repair. Minerva Cardioangiol 57: 185–202, 2009 [PubMed] [Google Scholar]
- 7.Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood 82: 2031–2037, 1993 [PubMed] [Google Scholar]
- 8.Cristante AF, Barros-Filho TE, Tatsui N, Mendrone A, Caldas JG, Camargo A, Alexandre A, Teixeira WG, Oliveira RP, Marcon RM. Stem cells in the treatment of chronic spinal cord injury: evaluation of somatosensitive evoked potentials in 39 patients. Spinal Cord 47: 733–738, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Csete M. Oxygen in the cultivation of stem cells. Ann NY Acad Sci 1049: 1–8, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Das R, Jahr H, van Osch GJ, Farrell E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev 16: 159–168, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev 13: 2905–2927, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Erwin GS, Crisostomo PR, Wang Y, Wang M, Markel TA, Guzman M, Sando IC, Sharma R, Meldrum DR. Estradiol-treated mesenchymal stem cells improve myocardial recovery after ischemia. J Surg Res 152: 319–324, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 9: 669–676, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Geng YJ. Molecular mechanisms for cardiovascular stem cell apoptosis and growth in the hearts with atherosclerotic coronary disease and ischemic heart failure. Ann NY Acad Sci 1010: 687–697, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J 20: 661–669, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun 358: 948–953, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Haack-Sorensen M, Bindslev L, Mortensen S, Friis T, Kastrup J. The influence of freezing and storage on the characteristics and functions of human mesenchymal stromal cells isolated for clinical use. Cytotherapy 9: 328–337, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Herrmann JL, Wang Y, Abarbanell AM, Weil BR, Tan J, Meldrum DR. Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock 33: 24–30, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Holzwarth C, Vaegler M, Gieseke F, Pfister SM, Handgretinger R, Kerst G, Muller I. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol 11: 11, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg 135: 799–808, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Hung SC, Pochampally RR, Hsu SC, Sanchez C, Chen SC, Spees J, Prockop DJ. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PloS One 2: e416, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin Y, Kato T, Furu M, Nasu A, Kajita Y, Mitsui H, Ueda M, Aoyama T, Nakayama T, Nakamura T, Toguchida J. Mesenchymal stem cells cultured under hypoxia escape from senescence via down-regulation of p16 and extracellular signal regulated kinase. Biochem Biophys Res Commun 391: 1471–1476, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Ju Z, Choudhury AR, Rudolph KL. A dual role of p21 in stem cell aging. Ann NY Acad Sci 1100: 333–344, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Jung YS, Qian Y, Chen X. Examination of the expanding pathways for the regulation of p21 expression and activity. Cell Signal 22: 1003–1012, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan M, Kutala VK, Vikram DS, Wisel S, Chacko SM, Kuppusamy ML, Mohan IK, Zweier JL, Kwiatkowski P, Kuppusamy P. Skeletal myoblasts transplanted in the ischemic myocardium enhance in situ oxygenation and recovery of contractile function. Am J Physiol Heart Circ Physiol 293: H2129–H2139, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan M, Kwiatkowski P, Rivera BK, Kuppusamy P. Oxygen and oxygenation in stem-cell therapy for myocardial infarction. Life Sci 87: 269–274, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lasala GP, Minguell JJ. Bone marrow-derived stem/progenitor cells: their use in clinical studies for the treatment of myocardial infarction. Heart Lung Circ 18: 171–180, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Lin Q, Kim Y, Alarcon RM, Yun Z. Oxygen and cell fate decisions. Gene Regul Syst Bio 2: 43–51, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association's Strategic Impact Goal Through 2020 and Beyond. Circulation 121: 586–613, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Lunde K, Solheim S, Forfang K, Arnesen H, Brinch L, Bjornerheim R, Ragnarsson A, Egeland T, Endresen K, Ilebekk A, Mangschau A, Aakhus S. Anterior myocardial infarction with acute percutaneous coronary intervention and intracoronary injection of autologous mononuclear bone marrow cells: safety, clinical outcome, and serial changes in left ventricular function during 12-months' follow-up. J Am Coll Cardiol 51: 674–676, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med 10: 494–501, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 22: 377–384, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Pons J, Huang Y, Arakawa-Hoyt J, Washko D, Takagawa J, Ye J, Grossman W, Su H. VEGF improves survival of mesenchymal stem cells in infarcted hearts. Biochem Biophys Res Commun 376: 419–422, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Ren H, Cao Y, Zhao Q, Li J, Zhou C, Liao L, Jia M, Zhao Q, Cai H, Han ZC, Yang R, Chen G, Zhao RC. Proliferation and differentiation of bone marrow stromal cells under hypoxic conditions. Biochem Biophys Res Commun 347: 12–21, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Shim WS, Jiang S, Wong P, Tan J, Chua YL, Tan YS, Sin YK, Lim CH, Chua T, Teh M, Liu TC, Sim E. Ex vivo differentiation of human adult bone marrow stem cells into cardiomyocyte-like cells. Biochem Biophys Res Commun 324: 481–488, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells 24: 1254–1264, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Taylor CT. Mitochondria and cellular oxygen sensing in the HIF pathway. Biochem J 409: 19–26, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Thum T, Bauersachs J, Poole-Wilson PA, Volk HD, Anker SD. The dying stem cell hypothesis: immune modulation as a novel mechanism for progenitor cell therapy in cardiac muscle. J Am Coll Cardiol 46: 1799–1802, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 105: 93–98, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Von Harsdorf R, Poole-Wilson PA, Dietz R. Regenerative capacity of the myocardium: implications for treatment of heart failure. Lancet 363: 1306–1313, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Wang JA, Chen TL, Jiang J, Shi H, Gui C, Luo RH, Xie XJ, Xiang MX, Zhang X. Hypoxic preconditioning attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Acta Pharmacol Sin 29: 74–82, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Wang JA, He A, Hu X, Jiang Y, Sun Y, Jiang J, Gui C, Wang Y, Chen H. Anoxic preconditioning: a way to enhance the cardioprotection of mesenchymal stem cells. Int J Cardiol 133: 410–412, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol 33: 907–921, 2001 [DOI] [PubMed] [Google Scholar]