Abstract
Enterotoxins elaborated by Vibrio cholerae and Escherichia coli cannot elicit fluid secretion in the absence of functional cystic fibrosis transmembrane conductance regulator (CFTR) chloride channels. After enterotoxin exposure, CFTR channels are rapidly recruited from endosomes and undergo exocytic insertion into the apical plasma membrane of enterocytes to increase the number of channels on the cell surface by at least fourfold. However, the molecular machinery that orchestrates exocytic insertion of CFTR into the plasma membrane is largely unknown. The present study used immunofluorescence, immunoblotting, surface biotinylation, glutathione S-transferase (GST) pulldown assays, and immunoprecipitation to identify components of the exocytic soluble N-ethylmaleimide (NEM)-sensitive factor attachment receptor (SNARE) vesicle fusion machinery in cyclic nucleotide-activated exocytosis of CFTR in rat jejunum and polarized intestinal Caco-2BBe cells. Syntaxin 3, an intestine-specific SNARE, colocalized with CFTR on the apical domain of enterocytes in rat jejunum and polarized Caco-2BBe cells. Coimmunoprecipitation and GST binding studies confirmed that syntaxin 3 interacts with CFTR in vivo. Moreover, heat-stable enterotoxin (STa) activated exocytosis of both CFTR and syntaxin 3 to the surface of rat jejunum. Silencing of syntaxin 3 by short hairpin RNA (shRNA) interference abrogated cyclic nucleotide-stimulated exocytosis of CFTR in cells. These observations reveal a new and important role for syntaxin 3 in the pathophysiology of enterotoxin-elicited diarrhea.
Keywords: cystic fibrosis transmembrane conductance regulator, intestine, secretory diarrhea, soluble N-ethylmaleimide-sensitive factor attachment protein receptor
despite widespread use of oral rehydration therapies, infectious diarrhea remains a major socioeconomic and global health problem that claims the lives of more than 2.5 million children each year (41). Rapid advances in the pace of biomedical research continue to benefit diseases that affect the developed world, but, sadly, this is not the case for underdeveloped countries. As a result, there is currently no effective drug therapy available for the treatment of secretory diarrhea (18).
Vibrio cholerae and Escherichia coli, the two major bacteria responsible for enterotoxigenic diarrhea, target the proximal small intestine, where they release enterotoxins. Once released, enterotoxins bind to receptors on the apical membrane of enterocytes and signal intracellular cAMP- and cGMP-dependent pathways that converge to activate cystic fibrosis transmembrane conductance regulator (CFTR) chloride channels on the apical membrane and mediate anion secretion (8, 28). The importance of CFTR to intestinal fluid secretion is underscored by the complete inability of enterotoxigenic bacteria to elicit fluid secretion in individuals lacking functional CFTR channels, as observed in the genetic disease cystic fibrosis (CF) (23).
We previously demonstrated (3, 4) that cAMP/protein kinase A (PKA)- and cGMP/protein kinase G (PKG)-elicited fluid secretion requires recruitment and insertion of CFTR from endosomes to the surface of enterocytes in rat small intestine. Fluid secretion elicited after E. coli heat-stable enterotoxin (STa) in the jejunum was observed after PKG-dependent increase in surface CFTR by at least fourfold. This scenario is possible because most (>50%) of the CFTR in enterocytes reside on membranes of subapical endosomes at steady state, a distribution required for agonist-stimulated exocytic recruitment in regulating fluid secretion (5).
Constitutive endocytic (clathrin-mediated) recycling to and from the plasma membrane efficiently controls the number of channels on the cell surface at steady state because CFTR has a long half-life (>20 h) in intestinal epithelial cells (12, 44, 48, 55). Endocytic recycling and regulated exocytosis directly modulate CFTR ion transport in the intestine in a cell type-specific manner in endogenous CFTR-expressing epithelial cells and tissues (9, 27). For example, the rab GTPase rab11a regulates apical recycling of CFTR in airway cells, but rab11b (not rab11a) regulates its apical recycling in the intestine (48, 50). Elucidation of the proteins that direct CFTR endocytosis and exocytosis in the intestine is critical for identifying therapeutic targets to treat secretory diarrhea and CF because both pathways are implicated in intestinal fluid secretion (2, 24).
Establishment of apical polarity requires precise targeting of proteins from the Golgi to the plasma membrane. This function is carried out by specific soluble N-ethylmaleimide (NEM)-sensitive factor attachment receptor (SNARE) proteins. SNARE membrane fusion machinery is essential for all membrane trafficking pathways. Vesicular cargo SNARES (v-SNAREs) interact with target membrane SNAREs (t-SNAREs) to mediate membrane fusion (30, 35). In epithelial cells, t-SNAREs of the syntaxin family localize to distinct compartments, where they direct trafficking of apical or basolateral proteins. It is this property that enables the cell to exert its specific function in epithelial tissues (31, 34, 46).
Syntaxin 3 is involved in apical recycling and in biosynthetic traffic from the trans-Golgi network (TGN) to the apical surface of epithelial cells, and its distribution in the small intestine resembles that of CFTR (1, 21, 34, 45, 47). In gastric parietal cells of the stomach, syntaxin 3 regulates acid secretion by recruitment of H+-K+-ATPase proton pumps from subapical vesicles to the apical membrane (6, 38, 47, 51). The behavior of syntaxin 3 in gastric parietal cells and its localization in the intestine suggested that it could play a similar role in second messenger-regulated exocytosis of CFTR to the surface of enterocytes.
In the present study, we examined whether syntaxin 3 is necessary for regulated exocytosis of CFTR in the small intestine. The data are consistent with a requirement for syntaxin 3 in second messenger-dependent recruitment of CFTR to the cell surface and a role in the pathophysiology of enterotoxin-mediated diarrhea. We also characterized a polarized cell model of differentiated enterocytes (Caco-2BBe) to examine apical recycling and regulated trafficking of CFTR in villus enterocytes of the small intestine. This was important because villus enterocytes are increasingly recognized as important sites for CFTR anion secretion and regulated trafficking (24, 53), but there are no validated intestinal cell models to study endocytic recycling and regulated exocytosis of endogenous CFTR in this compartment. We recently demonstrated (15) that Caco-2BBe cells are a useful model for studies of clathrin-mediated endocytosis of CFTR. Here, we extend the characterization of this cell model and show that Caco-2BBe cells express the machinery to regulate apical recycling and regulated recruitment of CFTR to the cell surface similar to its behavior in villus enterocytes of rat jejunum.
MATERIALS AND METHODS
Reagents and antibodies.
The following antibodies were used in this study: CFTR (M3A7) (Chemicon International, Temecula, CA), CFTR 217 (Cystic Fibrosis Foundation Therapeutics, Bethesda, MD), AME 4991 CFTR (2), alkaline phosphatase (ALP) (BYA1191) (Accurate Chemicals, Westbury, NY), syntaxin 3 (Alomone Labs, Jerusalem, Israel), rme-1 (gift from Barth Grant, Rutgers University), NKCC1 (gift from Dr. Chris Lytle, University of California-Riverside), EEA-1 (BD Transduction Laboratories, San Jose, CA), myosin VI (Sigma-Aldrich, St. Louis, MO), SNAP-23 (United States Biological, Swampscott, MA), munc-18, rab11 (BD Transduction Laboratories), β-actin (Sigma, St. Louis, MO), syntaxin 2 (Synaptic Systems), and mouse and rabbit IgG (Sigma). Rhodamine-labeled phalloidin and all other fluorescent secondary antibodies were purchased from Molecular Probes (Eugene, OR). Horseradish peroxidase-conjugated goat anti-rabbit IgG and goat anti-mouse IgG were obtained from BD Biosciences. EZ-Link sulfo-NHS-SS-biotin and Immunopure immobilized streptavidin-agarose were obtained from Pierce Biotechnology (Rockford, IL). STa and N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate sodium salt (DbcAMP) were purchased from Sigma.
Animal preparation.
Rodent studies were performed with the approval of Institutional Animal Care and Use Committees of the University of Pittsburgh and Yale University School of Medicine. Sprague-Dawley rats (250–300 g, Charles River Laboratories) were anesthetized with Inactin (120 mg/kg) by intraperitoneal injection. The abdomen was opened, segments of jejunum were identified, and loops were created with sutures and filled with 0.5 ml of freshly prepared warm (37°C) STa (0.5 μM) or saline (NS). The abdomen was closed, and animals were kept warm on a heating pad at 37°C. Jejunal loops were excised 30 min later and prepared for surface biotinylation or immunofluorescence microscopy. Adult male BALB/c mice were anesthetized with Avertin (2,2,2-tribromoethanol, Sigma) (180 mg/kg) administered by intraperitoneal injection. Intestinal tissues were removed, and rats were euthanized with an overdose of Inactin.
Cells.
Human embryonic kidney (HEK-293) cells were a gift from Dr. Neil Bradbury (Chicago Medical School). Cells were grown in DMEM with 10% fetal bovine serum (FBS, GIBCO, Glasgow, UK) on 35-mm dishes before Western blot analysis. Caco-2BBe cells were obtained from American Type Culture Collection (ATCC; Manassas, VA) and grown at 37°C in 5% CO2-90% air atmosphere in high glucose with l-glutamine Dulbecco's modified Eagle's medium (GIBCO), supplemented with 10% FBS, 10 μg/ml apo-transferrin (Chemicon International), 1 mM sodium pyruvate (Sigma), 1% penicillin-streptomycin (GIBCO), 1 μg/ml Fungizone (GIBCO), and 5 μg/ml Plasmocin (Invitrogen, San Diego CA). Caco-2BBe cells were seeded at 1 × 105 cells/cm2 on 100-mm cell culture dishes (Corning, Corning, NY) and passaged when 70% confluent onto a 75-mm Transwell (Costar, Cambridge, MA). Confluent monolayers of Caco-2BBe cells were used for immunofluorescence labeling, Western blot analysis, and surface biotinylation experiments. Surface biotinylation was performed on Caco-2BBe cells after 120 h in culture in all short hairpin (shRNA)-induced silencing studies.
Immunofluorescence microscopy of cells.
Confluent monolayers of polarized Caco-2BBe cells were treated with 1 mM DbcAMP or PBS for 30 min before fixation. Caco-2BBe cells were fixed in 2% paraformaldehyde (PFA)-PBS for 10 min at room temperature. Cells were permeabilized with 0.1% Triton X-100 in PBS for 10 min, followed by incubation with 0.5% BSA and 0.15% glycine in PBS for 45 min to block nonspecific binding. Primary antibodies were prepared in blocking solution containing 0.1% Triton X-100 and applied overnight at 4°C. Cells were washed and incubated with the appropriate secondary antibodies for 1 h at room temperature, and nuclear stain (Draq 5 or DAPI) was applied. Filter-grown labeled Caco-2BBe cells were mounted with Slow Fade medium and/or embedded in optimum cutting temperature (OCT) medium and sectioned in the vertical plane before examination by confocal microscopy.
Western blot analysis.
Caco-2BBe cells, HEK-293 cells, or intestinal mucosal scrapings were homogenized in TGH lysis buffer [25 mM HEPES, 10% (vol/vol) glycerol, 1% (vol/vol) Triton-X 100 pH 7.4] containing protease inhibitor cocktail [10 nM iodoacetamide, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 2 μg/ml leupeptin] for 30 min on ice. Homogenates were centrifuged at 14,000 rpm for 15 min at 4°C, and supernatants were recovered. Protein concentration of the supernatant was determined with Coomassie Protein Assay Reagent (Pierce). Samples were prepared in 2× SDS sample buffer and boiled for 5 min, except when detecting CFTR, in which case the samples were warmed at 37°C for 5 min. Proteins (20 μg) were resolved by SDS-PAGE. Gels were transferred onto Immun-Blot polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA) and incubated with primary antibodies diluted in blocking buffer (5% dry fat milk, 0.1% Tween 20, Tris-buffered saline, pH 7.5) for 1 h at room temperature. Membranes were incubated with secondary antibodies diluted in blocking buffer for 1 h at room temperature, and proteins were detected by chemiluminescence (Pierce Biotechnology) with Kodak Biomax Light Film (VWR).
Brush border membrane isolation and preparation.
Enterocytes from rat jejunum and polarized intestinal cells were isolated in NET buffer (0.13 M NaCl, 5 mM EDTA, 10 mM Tris base, pH 7.4) containing protease inhibitors and centrifuged at 500 g for 10 min. Cell pellets were resuspended in buffer 1 (0.298 M mannitol, 12 mM Tris, 1.5 mM Na azide, pH 7.4 containing protease inhibitors) and sonicated to break vesicular cell membranes on ice. Samples were incubated with 10 mM MgCl2 at 4°C for 15 min and centrifuged at 5,000 rpm at 4°C. The pellets were discarded, and the supernatants were centrifuged again at 13,000 rpm and 4°C, and the cycle was repeated until pure brush border membrane vesicle preparations were obtained and verified by microscopic evidence of enriched microvillar profiles with few cytoplasmic contaminants. Pellets were stored at −80°C overnight and resuspended in 200 μl of buffer 1, transferred to 1.5-ml Eppendorf tubes, and centrifuged at maximum speed for 30 min. Pellets were lysed in TGH buffer containing protease inhibitors on ice, and samples were analyzed by Western blot.
Immunofluorescence microscopy of tissue sections and image quantification.
Segments of jejunum from rats or mice were embedded in Tissue-Tek OCT embedding medium (Miles Laboratory, Elkhardt, IN) and frozen in isopentene precooled with liquid nitrogen. Cryosections were prepared as previously described (2). Briefly, frozen tissue sections were fixed with 2% PFA for 10 min and rehydrated with PBS, and nonspecific labeling was blocked with normal goat serum (1:20) for 45 min. Primary antibodies were applied for 1–2 h at room temperature or overnight at 4°C. Sections were washed with PBS containing 0.15% glycine, and secondary antibodies were applied for 1 h. After secondary antibody application, rhodamine-phalloidin (Invitrogen) was applied for 20 min to stain F-actin. Nuclei were stained with Draq 5 (Biostatus) or Hoechst stain. Immunofluorescence images were acquired on a Olympus Fluoview 500 confocal microscope using either ×60 oil or ×100 oil objectives. Images of NS- and STa-treated rat jejunum were taken at ×60 magnification with constant settings for laser, photomultiplier tube (PMT) gain, and offset at medium scan rate. Fluorescence intensities of CFTR and syntaxin 3 labeling were determined in images taken from fields containing an average of six to eight crypts and villi. Fluorescence intensities were obtained in a region within a 1.5-μm distance beneath the apical border facing the lumen, and the pixel intensity within the region was normalized to area (μm2). Analysis was performed with Metamorph software (UIC, Downington, PA) as described previously (2). Data are expressed as means + SE, and significance in mean values was determined by two-tailed Student's t-test. P < 0.05 was considered significant.
Surface biotinylation in rat jejunum and cells.
Surface biotinylation was performed as previously described (2, 3). Thirty minutes after enterotoxin treatment of jejunum, intestinal loops were excised and immediately placed on ice, the lumen was gently flushed with cold saline, and ends were resecured with ligatures. Freshly prepared sulfo-NH-SS-biotin (1 mg/ml) in PBS containing 0.1 mM CaCl2 and 1.0 mM MgCl2 (PBS-CM) was introduced into the lumen, and loops were incubated in the cold for 30 min (24). In separate experiments, PBS- and 1 mM DbcAMP-treated Caco-2BBe cells were incubated with NHS-SS-biotin for 30 min on ice. After surface biotinylation labeling, cells or mucosal scrapings were lysed in TGH buffer containing protease inhibitors. Equivalent amounts of protein were incubated with Immunopure immobilized streptavidin-agarose overnight at 4°C on a rotator. The following day, the biotinylated proteins were dissociated from streptavidin-agarose by 2× SDS sample buffer. Cell lysates (20 μg protein) and biotinylated samples were resolved by SDS-PAGE to detect CFTR and/or syntaxin 3 by Western blot analysis. Quantification of surface-labeled proteins was performed with a Biorad Fluor S-Multi-imager and Quantity one image analysis software. Data are expressed as means + SE. Significance in mean values was determined by two-tailed Student's t-test. P < 0.05 was considered significant.
Immunoprecipitation.
Mucosal scrapings from rat jejunum were lysed in TGH buffer containing protease inhibitors, and samples were spun at 15,000 rpm for 15 min at 4°C. Supernatants were precleared by incubation with 25 μl of protein A beads for 20 min on ice. Samples were centrifuged for 30 s at maximum speed, and supernatants were incubated with 1 μg of specific antibody (anti-CFTR) or IgG control antibody on ice for 90 min and then incubated with 20 μl of 50% protein A beads and rocked for 1 h at 4°C. After centrifugation (14,000 rpm), protein-antibody-bead complexes were washed with 1× RIPA buffer (500 mM HEPES, 150 mM NaCl, 1% Triton X-100, and 1 mM EDTA), and samples were eluted with 5× SDS sample buffer before Western blot analysis (2, 24).
DNA constructs.
Glutathione S-transferase (GST) was provided by Dr. Linton Traub (Dept. of Cell Biology, University of Pittsburgh). GST-full-length human syntaxin 3 (GST-STX3, 1–290 aa) (Open Biosystems) was cloned into pGEX4T-1 with EcoRI and XhoI sites, and clones were verified by sequencing.
Protein and peptide preparation.
Expression and purification of GST fusion proteins were performed as previously described (15, 29, 36). GST and GST-STX3 fusion proteins produced in E. coli BL21 cells were grown in 20 ml of LB broth (Invitrogen) plus ampicillin (200 mg/ml; Fisher Scientific) overnight at 37°C with constant shaking to create a starter culture. From the starter culture, bacteria were diluted 1:50 in LB plus ampicillin (200 mg/ml), grown to an absorbance at 600 nm (A600) of 0.6 at 37°C with constant shaking, and induced by adding isopropyl-1-thio-β-d-galactopyranoside (100 μM). After 3–5 h with constant shaking at room temperature or A600 of 1.3–1.4, the bacteria were recovered by centrifugation at 15,000 rpm (JA-14 rotor) at 4°C for 15 min and pellets were stored at −80°C. Pellets were resuspended in bacterial lysis sonification buffer (50 mM Tris·HCl, pH 8.0, 300 mM NaCl, 0.2% Triton X-100, and 100 mM 2-mercaptoethanol) containing 100 mM PMSF, sonicated, and centrifuged at 14,000 rpm (JA-20 rotor) at 4°C for 15 min. Supernatants were incubated with 1 ml of 75% glutathione Sepharose 4B bead slurry (Amersham, Piscataway, NJ). Beads were collected by centrifugation at 500 g for 5 min at 4°C. GST fusion proteins bound to beads were washed in PBS, and proteins were eluted with glutathione elution buffer (in mM: 25 Tris·HCl, pH 8.0, 200 NaCl, and 10 glutathione) with 1 mM DTT on ice. Eluted fusion proteins were pooled, dialyzed in PBS, and stored at −80°C before binding assays were performed.
Binding assays.
Binding assays were performed as previously described (37). Mucosal scrapings from rat jejunum were collected with a glass slide and homogenized in freshly prepared TGH buffer containing protease inhibitors. GST and GST-STX3 (200 μg) were bound to lysate supernatants and 1 ml of PBS and incubated at 4°C for 1 h while continuously rotating. After 1 h, 75 μl of glutathione Sepharose 4B beads was added to the complex of bound proteins/supernatants and incubated at 4°C overnight with continuous rotation. The GST fusion proteins and bead complex were centrifuged at 10,000 rpm at 4°C for 1 min, and supernatant was discarded. The GST fusion protein and bead complex were washed with cold PBS, and pellets were resuspended in 30 μl of 5× SDS sample buffer. Gels were stained with Coomassie blue (Bio-Rad), and samples were separated by SDS-PAGE and analyzed by Western blot.
Syntaxin 3 silencing in Caco-2BBe cells.
Syntaxin 3 mRNA was targeted with shRNA delivered by a lentiviral system based on a pLKO.1-Puro vector. Cells were transduced to stably express scrambled or syntaxin 3 shRNA. Syntaxin 3-targeting shRNA (5′-ggaacaaactgaagagcat-3′) was designed with the AsiDesigner online tool (http://sysbio.kribb.re.kr:8080/AsiDesigner/menuDesigner.jsf) and subcloned into AgeI and EcoRI restriction sites of pLKO.1-TRC vector (Addgene no. 10878), immediately downstream from the U6 promoter. Scrambled shRNA in the pLKO.1-Puro vector was obtained from Addgene (no. 1864). The HEK-293 t/17 (ATCC) packaging cell line was transfected with the aforementioned constructs and FuGENE 6 transfection reagent (Roche Diagnostics) according to Addgene's protocol. Cells were plated in 25-ml flasks at 1.5 × 105 cells/ml and transfected the following day at ∼60–70% confluence with 1 μg of shRNA-containing vector, 0.75 μg of packaging plasmid (psPAX2, Addgene no. 12260), and 0.25 μg of envelope plasmid (pMD2.G, Addgene no. 12259). Medium was replaced after 15 h at 37°C. Virus-containing medium was collected 24 and 48 h after transfection and titrated for optimal multiplicity of infection (MOI). Caco-2BBe cells seeded at 4 × 105/ml were transduced at 60–70% confluence with Polybrene (Millipore) at 5 μg/ml final concentration. After transduction, cells were selected with 6 μg/ml puromycin (Sigma). Efficiency of shRNA silencing was determined by Western blot. Surface biotinylation was performed on cells as described above.
Cell viability.
Controls (nontransduced or scrambled shRNA transduced) and syntaxin 3 shRNA-transduced Caco-2BBe cells were grown in 60-mm tissue culture dishes. Cells were scraped into 500 μl of complete medium without serum at a concentration of 1 × 100 cells/ml. One hundred microliters of 0.4% Trypan blue stain (Invitrogen) was added to the cell suspension, mixed, and incubated at room temperature for 5 min. Samples (10–20 μl) were added to a hemocytometer and viewed under a microscope to observe nonviable (stained) and viable (unstained) cells. Cell viability as determined by Trypan blue exclusion was >95%. No difference in viability was detected between controls and syntaxin 3 shRNA-transduced cells.
Surface CFTR detection in Caco-2BBe cells after silencing of syntaxin 3.
Control (nontransduced and scrambled shRNA transduced) or syntaxin 3 shRNA-transduced Caco-2BBe cells were grown in 60-mm tissue culture dishes. Five days after transduction, cells were divided into two groups and treated with either PBS or 1 mM DbcAMP for 10 min at 37°C. After treatment, cells were immediately placed on ice, and surface biotinylation assays were performed. Western blots were analyzed with CFTR, syntaxin 3, and β-actin antibodies.
Statistical analysis.
Data are expressed as means ± SE. The significance of differences in mean values was determined by the two-tailed Student's t-test. P values <0.05 were considered significant.
RESULTS
Polarized Caco-2BBe cells express the machinery to regulate endocytic recycling and CFTR anion transport.
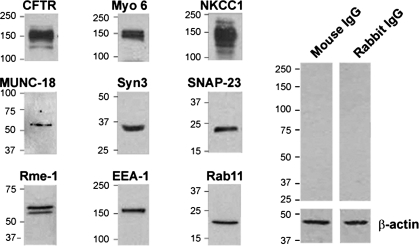
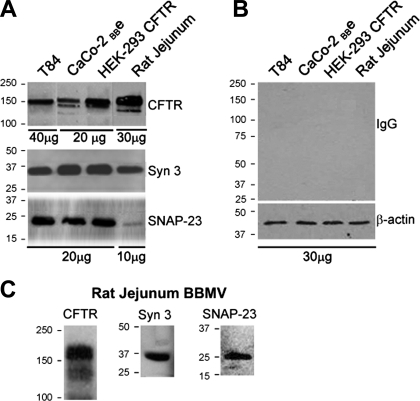
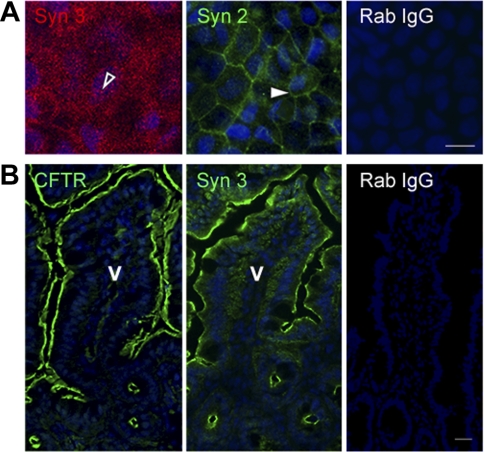
Because cAMP and cGMP can regulate exocytic insertion of CFTR to the surface of villus enterocytes and fluid secretion, we characterized an intestinal cell model that resembles villus enterocytes of the small intestine for the present studies of syntaxin 3 and regulated trafficking of CFTR to the cell surface. Caco-2BBe cells consist of differentiated enterocytes possessing a well-developed brush border (39, 40). We recently used this model to examine endocytic adaptors in clathrin-mediated endocytosis of CFTR in the intestine (15). Immunoblots of Caco-2BBe cell lysates (Fig. 1) revealed endogenous expression of endocytic (EEA-1, myosin VI), apical recycling (rme-1, rab11), exocytic proteins involved in vesicle fusion (SNAP 23, munc-18, syntaxin 3) basolateral chloride entry transport (NKCC1) and apical exit (CFTR) proteins necessary for endocytic recycling, exocytosis, and CFTR-mediated ion transport. Western blot analysis of brush border membranes from cultured T84 cells, rat jejunum, and HEK-293 CFTR-expressing cells also confirmed expression of CFTR, syntaxin 3, and SNAP 23 (Fig. 2). Syntaxin 2, another Q-SNARE family member, is expressed apically in polarized pancreatic acinar and MDCK II cells. We compared the distribution of syntaxin 2 and syntaxin 3 to determine which syntaxin predominates in the apical domain of polarized Caco-2BBe cells. Immunofluorescence labeling of Caco-2BBe cell monolayers (Fig. 3A) revealed apical staining for syntaxin 3, while syntaxin 2 was predominantly confined to the basolateral membranes. Immunofluorescence labeling of cryosections from mouse jejunum corroborated the observations in Caco-2BBe cells that the distribution of syntaxin 3 was apical and mirrored that of CFTR in native epithelial tissues (Fig. 3B).
Fig. 1.
Western blot analysis of endogenous cystic fibrosis transmembrane conductance regulator (CFTR) and apical recycling, exocytic, and ion transport proteins in polarized Caco-2BBe cells. Caco-2BBe cell lysates (20 μg) were resolved by SDS-PAGE, and proteins were detected by Western blot. Bands show endogenous expression of endocytic (EEA-1, myosin VI) and apical recycling (rme-1, rab11) proteins and exocytic proteins involved in vesicle fusion (SNAP 23, munc-18, syntaxin 3), basolateral chloride entry transport (NKCC1), and apical exit (CFTR). Control immunoblots probed with relevant IgG and β-actin loading are shown. Molecular mass standards (kDa) are indicated.
Fig. 2.
Western blot analysis of soluble N-ethylmaleimide (NEM)-sensitive factor attachment receptor (SNARE) proteins and CFTR in cultured cells and rat jejunum. Brush border membrane vesicle (BBMV) preparations and cell lysates (10–40 μg protein) were resolved by SDS-PAGE, and proteins were detected by immunoblotting. A: bands show endogenous expression of CFTR, syntaxin 3 (Syn 3), and SNAP-23 in T84, Caco-2BBe, and HEK-293 cells and rat jejunum. B: control immunoblot probed with relevant IgG antibodies and β-actin loading controls are shown. C: bands show CFTR, syntaxin 3, and SNAP-23 detected in BBMV preparations from rat jejunum. Molecular mass standards (kDa) are indicated.
Fig. 3.
Polarized distribution of syntaxin 2 and 3 in the intestine. Confluent polarized Caco-2BBe cells (A) and cryostat sections of mouse jejunum (B) were fixed and immunofluorescence performed as described in materials and methods. A: en face image taken at the level of the brush border shows the apical distribution of syntaxin 3 (open arrowhead). Image taken at basal level shows distribution of syntaxin 2 (Syn 2, solid arrowhead). B: staining for CFTR (green) and syntaxin 3 (green) in the apical domain of crypt and villus sections of mouse jejunum. Control labeling of cells (A) and tissue sections (B) with rabbit IgG (Rab IgG) antibody is shown. Hoechst nuclear stain labels the nuclei (blue) V, villus. Scale bar, 10 μm.
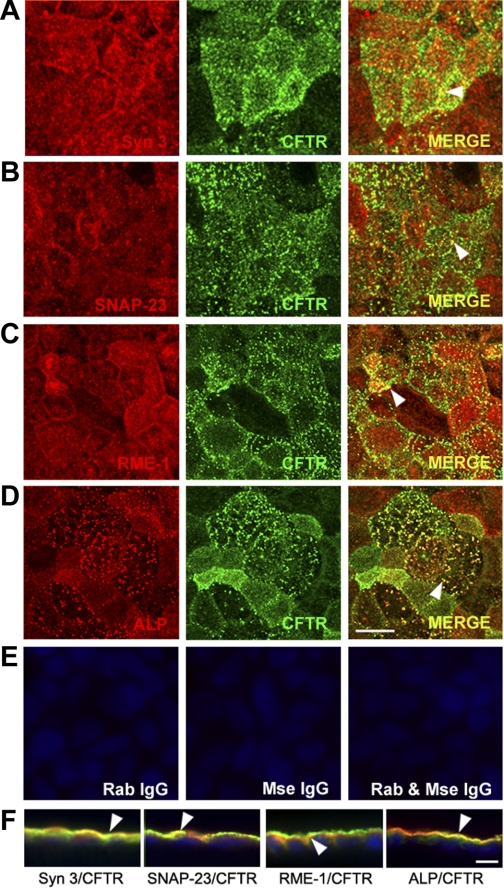
Distribution of apical recycling and exocytic vesicle SNARE machinery in polarized Caco-2BBe cells.
Using confocal microscopy, we examined the distribution of components of the apical recycling and syntaxin 3 SNARE machinery to understand their relationship to endogenous CFTR in polarized monolayers of Caco-2BBe cells. Images of views from en face and vertical sections of immunolabeled cells are shown in Fig. 4. The distribution of syntaxin 3 mirrored that of CFTR in the apical domain, as evidenced by their colocalization (Fig. 4, A and F, arrowheads). Although less abundant than syntaxin 3, the plasma membrane-associated SNARE protein SNAP 23 colocalized with CFTR on the apical membrane (Fig. 4, B and F), consistent with its role as a syntaxin 3-associated t-SNARE in polarized cells (56). The recycling marker rme-1 also localized to the apical domain and colocalized with CFTR (Fig. 4, C and F). Labeling for the brush border hydrolase ALP confirmed its localization on microvillar membranes (Fig. 4, D and F), where it colocalizes with CFTR. Control labeling with the relevant IgG confirmed specificity of antibody staining (Fig. 4E).
Fig. 4.
Subcellular distribution of endogenous CFTR and apical recycling and exocytic proteins in polarized Caco-2BBe cells. Confluent polarized Caco-2BBe cells were fixed, double-label immunofluorescence was performed, and cells were examined by confocal microscopy. A–D: en face images taken just above the level of the brush border show the distribution of syntaxin 3 (red, A), SNAP-23 (red, B), rme-1 (red, C), alkaline phosphatase (ALP, red, D), and CFTR (green, A–D). Merged images show colocalization (merge, yellow, arrowhead). E: control labeling with rabbit (Rab) and/or mouse (Mse) IgG antibodies. F: merged images of xz vertical sections of immunolabeled cells. Arrowheads indicate colocalization. Hoechst nuclear stain labels nuclei blue. Scale bar, 10 μm.
cAMP regulates recruitment of CFTR and syntaxin 3 to the surface of polarized Caco-2BBe cells.
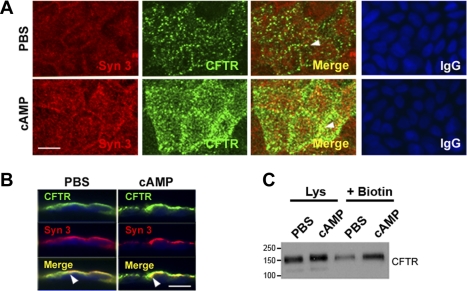
To characterize the Caco-2BBe cell model for studies of agonist-stimulated trafficking of CFTR in the villus compartment, we used two independent approaches to examine changes in the distribution of endogenous CFTR and syntaxin 3 in the apical domain following cAMP stimulation. Cells were treated with PBS or a cAMP agonist for 30 min and then were placed on ice, fixed and immunolabeled, and examined by confocal microscopy. Surface proteins were analyzed by cell surface biotinylation and Western blot. The distributions of syntaxin 3 and CFTR fluorescence are shown in Fig. 5, A and B. En face views of cells labeled for syntaxin 3 and CFTR and merged images of the apical surface of PBS- or cAMP-treated cells (Fig. 5A) reveal increased intensity of labeling for both proteins on the surface following cAMP treatment. Views of vertical sections (Fig. 5B) confirmed the increased labeling intensity and colocalization of syntaxin 3 and CFTR following cAMP treatment. The apical recruitment of CFTR following cAMP resembled its behavior in villus enterocytes of rat jejunum (24). Interestingly, apical labeling for syntaxin 3 also increased after cAMP treatment (Fig. 5, A and B). Surface biotinylation confirmed that cAMP stimulated trafficking of CFTR to the cell surface in Caco-2BBe cells (Fig. 5C), similar to what we observed in native intestinal tissues (3, 24).
Fig. 5.
cAMP stimulates apical recruitment of syntaxin 3 and CFTR in Caco-2BBe cells. Confluent monolayers of Caco-2BBe cells were treated with PBS or 1 mM cAMP. Cells were fixed, immunolabeled, and examined by confocal microscopy. A: en face views show the distribution of syntaxin 3 (red) and CFTR (green), and merged images show areas of colocalization (yellow) in PBS (top)- or cAMP (bottom)-treated cells. Control staining with relevant IgG antibodies is shown. B: images of vertical xz sections of immunolabeled cells show CFTR (green) and syntaxin 3 (red), and merged images (yellow) show colocalization (arrowheads). Scale bar, 10 μm. C: after PBS or cAMP treatment surface proteins were detected by sulfo-NHS-SS-biotin labeling and analyzed by Western blot as described in materials and methods. Cell lysates (Lys, 20 μg protein) and equivalent loads of surface biotinylated (+Biotin) proteins were resolved by SDS-PAGE and immunoblotted to detect CFTR. Molecular mass standards (kDa) are indicated.
Heat-stable enterotoxin increases syntaxin 3 and CFTR in the apical domain of rat jejunum.
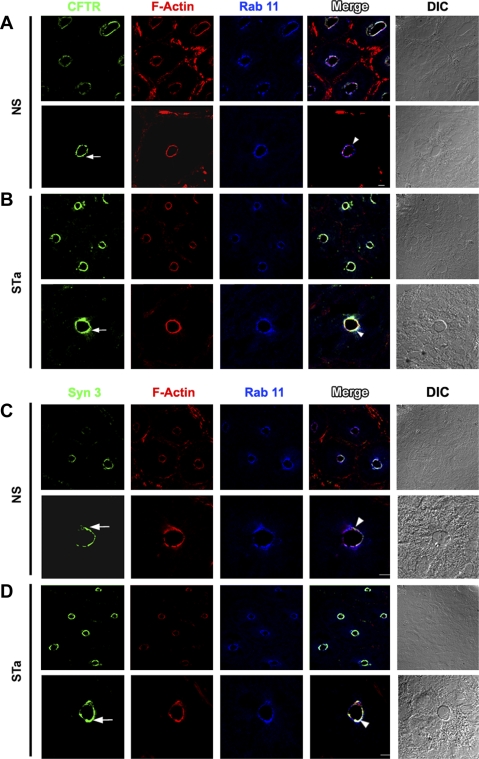
The cGMP agonist STa elicits massive fluid secretion and robust exocytosis of CFTR to the surface of rat jejunum (24, 54). To determine whether syntaxin 3 or the apical recycling protein rab11 was involved in STa-dependent exocytosis of CFTR, we immunolabeled sections of rat jejunum after STa (0.5 μM) or saline treatment as before and examined the sections by confocal microscopy to detect changes in their distribution in vivo. As predicted, STa treatment resulted in increased CFTR fluorescence in the apical domain (Fig. 6, A and B, arrows). The distribution of the apical recycling marker rab11 did not appear to be altered after STa treatment (Fig. 6), consistent with its role in constitutive apical recycling. However, similar to what we observed after cAMP treatment of Caco-2BBe cells, STa treatment resulted in a 50% increase in syntaxin 3 fluorescence labeling intensity in the apical domain of rat jejunum (Fig. 7, A and C). These results indicate that stimulation with CFTR second messengers simultaneously recruits syntaxin 3 to the apical domain in the intestine.
Fig. 6.
Apical distribution of CFTR, syntaxin 3, and rab11 in rat jejunum after heat-stable enterotoxin (STa). Cryostat sections of rat jejunum treated with normal saline (NS) or STa were immunolabeled and examined by confocal microscopy as described in materials and methods. Low- and high-magnification images of crypt sections are shown. Images of immunolabeled sections from rat jejunum after NS (A and C) or STa (B and D) show the distribution of CFTR (green, arrow, A and B), F-actin (red, A–D), rab11 (blue, A–D), and syntaxin 3 (green, arrow, C and D). Merged images show areas of colocalization (white; arrowhead). DIC, differential interference contrast. Scale bars, 10 μm and 100 μm.
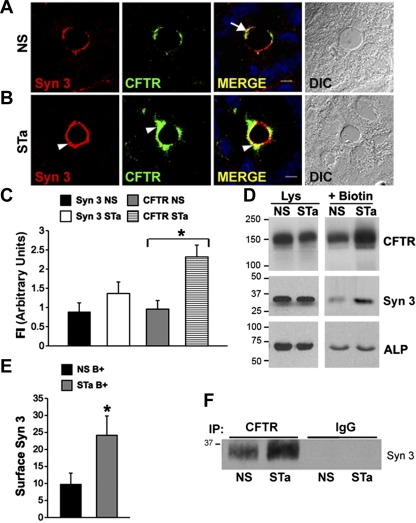
Fig. 7.
STa stimulates recruitment of CFTR and syntaxin 3 to the surface of rat jejunum. Cryostat sections of NS- or STa-treated rat jejunum were immunolabeled and examined by confocal microscopy. A and B: high-magnification images of crypt from NS (A)- or STa (B)-treated tissues show the apical distribution of syntaxin 3 (red) and CFTR (green) and merge (yellow). Increased apical staining for syntaxin 3 (B, arrowhead) and CFTR (green, arrowhead) after STa is shown. Merged images show areas of colocalization of CFTR and syntaxin 3 in NS (A, arrow)- and STa (B, arrowhead)-treated jejunum. Nuclei are stained with Hoechst stain (blue), Scale bar, 10 μm. C: quantification of fluorescence intensity (FI) of syntaxin 3 and CFTR label in the apical domain of NS- and STa-treated rat jejunum. Data represent means + SE (n > 10). *P < 0.01. D: after NS or STa treatment, surface proteins were labeled with sulfo-NHS-SS biotin in vivo, cells were lysed, and proteins were bound to streptavidin-agarose. Equivalent (20 μg) loads of protein from cell lysates and surface biotinylated samples were resolved by SDS-PAGE and immunoblotted to detect CFTR, syntaxin 3, and alkaline phosphatase. E: quantification (mean pixel values) of surface labeled (B+) syntaxin 3 (Syn 3) in NS- and STa-treated enterocytes. Data represent means + SE (n > 4). *P = 0.05. F: Western blot of CFTR coimmunoprecipitates from NS- and STa-treated jejunum detects increased syntaxin 3 in STa-treated jejunum. Equivalent starting proteins from tissue lysates were immunoprecipitated with either anti-CFTR or control rabbit IgG antibodies and bound to protein A beads, and samples were separated by SDS-PAGE and immunoblotted with anti-syntaxin 3 antibodies. Molecular mass standards (kDa) are indicated.
Heat-stable enterotoxin increases surface expression of syntaxin 3 in rat jejunum.
The increase in syntaxin 3 fluorescence in the apical domain of rat jejunum following STa treatment (Figs. 6 and 7) was surprising because, unlike CFTR, syntaxin 3 or members of the SNARE family are not regulated by PKA or PKG (49). To confirm that the observed increase in syntaxin 3 labeling in the apical domain reflected increased abundance of syntaxin 3 on the surface of enterocytes, we performed cell surface biotinylation and analyzed syntaxin 3 or CFTR surface expression by Western blot (Fig. 7D). Consistent with our previous results (24), STa stimulated a robust increase in surface CFTR expression in rat jejunum. Surface biotinylation also confirmed that indeed STa also increased syntaxin 3 surface expression by ∼50% (Fig. 7E), consistent with the increase in apical fluorescence intensities (Fig. 7, A and B). The observation that the CFTR agonist STa recruited both syntaxin 3 and CFTR to the cell surface and that both colocalized in subapical vesicles strongly suggested that syntaxin 3 and CFTR share a common endosomal compartment that undergoes agonist-stimulated recruitment to the plasma membrane. To determine whether both proteins physically interact in vivo, we performed coimmunoprecipitation experiments in rat jejunum after saline or STa treatment, using equivalent amounts of protein (6 mg) from each condition and a rat-specific anti-CFTR antibody. Immunoprecipitates from control and STa-treated jejunum were analyzed by Western blot to detect syntaxin 3. CFTR coimmunoprecipitated with syntaxin 3 in both control and STa-treated tissues, but there was a 1.6-fold increase in syntaxin 3 detected in immunoprecipitates from STa-treated samples (Fig. 7F). These results suggest that both proteins traffic together in their exocytic route to the apical membrane in the intestine.
CFTR interacts with syntaxin 3 in intestine.
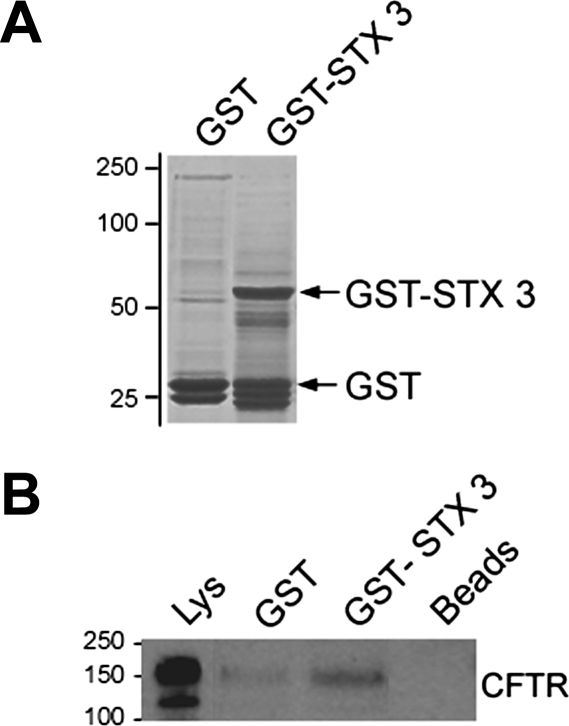
The results of our immunoprecipitation, morphological, and surface biotinylation experiments strongly suggested that CFTR interacts with syntaxin 3 to regulate apical exocytosis in the native intestine. Since second messenger-stimulated recruitment of CFTR and syntaxin 3 could be replicated in tissues and polarized Caco-2BBe cells, we used independent approaches of GST binding assays and lysates from polarized monolayers of Caco-2BBe cells to determine whether endogenous CFTR is a binding partner for syntaxin 3 (Fig. 8). The Coomassie-stained gel (Fig. 8A) shows bands of either purified GST or GST-STX3 fusion proteins. GST or GST-STX3 was allowed to interact with cell supernatants, and protein complexes were analyzed by Western blot to detect the CFTR-syntaxin 3 interaction (Fig. 8B). These results indicated that ∼14% CFTR interacts with syntaxin 3 in the intestine.
Fig. 8.
Glutathione S-transferase (GST) pulldown assay detects CFTR interaction with syntaxin 3 in rat jejunum. GST or GST + syntaxin 3 (GST-STX3) (200 μg) of were bound to glutathione Sepharose beads and incubated with rat jejunum lysates. Lysate and GST samples were resolved on SDS-PAGE gels and stained with Coomassie blue (A) or transferred to polyvinylidene difluoride nitrocellulose (B) and immunoblotted with anti-CFTR antibody. Molecular mass standards (kDa) are indicated.
Introduction of syntaxin 3 shRNA interrupts cAMP-dependent recruitment of CFTR to cell surface.
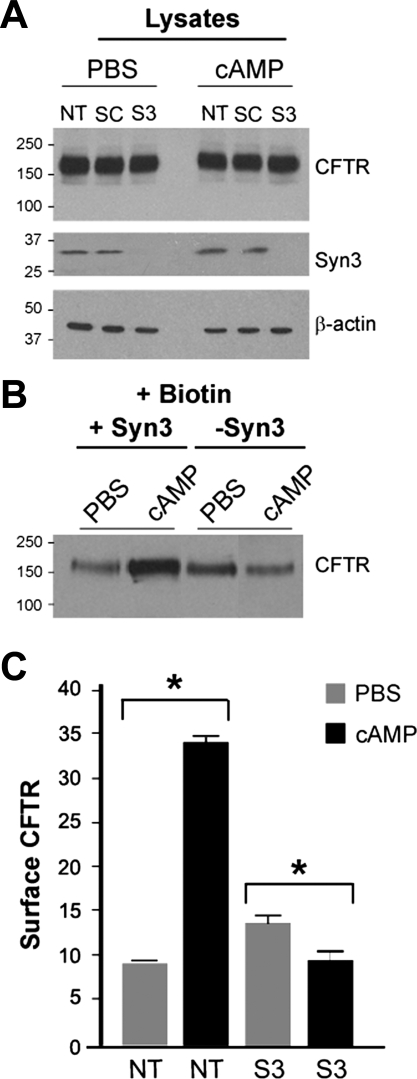
Finally, to determine whether syntaxin 3 is necessary for agonist-stimulated exocytosis of CFTR to the plasma membrane, surface CFTR was examined in Caco-2BBe cells lacking syntaxin 3. Controls (nontransduced or transduced with scrambled shRNA) and cells transduced with syntaxin 3 shRNA were subjected to surface biotinylation 30 min after treatment with PBS or cAMP. Immunoblot analysis of cell lysates revealed that the total amount of CFTR in PBS- or cAMP-treated cells remained unchanged (Fig. 9A). Immunoblot analysis of PBS- or cAMP-treated control cells showed similar levels of syntaxin 3, while transduction with syntaxin 3 shRNA efficiently reduced (∼100%) its expression in PBS- or cAMP-treated cells. cAMP increased surface levels of CFTR 3.5-fold in control cells. In cells lacking syntaxin 3, treatment with cAMP reduced surface CFTR by ∼30% to levels similar to PBS-treated controls (Fig. 9C). These results indicate a requirement for syntaxin 3 in the exocytosis of CFTR.
Fig. 9.
Short hairpin RNA (shRNA) silencing of syntaxin 3 reduces surface CFTR in cAMP-stimulated Caco-2BBe cells. A: immunoblot analysis of Caco-2BBe cell lysates from control [nontransduced (NT) or scrambled shRNA (SC)] or after transduction with lentiviral particles containing syntaxin 3 (S3). Cells were treated with PBS or 1 mM DbcAMP (cAMP) for 30 min. Lysates (20 μg) were separated on SDS-PAGE gels and analyzed by Western blot using CFTR, syntaxin 3, or β-actin antibodies. Syntaxin 3 shRNA reduced syntaxin 3 expression by ∼100%. β-Actin loading control bands are shown. B: surface-biotinylated CFTR in PBS- or cAMP-stimulated cells transduced with syntaxin 3 shRNA. After PBS or 1 mM DbcAMP treatment, surface proteins were labeled with sulfo-NHS-SS-biotin. Cells were lysed, and proteins were bound to streptavidin-agarose. Equivalent biotinylated protein samples were resolved by SDS-PAGE and immunoblotted to detect CFTR. C: quantification (mean pixel values) of surface CFTR in control and syntaxin 3 shRNA (S3)-transduced Caco-2BBe cells. Data represent means + SE (n > 3).*P = 0.05. Molecular mass standards (kDa) are indicated.
DISCUSSION
We previously identified (24) cAMP/PKA- and cGMP/PKG-regulated recruitment of CFTR from subapical vesicles to the surface of enterocytes as a critical step in enterotoxin-elicited fluid secretion in the small intestine, but the proteins regulating recruitment of CFTR from endosomes to the cell surface were unknown. In this study we identified apical SNARE (syntaxin 3, SNAP 23, munc-18) and recycling (rme-1 and rab11) proteins in CFTR-positive endosomes in the apical domain of enterocytes, indicating a close association of the apical recycling and regulated exocytic trafficking machinery in vivo. With an antibody that recognizes both a and b isoforms of rab11, apical staining remained unchanged after cGMP stimulation with STa, suggesting that rab11 is not involved in cGMP-regulated recruitment in the native intestine. rme-1, a basolateral recycling protein in the Caenorhabditis elegans intestine, regulates CFTR recycling in nonpolarized cells, but before the present study there was no evidence for apical localization of rme-1 in native CFTR-expressing epithelia (26, 42). The identification of rme-1 in subapical endosomes supports its role in apical recycling of CFTR in higher species. Furthermore, the distribution and expression of the proteins examined here are consistent with their role in apical trafficking and transport in epithelial cells (7, 32, 45).
We focused on syntaxin 3 because of its importance in apical targeting, recycling, and vesicle fusion in intestinal cells (13, 21, 47). Importantly, syntaxin 3 was shown to regulate second messenger-stimulated apical recruitment and function of proton transport in gastric epithelial cells of the stomach (6, 38). Gastric parietal cells resemble the subpopulation of CFTR High Expresser (CHE) cells that we first used to demonstrate regulated trafficking of CFTR to the apical domain of the intestine. Like parietal cells, CHE cells also possess a prominent subapical endosomal pool of CFTR-positive vesicles, but more is known about the proteins that regulate apical recycling and traffic in gastric parietal cells compared with CHE cells (3, 32). Although the subapical endosomal pool is not as prominent in crypt and villus enterocytes, regulated trafficking of CFTR is observed in both cell types.
In the gastrointestinal tract, syntaxin 2 and 3 are implicated in apical exocytosis of zymogen granules in pancreatic acinar cells (43). In cultured intestinal epithelial cells, agonist-dependent trafficking of the adenosine 2b receptor to the apical domain was shown to involve the SNARE machinery vesicle-associated membrane protein VAMP-2 and SNAP-23, suggesting that syntaxin 3 may be involved, but it was not examined in that study (56). Surprisingly, a recent study in guinea pig duodenal Brunner's gland identified syntaxin 4 and SNAP-23 in cholinergic-stimulated apical exocytosis and dilation of the ductal lumen (17). In the rat and mouse duodenum, high levels of CFTR are in the apical domain of Brunner's gland acinar cells, but the expression and function of specific syntaxins in CFTR function in Brunner's gland have not been identified (1, 5).
The role of syntaxins (including syntaxin 3) in CFTR trafficking and transport has been examined, but this is the first evidence demonstrating the involvement of syntaxin 3 in CFTR trafficking. Studies of native CFTR-expressing airway epithelia identified endogenous syntaxin 3 and syntaxin 1A in the apical domain. However, in contrast to the intestine, where syntaxin 3 binds to CFTR and is recruited to the cell surface following CFTR agonists, in the airway CFTR binds to syntaxin 1A (but not syntaxin 3) and, rather than regulating trafficking, syntaxin 1A interacts with CFTR to modulate its gating (14, 37). These findings and those of the present study are in agreement with the absence of second messenger-regulated trafficking of CFTR in native airway epithelial cells (9). Other studies identified interactions of the endosomal SNARE proteins syntaxin 7 and 8 (but not syntaxin 3) with CFTR that modulated trafficking and function (10, 11). The failure to identify a CFTR-syntaxin 3 interaction and a role for syntaxin 3 trafficking in airway epithelial cells further supports the notion that apical trafficking directs CFTR function in native epithelia through its interaction with cell-specific proteins in the same manner that syntaxins display isoform specificity in regulation of exocytosis in epithelial cells (27, 33, 48, 50).
Because syntaxin 3 is critical to apical polarity, attempts to silence its expression in polarized Caco-2BBe cells proved challenging in our hands. These cells acquire apical and basolateral polarity following at least 2 wk of growth on permeable membrane supports. However, shRNA-induced silencing effectively (∼100%) reduced syntaxin 3 expression in Caco-2BBe cells at 120 h. Control cells grown under these conditions demonstrated a modest increase in surface CFTR following cAMP stimulation (∼3-fold), compared with the almost fivefold response observed in native tissues. Nevertheless, cells lacking syntaxin 3 demonstrated reduced exocytic response to cAMP and lower surface CFTR levels compared with controls. These data confirm an important role for syntaxin 3 in regulated trafficking and exocytosis of CFTR to the apical membrane of intestinal cells.
Investigators have emphasized CFTR's role in anion secretion in crypt cells since its transport activity is high in this intestinal compartment. Importantly, CFTR behavior in the crypt is replicated phenotypically and functionally in the T84 cell model (22). Villus enterocytes possess distinct phenotypic and functional characteristics compared with their immature crypt cell counterparts. Villus enterocytes are the major sites of CFTR-mediated bicarbonate secretion and participate in regulated trafficking of CFTR to the cell surface (24, 53), but there is no validated polarized intestinal cell model available that replicates CFTR behavior in villus enterocytes in the small intestine. We recently validated Caco-2BBe cells as a model for studies of clathrin-mediated endocytosis of endogenous CFTR in the intestine. The present study extends this characterization by demonstrating the usefulness of this model for studies of CFTR apical recycling and regulated trafficking to the plasma membrane. Although the present study did not examine CFTR ion transport in Caco-2BBe cells, we observed CFTR currents in this model when mounted in Ussing chambers. The identification of syntaxin 3 SNARE and associated apical recycling machinery in regulated exocytosis of CFTR is an important step toward elucidating the role of CFTR and villus cells in the pathogenesis of secretory diarrhea. These studies are important because before identification of the CFTR gene, investigators had long noted that both cholera toxin and STa from E. coli target villus enterocytes in the jejunum (19, 20). This suggests that molecular studies using this cell model could prove vital in further elucidating villus-specific pathways of CFTR regulation.
The recent surge in global health awareness has stimulated a burst of philanthropic efforts and investment in research to develop compounds to treat enterotoxin-elicited diarrhea. Some of these compounds target CFTR channels on the surface of the intestine (52), but these approaches are designed to inhibit CFTR channels that are already resident on the plasma membrane rather than exocytosis-based mechanisms that increase CFTR on the cell surface to elicit fluid secretion. The findings reported here suggest that identification of physiologically relevant targets of apical endocytosis and exocytosis into the plasma membrane of the intestine will prove vital for effective drug therapies to treat enterotoxin-elicited diarrheal disease.
GRANTS
National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-077065 to N. Ameen supported this work.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Sean Alber and Christine Goldbach of the Center for Biologic Imaging (University of Pittsburgh) for their excellent technical assistance.
Present addresses: J. Marathe, SSM St Mary's Health Center, St. Louis, MO; A. Collaco, Dept. of Pediatrics, Yale University School of Medicine, 333 Cedar St., Fitkin 408, New Haven, CT 06520; H. Kohnke, Centre for Medical Parasitology, Copenhagen University Hospital, Rigshospitalet 7602, 2100 København Ø, Denmark.
REFERENCES
- 1.Ameen N, Alexis J, Salas P. Cellular localization of the cystic fibrosis transmembrane conductance regulator in mouse intestinal tract. Histochem Cell Biol 114: 69– 75, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Ameen N, Apodaca G. Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic 8: 998– 1006, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Ameen NA, Marino C, Salas PJ. cAMP-dependent exocytosis and vesicle traffic regulate CFTR and fluid transport in rat jejunum in vivo. Am J Physiol Cell Physiol 284: C429– C438, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Ameen NA, Martensson B, Bourguinon L, Marino C, Isenberg J. CFTR channel insertion to the apical surface in rat duodenal villus epithelial cells is upregulated by VIP in vivo. J Cell Sci 112: 887– 894, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Ameen NA, van Donselaar E, Posthuma G, de Jonge H, McLaughlin G, Geuze HJ, Marino C, Peters PJ. Subcellular distribution of CFTR in rat intestine supports a physiologic role for CFTR regulation by vesicle traffic. Histochem Cell Biol 114: 219– 228, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Ammar DA, Zhou R, Forte JG, Yao X. Syntaxin 3 is required for cAMP-induced acid secretion: streptolysin O-permeabilized gastric gland model. Am J Physiol Gastrointest Liver Physiol 282: G23– G33, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Banerjee A, Li G, Alexander EA, Schwartz JH. Role of SNAP-23 in trafficking of H+-ATPase in cultured inner medullary collecting duct cells. Am J Physiol Cell Physiol 280: C775– C781, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol 62: 535– 572, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Bertrand CA, Frizzell RA. The role of regulated CFTR trafficking in epithelial secretion. Am J Physiol Cell Physiol 285: C1– C18, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Bilan F, Nacfer M, Fresquet F, Norez C, Melin P, Martin-Berge A, Costa de Beauregard MA, Becq F, Kitzis A, Thoreau V. Endosomal SNARE proteins regulate CFTR activity and trafficking in epithelial cells. Exp Cell Res 314: 2199– 2211, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Bilan F, Thoreau V, Nacfer M, Derand R, Norez C, Cantereau A, Garcia M, Becq F, Kitzis A. Syntaxin 8 impairs trafficking of cystic fibrosis transmembrane conductance regulator (CFTR) and inhibits its channel activity. J Cell Sci 117: 1923– 1935, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Bradbury NA, Cohn JA, Venglarik CJ, Bridges RJ. Biochemical and biophysical identification of the cystic fibrosis transmembrane conductance regulator chloride channels as components of endocytic clathrin-coated vesicles. J Biol Chem 269: 8296– 8302, 1994 [PubMed] [Google Scholar]
- 13.Breuza L, Fransen J, Le Bivic A. Transport and function of syntaxin 3 in human epithelial intestinal cells. Am J Physiol Cell Physiol 279: C1239– C1248, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Chang SY, Di A, Naren AP, Palfrey HC, Kirk KL, Nelson DJ. Mechanisms of CFTR regulation by syntaxin 1A and PKA. J Cell Sci 115: 783– 791, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Collaco A, Jakab R, Hegan P, Mooseker M, Ameen N. Alpha-AP-2 directs myosin VI-dependent endocytosis of cystic fibrosis transmembrane conductance regulator chloride channels in the intestine. J Biol Chem 285: 17177– 17187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cosen-Binker LI, Morris GP, Vanner S, Gaisano HY. Munc18/SNARE proteins' regulation of exocytosis in guinea pig duodenal Brunner's gland acini. World J Gastroenterol 14: 2314– 2322, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahl CA, Yamada T. Global health inequity: scientific challenges remain but can be solved. J Clin Invest 118: 1242– 1243, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jonge HR. The localization of guanylate cyclase in rat small intestinal epithelium. FEBS Lett 53: 237– 241, 1975 [DOI] [PubMed] [Google Scholar]
- 20.de Jonge HR. The response of small intestinal villus and crypt epithelium to cholera toxin in rat. Evidence against a specific role of the crypt cells in choleragen induced secretion. Biochim Biophys Acta 381: 128– 143, 1975 [DOI] [PubMed] [Google Scholar]
- 21.Delgrossi MH, Breuza L, Mirre C, Chavrier P, Le Bivic A. Human syntaxin 3 is localized apically in human intestinal cells. J Cell Sci 110: 2207– 2214, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Denning GM, Ostedgaard LS, Cheng SH, Smith AE, Welch MJ. Localization of the cystic fibrosis transmembrane conductance regulator in chloride secretory epithelia. J Clin Invest 89: 339– 349, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Field M, Semrad CE. Toxigenic diarrheas, congenital diarrheas and cystic fibrosis: disorders of intestinal ion transport. Annu Rev Physiol 55: 631– 655, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Golin-Bisello F, Bradbury N, Ameen N. STa and cGMP stimulate CFTR translocation to the surface of villus enterocytes in rat jejunum and is regulated by protein kinase G. Am J Physiol Cell Physiol 289: C708– C716, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Grant B, Zhang Y, Paupard MC, Lin SX, Hall DH, Hirsh D. Evidence that RME-1, a conserved C. elegans EH-domain protein, functions in endocytic recycling. Nat Cell Biol 3: 573– 579, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol 7: 426– 436, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Guttman JA, Finlay BB. Subcellular alterations that lead to diarrhea during bacterial pathogenesis. Trends Microbiol 16: 535– 542, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Jha A, Agostinelli NR, Mishra SK, Keyel PA, Hawryluk MJ, Traub LM. A novel AP-2 adaptor interaction motif initially identified in the long-splice isoform of synaptojanin 1, SJ170. J Biol Chem 279: 2281– 2290, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Lafont F, Verkade P, Galli T, Wimmer C, Louvard D, Simons K. Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells. Proc Natl Acad Sci USA 96: 3734– 3738, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lam PP, Cosen Binker LI, Lugea A, Pandol SJ, Gaisano HY. Alcohol redirects CCK-mediated apical exocytosis to the acinar basolateral membrane in alcoholic pancreatitis. Traffic 8: 605– 617, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Lapierre LA, Avant KM, Caldwell CM, Ham AJ, Hill S, Williams JA, Smolka AJ, Goldenring JR. Characterization of immunoisolated human gastric parietal cells tubulovesicles: identification of regulators of apical recycling. Am J Physiol Gastrointest Liver Physiol 292: G1249– G1262, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Li G, Alexander EA, Schwartz JH. Syntaxin isoform specificity in the regulation of renal H+-ATPase exocytosis. J Biol Chem 278: 19791– 19797, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Li X, Low SH, Miura M, Weimbs T. SNARE expression and localization in renal epithelial cells suggest mechanism for variability of trafficking phenotypes. Am J Physiol Renal Physiol 283: F1111– F1122, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Low SH, Chapin SJ, Wimmer C, Whiteheart SW, Komuves LG, Mostov KE, Weimbs T. The SNARE machinery is involved in apical plasma membrane trafficking in MDCK cells. J Cell Biol 141: 1503– 1513, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishra SK, Hawryluk MJ, Brett TJ, Keyel PA, Dupin AL, Jha A, Heuser JE, Fremont DH, Traub LM. Dual engagement regulation of protein interactions with the AP-2 adaptor alpha appendage. J Biol Chem 279: 46191– 46203, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Naren AP, Di A, Cormet-Boyaka E, Boyaka PN, McGhee JR, Zhou W, Akagawa K, Fujiwara T, Thome U, Engelhardt JF, Nelson DJ, Kirk KL. Syntaxin 1A is expressed in airway epithelial cells, where it modulates CFTR Cl− currents. J Clin Invest 105: 377– 386, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng XR, Yao X, Chow DC, Forte JG, Bennett MK. Association of syntaxin 3 and vesicle-associated membrane protein (VAMP) with H+/K+-ATPase-containing tubulovesicles in gastric parietal cells. Mol Biol Cell 8: 399– 407, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson MD, Bement WM, Mooseker MS. An in vitro model for the analysis of intestinal brush border assembly. II. Changes in expression and localization of brush border proteins during cell contact-induced brush border assembly in Caco-2BBe cells. J Cell Sci 105: 461– 472, 1993 [DOI] [PubMed] [Google Scholar]
- 40.Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J Cell Sci 102: 581– 600, 1992 [DOI] [PubMed] [Google Scholar]
- 41.Petri WA, Jr, Miller M, Binder HJ, Levine MM, Dillingham R, Guerrant RL. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest 118: 1277– 1290, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Picciano JA, Ameen N, Grant BD, Bradbury NA. Rme-1 regulates the recycling of the cystic fibrosis transmembrane conductance regulator. Am J Physiol Cell Physiol 285: C1009– C1018, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Pickett JA, Campos-Toimil M, Thomas P, Edwardson JM. Identification of SNAREs that mediate zymogen granule exocytosis. Biochem Biophys Res Commun 359: 599– 603, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Prince LS, Workman RB, Marchese RB. Rapid endocytosis of the cystic fibrosis transmembrane conductance regulator chloride channel. Proc Natl Acad Sci USA 91: 5192– 5196, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riento K, Galli T, Jansson S, Ehnholm C, Lehtonen E, Olkkonen VM. Interaction of Munc-18–2 with syntaxin 3 controls the association of apical SNAREs in epithelial cells. J Cell Sci 111: 2681– 2688, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Schwartz JH, Li G, Yang Q, Suri V, Ross JJ, Alexander EA. Role of SNAREs and H+-ATPase in the targeting of proton pump-coated vesicles to collecting duct cell apical membrane. Kidney Int 72: 1310– 1315, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Sharma N, Low SH, Misra S, Pallavi B, Weimbs T. Apical targeting of syntaxin 3 is essential for epithelial cell polarity. J Cell Biol 173: 937– 948, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silvis MR, Bertrand CA, Ameen N, Golin-Bisello F, Butterworth MB, Frizzell RA, Bradbury NA. Rab11b regulates the apical recycling of CFTR in polarized intestinal epithelial cells. Mol Biol Cell 20: 2337– 2350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sollner TH. Regulated exocytosis and SNARE function. Mol Membr Biol 20: 209– 220, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Swiatecka-Urban A, Talebian L, Kanno E, Moreau-Marquis S, Coutermarsh B, Hansen K, Karlson KH, Barnaby R, Cheney RE, Langford GM, Fukuda M, Stanton BA. Myosin Vb is required for trafficking of the cystic fibrosis transmembrane conductance regulator in Rab11a-specific apical recycling endosomes in polarized human airway epithelial cells. J Biol Chem 282: 23725– 23736, 2007 [DOI] [PubMed] [Google Scholar]
- 51.ter Beest MB, Chapin SJ, Avrahami D, Mostov KE. The role of syntaxins in the specificity of vesicle targeting in polarized epithelial cells. Mol Biol Cell 16: 5784– 5792, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tradtrantip L, Namkung W, Verkman AS. Crofelemer, an antisecretory antidiarrheal proanthocyanidin oligomer extracted from Croton lechleri, targets two distinct intestinal chloride channels. Mol Pharmacol 77: 69– 78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tuo B, Wen G, Zhang Y, Liu X, Wang X, Dong H. Involvement of phosphatidylinositol 3-kinase in cAMP- and cGMP-induced duodenal epithelial CFTR activation in mice. Am J Physiol Cell Physiol 297: C503–C515, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Vaandrager A, Bot A, De Jonge H. Guanosine 3′,5′-cyclic monophosphate-dependent protein kinase II mediates heat-stable enterotoxin-provoked chloride secretion in rat intestine. Gastroenterology 112: 437– 443, 1997 [DOI] [PubMed] [Google Scholar]
- 55.van Barneveld A, Stanke F, Ballmann M, Naim HY, Tummler B. Ex vivo biochemical analysis of CFTR in human rectal biopsies. Biochim Biophys Acta 1762: 393– 397, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Wang L, Kolachala V, Walia B, Balasubramanian S, Hall RA, Merlin D, Sitaraman SV. Agonist-induced polarized trafficking and surface expression of the adenosine 2b receptor in intestinal epithelial cells: role of SNARE proteins. Am J Physiol Gastrointest Liver Physiol 287: G1100– G1107, 2004 [DOI] [PubMed] [Google Scholar]