Abstract
A mouse embryonic stem (ES) cell line containing an inducible transgene for the proneural gene Neurog1 has been used to generate glutamatergic neurons at a high efficiency. The present study used in vitro electrophysiology to establish the timeline for acquiring a functional neuronal phenotype in Neurog1-induced cells exhibiting a neuronal morphology. TTX-sensitive action potentials could be evoked from over 80% of the cells after only 4.5 days in vitro (DIV). These cells uniformly showed rapidly adapting responses to current injection, firing one to three action potentials at the onset of the stimulus. In the absence of Neurog1, a limited number of ES cells adopted a neuronal morphology, but these cells displayed slow calcium depolarizations rather than sodium-based spikes. Voltage-gated Na+, K+, and Ca2+ currents were present in nearly all induced cells as early as 4.5 DIV. The voltage-dependent properties of these currents changed little from 4 to 12 DIV with half-activation voltage varying by <10 mV for any current type throughout the culture period. This study demonstrates that forced expression of proneural genes can induce ES cells to quickly acquire a functional neuronal phenotype with mature electrophysiological properties. Transient overexpression of Neurog1 may be used in neural repair strategies that require the rapid induction of functional neurons from pluripotent stem cells.
Keywords: neuronal differentiation, neurogenesis, electrophysiology, basic helix-loop-helix, voltage-gated, ion channel
the engineering of embryonic stem (ES) cells holds great promise for the treatment of neurodegenerative disease and neurological trauma, but many challenges remain. Major advancements will require new strategies for guiding lineage determination, differentiation, and integration into a highly organized, heterogeneous environment. One exciting approach involves the ex vivo induction of ES cells into postmitotic neural progenitors or mature neuronal subtypes before implantation (36). By first directing differentiation to a neuronal lineage, the risk of ongoing ES cell proliferation after transplantation is reduced. Predifferentiation also allows control over the exogenous and endogenous factors influencing the development of mature phenotypic traits. However, the ability to specify distinct axodendritic morphology, discharge patterns, synaptic structure, and neurotransmitter systems remains a distant goal. Therefore, an essential step toward developing viable stem cell therapies is a greater understanding of the proneural signaling cascades that end in a desired structure and function.
Neural commitment can be induced in vitro by exposure to media supplements or coculture with feeder cell layers (for review, see Ref. 35). Alternatively, a neural fate can be forced by overexpression of proneural genes (34, 40). In a previous study, forced expression of the proneural gene neurogenin-1 (Neurog1) in mouse ES cells rapidly and efficiently produced β-tubulin-positive cells with markers for glutamatergic neurochemistry (34). Neurog1 is a basic helix-loop-helix transcription factor that promotes determination of a neuronal lineage while inhibiting gliogenesis (41). Expression of Neurog1 is a key event in sensory system development (for review, see Ref. 21), where deletion of this gene leads to the loss of progenitor cells in the trigeminal and otic placodes (26) and contributes to the loss of sensory neurons of the dorsal root ganglia (25). In the auditory periphery, loss of Neurog1 results in the complete absence of afferent and efferent neurons of the spiral ganglion even though target cells in the cochlea are structurally normal (24).
Neurons derived from Neurog1-induced ES cells are ideal candidates to replace or regenerate sensory cranial nerves such as the vestibulocochlear ganglion. However, progression toward function requires events far downstream of Neurog1, since neither this gene nor its immediate effector NeuroD directly induces genes encoding neurotransmitters, neuroreceptors, or ion channels (38). In the present study, we characterized the excitability of Neurog1-induced ES cells to determine whether induction of Neurog1 alone is sufficient to drive functional maturation. Electrical properties were monitored in culture for up to 12 days to determine the rapidity of acquiring a stable pattern of excitability and to determine whether Neurog1 induction further specifies a unique, homogeneous subtype. Our results indicated that Neurog1 overexpression efficiently and rapidly produced excitable cells that adopted a neuronal morphology and expressed voltage-gated Na+, Ca2+, and K+ channels within 4 days after induction. The majority of these cells also were capable of firing sodium-based action potentials, making them attractive candidates for therapies requiring regeneration of glutamatergic sensory neurons.
MATERIALS AND METHODS
Cell culture and in vitro differentiation.
Neurons were derived from an ES cell line containing a tetracycline-inducible Neurog1 transgene and an enhanced green fluorescent protein cassette driven by the constitutive ubiquitin ligase C promoter, as described previously (34). Undifferentiated ES cells of this line were maintained in sterile-filtered DMEM (Invitrogen, Carlsbad, CA) with 10% stem cell-compatible fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 5% embryonic stem cell supplement (DMEM with 21.65 mg/ml HEPES buffer, 3.63 mg/ml l-glutamine, and 63.63 ng/ml β-mercaptoethanol) and 0.5 μg/ml leukemia inhibitory factor (recombinant human LIF, Millipore, Bedford, MA). ES cells were plated for in vitro differentiation in serum-free media consisting of 80% DMEM-F-12, 20% Neurobasal, and 10 mM sodium pyruvate (Invitrogen) supplemented with B27 and N2 (1×; Invitrogen). Cells were plated at a density of 5 × 105 cells per well in 6-well dishes filled with 13-mm diameter Thermanox cell culture coverslips (Nunc, Naperville, IL) coated with 0.1% gelatin (Sigma). Doxycycline (Dox) (1 μg/ml; Sigma, St. Louis, MO) was added to the differentiation media for 72 h in experimental groups. After 72 h, Dox was washed off with 1× Hanks' balanced salt solution (HBSS, Gibco) and exchanged for normal differentiation media without Dox. Control cells were simultaneously grown in differentiation media without the addition of Dox. All cultures were maintained in a humidity-controlled incubator at 37°C in the presence of 5% CO2.
Immunocytochemistry.
Cells were prepared for immunocytochemistry by fixing with 4% paraformaldehyde for 10 min at room temperature. After blocking with Universal Blocking Reagent (Bio Genex, San Ramon, CA), the cells were incubated overnight at 4°C with a rabbit polyclonal antibody to the neuronal marker TUJ1 (class III-tubulin, 1:300; Covance, Madison, WI), and a rabbit monoclonal antibody NaV1.6 (1:150; NeuroMab clone K87A/10, Davis, CA). The specificity of the NaV1.6 antibody has been confirmed using mutant mice devoid of NaV1.6 (31). Primary antibodies were diluted in 0.1% Triton X-100 in phosphate-buffered saline. AlexaFluor secondaries (1:500; Invitrogen) were applied for 2 h at room temperature to visualize primary labeling. Control preparations were treated in parallel, except for the exclusion of primary antibodies.
Electrophysiology.
Electrophysiological studies were performed on cells with multipolar or monopolar morphology (Fig. 1A) at 2–12 days in vitro (DIV). Whole cell current- and voltage-clamp recordings were performed at room temperature (21–23°C) using an Axopatch 200B amplifier with a Digidata 1200 or 1322A digitizer (Molecular Devices, Sunnyvale, CA). Data were acquired using pCLAMP 9 software (Molecular Devices). Glass electrodes were pulled from borosilicate capillaries (1B100F-4, World Precision Instruments, Sarasota, FL) to achieve an electrical resistance of 3–6 MΩ. Electrodes were coated with R6101 (Dow Corning, Midland, MI) to reduce stray capacitance. Electrodes were filled with an internal solution containing (in mM) 112 KCl, 2 MgCl2, 0.1 CaCl2, 11 EGTA, 10 HEPES, and 5 Na2ATP and buffered to pH 7.2 with KOH. The external solution contained (in mM) 137 NaCl, 5 KCl, 1.7 CaCl2, 1 MgCl2, 10 HEPES, and 16 glucose and was buffered to pH 7.2 with NaOH. Voltage-dependent properties of Na+, Ca2+ , and K+ channels were measured under voltage-clamp mode. To record Na+ or Ca2+ currents, KCl in the internal solution was replaced by CsCl (120 mM). In addition, 20 mM tetraethylammonium-Cl (TEA-Cl) was added in both internal and external solutions to completely block K+ currents. To further isolate Na+ or Ca2+ currents, the external solution contained 0.5 mM NiCl2 and 0.1 mM CdCl2 to block Ca2+ channels or 100 nM TTX to block Na+ channels, respectively. To record K+ current, 100 nM TTX, 0.5 mM NiCl2, and 0.1 mM CdCl2 were added to the external solution. All chemicals used in the electrophysiology studies were purchased from Sigma. In all configurations, data were sampled at 10–20 kHz and low-pass filtered with a 4-pole Bessel filter at a 5-kHz cutoff frequency. Leak currents and capacitative transients were subtracted on-line. A liquid junction potential of −4 mV was corrected in all figures. Uncompensated series resistance was not corrected. Averaged results were obtained by pooling cells collected over consecutive days (e.g., 4.5 DIV from cells recorded at days 4 and 5 after plating). Three different culture durations were tested: 4.5, 8.5, and 11.5 DIV.
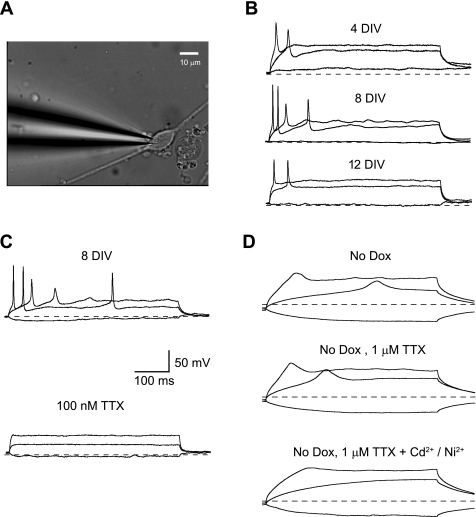
Fig. 1.
Firing properties of Neurog1-induced mouse embryonic stem (ES) cells. A: an example micrograph is shown to illustrate a typical cell targeted in electrophysiological studies. B: cells with a neuronal morphology were capable of firing action potentials for at least 9 days following 3 days of Neurog1 induction. Current-clamp recordings are shown for 3 cells during this culture period. Cells were held around −80 mV (dashed lines). Note that multiple action potentials were recorded in the cell at 8 days in vitro (DIV), but spikes were rapidly adapting in all cases. C: action potentials induced by Neurog1 were blocked by 100 nM TTX. D: uninduced cells spontaneously adopting a neuronal morphology were incapable of firing calcium-based action potentials. Exemplar current-clamp recordings are shown for an uninduced cell at 12 DIV. Slow depolarizations were elicited by injected currents (top). External application of 1 μM TTX had no effect (middle), whereas application of 0.1 mM Cd2+ and 0.5 mM Ni2+ blocked the active depolarizations (bottom). Scale shown in C applies to all traces in the figure. Dox, doxycycline.
Data analysis.
Peak and steady-state currents were analyzed in Clampfit, part of the pCLAMP 9 software suite. For Na+ and Ca2+ currents, the voltage sensitivity of activation was determined from conductance-voltage (G-V) curves. In these instances, conductance was determined from peak currents and estimates of the reversal potential for each current type. For K+ currents, activation curves were constructed from tail currents elicited by a final step to −24 mV. Normalized activation curves were fit to a single-order Boltzmann equation, G/Gmax or I/Imax = 1/{1 + exp[(V1/2 − V)/k]}, where V1/2 is the half-activation voltage, V is the voltage command, Imax is maximum current, and k is the Boltzmann slope. The voltage sensitivity of inactivation was determined from tail currents at −24 mV following 4-s activation steps. Normalized curves were fit to a single-order Boltzmann equation. Averages are presented as means ± SE. When comparing changes in various parameters across DIV groups, a one-way analysis of variance (ANOVA) was conducted with a Tukey-Kramer post hoc test for significant differences between the 4.5 DIV group and each of the older cultures. The criterion for a statistically reliable difference in any comparison was P < 0.05.
RESULTS
Excitability of Neurog1-induced cells.
Induction of Neurog1 for 72 h produced a high percentage of mouse ES cells acquiring a neuron-like morphology (Fig. 1A), as described previously (34). Current injection in those cells extending at least one long, thin process elicited fast action potentials as early as 4 DIV, and their excitability could be maintained for at least 12 DIV (Fig. 1B). Exposure to TTX eliminated these spikes, indicating that evoked action potentials were due to the activity of voltage-gated sodium channels (Fig. 1C).
The passive and active electrical properties of induced cells were assessed at three time points during culture (4.5, 8.5, and 11.5 DIV). These results are summarized in Table 1. Cell size, estimated from membrane capacitance (Cm), was relatively constant throughout this time period (P > 0.05). On average, tested neurons had resting potentials around −40 mV with no statistically reliable changes over time in culture. Intracellular chloride can have a large impact on cell resting potential and excitability (4, 42). Therefore, several cells at 4.5 DIV were examined using an alternate pipette solution, replacing 100 mM KCl with equal molar K-gluconate. The average resting potential was −40.4 ± 2.1 mV in KCl and −38.8 ± 1.3 mV in K-gluconate (n = 7). The difference in the means was statistically insignificant (P > 0.05), indicating that the relatively depolarized resting potential in these cells cannot be attributed to a resting chloride conductance. At early points in the culture, shortly after Neurog1 induction, 88% of the targeted cells were capable of generating action potentials. By 11.5 DIV, action potentials could be evoked in 100% of the tested cells. In most cases, a hyperpolarizing pre-step was required to elicit action potentials. At 4.5 and 11.5 DIV, 90% of the neurons fired only one action potential at any current step. There was a small increase in the average maximum number of action potentials at 8.5 DIV with over 70% of the neurons in this group firing multiple action potentials. Although these differences in spike number were statistically reliable (P < 0.05), the maximum number of spikes remained, on average, between 1 and 3. Therefore, in all culture groups, firing patterns were rapidly adapting (i.e., few spikes, occurring only at the onset of the current-clamp stimulus). In addition to time-dependent changes in maximum spike number, action potential threshold varied with DIV, becoming more positive with increasing time in culture (P < 0.05). Although the resting ionic currents appeared to be homogeneous and mature by 4 DIV, the conductances responsible for spike threshold and frequency were modulated over time.
Table 1.
Intrinsic properties and firing patterns of Neurog1-induced cells
| 4.5 DIV | 8.5 DIV | 11.5 DIV | |
|---|---|---|---|
| Cm, pF | 10.82 ± 0.45 (32) | 11.17 ± 0.62 (39) | 12.26 ± 0.99 (40) |
| Resting potential, mV | −40.4 ± 2.1 (22) | −41.0 ± 1.5 (25) | −40.2 ± 1.3 (24) |
| % of Cells capable of generating APs | 88.0 % (22/25) | 89.3 % (25/28) | 100 % (24/24) |
| % of Cells firing multiple APs | 9.1 % (2/22) | 76.0% (19/25) | 12.5 % (3/24) |
| Maximum number of APs | 1.6 ± 0.4 (22) | 3.0 ± 0.4 (25)* | 1.4 ± 0.3 (24) |
| AP threshold, mV | −38.2 ± 1.7 (22) | −35.9 ± 1.5 (25) | −33.4 ± 1.0 (25)* |
Values are means ± SE; nos. in parentheses are sample size. Cm, membrane capacitance; AP, action potential.
P < 0.05, using post hoc Tukey-Kramer test compared with 4.5 days in vitro (DIV).
In contrast, uninduced control cells rarely differentiated into neural-like cells with active voltage responses. A previous report noted that this ES cell line did not spontaneously differentiate into TUJ1-positive neurons within 5 DIV (34). Our cells were cultured for longer periods of time, so it was important to determine whether uninduced cells adopted neuronal traits after 5 DIV. The number of TUJ1-positive cells was assessed for control and induced cultures at 10 DIV. Similar to the selection rubric for electrophysiological assays, we counted only those cells with clearly defined neurites. On the basis of these data, only 1–5% of the neurons in induced cultures could be attributed to spontaneous differentiation of ES cells. The majority of control cells at 11–12 DIV exhibited passive responses to current steps (7 of 12 cells, 58%). Several others responded with small, broad depolarizations superimposed on passive membrane responses (4 of 12 cells, 33%; Fig. 1D). Half-widths for the active phase of these depolarizations exceeded 15 ms. External application of TTX (1 μM) had no effect, but Ca2+ channel antagonists (0.1 mM Cd2+ and 0.5 mM Ni2+) eliminated these depolarizations. Therefore, the active responses in these control cells were carried by Ca2+ current rather than Na+. Only one cell in the control group fired brief action potentials with half-widths <4 ms (data not shown). The shape, frequency, and latency associated with the response of this cell were consistent with those data from the TTX-sensitive Neurog1-induced cells, indicating that a small percentage of the control cells adopt a functional phenotype by 12 DIV (1 of 12, 8%). Combined with the lower frequency of spontaneous differentiation, these data imply that firing properties recorded from Dox-induced cells could be associated with Neurog1 overexpression with confidence.
Properties of Na+ currents.
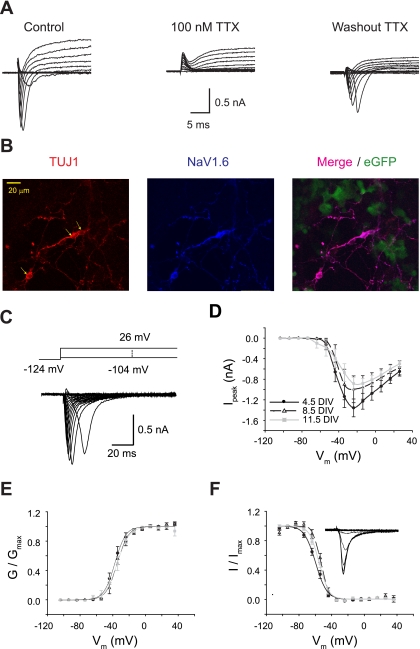
Under voltage-clamp conditions, fast-activating and inactivating inward currents in Neurog1-induced cells were completely blocked by the external application of 100 nM TTX (Fig. 2A; 5 of 5 cells at 4 DIV), indicating the dominance of TTX-sensitive sodium channel subtypes in these cells. Some preparations were stained with an antibody to NaV1.6, a sodium channel α-subunit common in neurons and responsible for fast-inactivating, TTX-sensitive currents (Fig. 2B). Overlap between TUJ1- and NaV1.6-positive cells was 100% (594 of 594 cells observed, 5 DIV), which correlates well with the high percentage of 4.5 DIV cells exhibiting Na+ currents and capable of generating action potentials (>80%). Moreover, NaV1.6 was absent from TUJ1-negative cells. Thus, the presence of NaV1.6 suggests that this channel contributes to the excitability of Neurog1-induced neurons.
Fig. 2.
Na+ channels in Neurog1-induced mouse ES cells. A: a voltage-clamp recording from a 5 DIV cell is shown, demonstrating the acquisition of both inward and outward currents from the progressively depolarizing voltage commands. TTX reversibly blocked transient inward currents. B: immunostaining of NaV1.6 and TUJ1 on Neurog1-induced cells was colocalized and exclusively found on cells extending thin processes. eGFP, enhanced green fluorescent protein. C: Na+ currents were isolated using K+ and Ca2+ channel blockers. Voltage-dependent fast-inactivating Na+ currents are shown for one cell recorded at 9 DIV. Voltage commands are shown above the current traces. D: averaged current-voltage (I-V) relationships for peak Na+ currents (Ipeak) are shown for each culture group: 4.5 DIV (n = 22), 8.5 DIV (n = 14), and 11.5 DIV (n = 12). Vm, cell membrane potential. E: the voltage dependence of Na+ channel activation was determined from the I-V relationships in D. Normalized curves from each culture group were overlapping. The averaged half-activation potentials (V1/2) from individual Boltzmann fits were −36.1 ± 2.3 mV at 4.5 DIV, −34.2 ± 2.3 mV at 8.5 DIV, and −34.5 ± 2.8 mV at 11.5 DIV. Gmax, maximum conductance. F: voltage-dependent inactivation was determined from tail currents (inset), averaged, and plotted for each culture group: 4.5 DIV (n = 20), 8.5 DIV (n = 12), or 11.5 DIV (n = 16). The small rightward shift in V1/2 across DIV was statistically insignificant. The averaged half-inactivation potentials for individual fits were −57.6 ± 1.9 mV at 4.5 DIV, −54.4 ± 1.4 mV at 8.5 DIV, and −52.7 ± 1.3 mV at 11.5 DIV. Imax, maximum current.
Sodium currents were isolated with potassium and calcium channel blockers and elicited from a preconditioning step of −124 mV (Fig. 2C). Current-voltage (I-V) relationships were obtained from peak Na+ currents and averaged for cells at 4.5, 8.5, and 11.5 DIV (Fig. 2D). Sodium currents activated near −50 mV and peaked at about −20 mV, regardless of time after differentiation. Average peak current appeared to decrease over time in culture, but a one-way ANOVA across all three culture groups did not reach the significance criterion. The voltage dependence of Na+ channel activation (Fig. 2E) and inactivation (Fig. 2F) were relatively constant over DIV. Differences in half-activation and half-inactivation voltage across culture duration were statistically insignificant (P > 0.05).
Properties of Ca2+ currents.
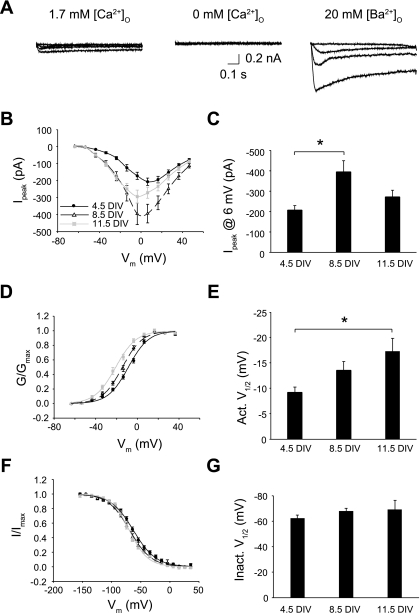
A slowly inactivating inward current was revealed by blocking both K+ channels and TTX-sensitive Na+ currents (Fig. 3A). This current was eliminated by removing external Ca2+ (5 of 5 cells) but was augmented when Ba2+ was used as the charge carrier. Thus, this residual inward current was attributed to voltage-gated Ca2+ channel activity. Peak Ba2+ current changed nonmonotonically during the 12-day culture period (Fig. 3B). Post hoc tests verified that the increase in current at 8.5 DIV compared with 4.5 was reliable (P < 0.05; Fig. 3C). Voltage-dependent activation curves shifted progressively to more negative values with time in culture (P < 0.05; Fig. 3, D and E). In contrast, the voltage dependence of inactivation was unchanged during the culture period (P > 0.05; Fig. 3, F and G).
Fig. 3.
Ca2+ channels in Neurog1-induced mouse ES cells. A: inward Ca2+ currents are shown for a recording from a 7 DIV cell. Recordings were made in normal external Ca2+ (1.7 mM) (left), in the absence of external Ca2+ (middle), and in 20 mM Ba2+/0 mM Ca2+ (right). [Ca2+]o, extracellular Ca2+ concentration; [Ba2+]o, extracellular Ba2+ concentration. B: peak Ba2+ currents were averaged and plotted against membrane voltage: 4.5 DIV (n = 23), 8.5 DIV (n = 33), and 11.5 DIV (n = 19). C: peak Ba2+ currents at steps to 6 mV are shown, revealing a nonmonotonic change in current level across culture duration. D: voltage-dependent activation was determined from peak currents. Conductance-voltage curves shifted leftward with increasing duration in culture (P < 0.05). E: averaged half-activation voltages (Act V) from individual Boltzmann fits were significantly different across DIV. F: voltage-dependent inactivation was determined from tail currents and averaged for each culture group: 4.5 DIV (n = 9), 8.5 DIV (n = 22), or 11.5 DIV (n = 18). G: the averaged half-inactivation voltage (Inact V) from individual curve fits was independent of DIV. *P < 0.05 between groups.
Properties of noninactivating K+ currents.
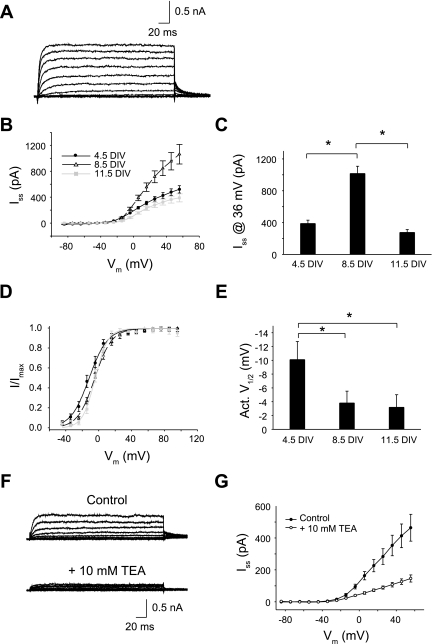
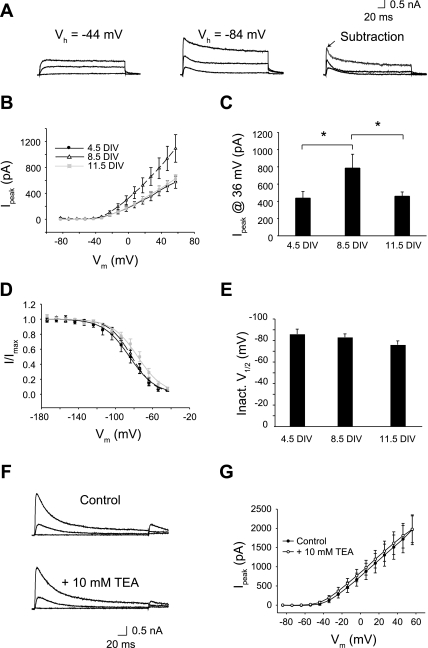
To characterize the properties of K+ currents, recordings were made in the presence of Na+ and Ca2+ channel blockers. Noninactivating K+ currents were recorded by stepping to command potentials from a holding of −44 mV. An example from a cell at 9 DIV is shown in Fig. 4A. Developmental changes in steady-state I-V curves revealed a time-dependent change in current response (Fig. 4B), which reached a maximum at 8.5 DIV [P < 0.05 for one-way ANOVA across DIV using steady-state currents (Iss) at 36 mV; Fig. 4C]. Activation curves were constructed from normalized tail currents (Fig. 4D). Currents activated above −40 mV regardless of DIV, but half-activation voltage shifted to more positive potentials with longer culture times (Fig. 4E). This shift was small—only about 7 mV—but reliable (P < 0.05). According to post hoc tests, differences between 4.5 DIV and each of the other longer time points were statistically significant, indicating a sudden shift in V1/2 after 4.5 DIV (P < 0.05; Fig. 4E).
Fig. 4.
Noninactivating K+ channels in Neurog1-induced mouse ES cells. A: noninactivating K+ currents are shown for one cell recorded at 9 DIV in response to depolarizing steps from a holding potential of −44 mV. B: I-V relationships of steady-state currents (Iss) were averaged for each culture group: 4.5 DIV (n = 14), 8.5 DIV (n = 13), and 11.5 DIV (n = 12). C: Iss changed nonmonotonically, peaking at 8.5 DIV. D: voltage-dependent activation was determined from normalized tail currents (I/Imax) in each culture group: 4.5 DIV (n = 12), 8.5 DIV (n = 19), or 11.5 DIV (n = 12). E: average half-activation voltages from individual Boltzmann fits revealed a significant rightward shift in voltage sensitivity over DIV. F: as shown for one cell at 11 DIV, noninactivating K+ currents were sensitive to tetraethylammonium (TEA). G: the averaged I-V relationships indicate that 10 mM TEA blocked 70% of noninactivating K+ currents (n = 8). *P < 0.05 between groups.
Noninactivating K+ currents were sensitive to external TEA (Fig. 4F). Steady-state currents at maximum conductance levels were 70% blocked by 10 mM TEA (Fig. 4G). Although the current amplitude was affected by TEA, the voltage-response of the I-V curves was similar between control and TEA solutions.
Properties of inactivating K+ currents.
Inactivating outward currents were isolated by subtracting currents elicited from a holding at −84 mV from those using a holding of −44 mV (Fig. 5A). Similar to noninactivating K+ currents, the peak amplitudes of the A-type currents were voltage dependent (Fig. 5B), with maximal values at 8.5 DIV (P < 0.05, post hoc test between 4.5 and 8.5 DIV at 36 mV; Fig. 5C). The voltage dependence of channel inactivation was tested with hyperpolarizing preconditioning steps. Despite a slight rightward shift in voltage dependence (Fig. 5D), half-inactivation voltage was unchanged over the culture period (P > 0.05; Fig. 5E). Inactivation rates were best fit with a double exponential curve. For currents elicited by the step to 96 mV, there were no statistically reliable differences in the fast or slow time constants across DIV (P > 0.05). When the data were pooled together, the average fast time constant was 14.6 ± 2.3 ms and the slow was 100.6 ± 13.5 ms. Unlike the noninactivating K+ currents, inactivating currents were insensitive to 10 mM external TEA (Fig. 5, F and G).
Fig. 5.
Inactivating K+ currents in Neurog1-induced mouse ES cells. A: inactivating K+ currents are shown for one cell at 9 DIV. Activation steps in this example included −14 mV, 16 mV, and 56 mV. Only noninactivating K+ currents were recorded from a holding of −44 mV (left). Inactivating currents were revealed when stepping from a holding of −84 mV (middle) and isolated by subtraction (right). Vh, holding potential. B: I-V relationships were determined from peak currents (arrow in A) and averaged for each culture group: 4.5 DIV (n = 13), 8.5 DIV (n = 10), and 11.5 DIV (n = 14). C: as with noninactivating K+ currents, the magnitude of inactivating K+ currents changed with duration in culture, peaking at 8.5 DIV. D: the voltage dependence of inactivation was determined from tail currents, normalized, and averaged for each culture group: 4.5 DIV (n = 9), 8.5 DIV (n = 13), or 11.5 DIV (n = 12). E: average half-inactivation voltages from individual Boltzmann fits were statistically independent of DIV. F: inactivating K+ currents were insensitive to TEA, as shown for one cell at 9 DIV. G: averaged I-V relationships constructed from peak inactivating K+ currents were overlapping in control saline and TEA (n = 8). *P < 0.05 between groups.
DISCUSSION
The results of this study show that transient, forced overexpression of Neurog1 is sufficient to rapidly drive ES cells from pluripotency to fate determination to function. By 4 DIV, all induced cells adopting a neuronal morphology acquired voltage-gated conductances and over 80% were capable of firing fast Na+ spikes. Although it remains to be seen whether these cells form patent synapses, our data join prior molecular and chemical evidence (34), indicating the adoption of a functional neuronal phenotype. Excitability was maintained for at least 12 days in culture, and the overall electrical properties of the cells were relatively homogeneous up to this time point.
In general, mature neurons are differentiated from ES cells by first coaxing these cells to adopt a neuronal lineage (1, 8, 46) followed by differentiation using exogenous factors or removal of mitogenic cues (35). Numerous studies have demonstrated the production of neuron-like cells using these methodologies, but assessment of a neuronal phenotype is typically limited to morphological and histological descriptions. When assessed for functionality, the outcome is highly variable. One study reported the absence of voltage-gated sodium channel activity in morphologically and biochemically mature “neurons” (6). In other reports, Na+ action potentials were elicited from a moderate to high percentage of stem cell-derived neurons (30–100%) (5, 12, 22). Overexpression of proneural genes is an attractive alternative approach for initiating neuronal differentiation. Although Neurog1 does not directly target genes involved in neuronal excitability (38), overexpression of this transcription factor in ES cells initiates a signaling program that ultimately leads to a neuronal phenotype without intermediate culture steps or specialized media.
Forced expression of Neurog1 also appeared to bypass some transitional steps in neurogenesis. In many neurons, early phases of development are characterized by spontaneous or evoked Ca2+ action potentials that guide maturation, synaptogenesis, and network formation (28, 39). Spontaneous differentiation of ES cells in the uninduced cultures produced neural-like cells that may have been following this maturation program, since many of these cells exhibited broad, slow, Ca2+-based responses by 12 DIV. In contrast, Na+ action potentials appeared shortly after Neurog1 induction, and Ca2+ action potentials were absent from induced neurons. Recordings from neurons during the 72-h induction phase showed initial acquisition of outward potassium currents followed by inward sodium currents and possibly small levels of calcium currents (data not shown). Although intrinsic excitability may be required for development of neuronal circuits, these transitional phases may not be necessary in applications that require mature phenotypes.
For future therapeutic strategies, it will be important to associate phenotypic traits with the transcriptional signaling cascades initiated by proneural genes. Therefore, we must identify the relevant ion channel genes induced by Neurog1 signaling. Ion channel families span a large number of genes, and their functional properties are diverse. The biophysical properties of many of these channels are cataloged by the International Union of Pharmacology in a series of compendia (for overview, see Ref. 45). Data from the compendia and other published literature were compared with that reported in the present study to identify classes of channels most likely involved in the excitability of our derived neurons. Conclusions drawn from voltage-clamp data focused on broad classes of ion channel types rather than specific configurations, since errors could be introduced by poor spatial control of the cell membrane potential. Space-clamp errors result from a delay in the activation of channels distant from the electrode site. In general, large notches or bumps were absent from voltage-clamp current traces, but subtle distortions could be seen in the Na+ currents at voltage steps near threshold (Fig. 2C). Some caution should be exercised when considering the kinetics and peak amplitudes of these currents. Poor spatial control would underestimate peak current and the steepness of voltage-dependent activation (7, 19). As a result, if space-clamp errors were large, differences in peak currents should be reflected in variable or skewed activation curves. However, peak Na+ currents varied considerably across DIV (Fig. 2D) while the shape of activation curves was unchanged (Fig. 2E). Therefore, space-clamp effects on channel kinetics, current amplitudes, and voltage sensitivity are expected to be small.
Voltage-gated Na+ channels can be divided into two categories based on their sensitivity or resistance to TTX. In our study, Na+ current was eliminated by TTX, indicating that resistant isoforms NaV1.5, NaV1.8, and NaV1.9 played no role in Neurog1-induced excitability. Many of the remaining TTX-sensitive subunits are found in sensory neurons of the dorsal root (9, 15), trigeminal (43), and vestibulocochlear ganglia (16, 20). NaV1.6 is common to each of these neural populations and appears to play an important role in Neurog1-induced ES cells as well (Fig. 2B). Further study is required to determine whether other NaV subunits are expressed and whether these also contribute to the excitability of our cells.
The noninactivating, or delayed rectifier, K+ currents were sensitive to high voltages, with a V1/2 near −3 mV in later cultures. Typically, high voltage-activated K+ channels shape the action potential waveform but play less of a role in governing spike threshold and latency. In our cells, the voltage activation range for these currents was unchanged after block with TEA, suggesting the presence of a single delayed rectifier with partial sensitivity to 10 mM TEA. Channels with these traits include the Kv2 family as well as Kv3.1 and Kv3.2. Most of these channels can be found in sensory neurons (10, 17, 32), and Kv2.1 and Kv3.1 are specifically expressed in the inner ear (2, 44). Therefore, Kv2 and Kv3 delayed rectifiers are strong candidates for governing the excitability of Neurog1-induced ES cells.
Our data also revealed inactivating, or A-type, K+ currents. In general, A-currents control neuronal excitability by adjusting spike rate and by altering spike duration during a sustained stimulus. In our study, these currents were insensitive to TEA, downplaying a role for the TEA-sensitive Kv3.3 and Kv3.4 subunits. Instead, the insensitivity to TEA and the kinetics of inactivation point to the involvement of Kv1.4 or channels in the Kv4 family, which also are found in nociceptive ganglia (14, 23), olfactory sensory neurons (18), and primary auditory neurons (2).
In addition to voltage-sensitive Na+ and K+ currents, induced neurons expressed a small voltage-gated Ca2+ current that could limit firing frequency by activating Ca2+-sensitive potassium channels or guide other processes including neurite growth, cell motility, and neurotransmitter release (30, 48). The high activation range of this current is inconsistent with the induction of T-type calcium channels. Other varieties (i.e., L, N, P, Q, or R) are all commonly found in sensory neurons, often segregating by type into different subcellular domains. To determine the putative role(s) of this current, it will be critical to localize contributing Ca2+ channels to specific axosomatic compartments.
While the voltage dependence of activation and inactivation changed little during the culture period, K+ and Ca2+ currents showed a nonmonotonic change in amplitude and Na+ currents decreased in amplitude during the culture. These changes in current density likely contributed to time-dependent changes in action potential number and spike threshold. These effects may reflect changes in channel density in individual neurons over time. Alternatively, differences in mean current levels may reflect sampling from different subpopulations of neurons with variable properties, rates of differentiation, and survivability. This alternative seems unlikely since time-dependent changes in K+ and Ca2+ current amplitudes were not mirrored by Na+ currents. These effects occur days after the transient induction of Neurog1. It is unclear whether the mechanism is intrinsic or extrinsic. The remodeling of channel density over time could result from the gradual unfolding of a long transcriptional program initiated by Neurog1 or the influence of a changing microenvironment, or both. It will be important to reexamine this effect over longer culture periods to identify the stable, mature electrical signature of these cells. Likewise, it will be important to determine the influence of trophic and tropic cues within the microenvironment. Such data will be essential for predicting how these cells will respond in in vivo applications.
Although we found at least some varieties of Na+, Ca2+, and K+ channels in differentiated cells, other key voltage-dependent ion channels were not detected, including several classes that play important roles in regulating cell resting potential. For example, in voltage-clamp recordings with steps from −174 to −44 mV, we did not find currents resembling inward rectifiers, low-voltage-activated K+ channels, or hyperpolarization-activated cyclic nucleotide-gated channels responsible for so-called H-currents. Each of these channel classes could impact resting potential as well as contribute to spike threshold, shape, and frequency. In our cells, the resting potential was near −40 mV, regardless of culture duration (Table 1). Since the threshold for Na+ channel activation was also about −40 mV, again regardless of culture duration, many of these channels were inactivated under resting conditions. As a result, induced neurons infrequently spiked from rest in response to injected currents. Spikes were apparent only after first hyperpolarizing the cell with a conditioning pre-step. This prehyperpolarization is a common requirement in stem cell-derived neurons (12, 22, 27). It is possible that a mature phenotype has not been reached, a required cofactor is missing, or the derived neurons are not in fact normal. Neurotrophic factors or further genetic manipulation could be used to drive resting to more negative potentials. These strategies also may allow customization of transmitter and firing subtypes, enabling a wide range of phenotypic traits. Alternatively, if ES cell-derived neurons are used in conjunction with biohybrid implant devices, stimulation paradigms could be adjusted to release Na+ channel inactivation and enable spike propagation.
Sensory neurons in the auditory periphery, trigeminal system, and dorsal root ganglia exhibit a variety of firing patterns, including bursting and repetitive firing, but strongly adapting responses are the most commonly reported discharge pattern (11, 13, 37, 47). Forced expression of Neurog1 in mouse ES cells resulted in neurons with a rapidly adapting discharge rate or a single spike at the onset of the stimulus. Therefore, these cells are excellent candidates for replacement of degenerating sensory neurons. In addition, the ionic properties of these cells are generally in accordance with neurons in the central nervous system (3, 29, 33), making them excellent candidates for treating a broad range of neurodegenerative diseases. To fully explore these possibilities, it will be important to examine the neurotransmitter systems and ligand-gated channels upregulated by Neurog1. Finally, the model system described in the present study is ideal for exploring the influences of trophic factors, topology, and host tissues. By understanding the transcriptional events leading from induction to neural specification, stem cell therapy can begin to reach its full potential.
GRANTS
This work was supported by grants from the National Institutes of Health (T32 DC005356 and P30 DC005188).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Drs. Gerhard Hill, III, and Mark Crumling for comments on early drafts of the manuscript. The authors extend special thanks to Drs. Josef Miller and K. Sue O'Shea for suggestions and comments during the course of this project. The ES cell line was a generous gift of Dr. K. Sue O'Shea.
REFERENCES
- 1.Abranches E, Silva M, Pradier L, Schulz H, Hummel O, Henrique D, Bekman E. Neural differentiation of embryonic stem cells in vitro: a road map to neurogenesis in the embryo. PloS One 4: e6286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson CL, Reid MA, Mo ZL, Bowne-English J, Davis RL. Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J Comparative Neurol 447: 331–350, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Ahlenius H, Visan V, Kokaia M, Lindvall O, Kokaia Z. Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. J Neurosci 29: 4408–4419, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez-Leefmans FJ, Gamino SM, Giraldez F, Nogueron I. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J Physiol 406: 225–246, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev Biol 168: 342–357, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Balasubramaniyan V, de Haas AH, Bakels R, Koper A, Boddeke HW, Copray JC. Functionally deficient neuronal differentiation of mouse embryonic neural stem cells in vitro. Neurosci Res 49: 261–265, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Bar-Yehuda D, Korngreen A. Space-clamp problems when voltage clamping neurons expressing voltage-gated conductances. J Neurophysiol 99: 1127–1136, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Bibel M, Richter J, Schrenk K, Tucker KL, Staiger V, Korte M, Goetz M, Barde YA. Differentiation of mouse embryonic stem cells into a defined neuronal lineage. Nat Neurosci 7: 1003–1009, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Black JA, Dib-Hajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG. Spinal sensory neurons express multiple sodium channel alpha-subunit mRNAs. Brain Res Mol Brain Res 43: 117–131, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Bocksteins E, Raes AL, Van de Vijver G, Bruyns T, Van Bogaert PP, Snyders DJ. Kv2.1 and silent Kv subunits underlie the delayed rectifier K+ current in cultured small mouse DRG neurons. Am J Physiol Cell Physiol 296: C1271–C1278, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brocard F, Verdier D, Arsenault I, Lund JP, Kolta A. Emergence of intrinsic bursting in trigeminal sensory neurons parallels the acquisition of mastication in weanling rats. J Neurophysiol 96: 2410–2424, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Carpenter MK, Inokuma MS, Denham J, Mujtaba T, Chiu CP, Rao MS. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol 172: 383–397, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Catacuzzeno L, Fioretti B, Pietrobon D, Franciolini F. The differential expression of low-threshold K+ currents generates distinct firing patterns in different subtypes of adult mouse trigeminal ganglion neurones. J Physiol 586: 5101–5118, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chien LY, Cheng JK, Chu D, Cheng CF, Tsaur ML. Reduced expression of A-type potassium channels in primary sensory neurons induces mechanical hypersensitivity. J Neurosci 27: 9855–9865, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djouhri L, Newton R, Levinson SR, Berry CM, Carruthers B, Lawson SN. Sensory and electrophysiological properties of guinea-pig sensory neurones expressing Nav 1.7 (PN1) Na+ channel alpha subunit protein. J Physiol 546: 565–576, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fryatt AG, Vial C, Mulheran M, Gunthorpe MJ, Grubb BD. Voltage-gated sodium channel expression in rat spiral ganglion neurons. Mol Cell Neurosci 42: 399–407, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Gravagna NG, Knoeckel CS, Taylor AD, Hultgren BA, Ribera AB. Localization of Kv2.2 protein in Xenopus laevis embryos and tadpoles. J Comparative Neurol 510: 508–524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han P, Lucero MT. Pituitary adenylate cyclase activating polypeptide reduces expression of Kv1.4 and Kv42 subunits underlying A-type K(+) current in adult mouse olfactory neuroepithelia. Neuroscience 138: 411–419, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Hartline DK, Castelfranco AM. Simulations of voltage clamping poorly space-clamped voltage-dependent conductances in a uniform cylindrical neurite. J Comput Neurosci 14: 253–269, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Hossain WA, Antic SD, Yang Y, Rasband MN, Morest DK. Where is the spike generator of the cochlear nerve? Voltage-gated sodium channels in the mouse cochlea. J Neurosci 25: 6857–6868, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korzh V, Strahle U. Proneural, prosensory, antiglial: the many faces of neurogenins. Trends Neurosci 25: 603–605, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Li H, Liu H, Corrales CE, Risner JR, Forrester J, Holt JR, Heller S, Edge AS. Differentiation of neurons from neural precursors generated in floating spheres from embryonic stem cells. BMC Neurosci 10: 122, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Simon SA. Modulation of IA currents by capsaicin in rat trigeminal ganglion neurons. J Neurophysiol 89: 1387–1401, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol 1: 129–143, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Q, Fode C, Guillemot F, Anderson DJ. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev 13: 1717–1728, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma QF, Chen ZF, Barrantes ID, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron 20: 469–482, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Monedero R, Yi E, Oshima K, Glowatzki E, Edge AS. Differentiation of inner ear stem cells to functional sensory neurons. Dev Neurobiol 68: 669–684, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Moe MC, Varghese M, Danilov AI, Westerlund U, Ramm-Pettersen J, Brundin L, Svensson M, Berg-Johnsen J, Langmoen IA. Multipotent progenitor cells from the adult human brain: neurophysiological differentiation to mature neurons. Brain 128: 2189–2199, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Moody WJ, Bosma MM. Ion channel development, spontaneous activity, and activity-dependent development in nerve and muscle cells. Physiol Rev 85: 883–941, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron 59: 861–872, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Osorio N, Cathala L, Meisler MH, Crest M, Magistretti J, Delmas P. Persistent Nav1.6 current at axon initial segments tunes spike timing of cerebellar granule cells. J Physiol 588: 651–670, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozaita A, Martone ME, Ellisman MH, Rudy B. Differential subcellular localization of the two alternatively spliced isoforms of the Kv3.1 potassium channel subunit in brain. J Neurophysiol 88: 394–408, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Ravin R, Hoeppner DJ, Munno DM, Carmel L, Sullivan J, Levitt DL, Miller JL, Athaide C, Panchision DM, McKay RD. Potency and fate specification in CNS stem cell populations in vitro. Cell Stem Cell 3: 670–680, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Reyes JH, O'Shea KS, Wys NL, Velkey JM, Prieskorn DM, Wesolowski K, Miller JM, Altschuler RA. Glutamatergic neuronal differentiation of mouse embryonic stem cells after transient expression of neurogenin 1 and treatment with BDNF and GDNF: in vitro and in vivo studies. J Neurosci 28: 12622–12631, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson MJ, Gip P, Schaffer DV. Neural stem cell engineering: directed differentiation of adult and embryonic stem cells into neurons. Front Biosci 13: 21–50, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Rossi F, Cattaneo E. Opinion: neural stem cell therapy for neurological diseases: dreams and reality. Nature Rev 3: 401–409, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Rusznak Z, Szucs G. Spiral ganglion neurones: an overview of morphology, firing behaviour, ionic channels and function. Pflügers Arch 457: 1303–1325, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Seo S, Lim JW, Yellajoshyula D, Chang LW, Kroll KL. Neurogenin and NeuroD direct transcriptional targets and their regulatory enhancers. EMBO J 26: 5093–5108, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spitzer NC. Electrical activity in early neuronal development. Nature 444: 707–712, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Sugimoto Y, Furuno T, Nakanishi M. Effect of NeuroD2 expression on neuronal differentiation in mouse embryonic stem cells. Cell Biol Int 33: 174–179, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Sun Y, Nadal-Vicens M, Misono S, Lin MZ, Zubiaga A, Hua X, Fan G, Greenberg ME. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell 104: 365–376, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci 20: 7531–7538, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai CW, Tseng JJ, Lin SC, Chang CY, Wu JL, Horng JF, Tsay HJ. Primary structure and developmental expression of zebrafish sodium channel Na(v)1.6 during neurogenesis. DNA Cell Biol 20: 249–255, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Varela-Ramirez A, Trujillo-Provencio C, Serrano EE. Detection of transcripts for delayed rectifier potassium channels in the Xenopus laevis inner ear. Hearing Res 119: 125–134, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev 57: 387–395, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol 19: 1129–1133, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Zhang XF, Zhu CZ, Thimmapaya R, Choi WS, Honore P, Scott VE, Kroeger PE, Sullivan JP, Faltynek CR, Gopalakrishnan M, Shieh CC. Differential action potentials and firing patterns in injured and uninjured small dorsal root ganglion neurons after nerve injury. Brain Res 1009: 147–158, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Zheng JQ, Poo MM. Calcium signaling in neuronal motility. Annu Rev Cell Dev Biol 23: 375–404, 2007 [DOI] [PubMed] [Google Scholar]