Abstract
We examined the effects of fluid shear stress on metallothionein (MT) gene and protein expression and intracellular free zinc in mouse aorta and in human umbilical vein endothelial cells (HUVECs). Immunostaining of the endothelial surface of mouse aorta revealed increased expression of MT protein in the lesser curvature of the aorta relative to the descending thoracic aorta. HUVECs were exposed to high steady shear stress (15 dyn/cm2), low steady shear stress (1 dyn/cm2), or reversing shear stress (mean of 1 dyn/cm2, 1 Hz) for 24 h. Gene expression of three MT-1 isoforms, MT-2A, and zinc transporter-1 was upregulated by low steady shear stress and reversing shear stress. HUVECs exposed to 15 dyn/cm2 had increased levels of free zinc compared with cells under other shear stress regimes and static conditions. The increase in free zinc was partially blocked with an inhibitor of nitric oxide synthesis, suggesting a role for shear stress-induced endothelial nitric oxide synthase activity. Cells subjected to reversing shear stress in zinc-supplemented media (50 μM ZnSO4) had increased intracellular free zinc, reduced surface intercellular adhesion molecule-1 expression, and reduced monocyte adhesion compared with cells exposed to reversing shear stress in normal media. The sensitivity of intracellular free zinc to differences in shear stress suggests that intracellular zinc levels are important in the regulation of the endothelium and in the progression of vascular disease.
Keywords: endothelial cells, vascular biology
atherosclerosis is primarily localized to the carotid artery sinus, the coronary arteries, the abdominal aorta, and the superficial femoral arteries (45). The vessel wall at these atheroprone locations is exposed to complex blood flow patterns including recirculation (reversing flow and low time-average wall shear stress) in contrast to the nonreversing shear stress that occurs in most of the arteries. Exposure to disturbed hemodynamics, compared with nonreversing arterial shear stress, alters the gene expression profile and ultimately the structure and function of endothelial cells, priming them for atherogenesis (8, 16).
Metallothioneins (MTs) are heavy-metal binding proteins that predominantly bind zinc in mammals and are responsible for regulating intracellular free zinc levels (27). We found that MTs, particularly the MT-1 family and MT-2A, are twofold downregulated by nonreversing pulsatile shear stress compared with steady shear stress (46). Our microarray studies (10), as well as those of Dai et al. (14), showed increased MT-1 and MT-2A mRNA expression in human umbilical vein endothelial cells (HUVECs) exposed to reversing shear stress compared with cells exposed to nonreversing shear stress. Regulation of MTs by shear stress was also reported by Ohura et al. (31) who showed downregulation of MT expression in cells exposed to turbulent flow compared with high steady laminar flow. These observations indicate that MT gene expression is highly sensitive to variations in shear stress.
The intracellular concentration of free (unbound) zinc is typically very low and highly regulated. Zinc binds with high affinity to metalloenzymes, structural proteins, and transcription factors (12). Zinc has been estimated to be required for the function of over 2,000 transcription factors and 300 enzymes, making many signaling pathways dependent on zinc (6). Large increases in extracellular zinc activate metal-responsive transcription factor-1 (MTF-1), which increases transcription of genes involved in zinc transport and binding, including several MT isoforms, and zinc transporter-1 (ZnT-1), which is responsible for the efflux of zinc across the cell membrane (12). Increases in free zinc were previously reported in endothelial cells stimulated by hydrogen peroxide (43) or hypoxia (7). Zinc deficiency has been shown to increase monocyte adhesion (37, 38) and upregulate nuclear factor-κB (NF-κB) in cultured endothelial cells (23), even though NF-κB does not contain structural zinc (22). Exposure to nitric oxide (NO) has been shown to increase free zinc in endothelial cells (44), and intracellular free zinc release by NO protects endothelial cells from H2O2-induced toxicity (11).
Epidemiological studies (2, 39) have shown that low serum zinc levels are associated with coronary artery disease. Zinc supplementation was found to reduce the development of atherosclerosis in rabbits (1, 26, 36) but not in apolipoprotein E (−/−) mice (33). LDL receptor (−/−) mice fed a zinc-deficient diet had increased serum levels of VLDL and HDL and had increased vascular cell adhesion molecule-1 (VCAM-1) in the thoracic aorta (35). Conflicting results on the role of zinc have been found in postmortum studies: one study (42) found decreased zinc in the abdominal aorta in patients who died of cardiovascular disease compared with other causes, while another study (40) found elevated zinc in advanced lesions that correlated with lesion calcification. Mechanistic insight from in vivo studies has been limited because of the inability to link indirect measurements of zinc (zinc dietary intake, zinc serum levels, or zinc mineralization) to changes in intracellular zinc levels in arteries.
We found that MT protein expression increased in atheroprone areas of the mouse aorta. In vitro, we found increased MT mRNA expression in HUVECs subjected to reversing shear stress, as well as low steady shear stress. We observed a decrease in free zinc in endothelial cells exposed to reversing shear stress relative to 15 dyn/cm2. In addition, the increased monocyte binding to HUVECs pretreated with reversing shear stress was reduced when the cells were pretreated with reversing shear stress in zinc-supplemented medium.
The mechanosensitivity of intracellular free zinc to differences in shear stress suggests that zinc levels may represent an important signaling pathway in the regulation of the endothelium and in the progression of vascular disease. Furthermore, our results suggest a new mechanism for the role of MT and zinc in atherosclerosis in which endothelial cells exposed to reversing shear stress have elevated MT gene expression resulting in reduced intracellular free zinc, possibly priming these cells towards atherosclerosis.
METHODS
Mouse aorta immunohistochemistry.
C57BL/6 mice (6- to 8 wk-old males; The Jackson Laboratory, Bar Harbor, ME) were euthanized by CO2 inhalation, and the aortas were perfusion fixed with 10% formalin. The aortas were carefully cleaned in situ, and the arches and thoracic aortas were dissected and stained with rabbit-anti-human MT-1G (1:100; Santa Cruz). Due to the strong protein sequence homology across MT family 1 and 2 isoforms (13, 28), it was assumed that the MT-1G antibody was reactive against all human and mouse MT family 1 and 2 isoforms. Immunoreactive MT-1G was detected with goat anti-rabbit IgG (1:250; Alexa Fluor 568; Invitrogen). Along with primary antibody, all samples were counterstained with antibody against CD31 using rat anti-mouse IgG (1:100; Santa Cruz), followed by counterstaining with goat anti-rat IgG (1:250; Alexa Fluor 488). The aortas were opened and separated into regions of lesser curvature, greater curvature, and thoracic artery. En face images were collected with a LSM 510 META confocal microscope (Zeiss, Jena, Germany). Laser, filter, and imaging conditions were the same in all images analyzed. Mean fluorescence intensity of representative fields from both thoracic and lesser curvature regions of three aortas were measured using Image J and analyzed for differences with a paired t-test. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, Revised 1996). Approval for use of animal studies was provided by the Emory University Institutional Animal Care and Use Committee.
Cell culture and shear stress.
HUVECs (Lonza, Walkersville, MD) were grown and subjected to shear stress as previously described (9, 10). Pooled HUVECs (Lonza), passage 3–5, were grown in M199 media (Mediatech) supplemented with 20% (vol/vol) FBS (Hyclone), 50 ug/ml endothelial mitogen (Biomedical Technologies, Stoughton, MA), 2 mM l-glutamine (Mediatech), 2.5 U/ml heparin sodium (American Pharmaceutical Partners), 50 U/ml penicillin, and 50 μg/ml streptomycin (Mediatech). Cells were seeded (20,000 cells/cm2) on glass slides and then after 36 to 48 h were subjected to 24 h of one of four conditions: reversing shear stress (nonharmonic, 11 dyn/cm2 maximum, −11 dyn/cm2 minimum, −1 dyn/cm2 average, 1 Hz), steady arterial shear stress (15 dyn/cm2), low steady shear stress (1 dyn/cm2), and static culture. In the indicated experiments, 100 μM NG-nitro-l-arginine methyl ester (l-NAME; Sigma) was added to the media at the beginning of application of shear stress to inhibit NO production. Nitrite/nitrate measurements were made with the nitrate/nitrite fluorometric assay kit (Cayman Chemical). In the zinc supplementation experiments, cell culture media were supplemented with 50 μM ZnSO4.
Quantitative real-time PCR.
Immediately after exposure to shear stress, total RNA and protein were extracted using TRI-zol (Invitrogen) and RNA was further purified with DNase (Qiagen, Valencia, CA) and RNeasy MinElute cleanup kit (Qiagen), according to manufacturers' instructions. The integrity and quantity of the RNA were verified with ultraviolet spectrophotometry, accepting only RNA with a 260/280 ratio >1.9.
For quantitative real-time PCR, total RNA was reverse transcribed into cDNA with SuperScript II (Invitrogen) according the manufacturer's instructions. The resulting cDNA was purified through Micro Bio-Spin P-30 chromatography columns (Bio-Rad, Hercules, CA) and diluted 1:20. quantitative (q)RT-PCR primers used were ZnT-1 (forward: 5′-tacatggaggtggctaaaacca-3′; reverse: 5′-tgtcccacaacattgcttcaaa-3′), MT-1E (forward: 5′-gcttgttcgtctcactggtg-3′; reverse: 5′-caggttgtgcaggttgttcta-3′), MT-1F (MT-1F predesigned PCR primer; Qiagen), MT-1G (forward: 5′-cttctcgcttgggaactcta-3′; reverse: 5′-aggggtcaagattgtagcaaa-3′), MT-2A (forward: 5′-ccgactctagccgcctctt-3′; reverse: 5′-gtggaagtcgcgttctttaca-3′), and 18S (QuantumRNA Classical II; Ambion). qRT-PCR reactions and analysis were performed on a MyiQ (Bio-Rad) using iQ SYBR Green Supermix (Bio-Rad) according to the manufacturer's instructions. The qRT-PCR reactions and analysis were performed on a MyiQ (Bio-Rad) with a 3-min initial denaturation step at 95°C; 45 cycles (30 cycles for 18 s) at 95°C for 5 s, 55°C for 10 s, 72°C for 60 s/kbp product; and a ramped melting cycle. Samples were normalized by 18S expression. Fold changes were determined using the ΔΔCt method.
Zinc staining.
Following shear stress, cells were rinsed with PBS and then incubated with 2.5 μM FluoZin-3-AM (Invitrogen), a zinc-sensitive cell-permeant dye (17, 18), in PBS for 15 min. Cells were rinsed with PBS and imaged directly on glass slides using a standard fluorescent microscope. For flow cytometry studies, following shear stress, cells were trypsinized, centrifuged, and then resuspended in 2.5 μM FluoZin-3-AM in PBS (without calcium) for 30 min, after which cells were centrifuged and resuspended in PBS. All samples were analyzed on a BD LSR flow cytometer (Becton-Dickinson, Franklin Lakes, NJ) using BD FACSDiVa software. A total of 10,000 events was collected on a log scale. Data presented are the normalized mean fluorescence intensity. To confirm uniform intracellular loading of FluoZin-3 across samples, cells were exposed to 200 μM ZnSO4 and 50 μM pyrithione (a zinc ionophore). To confirm zinc specificity of FluoZin-3-AM, cells were simultaneously incubated with 30 μM N,N,N′,N′-tetrakis-(2-pyridylmethyl)-ethylenediamine (TPEN), a zinc-specific chelator.
Monocyte adhesion assay.
Monocyte adhesion assays were performed on HUVECs pretreated for 24 h with reversing shear stress as previously described (10). CellTracker Green CMFDA (Invitrogen)-stained THP-1 (human acute monocytic leukemia cell line) cells (106 cells/ml) were perfused across the pretreated HUVECs at 1 dyn/cm2 for 2 min, flow was stopped for 30 s and then the cells were perfused with media for an additional 5 min at 1 dyn/cm2. The number of adherent cells was counted in 10 frames for each replicate.
Intercellular adhesion molecule-1 and VCAM-1 surface expression.
After exposure to shear stress, endothelial cells were trypsinized and resuspended in FACS buffer (110 mM NaCl, 10 mM KCl, 1 mM MgCl2, 10 mM glucose, 30 mM HEPES, and 1.5 mM CaCl2) containing 1.0% (vol/vol) human serum albumin (CSL Behring, Kankakee, IL). Endothelial cells were labeled with antibodies specific for intercellular adhesion molecule-1 (ICAM-1; FITC; R&D Systems) and VCAM-1 (AF688; Santa Cruz) at 4°C for 1 h in the dark. All samples were analyzed on a BD LSR flow cytometer (Becton-Dickinson) using BD FACSDiVa software. A total of 10,000 events was collected on a log scale. Data presented are the normalized mean fluorescence intensity.
Statistics.
In experiments with more than two conditions, samples were analyzed using one-way ANOVA, followed by Newman-Keuls multiple comparison test. Metallothionein mRNA measurements were first subjected to a log transformation before statistical analysis to equalize the uneven variance between shear stress treatments (see Fig. 2). In experiments with only two conditions, samples were analyzed using Student's paired t-test. In all statistical analysis, P < 0.05 was considered significant.
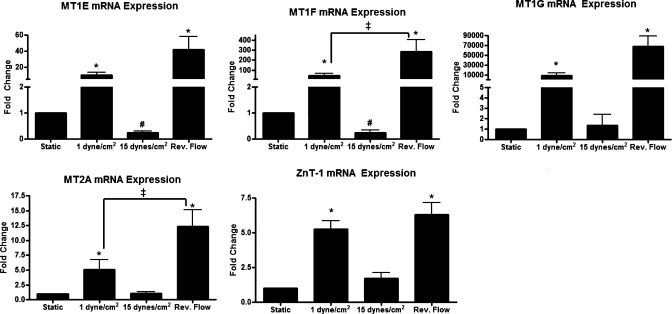
Fig. 2.
MT and zinc transporter-1 (Znt-1) expression is upregulated by reversing shear stress. mRNA expression of MT-1E, MT-1F, MT-1G, MT-2A, and ZnT-1 was increased in cells exposed to reversing shear stress and 1 dyn/cm2 (n = 5; #P < 0.05, compared with static culture; *P < 0.05, compared with both static and 15 dyn/cm2; ‡significant difference between indicated conditions).
RESULTS
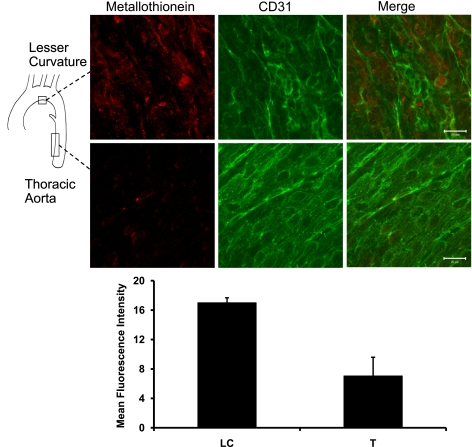
Immunofluorescent staining of MT on the endothelial surface of the mouse aorta (Fig. 1) revealed that MT protein expression was elevated in the region of lesser curvature, characterized by reversing flow patterns, compared with the thoracic aorta, which is characterized by laminar flow (41). Control samples (without primary antibody) showed no positive staining (data not shown). Mean fluorescence intensity was measured in three fields from each of three aortas, revealing a more than twofold increase in lesser curvature staining compared with thoracic aorta (data are means ± SE for the LC areas = 17.0 ± 0.68 and for T areas = 7.06 ± 2.54; P = 0.035).
Fig. 1.
Metallothionein (MT) protein expression in mouse aorta is elevated in the area of lesser curvature. Whole mouse aortas were stained for MT-1G (n = 3), and the lesser curvature and thoracic aorta were mounted en face. Endothelial cell integrity was confirmed with CD31 counterstaining. Scale bars are 20 μm. Mean intensity of representative fields from both thoracic (T) and lesser curvature (LC) areas of 3 aortas were measured using Image J and analyzed for differences using a paired t-test. Data are means ± SE for the LC areas = 17.0 ± 0.68 and for T areas = 7.06 ± 2.54 (P = 0.035).
In vitro, MT-1E, MT-1F, MT-1G, MT-2A, and ZnT-1 mRNA were most highly expressed in HUVECs exposed to reversing shear stress and were also elevated in endothelial cells exposed to 1 dyn/cm2 (Fig. 2). High steady shear stress (15 dyn/cm2) and static conditions had low gene expression of MT isoforms and ZnT-1. MT-1E and MT-1F were significantly decreased in cells exposed to 15 dyn/cm2 compared with static cells, whereas there were no significant differences between these conditions for MT-1G, MT-2A, and ZnT-1. A comparison of the relative expression of the four isoforms is shown in Supplemental Fig. 1 (Supplemental Material for this article is available online at the Am J Physiol-Cell Physiol website), in which MT-2A was the most expressed isoform in all three shear stress conditions and static culture.
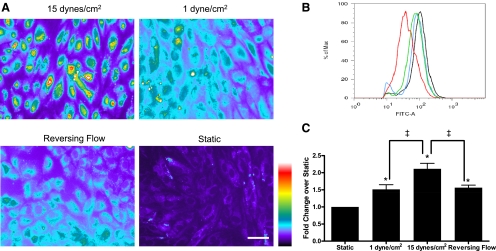
Intracellular free zinc levels, as measured by FluoZin-3-AM staining, were increased in cells exposed to high steady shear stress, reversing shear stress, and low steady shear stress compared with cells exposed to static culture (Fig. 3A; see Supplemental Fig. 2 for the original images). To quantitate free zinc levels, cells were stained with FluoZin-3-AM and analyzed by flow cytometry (Fig. 3, B and C). Consistent with the fluorescent images in Fig. 3A, cells exposed to 15 dyn/cm2 had the highest free zinc level, which was attenuated under 1 dyn/cm2 and reversing shear stress. All shear stressed cells had increased free zinc compared with cells grown in static culture. Similar fluorescence levels were observed across all experimental conditions when cells were exposed to exogenous zinc and the zinc ionophore pyrithione (Supplemental Fig. 3), confirming equal intracellular loading of FluoZin-3-AM. Specificity of FluoZin-3-AM to zinc was confirmed by reduced fluorescence in the presence of the zinc chelator TPEN (Supplemental Fig. 3).
Fig. 3.
Free zinc levels are increased in human umbilical vein endothelial cells (HUVECs) exposed to shear stress. HUVECs subjected to 24-h shear stress treatment were stained with FluoZin-3-AM. A: fluorescent images were converted to pseudocolor to show fluorescence intensity (scale bar is 50 μm). B: representative flow cytometry profile (red, static; blue, reversing flow; green, 1 dyn/cm2; black, 15 dyn/cm2). C: histograms showing mean fluorescence (n = 7; *P < 0.01, compared with static culture; ‡P < 0.01, between indicated conditions). Free zinc is highest under 15 dyn/cm2, attenuated under 1 dyn/cm2 and reversing flow, and lowest under static conditions.
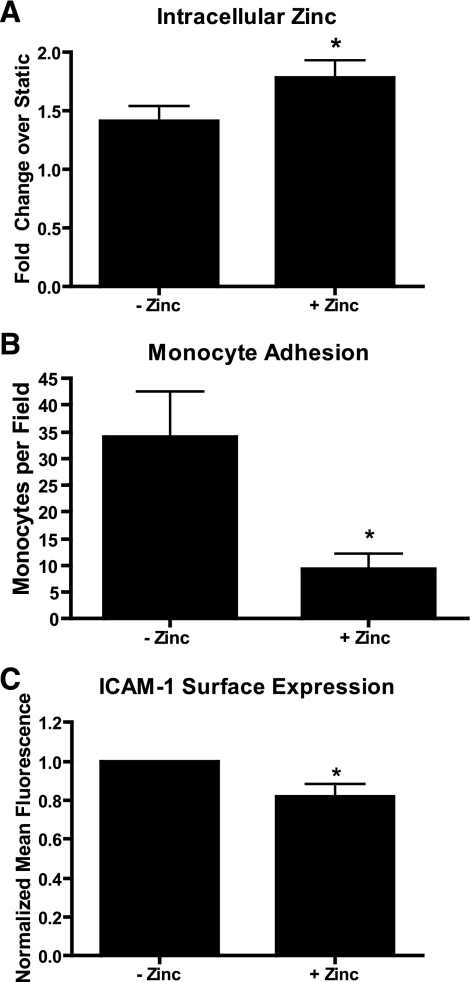
Cells were exposed to reversing shear stress in the presence of media supplemented with 50 μM ZnSO4, which is estimated to be a 10-fold increase over nonsupplemented media [FBS, the primary source of zinc in cell culture media, has a zinc concentration of ∼25 μM (12); thus the zinc concentration in media supplemented with 20% (vol/vol) FBS is 5 μM]. A previous study (12) showed there is no change in the viability of endothelial cells exposed to media supplemented with up to 200 μM ZnSO4. Cells in zinc supplemented media exhibited increased intracellular free zinc compared with cells exposed to reversing shear stress in nonsupplemented media (Fig. 4A). Zinc-supplemented cells also had reduced monocyte adhesion (Fig. 4B) and reduced ICAM-1 surface expression (Fig. 4C). Zinc supplementation did not affect VCAM-1 surface expression (data not shown).
Fig. 4.
Addition of extracellular zinc inhibits the effects of reversing shear stress. Cells were exposed to reversing shear stress in regular media or media supplemented 50 μM ZnSO4 (n = 5; *P < 0.05). Cells shear stressed in zinc supplemented media had significantly increased levels of free zinc (A), reduced monocyte adhesion (B), and reduced intercellular adhesion molecule-1 (ICAM-1) surface expression (C).
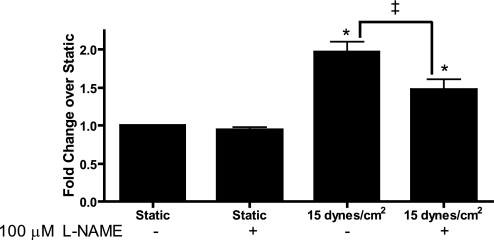
Free zinc levels were significantly attenuated in cells subjected to 15 dyn/cm2 shear stress the presence of 100 μM l-NAME, which inhibits NO synthesis, suggesting that NO may regulate increases in free zinc (Fig. 5). l-NAME completely blocked shear-stress mediated increases in NO as measured by nitrate and nitrite formation (data not shown).
Fig. 5.
Inhibition of nitric oxide attenuates the free zinc increase by shear stress. Cells were exposed to 24 h of either 15 dyn/cm2 or static culture in the presence or absence of 100 μM NG-nitro-l-arginine methyl ester (l-NAME) to inhibit nitric oxide (n = 4). Inhibiting nitric oxide resulted in decreased free zinc only in endothelial cells exposed to shear stress (*P < 0.05, between sample and static; ‡P < 0.05, between indicated conditions).
DISCUSSION
We have demonstrated increased levels of MT protein in an atheroprone area of the mouse aorta (Fig. 1) and increased MT isoform mRNA expression in endothelial cells subjected to atheroprone patterns of shear stress (1 dyn/cm2 or reversing shear stress; Fig. 2). We have shown in functional studies that relative to 15 dyn/cm2, intracellular free zinc levels are significantly lower under both reversing flow and 1 dyn/cm2 (Fig. 3). Furthermore, zinc supplementation decreases monocyte binding in endothelial cells subjected to reversing shear stress (Fig. 4B).
To our knowledge, this is the first report of both MT gene expression and intracellular free zinc changing in response to mechanical force in endothelial cells, suggesting that MTs represent a novel mechanotransduction signaling pathway. Previous reports have shown that intracellular zinc changes can affect transcription factor activity (12) and may be important in mediating protein/protein interactions (29). The increase in free zinc levels in endothelial cells subjected to all shear stress regimens (Fig. 3) may alter transcription factor and enzymatic activity, contributing to shear stress-mediated gene expression changes (10). Furthermore, the addition of l-NAME, a NO synthase inhibitor, to cells subjected to 15 dyn/cm2 caused a significant attenuation of the free zinc increase (Fig. 5), suggesting that shear stress-induced production of NO (34) is partially responsible for the increase in free zinc. However, l-NAME did not significantly affect the expression of MT in cells subjected to static or 15 dyn/cm2 shear stress (data not shown), further supporting the hypothesis that NO mediates zinc release from MT without affecting MT expression. Zinc release from MTs has previously been shown to be NO sensitive (7), suggesting that MTs may be the source of NO-induced zinc release under shear stress. l-NAME did not completely block the increase in free zinc observed under shear stress, suggesting there are additional unknown mechanism(s) that contribute to the increases in zinc.
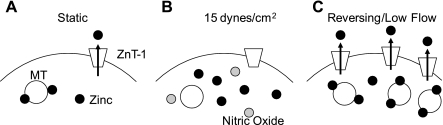
The atheroprone regimens of low steady or reversing shear stress upregulated gene expression of four MT isoforms and ZnT-1 compared with cells exposed to high steady shear stress (Fig. 2). Our finding that free zinc levels are reduced under reversing shear stress compared with high steady shear stress (Fig. 3) is consistent with the MT and ZnT-1 results, because it suggests that free zinc levels would be decreased in endothelial cells in atheroprone regions of the vasculature. The finding that reversing shear stress increases the gene expression of MTs and ZnT-1 (Fig. 2) offers a possible mechanism for this decrease. Additionally, it has been shown previously (48) that NO is reduced under oscillatory shear stress compared with cells exposed to arterial levels of unidirectional shear stress. Increases in MT expression and decreased NO could lead to increased protein binding of zinc, and increases in ZnT-1 could lead to increased cellular efflux of zinc, which together reduce the levels of intracellular free zinc (Fig. 6).
Fig. 6.
Proposed mechanism for zinc regulation in endothelial cells. A: under static culture conditions, zinc (black circles) is primarily bound to MT and effluxed out of the cell across the cell membrane by ZnT-1. B: under high shear stress, nitric oxide (gray circles) causes zinc release from MT. C: under reversing shear stress or low shear stress, there is increased expression of MT and ZnT-1, resulting in increased bound zinc and zinc efflux thereby lowering the levels of free zinc.
Changes in expression of multiple MT isoforms and in ZnT-1 (Fig. 2) suggest the possibility that the transcription factor MTF-1 is activated in cells exposed to reversing flow. In addition to activation by zinc, MTF-1 has been shown to be activated in response to increased oxidative stress (3). Since reversing shear stress leads to increased reactive oxygen species (25), it is possible that oxidative stress could be contributing to the upregulation of MT and ZnT-1, thereby affecting free zinc levels in the cell. The large increase in MT expression under reversing shear stress may be protective, as increased expression of MT is associated with resistance to oxidative stress (30).
Our in vitro results suggest that endothelial cells exposed to reversing wall shear stress in vivo (e.g., at sites prone to the development of atherosclerosis) have less intracellular free zinc. We observed increased MT protein expression in the lesser curvature of the mouse aortic arch compared with the thoracic aorta (Fig. 1), which strongly suggests that there would be regional differences in free zinc in the mouse aorta. The lesser curvature of the mouse aorta experiences reversing shear stress and is prone to the development of lipid deposits in mice lacking LDL receptors or apolipoprotein E (41). Supporting our results of increased MT expression at sites of disturbed flow, two previous studies (15, 21) also showed increased MT expression in smooth muscle cells of human atherosclerotic lesions, but neither study detected MT expression in the endothelium of the lesion. One possible explanation for the absence of MT expression in the lesion samples in these studies is that the endothelium was damaged or dysfunctional as a result of the advanced stage of atherosclerosis. Supporting our MT-positive endothelial immunostaining results, other studies have observed expression of MT in endothelial cells (4) and shown that MTs have important endothelial functions such as promoting angiogenesis (47) and protecting against ischemia-reperfusion injury in the heart (32).
The implications of changes in MTs and free zinc are not fully understood, but we suggest that changes could affect the regulation of zinc-sensitive transcription factors and enzymes. Recently, it was shown that nuclear factor (erythroid-derived 2)-like 2 (nrf-2), a transcription factor regulated by shear stress (24), is activated by increased free zinc (12). Zinc has also been shown to be important in reducing inflammation in endothelial cells in vivo (35) and in vitro (38) and atherosclerosis in rabbits (1, 26, 36). A possible explanation for the protective effects of zinc is that zinc supplementation could lead to increases in free zinc levels within the cell and thus counteracting decreases in free zinc in endothelial cells exposed to reversing flow. In an effort to better understand the zinc-related shear stress signaling pathways, we performed shear stress experiments in the presence of TPEN, a zinc specific chelator; however, 9 μM TPEN (the highest concentration at which cells survived 24 h shear stress) did not block the shear stress-induced intracellular zinc levels (data not shown). It is unclear if the cytotoxicity of TPEN arises from the chelation of zinc or results from other nonspecific effects.
In this study, we show that endothelial cells exposed to proatherogenic shear stress have decreased intracellular free zinc. Numerous studies (1, 2, 22, 23, 26, 33, 35, 36, 38–40, 42) have suggested that zinc is anti-atherogenic, but no clear mechanism has been postulated. We propose that increases in zinc-binding proteins, such as MTs, in endothelial cells subjected to reversing shear stress may prime these cells towards atherosclerosis by altering zinc-sensitive signaling pathways and gene expression. Although intracellular free zinc levels are highly regulated and are typically insensitive to minor changes in dietary zinc intake, we show that the addition of excess zinc to cells exposed to reversing shear stress resulted in a significant increase in intracellular free zinc (Fig. 4A), offering a possible explanation for the previously observed atheroprotective effect of increased dietary zinc (1, 2, 5, 26, 36, 39). We confirmed that the level of zinc supplementation under reversing flow did not change MT expression (data not shown), indicating that the protective effects of zinc supplementation are not due to changes in MT expression. Recent work (19, 20) has shown single nucleotide polymorphisms in MT-1A and MT-2A are more prevalent in patients with diabetes and are associated with diabetic-induced cardiovascular complications. Interestingly, the single nucleotide polymorphisms in MT-1A showed reduced zinc release in the presence of NO (19), suggesting that impaired zinc release may be associated with cardiovascular disease. Future studies should seek to examine the changes in and regulation of intracellular free zinc during the development and progression of cardiovascular disease.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-18672 and HL-70537.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Yumiko Sakurai for technical assistance and helpful discussion.
REFERENCES
- 1.Alissa EM, Bahijri SM, Lamb DJ, Ferns GA. The effects of coadministration of dietary copper and zinc supplements on atherosclerosis, antioxidant enzymes and indices of lipid peroxidation in the cholesterol-fed rabbit. Int J Exp Pathol 85: 265– 275, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alissa EM, Bahjri SM, Ahmed WH, Al-Ama N, Ferns GA. Trace element status in Saudi patients with established atherosclerosis. J Trace Elem Med Biol 20: 105– 114, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Andrews GK. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol 59: 95– 104, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Apostolova MD, Chen S, Chakrabarti S, Cherian MG. High-glucose-induced metallothionein expression in endothelial cells: an endothelin-mediated mechanism. Am J Physiol Cell Physiol 281: C899– C907, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Bao B, Prasad AS, Beck FW, Fitzgerald JT, Snell D, Bao GW, Singh T, Cardozo LJ. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: a potential implication of zinc as an atheroprotective agent. Am J Clin Nutr 91: 1634– 1641, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beattie JH, Kwun IS. Is zinc deficiency a risk factor for atherosclerosis? Br J Nutr 91: 177– 181, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Bernal PJ, Leelavanichkul K, Bauer E, Cao R, Wilson A, Wasserloos KJ, Watkins SC, Pitt BR, St Croix CM. Nitric-oxide-mediated zinc release contributes to hypoxic regulation of pulmonary vascular tone. Circ Res 102: 1575– 1583, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien S. Effects of disturbed flow on endothelial cells. Ann Biomed Eng 36: 554– 562, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conway DE, Sakurai Y, Weiss D, Vega JD, Taylor WR, Jo H, Eskin SG, Marcus CB, McIntire LV. Expression of CYP1A1 and CYP1B1 in human endothelial cells: regulation by fluid shear stress. Cardiovasc Res 81: 669– 677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conway DE, Williams MR, Eskin SG, McIntire LV. Endothelial cell responses to atheroprone flow are driven by two separate flow components: low time-average shear stress and fluid flow reversal. Am J Physiol Heart Circ Physiol 298: H367– H374, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortese-Krott MM, Suschek CV, Wetzel W, Kroncke KD, Kolb-Bachofen V. Nitric oxide-mediated protection of endothelial cells from hydrogen peroxide is mediated by intracellular zinc and glutathione. Am J Physiol Cell Physiol 296: C811– C820, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Cortese MM, Suschek CV, Wetzel W, Kroncke KD, Kolb-Bachofen V. Zinc protects endothelial cells from hydrogen peroxide via Nrf2-dependent stimulation of glutathione biosynthesis. Free Radic Biol Med 44: 2002– 2012, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci 59: 627– 647, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA., Jr Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci USA 101: 14871– 14876, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daskalopoulou SS, Daskalopoulos ME, Theocharis S, Kavantzas N, Perrea D, Karandrea D, Constantinides AG, Mikhailidis DP, Nicolaides AN, Liapis CD. Metallothionein expression in the high-risk carotid atherosclerotic plaque. Curr Med Res Opin 23: 659– 670, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Davies PF. Endothelial transcriptome profiles in vivo in complex arterial flow fields. Ann Biomed Eng 36: 563– 570, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Gee KR, Zhou ZL, Qian WJ, Kennedy R. Detection and imaging of zinc secretion from pancreatic beta-cells using a new fluorescent zinc indicator. J Am Chem Soc 124: 776– 778, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Gee KR, Zhou ZL, Ton-That D, Sensi SL, Weiss JH. Measuring zinc in living cells. A new generation of sensitive and selective fluorescent probes. Cell Calcium 31: 245– 251, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Giacconi R, Bonfigli AR, Testa R, Sirolla C, Cipriano C, Marra M, Muti E, Malavolta M, Costarelli L, Piacenza F, Tesei S, Mocchegiani E. +647 A/C and +1245 MT1A polymorphisms in the susceptibility of diabetes mellitus and cardiovascular complications. Mol Genet Metab 94: 98– 104, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Giacconi R, Cipriano C, Muti E, Costarelli L, Maurizio C, Saba V, Gasparini N, Malavolta M, Mocchegiani E. Novel -209A/G MT2A polymorphism in old patients with type 2 diabetes and atherosclerosis: relationship with inflammation (IL-6) and zinc. Biogerontology 6: 407– 413, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Gobel H, van der Wal AC, Teeling P, van der Loos CM, Becker AE. Metallothionein in human atherosclerotic lesions: a scavenger mechanism for reactive oxygen species in the plaque? Virchows Arch 437: 528– 533, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Hainaut P, Mann K. Zinc binding and redox control of p53 structure and function. Antioxid Redox Signal 3: 611– 623, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Hennig B, Meerarani P, Toborek M, McClain CJ. Antioxidant-like properties of zinc in activated endothelial cells. J Am Coll Nutr 18: 152– 158, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Hosoya T, Maruyama A, Kang MI, Kawatani Y, Shibata T, Uchida K, Warabi E, Noguchi N, Itoh K, Yamamoto M. Differential responses of the Nrf2-Keap1 system to laminar and oscillatory shear stresses in endothelial cells. J Biol Chem 280: 27244– 27250, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of O2- from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem 278: 47291– 47298, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Jenner A, Ren M, Rajendran R, Ning P, Huat BT, Watt F, Halliwell B. Zinc supplementation inhibits lipid peroxidation and the development of atherosclerosis in rabbits fed a high cholesterol diet. Free Radic Biol Med 42: 559– 566, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kang YJ. Metallothionein redox cycle and function. Exp Biol Med (Maywood) 231: 1459– 1467, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Laukens D, Waeytens A, De Bleser P, Cuvelier C, De Vos M. Human metallothionein expression under normal and pathological conditions: mechanisms of gene regulation based on in silico promoter analysis. Crit Rev Eukaryot Gene Expr 19: 301– 317, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Maret W. Molecular aspects of human cellular zinc homeostasis: redox control of zinc potentials and zinc signals. Biometals 22: 149– 157, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Nath R, Kumar D, Li T, Singal PK. Metallothioneins, oxidative stress and the cardiovascular system. Toxicology 155: 17– 26, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Ohura N, Yamamoto K, Ichioka S, Sokabe T, Nakatsuka H, Baba A, Shibata M, Nakatsuka T, Harii K, Wada Y, Kohro T, Kodama T, Ando J. Global analysis of shear stress-responsive genes in vascular endothelial cells. J Atheroscler Thromb 10: 304– 313, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Oshima Y, Fujio Y, Nakanishi T, Itoh N, Yamamoto Y, Negoro S, Tanaka K, Kishimoto T, Kawase I, Azuma J. STAT3 mediates cardioprotection against ischemia/reperfusion injury through metallothionein induction in the heart. Cardiovasc Res 65: 428– 435, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Paul A, Calleja L, Joven J, Vilella E, Ferre N, Camps J, Girona J, Osada J. Supplementation with vitamin E and/or zinc does not attenuate atherosclerosis in apolipoprotein E-deficient mice fed a high-fat, high-cholesterol diet. Int J Vitam Nutr Res 71: 45– 52, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Ranjan V, Xiao Z, Diamond SL. Constitutive NOS expression in cultured endothelial cells is elevated by fluid shear stress. Am J Physiol Heart Circ Physiol 269: H550– H555, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Reiterer G, MacDonald R, Browning JD, Morrow J, Matveev SV, Daugherty A, Smart E, Toborek M, Hennig B. Zinc deficiency increases plasma lipids and atherosclerotic markers in LDL-receptor-deficient mice. J Nutr 135: 2114– 2118, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Ren M, Rajendran R, Ning P, Tan Kwong Huat B., Choon Nam O., Watt F., Jenner A., Halliwell B. Zinc supplementation decreases the development of atherosclerosis in rabbits. Free Radic Biol Med 41: 222– 225, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Shen H, Arzuaga X, Toborek M, Hennig B. Zinc nutritional status modulates expression of AhR-responsive P450 enzymes in vascular endothelial cells. Environ Toxicol Pharmacol 25: 197– 201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen H, Oesterling E, Stromberg A, Toborek M, MacDonald R, Hennig B. Zinc deficiency induces vascular pro-inflammatory parameters associated with NF-kappaB and PPAR signaling. J Am Coll Nutr 27: 577– 587, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Singh RB, Gupta UC, Mittal N, Niaz MA, Ghosh S, Rastogi V. Epidemiologic study of trace elements and magnesium on risk of coronary artery disease in rural and urban Indian populations. J Am Coll Nutr 16: 62– 67, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Stadler N, Stanley N, Heeneman S, Vacata V, Daemen MJ, Bannon PG, Waltenberger J, Davies MJ. Accumulation of zinc in human atherosclerotic lesions correlates with calcium levels but does not protect against protein oxidation. Arterioscler Thromb Vasc Biol 28: 1024– 1030, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Suo J, Ferrara DE, Sorescu D, Guldberg RE, Taylor WR, Giddens DP. Hemodynamic shear stresses in mouse aortas: implications for atherogenesis. Arterioscler Thromb Vasc Biol 27: 346– 351, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Vlad M, Caseanu E, Uza G, Petrescu M. Concentration of copper, zinc, chromium, iron and nickel in the abdominal aorta of patients deceased with coronary heart disease. J Trace Elem Electrolytes Health Dis 8: 111– 114, 1994 [PubMed] [Google Scholar]
- 43.Wiseman DA, Wells SM, Hubbard M, Welker JE, Black SM. Alterations in zinc homeostasis underlie endothelial cell death induced by oxidative stress from acute exposure to hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol 292: L165– L177, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Wiseman DA, Wells SM, Wilham J, Hubbard M, Welker JE, Black SM. Endothelial response to stress from exogenous Zn2+ resembles that of NO-mediated nitrosative stress, and is protected by MT-1 overexpression. Am J Physiol Cell Physiol 291: C555– C568, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Wootton DM, Ku DN. Fluid mechanics of vascular systems, diseases, and thrombosis. Annu Rev Biomed Eng 1: 299– 329, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Yee A, Bosworth KA, Conway DE, Eskin SG, McIntire LV. Gene expression of endothelial cells under pulsatile nonreversing vs. steady shear stress; comparison of nitric oxide production. Ann Biomed Eng 36: 571– 579, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Zbinden S, Wang J, Adenika R, Schmidt M, Tilan JU, Najafi AH, Peng X, Lassance-Soares RM, Iantorno M, Morsli H, Gercenshtein L, Jang GJ, Epstein SE, Burnett MS. Metallothionein enhances angiogenesis and arteriogenesis by modulating smooth muscle cell and macrophage function. Arterioscler Thromb Vasc Biol 30: 477– 482, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Ziegler T, Bouzourene K, Harrison VJ, Brunner h, Hayoz D. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler Thromb Vasc Biol 18: 686– 692, 1998 [DOI] [PubMed] [Google Scholar]