Abstract
Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) is a member of the immunoglobulin superfamily of cell adhesion molecules with important roles in angiogenesis and inflammation. However, the molecular and cellular mechanisms, and the role that specific PECAM-1 isoforms play in these processes, remain elusive. We recently showed attenuation of retinal vascular development and neovascularization in PECAM-1-deficient (PECAM-1−/−) mice. To gain further insight into the role of PECAM-1 in these processes, we isolated primary retinal endothelial cells (EC) from wild-type (PECAM-1+/+) and PECAM-1−/− mice. Lack of PECAM-1 had a significant impact on endothelial cell-cell and cell-matrix interactions, resulting in attenuation of cell migration and capillary morphogenesis. Mechanistically these changes were associated with a significant decrease in expression of endothelial nitric oxide synthase (eNOS) and nitric oxide (NO) bioavailability in PECAM-1−/− retinal EC. PECAM-1−/− retinal EC also exhibited a lower rate of apoptosis under basal and challenged conditions, consistent with their increased growth rate. Furthermore, reexpression of PECAM-1 was sufficient to restore migration and capillary morphogenesis of null cells in an isoform-specific manner. Thus PECAM-1 expression modulates proangiogenic properties of EC, and these activities are significantly influenced by alternative splicing of its cytoplasmic domain.
Keywords: angiogenesis, retinal endothelial cells, CD31, osteopontin, thrombospondins, tenascin-C, adherens junctions, endothelial nitric oxide synthase
platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) is a 130-kDa transmembrane glycoprotein expressed on the surface of endothelial cells (EC), platelets, and some hematopoietic cells (24, 31, 38). PECAM-1 is generally localized to sites of cell-cell contact in confluent EC monolayers with important roles in vascular permeability, coagulation, and transmigration of leukocytes (24, 31). PECAM-1 is a member of the immunoglobulin (Ig) superfamily and is composed of six extracellular Ig domains, a transmembrane domain, and a relatively long cytoplasmic domain. Exons 10 through 16 encode for the PECAM-1 cytoplasmic domain whose alternative splicing generates eight isoforms, which only differ in the length of their cytoplasmic domain. These isoforms include full-length PECAM-1 and isoforms lacking exons 12 (Δ12), 14 (Δ14), 15 (Δ15), 12 and 14 (Δ12&14), 12 and 15 (Δ12&15), 14 and 15 (Δ14&15), and 12, 14, and 15 (Δ12,14&15). We have shown that multiple isoforms of PECAM-1 are expressed in vascular beds of different tissues (40), and their expression pattern is regulated during development and angiogenesis (40, 48, 49, 52, 53). Although full-length PECAM-1 is the predominant isoform expressed in human endothelium and hematopoietic cells, Δ14&15 PECAM-1 is the predominant isoform expressed in murine endothelium and hematopoietic cells. However, the isoform-specific function of PECAM-1 in angiogenesis and inflammation requires further investigation.
PECAM-1 cytoplasmic domain lacks intrinsic kinase activity, but it contains a number of tyrosine residues encoded in individual exons. These tyrosine residues can be phosphorylated under various conditions, including shear stress and adhesion to specific matrix proteins, providing docking sites for intracellular signaling proteins with SH2 domain (24, 31). A number of such proteins including Src family kinases, phosphatidylinositol 3-kinase (PI3-kinase), and SHP-2 associate with PECAM-1 and modulate its signaling capacity and EC phenotype. Thus inclusion and/or exclusion of tyrosine-encoding exons from PECAM-1 cytoplasmic domain will have significant impact on its signaling capacity. We have shown (8, 39, 50) different potential for specific isoforms of PECAM-1 in activation of various intracellular signaling pathways, including Rho GTPases and MAP kinase pathways, impacting cell adhesive and migratory properties. In addition, junctional localization of PECAM-1 is dependent on the ability of the cells that express PECAM-1 to form adherens junctions. The disruption of adherens junction formation results in failure of PECAM-1 to localize to sites of cell-cell contact (39, 55).
In EC, nitric oxide (NO) is mainly produced by endothelial nitric oxide synthase (eNOS) and functions as a key player in regulation of regional blood flow, modulation of leukocyte endothelial interactions, and organization of the intercellular junctions (1, 10, 13, 27). PECAM-1 associates with eNOS at the intercellular junctions and may regulate eNOS activation in response to rapid changes in shear stress (12, 14, 17). Mice deficient in PECAM-1 (PECAM-1−/−) exhibit abnormal vasodilatory activity, further supporting a role for PECAM-1 in regulation of eNOS activity and NO bioavailability (2). However, the mechanisms involved and the role that PECAM-1 isoforms play in these processes remain elusive.
PECAM-1−/− mice also exhibit defects in diapedesis of leukocytes (11) and various angiogenesis-dependent pathologies, thus supporting an important role for PECAM-1 in inflammation and angiogenesis (6, 18, 42). We recently showed (9) abnormal development of retinal vasculature and attenuation of retinal neovascularization during oxygen-induced ischemic retinopathy in PECAM-1−/− mice. To gain insight into the roles that PECAM-1 and its isoforms play in these processes, we have isolated primary retinal EC from PECAM-1+/+ and PECAM-1−/− mice. Here we show that lack of PECAM-1 has significant impact on endothelial cell-cell and cell-matrix interactions impacting EC migration and capillary morphogenesis. Mechanistically these alterations were associated with reduced eNOS expression and NO bioavailability. In addition, lack of PECAM-1 was associated with increased rate of growth, which was mainly attributed to a reduced level of apoptosis in these cells. Furthermore, we show that the defects in migration and capillary morphogenesis of PECAM-1−/− retinal EC can be restored by expression of PECAM-1 in an isoform-specific manner.
MATERIALS AND METHODS
Experimental animals.
All experiments were carried out in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care Committee of the University of Wisconsin School of Medicine and Public Health. Immortomice expressing a temperature-sensitive SV40 large T antigen were obtained from Charles River Laboratories (Wilmington, MA). PECAM-1−/− mice were generated as previously described by Duncan et al. (11) and obtained from Dr. T. W. Mak (Amgen Institute, Toronto, ON, Canada). PECAM-1−/− mice were crossed with Immortomice, and the Immorto/PECAM-1−/− mice (all on C57BL/6 background) were identified by PCR analysis of DNA isolated from tail biopsies. The PCR primer sequences were as follows: Immorto forward: 5′-CCT CTG AGC TAT TCC AGA AGT AGT G-3′, Immorto reverse: 5′-TTA GAG CTT TAA ATC TCT GTA GGT AG-3′; Neo forward: 5′-TGC TCT CCA TCT GCA CGA GAC TAG-3′, Neo reverse: 5′-GAG TT GCT TGT GGT GAA CGC TCA G-3′; and PECAM-1 forward: 5′-TGC TCT CGA AGC CCA GTA TT-3′, PECAM-1 reverse: 5′-CGC TGA ACA CCG CGG GGT GGG AAT GGC-3′.
Preparation of lectin-coated magnetic beads.
Dynabeads M-450 Epoxy (30 mg; Invitrogen, Carlsbad, CA) were washed three times with 1 ml of wash buffer (19 mM NaH2PO4, 80 mM Na2HPO4, pH 7.7). Approximately, 5 mg of lectin from Bandeiraea simplicifolia (B4 lectin; Sigma, St. Louis, MO) was dissolved in 1 ml of wash buffer, mixed with the magnetic beads, and incubated overnight at 4°C on a rocker. B4 lectin-coated magnetic beads were then washed with 1 ml of phosphate-buffered saline (PBS; 3 times), resuspended in 1 ml of Tris-buffered saline (TBS; 20 mM Tris·HCl, 150 mM NaCl, pH 7.6) containing 1% bovine serum albumin (BSA), and stored at 4°C.
Isolation and culture of PECAM-1−/− retinal EC.
Retinal EC were isolated from one litter (6 or 7 pups) of 4-wk-old PECAM-1+/+ and PECAM-1−/− Immortomice by collection of retinas under a dissecting microscope. Retinas were rinsed with serum-free Dulbecco's modified Eagle's medium (DMEM), minced into small pieces with a sterilized razor blade in a 60-mm tissue culture dish, and digested with 2 ml of collagenase type I (1 mg/ml in serum-free DMEM; Worthington, Lakewood, NJ) at 37°C for 45 min. After digestion, cells were rinsed with 5 ml of DMEM containing 10% fetal bovine serum (FBS), centrifuged for 5 min at 400 g, and resuspended in 5 ml of DMEM with 10% FBS. After filtration through a double layer of sterile 40-μm nylon mesh (Sefar America, Fisher Scientific, Hanover Park, IL), cells were centrifuged at 400 g for 10 min and rinsed twice with DMEM containing 10% FBS. Cells were resuspended in 1 ml of DMEM with 10% FBS and incubated with 10 μl of B4 lectin-coated beads for 1 h at 4°C on a rocker. After incubation, cells bound to magnetic beads were collected with a magnetic tube holder and washed six times with 1 ml of DMEM containing 10% FBS, and bound cells were plated in a single well of a 24-well plate coated with fibronectin (2 μg/ml in serum-free DMEM; BD Biosciences, Bedford, MA) in 0.5 ml of EC growth medium and incubated in a tissue culture incubator at 33°C and 5% CO2. EC were grown in DMEM containing 10% FBS, 2 mM l-glutamine, 2 mM sodium pyruvate, 20 mM HEPES, 1% nonessential amino acids, 100 μg of streptomycin, 100 U/ml penicillin, 55 U/ml heparin (Sigma), 100 μg/ml endothelial growth supplement (Sigma), and 44 U/ml recombinant murine interferon-γ (R&D Systems, Minneapolis, MN). Cells were incubated at 33°C with 5% CO2 and progressively passaged to larger plates and maintained on 1% gelatin-coated 60-mm tissue culture dishes.
FACS analysis.
Monolayers of retinal EC on 60-mm culture dishes were washed once with PBS containing 0.04% EDTA and incubated with 3 ml of cell dissociation solution (TBS containing 2 mM EDTA and 0.05% BSA) to collect the cells from the plate. Cells were washed once with DMEM containing 10% FBS and blocked in 0.5 ml of TBS with 1% goat serum for 20 min on ice. Cells were pelleted, resuspended in 0.5 ml of TBS with 1% BSA containing an appropriate dilution of primary antibody (recommended by the supplier), and incubated on ice for 30 min. For vascular EC markers, cells were incubated with anti-PECAM-1, anti-endoglin, anti-ICAM-2 (all from BD Biosciences), anti-VE-cadherin, anti-ICAM-1 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-VCAM-1 (Chemicon, Billerica, MA), anti-VEGF receptor (VEGFR)1 and VEGFR2 (R&D Systems), or FITC-conjugated B4 lectin. For intracellular detection cells were fixed with 0.5 ml of 2% paraformaldehyde and 0.1% Triton X-100 in TBS for 15 min on ice, washed with TBS containing 1% BSA, and incubated with primary antibodies (prepared in 0.5 ml of TBS with 1% BSA, 0.1% Triton X-100) for 30 min on ice. For integrin expression analysis, anti-α1-integrin (BD Biosciences), anti-α2-, α3-, α5-, αv-, β1-, and β8-integrin (Santa Cruz), and anti-β3-, α5β1-, and αvβ3-integrin (Millipore) antibodies were used. After incubation with primary antibody, cells were washed twice with TBS containing 1% BSA and then incubated with appropriate FITC-conjugated secondary antibody (Pierce, Rockford, IL; 1:200 dilution in 0.5 ml of TBS with 1% BSA for 30 min on ice). The stained cells were washed twice with TBS containing 1% BSA, resuspended in 0.5 ml of TBS with 1% BSA, and analyzed with a FACScan caliber flow cytometer (Becton Dickinson).
Cell proliferation.
The cell proliferation assay was performed by plating cells in 60-mm tissue culture dishes. The cell numbers were counted every other day in triplicate for 2 wk. Cells (1 × 104) were plated on gelatin-coated 60-mm tissue culture dishes and counted the next day for day 1. Cells were then fed every other day, and counted on the days that they were not fed, for 2 wk.
The rate of DNA synthesis was measured with the Click-iT EdU Alexa Fluor 488 kit (Invitrogen) as recommended by the supplier. The assay measures incorporation of 5-ethynyl-2′-deoxyuridine (EdU), a nucleoside analog of thymidine, during cell proliferation. PECAM-1+/+ and PECAM-1−/− retinal EC (5 × 105) were plated on 60-mm tissue culture dishes and were incubated with 10 μM EdU in culture medium for 24 h at 33°C. DNA synthesis was analyzed by measuring incorporated EdU with a FACSscan caliber flow cytometer (Becton Dickinson).
Apoptosis and cell viability.
Apoptosis was determined by measuring caspase activation with the Caspase-Glo 3/7 assay system (Promega, Madison, WI) as recommended by the supplier. The assay provides caspase-3/7 DEVD-aminoluciferin substrate, and the caspase 3/7 activity is detected by luminescent signal. For the assay, retinal EC (5 × 103) from PECAM-1+/+ and PECAM-1−/− mice were plated in 96-well plates. As an apoptotic stimulus, retinal EC were incubated with 1.75 mM hydrogen peroxide (H2O2; Fisher Scientific, Fair Lawn, NJ) or 2 μM staurosporine (Invitrogen) in EC growth medium for 8 h at 33°C. Caspase activity was detected by a luminescent microplate reader (Victa2 1420 Multilabel Counter, PerkinElmer, Waltham, MA).
Cellular viability of retinal EC was demonstrated by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (Promega). Plated retinal EC (4 × 103) on 96-well plates were incubated with 1.75 mM H2O2 for 2 days at 33°C and incubated further with MTS solution for 3 h. Viability was determined by measuring absorbance at 490 nm with a microplate reader (Thermomax, Molecular Devices, Sunnyvale, CA) and determined as percentage of control untreated cells. All samples were prepared in triplicate and repeated twice.
Indirect immunofluorescence.
Cells (1 × 105) were plated on fibronectin-coated glass chamber slides until confluent (1–2 days), washed in PBS, fixed and permeabilized with methanol for 15 min on ice, and blocked with 1% ovalbumin in TBS at 37°C for 20 min. Slides were washed with TBS and incubated with anti-VE-cadherin, N-cadherin, β-catenin (BD Biosciences), ZO-1 (Zymed, Carlsbad, CA), vinculin, and FITC-conjugated phalloidin (Sigma) in TBS containing 1% ovalbumin at 37°C for 40 min. After washing with TBS, cells were incubated with appropriate Cy3-conjugated secondary antibody (1:800 dilution in TBS containing 1% ovalbumin) at 37°C for 40 min. Cells were washed with TBS five times and analyzed with a fluorescent microscope (Carl Zeiss Optical, Germany), and images were captured in digital format.
Scratch wound assays.
Cells (6 × 105) were plated in 60-mm tissue culture dishes and allowed to reach confluence (2–3 days). Cell monolayers were wounded with a 1-ml micropipette tip, rinsed with DMEM containing 10% FBS twice, and fed with EC growth medium containing 1 μM 5-fluorouracil (Sigma) to exclude potential contribution of cell proliferation to wound closure. The wound closure was monitored and photographed at 0, 24, and 48 h with a phase microscope in digital format. For quantitative assessment, the distances migrated as percentage of total distance were determined as described previously (8).
Transwell migration assays.
The Transwell migration assay was conducted as previously described (45). Briefly, the bottom of Costar Transwells with 8-μm pore size (Fisher) were coated with fibronectin (2 μg/ml in PBS) at 4°C overnight. After being rinsed with PBS, the bottom side of the Transwell was blocked with 2% BSA in PBS for 1 h at room temperature. Retinal EC were trypsinized and resuspended in serum-free DMEM, and 1 × 105 cells in 0.1 ml were added to the top of the Transwell membrane. Cells were incubated for 3 h at 33°C, fixed with 2% paraformaldehyde (PFA) for 10 min at room temperature, and stained with hematoxylin and eosin. The stained membranes were mounted on a glass slide, and the number of migrated cells through the membrane, which attached to the bottom, were determined by counting 10 high-power fields (×200).
Cell adhesion to various extracellular matrix proteins.
Cell adhesion assays were performed in 96-well flat-bottom plates (Nunc Immunoplate Maxisorp, Fisher Scientific) coated with various concentrations of matrix proteins or BSA as control. Fibronectin, vitronectin, collagen I, and collagen IV (BD Biosciences) were serially diluted in TBS containing 2 mM CaCl2 and 2 mM MgCl2 (TBS with Ca/Mg) and coated (50 μl) the wells of the 96-well plates overnight at 4°C. Plates were washed four times with 200 μl of TBS with Ca/Mg and then blocked with 200 μl of TBS with Ca/Mg containing 1% BSA at room temperature for 1 h. Cells were removed from the tissue culture plates with 3 ml of cell dissociation buffer (2 mM EDTA, 0.05% BSA in TBS), washed with TBS, and resuspended in cell binding buffer (20 mM HEPES, 150 mM NaCl, 4 mg/ml BSA, pH 7.4) at ∼6 × 105 cells/ml. The plate were washed once with TBS with Ca/Mg and incubated with 50 μl of TBS with Ca/Mg and 50 μl of cell suspension for 2 h at 37°C in a humidified incubator. After incubation, the plates were washed with 200 μl of TBS with Ca/Mg to remove nonadherent cells until no cells were left in the BSA-coated wells. For quantification of the number of adherent cells, the levels of intracellular acid phosphatase were measured by lysing the adherent cells in 100 μl of lysis buffer (50 mM sodium acetate pH 5.0, 1% Triton X-100, 6 mg/ml p-nitrophenylphosphate) and incubating at 4°C overnight. The reaction was neutralized by addition of 50 μl of 1 M NaOH, and the absorbance was determined at 405 nm with a microplate reader (Thermomax, Molecular Devices). All samples were done in triplicate and repeated twice.
Western blot analysis.
Cells (6 × 105) were plated on 60-mm culture dishes and allowed to reach ∼90% confluence. The cells were then rinsed once with serum-free DMEM and incubated in EC growth medium without serum for 2 days. Conditioned medium (CM) was collected and centrifuged to remove cell debris. The cells were also lysed in 0.1 ml of lysis buffer [in mM: 50 HEPES pH 7.5, 100 NaCl, 0.1 EDTA, 1 CaCl2, and 1 MgCl2, with 1% Triton X-100, 1% NP-40, and protease inhibitor cocktail (Roche Biochemicals, Mannheim, Germany)]. To detect phospho-eNOS, cells were serum starved for 2 days and stimulated with serum-containing medium for 30 min. After incubation, cells were rinsed with cold PBS containing 1 mM Na3OV4 (twice) and lysed in 0.1 ml of lysis buffer containing 3 mM Na3OV4 and 5 mM NaF. Protein concentrations were determined with BCA protein assay (Bio-Rad, Hercules, CA); samples were adjusted for protein content, mixed with an appropriate volume of 6× SDS-sample buffer, and analyzed by SDS-PAGE (4–20% Tris-glycine gels, Invitrogen). Proteins were transferred to nitrocellulose membrane, and the membrane was blocked with blocking buffer (0.05% Tween 20 and 5% skim milk in TBS). Anti-tenacin-C (Chemicon), fibronectin (Santa Cruz Biotechnology), osteopontin (R&D Systems), thrombospondin (TSP)1 (A6.1, Neo Markers, Fremont, CA), TSP2 (R&D Systems), eNOS (Santa Cruz Biotechnology), phospho-eNOS (Cell Signaling), p120-catenin, paxillin (BD Transduction), focal adhesion kinase (FAK; Santa Cruz Biotechnology), and β-actin (Sigma) antibodies were diluted to 1:1,000 in blocking buffer and incubated with the membrane for 2 h at room temperature. Other antibodies used for Western blot analysis were the same as those used for FACS. Blots were washed with TBS with 0.05% Tween 20 (TBST) and incubated with appropriate secondary horseradish peroxidase-conjugated antibody. The blots were then washed with TBST and developed with enhanced chemiluminescence (Fisher Scientific). The blot was stripped and incubated with anti-β-actin (Sigma) antibody for loading control.
Capillary morphogenesis assays.
Tissue culture plates (35 mm) were coated with 0.5 ml of Matrigel (10 mg/ml, BD Biosciences) and allowed to harden by incubating at 37°C for at least 30 min. Cells were removed by trypsin-EDTA, washed with DMEM containing 10% FBS, and resuspended at 1 × 105 cells/ml in EC growth medium without FBS. Cells (2 × 105) in 2 ml were applied to the Matrigel-coated plates, incubated at 33°C, photographed after 18 h with a Nikon microscope in digital format. For quantitative assessment of the data, the mean numbers of branch points were determined by counting the number of branch points in five high-power fields (×100). Longer incubation times did not further improve the degree of capillary morphogenesis.
Aortic ring sprouting assays.
Aortas were dissected from CO2-euthanized 3-wk-old PECAM-1+/+ or PECAM-1−/− mice. Freshly isolated aortas were transferred to cold serum-free DMEM and washed three times. Adipose tissues were carefully removed with microdissecting forceps and iridectomy scissors, and aortic rings (1–1.5 mm long, 5 per aorta) were sectioned. The aortic rings were embedded on 0.3 ml of Matrigel gel-coated wells of a 12-well tissue culture plate (Fisher) and 0.5 ml of DMEM containing 1% serum, 100 of μg streptomycin, and 100 U/ml penicillin was applied for 2 days at 37°C. The medium was then replaced with EC growth medium, and the angiogenic response of aortic cultures was photographed after 4 days. For quantitative assessment of sprouting, the areas of sprouting per millimeter of tissue edge were assessed with Image J software (National Institutes of Health; http://rsb.info.nih.gov/ij).
Construction of adenoviruses expressing various PECAM-1 isoforms.
The adenoviruses expressing a specific isoform of PECAM-1 were prepared as previously described (26). Briefly, the PECAM-1 isoform cDNAs were obtained as HindIII/NotI fragments, blunted, and ligated with the intermediate plasmid (pShuttle; Stratagene, La Jolla, CA) digested with EcoRV. The ligation was transformed into Escherichia coli DH5α-competent cells (Invitrogen), and positive clones were confirmed by restriction digestion and DNA sequencing. The quality and efficiency of the constructs was confirmed by expressing in 293 cells and Western blotting. For construction of a recombinant adenovirus, pShuttle/PECAM-1 isoform was linearized by PacI and introduced into E. coli BJ5183-AD-1 (Stratagene), which contains the viral plasmid, and the recombinant clones were screened by PmeI digestion. The isolated recombinant viral plasmid DNA was then introduced into 293 cells for viral packaging and amplification. The amplified virus was then titered and used for expression studies.
To reexpress PECAM-1 in PECAM-1−/− retinal EC, cells (4 × 105) were plated on 60-mm tissue culture dishes. The next day, adenoviruses encoding a specific isoform of PECAM-1 or empty vector (50 pfu/cell) and Lipofectin (15 μl; Invitrogen) were diluted with 0.75 ml of opti-MEM (Invitrogen) separately and incubated for 30 min at room temperature. After incubation, the diluted adenoviruses and Lipofectin were gently mixed and allowed to incubate at room temperature for 10 min. After incubation, the tissue culture plates were removed from the incubator, washed in serum-free DMEM (twice), and incubated with 1.5 ml of the adenovirus-Lipofectin mixture overnight at 33°C. The next day, the adenovirus-Lipofectin-containing medium was removed, and cells were gently washed with DMEM containing 10% FBS (twice) and incubated with EC growth medium for 2 days before they were used for experiments.
Intracellular NO measurements.
The intracellular NO level of PECAM-1+/+ and PECAM-1−/− retinal EC was determined with 4-amino-5-methylamino-2,7-difluorofluorescein diacetate (DAF-FM diacetate; Invitrogen). DAF-FM diacetate is a cell-permeant molecule that passively defuses into the cell and becomes deacetylated by intracellular esterases, forming DAF-FM. Fluorescence of DAF-FM increases significantly after it reacts with NO and can be detected with a fluorescein filter. Cells (5 × 103 cells/0.1 ml) were plated on gelatin-coated 96-well black/clear-bottom plates (BD Falcon) and incubated overnight. The next day, medium was removed and fresh EC growth medium containing 30 μM DAF-FM diacetate (0.1 ml/well) was added and incubated for 40 min. After incubation, the medium was removed and replaced with fresh EC growth medium without DAF-FM (0.1 ml/well). The samples were incubated for 30 min and washed with TBS (0.1 ml/well, twice), and DAF-FM fluorescence of cells in TBS was detected at an excitation of 485 nm and an emission of 535 nm with a fluorescent microplate reader (Victa2 1420 Multilabel Counter, PerkinElmer). These assays were performed three times in triplicate, and results were normalized for cell number.
Secreted VEGF measurements.
The amount of secreted VEGF produced by PECAM-1+/+ and PECAM-1−/− retinal EC was determined with the Mouse VEGF Immunoassay Kit (R&D Systems). Cells (6 × 105) were plated on 60-mm tissue culture dishes and allowed to reach ∼90% confluence. The cells were then rinsed once with serum-free DMEM and incubated with 2 ml of EC growth medium without serum for 2 days. The CM was centrifuged to remove cell debris, and the secreted VEGF in CM was analyzed according to manufacturer's instructions. The amount of VEGF was determined with a standard curve generated with known amounts of VEGF in the same experiment.
Statistical analysis.
Statistical differences between control and treated samples were evaluated with Student's unpaired t-test (2-tailed) or two-way ANOVA with Bonferroni correction for multiple comparisons when appropriate. Means ± SD are shown. P values ≤ 0.05 were considered significant.
RESULTS
Isolation and characterization of PECAM-1−/− retinal EC.
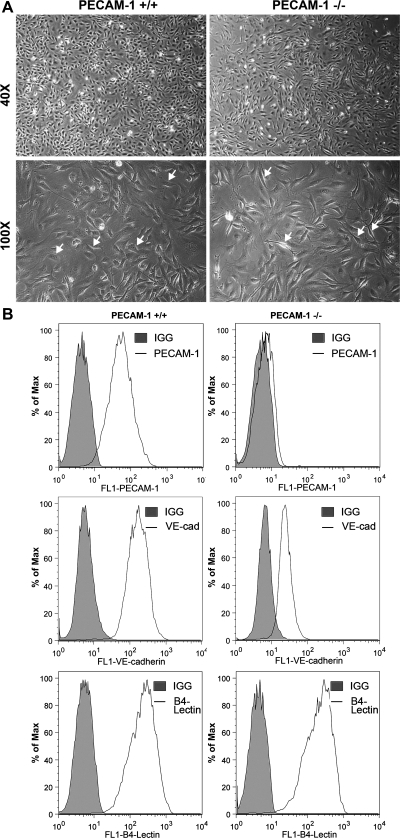
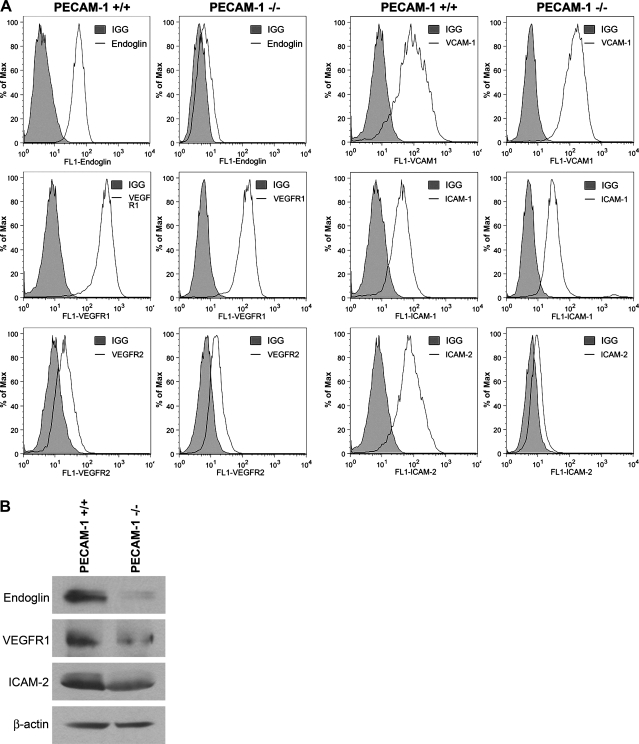
Retinal EC were prepared from PECAM-1+/+ and PECAM-1−/− Immortomice with B4 lectin-coated magnetic beads. We were the first to report successful culture of primary EC from mouse retina (43). Figure 1A shows that PECAM-1−/− retinal EC are more elongated, spindly, and less spread out compared with PECAM-1+/+ retinal EC when plated on gelatin-coated tissue culture dishes. PECAM-1−/− retinal EC also failed to form a closely apposed monolayer when cells reached confluence, suggesting defects in formation of adherens junctions. We next determined the expression of EC markers in these cells by FACS analysis (Fig. 1B). PECAM-1+/+ EC exhibited significant expression of PECAM-1 and VE-cadherin, while PECAM-1−/− EC lacked PECAM-1 expression as expected and had a significantly lower level of VE-cadherin expression. Lack of PECAM-1 did not affect the expression level of B4 lectin in retinal EC. In addition, the expression of VCAM-1, VEGFR2, and ICAM-1 were comparable in PECAM-1+/+ and PECAM-1−/− EC. However, a significant decrease in expression of endoglin, VEGFR1, and ICAM-2 was observed in PECAM-1−/− EC compared with PECAM-1+/+ EC (Fig. 2A). These results were confirmed by Western blot (Fig. 2B).
Fig. 1.
Isolation and characterization of mouse retinal endothelial cells (EC). Platelet endothelial cell adhesion molecule (PECAM)-1+/+ and PECAM-1−/− retinal EC were prepared as described in materials and methods and cultured on gelatin-coated plates in 60-mm dishes. A: cells were photographed in digital format at ×40 and ×100 magnification. Note the elongated, spindly morphology of PECAM-1−/− cells compared with PECAM-1+/+ cells (arrows). B: expression of vascular EC markers in EC. Retinal EC were examined for expression of PECAM-1, VE-cadherin (VE-cad), and B4 lectin by FACS analysis. Shaded areas show control IgG staining. These experiments were repeated with 3 isolations of EC, with similar results.
Fig. 2.
Expression of other EC markers in PECAM-1+/+ and PECAM-1−/− retinal EC. A: expression of endoglin, VEGF receptor (VEGFR)1, VEGFR2, VCAM-1, ICAM-1, and ICAM-2 was determined by FACS analysis of PECAM-1+/+ and PECAM-1−/− retinal EC using specific antibodies. Shaded areas show control IgG staining. Note decreased expression of endoglin, VEGFR1, and ICAM-2 in PECAM-1−/− EC. B: changes in the levels of these proteins were further confirmed by Western blot analysis. These experiments were repeated with 3 isolations of EC, with similar results.
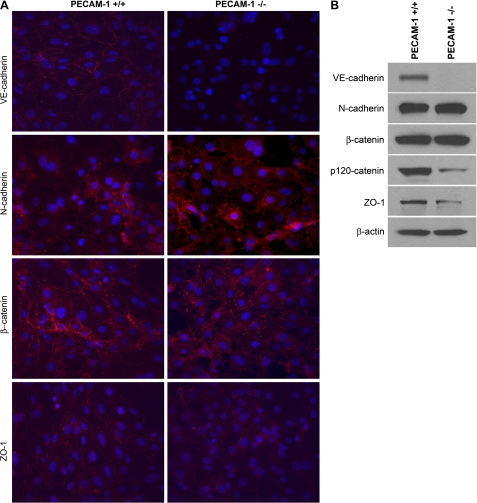
VE-cadherin is a major component of the vascular EC adherens junction complexes with important roles in vascular development and angiogenesis (4). We next examined expression and localization of VE-cadherin by indirect immunofluorescence staining of PECAM-1+/+ and PECAM-1−/− retinal EC. We observed very low expression of VE-cadherin in PECAM-1−/− EC, which lacked junctional localization compared with PECAM-1+/+ EC (Fig. 3A). We observed significant expression of VE-cadherin in PECAM-1+/+ EC with junctional localization as previously shown in other types of EC. These results were consistent with the FACS data (Fig. 1B).
Fig. 3.
Cellular localization and expression level of VE-cadherin, N-cadherin, β-catenin, and ZO-1. A: PECAM-1+/+ and PECAM-1−/− retinal EC were grown on fibronectin-coated chamber slides to confluence and stained as described in materials and methods with specific antibodies. No staining was observed when primary antibody was left out. Note reduced expression of VE-cadherin and ZO-1 and their absence from the sites of cell-cell contact. Although the levels of N-cadherin were comparable, the junctional localization of N-cadherin was more prominent in PECAM-1−/− retinal EC compared with PECAM-1+/+ EC. B: Western blot analysis of cell lysates for junctional proteins. Cell lysates prepared from PECAM-1+/+ and PECAM-1−/− retinal EC were analyzed for expression of different junction proteins including VE-cadherin, N-cadherin, β-catenin, p120-catenin, ZO-1, and β-actin by Western blotting. Note significant decrease in protein levels of VE-cadherin and ZO-1 in PECAM-1−/− retinal EC. The levels of β-catenin and actin were similar. These experiments were repeated twice with 2 isolations of these cells, with similar results.
N-cadherin is a member of the cadherin family of proteins with important roles in angiogenesis and vascular stabilization (30, 32). VE-cadherin competes with N-cadherin for formation of adherens junctions in EC and generally localizes to the site of cell-cell contact. We next determined expression and localization of N-cadherin in PECAM-1+/+ and PECAM-1−/− retinal EC. Although a similar level of N-cadherin was detected in these cells, its junctional localization was more prominent in PECAM-1−/− EC (Fig. 3A). The localization of β-catenin, another component of adherens junctions, was also affected in PECAM-1−/− EC. β-Catenin showed a punctate staining pattern in PECAM-1−/− EC compared with PECAM-1+/+ EC, which showed a more uniform junctional localization (Fig. 3A). Another protein with important function in formation of tight junctions is ZO-1, whose junctional localization is VE-cadherin dependent. We observed a decrease in ZO-1 staining and lack of ZO-1 junctional localization in PECAM-1−/− EC (Fig. 3A). The expression levels of the junctional proteins were further confirmed by Western blot analysis of cell lysates (Fig. 3B). A significant decrease in the levels of VE-cadherin, p120-catenin (a cytoplasmic binding partner that regulates cadherin stability) (41), and ZO-1 was observed in PECAM-1−/− retinal EC compared with PECAM-1+/+ cells. We also observed reduced levels of VE-cadherin in retinal extracts prepared from PECAM-1−/− mice compared with PECAM-1+/+ mice, and these results were further confirmed by quantitative real-time PCR (not shown). However, the decrease in VE-cadherin was more dramatic in PECAM-1−/− EC, perhaps as a result of culture in vitro. Although localization of β-catenin and N-cadherin was altered in the absence of PECAM-1, their protein levels were very similar. Thus lack of PECAM-1 had significant impact on expression and/or localization of EC junctional proteins impacting cell-cell interactions.
PECAM-1−/− retinal EC grew at faster rate and exhibited reduced levels of apoptosis.
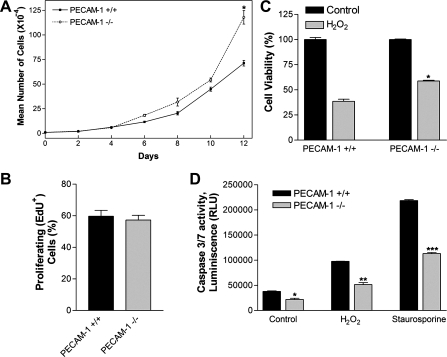
The effect of PECAM-1 deficiency on the growth rate of retinal EC was determined by counting the number of cells for 2 wk. Figure 4A shows a significant increase in the growth rate of PECAM-1−/− EC compared with PECAM-1+/+ cells. At 12 days of culture, the cell number for PECAM-1+/+ EC was ∼65% of PECAM-1−/− EC (P < 0.05; n = 3). These results are consistent with the increased growth rate we previously reported (33) in polyomavirus middle-T-transformed PECAM-1−/− brain EC compared with PECAM-1+/+ brain EC. To determine whether the increased growth rate was due to an increased rate of DNA synthesis, we determined the percentage of cells undergoing active DNA synthesis by labeling with EdU, a synthetic nucleoside analog. PECAM-1−/− retinal EC showed a level of DNA synthesis comparable with PECAM-1+/+ retinal EC (Fig. 4B; P > 0.05; n = 4). Thus the increased growth rate may be attributed to a decreased level of apoptosis in PECAM-1−/− retinal EC.
Fig. 4.
Altered proliferation and apoptosis of PECAM-1−/− retinal EC. A and B: the rate of retinal EC proliferation was determined by counting the number of cells in triplicate after different days in culture as described in materials and methods (A) and by analyzing the rate of DNA synthesis by FACScan flow cytometer analysis (B; P > 0.05; n = 4). EdU, 5-ethynyl-2′-deoxyuridine. C: hydrogen peroxide (H2O2) toxicity of retinal EC was measured with the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay method. Retinal EC were incubated with 1.75 mM H2O2 in EC growth medium for 2 days, and the level of toxicity was determined by measuring colorimetric change of MTS in cells on 96-well plates. PECAM-1−/− retinal EC were significantly less sensitive to cytotoxic effect of H2O2 (*P < 0.05; n = 5). D: rate of apoptosis was determined by measuring caspase activity with luminescent signal from caspase-3/7 DEVD-aminoluciferin substrate, as recommended by the supplier. As an apoptotic stimulus, H2O2 and staurosporine in EC growth media were added for 8 h. Note the significant decrease in the rate of apoptosis in PECAM-1−/− retinal EC compared with PECAM-1+/+ retinal EC (*,**,***P < 0.05; n = 5). RLU, relative light units.
The cytotoxicity of H2O2 toward retinal EC was evaluated with the MTS cytotoxicity assay. PECAM-1+/+ and PECAM-1−/− retinal EC plated on gelatin-coated 96-well plate were incubated with different concentrations of H2O2 (0–2 mM) for 2 days. Cell viability was decreased in a concentration-dependent manner in both PECAM-1+/+ and PECAM-1−/− retinal EC (not shown). Incubation with 1.75 mM H2O2 decreased viability of PECAM-1+/+ EC by 60%, while that of PECAM-1−/− EC was decreased by 40% (Fig. 4C; P < 0.05; n = 5). Thus PECAM-1−/− retinal EC were less sensitive to H2O2-mediated cytotoxicity compared with PECAM-1+/+ cells.
We next determined the levels of apoptosis in PECAM-1+/+ and PECAM-1−/− retinal EC under steady-state culture conditions. Apoptotic cell death was determined by evaluation of the activation status of caspase 3/7. PECAM-1−/− EC consistently showed a 40% decrease in the rate of apoptosis compared with PECAM-1+/+ cells (Fig. 4D; P < 0.05; n = 5). H2O2, a highly reactive oxygen species, is a potent inducer of apoptosis in EC. We thus determined the level of H2O2-induced apoptosis in PECAM-1+/+ and PECAM-1−/− cells. Retinal EC were incubated with 1.75 mM H2O2 in culture medium for 8 h, and the level of apoptosis was determined as described above. H2O2-induced apoptosis in PECAM-1−/− EC was reduced by ∼50% compared with PECAM-1+/+ EC (Fig. 4D). Similar results were observed with staurosporine (2 μM; Fig. 4D; P < 0.05; n = 5).
PECAM-1−/− retinal EC were less migratory.
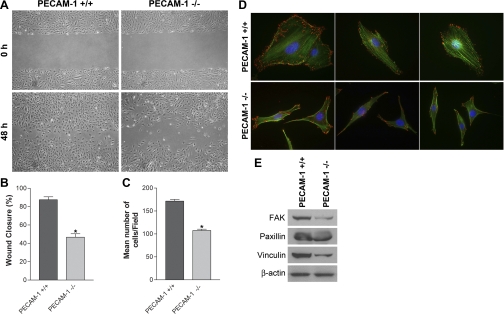
Cell migration is fundamental to the ability of EC to undergo capillary morphogenesis during angiogenesis. A scratch wound assay was performed to investigate migratory properties of retinal EC. Confluent monolayers of PECAM-1+/+ or PECAM-1−/− EC were wounded, and wound closure by cell migration was monitored with still photography. To eliminate the impact of cell proliferation on migration and wound closure these experiments were performed in the presence of a low concentration of 5-fluorouracil (1 μM). We observed that a significant area of wound was closed in PECAM-1+/+ EC by 48 h. In contrast, wound closure was significantly delayed in PECAM-1−/− EC (Fig. 5A). The quantitative assessment of the data is shown in Fig. 5B (P < 0.05; n = 3). Similar results were observed with a Transwell migration assay (Fig. 5C; P < 0.05; n = 3). The defective migratory phenotype of PECAM-1−/− retinal EC was also confirmed by observation of fewer actin stress fibers and reduced focal adhesion complex formation following staining with phalloidin (actin filaments) and vinculin (focal adhesions) (Fig. 5D). In addition, the majority of focal adhesions were localized to the edges of PECAM-1+/+ cells, while in PECAM-1−/− cells they were mainly localized to the basal surfaces. These results were further supported by decreased levels of FAK, paxillin, and vinculin in lysates prepared from PECAM-1−/− EC compared with PECAM-1+/+ EC (Fig. 5E).
Fig. 5.
PECAM-1−/− retinal EC are less migratory. A: cell migration was determined by scratch wound assay of the retinal EC monolayer on gelatin-coated plates. Wound closure was monitored by photography within 48 h. B: quantitative assessment of the data (*P < 0.05, n = 3). C: rate of cell migration was also determined with a Transwell migration assay (*P < 0.05, n = 3). D: the migratory phenotype of retinal EC was further confirmed by staining the cells with phalloidin (green, actin) and vinculin (red, focal adhesions). Note increased actin stress fibers and focal adhesions in PECAM-1+/+ cells. Fewer focal adhesions were detected in PECAM-1−/− cells, which mainly localized to the basal surface of the cells. E: decreased expression of focal adhesion proteins including focal adhesion kinase (FAK), paxillin, and vinculin was detected in PECAM-1−/− EC. β-Actin was used as loading control. These experiments were repeated with 3 isolations of cells, with similar results. Note the flatter morphology and peripheral actin localization in PECAM-1−/− EC.
PECAM-1−/− retinal EC were more adherent.
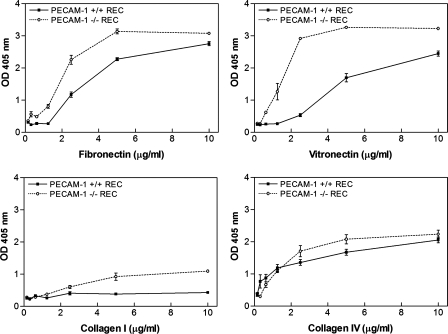
The defect in migration of PECAM-1−/− retinal EC suggested alterations in adhesion properties. We next examined adhesion of PECAM-1+/+ and PECAM-1−/− EC to various ECM proteins. Figure 6 shows that PECAM-1−/− EC adhered more strongly to fibronectin, vitronectin, collagen I, and collagen IV compared with PECAM-1+/+ EC. Neither PECAM-1−/− nor PECAM-1+/+ cells adhered well to laminin (not shown). Thus PECAM-1 deficiency had a significant impact on adhesion of retinal EC to various extracellular matrix (ECM) proteins, and it is consistent with their reduced migration.
Fig. 6.
Enhanced adhesion of PECAM-1−/− retinal EC (REC) to various extracellular matrix (ECM) proteins. Adhesion of retinal EC to fibronectin, vitronectin, collagen I, and collagen IV was determined as described in materials and methods. Note strong adhesion of PECAM-1−/− retinal EC to fibronectin and vitronectin. These experiments were performed at least twice with 2 different isolations of retinal EC. OD, optical density.
Alterations in production of ECM proteins and their receptors in PECAM-1−/− retinal EC.
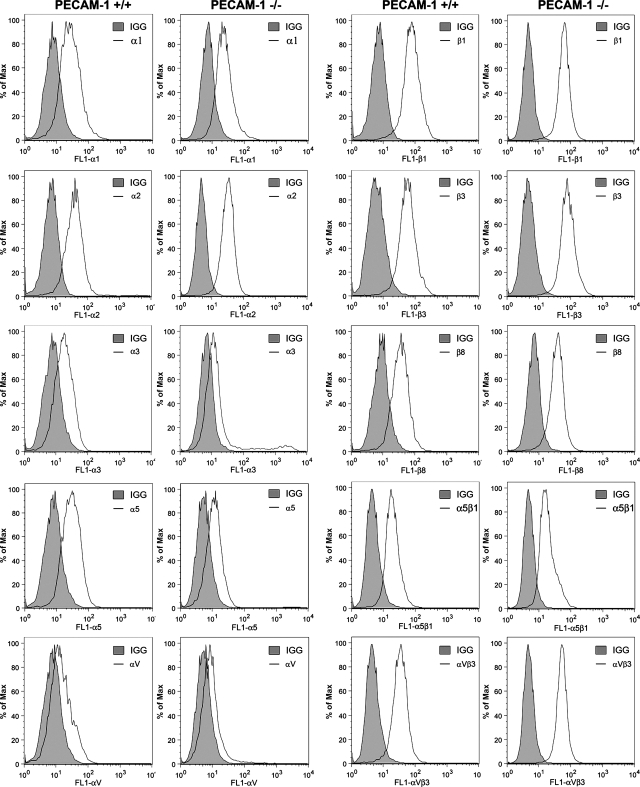
The process of EC adhesion and migration involves binding of ECM proteins through specific cell surface integrins, a critical component of the angiogenic response (5, 22, 23). To determine whether the defects observed in adhesion and migration of PECAM-1−/− retinal EC are due in part to altered integrin expression, we analyzed the expression of various integrins on the surface of retinal EC by FACS analysis. The expression levels of α1-, α2-, α3-, α5-,αv-, β1-, β3-, β8-, and α5β1-integrin showed no significant differences between PECAM-1+/+ and PECAM-1−/− EC (Fig. 7). However, PECAM-1−/− EC showed a modest increase in the level of αvβ3-integrin, consistent with their enhanced adhesion to vitronectin and fibronectin.
Fig. 7.
Expression of integrins in retinal EC. α1-, α2, α3-, α5-, αv-, β1-, β3-, β8-, α5β1-, and αvβ3-integrin expression on retinal EC was determined by FACS analysis as described in materials and methods. These experiments were repeated with 2 isolations of cells, with similar results. Note similar expression of integrins in both cell types.
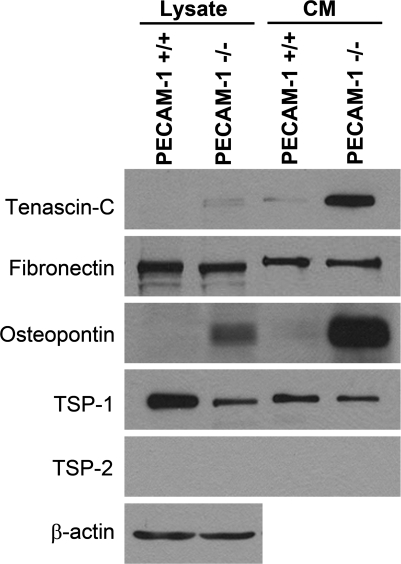
Fibronectin, tenascin-C, and osteopontin are major components of the ECM and play important roles in cell migration, wound repair, and inflammation (29, 35, 47). We next examined the levels of these proteins in PECAM-1+/+ and PECAM-1−/− retinal EC by Western blotting of the CM and cell lysates. PECAM-1+/+ and PECAM-1−/− EC produced comparable levels of fibronectin. A significant amount of tenascin-C was only detected in the CM prepared from PECAM-1−/− retinal EC. Osteopontin was detected in both cell lysate and CM prepared from PECAM-1−/− EC at significantly higher levels compared with PECAM-1+/+ EC (Fig. 8). TSP1 is an endogenous inhibitor of angiogenesis with a significant impact on EC proangiogenic properties (54). PECAM-1−/− EC produced a decreased level of TSP1 in both cell lysate and CM compared with PECAM-1+/+ EC (Fig. 8). This is consistent with enhanced proliferation and reduced apoptosis observed in PECAM-1−/− EC (54). PECAM-1+/+ and PECAM-1−/− EC did not produce detectable levels of TSP2, a family member closely related to TSP1 with antiangiogenic activity.
Fig. 8.
Altered expression of ECM proteins in PECAM-1−/− retinal EC. Retinal EC from PECAM-1+/+ and PECAM-1−/− mice were incubated for 2 days in serum-free medium. The collected conditioned medium (CM) and cell lysates were analyzed by Western blotting for tenascin-C, fibronectin, osteopontin, thrombospondin (TSP)1, and TSP2 with specific antibodies. These experiments were repeated with 2 different isolations, with similar results. Note significant upregulation of tenascin-C and osteopontin in PECAM-1−/− retinal EC.
Attenuation of capillary morphogenesis in PECAM-1−/− retinal EC.
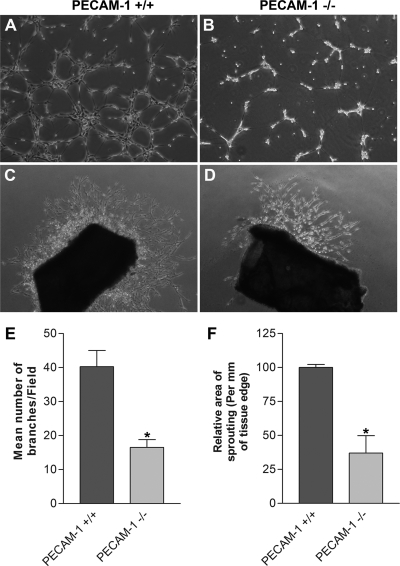
Angiogenesis is led by migration and capillary morphogenesis of EC. The ability to form capillary-like structures is an important feature of EC distinguished from other cell types. Most EC form and organize into a capillary-like network in Matrigel. We investigated whether PECAM-1 expression affects capillary morphogenesis of retinal EC. PECAM-1+/+ EC formed well-organized capillary structures in Matrigel (Fig. 9A). However, the ability of PECAM-1−/− EC to form a capillary-like network was attenuated (Fig. 9B), as previously shown in EC without PECAM-1 expression (37). A quantitative assessment of the data is shown in Fig. 9E (P < 0.05; n = 5).
Fig. 9.
Attenuation of capillary morphogenesis of retinal EC and sprouting of aortas from PECAM-1−/− mice. A and B: PECAM-1+/+ (A) and PECAM-1−/− (B) retinal EC were plated on Matrigel, and capillary morphogenesis was monitored for 3 days. The photographs were taken in digital format after 18 h when optimal capillary morphogenesis was observed. C and D: aortas prepared from PECAM-1+/+ (C) and PECAM-1−/− (D) mice were cultured on Matrigel, and sprouting was monitored for 6 days and photographed in digital format. Quantitative assessments of the data are shown in E and F, respectively. Data are the mean number of branch points from 5 high-power fields (×100). Note a significant decrease in capillary morphogenesis of PECAM-1−/− EC compared with PECAM-1+/+ cells (*P < 0.05; n = 5). Also note a significant decrease in sprouting of aortas from PECAM-1−/− mice compared with PECAM-1+/+ mice (*P < 0.05; n = 6). These experiments were repeated with 3 different isolations of retinal EC and aortas from 5 different mice, with similar results.
Attenuation of ex vivo sprouting of aortas from PECAM-1−/− mice.
We next investigated the effect of PECAM-1 expression on vascular sprouting with the aortic ring ex vivo angiogenesis assay. Aortic rings were isolated from 3-wk-old PECAM-1+/+ and PECAM-1−/− mice and cultured on Matrigel. We observed a significant decrease in sprouting/outgrowths of aortic rings from PECAM-1−/− mice (Fig. 9, C and D). A quantitative assessment of the data is shown in Fig. 9F (P < 0.05; n = 6). Thus vascular cell sprouting ability is significantly reduced in the absence of PECAM-1.
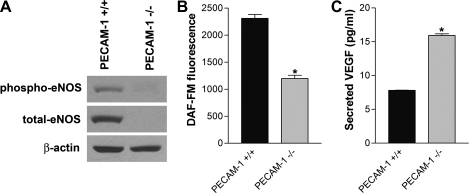
Decreased eNOS expression and NO bioavailability in absence of PECAM-1.
Angiogenic factors, such as VEGF, modulate vascular development and angiogenesis by activation of eNOS (15, 36). We determined the expression and phosphorylation level of eNOS in PECAM-1+/+ and PECAM-1−/− retinal EC by Western blot analysis. PECAM-1+/+ EC expressed significant amounts of total and phosphorylated eNOS, whereas PECAM-1−/− EC showed a significant decrease in eNOS expression and phosphorylation (Fig. 10A). To demonstrate the intracellular NO level produced by eNOS activation in retinal EC, we determined NO levels with the use of DAF-FM diacetate, which reflects the intracellular NO level with fluorescence. Figure 10B shows a significant decrease in the level of intracellular NO in PECAM-1−/− EC (P < 0.05; n = 3). The basal amount of NO in PECAM-1−/− EC was 50% of the NO produced in PECAM-1+/+ EC. We also examined the level of VEGF secreted in the medium collected from PECAM-1+/+ and PECAM-1−/− retinal EC. We observed a significantly higher VEGF level in PECAM-1−/− EC (Fig. 10C; P < 0.05; n = 3).
Fig. 10.
Altered expression and phosphorylation of endothelial nitric oxide synthase (eNOS) and VEGF expression in PECAM-1−/− retinal EC. A: phospho-eNOS and total eNOS in cell lysates were analyzed by Western blotting. β-Actin was used for loading control. B: intracellular nitric oxide (NO) level in retinal EC was measured with 4-amino-5-methylamino-2,7-difluorofluorescein (DAF-FM) as described in materials and methods. Note a significant decrease in intracellular NO level in PECAM-1−/− retinal EC compared with PECAM-1+/+ retinal EC (*P < 0.05; n = 3). C: secreted level of VEGF in retinal EC was determined with an immunoassay as described in materials and methods. Note a significant increase in the level of VEGF secreted by PECAM-1−/− retinal EC compared with PECAM-1+/+ EC (*P < 0.05; n = 3). These experiments were repeated with 3 isolations of cells, with similar results.
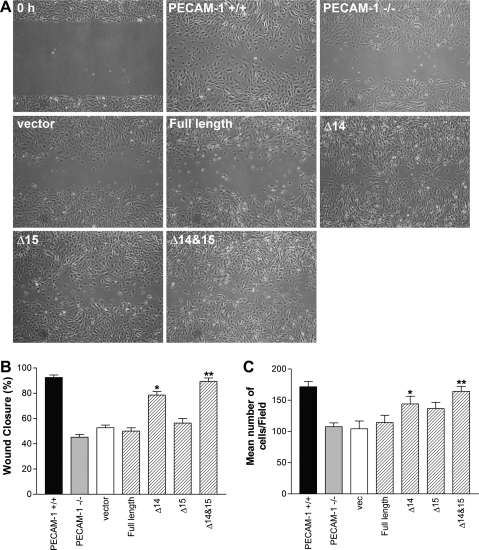
Expression of PECAM-1 restores migration and capillary morphogenesis of PECAM-1−/− retinal EC in isoform-specific manner.
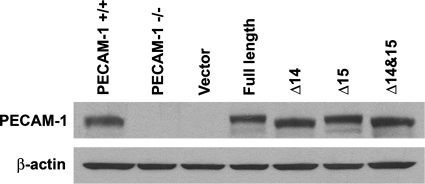
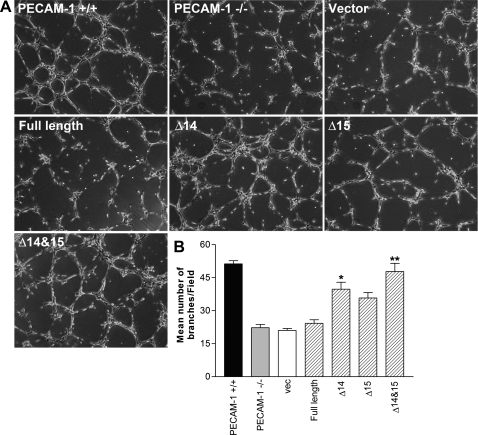
PECAM-1−/− retinal EC showed reduced migration in scratch wound and Transwell migration assays (Fig. 5, A–C) and failed to undergo capillary morphogenesis in Matrigel (Fig. 9, A, B, and E). To determine whether PECAM-1 expression is sufficient to restore migration and capillary morphogenesis, we expressed a specific PECAM-1 isoform, including full-length, Δ14, Δ15, or Δ14&15 PECAM-1, in PECAM-1−/− retinal EC. Figure. 11 shows successful expression of these isoforms in PECAM-1−/− EC exhibiting very similar levels of PECAM-1. We next determined the ability of a specific PECAM-1 isoform to restore migration of PECAM-1−/− retina EC. Only cells expressing the Δ14&15 isoform restored cell migration of null cells to the level seen in PECAM-1+/+ cells (Fig. 12A). A significant enhancement of migration was also observed in null cells expressing the Δ14 isoform. A quantitative assessment of the data is shown in Fig. 12B (P < 0.05; n = 3). A similar result was observed in the Transwell migration assay (Fig. 12C; P < 0.05; n = 3). We next determined the impact of various PECAM-1 isoforms on capillary morphogenesis of PECAM-1−/− cells in Matrigel. Expression of the Δ14&15 isoform also restored capillary morphogenesis of null cells to levels comparable to PECAM-1+/+ cells (Fig. 13A). The quantitative presentation of data is shown in Fig. 13B (P < 0.05; n = 3). These results are consistent with detection of Δ14&15 PECAM-1 as the predominant isoform expressed in murine endothelial and hematopoietic cells (48, 49).
Fig. 11.
Expression of various PECAM-1 isoforms in PECAM-1−/− retinal EC. Adenoviruses encoding empty vector or a specific PECAM-1 isoform [full length or isoform lacking exon 14 (Δ14), 15 (Δ15), or 14 and 15 (Δ14&15)] were used to infect PECAM-1−/− retinal EC as described in materials and methods. The level of PECAM-1 was determined by Western blotting of cell lysates. Note similar expression level of PECAM-1 in PECAM-1−/− retinal EC compared with PECAM-1+/+ retinal EC.
Fig. 12.
Reexpression of PECAM-1 restored migration of PECAM-1−/− retinal EC in an isoform-specific manner. A: cell migration was determined by scratch wound assay of monolayer of retinal EC expressing the empty vector or a specific PECAM-1 isoform. Wound closure was monitored by photography within 48 h and quantified relative to time zero. B: quantitative assessment of the data (*,**P < 0.05, n = 3). C: similar results were observed with a Transwell migration assay (*,**P < 0.05, n = 3).
Fig. 13.
Reexpression of PECAM-1 restored capillary morphogenesis of PECAM-1−/− retinal EC in an isoform-specific manner. A: capillary morphogenesis was determined by plating retinal EC expressing the empty vector or a specific PECAM-1 isoform on Matrigel and photographed after 18 h. B: quantitative assessment of data. Data are the mean number of branch points from 5 high-power fields (×100). Note the significant restoration of capillary morphogenesis in PECAM-1−/− retinal EC expressing Δ14&15 PECAM-1 isoform. These experiments were repeated with 2 different isolations of retinal EC. *,**P < 0.05; n = 3.
DISCUSSION
PECAM-1/CD31 is an EC adhesion molecule with important roles in angiogenesis and inflammation. These activities of PECAM-1 are further regulated by alternative splicing of its cytoplasmic domain. However, the role of PECAM-1 isoforms and their potential signaling capacity in regulation of these processes require further study. The study of the PECAM-1 isoform-specific function in primary cultures of EC has been very limited. Here we isolated retinal EC from wild-type and PECAM-1−/− mice. We show a significant impact for lack of PECAM-1 on endothelial cell-cell and cell-matrix interactions, proliferation, migration, capillary morphogenesis, and eNOS activity and NO bioavailability. We also show that the migration and capillary morphogenesis of null cells can be restored by reexpression of PECAM-1 in an isoform-specific manner.
The levels of VE-cadherin, endoglin, and ICAM-2 were significantly reduced in PECAM-1−/− EC compared with PECAM-1+/+ EC. We also noted that PECAM-1−/− EC are more elongated and spindly and do not form a closely packed cell layer as seen in PECAM-1+/+ cells (Fig. 1A). These results suggested potential alterations in formations of cell-cell contacts in PECAM-1−/− EC, a process that is dependent on VE-cadherin expression (4). We observed a significant decrease in the staining and junctional localization of VE-cadherin in PECAM-1−/− EC (Figs. 1B, 3A). The decrease in VE-cadherin level in the absence of PECAM-1 was further confirmed by Western blot analysis of EC and retinal extracts using antibodies from multiple sources and real-time PCR (Fig. 3B and data not shown). VE-cadherin expression is also essential for formation of tight junctions and localization of ZO-1 to sites of cell-cell contact (28). A significant decrease in staining, junctional localization, and levels of ZO-1 was also observed in PECAM-1−/− retinal EC (Fig. 3). In addition, junctional localization of β-catenin in PECAM-1−/− EC was discontinuous and more punctate. These observations are consistent with the important role proposed for PECAM-1 in interaction with components of the adherens junctions and modulation of junctional stability (24).
The expressions of VE-cadherin and N-cadherin are essential components of the developing vasculature (4, 30, 32). Retinal EC also expressed N-cadherin as previously demonstrated in other EC (34). The absence of PECAM-1 had minimal effect on the level of N-cadherin in these cells (Fig. 3B). VE-cadherin normally competes with N-cadherin in formation of adherens junctions and junctional localization in EC (30). We observed a significant increase in junctional localization of N-cadherin in PECAM-1−/− EC with reduced level of VE-cadherin (Fig. 3A). Thus the presence of N-cadherin may compensate for the lack of VE-cadherin in formation of adherens junctions, allowing vascular development and angiogenesis to proceed.
ICAM-2 is constitutively expressed on the surface of EC and is shown to be important in angiogenesis and transendothelial migration of neutrophils (20, 21). Although transendothelial migration occurs independent of PECAM-1, ICAM-2 and PECAM-1 appear to act in the same molecular pathway to modulate the diapedesis process in a cytokine-specific manner, and perhaps angiogenesis (21, 46). Thus decreased ICAM-2 level in PECAM-1−/− retinal EC may impact on and contribute to their proangiogenic and inflammatory defects.
A significant increase in the growth rate of PECAM-1−/− retinal EC was mainly attributed to a decreased level of apoptosis in these cells. Although these observations are in contrast to the results reported by Gao et al. (16), they are consistent with the results reported by Bergom et al. (3), who showed that an isoform of PECAM-1 that lacks exon 15 was not protective against mitochondrion-dependent apoptosis. Unfortunately, the authors did not determine whether their exon 15-specific antibody detects other PECAM-1 isoforms that lack exon 15, including Δ12&15, Δ14&15, and Δ12,14&15 in EC, and how they may affect apoptosis of PECAM-1-null EC. Thus the decreased sensitivity of PECAM-1−/− cells to apoptotic stimuli compared with PECAM-1+/+ cells could be due to the predominant expression of PECAM-1 isoforms lacking exon 15 (Δ15 and Δ14&15) (48, 53). How the presence and/or absence of exon 15 may contribute to prosurvival and/or proapoptotic activities of PECAM-1 in response to mitochondrion-dependent apoptosis pathways remains unknown.
We previously showed (8) that brain microvascular EC prepared from PECAM-1−/− mice are less migratory. Here we showed that retinal EC from PECAM-1−/− mice are also less migratory (Fig. 5). These observations are consistent with the reduced actin stress fibers and focal adhesions in PECAM-1-null cells and the reported role of PECAM-1 in modulation of Rho GTPases (19, 50). PECAM-1−/− retinal EC were more adherent on fibronectin and vitronectin. The alterations in the adhesion of PECAM-1−/− cells were minimally impacted by changes in integrin expression levels (Fig. 7). However, we cannot rule out the possibility of alteration in the activation state of integrin in the absence of PECAM-1. We have shown (50) that PECAM-1 associates with αvβ3-integrin in an isoform-specific manner. Alternatively, the alteration in adhesive and migratory properties of PECAM-1−/− EC may be explained, at least in part, by increased production of tenascin-C and osteopontin (Fig. 8), with significant roles in modulation of angiogenesis and inflammation. The capillary morphogenesis of retinal EC and sprouting of aortic rings prepared from PECAM-1−/− mice were also attenuated compared with PECAM-1+/+ mice (Fig. 9). These observations are consistent with the reported attenuation of retinal neovascularization and lung alveolarization (an angiogenesis-dependent process) in PECAM-1−/− mice (7, 9). Thus expression of PECAM-1 has a significant impact on adhesive and migratory properties of EC.
eNOS plays a significant role in angiogenesis, and its activity is modulated by PECAM-1 (14). We previously reported (9) a decreased level of eNOS in the retinal vasculature of PECAM-1−/− mice. Here we observed a significant decrease in the amount of total and active phospho-eNOS in PECAM-1−/− retinal EC. This was concomitant with a reduced intracellular NO level in these cells. Since the major angiogenic activity of VEGF is mediated through NO production (36), we also determined the level of VEGF produced by these cells. PECAM-1−/− retinal EC produced a significantly higher level of VEGF compared with PECAM-1+/+ EC. Thus expression of VEGF by itself was not sufficient to activate eNOS and enhance NO production and angiogenesis. These observations are consistent with our recent studies (44) in which we showed that incubation of PECAM-1+/+ retinal EC with nitro-l-arginine methyl ester (l-NAME, eNOS inhibitor), but not nitro-d-arginine methyl ester (d-NAME, inactive analog), attenuates capillary morphogenesis. Thus appropriate expression and activity of eNOS and NO bioavailability are significantly influenced by PECAM-1 expression and/or activity.
Alternative splicing of PECAM-1 was previously recognized (25), and the presence of multiple isoforms of PECAM-1 in whole mouse embryos was reported (56). However, we were the first to demonstrate (40) not only that multiple isoforms of PECAM-1 are present in vascular beds of different tissues but also that their expression pattern changes during development, using an exon 14-specific antibody. We later showed (48, 49, 51–53) that multiple isoforms of PECAM-1 are also present in the endothelium of various human tissues, hematopoietic cells, and platelets. Although full-length PECAM-1 is the major isoform expressed in human endothelium, the Δ14&15 isoform is the predominant isoform in murine endothelium. Unfortunately, many investigations of PECAM-1 function, especially the role of immunoreceptor tyrosine-based inhibitory motif (ITIM; expands exons 13 and 14), have overlooked the fact that the predominant isoform of PECAM-1 in murine endothelium (Δ14&15) lacks the intact ITIM. These studies are difficult to interpret when human full-length PECAM-1 and/or non-EC are used to address PECAM-1 function in the mouse endothelium. As a result, many contradicting observations have been reported, which are difficult to reconcile and need reevaluation using appropriate cells and/or PECAM-1 isoforms. We observed that expression of Δ14&15 PECAM-1 was sufficient to restore migration and capillary morphogenesis of null cells to wild-type levels. Full-length PECAM-1 had no effect on these cellular properties. These observations are consistent with the expression of Δ14&15 PECAM-1 as the predominant isoform in murine endothelium and the potential role of ITIM in regulation of EC proangiogenic phenotype. The identity of isoform-specific signaling pathways involved, and their impact on eNOS expression and/or activity, remain elusive and are the subject of current investigation.
In summary, PECAM-1 is an important modulator of proangiogenic activity of EC such that in its absence angiogenesis is attenuated both in vivo and in vitro. These activities are mediated through modulation of endothelial cell-cell and cell-matrix interactions impacting EC proliferation, apoptosis, and migration. The study of PECAM-1 isoform-specific function in PECAM-1-null EC will be informative and will enhance our understanding of PECAM-1's function in the endothelium.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants EY-16995 and EY-18179 (Sheibani), P30-CA-014520 University of Wisconsin Paul P. Carbone Cancer Center support grant, and P30-EY-16665 and an unrestricted departmental award from Research to Prevent Blindness. N. Sheibani is a recipient of a Research Award from the American Diabetes Association (1-10-BS-160) and the Retina Research Foundation. C. M. Sorenson is a recipient of an American Heart Association research award (0950057G).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J 357: 593– 615, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagi Z, Frangos JA, Yeh JC, White CR, Kaley G, Koller A. PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arterioscler Thromb Vasc Biol 25: 1590– 1595, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Bergom C, Paddock C, Gao C, Holyst T, Newman DK, Newman PJ. An alternatively spliced isoform of PECAM-1 is expressed at high levels in human and murine tissues, and suggests a novel role for the C-terminus of PECAM-1 in cytoprotective signaling. J Cell Sci 121: 1235– 1242, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98: 147– 157, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Cheresh DA, Stupack DG. Regulation of angiogenesis: apoptotic cues from the ECM. Oncogene 27: 6285– 6298, 2008 [DOI] [PubMed] [Google Scholar]
- 6.DeLisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, Kerr JS, Garlanda C, Merwin JR, Madri JA, Albelda SM. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol 151: 671– 677, 1997 [PMC free article] [PubMed] [Google Scholar]
- 7.DeLisser HM, Helmke BP, Cao G, Egan PM, Taichman D, Fehrenbach M, Zaman A, Cui Z, Mohan GS, Baldwin HS, Davies PF, Savani RC. Loss of PECAM-1 function impairs alveolarization. J Biol Chem 281: 8724– 8731, 2006 [DOI] [PubMed] [Google Scholar]
- 8.DiMaio TA, Sheibani N. PECAM-1 isoform-specific functions in PECAM-1-deficient brain microvascular endothelial cells. Microvasc Res 75: 188– 201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimaio TA, Wang S, Huang Q, Scheef EA, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization in PECAM-1-deficient mice. Dev Biol 315: 72– 88, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol 46: 235– 276, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, Luis de la Pompa J, Elia A, Wakeham A, Karan-Tamir B, Muller WA, Senaldi G, Zukowski MM, Mak TW. Genetic evidence for functional redundancy of platelet/endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol 162: 3022– 3030, 1999 [PubMed] [Google Scholar]
- 12.Dusserre N, L'Heureux N, Bell KS, Stevens HY, Yeh J, Otte LA, Loufrani L, Frangos JA. PECAM-1 interacts with nitric oxide synthase in human endothelial cells: implication for flow-induced nitric oxide synthase activation. Arterioscler Thromb Vasc Biol 24: 1796– 1802, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Fischer S, Clauss M, Wiesnet M, Renz D, Schaper W, Karliczek GF. Hypoxia induces permeability in brain microvessel endothelial cells via VEGF and NO. Am J Physiol Cell Physiol 276: C812– C820, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci 118: 4103– 4111, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA 98: 2604– 2609, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao C, Sun W, Christofidou-Solomidou M, Sawada M, Newman DK, Bergom C, Albelda SM, Matsuyama S, Newman PJ. PECAM-1 functions as a specific and potent inhibitor of mitochondrial-dependent apoptosis. Blood 102: 169– 179, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Govers R, Bevers L, de Bree P, Rabelink TJ. Endothelial nitric oxide synthase activity is linked to its presence at cell-cell contacts. Biochem J 361: 193– 201, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, Ruddle NH, Engelhardt B, Madri JA. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest 109: 383– 392, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gratzinger D, Canosa S, Engelhardt B, Madri JA. Platelet endothelial cell adhesion molecule-1 modulates endothelial cell motility through the small G-protein Rho. FASEB J 17: 1458– 1469, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Huang MT, Larbi KY, Scheiermann C, Woodfin A, Gerwin N, Haskard DO, Nourshargh S. ICAM-2 mediates neutrophil transmigration in vivo: evidence for stimulus specificity and a role in PECAM-1-independent transmigration. Blood 107: 4721– 4727, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Huang MT, Mason JC, Birdsey GM, Amsellem V, Gerwin N, Haskard DO, Ridley AJ, Randi AM. Endothelial intercellular adhesion molecule (ICAM)-2 regulates angiogenesis. Blood 106: 1636– 1643, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Hynes RO. Cell-matrix adhesion in vascular development. J Thromb Haemost 5, Suppl 1: 32– 40, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Hynes RO. The extracellular matrix: not just pretty fibrils. Science 326: 1216– 1219, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ilan N, Madri JA. PECAM-1: old friend, new partners. Curr Opin Cell Biol 15: 515– 524, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Kirschbaum NE, Gumina RJ, Newman PJ. Organization of the gene for human platelet/endothelial cell adhesion molecule-1 shows alternatively spliced isoforms and a functionally complex cytoplasmic domain. Blood 84: 4028– 4037, 1994 [PubMed] [Google Scholar]
- 26.Kondo S, Scheef EA, Sheibani N, Sorenson CM. PECAM-1 isoform-specific regulation of kidney endothelial cell migration and capillary morphogenesis. Am J Physiol Cell Physiol 292: C2070– C2083, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kuhlencordt PJ, Rosel E, Gerszten RE, Morales-Ruiz M, Dombkowski D, Atkinson WJ, Han F, Preffer F, Rosenzweig A, Sessa WC, Gimbrone MA, Jr, Ertl G, Huang PL. Role of endothelial nitric oxide synthase in endothelial activation: insights from eNOS knockout endothelial cells. Am J Physiol Cell Physiol 286: C1195– C1202, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Liebner S, Cavallaro U, Dejana E. The multiple languages of endothelial cell-to-cell communication. Arterioscler Thromb Vasc Biol 26: 1431– 1438, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Lund SA, Giachelli CM, Scatena M. The role of osteopontin in inflammatory processes. J Cell Commun Signal 3: 311– 322, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo Y, Radice GL. N-cadherin acts upstream of VE-cadherin in controlling vascular morphogenesis. J Cell Biol 169: 29– 34, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol 23: 953– 964, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Paik JH, Skoura A, Chae SS, Cowan AE, Han DK, Proia RL, Hla T. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev 18: 2392– 2403, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothermel TA, Engelhardt B, Sheibani N. Polyoma virus middle-T-transformed PECAM-1 deficient mouse brain endothelial cells proliferate rapidly in culture and form hemangiomas in mice. J Cell Physiol 202: 230– 239, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Sabatini PJB, Zhang M, Silverman-Gavrila R, Bendeck MP, Langille BL. Homotypic and endothelial cell adhesions via N-cadherin determine polarity and regulate migration of vascular smooth muscle cells. Circ Res 103: 405– 412, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Sabet MD, Gordon SR. Ultrastructural immunocytochemical localization of fibronectin deposition during corneal endothelial wound repair. Evidence for cytoskeletal involvement. Biol Cell 65: 171– 179, 1989 [PubMed] [Google Scholar]
- 36.Sessa WC. Regulation of endothelial derived nitric oxide in health and disease. Mem Inst Oswaldo Cruz 100, Suppl 1: 15– 18, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Sheibani N, Frazier WA. Down-regulation of platelet endothelial cell adhesion molecule-1 results in thrombospondin-1 expression and concerted regulation of endothelial cell phenotype. Mol Biol Cell 9: 701– 713, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheibani N, Frazier WA. Thrombospondin-1, PECAM-1, and regulation of angiogenesis. Histol Histopathol 14: 285– 294, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Sheibani N, Sorenson CM, Frazier WA. Differential modulation of cadherin-mediated cell-cell adhesion by platelet endothelial cell adhesion molecule-1 isoforms through activation of extracellular regulated kinases. Mol Biol Cell 11: 2793– 2802, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheibani N, Sorenson CM, Frazier WA. Tissue specific expression of alternatively spliced murine PECAM-1 isoforms. Dev Dyn 214: 44– 54, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Smalley-Freed WG, Efimov A, Burnett PE, Short SP, Davis MA, Gumucio DL, Washington MK, Coffey RJ, Reynolds AB. p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J Clin Invest 120: 1824– 1835, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solowiej A, Biswas P, Graesser D, Madri JA. Lack of platelet endothelial cell adhesion molecule-1 attenuates foreign body inflammation because of decreased angiogenesis. Am J Pathol 162: 953– 962, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su X, Sorenson CM, Sheibani N. Isolation and characterization of murine retinal endothelial cells. Mol Vis 9: 171– 178, 2003 [PubMed] [Google Scholar]
- 44.Tang Y, Scheef EA, Gurel Z, Sorenson CM, Jefcoate CR, Sheibani N. CYP1B1 and endothelial nitric oxide synthase combine to sustain proangiogenic functions of endothelial cells under hyperoxic stress. Am J Physiol Cell Physiol 298: C665– C678, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang Y, Scheef EA, Wang S, Sorenson CM, Marcus CB, Jefcoate CR, Sheibani N. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood 113: 744– 754, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson RD, Noble KE, Larbi KY, Dewar A, Duncan GS, Mak TW, Nourshargh S. Platelet-endothelial cell adhesion molecule-1 (PECAM-1)-deficient mice demonstrate a transient and cytokine-specific role for PECAM-1 in leukocyte migration through the perivascular basement membrane. Blood 97: 1854– 1860, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Tucker RP. The distribution of J1/tenascin and its transcript during the development of the avian cornea. Differentiation 48: 59– 66, 1991 [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Repyak K, Sheibani N. Expression pattern of alternatively spliced PECAM-1 isoforms in retinal vasculature. Mol Vis 10: 103– 111, 2004 [PubMed] [Google Scholar]
- 49.Wang Y, Sheibani N. Expression pattern of alternatively spliced PECAM-1ba isoforms in hematopoietic cells and platelets. J Cell Biochem 87: 424– 438, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Sheibani N. PECAM-1 isoform-specific activation of MAPK/ERKs and small GTPases: implications in inflammation and angiogenesis. J Cell Biochem 98: 451– 468, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Su X, Sorenson CM, Sheibani N. Modulation of PECAM-1 expression and alternative splicing during differentiation and activation of hematopoietic cells. J Cell Biochem 88: 1012– 1024, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Su X, Sorenson CM, Sheibani N. Tissue-specific distributions of alternatively spliced human PECAM-1 isoforms. Am J Physiol Heart Circ Physiol 284: H1008– H1017, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Su X, Wu Z, Sheibani N. Thrombospondin-1 deficient mice exhibit an altered expression pattern of alternatively spliced PECAM-1 isoforms in retinal vasculature and endothelial cells. J Cell Physiol 204: 352– 361, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Wang S, Sheibani N. Enhanced proangiogenic signaling in thrombospondin-1-deficient retinal endothelial cells. Microvasc Res 71: 143– 151, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Wu J, Sheibani N. Modulation of VE-cadherin and PECAM-1 mediated cell-cell adhesions by mitogen-activated protein kinases. J Cell Biochem 90: 121– 137, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Yan HC, Baldwin HS, Sun J, Buck CA, Albelda SM, DeLisser HM. Alternative splicing of a specific cytoplasmic exon alters the binding characteristics of murine platelet/endothelial cell adhesion molecule-1 (PECAM-1). J Biol Chem 270: 23672– 23680, 1995 [DOI] [PubMed] [Google Scholar]