Abstract
Pressure overload-induced cardiac hypertrophy results in a pathological type of hypertrophy with activation of signaling cascades like the extracellular signal-regulated kinase (ERK) pathway, which promotes negative cardiac remodeling and decreased contractile function. In contrast, thyroid hormone mediates a physiological type of hypertrophy resulting in enhanced contractile function. In addition, thyroid hormone action is diminished in pressure overload-induced cardiac hypertrophy. We hypothesized that thyroid hormone status modulates ERK activity and that administration of thyroid hormone could alter the activity of this kinase in cardiac hypertrophy induced by pressure overload. ERK is activated by phosphorylation; accordingly, we investigated phosphorylation of ERK in hearts of control, hypothyroid, and hyperthyroid mice. In addition, the effect of T3 treatment on ERK phosphorylation in hypertrophied hearts from transverse aortic-constricted (TAC) mice was investigated. Results showed that phosphorylated ERK (p-ERK) was decreased by 25% in hyperthyroid mice. In contrast, hypothyroid mice presented increased p-ERK by 80%. TAC mice presented a greater than fourfold increase of p-ERK compared with control mice. Interestingly, T3 administration dramatically canceled TAC-induced ERK phosphorylation (36% lower compared with control). Raf-1 is upstream of the ERK pathway. TAC mice presented a 45% increase in phospho-Raf-1 (Ser338). T3 treatment inhibited this effect of pressure overload and further decreased p-Raf-1 (Ser338) by 37%, compared with control. Overexpression of thyroid hormone receptor-α in cultured cardiomyocytes potentiated the inhibitory effect of T3 on ERK phosphorylation. We concluded that thyroid hormone has an inhibitory effect on the Raf-1/ERK pathway. Furthermore, treatment of TAC mice with T3 inhibited Raf-1/ERK pathway by a thyroid hormone receptor-dependent mechanism.
Keywords: MAPK, thyroid hormone receptors
cardiac hypertrophy occurs in response to physiological or pathological stimuli. The heart responds to overload with mechanisms that induce myocyte growth to enhance contractile function, reduce ventricular wall stress, and/or compensate for the increase in hemodynamic demand. However, sustained overload results in decompensation and end-stage heart failure (6). Physiological hypertrophy (compensated) can be induced by exercise or by increased thyroid hormone action. In contrast, pathological hypertrophy is induced by arterial hypertension, myocardial infarction, or pressure overload.
Signaling transduction pathways that mediate cardiac hypertrophy are different between physiological and pathological hypertrophy. For physiological hypertrophy, one of the models proposes that insulin-like growth factor-1 binds to its receptor on the cardiac myocyte. This stimulates the activation of phosphatidylinositol 3-kinase (PI3K), leading to AKT phosphorylation, which then initiates changes in gene expression that are compatible with the physiological cardiac hypertrophy phenotype (23). In contrast, the activation of mitogen-activated protein kinase (MAPK) cascades plays a major role in pathological cardiac hypertrophy and heart failure. The main branches of MAPKs include extracellular signal-regulated kinase (ERK1/2), stress-activated p38, MAPK, and c-Jun NH2-terminal kinase (JNK1/2/3) (22). ERK activation involves the Ras/Raf/mitogen-activated protein-ERK kinase (MEK)/ERK cascade, which has been considered the most important signaling pathway for maladaptive cardiac hypertrophy (21). Because interactions between physiological and pathological hypertrophy occur, a clear separation between these two processes cannot be made and has been discussed in recent reviews (4). Over time, a compensated physiological hypertrophy may develop into dilated cardiomyopathy and a pathological type of hypertrophy (4). Therefore, understanding the mechanisms that determine physiological or pathological hypertrophy is necessary.
Thyroid hormone action can induce physiological hypertrophy by mechanisms not well understood. However, the beneficial effects and potential therapeutic implications of thyroid hormone on cardiac diseases have extensively been investigated (for review see Refs. 3 and 24).
Thyroid hormone action occurs mainly by binding thyroid hormone to nuclear thyroid hormone receptors (TRs) (30) although rapid nongenomic effects have also been recognized (1, 2).
TRs are encoded by two genes, TR-α and TR-β (19), and at least two TR-α isoforms and three TR-β isoforms have been identified. TR-α1, TR-β1, TR-β2, and TR-β3 isoforms bind to T3, whereas TR-α2 does not bind to T3 and functions, at least in vitro, as a TR-α1 and TR-β1 antagonist (16). TR-α1 is dominant in the mouse heart. In mouse hearts, TR-α1 represents 70% of total cardiac TR mRNA, and TR-β1 represents 30% (7, 15). Studies in TR-α1 knockout (KO) mice show that TR-α1 action is predominant in the heart (7, 15). Signaling mechanisms implicated in thyroid hormone-induced physiological cardiac hypertrophy are less studied. It has been reported that thyroid hormone stimulates protein synthesis in the cultured cardiomyocyte by activating the AKT/mammalian target of rapamycin (mTOR) and the S6 kinase (p70S6K) pathways (17), and this effect can be blocked by specific PI3K inhibitors (17). In addition, increased phosphorylation of AKT, p70S6K, and mTOR in hearts of thyroxine-treated rats has been demonstrated (18). These data support a role for the PI3K/AKT/mTOR signaling pathway in T3-induced physiological cardiac hypertrophy; however, thyroid hormone is able to activate ERK kinase signaling in a short-term fashion (only in the first 8 min) in neonatal rat cardiac myocytes (25). This result would be in conflict with the idea of thyroid hormone inducing physiological hypertrophy because ERK signaling has been recognized as the most important signaling pathway for pathological hypertrophy. The interaction between thyroid hormone actions on ERK signaling in the hypertrophied heart has not been explored.
In this study, we sought to investigate the effects of thyroid status and pressure overload-induced cardiac hypertrophy on the ERK signaling pathway. In addition, we explored the effect of thyroid hormone treatment on ERK signaling in the heart of mice undergoing cardiac hypertrophy. Interactions with the PI3K/AKT pathway were also explored. Our results showed, for the first time, that thyroid hormone blocks ERK activation in hypertrophied hearts in part by modulating the PI3K/AKT pathway.
MATERIALS AND METHODS
All animal protocols were approved by the University of California, San Diego, Institutional Animal Care and Use Committee and conform to the Guide for the Care and Use of Laboratory Animals as outlined by the National Institutes of Health.
Isolation and culture of neonatal rat cardiac myocytes.
Primary cultures of neonatal rat cardiomyocytes were prepared as described previously (9). Cells (3 × 106 cells per 10-cm plate) were plated onto gelatin-coated culture dishes. Medium consisted of 4.25:1 DMEM:M199, 10% horse serum, 5% fetal bovine serum, 1% penicillin/streptomycin/fungizone, and 5.5 mmol/l d-glucose. Cells were allowed to adhere to the plates for at least 24 h before treatments. Subsequently, cells were maintained in T3-free media during treatments. To mimic the hypertrophic condition in cell culture, we used phorbol 12-myristate 13 acetate (PMA; Sigma-Aldrich, St. Louis, MO) as reported before (9, 12). PMA was added in a final concentration of 300 nM, and LY294002 (LY) was 50 μM.
Transfection of TR-α1 into neonatal rat cardiomyocytes.
The sequences coding for the rat TR-α1 (a kind gift of Dr. Howard Towle, Michigan State University, Minneapolis, MN) were cloned into a replication-deficient adenoviral vector under control of the promoter-enhancer region of the human cytomegalovirus (Adv-TR-α1). An adenovirus containing the catalytic domain of Raf-1 was used to express an activated form of Raf (13). Adenovirus encoding the activated Raf was a gift from Dr. Kevin M. Pumiglia (Albany Medical College, Albany, NY). The general procedure was described previously (10). An empty adenovirus without transgene (Adv-control) was used in the control group. A concentration of 20 plaque-forming units/cell was used for each adenovirus. Cells were analyzed 72 h after transfection.
Transverse aortic constriction.
Pressure overload was created in male mice (NIH Swiss, 6 wk old; Harlan Sprague Dawley, Indianapolis, IN) by transverse aortic constriction (TAC) as previously described (15, 23) with modifications. For TAC model, the band is placed on the aortic arch between the innominate and left carotid arteries. Mice were anesthetized with a mixture of ketamine (100 mg/kg ip) and xylazine (5 mg/kg ip) before surgery. All data were obtained from mice at 10 wk after TAC. T3 administration was started at 8 wk after TAC and continued for 2 wk (3.5 ng/g body wt ip daily). Plasma T3 levels were 87.9 ± 4 ng/dl in control, 65.6 ± 1 ng/dl in TAC (P < 0.05 vs. control), and 80.7 ± 3 ng/dl in TAC + T3 group.
Preparation of hypo- and hyperthyroid mice.
Mice were made hypothyroid by being fed a diet of iodine-free/0.15% 6-n-propil-2-thiouracil from Harlan Teklad (Madison, WI). This diet regimen was maintained for 4 wk. The hypothyroid status in these mice (n = 10) was confirmed by both lower serum T3 levels and higher thyroid-stimulating hormone (TSH) values compared with control mice (n = 6) (T3: 46.65 ± 3.84 vs. 80.31 ± 3.39 ng/dl, determined by radioimmunoassay with the coat-A-count total T3 kit, Diagnostic Products; TSH: 2960 ± 440 vs. 70 ± 10 ng/ml, determined by Ani-Lytics, Gaithersburg, MD; P < 0.01). Hyperthyroidism was induced in mice by daily intraperitoneal injection of l-thyroxine (T4) at a dose of 1 μg/g body wt for 2 wk. Serum T3 levels in these mice (n = 8) were ∼10-fold higher than those in control mice (n = 13) (872.66 ± 112.53 vs. 78.42 ± 4.95 ng/dl, respectively, P < 0.01). Because of the low baseline TSH value in normal mice, no further inhibition of TSH was detectable in hyperthyroid mice (70 ± 10 vs. 70 ± 10 ng/ml, P > 0.05).
Western blot analysis.
Ventricular tissues or neonatal cardiac myocytes were homogenized in lysis buffer (20 mM Tris, pH 7.4, 20 mM NaCl, 0.1 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 1 mM dithiothreitol) with a polytron homogenizer. Protein content was measured by the Bradford method (Bio-Rad, Hercules, CA) and adjusted for equal loading. Protein extracts from heart tissue (50 μg) were separated by a 4–12% Bis-Tris·HCl-buffered polyacrylamide gel (Invitrogen, Carlsbad, CA) and transferred to a nitrocellulose membrane. Membranes were probed for phospho-Raf-1 (Ser338, activator site), phospho-Raf-1 (Ser259, inhibitory site), Raf-1, phospho-ERK1/2 (Thr202/Tyr204), ERK1/2, MEK, and phospho-MEK (Ser217) (Cell Signaling Technology, Danvers, MA). Secondary antibodies were anti-rabbit IgG-horseradish peroxidase (HRP) conjugate (Cell Signaling Technology), anti-mouse IgG-HRP conjugate (Amersham, Piscataway, NJ), and anti-goat IgG-HRP conjugate (Santa Cruz Biotechnology, Santa Cruz, CA). Blots were also probed with a rabbit polyclonal anti-voltage-dependent-anion channel (Cell Signaling Technology) or mouse monoclonal anti-α-actin (Santa Cruz Biotechnology) antibodies as internal control to ensure equivalent protein loading and protein integrity. The immunoblots were detected with Western Lightning enhanced chemiluminescence detection reagent (Perkin Elmer, Norton, OH) and exposed to film. Protein bands were quantified from scanned images using ImageJ software (NIH) and expressed in arbitrary units.
Statistical analysis.
Results are presented as means ± SE from at least three different experiments. Statistical significance between different groups was evaluated using one-way ANOVA with Bonferroni's multiple-comparison posttest. P value <0.05 denotes the presence of a statistically significant difference.
RESULTS
General.
Mice subjected to TAC for 10 wk developed cardiac hypertrophy presenting increased heart weight/body weight ratios by 73% (Table. 1). Cardiac function evaluated by echocardiography was impaired in TAC mice, which presented a 20% decrease in fractional shortening (Table. 1). The sarco(endo)plasmic reticulum Ca2+-ATPase was decreased in hearts from TAC mice, which is consistent with a decreased contractility. In addition, TAC mice have lower T3 serum levels and TR levels compared with control mice (Table 1).
Table 1.
General characteristics of transverse aortic-constricted mice
| Control | Banded TAC | Effect | |
|---|---|---|---|
| BW, g | 31.53 ± 0.73 | 32.50 ± 0.95 | none |
| HW/BW, mg/g | 3.83 ± 0.19 | 6.65 ± 0.15* | +73% |
| T3 serum levels | 87.85 ± 3.56 | 65.62 ± 1.57* | −26% |
| Heart | |||
| SERCA2a protein, AU | 100 ± 0.02 | 87 ± 0.01* | −23% |
| SERCA2a mRNA, AU | 1.30±.02 | 0.71 ± 0.09* | −45% |
| Heart | |||
| Thyroid receptor | 1.20 ± 0.038 | 0.91 ± 0.054* | −25% |
| mRNA, AU | |||
| Fractional shortening, % Echo | 41 ± 3.9 | 33 ± 1.8* | −20% |
Applicable values are means ± SE. TAC, transverse aortic constriction
BW, body weight; HW, heart weight; SERCA2a, sarco(endo)plasmic reticulum Ca2+-ATPase; AU, arbitrary units; Echo, echocardiography.
Influence of thyroid hormone status and TAC on the Raf/ERK pathway.
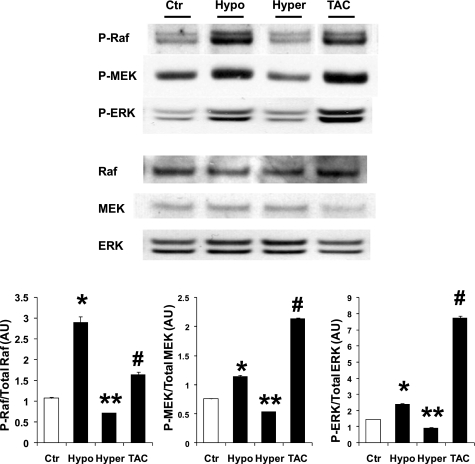
Changes in thyroid hormone levels in vivo modulate ERK activation (Fig. 1). ERK phosphorylation was increased by 80% in mice undergoing hypothyroidism for 1 mo compared with control mice. In contrast, hyperthyroidism decreased phospho-ERK by 25% from normal levels (Fig. 1). Aortic-constricted mouse hearts had a dramatic increase in phospho-ERK (more than 4-fold higher than control). Upstream of the Raf/MEK/ERK pathway, changes in thyroid hormone status or TAC produced the same trend observed in ERK phosphorylation (Fig. 1). MEK phosphorylation was increased in hypothyroidism by 51% and decreased by hyperthyroidism by 30%. TAC mice presented increased phospho-MEK more than twofold. Phosphorylated Raf (Ser338) showed a similar pattern (Fig. 1). Hypothyroidism increased p-Raf (Ser338) more than twofold vs. control levels. Hyperthyroidism lowered p-Raf (Ser338) by 60%, and TAC hearts showed an increase of 52% over control levels (Fig. 1).
Fig. 1.
MAPK/Raf/MEK/ERK pathway activity is modulated by thyroid status and pressure overload. Phosphorylated Raf, MEK, and ERK in mouse hearts from control (Ctr), hypothyroid (Hypo; 6-n-propil-2-thiouracil food 1 mo), hyperthyroid (Hyper; T4 for 2 wk), and transverse aortic constricted (TAC; 10 wk). Western blots show increased phospho-Raf/MEK/ERK in Hypo and TAC mice. Phospho-Raf/MEK/ERK are reduced in hyperthyroidism. Levels of phospho-active kinases relative to total kinases are shown in the bar graphs. Values are means ± SE. *P < 0.05 vs. Ctr, Hyper, TAC; **P < 0.05 vs. Ctr, Hypo, TAC; #P < 0.05 vs. Ctr, Hypo, Hyper; n = 8. AU, arbitrary units.
T3 treatment inhibits ERK activation in TAC mouse hearts.
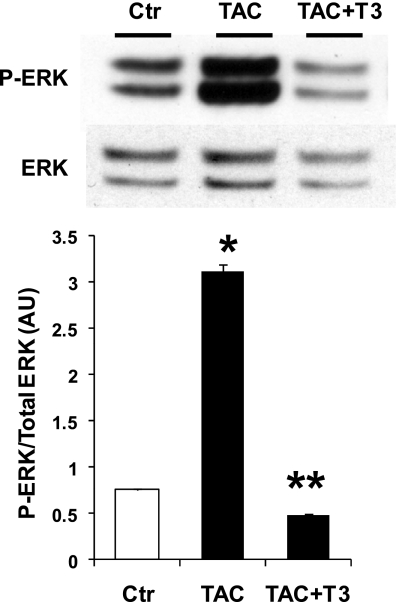
After 8 wk of TAC, mice were treated with daily intraperitoneal injections of T3 for 2 wk and then euthanized, and the hearts were analyzed. Results of ERK phosphorylation are shown in Fig. 2. TAC hearts presented an increase in phospho-ERK, as shown before. T3 treatment dramatically reduced phospho-ERK to values even lower than control mice (37% lower than control).
Fig. 2.
T3 inhibits pressure overload-induced ERK activation. Phosphorylated ERK in mouse hearts from control, TAC (10 wk), and TAC-treated with T3 for 2 wk. Western blot shows increased p-ERK in TAC mice. T3 treatment markedly reduced p-ERK. Levels of phospho-ERK relative to total ERK are shown in the bar graph. Values are means ± SE. *P < 0.05 vs. Ctr, TAC + T3; **P < 0.05 vs. Ctr, TAC; n = 4.
Thyroid hormone effect inhibiting ERK phosphorylation is TR dependent.
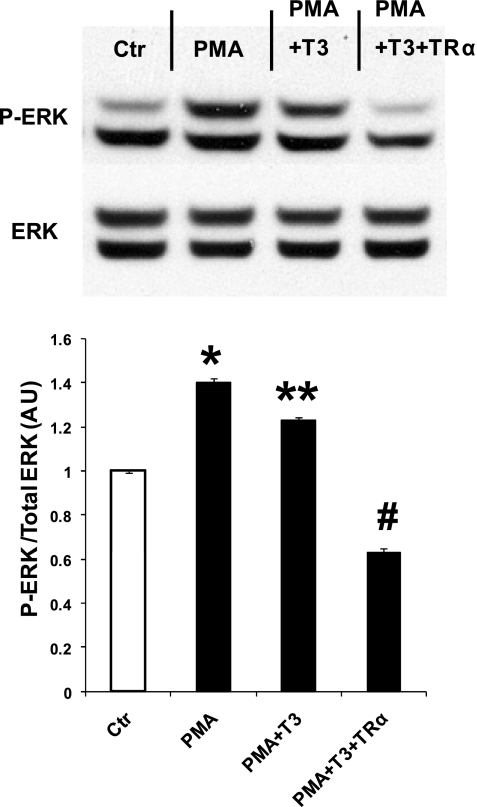
To determine whether thyroid hormone action on ERK phosphorylation was a TR-mediated effect, we overexpressed TR-α1, which is the predominant TR in the heart, in cultured neonatal rat cardiac myocytes. Figure 3 shows that PMA-activated ERK phosphorylation can be decreased by 50% with 1 nM T3 (PMA vs. PMA + T3). This effect was considerably amplified by TR-α1 overexpression, which completely suppressed the activating PMA effect and reduced phospho-ERK 40% below control values (Fig. 3).
Fig. 3.
Influence of adenoviral overexpression of thyroid hormone receptor (TR)-α1 in neonatal rat cardiomyocytes on phorbol 12-myristate 13 acetate (PMA)-induced ERK phosphorylation. Western blot shows analysis of ERK phosphorylation in cells cultured with T3-free serum only (Ctr), PMA (300 nM) added, PMA plus 1 nM T3, and PMA plus 1 nM T3 and adenovirus expressing TR-α1. Overexpression of TR-α1 reduced phospho-ERK to levels lower than control. Values are means ± SE. *P < 0.05 vs. Ctr, PMA + T3, PMA + T3 + TR-α; **P < 0.05 vs. Ctr, PMA + T3 + TR-α; #P < 0.05 vs. Ctr, PMA, PMA + T3; n = 6 from 3 experiments.
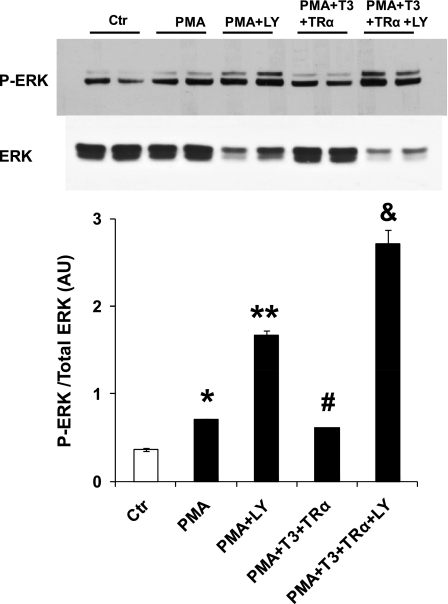
PI3K has been shown to be involved in the signaling mechanisms of thyroid hormone in physiological cardiac hypertrophy. Therefore, we investigated the effect of a PI3K inhibitor, LY, on thyroid hormone effects. Figure 4 shows the effects of LY and thyroid hormone on neonatal rat cardiac myocytes stimulated with PMA. PI3K inhibition further increased ERK phosphorylation induced by PMA. TR-α1 overexpression failed to decrease ERK phosphorylation when LY was present, indicating that PI3K activation is necessary for the inhibitory effect of thyroid hormone of ERK (Fig. 4).
Fig. 4.
Inhibition of phosphatidylinositol 3-kinase (PI3K) annuls the effect of T3 or TR-α1 overexpression on ERK phosphorylation. Western blot shows analysis of ERK phosphorylation in cells cultured with T3-free serum only (Ctr), PMA (300 nM) added, PMA plus LY294002 (LY, 50 μM), PMA plus 1 nM T3 and adenovirus expressing TR-α1, and PMA plus 1 nM T3 and adenovirus expressing TR-α1 and LY. Inhibition of PI3K blocked the inhibitory effect of thyroid hormone action on ERK phosphorylation. Values are means ± SE. *P < 0.05 vs. Ctr, PMA + LY, PMA + T3 + TR-α +LY; **P < 0.05 vs. Ctr, PMA, PMA + T3 + TR-α + LY; *P < 0.05 vs. PMA + LY, PMA + T3 + TR-α + LY; &P < 0.05 vs. Ctr, PMA, PMA + LY, PMA + T3 + TR-α; n = 6 from 3 experiments.
Thyroid hormone activity affects Raf phosphorylation at two sites.
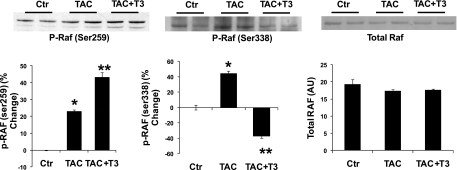
PI3K can affect the Raf/ERK pathway by activating AKT, which in turn can phosphorylate an inhibitory Ser259 in Raf, thereby inhibiting ERK phosphorylation (31). We analyzed AKT activation in mouse hearts subjected to aortic constriction in a separate group of experiments. Results in Fig. 5 demonstrate that phosphorylation of the inhibitory site in Raf was increased by thyroid hormone action. Surprisingly, phosphorylation of the Raf activator site (Ser338) was decreased by thyroid hormone action as well.
Fig. 5.
T3 inhibits ERK phosphorylation by affecting 2 phosphorylation sites in Raf. Western blots from mouse hearts. Groups were control, TAC, and TAC mice treated with T3 for 2 wk. Westerns blot show phosphorylated Raf at two different sites. Phosphorylation of Ser259 inhibits Raf. Phosphorylation of Ser338 activates Raf. T3 treatment decreased Raf activation and increased inactivation in the TAC hearts. Summarized results are shown in the bar graphs. Values are means ± SE. *P < 0.05 vs. Ctr, TAC + T3; **P < 0.05 vs. Ctr, TAC; n = 4.
In another group of experiments, we investigated whether thyroid hormone could act directly downstream of Raf. We expressed an activated form of Raf (ΔRaf) (27) in neonatal rat cardiomyocytes. TR-α1 overexpression did not affect ΔRaf-induced ERK activation, confirming that thyroid hormone action is located at the level of Raf or upstream (Fig. 6).
Fig. 6.
Thyroid hormone action has no effect on ERK phosphorylation after expression of activated Raf. An activated Raf (ΔRaf) was expressed in neonatal rat cardiomyocytes, and the effect of thyroid hormone action was tested. Western blot shows phospho-ERK in control cells cultured in T3-free media. ΔRaf expression increased phospho-ERK. TR-α1 in the presence of high T3 concentration failed to inhibit ERK phosphorylation.
DISCUSSION
In the present study, we investigated the influence of thyroid hormone on ERK phosphorylation in the hypertrophied mouse heart. Our results demonstrate that T3 treatment of mice with pressure overload-induced hypertrophy was able to reverse the activation of the ERK signaling cascade in the heart. This effect was partially mediated by activation of the PI3K/AKT pathway.
There is a longstanding debate in the literature as to whether physiological cardiac hypertrophy can be separated from pathological hypertrophy (4, 29). Nevertheless, thyroid hormone is considered to induce physiological hypertrophy, and its therapeutic effects limiting ischemic injury (11), attenuating cardiac remodeling, and improving cardiac hemodynamics (26) have been recently reviewed (24). Although signaling mechanisms in cardiac hypertrophy and in thyroid hormone-induced hypertrophy have been investigated, the influences of thyroid hormone on signaling patterns in pressure overload-induced hypertrophy are unknown. For that reason, we focused on the effects of thyroid hormone on the ERK pathway, which is believed to be the main player in the pathological hypertrophy, and on the PI3K/AKT pathway, which is involved with physiological hypertrophy and has been proposed as the target of thyroid hormone action.
We first investigated the pattern of ERK phosphorylation during hypothyroidism, hyperthyroidism, and in mouse hearts with 10 wk of TAC. Our results showed that hypothyroidism is associated with increased cardiac phospho-ERK levels compared with control mice. In contrast, hyperthyroidism presented decreased phospho-ERK levels with respect to control mice. These results suggest that thyroid hormone may inhibit ERK activation. Increased cardiac phospho-ERK levels have been demonstrated in mouse models of pressure overload-induced heart failure (5, 28) and also in humans with heart failure (8). In this study, mice with cardiac hypertrophy and heart failure induced by TAC for 10 wk presented dramatically increased phospho-ERK values compared with control mice and even with hypothyroid mice with already higher than control values.
Upstream of the ERK cascade, phosphorylated Raf was affected in the same direction as phospho-ERK by thyroid status or by pressure overload, indicating that activation of the Raf/ERK pathway is involved. ERK activation in pressure-overloaded hearts can be the result of multiple factors (20, 22); however, the role of thyroid hormone on ERK activation in cardiac hypertrophy and/or heart failure has not been reported before. Our results showed that thyroid hormone levels are inversely associated with ERK activation. Some reports have drawn a connection between heart failure and ischemia decreasing thyroid hormone signaling activity (14). Therefore, it can be assumed that a subclinical hypothyroid status in heart failure will lead to increased ERK phosphorylation. In support of this hypothesis, our results showed that administration of a physiological dose of T3, enough only to return T3 to control values, was able to suppress pressure overload-induced ERK activation. The PI3K/AKT pathway was also affected by thyroid hormone, as demonstrated by an increase in phospho-AKT after T3 treatment. We further investigated whether the thyroid hormone effect was TR mediated. We overexpressed TR-α1 in neonatal rat cardiac myocytes stimulated with PMA, which mimics a hypertrophic stimulus (9, 12). PMA-induced ERK phosphorylation was modest compared with the in vivo results obtained after aortic constriction. These results indicate that the model of cardiomyocytes treated with PMA lacks of additional factors that contribute to ERK phosphorylation in vivo as may be expected. Nevertheless, we were able to prove in this model that TR-α1 overexpression inhibited ERK activation, indicating that the ability of thyroid hormone to inhibit ERK phosphorylation is receptor mediated. Furthermore, because thyroid hormone affected both the Raf/ERK and PI3K/AKT pathways, we investigated the existence of an interaction between these two pathways in response to thyroid hormone action. Blockade of PI3K also blocked the inhibitory effect of thyroid hormone on ERK phosphorylation. One link between PI3K/AKT and Raf/ERK pathways is Raf, which can be phosphorylated by AKT at an inhibitory site (Ser259). Analysis of thyroid hormone action in vivo on Raf phosphorylation showed that hearts from TAC mice treated with T3 exhibited increased phosphorylation of the inhibitory site in Raf, whereas the activating site (Ser338) was less phosphorylated after T3 treatment. These results are in agreement with a PI3K/AKT-mediated thyroid hormone effect on ERK phosphorylation. However, because the Raf-activating site was also affected by thyroid hormone, an effect of the hormone upstream of Raf is also involved.
Pathological hypertrophy signaling is mediated by ERK activation, whereas physiological hypertrophy is mediated by PI3K/AKT. Our results show for the first time that thyroid hormone can switch from an established pathological hypertrophy signaling to a physiological signaling pattern, explaining in part the therapeutic effects of thyroid hormone on heart failure.
In summary, we demonstrated that ERK phosphorylation is inhibited by thyroid hormone by inhibiting the MAPK Raf/ERK pathway. This inhibition is partially mediated by stimulation of the PI3K/AKT pathway. In addition, the effects observed here are mediated by TR-α1.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-89938 (to W. H. Dillmann).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Davis PJ, Leonard JL, Davis FB. Mechanisms of nongenomic actions of thyroid hormone. Front Neuroendocrinol 29: 211–218, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Davis PJ, Zhou M, Davis FB, Lansing L, Mousa SA, Lin HY. Mini-review: Cell surface receptor for thyroid hormone and nongenomic regulation of ion fluxes in excitable cells. Physiol Behav 99: 237–239, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Dillmann W. Cardiac hypertrophy and thyroid hormone signaling. Heart Fail Rev 15: 125–132, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorn GW., Jr The fuzzy logic of physiological cardiac hypertrophy. Hypertension 49: 962–970, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Esposito G, Prasad SVN, Rapacciuolo A, Mao L, Koch WJ, Rockman HA. Cardiac overexpression of a Gq inhibitor blocks induction of extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activity in in vivo pressure overload. Circulation 103: 1453–1458, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol 65: 45–79, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Gloss B, Trost SU, Bluhm WF, Swanson EA, Clark R, Winkfein R, Janzen KM, Giles W, Chassande O, Samarut J, Dillmann WH. Cardiac ion channel expression and contractile function in mice with deletion of thyroid hormone receptor α or β. Endocrinology 142: 544–550, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Haq S, Choukroun G, Lim H, Tymitz KM, del Monte F, Gwathmey J, Grazette L, Michael A, Hajjar R, Force T, Molkentin JD. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation 103: 670–677, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Hartong R, Villarreal FJ, Giordano F, Hilal-Dandan R, McDonough PM, Dillmann WH. Phorbol myristate acetate-induced hypertrophy of neonatal rat cardiac myocytes is associated with decreased sarcoplasmic reticulum Ca2+ATPase (SERCA2) gene expression and calcium reuptake. J Mol Cell Cardiol 28: 2467–2477, 1996 [DOI] [PubMed] [Google Scholar]
- 10.He H, Meyer M, Martin JL, McDonough PM, Ho P, Lou X, Lew WY, Hilal-Dandan R, Dillmann WH. Effects of mutant and antisense RNA of phospholamban on SR Ca(2+)-ATPase activity and cardiac myocyte contractility. Circulation 100: 974–980, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Henderson KK, Danzi S, Paul JT, Leya G, Klein I, Samarel AM. Physiological replacement of T3 improves left ventricular function in an animal model of myocardial infarction-induced congestive heart failure. Circ Heart Fail 2: 243–252, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Henrich CJ, Simpson PC. Differential acute and chronic response of protein kinase C in cultured neonatal rat heart myocytes to α1-adrenergic and phorbol ester stimulation. J Mol Cell Cardiol 20: 1081–1085, 1988 [DOI] [PubMed] [Google Scholar]
- 13.Ho PD, Zechner DK, He H, Dillmann WH, Glembotski CC, McDonough PM. The Raf-MEK-ERK cascade represents a common pathway for alteration of intracellular calcium by Ras and protein kinase C in cardiac myocytes. J Biol Chem 273: 21730–21735, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Iervasi G, Pingitore A, Landi P, Raciti M, Ripoli A, Scarlattini M, L'Abbate A, Donato L. Low-T3 syndrome: a strong prognostic predictor of death in patients with heart disease. Circulation 107: 708–713, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Johansson C, Vennstrom B, Thoren P. Evidence that decreased heart rate in thyroid hormone receptor-α 1-deficient mice is an intrinsic defect. Am J Physiol Regul Integr Comp Physiol 275: R640–R646, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Katz D, Lazar MA. Dominant negative activity of an endogenous thyroid hormone receptor variant (alpha 2) is due to competition for binding sites on target genes. J Biol Chem 268: 20904–20910, 1993 [PubMed] [Google Scholar]
- 17.Kenessey A, Ojamaa K. Thyroid hormone stimulates protein synthesis in the cardiomyocyte by activating the Akt-mTOR and p70S6K pathways. J Biol Chem 281: 20666–20672, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Kuzman JA, Vogelsang KA, Thomas TA, Gerdes AM. l-Thyroxine activates Akt signaling in the heart. J Mol Cell Cardiol 39: 251–258, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Lazar MA. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev 14: 184–193, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Lorenz K, Schmitt JP, Vidal M, Lohse MJ. Cardiac hypertrophy: targeting Raf/MEK/ERK1/2-signaling. Int J Biochem Cell Biol 41: 2351–2355, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Mitchell S, Ota A, Foster W, Zhang B, Fang Z, Patel S, Nelson SF, Horvath S, Wang Y. Distinct gene expression profiles in adult mouse heart following targeted MAP kinase activation. Physiol Genomics 25: 50–59, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Muslin AJ. MAPK signalling in cardiovascular health and disease: molecular mechanisms and therapeutic targets. Clin Sci (Lond) 115: 203–218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol 37: 449–471, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Pantos C, Mourouzis I, Xinaris C, Papadopoulou-Daifoti Z, Cokkinos D. Thyroid hormone and “cardiac metamorphosis”: Potential therapeutic implications. Pharmacol Ther 118: 277–294, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Pantos C, Xinaris C, Mourouzis I, Malliopoulou V, Kardami E, Cokkinos DV. Thyroid hormone changes cardiomyocyte shape and geometry via ERK signaling pathway: potential therapeutic implications in reversing cardiac remodeling? Mol Cell Biochem 297: 65–72, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Pingitore A, Galli E, Barison A, Iervasi A, Scarlattini M, Nucci D, L'Abbate A, Mariotti R, Iervasi G. Acute effects of triiodothyronine (T3) replacement therapy in patients with chronic heart failure and low-T3 syndrome: a randomized, placebo-controlled study. J Clin Endocrinol Metab 93: 1351–1358, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Pumiglia KM, Decker SJ. Cell cycle arrest mediated by the MEK/mitogen-activated protein kinase pathway. Proc Natl Acad Sci USA 94: 448–452, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purcell NH, Wilkins BJ, York A, Saba-El-Leil MK, Meloche S, Robbins J, Molkentin JD. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci USA 104: 14074–14079, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wakatsuki T, Schlessinger J, Elson EL. The biochemical response of the heart to hypertension and exercise. Trends Biochem Sci 29: 609–617, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Yen PM, Ando S, Feng X, Liu Y, Maruvada P, Xia X. Thyroid hormone action at the cellular, genomic and target gene levels. Mol Cell Endocrinol 246: 121–127, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B). Science 286: 1741–1744, 1999 [DOI] [PubMed] [Google Scholar]