Abstract
Hypogelsolinemia is observed in patients with different states of acute or chronic inflammation such as sepsis, rheumatoid arthritis, and multiple sclerosis. In animal models of sepsis, repletion of plasma gelsolin reduces septic mortality. However, the functions of extracellular gelsolin and the mechanisms leading to its protective nature are poorly understood. Potential mechanisms involve gelsolin's extracellular actin scavenging function or its ability to bind bioactive lipids or proinflammatory mediators, which would limit inflammatory responses and prevent tissue damage. Here we report that human plasma gelsolin binds to sphingosine 1-phosphate (S1P), a pleiotropic cellular agonist involved in various immune responses, and to its synthetic structural analog FTY720P (Gilenya). The fluorescence intensity of a rhodamine B-labeled phosphatidylinositol 4,5-bisphosphate binding peptide derived from gelsolin and the optical density of recombinant human plasma gelsolin (rhpGSN) were found to decrease after the addition of S1P or FTY720P. Gelsolin's ability to depolymerize F-actin also decreased progressively with increasing addition of S1P. Transient increases in phosphorylation of extracellular signal-regulated kinase in bovine aortic endothelial cells (BAECs) after S1P treatment were inhibited by rhpGSN. The ability of S1P to increase F-actin content and the elastic modulus of primary astrocytes and BAECs was also prevented by rhpGSN. Evaluation of S1P and gelsolin levels in cerebrospinal fluid reveals a low concentration of gelsolin and a high concentration of S1P in samples obtained from patients suffering from lymphatic meningitis. These findings suggest that gelsolin-mediated regulation of S1P bioactivity may be important to maintain immunomodulatory balance at inflammatory sites.
Keywords: binding, inflammation, FTY720, astrocytes, endothelial cells
gelsolin is a multifunctional actin-binding, phosphatidylinositol 4,5-bisphosphate (PIP2)-regulated protein present both in extracellular compartments and in the cytoplasm (5, 50). Intracellular gelsolin is involved in the regulation of actin cytoskeleton organization, while its extracellular isoform, plasma gelsolin, functions as a part of an extracellular actin scavenger system. This system is designed to eliminate actin released from injured cells in order to prevent increased blood viscosity and possible toxicity of F-actin (23, 25). Gelsolin's PIP2 binding domain resides in two sequences located near its NH2-terminal domain, and peptides based on those sequences show preferential interaction with several acidic lipid signaling molecules including PIP2, lysophosphatidic acid (LPA), lipoteichoic acid (LTA), and lipopolysaccharide (LPS) (2–4, 15, 27). The interaction of plasma gelsolin with bioactive lipids in extracellular fluids may have several important consequences. Gelsolin complexed with lipids lacks the ability to bind to and sever actin filaments, and the ability of lipid molecules to act as cell agonists can be either compromised or augmented depending on the gelsolin-to-lipid ratio and the manner in which the bioactive lipid functions. The ability of LPS or LTA to activate Toll-like receptors (TLRs) or of LPA to activate endothelial differentiation gene receptors (EDG) is compromised by recombinant human plasma gelsolin (rhpGSN) (2, 12, 13, 44).
Clinically, an increasing number of studies strongly suggest that plasma gelsolin depletion precedes and therefore might predict secondary inflammation and tissue injury, and that hypogelsolinemia can be an indicator of poor prognosis or critical care complication (5, 9). For example, low levels of gelsolin in trauma patients or critically ill surgical patients predict a negative clinical outcome (21, 22, 31). Bone marrow transplant patients with lower plasma gelsolin levels were more likely to develop respiratory complication and death (10). A decrease in plasma gelsolin was also observed in the blood of patients suffering from chronic inflammatory diseases such as rheumatoid arthritis (35) and multiple sclerosis (19).
Sphingosine 1-phosphate (S1P) is a potent and pleiotropic bioactive lysosphingolipid mostly released by hematopoietic cells. It acts on target cells through G protein-coupled receptors S1P1–S1P5 (39). In circulating blood, S1P is present at a concentration between 200 and 900 nM, and its free amount is sufficient to provide tonic stimulation to endothelial cells. S1P serves as a first messenger and is involved in various cellular responses including cell migration, adherens junction assembly, platelet aggregation, and smooth muscle contraction (11, 28). Opposing functions of sphingosine and S1P have been linked to a “rheostat” that determines the balance between cell survival and death pathways (46). In addition to serum, S1P is present in other compartments, especially at inflammatory sites, where its cellular release was found to increase. Accordingly, the human cerebrospinal fluid (CSF) HDL fraction was found to induce rat astrocyte migration in a manner sensitive to the S1P receptor antagonist VPC23019, indicating that S1P accumulates in CSF lipoproteins and mediates some lipoprotein-induced neural cell functions in the central nervous system (42). Additionally, S1P has a potent chemoattractant activity for neural stem/progenitor cells, stimulating their migration toward a damaged area of the central nervous system (17). On the other hand, S1P can also promote reactive astrogliosis (45). S1P cell activation requires an effective control, as acute changes in its plasma concentration are sufficient to induce sudden cardiac death through an S1P3 receptor-mediated mechanism (38, 40). At the cellular level, S1P activation results in engagement of various G proteins and subsequent activation of signaling pathways involving phospholipase C, Ras, phosphatidylinositol 3-kinase (PI3K), Akt, ERK, and protein kinase C (1).
In this article we report that rhpGSN, in addition to its previously reported binding to LPS, LTA, LPA, and platelet-activating factor (PAF) (3, 4, 30, 34), binds and attenuates certain cellular effects of S1P. rhpGSN also binds to the synthetic S1P analog FTY720P, which exerts a significantly different biological activity compared with S1P. This finding indicates that in the extracellular environment plasma gelsolin may act as a universal scavenger or carrier of bioactive lipids, and its function may include interference with the multifunctional immunomodulatory actions of S1P. Such action is potentially of importance in settings where the concentrations of plasma gelsolin and/or S1P change over their homeostatic ranges. One example of such a condition was identified on the basis of S1P and gelsolin analysis in human samples of CSF. In patients with lymphatic meningitis, S1P and gelsolin concentrations showed a tendency to increase and decrease, respectively, compared with other neurological disorders.
MATERIALS AND METHODS
Materials.
QRLFQVKGRR (gelsolin residues 160–169) and QRL peptides from gelsolin were prepared by solid-phase peptide synthesis and fluorescently labeled at their NH2 termini by reaction with the succinimidyl ester of rhodamine B as previously described (8). FTY720 and (S)-FTY720P were from Echelon Biosciences (Salt Lake City, UT). FTY720P functions as an activator of four S1P receptors (S1P1,3,4,5), but its immunomodulatory activity is associated with downregulation of S1P1 (1). Sphingosylphosphorylcholine (SPC), S1P, lysophosphatidylcholine (lyso-PC) from bovine brain, d-erythro-dihydrosphingosine 1-phosphate (dhS1P), and LPS from Escherichia coli (serotype O26:B6) were purchased from Sigma (St. Louis, MO). PAF (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine), N-octanoyl-ceramide 1-phosphate (C1P), and ceramide from porcine brain were obtained from Avanti Polar Lipids (Alabaster, AL). rhpGSN was obtained from Biogen-Idec (Cambridge, MA). S6 ribosomal protein (S6rp) clone 5G10 rabbit monoclonal antibodies were from Cell Signaling (Santa Cruz, CA). Stock solutions of S1P were prepared in 0.3 M NaOH, since pH strongly affects the aggregation behavior of S1P (41). Various concentrations of S1P were prepared by mixing its stock solution [sonicated for 10 min at room temperature (RT)] with the buffer required for a particular experiment. Stock solutions of dhS1P were prepared in 60% methanol-30% tetrahydrofuran-10% water. From this solution, a lipid film was prepared by evaporating the solvent with a stream of nitrogen, and the dry film was hydrated with 20 mM Tris buffer, pH 7.4. When required, the lipid film was hydrated with a solution of fatty acid-free bovine albumin in Tris buffer (4 mg/ml). Gelsolin denaturation (a modification in conformation not accompanied by rupture of peptide bonds) was achieved by heating its stock solution at 55°C for 1 h.
Interaction of S1P with PBP10 peptide and gelsolin.
The fluorescence intensity of PBP10, a peptide from the PIP2 binding domain of gelsolin (residues 160–169) linked to rhodamine B, was measured with a SL-5B spectrofluorometer (Perkin Elmer, Waltham, MA) (excitation wavelength 560 nm, emission wavelength 590 nm). Fluorescence intensity was measured 15 min after addition and vortexing of various concentrations of lipids with 2 μM peptide solutions in buffer A (10 mM Tris, 10 mM MES, pH 7.45). To determine the binding of lipids to gelsolin, the optical density at 280 nm was measured in solutions containing different amounts of lipids added to 0.1 mg/ml rhpGSN in PBS. A decrease in tyrosine and tryptophan fluorescence, due to decreased absorbance, has been documented as an assay for PIP2 binding to gelsolin (6).
Protein-lipid overlay assay.
PIP2, S1P, and phosphatidylserine (PS) were reconstituted in 1:1 chloroform-methanol and diluted in 1:2:0.8 chloroform-methanol-water at concentrations of 0.1, 1, 10, and 100 μM. A volume of 1 μl of each solution was spotted on a Hybond P-PVDF membrane (Amersham Biosciences, Little Chalfont, UK) and allowed to dry for 1 h (48). The membrane was then placed in blocking buffer for 30 min, incubated with 10 nM rhpGSN, rinsed in TBS-T buffer (150 mM NaCl, 50 mM Tris, 0.05% Tween 20, pH 7.4), incubated in primary antibody against human gelsolin (1:10,000 dilution) for 1 h, rinsed, and incubated in horseradish peroxidase (HRP)-conjugated secondary anti-mouse antibody for 1 h (1:16,000). Immunoblots were developed with the Fuji Film LAS-300 system and ECL Plus HRP-targeted chemiluminescent substrate (Amersham Biosciences).
F-actin preparation and severing activity of gelsolin.
Monomeric G-actin was prepared from rabbit skeletal muscle (rabbit muscle acetone powder, Pre-Freez Biologicals, Rogers, AR) (47) and labeled with pyrene-iodoacetamide (18). The nonpolymerizing solution contained (in mM) 2 Tris, 0.2 CaCl2, 0.5 ATP, and 0.2 DTT, pH 7.4. Actin was polymerized by addition of 150 mM KCl and 2 mM MgCl2 and incubation for 1 h at RT. rhpGSN-severing activity was measured in 0.4 μM pyrene-labeled F-actin samples after addition of gelsolin, alone or in combination with S1P or other lipids. The fluorescence intensity of F-pyrene actin was monitored for 3 min. The severing activity was calculated from the rate of fluorescence intensity decrease as described previously (16).
Cell culture.
Bovine aortic endothelial cells (BAECs) were purchased from Clonetics (San Diego, CA) and were grown in an incubator at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) with low-glucose-containing 10% fetal bovine serum (Cambrex BioScience, Walkersville, MD). Rat primary astrocytes (a gift from Dr. David Meaney, University of Pennsylvania) were obtained from prenatal rats according to a protocol approved by the Office of Regulatory Affairs, University of Pennsylvania, and maintained for 14 days in culture before use. Embryos (embryonic days 17–19) were removed by cesarean section from a Sprague-Dawley rat, and the hippocampi were surgically removed. The tissue was digested in trypsin-DNase at 37°C, centrifuged (1,000 g × 5 min), and filtered to derive suspensions from each pup. Cells were grown in an incubator at 37°C and 5% CO2 in DMEM (BioWhittaker, Walkersville, MD) supplemented with Ham's F-12 (Sigma) and 5% fetal bovine serum (Hyclone, Logan, UT) for 7 days, followed by an additional 7 days in neurobasal medium (Invitrogen, Carlsbad, CA) also supplemented with 5% fetal bovine serum, 2 mM l-glutamine, 50 μg/ml streptomycin, and 50 U/ml penicillin. All cells were maintained under standard cell culture conditions at 37°C in humidified air with 5% CO2.
Evaluation of ERK1/2 activation.
After the indicated time for activation with S1P (0–10 min), BAECs were washed in phosphate-buffered saline (PBS) and lysed in radioimmunoprecipitation assay buffer supplemented with 0.5 mM phenylmethylsulfonyl fluoride, phosphatase inhibitor cocktails I and II (Sigma), and protease inhibitor cocktail from Roche (Basel, Switzerland). For normalization of gel loading, the protein extracts were assayed by the Lowry method (Bio-Rad, Hercules, CA; DC protein assay). Typically, 25 μg of protein per lane was loaded. To examine protein phosphorylation, the membranes were incubated with anti-ERK1/2 antibodies (Cell Signaling, Danvers, MA). Next, the membranes were incubated with the appropriate secondary peroxidase-conjugated antibodies (GE Healthcare). The blots were developed with the ECL Plus System (Amersham, Pittsburgh, PA). Western blot analysis of S6rp, a downstream target of the PI3K/Akt/mammalian target of rapamycin (mTOR) pathway (24), was used as an additional loading control in which a respective membrane was reblotted with anti-S6rp antibody after the removal of primary anti-ERK1/2 and secondary antibodies from a membrane with reblot buffer from Chemicon International (Billerica, MA).
Microscopic evaluation of actin cytoskeleton in rat astrocytes after S1P treatment.
Rat astrocytes after S1P activation (1 μM) were fixed with 4% glutaraldehyde, and actin was stained with phalloidin-FITC after cell permeabilization with 0.1% Triton X-100. Images from ×40 magnification with a Leica microscope were captured with a Cool SNAP (HQ) camera. In addition to microscopic observation, we determined the phalloidin fluorescence intensity with ImageJ software, indicative of F-actin concentration (fluorescence/area) for several cells (∼10) in each condition.
Elastic modulus.
The stiffness (elastic modulus) of rat astrocytes and BAECs was measured at 1 Hz by atomic force microscopy (AFM) with a DAFM-2X Bioscope (Veeco, Woodbury, NY) mounted on an Axiovert 100 microscope (Zeiss, Thornwood, NY) using silicon nitride cantilevers (196 mm long, 23 mm wide, 0.6 mm thick) with a pyramidal tip (40-nm diameter) for indentation as described previously (49). The spring constant of the cantilever, calibrated by resonance measurements, was typically 0.06 N/m (Veeco, Chadds Ford, PA).
Evaluation of S1P and FTY720P effects on BAEC NF-κB localization.
In BAEC cultures, NF-κB translocation was monitored after a 2-h incubation in serum-free medium containing 10 ng/ml LPS (positive control), 0.1–5 μM S1P, or 0.1–10 μM FTY720P. A monoclonal antibody to the NF-κB/subunit p65 (Santa Cruz Biotechnology) was used for visualization after treatments. Individual cells were counted to assess NF-κB localization (200–600 cells/treatment).
Evaluation of S1P in CSF samples.
CSF samples were obtained from individuals admitted to the Department of Neurology at the Medical University of Białystok and undergoing lumbar puncture for diagnostic purposes. Samples of CSF after collection were centrifuged (2,000 g, 20 min), and the supernatants of CSF were subjected to total protein analysis and frozen. The Medical University of Białystok Ethics Committee for Research on Humans and Animals approved the study, and written consent was obtained from all subjects. CSF concentration of S1P was measured as described previously (20, 29) with a HPLC system (ProStar, Varian) equipped with a fluorescence detector and a C18 reverse-phase column (OmniSpher 5, 4.6 × 150 mm). Isocratic elution with acetonitrile and water (9:1 vol/vol) and a flow rate of 1 ml/min was used. The column temperature was maintained at 33°C.
Immunoblotting of gelsolin in cerebrospinal fluid.
Gel sample buffer was added to freshly thawed CSF samples, which were then boiled and subjected to electrophoresis on 10% polyacrylamide gels. rhpGSN was loaded as a standard in each gel in a concentration range comparable to the gelsolin concentration in the samples. After electrophoresis, proteins were transferred to polyvinylidene difluoride membranes (Amersham Biosciences), which were blocked by incubation in 5% (wt/vol) nonfat dry milk dissolved in TBS-T. After transfer, proteins were probed with a monoclonal anti-human gelsolin antibody used at a 1:10,000 dilution in TBS-T. HRP-conjugated secondary antibodies were used at a 1:20,000 dilution in TBS-T. Immunoblots were developed with the Fuji Film LAS-300 system and an ECL Plus HRP-targeted chemiluminescent substrate (Amersham Biosciences). Densitometry analysis was performed with Image Gauge software (version 4.22, Fuji Photo Film).
Statistical analysis.
Data are reported as means ± SD from three to six experiments. Differences between means were evaluated for statistical significance with the unpaired Student's t-test. P values of <0.05 were considered significant.
RESULTS
S1P interacts with gelsolin's PIP2 binding sequence (residues 160–169) and recombinant human plasma gelsolin.
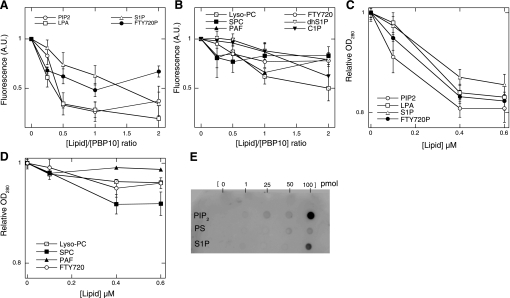
As previously reported (3), upon specific interaction of PBP10 with LPS there is an initial decrease in fluorescence at low LPS-to-peptide ratios. At the molar ratios tested in the present experiment, only the initial stage of fluorescence decrease was observed with PIP2, LPA, S1P, C1P, and FTY720P. The fluorescence intensity of PBP10 decreased to the same extent upon addition of PIP2, LPA, and S1P at lipid-to-PBP10 molar ratios of 2:1. C1P and FTY720P had a weaker effect on PBP10 fluorescence (Fig. 1, A and B). There was no significant fluorescence change after addition of PAF, lyso-PC, dhS1P, or FTY720 to PBP10 at the lipid-to-peptide ratios used. No decrease of PBP10 fluorescence intensity was observed after ceramide addition (data not shown). Changes in PBP10 fluorescence intensity suggest that gelsolin, in addition to binding S1P, may also bind to C1P, a phosphorylated bioactive sphingolipid involved in a multitude of cellular activities including the regulation of eicosanoid synthesis (7), but not to ceramide. This result underscores the importance of phosphate groups in the binding of bioactive lipids to gelsolin. No change in fluorescence was observed on addition of S1P, FTY720P, LPA, C1P, dhS1P, and SPC to a control peptide with the sequence rhodamine B-QRL (RhB-QRL) (data not shown). Binding of S1P to intact gelsolin was evident from both a change in UV absorbance (Fig. 1, C and D) and data from a protein lipid overlay assay in which gelsolin binding to immobilized S1P was detected with a specific anti-gelsolin antibody (Fig. 1E). S1P decreased the absorbance of gelsolin, with a maximal decrease of ∼15%, similar to that found with PIP2. A decrease of gelsolin's absorbance was also observed after addition of LPA and FTY720P, but not with PAF, lyso-PC, SPC, or FTY720 (Fig. 1, C and D).
Fig. 1.
Human plasma gelsolin interacts with various phospho- and lysolipids. A and B: rhodamine B fluorescence changes of RhB-QRLFQVKGRR (PBP10) peptide (2 μM) in the presence of different lipids. AU, arbitrary units. C and D: optical density at 280 nm (OD280) of gelsolin (0.1 μM) in solutions containing varying amount of lipids (a decrease indicates interaction of gelsolin with these compounds). Lipid symbols are as follows: PIP2, phosphatidylinositol 4,5-bisphosphate; S1P, sphingosine 1-phosphate; LPA, lysophosphatidic acid, lyso-PC, lysophosphatidylcholine; SPC, sphingosylphosphorylcholine, also called lysosphingomyelin; PAF, platelet-activating factor; FTY720, fingolimod; FTY720P, (S)-FTY720 phosphate; dhS1P, d-erythro-dihydrosphingosine 1-phosphate; C1P, N-octanoyl-ceramide 1-phosphate. Addition of lipids to the buffer used to prepare the PBP10 solution did not interfere with fluorescence reading. Similarly, the different lipids used in this study at concentrations up to 0.6 μM do not interfere with the absorbance at 280 nm of the buffer in which the gelsolin solution was prepared. All data in A–D were normalized to the reading of PBP10 or gelsolin solution before addition of lipids. E: protein-lipid overlay assay. A small volume (1 μl) of each lipid solution was spotted on a Hybond P-PVDF membrane, which was incubated with recombinant human plasma gelsolin (rhpGSN) solution and after extensive rinsing immunoblots with anti-human plasma gelsolin antibody. A dark (ECL positive) spot identified the area of specific interaction between gelsolin and PIP2 or S1P. Data shown are means ± SD of 3–4 experiments. PS, phosphatidylserine.
S1P inhibits gelsolin's actin filament severing activity.
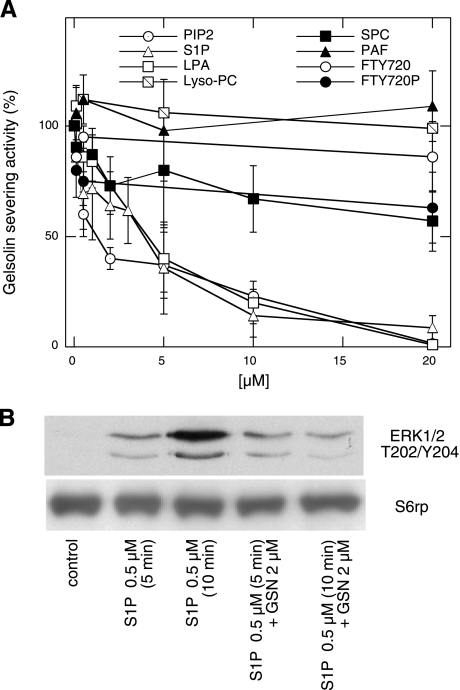
When added to 0.05 μM rhpGSN, 5 and 20 μM S1P inhibit ∼60% and 80% of gelsolin's severing activity, respectively (Fig. 2A). On a molar basis, S1P has a potency similar to that of PIP2 and LPA (3). Figure 2A shows that gelsolin's severing activity was weakly reduced by FTY720P and SPC. These results indicate that among acidic lysolipids only LPA and S1P significantly inhibit gelsolin's ability to depolymerize F-actin.
Fig. 2.
Modification of gelsolin and lipid functionality upon their binding. A: effect of different lipids on actin filament severing activity of rhpGSN (50 nM). B: rhpGSN prevents S1P-mediated activation of ERK in bovine aortic endothelial cells (BAECs). For electrophoresis, equal amounts of protein were applied to each lane based on the total protein content in the cell lysates. Evaluation of S6 ribosomal protein (S6rp) served as a loading control. Data are means ± SD of 3–4 experiments or represent 1 of 3 experiments.
Gelsolin prevents S1P-mediated activation of ERK.
ERK1 and 2 (p44 and p42 MAP kinases) function in a protein kinase cascade. Earlier studies have shown that S1P enhanced the threonine/tyrosine phosphorylation of ERK in a time- and dose-dependent manner, indicating activation of Gi/o downstream signaling pathways in astrocytes (26, 45). The level of phosphorylated ERK in resting BAECs was undetectable. S1P (0.5 μM) activation for 5 and 10 min increased the phosphorylated forms of ERK. This effect was not observed when BAEC activation with S1P was performed in the presence of rhpGSN (2 μM) for 5 and 10 min (Fig. 2B).
Gelsolin prevents S1P-induced actin remodeling and cell stiffening.
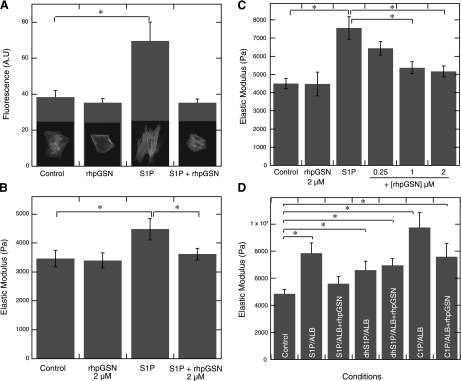
In cultured rat astrocytes, significant remodeling of the actin cytoskeleton was observed upon S1P activation. S1P (1 μM) rapidly induced assembly of cortical actin and stress fibers, as seen in the pronounced phalloidin staining compared with that seen in the control cells that had few, sparse actin filament bundles. The changes in actin staining are in agreement with changes in the shape of astrocytes, which upon S1P activation become more elongated. However, when first incubated with rhpGSN, S1P had no effect on the actin cytoskeleton and shape of astrocytes (Fig. 3A). AFM measurements of rat astrocyte and BAEC stiffness revealed an increase in the elastic modulus from 3,500 to 4,500 Pa and from 4,800 to 7,500 Pa, respectively, after addition of 1 μM S1P (Fig. 3, B and C). Increased cell stiffness after S1P stimulation was significantly reduced by gelsolin (0.25–2 μM) in both rat astrocytes and BAECs. rhpGSN (0.5–5 μM) alone had no effect on primary rat astrocyte or BAEC stiffness (Fig. 3, B and C, and data not shown). The S1P-induced increase in astrocyte stiffness was not inhibited when S1P was preincubated with denatured rhpGSN, suggesting that the binding of S1P to gelsolin requires the native conformation of the protein (data not shown). When BAECs were subjected to S1P, dhS1P, or C1P activation (1 μM each), using stock solutions prepared in buffers containing fatty acid-free bovine albumin (4 mg/ml), we observed a significant increase in cell stiffness that reached on average 7,800, 6,600, and 9,700 Pa upon S1P, dhS1P, and C1P addition, respectively (Fig. 3D). In the presence of 2 μM rhpGSN, the effect of S1P but not dhS1P was significantly reduced. rhpGSN also appeared to decrease cell stiffening induced by C1P, but this reduction was not statistically significant.
Fig. 3.
Gelsolin inhibits S1P-induced increase in cell stiffness. A: quantification of fluorescence staining for F-actin with phalloidin in rat primary astrocytes was used to demonstrate the blocking by plasma gelsolin (2 μM) of S1P (1 μM)-induced actin cytoskeleton assembly and cell shape changes. B and C: rat primary astrocyte (B) and BAEC (C) stiffness (elastic modulus) under S1P treatment was measured by atomic force microscopy (AFM). To evaluate gelsolin's effect on S1P-induced increase in cell stiffness, rat astrocytes and BAECs were treated with S1P (1 μM) in the presence of rhpGSN (0.25–2 μM). D: increase in BAEC stiffness upon S1P, dhS1P, and C1P addition (1 μM each) in the presence of albumin (Alb) or albumin and rhpGSN. Error bars in B–D represent SD from measurements on larger populations of cells (n = 30–50). *Significantly different.
Gelsolin binds lipids with opposite cellular activity.
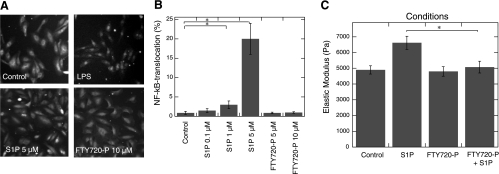
LPS activates the transcription factor NF-κB, which triggers the transcription of proinflammatory cytokine genes in various cell types, and gelsolin blocks this effect of LPS (3). In untreated BAECs, NF-κB was located in the cytoplasm (Fig. 4A); activation by LPS or S1P resulted in its translocation to the nucleoplasm. However, at the concentration of S1P used in this study (5 μM) the nuclear localization of NF-κB was observed in a limited population of BAECs (20%) compared with the LPS-mediated effect, which at 0.1 μg/ml induces translocation in ∼80% of BAECs. We were unable to evaluate NF-κB translocation at higher concentrations of S1P because of its toxicity, as determined by BAEC morphology and lactate dehydrogenase release from cells treated with S1P at concentrations exceeding 5 μM (data not shown). The S1P-induced translocation of NF-κB was blocked by its preincubation with rhpGSN (data not shown). In this system, FTY720P (up to 10 μM) had no effect on NF-κB translocation with or without physiological concentrations of gelsolin. In another set of experiments, we observed an inhibitory effect of FTY720P on the S1P-induced increase in BAEC stiffness (Fig. 4C).
Fig. 4.
FTY720P inhibits S1P-induced cell activation. A: representative image of BAECs immunostained with antibody against NF-κB/subunit p65. In BAECs, addition of LPS (10 ng/ml) or S1P but not FTY720P caused NF-κB translocation to the nucleus. B: quantification of NF-κB translocation in BAECs after S1P and FTY720P treatment. C: FTY720P (1 μM) prevents increase in BAEC stiffness induced with S1P (1 μM). Experiments presented in A were repeated 3 or 4 times, and data from 1 representative experiment are shown. Data shown in B are means ± SD of 3 or 4 experiments. In C, error bars represent SD from measurements on larger populations of cells (n = 30–50). *Significantly different.
Gelsolin and S1P concentration in cerebrospinal fluid samples.
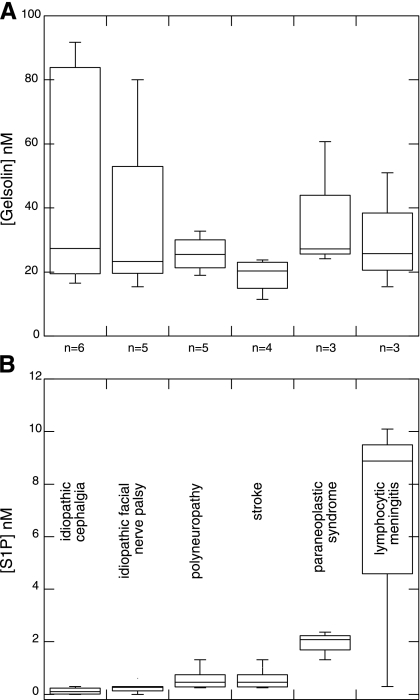
Twenty-six specimens of CSF obtained from patients with various neurological symptoms were analyzed by HPLC and quantitative Western blot analysis to determine S1P and gelsolin concentrations, respectively. As shown in Fig. 5, samples were obtained from patients diagnosed with six different conditions. The range of gelsolin and S1P concentrations in these samples was 15.1–88.7 nM and 0–10.1 nM, respectively. The lowest gelsolin and S1P concentrations were observed in patients diagnosed with stroke and idiopathic cephalgia, respectively. Higher gelsolin and S1P concentrations were found in patients diagnosed with idiopathic cephalgia and lymphocytic meningitis. The largest ratio of S1P to gelsolin was found in lymphocytic meningitis. The importance of this observation is uncertain because of the limited number of CSF samples currently analyzed, but the large variance in gelsolin levels compared with assumedly relatively constant levels in normal samples suggests that further studies are warranted.
Fig. 5.
Gelsolin (A) and S1P (B) concentrations in cerebrospinal fluid (CSF) samples obtained from subjects with various neurological disorders.
DISCUSSION
Gelsolin and S1P are both among an extremely small fraction of proteins and lipids that function in both extracellular and intracellular compartments. In the extracellular environment, plasma gelsolin functions in part as an actin scavenger, while S1P acts as an agonist for numerous cell types. Our findings show that human plasma gelsolin binds to S1P. This interaction is characterized by S1P-mediated inhibition of gelsolin's F-actin severing activity and by gelsolin's interference with S1P-mediated cell activation. Gelsolin severing activity decreases when gelsolin binds to S1P, FTY720P, SPC, or LPA. At the molecular level, these lipids bind gelsolin by interacting with its PIP2 binding site (30), as demonstrated by a decrease of rhodamine B fluorescence of PBP10 peptide (Fig. 1A) and conformational changes in gelsolin structure (as monitored by absorbance measurements at 280 nm; Fig. 1C), resulting in changes of gelsolin's ability to bind F-actin. This result is consistent with previous data showing that LPS and LTA, which share some structural similarities to S1P such as a high negative charge density and the presence of phosphomonoesters, disrupt gelsolin's ability to sever F-actin (2, 3).
It is possible that systemic administration of gelsolin in pathological conditions associated with increasing concentrations of circulating bioactive lipids may act as a buffer that could neutralize these factors and lower their bioavailability (33). However, preferential binding of gelsolin with bioactive lipids does not correlate with lipid toxicity or proinflammatory potency. From the family of lysophospholipids represented by LPA, lyso-PC, SPC, and S1P, LPA and S1P have the strongest effect on gelsolin severing activity. Notably, lyso-PC and SPC, which effectively augment inflammation through effects on adhesion molecules, monocytes, and macrophages, have a very limited effect on gelsolin function. The tight binding of gelsolin or other components of plasma to S1P and other bioactive lipids might interfere with binding to their receptors and thereby attenuate the lipid-receptor-mediated cell activation (32). Therefore, in the case of S1P, a concern arises about the side effects of neutralizing all of the S1P in the periphery. However, the S1P level in human plasma far exceeds the KD (8–50 nM) for S1P receptors (43), and nontoxicity or no pathological changes were observed in animals treated with high doses of anti-S1P antibody (33).
This reciprocal gelsolin-S1P interaction suggests multiple possible physiological effects. Gelsolin might impair the S1P-S1P1 receptor system that regulates lymphocyte distribution within lymphoid organs and egress to the blood (14, 37) and involves S1P-mediated modulation of endothelial cell barrier function. This hypothesis is consistent with our data showing that binding of gelsolin to S1P prevents its ability to regulate endothelial stiffness. In addition, this interaction can reduce the effects of S1P-regulated endothelial permeability such as the prevention of fluid accumulation in tissues. Since activation of sphingosine kinase 1 (SphK1) by a variety of cytokines and concomitant formation of S1P are important for various inflammatory responses, it is also likely that gelsolin-S1P interaction controls several steps of inflammation (7). This possibility supports a recent finding showing that ceramide, sphingosine, and S1P all induced cyclooxygenase-2 in A549 human lung cells, and in response to TNF-α treatment an increase in the levels of S1P was observed (36).
At the cellular level, immune modulation by synthetic monoclonal antibodies directed against S1P results in inhibition of S1P-mediated release of proangiogenic and prometastatic cytokines from human ovarian carcinoma cells (33), indicating a potential therapeutic benefit of gelsolin-mediated S1P functional sequestration. This possibility is consistent with our observation that rhpGSN reduced S1P-induced activation of cells as determined by inhibition of ERK phosphorylation (Fig. 2B).
Assuming that disruption of the homeostatic balance that controls gelsolin and S1P concentrations in the extracellular space can have pathological consequences, the restoration of such balance may offer a therapeutic solution, especially in situations where the levels of S1P and gelsolin change in opposite directions. Since lymphocytic meningitis is characterized by an increase in S1P levels and lowered gelsolin in CSF (Fig. 5), the potential benefit of administration of gelsolin deserves further investigation.
GRANTS
This work was supported by National Institutes of Health Grants HL-67286, GM-83272, and HL-083187 and Medical University of Bialystok Grants 3-18609L and 3-44977L.
DISCLOSURES
In 2008, P. A. Janmey and R. Bucki were involved in a sponsored research agreement with Critical Biologics, Inc. in a project directed at evaluating the potential clinical use of gelsolin, but not otherwise related to the present study. None of the research reported in this article was supported by Critical Biologics, Inc. or by any other corporate entity. The other authors make no declarations.
ACKNOWLEDGMENTS
We thank A. Sadzyñski (Medical University of Białystok) for facilitating the experimental work.
REFERENCES
- 1.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther 115: 84–105, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bucki R, Byfield FJ, Kulakowska A, McCormick ME, Drozdowski W, Namiot Z, Hartung T, Janmey PA. Extracellular gelsolin binds lipoteichoic acid and modulates cellular response to proinflammatory bacterial wall components. J Immunol 181: 4936–4944, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bucki R, Georges PC, Espinassous Q, Funaki M, Pastore JJ, Chaby R, Janmey PA. Inactivation of endotoxin by human plasma gelsolin. Biochemistry 44: 9590–9597, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Bucki R, Janmey PA. Interaction of the gelsolin-derived antibacterial PBP 10 peptide with lipid bilayers and cell membranes. Antimicrob Agents Chemother 50: 2932–2940, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bucki R, Levental I, Kulakowska A, Janmey PA. Plasma gelsolin: function, prognostic value, and potential therapeutic use. Curr Protein Pept Sci 9: 541–551, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Burtnick LD, Koepf EK, Grimes J, Jones EY, Stuart DI, McLaughlin PJ, Robinson RC. The crystal structure of plasma gelsolin: implications for actin severing, capping, and nucleation. Cell 90: 661–670, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Chalfant CE, Spiegel S. Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. J Cell Sci 118: 4605–4612, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Cunningham CC, Vegners R, Bucki R, Funaki M, Korde N, Hartwig JH, Stossel TP, Janmey PA. Cell permeant polyphosphoinositide-binding peptides that block cell motility and actin assembly. J Biol Chem 276: 43390–43399, 2001 [DOI] [PubMed] [Google Scholar]
- 9.DiNubile MJ. Plasma gelsolin: in search of its raison d'etre. Focus on “Modifications of cellular responses to lysophosphatidic acid and platelet-activating factor by plasma gelsolin.” Am J Physiol Cell Physiol 292: C1240–C1242, 2007 [DOI] [PubMed] [Google Scholar]
- 10.DiNubile MJ, Stossel TP, Ljunghusen OC, Ferrara JL, Antin JH. Prognostic implications of declining plasma gelsolin levels after allogeneic stem cell transplantation. Blood 100: 4367–4371, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Gardell SE, Dubin AE, Chun J. Emerging medicinal roles for lysophospholipid signaling. Trends Mol Med 12: 65–75, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Goetzl EJ. Pleiotypic mechanisms of cellular responses to biologically active lysophospholipids. Prostaglandins Other Lipid Mediat 64: 11–20, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Goetzl EJ, Lee H, Azuma T, Stossel TP, Turck CW, Karliner JS. Gelsolin binding and cellular presentation of lysophosphatidic acid. J Biol Chem 275: 14573–14578, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Gollmann G, Neuwirt H, Tripp CH, Mueller H, Konwalinka G, Heufler C, Romani N, Tiefenthaler M. Sphingosine-1-phosphate receptor type-1 agonism impairs blood dendritic cell chemotaxis and skin dendritic cell migration to lymph nodes under inflammatory conditions. Int Immunol 20: 911–923, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Janmey PA, Lamb J, Allen PG, Matsudaira PT. Phosphoinositide-binding peptides derived from the sequences of gelsolin and villin. J Biol Chem 267: 11818–11823, 1992 [PubMed] [Google Scholar]
- 16.Janmey PA, Lind SE. Capacity of human serum to depolymerize actin filaments. Blood 70: 524–530, 1987 [PubMed] [Google Scholar]
- 17.Kimura A, Ohmori T, Ohkawa R, Madoiwa S, Mimuro J, Murakami T, Kobayashi E, Hoshino Y, Yatomi Y, Sakata Y. Essential roles of sphingosine 1-phosphate/S1P1 receptor axis in the migration of neural stem cells toward a site of spinal cord injury. Stem Cells 25: 115–124, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Kouyama T, Mihashi K. Fluorimetry study of N-(1-pyrenyl)iodoacetamide-labelled F-actin. Local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur J Biochem 114: 33–38, 1981 [PubMed] [Google Scholar]
- 19.Kulakowska A, Drozdowski W, Sadzynski A, Bucki R, Janmey PA. Gelsolin concentration in cerebrospinal fluid from patients with multiple sclerosis and other neurological disorders. Eur J Neurol 15: 584–588, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Kulakowska A, Zendzian-Piotrowska M, Baranowski M, Kononczuk T, Drozdowski W, Gorski J, Bucki R. Intrathecal increase of sphingosine 1-phosphate at early stage multiple sclerosis. Neurosci Lett 477: 149–152, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Lee PS, Drager LR, Stossel TP, Moore FD, Rogers SO. Relationship of plasma gelsolin levels to outcomes in critically ill surgical patients. Ann Surg 243: 399–403, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee PS, Patel SR, Christiani DC, Bajwa E, Stossel TP, Waxman AB. Plasma gelsolin depletion and circulating actin in sepsis: a pilot study. PLoS ONE 3: e3712, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee WM, Galbraith RM. The extracellular actin-scavenger system and actin toxicity. N Engl J Med 326: 1335–1341, 1992 [DOI] [PubMed] [Google Scholar]
- 24.Lepin EJ, Zhang Q, Zhang X, Jindra PT, Hong LS, Ayele P, Peralta MV, Gjertson DW, Kobashigawa JA, Wallace WD, Fishbein MC, Reed EF. Phosphorylated S6 ribosomal protein: a novel biomarker of antibody-mediated rejection in heart allografts. Am J Transplant 6: 1560–1571, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Lind SE, Smith DB, Janmey PA, Stossel TP. Role of plasma gelsolin and the vitamin D-binding protein in clearing actin from the circulation. J Clin Invest 78: 736–742, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuo Y, Miura S, Kawamura A, Uehara Y, Rye KA, Saku K. Newly developed reconstituted high-density lipoprotein containing sphingosine-1-phosphate induces endothelial tube formation. Atherosclerosis 194: 159–168, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Meerschaert K, De Corte V, De Ville Y, Vandekerckhove J, Gettemans J. Gelsolin and functionally similar actin-binding proteins are regulated by lysophosphatidic acid. EMBO J 17: 5923–5932, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta D, Konstantoulaki M, Ahmmed GU, Malik AB. Sphingosine 1-phosphate-induced mobilization of intracellular Ca2+ mediates rac activation and adherens junction assembly in endothelial cells. J Biol Chem 280: 17320–17328, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Min JK, Yoo HS, Lee EY, Lee WJ, Lee YM. Simultaneous quantitative analysis of sphingoid base 1-phosphates in biological samples by o-phthalaldehyde precolumn derivatization after dephosphorylation with alkaline phosphatase. Anal Biochem 303: 167–175, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Mintzer E, Sargsyan H, Bittman R. Lysophosphatidic acid and lipopolysaccharide bind to the PIP2-binding domain of gelsolin. Biochim Biophys Acta 1758: 85–89, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Mounzer KC, Moncure M, Smith YR, Dinubile MJ. Relationship of admission plasma gelsolin levels to clinical outcomes in patients after major trauma. Am J Respir Crit Care Med 160: 1673–1681, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Murata N, Sato K, Kon J, Tomura H, Yanagita M, Kuwabara A, Ui M, Okajima F. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem J 352: 809–815, 2000 [PMC free article] [PubMed] [Google Scholar]
- 33.O'Brien N, Jones ST, Williams DG, Cunningham HB, Moreno K, Visentin B, Gentile A, Vekich J, Shestowsky W, Hiraiwa M, Matteo R, Cavalli A, Grotjahn D, Grant M, Hansen G, Campbell MA, Sabbadini R. Production and characterization of monoclonal anti-sphingosine-1-phosphate antibodies. J Lipid Res 22: 222–223, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osborn TM, Dahlgren C, Hartwig JH, Stossel TP. Modifications of cellular responses to lysophosphatidic acid and platelet-activating factor by plasma gelsolin. Am J Physiol Cell Physiol 292: C1323–C1330, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Osborn TM, Verdrengh M, Stossel TP, Tarkowski A, Bokarewa M. Decreased levels of the gelsolin plasma isoform in patients with rheumatoid arthritis. Arthritis Res Ther 10: R117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, Chalfant CE, Obeid LM, Hannun YA. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J 17: 1411–1421, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Rosen H, Sanna G, Alfonso C. Egress: a receptor-regulated step in lymphocyte trafficking. Immunol Rev 195: 160–177, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Rosen H, Sanna MG, Cahalan SM, Gonzalez-Cabrera PJ. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol 28: 102–107, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Rouach N, Pebay A, Meme W, Cordier J, Ezan P, Etienne E, Giaume C, Tence M. S1P inhibits gap junctions in astrocytes: involvement of G and Rho GTPase/ROCK. Eur J Neurosci 23: 1453–1464, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, Rosen H. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem 279: 13839–13848, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Sasaki H, Arai H, Cocco MJ, White SH. pH dependence of sphingosine aggregation. Biophys J 96: 2727–2733, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato K, Malchinkhuu E, Horiuchi Y, Mogi C, Tomura H, Tosaka M, Yoshimoto Y, Kuwabara A, Okajima F. HDL-like lipoproteins in cerebrospinal fluid affect neural cell activity through lipoprotein-associated sphingosine 1-phosphate. Biochem Biophys Res Commun 359: 649–654, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Scherer M, Schmitz G, Liebisch G. High-throughput analysis of sphingosine 1-phosphate, sphinganine 1-phosphate, and lysophosphatidic acid in plasma samples by liquid chromatography-tandem mass spectrometry. Clin Chem 55: 1218–1222, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Sezen D, Bongiovanni AM, Gelber S, Perni U, Hutson JM, Skupski D, Witkin SS. Gelsolin down-regulates lipopolysaccharide-induced intraamniotic tumor necrosis factor-alpha production in the midtrimester of pregnancy. Am J Obstet Gynecol 200: 191e191–e194, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Sorensen SD, Nicole O, Peavy RD, Montoya LM, Lee CJ, Murphy TJ, Traynelis SF, Hepler JR. Common signaling pathways link activation of murine PAR-1, LPA, and S1P receptors to proliferation of astrocytes. Mol Pharmacol 64: 1199–1209, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Spiegel S, Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem 277: 25851–25854, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Spudich JA, Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem 246: 4866–4871, 1971 [PubMed] [Google Scholar]
- 48.Thomas AM, Tinker A. Determination of phosphoinositide binding to K+ channel subunits using a protein-lipid overlay assay. Methods Mol Biol 491: 103–111, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Winer JP, Janmey PA, McCormick ME, Funaki M. Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng Part A 15: 147–154, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Yin HL, Stossel TP. Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature 281: 583–586, 1979 [DOI] [PubMed] [Google Scholar]