Abstract
Regulator of G protein signaling 4 (RGS4) regulates the strength and duration of G protein signaling and plays an important role in smooth muscle contraction, cardiac development, and psychiatric disorders. Little is known about the posttranscriptional regulation of RGS4 expression. We cloned the full-length cDNA of rabbit RGS4, which contains a long 3′-untranslated region (UTR) with several AU-rich elements (AREs). We determined whether RGS4 mRNA stability is mediated by the RNA-binding protein human antigen R (HuR) and contributes to IL-1β-induced upregulation of RGS4 expression. We show that IL-1β treatment in colonic smooth muscle cells doubled the half-life of RGS4 mRNA. Addition of RGS4 3′-UTR to the downstream of Renilla luciferase reporter induced dramatic reduction in the enzyme activity and mRNA expression of luciferase, which was attenuated by the site-directed mutation of the two 3′-most ARE sites. IL-1β increased luciferase mRNA stability in a UTR-dependent manner. Knockdown of HuR significantly aggravated UTR-mediated instability of luciferase and inhibited IL-1β-induced upregulation of RGS4 mRNA. In addition, IL-1β increased cytosolic translocation and RGS4 mRNA binding of HuR. These findings suggest that 3′-most ARE sites within RGS4 3′-UTR are essential for the instability of RGS4 mRNA and IL-1β promotes the stability of RGS4 mRNA through HuR.
Keywords: regulators of G protein signaling, Interleukin, AU-rich element
regulator of g protein signaling (RGS) proteins belong to a large family of highly diverse, multifunctional signaling proteins, which share a conserved signature domain (RGS domain) that binds directly to the activated Gα subunits. RGS terminates G protein signaling by accelerating intrinsic GTPase activity of Gα and thereby fostering reassociation of the Gαβγ trimer (61). Over 30 mammalian family members have been described so far and classified into seven subfamilies based on sequence identity and functional similarities (70, 72). RGS4 is one of the most extensively studied RGS proteins (44, 61, 62, 70, 72) and regulates the strength and duration of the Gαi/o and Gαq/11 family members (26, 36). RGS4 plays an important role in promoting cardiac development, regulating neuronal plasticity, and modulating smooth muscle contraction (2). In cardiomyocytes, RGS4 expression is induced by endotoxin and interleukin (IL)-1β (57, 58) and contributes to the loss of Gαq-mediated activation of phospholipase C (PLC) by endothelin-1 (51). In the central nervous system, RGS4 is linked to schizophrenia (6, 14, 27, 44, 46), Alzheimer disease (13), and Huntington disease (63). RGS4 regulates the pain process (17, 25) and morphine tolerance (25, 31) through modulation of opioid receptor signaling (43, 69). In gastrointestinal smooth muscle, RGS4 negatively regulates Gαq signaling activated by M3 or motilin receptors (37, 38) and thus inhibits agonist-induced initial contraction (33, 35, 36). Recently, we demonstrated (35) that treatment of colonic smooth muscle cells with the proinflammatory cytokine IL-1β inhibits acetylcholine-stimulated initial contraction through increasing the expression of RGS4. Upregulation of RGS4 by IL-1β is mediated by NF-κB signaling (33) and modulated by MAP kinases (MAPKs) and a phosphatidylinositol 3-kinase (PI3-kinase)/Akt/GSK3β pathway (34).
Inflammatory mediators regulate gene expression via transcriptional and posttranscriptional mechanisms. The steady status of the target gene transcripts depends on both synthesis and degradation. One of the important mechanisms to regulate gene expression is mRNA stability, which is tightly controlled by the interaction of specific mRNA sequences (cis-elements) with specific trans-acting factors such as RNA-binding proteins (RBPs). AU-rich elements (AREs) are the critical, albeit not exclusive, cis-acting elements in the 3′-untranslated regions (3′-UTRs) of many short-lived genes such as cytokines, growth factors, transcription factors, and protooncogenes (8, 49, 65). Selective binding of RBPs to AREs of target mRNAs leads to either increase or decrease in the half-life of target transcript. A number of ARE binding proteins have been identified, including ELAV (embryonic lethal abnormal vision in Drosophila melanogaster) protein family members such as human antigen R (HuR) (8), tristetraprolin (4), AU-rich binding/degradation factor (AUF1) (75), the KH-type splicing regulatory protein (KSRP) (19), and T cell intracellular antigen-1 (TIA-1) (47). HuR is a ubiquitously expressed member of the ELAV family and is increasingly recognized as an important posttranscriptional regulator for gene expression (30). HuR is implicated in a large variety of pathologies in which destabilization of many labile key mRNAs is causally linked with the onset and course of diseases (12). HuR has been shown to stabilize ARE-containing mRNAs of several inducible genes such as c-fos, c-myc, cyclooxygenase-2, tumor necrosis factor-α, β-catenin, cyclin D1, p21, p53, etc. (1, 8, 30). HuR is present predominantly in the nucleus of unstimulated cells but translocates to the cytoplasm in response to various stress stimuli and binds with great affinity and specificity to AREs in a variety of mRNAs (12). It becomes clear that HuR subcellular localization is closely linked to its effects on target transcripts.
Cytokines, growth factors, and others have been shown to regulate RGS4 expression in different types of cells. However, the molecular mechanisms for RGS4 regulation remain poorly understood. At the protein level, RGS4 is regulated by the posttranslational N-end rule mechanism (5). At the mRNA level, RGS4 is regulated by a neural type-specific transcription factor, Phox2b (22). In our previous studies (33, 45), we demonstrated the important contribution of the transcriptional mechanism to IL-1β-induced upregulation of RGS4 expression. In the present study, we explored the importance of the posttranscriptional mechanism underlying the upregulation of RGS4 expression. We cloned the full length of rabbit RGS4 3′-UTR, which is enriched with ARE sites. Using reporter gene assay as well as deletion and site-directed mutation assay, we demonstrated that the two 3′-most ARE sites are critical to mediate or regulate the instability of RGS4 mRNA. IL-1β stabilizes RGS4 mRNA through 3′-UTR by promoting cytosolic translocation and the 3′-UTR binding of HuR. This study provides the first evidence for the mRNA-stabilizing mechanism in RGS4 regulation.
MATERIALS AND METHODS
Cell culture.
Animal protocols were reviewed and approved by the Temple University Institutional Animal Care and Use Committee (IACUC). Rabbit colonic smooth muscle cells were isolated and cultured as previously described (35, 52). Briefly, distal colon from euthanized New Zealand White rabbits (2–2.5 kg) was placed in HEPES-buffered smooth muscle medium. The circular and longitudinal smooth muscle layers were dissected under the stereomicroscope and treated with 0.1% collagenase (type II) and 0.1% soybean trypsin inhibitor for 30 min at 31°C. Isolated single muscle cells after several rounds of spontaneous dispersion were harvested by filtration through 500-μm Nitex and centrifuged twice at 350 g for 10 min. The isolated smooth muscle cells were plated in 10-cm dishes with DMEM containing 10% fetal bovine serum and 1% antibiotics and antimycotics. After 10–14 days, the smooth muscle cells attained confluence and were then passaged once for use in various experiments.
Rapid amplification of cDNA ends.
The coding sequence (cds) and 5′-UTR of rabbit RGS4 were cloned by degenerative reverse transcription (RT)-PCR and 5′-rapid amplification of cDNA ends (RACE) as described previously (45). The 3′-UTR of rabbit RGS4 transcript was identified by using the SMART (Switching Mechanism At 5′ end of RNA Transcript) RACE cDNA amplification kit (BD Biosciences, Clontech, Palo Alto, CA) according to the manufacturer's instructions. The cDNA was generated from total RNA of rabbit colonic smooth muscle cells. PCR was carried out with the forward primer (5′-ATGTGCAAAGGACTTGCAGGTC-3′) from rabbit RGS4-cds and the universal primer provided in the kit. The PCR product of 2.5 kb was gel purified and cloned into the pCRII-TOPO T-A vector (Invitrogen). Positive clones were identified by restriction digestion with EcoRI, and the nucleotide sequence of the two ends of the insert was determined by sequencing with universal T7 and SP6 primers. The sequence of the middle region of the insert was determined by sequencing with a customer-designed primer based on the sequence determined by the T7 primer.
Construction of various vectors.
In the preliminary studies, we placed the full-length (fl) 3′-UTR (2,092 bp) of rabbit RGS4 downstream of the luciferase reporter in RGS4 promoter (1.5 kb)-driven secreted Renilla luciferase (rLuc) vector at AgeI/BstBI digestion sites. Reporter assay showed that the luciferase signal was undetectable, perhaps because of the removal of partial poly(A) signal and/or the weak promoter activity. Thus we harvested the insert encoding secreted Renilla luciferase plus RGS4 3′-UTR(fl) from the above vector by BstBI digestion, blunting, and KpnI digestion followed by gel purification. The insert was cloned through KpnI/EcoRV double digestion into pcDNA3 vector, which contains cytomegalovirus (CMV) promoter and bovine growth hormone (BGH) poly(A) signal. This vector was designated as pcDNA3-rLuc-RGS4-UTR(fl). The parent vector pcDNA3-rLuc was generated by inserting Renilla luciferase reporter fragment from pMluc-3 AccepTor vector (EMD Bioscience/Novagen) through KpnI and blunted AgeI into pcDNA3 vector at KpnI/EcoRV. All vectors were validated by restriction digestion and sequencing with the universal T7 and SP6 primers.
Various deletion constructs of pcDNA3-rLuc-RGS4-UTR(fl) were generated through digestion, blunting, and ligation by analyzing and combining the digestion sites within the insert and the backbone vector. The NH2-terminal deletion mutant (deleting nucleotides 1–607 of RGS4 3′-UTR) designated pcDNA3-rLuc-RGS4-UTR(Δnd) containing the sites of ARE-3 to ARE-6 was generated by double digestion with AgeI/EcoRV followed by blunting and ligation. The COOH-terminal deletion mutant (deleting nucleotides 607–2092) designated pcDNA3-rLuc-RGS4-UTR(Δcd) containing ARE-1 and ARE-2 sites was generated by single digestion with EcoRV and ligation.
The classical ARE motif (AUUUA) was used to search against the RGS4 3′-UTR full length, identifying six ARE sites. Because RGS4-UTR(Δcd) prevented UTR-mediated mRNA instability and ARE-5 and ARE-6 on the COOH-terminal region are highly conserved, their site-directed mutation constructs were generated by site-directed mutagenesis using pcDNA3-rLuc-RGS4-UTR(fl) vector as template according to the protocol of the QuikChange kit (Stratagene). The mutagenic primer for RGS4-UTR-ΔARE-6 is shown here as a representative, and the underlined nucleotides are the mutated ARE site (forward): 5′-TTGAAAATTTCTCATTTATGTTGCTCGTGATGTTGTTTTGTAC-3′. The final mutation was verified by nucleotide sequencing.
The cds of rabbit RGS4 was cloned into the downstream of Renilla luciferase reporter at the AgeI site of the pcDNA3-rLuc vector with the in-fusion advantage PCR cloning kit (Clontech).
HuR short hairpin RNA (shRNA) expression vectors were generated as previously described (32). Briefly, two shRNA encoding sequences, HuR-A and HuR-B, were designed based on 100% homology between human, mouse, and rat HuR and targeted nucleotides 117–137 and 429–450 of mouse HuR (AK161667). The shRNA expression cassette was generated through two consequential rounds of PCR and cloned into the pLL3.7 lentiviral vector, which contains a CMV-promoted enhanced green fluorescent protein (EGFP) marker as internal control (32). The sequence of each shRNA expression cassette in the vector was confirmed by restriction enzyme digestion and DNA sequencing. The nucleotide sequences for HuR shRNA are 5′-GCAGGACACAGCTTGGGCTAC-3′ for HuR-A and 5′-GAAGAGGCAATTACCAGTTTCA-3′ for HuR-B.
Rabbit HuR sequence was obtained by RT-PCR using RNA from rabbit colonic smooth muscle cells and the degenerative primers obtained by comparing the cds of HuR from human, mouse, and rat. The sequence of rabbit HuR was deposited in GenBank (accession no. HM037267). The Flag-tagged rabbit HuR expression vector was generated by standard PCR cloning into pRK-Flag vector.
Transfection of cultured colonic smooth muscle cells.
All the vectors for mammalian expression were prepared with the EndoFree Plasmid Maxi kit (Qiagen). Confluent smooth muscle cells in first passage were transiently transfected with the indicated vectors with Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen). Fluorescent analysis of EGFP or immunocytochemical staining with anti-Flag antibody showed a transfection efficiency of 60–70%.
Reporter assays.
Cells (2 × 104/well) were cultured on 96-well plates. After transfection and incubation for the indicated time periods the media were harvested for measurement of Renilla luciferase activity, and at the end the cell lysate was used for measurement of firefly luciferase activity. The Renilla luciferase was determined with a Renilla luciferase assay kit (Promega). The firefly luciferase was determined with the ONE-Glo luciferase assay system (Promega). Luminescence was measured with the Envision Luminescence plate reader (Perkin Elmer). Data are normalized by dividing Renilla luciferase activity by the corresponding firefly luciferase activity in each of four to six wells. Experiments were performed at least three times.
Immunofluorescent cytochemistry and quantitative image analysis.
Cells were seeded on eight-well glass chamber slides (Nalge Nunc, Lab-Tek, Rochester, NY) and cultured until full confluence. After 24-h serum starvation, cells were treated with IL-1β for different time periods, followed by fixation with 4% paraformaldehyde-PBS for 30 min. After washing with PBS, cells were permeabilized with 0.5% Triton X-100 for 30 min, blocked with 10% normal donkey serum for 1 h, and incubated with the primary anti-HuR polyclonal antibody (Santa Cruz, 1:400) overnight at 4°C. After washing, the Alexa Fluor 488 (green)-linked secondary donkey anti-rabbit (Invitrogen, 1:200) antibody was applied for 1 h. Staining specificity was determined by omitting the primary antibody. Hoechst 33258 was used for counterstaining of nuclei. The slides were coverslipped with antifading aqueous mounting medium. The fluorescences of HuR and Hoechst staining were captured by sequential acquisition under the fluorescent inverted microscope (Nikon, Japan) with IPLab software. Quantitative analysis of HuR nuclear-to-cytosolic translocation was performed with NIH ImageJ 1.43q software, following the methods used for p65 nuclear translocation (10, 18, 34, 55). Briefly, a median filter (3 × 3 pixel radius) was applied to the Hoechst-stained image to remove noise and to approximate the distribution of staining intensity to a median value. A binary mask containing all the fluorescence above background was made. The nuclear mask was generated by 1-pixel erosion to avoid the cytosolic contamination. The cytosolic mask was generated by using a 4-pixel dilation of the nuclear mask and subtraction. Each mask was then applied to the original HuR-positive immunofluorescent image after 3-pixel median filtering to obtain the integrated optical density. The ratio of nuclear and cytosolic integrals was used to normalize the data and exclude variations in size and shape of the cell and nucleus. The ratios were averaged for 4–6 fields per well under a ×40 oil objective with 30–50 cells per field. Measurements were repeated in triplicate (3 wells), and data are presented as means ± SE of four separate experiments.
Western blot analysis.
Cells were cultured in six-well plates. Nuclear and cytosolic extracts were prepared with the NE-PER kit (Pierce). Western blot analysis was performed as previously described (32). Briefly, equal amounts of proteins were fractionated by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membrane (Bio-Rad). Blots were blocked in 5% nonfat dry milk-Tris-buffered saline (pH 7.6) plus 0.1% Tween-20 (TBS-T) for 1 h and incubated overnight at 4°C with various primary antibodies in TBS-T plus 1% milk. After incubation for 1 h with horseradish peroxidase-conjugated corresponding secondary antibody (1/2,000, 10 μg/ml; Pierce) in TBS-T plus 1% milk, immunoreactive proteins were visualized with the SuperSignal Femto maximum sensitivity substrate kit (Pierce). All washing steps were performed with TBS-T.
Reverse transcription and real-time PCR.
Cells were seeded on six-well plates. Total RNA was extracted with the TRIzol reagent (Invitrogen). Potentially contaminated genomic DNA was removed by TURBO DNase (Ambion). One microgram of RNA was used to synthesize cDNA with SuperScript III reverse transcriptase (Invitrogen) with random hexanucleotide primer. Real-time PCR analysis was carried out on a LightCycler480 (Roche). Expression of rabbit RGS4 mRNA was analyzed with the TaqMan PCR Master Mix Reagents Kit (Applied Biosystems) as described previously (35). The mRNA expressions of reporter luciferase and rabbit GAPDH were determined by using the SYBR Green I kit (Roche). The sequences for luciferase primers are forward 5′-AATCTTTCTGCACGGCAACG-3′ and reverse 5′-TGCCGATCAGATCAGGAATGA-3′. Rabbit GAPDH primers are forward 5′-CGCCTGGAGAAAGCTGCTAA-3′ and reverse 5′-CGACCTGGTCCTCGGTGTAG-3′. Each sample was tested in triplicate. Cycle threshold (Ct) values were obtained graphically for RGS4 and GAPDH. The difference in Ct values between GAPDH and RGS4 are represented as ΔCt values. ΔΔCt values were obtained by subtracting the ΔCt values of the control samples from that of the treated samples. Relative fold change in gene expression was calculated as 2−ΔΔCt. In some cases (see Fig. 5B), absolute quantification was performed with RGS4 plasmid as the standard.
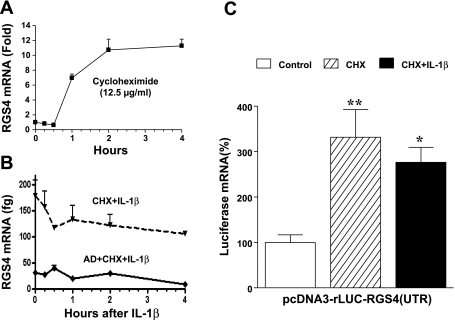
Fig. 5.
RGS4-UTR-dependent mRNA instability involves new protein synthesis. A: translation inhibitor cycloheximide (CHX) alone induced a significant increase in mRNA expression of endogenous RGS4. Cultured colonic smooth muscle cells after serum starvation were treated with CHX (12.5 μg/ml) for indicated time periods, and RGS4 mRNA level was determined by real-time RT-PCR. The fold change was relative to the level before CHX treatment. B: transcription inhibitor actinomycin D (AD) prevented CHX-induced upregulation of RGS4 mRNA, while CHX prevented IL-1β-induced upregulation of RGS4 mRNA. Cells were pretreated with AD (10 μM) 1 h and CHX 30 min before IL-1β treatment for indicated time periods, and RGS4 mRNA level was determined by absolute quantitative real-time RT-PCR using rabbit RGS4 plasmid as standard. C: RGS4 3′-UTR-dependent instability of luciferase was alleviated by pretreatment with CHX. Cells were transfected with pcDNA3-rLuc-RGS4-UTR(fl) for 24 h, serum starved for 24 h, and treated with vehicle control, CHX, and IL-1β 10 ng/ml for 24 h. Renilla luciferase was measured. Data represent means ± SE of 4 separate experiments. *P < 0.05 and **P < 0.01, statistically significant increase by ANOVA compared with corresponding control.
Biotin RNA pull-down assay.
The coding region and 3′-UTR of rabbit RGS4 were obtained by PCR TA cloning into pCR-II vector that contains T7 and SP6 RNA polymerase promoter. The orientation of PCR inserts was verified by sequencing. After lineation by digestion with NotI, the vector was purified by phenol-chloroform extraction. The DNA was used as template for the synthesis of the corresponding biotinylated RNA with the MEGAscript SP6 in vitro transcription kit (Ambion) and biotin-11-UTP. The biotinylated transcript (15 pmol) was incubated with precleared cytosolic (100 μg) or nuclear (50 μg) extract for 30 min at room temperature. The complexes were isolated with streptavidin-conjugated Dynabeads (Dynal), and bound proteins in the pull-down material were analyzed by Western blotting using antibodies against HuR or IKK1 (Santa Cruz).
PLC-β activity assay.
PLC-β activity was determined in cultured smooth muscle cells by measuring the formation of inositol phosphates by ion-exchange chromatography as described previously (33, 53). Transfected cells labeled with myo-[3H]inositol (0.5 μCi/ml) for 24 h in inositol-free DMEM without FBS were washed with PBS and treated with acetylcholine (0.1 μM) plus methoctramine (0.1 μM) for 0.5 min in 1 ml of HEPES-buffered solution (pH 7.4). The reaction was terminated by the addition of 940 μl of chloroform-methanol-HCl (50:100:1). The aqueous phase after extraction and centrifugation was applied to a Dowex AG-1 column, and [3H]inositol phosphates were eluted with 0.8 M ammonium formamate plus 0.1 M formic acid. Radioactivity was determined by liquid scintillation and expressed as counts per minute (cpm).
Statistical analysis.
For assessment of mRNA half-life, the RGS4 mRNA level after normalization over the time period after addition of actinomycin D was analyzed by the one-phase exponential decay of nonlinear regression with GraphPad Prism. Data are expressed as means ± SE of three or four separate experiments. Statistical significance between different groups was determined by Student's t-test or one-way analysis of variance (ANOVA) with Newman-Keuls comparison.
RESULTS
RGS4 mRNA half-life is increased by IL-1β.
The steady-state levels of RGS4 mRNA reflect the balance between synthesis and degradation. Our previous studies (33) demonstrated that IL-1β induces a rapid increase in RGS4 mRNA expression, which is blocked by preaddition of transcription inhibitor actinomycin D. To explore the potential posttranscriptional mechanisms for IL-1β-induced upregulation of RGS4 mRNA, we measured the stability of RGS4 mRNA by determining the time course of RGS4 mRNA remaining after inhibition of transcription by actinomycin D in the presence or absence of IL-1β treatment. The constitutive level of RGS4 mRNA without IL-1β (and no serum) was lower but detectable (taken as 100%) and gradually reduced after transcription inhibition (Fig. 1, A and B). When IL-1β and actinomycin D were added simultaneously (0 h, 100%), IL-1β induced a rapid increase within 30 min before the actinomycin D took effect and doubled the half-life of RGS4 mRNA (Fig. 1A). When IL-1β was added after pretreatment with actinomycin D for 30 min, IL-1β produced a similar effect on RGS4 mRNA decay (Fig. 1B). Under the higher level of RGS4 mRNA (100%) after IL-1β induction for 3 h (Fig. 1C) or 24 h (Fig. 1D), as reported previously (33, 35), addition of actinomycin D without IL-1β (by changing the culture medium) produced a faster degradation of RGS4 mRNA, with a half-life of 44–48 min. Continuous exposure of IL-1β after transcription inhibition also doubled the half-life of RGS4 mRNA (Fig. 1, C and D). These data suggest that IL-1β has a dual effect on RGS4 mRNA through increasing transcription and decreasing mRNA decay. The decay rate of RGS4 mRNA relies on the initial level, and IL-1β treatment stabilizes RGS4 mRNA independently of initial level in rabbit colonic smooth muscle cells.
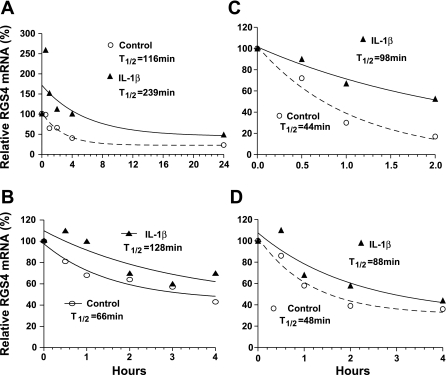
Fig. 1.
Interleukin (IL)-1β extends the half-life (T1/2) of regulator of G protein signaling 4 (RGS4) mRNA in rabbit colonic smooth muscle cells. A: cultured cells after serum starvation for 24 h were treated simultaneously without (Control) or with IL-1β (10 ng/ml) + actinomycin D (10 μM) for indicated time periods. B: cells were pretreated with actinomycin D for 30 min and treated without (Control) or with IL-1β for indicated periods. C and D: cells were treated without (Control) or with IL-1β for 3 h (C) or 24 h (D) before addition of actinomycin D. Total RNA extracted with TRIzol reagent was treated with DNase I before real-time RT-PCR analysis. After normalization with GAPDH, RGS4 mRNA levels at each time point were compared with the initial level (100%) before addition of actinomycin D (A, C, D) or IL-1β (B). A nonlinear regression analysis for the half-life of mRNA decrease was conducted by 1-phase exponential decay software from GraphPad Prism.
Rabbit RGS4 cDNA contains a long 3′-UTR.
We have cloned the 5′-UTR of rabbit RGS4 by 5′-RACE (45). To understand the mechanism for RGS4 mRNA stability, we cloned the 3′-UTR of rabbit RGS4 by 3′-RACE. The PCR product from 3′-RACE presented a single band, and sequencing analysis revealed a long 3′-UTR of 2,092 bp. Combining the previously identified cds and 5′-UTR sequence generated the full length (2,830 bp) of rabbit RGS4 cDNA as shown in deposited GenBank accession number GQ848313GQ848313. This full-length cDNA is consistent with the result from Northern blot analysis detecting a single band of 2.8 kb of mature RGS4 mRNA in rabbit intestine (45). The genomic structure of rabbit RGS4 gene as shown in Fig. 2A contains five exons, and the fifth exon includes all the sequence of 3′-UTR. This feature of rabbit RGS4 genomic structure is shared by RGS4 genes from other species such as human (NM_001102445), mouse (NM_009062), and rat (NM_017214).
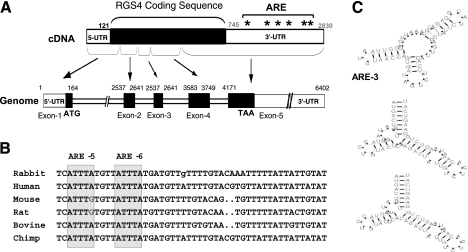
Fig. 2.
Cloning and homology analysis of rabbit RGS4 3′-untranslated region (UTR). A: full-length cDNA and genome of rabbit RGS4. Sequence of cDNA was built from 5′- and 3′-rapid amplification of cDNA ends (RACE), and 6 canonical AU-rich element (ARE) sites on 3′-UTR were labeled. Intron/exon structure was deduced through GenomeBLAST against rabbit genome. B: homology analysis of AREs between species. Selected regions containing highly conserved ARE-5/6 are shown. C: decay motifs in rabbit RGS4 3′-UTR. The structure and score of the decay motif were predicted by the RNApromo program.
Sequence analysis of the 3′-UTR of rabbit RGS4 revealed a context of uridine-rich and adenosine-rich elements and six putative AREs with canonical AUUUA sequences that may serve as determinants for RGS4 mRNA stability. Searching for noncanonical NNUUNNUUU sequence against RGS4 3′-UTR identified eight sites. Clustal W alignment of RGS4 3′-UTR from various species demonstrated that rabbit RGS4 3′-UTR shares 74% identity with human and primate and 62% identity with rodents. The fifth and sixth canonical ARE sites are highly conserved (with ARE-6 sharing 100% identity among all species; Fig. 2B), implicating their potential roles in regulating RGS4 mRNA stability.
Computational RNA motif prediction analysis of RGS4 3′-UTR full length (2,092 bp) using a newly developed RNApromo (60) identified three RNA motifs that may regulate mRNA decay (Fig. 2C). The first locus is at nucleotides 700–767 (CCAUGGAUCUUUCUGGAAAAGCAUCCAAGCAAAUUCAUGGUUAAUUUAACCAGUGAUAGUCUUCACAUUGAGCUCUAUUCUACGGAGAGAAAAAUC), with motif score 65.4. The second and third loci are at the same nucleotides 1926–1987 (AUGACUUUGAUAUGGUACCUGUACUCACAGACUAUUGUCUCACAAAUAAGUCUGGAAGUCAU), with scores of 85.4 and 82.5 respectively, showing minor differences of structure in the loop (Fig. 2C). These motif structures, although not related to ARE-5 and ARE-6, suggest that RGS4 3′-UTR may play a role in regulating mRNA stability.
3′-UTR of rabbit RGS4 negatively regulates RGS4 expression and mRNA stability.
The presence of a long 3′-UTR with decay motif structures and ARE sites as shown above implies a potential posttranscriptional mechanism. To gain experimental evidence for the function of RGS4 3′-UTR, we prepared the mammalian expression construct pcDNA3-rLuc-RGS4-UTR encoding a chimeric transcript containing a secreted Renilla luciferase reporter gene fused to RGS4 3′-UTR plus vector-derived BGH poly(A) signal. In our preliminary studies, removal of the poly(A) signal led to entire loss of luciferase activity of the parent construct pcDNA3-rLuc, suggesting that the poly(A) signal is required to maintain luciferase mRNA. As shown in Fig. 3, addition of RGS4 3′-UTR to the downstream of luciferase reporter gene induced >80% reduction in the activity and 70% reduction in the mRNA expression of luciferase, suggesting that the 3′-UTR of RGS4 is capable of modulating the instability of a heterologous mRNA.
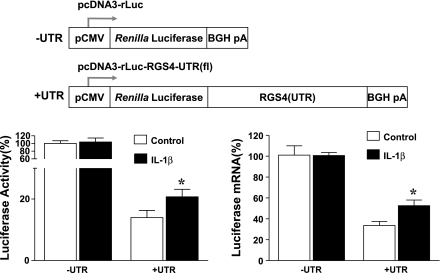
Fig. 3.
Insertion of RGS4 3′-UTR remarkably inhibited cytomegalovirus (CMV)-promoted luciferase activity (left) and mRNA expression (right). IL-1β treatment for 3 h significantly prevented RGS4 UTR-dependent mRNA decay of luciferase reporter gene. Cultured colonic smooth muscle cells were cotransfected with indicated vectors (top) carrying secreted Renilla luciferase (rLuc) and pGL4-CMV vector carrying firefly luciferase (for normalization). After 24 h, cells were serum starved for 24 h and treated with vehicle control or IL-1β (10 ng/ml) for 24 h. The Renilla and firefly luciferases were measured separately in quadruplicate, and mRNA levels of Renilla luciferase were determined by real-time RT-PCR in triplicate. Data represent means ± SE of 3 independent experiments. *P < 0.05, statistically significant increase by Student's t-test compared with corresponding control. BGH pA, bovine growth hormone poly(A).
To address whether 3′-UTR mediates IL-1β-induced upregulation of RGS4 expression, smooth muscle cells transfected with parent luciferase reporter or luciferase-RGS4-UTR reporter vectors were treated with IL-1β for 24 h and luciferase activity and mRNA level were determined. In the absence of 3′-UTR sequence of RGS4, treatment with IL-1β had no significant effect on the enzyme activity and mRNA expression. However, in the presence of 3′-UTR IL-1β treatment significantly attenuated the reduction in both activity and mRNA level of luciferase reporter (Fig. 3). These data suggest that IL-1β stabilizes the mRNA transcript and protein product of the reporter gene via 3′-UTR of RGS4.
The two 3′-most ARE sites are critical for RGS4 mRNA destabilization.
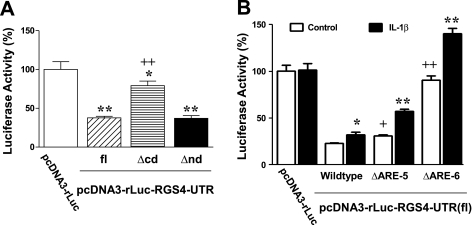
Six putative canonical AREs are distributed throughout the full-length (2,092 bp) 3′-UTR of rabbit RGS4, with the two 3′-most AREs (Fig. 2B) situated in a highly conserved, AU-rich context. To determine the roles of specific AREs in regulating RGS4 mRNA stability, deletion mutants of the chimeric luciferase-RGS4-UTR vector as described above were prepared and analyzed by luciferase reporter assay. Deletion of 3′-UTR NH2-terminal domain (nd; nucleotides 1–607, containing ARE-1/2) had no effect on the luciferase activity mediated by the full-length UTR (Fig. 4A). However, deletion of the COOH-terminal domain (cd; nucleotides 607–2092, containing ARE-3–6) significantly attenuated UTR-induced reduction of luciferase activity, implying that the COOH-terminal region is critical for RGS4 mRNA instability.
Fig. 4.
COOH-terminal domain (A) and in particular ARE-5/6 sites (B) of rabbit RGS4 3′-UTR play an important role in regulating RGS4 mRNA instability. Cultured colonic smooth muscle cells were cotransfected with indicated vectors + normalization pGL4-CMV vector for 24 h. After serum starvation, cells were treated with vehicle control or IL-1β (10 ng/ml) for 24 h. Renilla and firefly luciferases were measured separately. Percentage changes in Renilla luciferase relative to the pcDNA3-rLuc without RGS4 3′-UTR were calculated. Data represent means ± SE of 4–6 separate experiments. *P < 0.05 and **P < 0.01, statistically significant changes by 1-way ANOVA and Newman-Keuls test compared with the pcDNA3-rLuc (A) or Student's t-test compared with the corresponding control (B). +P < 0.05 and ++P < 0.01, significant changes by ANOVA compared with wild type of pcDNA3-rLuc-RGS4-UTR(fl). Δcd and Δnd indicate the deletion of COOH-terminal and NH2-terminal domains of RGS4 3′-UTR.
To identify which ARE sites are responsible for the instability of RGS4 mRNA, we performed a site-directed mutagenesis assay using pcDNA3-rLuc-RGS4-UTR(fl) as parent vector. As shown in Fig. 4B, mutation of ARE-5 significantly attenuated UTR full length-induced reduction of luciferase activity, while mutation of ARE-6 abolished it, implying that ARE-6 is most critical in mediating RGS4 mRNA instability. Thus both ARE-5 and ARE-6 are critical in mediating the instability of RGS4 mRNA, and ARE-6 is most effective. This is also consistent with the potential role of ARE-6 and ARE-5 as predicted by the homology analysis.
To further delineate the role of ARE-5 and ARE-6 in mediating IL-1β-induced upregulation of RGS4 mRNA, we treated cells expressing site-directed mutants with IL-1β. As described above (Fig. 2), IL-1β treatment induced a significant increase in the luciferase activity of the RGS4-UTR full-length (wild type)-mediated reporter luciferase (Fig. 4B). Unexpectedly, mutation of ARE-5 or ARE-6 did not prevent IL-1β-induced increase of the reporter luciferase activity. In contrast, they all produced further increase of luciferase activity induced by IL-1β (Fig. 4B). These data suggest that IL-1β stabilizes RGS4 mRNA through unknown mechanisms, while the fifth and sixth ARE sites alleviate the effect of IL-1β by regulating the instability of RGS4 mRNA.
RGS4 UTR-dependent mRNA instability involves new protein synthesis.
In an attempt to determine the transcriptional and posttranscriptional mechanisms for IL-1β-induced upregulation of RGS4, we observed that inhibition of protein biosynthesis by cycloheximide (CHX, 12.5 μg/ml) in cultured serum-starved colonic smooth muscle cells induced a dramatic and time-dependent increase in the mRNA level of RGS4 as determined by real-time RT-PCR (Fig. 5A). Pretreatment with CHX 30 min before IL-1β treatment prevented further increase in RGS4 mRNA by IL-1β (Fig. 5B), indicating that new protein synthesis is involved in IL-1β-induced upregulation of RGS4 mRNA expression. The increase of RGS4 mRNA induced by CHX or IL-1β alone or their combination was inhibited by pretreatment with the transcription inhibitor actinomycin D (Fig. 5B), implying that the transcriptional mechanisms are involved in CHX- and IL-1β-induced upregulation of RGS4 mRNA (33). However, it may also result from the increased mRNA stability. To validate the role of new protein synthesis in regulating RGS4 mRNA instability, we transfected smooth muscle cells with luciferase-RGS4-UTR reporter vector and treated the cells with CHX. As shown in Fig. 5C, CHX treatment prevented UTR-mediated decrease in luciferase mRNA expression, implying that the increased UTR-mediated RGS4 mRNA instability involves synthesis of new proteins, preferentially the destabilizing proteins. Either IL-1β or CHX alone stabilized luciferase mRNA. However, no additive or superinductive effect of IL-1β plus CHX was observed by luciferase reporter assay (Fig. 5C), suggesting that IL-1β stabilizes mRNA via a process of new protein synthesis like mRNA-stabilizing proteins (65). Similarly, either IL-1β or CHX alone dramatically upregulated the mRNA expression of endogenous RGS4 in a time-dependent manner, but both together did not induce further increases (Fig. 5B). In contrast, the RGS4 mRNA in the presence of both IL-1β and CHX displayed a gradual decay (Fig. 5B). Altogether, these data imply that new protein synthesis is implicated in UTR-mediated RGS4 mRNA instability and IL-1β-stimulated RGS4 mRNA stabilization.
HuR regulates RGS4 expression in a 3′-UTR-dependent manner.
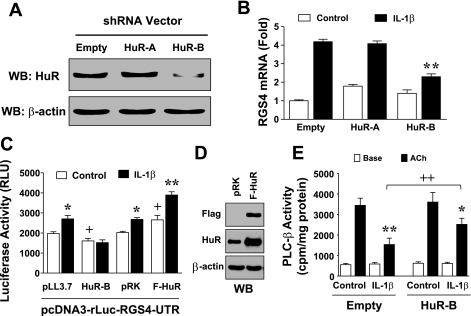
As demonstrated above, IL-1β treatment stabilized RGS4 mRNA through the 3′-UTR, but the mechanism and signaling pathways remain unknown. HuR is a RBP that stabilizes ARE-containing mRNAs. Deletion analysis of the RGS4 3′-UTR identified the important role of ARE sites within the COOH-terminal region in regulating the instability of RGS4 mRNA. We hypothesized that the mRNA-stabilizing protein HuR may mediate IL-1β-induced upregulation of RGS4 mRNA. To test this, we performed loss-of-function studies. Silencing efficiency and specificity of HuR shRNA expression constructs were determined by Western blot and RT-PCR analysis in cultured rabbit colonic smooth muscle cells. As shown in Fig. 6A, HuR-B shRNA effectively inhibited the protein expression of endogenous HuR, but HuR-A was ineffective in rabbit smooth muscle cells, although the shRNA-encoding sequence of HuR-A has been reported for its efficiency to silence HuR expression in mouse (77). Further analysis of HuR-A sequence against the newly sequenced rabbit RGS4 identified a mismatch of the last nucleotide (C) to rabbit RGS4 (T), which attests to the sequence-specific silencing efficiency of RNA interference. Accordingly, the effective HuR-B shRNA significantly (∼2-fold) inhibited IL-1β-induced upregulation of endogenous RGS4 mRNA under transcriptional inhibition as determined by real-time PCR, whereas the ineffective HuR-A shRNA and the control empty vector had no effect on IL-1β-induced upregulation of RGS4 mRNA expression (Fig. 6, A and B). These data suggest that HuR mediates IL-1β-induced upregulation of RGS4 mRNA expression through a posttranscriptional mechanism in rabbit colonic smooth muscle cells. The approximately twofold effect of HuR silencing on mRNA expression is consistent with the effect of IL-1β on RGS4 mRNA half-life (Fig. 1), supporting the notion that HuR may preferentially affect RGS4 mRNA stability, although we cannot rule out the possibility that other factors or signaling components required for RGS4 transcription such as NF-κB and MAPK may be affected by HuR silencing.
Fig. 6.
Human antigen R (HuR) mediates IL-1β-induced upregulation of RGS4 and inhibition of phospholipase C (PLC)-β activation. A and B: effective HuR-B short hairpin RNA (shRNA) inhibited IL-1β-induced upregulation of endogenous RGS4 mRNA expression. Smooth muscle cells were transfected with empty vector or HuR shRNA expression vectors. After 48 h, cells were pretreated with actinomycin D (10 μM) for 30 min and treated with vehicle control or IL-1β (10 ng/ml) for 3 h, followed by Western blotting (WB) with anti-HuR or anti-β-actin antibodies and real-time RT-PCR analysis. Relative fold changes in RGS4 mRNA are expressed as means ± SE of 3 separate experiments. **P < 0.01, statistically significant decrease by ANOVA compared with empty vector. C: HuR knockdown increased but HuR overexpression decreased RGS4 3′-UTR-mediated instability of reporter luciferase. Cultured colonic smooth muscle cells were cotransfected with indicated vectors and pGL4-CMV vector carrying firefly luciferase (F-HuR Flag-tagged HuK, for normalization). After 72 h, the Renilla and firefly luciferases were measured separately. Data represent means ± SE of 4 separate experiments. *P < 0.05 and **P < 0.01, statistically significant increase by Student's t-test compared with control group. +P < 0.05, significant change by Student's t-test compared with corresponding empty vector. RLU, relative fluorescence unit. D: relative level of overexpressed HuR was validated by Western blot. E: HuR knockdown attenuated IL-1β-induced inhibition of PLC-β activity. Cultured cells were transfected with empty vector or effective HuR-B shRNA for 2 days and labeled with myo-[3H]inositol in serum-free medium for 1 day, pretreated with IL-1β (10 ng/ml) for 3 h, and stimulated with acetylcholine (ACh, 0.1 μM) + methoctramine (0.1 μM) for 0.5 min. [3H]inositol phosphate was determined as described in materials and methods and expressed as counts per minute (cpm). Values are means ± SE of 3 experiments. *P < 0.05 and **P < 0.01, significant decrease by IL-1β in ACh-induced PLC-β activity compared with corresponding control. ++P < 0.05, significant increase in ACh-induced PLC-β activity compared with corresponding empty vector.
To further determine the potential role of HuR in the UTR-dependent regulation of RGS4 mRNA stability, colonic smooth muscle cells were cotransfected with luciferase-RGS4-UTR reporter vector and Flag-tagged rabbit HuR expression vector or HuR-B shRNA vector. As shown in Fig. 6C, the dramatic reduction in luciferase activity by the addition of RGS4–3′-UTR into the downstream luciferase reporter was aggravated by effective HuR-B shRNA while attenuated by overexpression of HuR (Fig. 6D). Thus HuR plays a direct role in stabilizing RGS4 mRNA via its 3′-UTR. To delineate the functional connection between IL-1β and HuR signaling pathways, we treated HuR knockdown or overexpression cells with IL-1β and examined the RGS4–3′-UTR-dependent luciferase reporter activity. HuR knockdown blocked IL-1β-induced upregulation of 3′-UTR-dependent reporter activity, whereas HuR overexpression significantly enhanced the IL-1β effect (Fig. 6C). These data suggest that HuR mediates IL-1β-induced stabilization of RGS4 mRNA via 3′-UTR in colonic smooth muscle cells.
HuR knockdown attenuates IL-1β-induced inhibition of acetylcholine-stimulated PLC-β activation during initial phase of contraction.
Using PLC-β activity as a marker to reflect the initial contractile response of smooth muscle cells (37, 53), we demonstrated previously that RGS4 mediates IL-1β-induced inhibition of acetylcholine-stimulated contraction. To investigate the functional significance of HuR-mediated RGS4 mRNA stabilization, we evaluated the effect of HuR shRNA knockdown on IL-1β-induced inhibition of PLC-β activity. Consistent with previous studies (35), IL-1β treatment inhibited acetylcholine-stimulated PLC-β activation. Knockdown of HuR by the effective HuR-B shRNA significantly prevented IL-1β-induced reduction in acetylcholine-stimulated PLC-β activation (Fig. 6E).
IL-1β induced rapid cytosolic translocation of HuR.
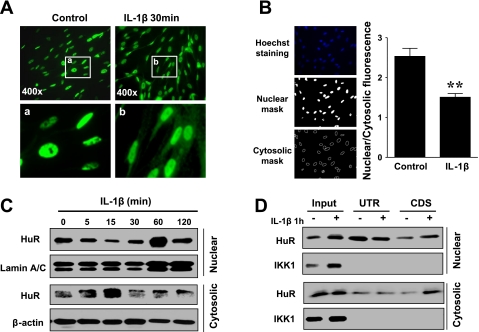
Cytosolic translocation of HuR has been shown to increase mRNA stability in several genes and various cell types (12, 16). To determine whether IL-1β affects HuR cytosolic translocation in rabbit colonic smooth muscle cells, we examined the time course of HuR subcellular distribution by fluorescent immunocytochemistry and Western blot analysis. As shown in Fig. 7, A and B, robust nuclear staining of HuR was constitutively detected in the serum-starved smooth muscle cells, while lower-intensity, but still detectable, HuR staining was evident in the cytosol in some cells. After treatment with IL-1β, the nuclear staining was reduced and evident cytosolic staining was detected in the majority of cells (Fig. 7A). Quantitative image analysis identified the significant reduction in the ratio of nuclear to cytosolic HuR immunofluorescence after IL-1β treatment (Fig. 7B). To determine the dynamic changes in HuR nuclear/cytosolic translocation, Western blot analyses of cytosolic and nuclear extracts after IL-1β treatment for different time periods were performed. Consistently, HuR expression was predominantly localized in the nucleus but also detectable in the cytosol under constitutive conditions (Fig. 7, C and D). IL-1β exposure induced rapid (5–15 min) reduction in HuR nuclear expression (Fig. 7C). In addition, IL-1β treatment increased the nuclear expression of HuR (Fig. 7, C and D) at 1 h, which reduced again at 2 h, implying the nuclear-cytosolic shuttling of HuR as described previously (12). Lamin A/C was used as loading control. These data suggest that IL-1β promotes HuR cytosolic translocation.
Fig. 7.
IL-1β induced nuclear-to-cytosolic translocation of HuR and increased the binding of HuR to RGS4 coding region in rabbit colonic smooth muscle cells. A: representative immunofluorescent micrographs of HuR nuclear-to-cytosolic translocation in cultured cells after IL-1β treatment for 30 min. B: typical example of HuR fluorescent image analysis and quantitative data after Il-1β treatment for 30 min. **P < 0.01, statistically significant decrease by Student's t-test compared with control. C: dynamic changes in HuR protein expression in nuclear and cytosolic fractions determined by Western blot analysis. D: biotin-RNA pull-down assay showing that both UTR and coding sequence (cds) of rabbit RGS4 specifically bind to HuR and IL-1β stimulation increases binding activity of both cytosolic and nuclear HuR to RGS4 coding region.
IL-1β promotes binding of HuR to RGS4 mRNA.
Sequence analysis of RGS4 3′-UTR predicted the presence of ARE motifs that potentially bind to mRNA-stabilizing or -destabilizing proteins. To validate the direct binding activity of HuR within the ARE motif of RGS4, we performed a biotinylated RNA pull-down assay. The in vitro-transcribed biotinylated RNA probe from RGS4 3′-UTR detected strong binding of HuR in both nuclear and cytosolic extracts from cultured colonic smooth muscle cells. The amount of pull-down nuclear HuR relative to the input (1:10) was enriched more than that of cytosolic HuR, implying that the 3′-UTR binding of nuclear HuR was stronger than that of cytosolic HuR (Fig. 7D). IL-1β stimulation increased 3′-UTR binding of cytosolic HuR but decreased 3′-UTR binding of nuclear HuR, supporting the notion that IL-1β promotes HuR cytosolic translocation. Unexpectedly, the biotinylated RNA probe from RGS4-cds originally designed as a negative control also detected strong binding of both nuclear and cytosolic HuR. In addition, IL-1β increased the binding activity of the cds probe to the nuclear and, in particular, the cytosolic HuR. For a control experiment, we examined the expression and possible mRNA binding of IKK1 in the same blot membrane. Consistent with previous reports (20), IKK1 expression is predominantly located in the cytosol and IL-1β treatment increased the expression of IKK1 in both cytosol and nucleus. However, the biotinylated RNA probe from either RGS4–3′-UTR or cds did not pull down IKK1 in both cytosol and nucleus. These data suggest that both 3′-UTR and cds of rabbit RGS4 specifically bind to HuR, whereas IL-1β stimulation promotes HuR translocation from nucleus to cytosol and increases the cds binding activity of both cytosolic and nuclear HuR.
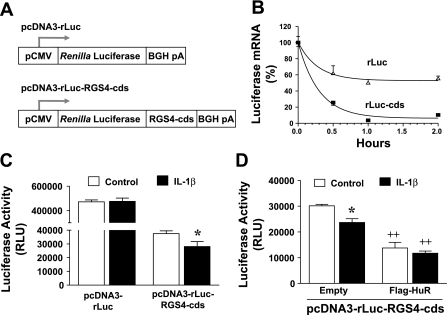
HuR binding of RGS4-cds implies that the coding sequence may regulate RGS4 mRNA stability. To test this, we cloned RGS4-cds into the downstream of CMV-promoted Renilla luciferase reporter (Fig. 8A) and measured the decay curve of luciferase mRNA after transcription inhibition by actinomycin D. Addition of RGS4-cds dramatically promoted the mRNA decay of luciferase reporter gene (Fig. 8B) and induced >90% reduction in luciferase activity (Fig. 8C). Unexpectedly, IL-1β treatment further decreased luciferase activity (Fig. 8, C and D). Overexpression of HuR significantly decreased RGS-cds-mediated luciferase activity (Fig. 8D). These data suggest that IL-1β-induced RGS4-cds binding of HuR negatively regulates RGS4 mRNA stability.
Fig. 8.
IL-1β promotes RGS4-cds-mediated mRNA instability via increasing HuR binding to RGS4-cds. A: cloning of RGS4-cds reporter vectors. B: RGS4-cds destabilizes mRNA of luciferase reporter gene. Cultured colonic smooth muscle cells were transfected with pcDNA3-rLuc or pcDNS3-rLuc-RGS4-cds vectors. After 24 h, cells were serum starved for 24 h and treated with actinomycin D for indicated time periods. The mRNA levels of Renilla luciferase were determined by real-time RT-PCR in triplicate. C: IL-1β promotes RGS4-cds-mediated reduction of luciferase activity. Cells were cotransfected with indicated vectors + firefly luciferase vector (normalization), followed by serum starvation (24 h) and IL-1β treatment (24 h). Renilla and firefly luciferases were measured separately in quadruplicate. Data represent means ± SE of 3 independent experiments. *P < 0.05, statistically significant decrease by Student's t-test compared with corresponding control. D: HuR overexpression promotes RGS4-cds-mediated reduction of luciferase activity. Cells were cotransfected and treated as described in C. *P < 0.05, statistically significant decrease by Student's t-test compared with corresponding control. ++P < 0.01, statistically significant decrease compared with corresponding empty vector.
DISCUSSION
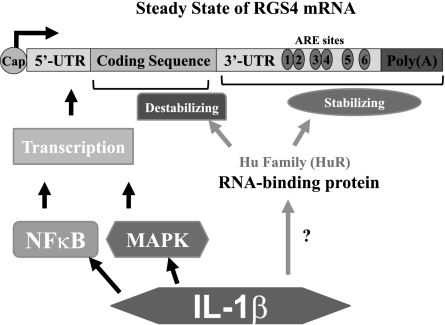
RGS4 is one of seven members of the classic R4 RGS protein family that accelerates the GTPase activity of the Gαi/o and Gαq/11 family members. The expression and function of RGS4 have been well studied in smooth muscle cells (28, 35, 67), cardiomyocytes (26, 42, 50), nerve tissue (41), and cancer cells (39, 54, 73). However, the regulatory mechanisms for RGS4 expression have not yet been well elucidated. Our previous studies (35) demonstrated that IL-1β significantly upregulates RGS4, leading to an inhibitory effect on agonist-stimulated initial contraction of gut smooth muscle. We also demonstrated (33, 34) that NF-κB, MAPK, and PI3-kinase/Akt/GSK3β signaling pathways are involved in IL-1β-induced upregulation of RGS4 expression. In a parallel study (45), we cloned the promoter of rabbit RGS4 and identified the transcriptional mechanism responsible for RGS4 upregulation. In the present study, we cloned the long 3′-UTR of rabbit RGS4 and demonstrated that the two 3′-most ARE sites are critical to mediate the mRNA-destabilizing effect of RGS4 3′-UTR. IL-1β treatment promotes cytosolic translocation and mRNA binding of HuR. Gain- or loss-of-function studies confirmed that HuR is required for IL-1β-induced upregulation of RGS4. Therefore, transcriptional and posttranscriptional mechanisms contribute to the regulation of RGS4 expression in rabbit colonic smooth muscle cells (Fig. 9).
Fig. 9.
IL-1β upregulates RGS4 mRNA expression in colonic smooth muscle cells via transcriptional and posttranscriptional mechanisms. HuR stabilizes the 3′-UTR while destabilizing the coding region of RGS4.
RGS proteins play critical roles in regulating G protein signaling, which is well known to affect a large array of cellular functions. Most studies have been done on the protein stability of RGS, but little is known about the mRNA stability and the underlying mechanisms of RGS proteins (15). Sequence analysis of RGS proteins identified a long 3′-UTR with variable numbers of ARE sites in most members of the RGS family such as RGS4, RGS5, RGS6, RGS12, RGS16, and RGS17. Here, we provide the first experimental evidence that the long 3′-UTR of RGS4 is critical in regulating or mediating mRNA instability. We predict that the long 3′-UTR in other members of the RGS family is also critical in regulating the stability of corresponding mRNA. This is also consistent with the fact that most RGS proteins are short-lived.
Gene expression is tightly regulated by transcription factors. Recent studies have revealed that the posttranscriptional mechanism triggered by RBPs and microRNA (miRNA) is also essential to maintain a precise and transient elevation of a gene product and ensure its function (8, 49). About 10% of known genes have been shown to be regulated by degradation or stabilization of their mRNA (24). In our previous studies (33, 35), we showed that IL-1β consistently induced over 10- to 20-fold increase in mRNA expression of endogenous RGS4 in colonic smooth muscle cells. However, a reporter gene assay using RGS4 promoter detected only one- to twofold induction by IL-1β in rabbit colonic smooth muscle cells (45). This prompted us to investigate the potential contribution of posttranscriptional regulation (mRNA stability) to IL-1β-induced upregulation of RGS4. The presence of highly conserved ARE sites within RGS4 3′-UTR suggests that a posttranscriptional mechanism mediated by ARE-binding proteins may control RGS4 mRNA stability. Many ARE-binding proteins have been identified, including HuR (8, 49). The end point effects of these ARE-binding proteins rely on cell types and pathophysiological conditions (24, 49). In most cases, the ubiquitously expressed HuR functions by stabilizing the mRNA of target genes. In the present study, we show that HuR stabilizes RGS4 mRNA through 3′-UTR. IL-1β stimulation promotes HuR cytosolic translocation and increases HuR binding to RGS4 3′-UTR. Therefore, during RGS4 mRNA nucleocytosolic transport and subsequent translation, HuR binding aids in protecting RGS4 mRNA from decaying, and IL-1β increases RGS4 mRNA level by enhancing cytosolic HuR binding activity, leading to more stabilization.
Another finding is that HuR also strongly binds to the coding region of RGS4. In contrast to 3′-UTR binding, RGS4-cds binding of HuR negatively modulates mRNA stability, implying an intricate mechanism for RGS4 mRNA regulation. The experimental evidence of HuR binding within the cds has been reported previously in a few genes such as IL-4 (74), CD83 (59), XIAP (76), c-fos (23, 68), c-myc (71), and others (9). Although these genes do not contain the canonical ARE sites in the cds, the presence of the noncanonical HuR binding motif (NNUUNNUUU) supports the experimental data (48). Sequence analysis of RGS4-cds did not show any sites for the canonical AUUUA sequence but did identify one site for the noncanonical NNUUNNUUU sequence in the sense strand and two sites in the antisense strand. Our data are supported by the previous observation that HuR binding to CD83-cds does not stabilize mRNA (59). In contrast, CD83-cds binding of HuR affects the nucleocytosolic translocation of CD83 mRNA. The nucleocytosolic trafficking of HuR and its target transcripts is mediated by the HuR ligands pp32 (ANP32A) and APRIL (ANP32B) (7, 16). Thus IL-1β may promote the nuclear-to-cytosolic trafficking of RGS4 mRNA by increasing the binding of RGS4-cds to both nuclear and cytosolic HuR. The underlying mechanism and signaling pathways for IL-1β-induced nucleocytosolic trafficking of RGS4 mRNA will be an interesting project in the future.
Although six canonical ARE sites are present in the 3′-UTR of rabbit RGS4, only the two 3′-most ARE sites are evolutionarily conserved and functional at mediating mRNA instability. Site-directed mutation of ARE-5 and particularly ARE-6 reversed UTR-mediated instability, implying that ARE-5/6 is essential for RGS4 decay. However, it did not prevent the IL-1β-induced mRNA-stabilizing effect on the luciferase reporter, suggesting that ARE-5/6 is not involved in the mRNA stabilization in response to IL-1β. Therefore, ARE-5/6 may preferentially bind to the destabilizing protein and promote or mediate mRNA instability, whereas the stabilizing protein HuR preferentially binds to other regions outside of the ARE-5/6 context and mediates the inducible effect of IL-1β on RGS4 mRNA stability.
It is noteworthy that the translation inhibitor CHX alone induced dramatic upregulation of RGS4 mRNA expression. The molecular mechanisms remain poorly understood but may involve transcriptional and posttranscriptional events. The presence of short-lived labile repressor protein during the transcription process has been documented for CHX superinduction (64). The implication of transcriptional regulation is supported by the fact that actinomycin D significantly prevented CHX-induced upregulation of RGS4 mRNA. It is also supported by previous studies in many other genes such as thrombomodulin (11), c-fos(21), ICAM-1 (56), and G-CSF receptor (66), etc. Another possibility is RGS4 mRNA stability and cotranslational regulation. The present data support the concept that CHX may induce RGS4 upregulation by inhibiting the synthesis of destabilizing proteins involved in RGS4 mRNA instability. However, the “superinduction” effect by CHX in many other genes (3, 29, 40, 64) was not observed for IL-1β-induced RGS4 mRNA upregulation, implying a gene-specific mechanism for superinduction. In contrast, RGS4 mRNA in the presence of both IL-1β and CHX displayed a gradual decay. These data suggest that new protein synthesis is implicated in the IL-1β-induced stabilizing effect on RGS4 mRNA.
In conclusion, the present study provides the first evidence for the critical role of 3′-UTR in regulating mRNA instability of RGS proteins. We also demonstrated for the first time that IL-1β upregulates RGS4 mRNA expression by promoting HuR cytosolic translocation and mRNA binding. RGS4 is a novel target of HuR. HuR plays a pivotal role in regulating many genes related to inflammation and neural development. RGS4 is widely implicated in neural disorders, cardiovascular diseases, and inflammatory diseases. Therefore, HuR-mediated posttranscriptional regulation of RGS proteins will provide a new basis to understand the biological relevance and pharmaceutical development of targeting RGS proteins. Identification of the signaling pathways of HuR activation and function will advance our understanding of how inflammatory responses regulate smooth muscle contraction and neural behavior.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-075964 and DK-015564.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Abdelmohsen K, Lal A, Kim HH, Gorospe M. Posttranscriptional orchestration of an anti-apoptotic program by HuR. Cell Cycle 6: 1288– 1292, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Abramow-Newerly M, Roy AA, Nunn C, Chidiac P. RGS proteins have a signalling complex: interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell Signal 18: 579– 591, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bi Y, Lin GX, Millecchia L, Ma Q. Superinduction of metallothionein I by inhibition of protein synthesis: role of a labile repressor in MTF-1 mediated gene transcription. J Biochem Mol Toxicol 20: 57– 68, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Blackshear PJ. Tristetraprolin and other CCCH tandem zinc-finger proteins in the regulation of mRNA turnover. Biochem Soc Trans 30: 945– 952, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Bodenstein J, Sunahara RK, Neubig RR. N-terminal residues control proteasomal degradation of RGS2, RGS4, and RGS5 in human embryonic kidney 293 cells. Mol Pharmacol 71: 1040– 1050, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Bowden NA, Scott RJ, Tooney PA. Altered expression of regulator of G-protein signalling 4 (RGS4) mRNA in the superior temporal gyrus in schizophrenia. Schizophr Res 89: 165– 168, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Chemnitz J, Pieper D, Gruttner C, Hauber J. Phosphorylation of the HuR ligand APRIL by casein kinase 2 regulates CD83 expression. Eur J Immunol 39: 267– 279, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Cherry J, Karschner V, Jones H, Pekala PH. HuR, an RNA-binding protein, involved in the control of cellular differentiation. In Vivo 20: 17– 23, 2006 [PubMed] [Google Scholar]
- 9.Davis CA, Monnier JM, Nick HS. A coding region determinant of instability regulates levels of manganese superoxide dismutase mRNA. J Biol Chem 276: 37317– 37326, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Ding GJ, Fischer PA, Boltz RC, Schmidt JA, Colaianne JJ, Gough A, Rubin RA, Miller DK. Characterization and quantitation of NF-kappaB nuclear translocation induced by interleukin-1 and tumor necrosis factor-alpha. Development and use of a high capacity fluorescence cytometric system. J Biol Chem 273: 28897– 28905, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Dittman WA, Kumada T, Majerus PW. Transcription of thrombomodulin mRNA in mouse hemangioma cells is increased by cycloheximide and thrombin. Proc Natl Acad Sci USA 86: 7179– 7182, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doller A, Pfeilschifter J, Eberhardt W. Signalling pathways regulating nucleo-cytoplasmic shuttling of the mRNA-binding protein HuR. Cell Signal 20: 2165– 2173, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Emilsson L, Saetre P, Jazin E. Low mRNA levels of RGS4 splice variants in Alzheimer's disease: association between a rare haplotype and decreased mRNA expression. Synapse 59: 173– 176, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Erdely HA, Tamminga CA, Roberts RC, Vogel MW. Regional alterations in RGS4 protein in schizophrenia. Synapse 59: 472– 479, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Frevel MA, Bakheet T, Silva AM, Hissong JG, Khabar KS, Williams BR. p38 Mitogen-activated protein kinase-dependent and -independent signaling of mRNA stability of AU-rich element-containing transcripts. Mol Cell Biol 23: 425– 436, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fries B, Heukeshoven J, Hauber I, Gruttner C, Stocking C, Kehlenbach RH, Hauber J, Chemnitz J. Analysis of nucleocytoplasmic trafficking of the HuR ligand APRIL and its influence on CD83 expression. J Biol Chem 282: 4504– 4515, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Garnier M, Zaratin PF, Ficalora G, Valente M, Fontanella L, Rhee MH, Blumer KJ, Scheideler MA. Up-regulation of regulator of G protein signaling 4 expression in a model of neuropathic pain and insensitivity to morphine. J Pharmacol Exp Ther 304: 1299– 1306, 2003 [DOI] [PubMed] [Google Scholar]
- 18.George TC, Fanning SL, Fitzgeral-Bocarsly P, Medeiros RB, Highfill S, Shimizu Y, Hall BE, Frost K, Basiji D, Ortyn WE, Morrissey PJ, Lynch DH. Quantitative measurement of nuclear translocation events using similarity analysis of multispectral cellular images obtained in flow. J Immunol Methods 311: 117– 129, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Gherzi R, Lee KY, Briata P, Wegmuller D, Moroni C, Karin M, Chen CY. A KH domain RNA binding protein, KSRP, promotes ARE-directed mRNA turnover by recruiting the degradation machinery. Mol Cell 14: 571– 583, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Gloire G, Dejardin E, Piette J. Extending the nuclear roles of IkappaB kinase subunits. Biochem Pharmacol 72: 1081– 1089, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Greenberg ME, Hermanowski AL, Ziff EB. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol 6: 1050– 1057, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grillet N, Dubreuil V, Dufour HD, Brunet JF. Dynamic expression of RGS4 in the developing nervous system and regulation by the neural type-specific transcription factor Phox2b. J Neurosci 23: 10613– 10621, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grosset C, Chen CY, Xu N, Sonenberg N, Jacquemin-Sablon H, Shyu AB. A mechanism for translationally coupled mRNA turnover: interaction between the poly(A) tail and a c-fos RNA coding determinant via a protein complex. Cell 103: 29– 40, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Halees AS, El-Badrawi R, Khabar KS. ARED Organism: expansion of ARED reveals AU-rich element cluster variations between human and mouse. Nucleic Acids Res 36: D137– D140, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han MH, Renthal W, Ring RH, Rahman Z, Psifogeorgou K, Howland D, Birnbaum S, Young K, Neve R, Nestler EJ, Zachariou V. Brain region specific actions of regulator of G protein signaling 4 oppose morphine reward and dependence but promote analgesia. Biol Psychiatry 67: 761– 769, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hao J, Michalek C, Zhang W, Zhu M, Xu X, Mende U. Regulation of cardiomyocyte signaling by RGS proteins: differential selectivity towards G proteins and susceptibility to regulation. J Mol Cell Cardiol 41: 51– 61, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry 10: 40– 68; image 5, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Hendriks-Balk MC, van Loenen PB, Hajji N, Michel MC, Peters SL, Alewijnse AE. S1P receptor signalling and RGS proteins; expression and function in vascular smooth muscle cells and transfected CHO cells. Eur J Pharmacol 600: 1–9, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Hershko DD, Robb BW, Wray CJ, Luo GJ, Hasselgren PO. Superinduction of IL-6 by cycloheximide is associated with mRNA stabilization and sustained activation of p38 map kinase and NF-kappaB in cultured caco-2 cells. J Cell Biochem 91: 951– 961, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Hinman MN, Lou H. Diverse molecular functions of Hu proteins. Cell Mol Life Sci 65: 3168– 3181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooks SB, Martemyanov K, Zachariou V. A role of RGS proteins in drug addiction. Biochem Pharmacol 75: 76– 84, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Hu W, Huang J, Mahavadi S, Li F, Murthy KS. Lentiviral siRNA silencing of sphingosine-1-phosphate receptors S1P1 and S1P2 in smooth muscle. Biochem Biophys Res Commun 343: 1038– 1044, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Hu W, Li F, Mahavadi S, Murthy KS. Interleukin-1beta up-regulates RGS4 through the canonical IKK2/IkappaBalpha/NF-kappaB pathway in rabbit colonic smooth muscle. Biochem J 412: 35– 43, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu W, Li F, Mahavadi S, Murthy KS. Upregulation of RGS4 expression by IL-1beta in colonic smooth muscle is enhanced by ERK1/2 and p38 MAPK and inhibited by the PI3K/Akt/GSK3beta pathway. Am J Physiol Cell Physiol 296: C1310– C1320, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu W, Mahavadi S, Li F, Murthy KS. Upregulation of RGS4 and downregulation of CPI-17 mediate inhibition of colonic muscle contraction by interleukin-1beta. Am J Physiol Cell Physiol 293: C1991– C2000, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang C, Hepler JR, Gilman AG, Mumby SM. Attenuation of Gi- and Gq-mediated signaling by expression of RGS4 or GAIP in mammalian cells. Proc Natl Acad Sci USA 94: 6159– 6163, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang J, Zhou H, Mahavadi S, Sriwai W, Lyall V, Murthy KS. Signaling pathways mediating gastrointestinal smooth muscle contraction and MLC20 phosphorylation by motilin receptors. Am J Physiol Gastrointest Liver Physiol 288: G23– G31, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Huang J, Zhou H, Mahavadi S, Sriwai W, Murthy KS. Inhibition of Galphaq-dependent PLC-beta1 activity by PKG and PKA is mediated by phosphorylation of RGS4 and GRK2. Am J Physiol Cell Physiol 292: C200– C208, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Hurst JH, Mendpara N, Hooks SB. Regulator of G-protein signalling expression and function in ovarian cancer cell lines. Cell Mol Biol Lett 14: 153– 174, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joiakim A, Mathieu PA, Elliott AA, Reiners JJ., Jr Superinduction of CYP1A1 in MCF10A cultures by cycloheximide, anisomycin, and puromycin: a process independent of effects on protein translation and unrelated to suppression of aryl hydrocarbon receptor proteolysis by the proteasome. Mol Pharmacol 66: 936– 947, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Krumins AM, Barker SA, Huang C, Sunahara RK, Yu K, Wilkie TM, Gold SJ, Mumby SM. Differentially regulated expression of endogenous RGS4 and RGS7. J Biol Chem 279: 2593– 2599, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Lee MJ, Tasaki T, Moroi K, An JY, Kimura S, Davydov IV, Kwon YT. RGS4 and RGS5 are in vivo substrates of the N-end rule pathway. Proc Natl Acad Sci USA 102: 15030– 15035, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leontiadis LJ, Papakonstantinou MP, Georgoussi Z. Regulator of G protein signaling 4 confers selectivity to specific G proteins to modulate mu- and delta-opioid receptor signaling. Cell Signal 21: 1218– 1228, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Levitt P, Ebert P, Mirnics K, Nimgaonkar VL, Lewis DA. Making the case for a candidate vulnerability gene in schizophrenia: convergent evidence for regulator of G-protein signaling 4 (RGS4). Biol Psychiatry 60: 534– 537, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Li F, Murthy KS, Khalili K, Hu W. Cloning and characterization of rabbit Rgs4 promoter in gut smooth muscle. Gene 451: 45– 53, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lipska BK, Mitkus S, Caruso M, Hyde TM, Chen J, Vakkalanka R, Straub RE, Weinberger DR, Kleinman JE. RGS4 mRNA expression in postmortem human cortex is associated with COMT Val158Met genotype and COMT enzyme activity. Hum Mol Genet 15: 2804– 2812, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Lopez de Silanes I, Galban S, Martindale JL, Yang X, Mazan-Mamczarz K, Indig FE, Falco G, Zhan M, Gorospe M. Identification and functional outcome of mRNAs associated with RNA-binding protein TIA-1. Mol Cell Biol 25: 9520– 9531, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopez de Silanes I, Zhan M, Lal A, Yang X, Gorospe M. Identification of a target RNA motif for RNA-binding protein HuR. Proc Natl Acad Sci USA 101: 2987– 2992, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mbonye UR, Song I. Posttranscriptional and posttranslational determinants of cyclooxygenase expression. BMB Rep 42: 552– 560, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Mittmann C, Chung CH, Hoppner G, Michalek C, Nose M, Schuler C, Schuh A, Eschenhagen T, Weil J, Pieske B, Hirt S, Wieland T. Expression of ten RGS proteins in human myocardium: functional characterization of an upregulation of RGS4 in heart failure. Cardiovasc Res 55: 778– 786, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Mittmann C, Schuler C, Chung CH, Hoppner G, Nose M, Kehrl JH, Wieland T. Evidence for a short form of RGS3 preferentially expressed in the human heart. Naunyn Schmiedebergs Arch Pharmacol 363: 456– 463, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Murthy KS, Makhlouf GM. Differential coupling of muscarinic m2 and m3 receptors to adenylyl cyclases V/VI in smooth muscle. Concurrent M2-mediated inhibition via Galphai3 and m3-mediated stimulation via Gbetagammaq. J Biol Chem 272: 21317– 21324, 1997 [DOI] [PubMed] [Google Scholar]
- 53.Murthy KS, Makhlouf GM. Opioid mu, delta, and kappa receptor-induced activation of phospholipase C-beta 3 and inhibition of adenylyl cyclase is mediated by Gi2 and G(o) in smooth muscle. Mol Pharmacol 50: 870– 877, 1996 [PubMed] [Google Scholar]
- 54.Nikolova DN, Zembutsu H, Sechanov T, Vidinov K, Kee LS, Ivanova R, Becheva E, Kocova M, Toncheva D, Nakamura Y. Genome-wide gene expression profiles of thyroid carcinoma: identification of molecular targets for treatment of thyroid carcinoma. Oncol Rep 20: 105– 121, 2008 [PubMed] [Google Scholar]
- 55.Noursadeghi M, Tsang J, Haustein T, Miller RF, Chain BM, Katz DR. Quantitative imaging assay for NF-kappaB nuclear translocation in primary human macrophages. J Immunol Methods 329: 194– 200, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohh M, Takei F. Regulation of ICAM-1 mRNA stability by cycloheximide: role of serine/threonine phosphorylation and protein synthesis. J Cell Biochem 59: 202– 213, 1995 [DOI] [PubMed] [Google Scholar]
- 57.Patten M, Bunemann J, Thoma B, Kramer E, Thoenes M, Stube S, Mittmann C, Wieland T. Endotoxin induces desensitization of cardiac endothelin-1 receptor signaling by increased expression of RGS4 and RGS16. Cardiovasc Res 53: 156– 164, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Patten M, Stube S, Thoma B, Wieland T. Interleukin-1beta mediates endotoxin- and tumor necrosis factor alpha-induced RGS16 protein expression in cultured cardiac myocytes. Naunyn Schmiedebergs Arch Pharmacol 368: 360– 365, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Prechtel AT, Chemnitz J, Schirmer S, Ehlers C, Langbein-Detsch I, Stulke J, Dabauvalle MC, Kehlenbach RH, Hauber J. Expression of CD83 is regulated by HuR via a novel cis-active coding region RNA element. J Biol Chem 281: 10912– 10925, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Rabani M, Kertesz M, Segal E. Computational prediction of RNA structural motifs involved in posttranscriptional regulatory processes. Proc Natl Acad Sci USA 105: 14885– 14890, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riddle EL, Schwartzman RA, Bond M, Insel PA. Multi-tasking RGS proteins in the heart: the next therapeutic target? Circ Res 96: 401– 411, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Roman DL, Talbot JN, Roof RA, Sunahara RK, Traynor JR, Neubig RR. Identification of small-molecule inhibitors of RGS4 using a high-throughput flow cytometry protein interaction assay. Mol Pharmacol 71: 169– 175, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Runne H, Regulier E, Kuhn A, Zala D, Gokce O, Perrin V, Sick B, Aebischer P, Deglon N, Luthi-Carter R. Dysregulation of gene expression in primary neuron models of Huntington's disease shows that polyglutamine-related effects on the striatal transcriptome may not be dependent on brain circuitry. J Neurosci 28: 9723– 9731, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sakata Y, Yoshioka W, Tohyama C, Ohsako S. Internal genomic sequence of human CYP1A1 gene is involved in superinduction of dioxin-induced CYP1A1 transcription by cycloheximide. Biochem Biophys Res Commun 355: 687– 692, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell 46: 659– 667, 1986 [DOI] [PubMed] [Google Scholar]
- 66.Steinman RA, Tweardy DJ. Granulocyte colony-stimulating factor receptor mRNA upregulation is an immediate early marker of myeloid differentiation and exhibits dysfunctional regulation in leukemic cells. Blood 83: 119– 127, 1994 [PubMed] [Google Scholar]
- 67.Takata Y, Liu J, Yin F, Collins AR, Lyon CJ, Lee CH, Atkins AR, Downes M, Barish GD, Evans RM, Hsueh WA, Tangirala RK. PPARdelta-mediated antiinflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis. Proc Natl Acad Sci USA 105: 4277– 4282, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Veyrune JL, Carillo S, Vie A, Blanchard JM. c-fos mRNA instability determinants present within both the coding and the 3′ non coding region link the degradation of this mRNA to its translation. Oncogene 11: 2127– 2134, 1995 [PubMed] [Google Scholar]
- 69.Wang Q, Liu-Chen LY, Traynor JR. Differential modulation of mu- and delta-opioid receptor agonists by endogenous RGS4 protein in SH-SY5Y cells. J Biol Chem 284: 18357– 18367, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Willars GB. Mammalian RGS proteins: multifunctional regulators of cellular signalling. Semin Cell Dev Biol 17: 363– 376, 2006 [DOI] [PubMed] [Google Scholar]
- 71.Wisdom R, Lee W. The protein-coding region of c-myc mRNA contains a sequence that specifies rapid mRNA turnover and induction by protein synthesis inhibitors. Genes Dev 5: 232– 243, 1991 [DOI] [PubMed] [Google Scholar]
- 72.Xie GX, Palmer PP. How regulators of G protein signaling achieve selective regulation. J Mol Biol 366: 349– 365, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie Y, Wolff DW, Wei T, Wang B, Deng C, Kirui JK, Jiang H, Qin J, Abel PW, Tu Y. Breast cancer migration and invasion depend on proteasome degradation of regulator of G-protein signaling 4. Cancer Res 69: 5743– 5751, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yarovinsky TO, Butler NS, Monick MM, Hunninghake GW. Early exposure to IL-4 stabilizes IL-4 mRNA in CD4+ T cells via RNA-binding protein HuR. J Immunol 177: 4426– 4435, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Zhang W, Wagner BJ, Ehrenman K, Schaefer AW, DeMaria CT, Crater D, DeHaven K, Long L, Brewer G. Purification, characterization, and cDNA cloning of an AU-rich element RNA-binding protein, AUF1. Mol Cell Biol 13: 7652– 7665, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang X, Zou T, Rao JN, Liu L, Xiao L, Wang PY, Cui YH, Gorospe M, Wang JY. Stabilization of XIAP mRNA through the RNA binding protein HuR regulated by cellular polyamines. Nucleic Acids Res 37: 7623– 7637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou H, Jarujaron S, Gurley EC, Chen L, Ding H, Studer E, Pandak WM, Jr, Hu W, Zou T, Wang JY, Hylemon PB. HIV protease inhibitors increase TNF-alpha and IL-6 expression in macrophages: involvement of the RNA-binding protein HuR. Atherosclerosis 195: e134– e143, 2007 [DOI] [PubMed] [Google Scholar]