Abstract
To identify the genetic locus responsible for malignant hyperthermia susceptibility (MHS) in an Italian family, we performed linkage analysis to recognized MHS loci. All MHS individuals showed cosegregation of informative markers close to the voltage-dependent Ca2+ channel (CaV) α1S-subunit gene (CACNA1S) with logarithm of odds (LOD)-score values that matched or approached the maximal possible value for this family. This is particularly interesting, because so far MHS was mapped to >178 different positions on the ryanodine receptor (RYR1) gene but only to two on CACNA1S. Sequence analysis of CACNA1S revealed a c.4060A>T transversion resulting in amino acid exchange T1354S in the IVS5-S6 extracellular pore-loop region of CaVα1S in all MHS subjects of the family but not in 268 control subjects. To investigate the impact of mutation T1354S on the assembly and function of the excitation-contraction coupling apparatus, we expressed GFP-tagged α1ST1354S in dysgenic (α1S-null) myotubes. Whole cell patch-clamp analysis revealed that α1ST1354S produced significantly faster activation of L-type Ca2+ currents upon 200-ms depolarizing test pulses compared with wild-type GFP-α1S (α1SWT). In addition, α1ST1354S-expressing myotubes showed a tendency to increased sensitivity for caffeine-induced Ca2+ release and to larger action-potential-induced intracellular Ca2+ transients under low (≤2 mM) caffeine concentrations compared with α1SWT. Thus our data suggest that an additional influx of Ca2+ due to faster activation of the α1ST1354S L-type Ca2+ current, in concert with higher caffeine sensitivity of Ca2+ release, leads to elevated muscle contraction under pharmacological trigger, which might be sufficient to explain the MHS phenotype.
Keywords: excitation-contraction coupling, calcium channels, skeletal muscle disease, caffeine-induced Ca2+ release, channelopathy
malignant hyperthermia (MH) is a potentially lethal pharmacogenetic disorder of skeletal muscle inherited in an autosomal dominant mode. Clinical MH episodes are triggered in MH susceptible (MHS) individuals by exposure to volatile anesthetics and depolarizing skeletal muscle relaxants (e.g., succinylcholine; Refs. 8, 19). MH episodes are characterized by uncontrolled increase of muscle activity and heat production. This results in a progressive hyperthermia and muscle rigidity associated with tachycardia, metabolic acidosis, hyperkalemia, and hypoxia that can be deadly, if not treated immediately. Apart from pharmacologically induced episodes, MH patients do not present clinically relevant symptoms.
Because of its subclinical nature, MHS has to be usually diagnosed by an invasive in vitro contracture test (IVCT) to avoid life-threatening incidents during anesthesia in patients from susceptible families. According to the European standardized protocol (37, 31), the contracture responses of muscle tissue upon exposure to halothane and caffeine discriminate individuals as MHS, MH equivocal (MHE), and MH normal (MHN). All symptoms of a MH episode are relevant consequences of aberrant excitation-contraction (EC) coupling that results in abnormal and uncontrolled Ca2+ release from sarcoplasmic reticulum (SR) stores in skeletal muscle cells after administration of anesthetics and muscle relaxants (25).
Skeletal muscle EC coupling is known as a signaling mechanism (10) between the sarcolemmal voltage-gated L-type Ca2+ channel and the SR Ca2+ release channel or ryanodine receptor type-1 (RyR1). Membrane depolarization induces conformational changes in the voltage sensing and pore forming CaVα1S-subunit of the L-type Ca2+ channel, which opens the RyR1 via protein-protein interaction. Consequently, Ca2+ released from the SR stores activates the contractile apparatus of the muscle fibers. Genes encoding the key proteins of the EC coupling machinery are the molecular suspects for MH susceptibility. Linkage studies established the RyR1 gene (RYR1) as the main relevant gene (23, 24) that accounts for MHS in >50% of MH families. So far, a genome-wide search has identified additional MHS loci on chromosomes 1q32 (26) (MHS5, OMIM 601887) where the gene coding for the CaVα1S-subunit is located, 7q21–22 (18) (MHS3, OMIM 154276) the gene locus of the accessory CaVα2δ-1-subunit, 17q11–24 (22) (MHS2, OMIM 154275) the gene locus of the voltage-gated Na+ channel NaV1.4α-subunit, chromosomes 3q13.1 (36) (MHS4, OMIM 600467) and 5p (34) (MHS6, OMIM 601888). However, missense mutations were so far only discovered in two proteins associated with these MHS loci. More than 178 missense mutations and deletions are described for RyR1, out of which 29 have been functionally characterized (33). In contrast, only two MH-causing mutations were hitherto identified in the second locus, MHS5, mapped to gene CACNA1S (5, 26) encoding the CaVα1S-subunit. The first identified mutation, R1086H in the intracellular loop connecting homologous repeats III and IV of CaVα1S, was later also physiologically characterized (41).
Here we report about a south Italian MH family pedigree where the MHS phenotype is again associated with the CACNA1S gene. Specifically, we identified a threonine to serine exchange at position 1354 in the S5-S6 extracellular pore-loop region of homologous repeat IV of CaVα1S. We introduced this T1354S mutation into the well-characterized GFP-tagged rabbit α1S-subunit (16) and expressed construct α1ST1354S in dysgenic (α1S-null) mouse myotubes to study the influence of this mutation on EC coupling and caffeine-induced SR Ca2+ release. Our results demonstrate that mutation T1354S accelerates L-type Ca2+ current kinetics and also contributes to a more pronounced caffeine sensitivity of SR Ca2+ release. We conclude that the accelerated Ca2+ channel activation kinetics together with a higher caffeine-induced Ca2+ release in α1ST1354S-expressing myotubes contribute to amplification of intracellular Ca2+ release and thus are connected with the MH phenotype.
MATERIALS AND METHODS
In Vitro Contracture Test and Linkage Analysis
A patient that showed symptoms of an MH episode and 10 other family members (family NA-6) were subjected to the standardized European IVCT (37, 31) (Supplemental Table 1; Supplemental Material for this article is available online at the Am J Physiol-Cell Physiol website). Briefly, force production of standardized muscle biopsy material at concentrations of 2% halothane or 2 mM caffeine was determined. Samples that generated a force of ≥0.2 g for both triggering agents were judged MHS. Samples with this force produced by only one of the triggering agents were classified as MHE.
Genomic DNA was extracted from blood samples with the “Nucleon” procedure (Amersham). Microsatellite repeat marker alleles for MHS loci 1–6 were PCR amplified, labeled with 32P, and fractionated on 4% polyacrylamide sequencing gels for subsequent visualization by autoradiography or were labeled with 6-FAM, HET, HEX, and NED fluorochromes and separated by capillary electrophoresis with an ABI PRISM 3130 Genetic Analyzer (Applied Biosystems). Linkage analysis was performed using the MLINK program of the LINKAGE package (version 5.1) as implemented in FASTLINK (21, 7). Two-point logarithm of odds (LOD) scores were calculated between each marker locus and MHS, MHE, and MHN characteristics under the standard genetic model defined by the European MH Group genetics section as reported earlier (9, 35). Disease allele frequency was 1/10,000, penetrance of the index case was taken as 1.0, penetrance of the MHS heterozygote was set at 0.98, the phenocopy rate was set at 0.02, and MHE individuals were assigned unknown status and did not contribute to the LOD scores of the pedigree. The maximum two-point LOD score of family NA-6 was calculated under the same genetic model by using an anonymous marker with five alleles, heterozygosity of 0.8, and recombination fraction θ = 0.
Sequence Analysis
PCR primers for the amplification of the 44 exons of gene CACNA1S and of their intron boundaries were designed according to the human CACNA1S sequence (OMIM 114208, primer sequence on request to carsana@unina.it). Amplicons were directly sequenced with dye-terminator chemistry (Applied Biosystems) using a PRISM 3130 Genetic Analyzer (Applied Biosystems). Nucleotide substitutions in the coding sequence were numbered according to the CaVα1S cDNA sequence (GenBank Accession No. NM_000069).
Construction of Mutant CaVα1S cDNAs
Nucleotide numbers (nt) are given below in parentheses and asterisks indicate restriction enzyme (RE) sites introduced by PCR using proofreading Pfu Turbo DNA polymerase (Stratagene). All sequences generated and modified by PCR were checked for integrity by sequence analysis (Eurofins MWG, Ebersberg, Germany).
α1ST1354S.
To substitute Thr1354 by Ser in wild-type rabbit GFP-α1S (16), a silent AccI* RE site (nt 4049) and a “helper” SpeI* site (nt 4520), together with an A to T transversion (nt 4060) that created triplet TCC coding for Ser instead of ACC for Thr, were introduced by PCR primers. The AatII-SpeI* fragment (nt 3591–4520) produced by a fusion PCR step was coligated with the XhoI-AatII fragment of clone GFP-α1S (nt 2654–3591) into the corresponding XhoI/SpeI polylinker sites of pBluescript (Stratagene). Subsequently, the XhoI-BglII fragment (nt 2654–4488) of this subclone was inserted into the corresponding RE sites of GFP-α1S. For simplicity, we refer to this GFP-tagged construct as α1ST1354S.
α1SΔ29.
CaVα1S splice variant α1SΔ29, lacking exon 29 (coding for 19 of the 32 residues of the extracellular loop linking transmembrane segments IVS3 and IVS4) was found to be expressed at low levels in differentiated human and mouse muscle and up to 80% in cultured myotubes (38). To generate clone α1SΔ29, nts 3609–3666 that code for exon 29 were deleted from rabbit GFP-α1S (16) by PCR splicing as described in Tuluc et al. (38).
Cell Culture and Transfections
Myotubes of the homozygous CaVα1S-null (dysgenic) mouse cell line GLT were cultured on 35-mm culture dishes as described previously (32). GLT myotubes were transfected with GFP-tagged α1S cDNA constructs at the onset of myoblast fusion using FuGENE 6 transfection reagent according to the manufacturer's protocols (Roche Diagnostics). Three to four days after transfection, myotubes were either fixed for immunocytochemistry (14) or were analyzed in combined patch-clamp and intracellular Ca2+ measurements. Additionally, myotubes generated from primary myoblasts (referred as MDG) derived from newborn dysgenic mice (12) were cultured and transfected with FuGENE, following the same protocol as described previously (29). Cells were cultured until day 9 for complete differentiation and were used for caffeine-induced and electrically elicited Ca2+ release in perfusion and field stimulation experiments (see below).
Immunofluorescence Analysis
Immunofluorescence analysis was performed as previously described (14). To stain the CaVα1S-subunit protein, monoclonal antibody mAb 1A (28, 20) (1:2,000) or a rabbit affinity-purified anti-GFP antibody (1:5,000; Molecular Probes) was used together with an Alexa-488-conjugated secondary antibody so that the intrinsic GFP signal and the antibody label were both recorded in the green channel. Affinity-purified antibody 162 against RyR1 (15) (1:2,000) and monoclonal antibody mAb 20A (27) against the α2δ-1-subunit (1:1,000) were used in combination with Alexa-594-conjugated secondary antibody. Images were recorded on a Zeiss Axiophot fluorescence microscope with a cooled charge-coupled device camera and MetaView image-processing software (Universal Imaging, West Chester, PA).
Patch-Clamp Analysis and Intracellular Ca2+ Measurements
Ca2+ currents were monitored simultaneously with intracellular Ca2+ transients in GLT myotubes on days 7 or 8 after passaging. Myotubes were cultured in 35-mm culture dishes and mounted on an Olympus IX70 inverted microscope equipped with Hoffmann modulation contrast. L-type Ca2+ currents were recorded using the whole cell patch-clamp technique from myotubes bathed in an external solution that contained 145 mM TEA-Cl, 10 mM CaCl2, and 10 mM HEPES (pH 7.4 with TEA-OH). Patch pipettes were pulled from borosilicate glass (Harvard Apparatus) and fire-polished (Microforge MF-830; Narishige), and they had resistances of 1.5–2.5 MΩ when filled with a solution consisting of 145 mM Cs-aspartate, 2 mM MgCl2, 10 mM HEPES, 0.1 mM Cs-EGTA, and 2 mM Mg-ATP (pH 7.4 with CsOH). Fluorescence signals of depolarization-induced intracellular Ca2+ transients were collected during whole cell recordings from a small area of the patch-clamped myotubes with a photomultiplier detection system (Photon Technology International, South Brunswick, NJ) by including 0.2 mM Fluo-4 pentapotassium salt (Molecular Probes, Eugene, OR) to the pipette solution. All recordings were performed with an Axopatch 200A amplifier controlled by pClamp 7.0 software. Currents were elicited with 200-ms depolarizing steps from a holding potential of −80 mV to test potentials between −50 and +80 mV in 10-mV increments. Each test pulse was preceded by a 1-s prepulse to −50 mV to inactivate low voltage-activated T-type currents. Linear leak and capacitative currents were digitally subtracted with a P/4 prepulse protocol (1). Recordings were low-pass Bessel filtered at 2 kHz and sampled at 5 kHz. Ca2+ currents were normalized by linear cell capacitance (expressed in pA/pF).
Voltage dependence of intracellular Ca2+ release (ΔF/F) was fitted according to a Boltzmann distribution:
| (1) |
where V is the membrane potential, V1/2 is the potential at which ΔF/F0 = (ΔF/F0)max/2, and k is a slope factor.
The activation phase of the Ca2+ current (from current level 0 to 98% of peak current amplitude) was fitted with the following single (Eq. 2) and double exponential (Eq. 3) functions:
| (2) |
| (3) |
where I(t) is the current at time t after depolarization; Amono, Afast, and Aslow are the steady-state current amplitudes of each component with their respective time constants of activation (τmono, τfast, and τslow); and C represents the steady-state peak current.
All recordings were performed at room temperature. Only currents with a maximal voltage error <5 mV due to series resistance were analyzed with Clampfit 8.0 (Axon Instruments, Foster City, CA) and SigmaPlot 8.0 (SPSS) software. Data are given as means ± SE. Statistical significance was determined by unpaired Student's t-test.
Measurements of Caffeine-Induced and Electrically Elicited Ca2+ Release
Caffeine sensitivity of intracellular Ca2+ release was analyzed in MDG myotubes that proved more stable under field stimulation conditions as GLT myotubes. MDG cells were cultured for 9 days until complete differentiation on glass coverslips coated with carbon and gelatin. Caffeine response curves were monitored from myotubes loaded with Indo-1, a ratiometric Ca2+ indicator. MDG cells were incubated for 1 h at room temperature with 6 μM Indo-1-AM (Invitrogen) and 0.12% Pluronic in colorless DMEM. Loaded myotubes were bathed in a normal rodent Ringer solution (145 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, and 10 mM HEPES, pH 7.4) and placed in the incubation chamber with a pair of electrodes on the two opposite sides of the chamber in a 6-mm distance. The chamber was mounted on a Zeiss Axiovert epifluorescence microscope and the sample was excited at 350 nm with an Hg 50W illumination system. Fluorescence emissions at 405 (F405) and 485 (F485) nm were collected with a 2 photomultiplier detection system, (Photon Technology International, S. Brunswick, NJ) and analyzed as ratio (R) of F405 to F485. Before each recording, a cell-free region of the glass coverslip was selected in the photomultiplier detection window to measure the background fluorescence (Rbackground), which was afterwards automatically subtracted. First, each cell that was preselected for CaVα1S expression by green fluorescence, was electrically stimulated three times (1.7 V per mm electrode distance for 2 ms) via the two stimulation electrodes to check for intact CaV-RyR1 coupling. Responding myotubes were sequentially exposed to 60-s applications of different concentrations of caffeine (0.5, 1.0, 3.0, 5.0, 10, 30, and 40 mM in Ringer solution) using an automated rapid perfusion system (VC-8 Eight Channel Perfusion Valve Control Systems, Warner Instrument, Hamden, CT). Every caffeine exposure was followed by a 60-s wash step with Ringer solution. Peak values of intracellular Ca2+ changes in response to agonist (caffeine) application were expressed as ΔR (Ragonist − Rbaseline). Data were analyzed with FeliX (Photon Technology, Princeton, NJ) and SigmaPlot 8.0 (SPSS, Chicago, IL) software packages. Caffeine concentration-response curves were fitted according to the following equation:
| (4) |
where Dmax is the maximal response, [D] is the caffeine concentration, nH is the Hill coefficient, and EC50 is the concentration of caffeine at which the response was half-maximal. Compared with similar experiments in an earlier study (41) MDG myotubes displayed a lower sensitivity to caffeine in the recent study, possibly due to higher passage numbers or slightly different culture condition.
To evaluate the action potential-elicited Ca2+ release from intracellular SR stores, transient elevations of intracellular Ca2+ were analyzed in Indo-1-AM-loaded myotubes, stimulated by three succeeding 2-ms depolarizing pulses with 1.7 V/mm electrode distance. Action-potential-induced Ca2+ transients were measured in single myotubes during a sequential application of 0, 1, and 2 mM caffeine dissolved in Ringer solution containing 0 and 2 mM Ca2+, respectively. Only for measurements in presence of 2 mM extracellular Ca2+ solution, myotubes were incubated between each stimulation step for 2 min with 10 mM Ca2+ and caffeine-free extracellular solution to ensure complete Ca2+ reloading of the SR stores. Fluorescence signals were detected by a photomultiplier system (PTI 814; Photon Technology International, Lawrenceville, NJ) and analyzed by FeliX and SigmaPlot 8.0 software.
RESULTS
MHS is Linked to the CACNA1S Gene in a South Italian Family
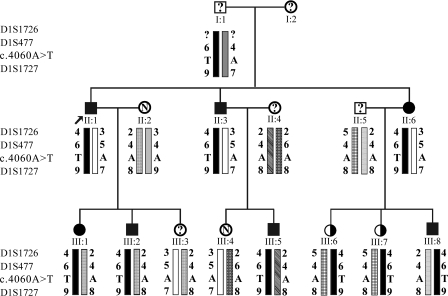
During anesthesia for tonsillectomy a patient (II:1, Fig. 1) developed tachycardia, hypercapnia, muscular rigidity, and a severe increase in body temperature, so that surgery had to be terminated. Twenty-four hours after this incident creatine kinase levels were elevated to 22.000–31.000 U/l (normal range 0–190 U/l). Linkage analysis of the family (NA-6) of this proband II:1 (Fig. 1) revealed a cosegregation of the MH phenotype with microsatellite markers for the MHS5 locus (MIM 601887), which contains the CACNA1S gene coding for the L-type Ca2+ channel subunit CaVα1S. In fact, LOD score values at a recombination fraction of θ = 0 were identical or close to the theoretical maximum value of +1.76 obtained only with markers in or adjacent to the MH5 locus (Table 1). Low or negative LOD score values obtained with markers for all other MHS loci indicate their irrelevance for the MHS phenotype in family NA-6 (Table 1).
Fig. 1.
Segregation of MHS5 microsatellite markers and of the c.4060A>T malignant hyperthermia susceptibility (MHS) mutation within the pedigree of family NA-6. I, II, and III indicate the 3 generations investigated; squares symbolize male and circles female family members. Black symbols denote individuals identified as MHS by invasive in vitro contracture test; white symbols marked with N denote those identified as MH normal (MHN); black/white symbols denote those identified as MH equivocal (MHE); and symbols with a question mark denote untested family members (disease status unknown). The index patient (II:1; indicated by an arrow) developed an MH crisis during anesthesia. All identical haplotypes are symbolized by bars in identical patterns. The haplotype 4–6-T-9 (black bar) is shared by all of the affected individuals (MHS and MHE) and by none of the unaffected individuals (MHN).
Table 1.
MHS loci, microsatellite markers, and two-point LOD scores in family NA-6
| Loci | LOD Score at θ = 0 |
|---|---|
| MHS1 19q13.1 | |
| D19S191 | +0.7 |
| D19S220 | +0.7 |
| D19S223 | +0.7 |
| MHS2 17q11.2-24 | |
| D17S1855 | −1.91 |
| D17S794 | n.i. |
| D17S1825 | −2.45 |
| MHS3 7q21-22 | |
| D7S669 | n.i. |
| D7S2443 | −2.1 |
| D7S440 | −1.67 |
| MHS4 3q13.1 | |
| D3S1281 | n.i. |
| D3S3638 | −1.39 |
| D3S695 | −3.21 |
| MHS5 1q32 | |
| D1S1726 | +1.76 |
| D1S477 | +1.16 |
| CACNL1A3.PCR | +1.28 |
| D1S1727 | +1.76 |
| MHS6 5p | |
| D5S418 | −1.61 |
| D5S398 | −1.52 |
Linkage analysis was performed in family NA-6 to specific microsatellite markers adjacent to each of the six known malignant hyperthermia susceptibility (MHS) loci. Logarithm of odds (LOD) score values calculated for each marker defined the genetic association (values close to the theoretical maximum value of +1.76) or the genetic exclusion (low or negative values) with the MHS phenotype. Genetic association with MHS was found only with locus MHS5 in NA-6. n.i., Not informative (the subjects of generation II are homozygous).
DNA sequencing of all CACNA1S exons and exon-intron boundaries from MHS subjects of family NA-6 ruled out the presence of a previously identified MHS5 mutation (26). Sequence analysis not only identified several already reported polymorphisms (6) but also new nucleotide substitutions (Supplemental Table 2) Only transversion c.4060A>T was found at a heterozygous status in all MHS and MHE subjects of the family (Fig. 1) but was absent in all MHN-tested family members and also in 268 chromosomal sequences derived from MHN-tested individuals unrelated to family NA-6.
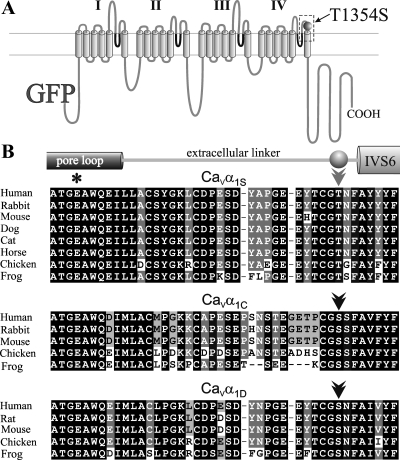
Mutation c.4060A>T results in the replacement of Thr1354 by a Ser residue in an extracellular portion of the L-type Ca2+ channel CaVα1S-subunit. Specifically, this position is located in the linker between the pore-forming segment (pore-loop) in homologous repeat IV and transmembrane segment IVS6, 3 amino acids before IVS6 (Fig. 2). Compared with sequences retrieved from National Center for Biotechnology Information databases, this particular Thr at the position 3 amino acids before IVS6 is conserved in all so far sequenced mammalian CaVα1S-subunits as well as in those from chicken and frog. Interestingly, however, in the faster activating and inactivating neuronal/cardiac CaVα1C and the neuroendocrine CaVα1D, this position is occupied by a conserved Ser (Fig. 2B).
Fig. 2.
Thr1354, 3 amino acids before segment IVS6, is highly conserved in all α1S-subunits but is replaced by Ser in the cardiac/neuronal CaVα1C- and CaVα1D-subunits. A: location of the mutation T1354S in a transmembrane domain model of GFP-α1S. I-IV indicate the 4 homologous repeats, and pore loop domains are indicated in dark gray. Double line indicates the sarcolemmal membrane. B: sequence alignments of the C-terminal half of the pore-forming IVS5-S6 loop of CaVα1S, CaVα1C, and CaVα1D from different species and their positions in the domain model. For each isoform, residues that are identical in all species are boxed in black. The selectivity filter Glu of pore loop domain IV (dark gray cylinder) is indicated by an asterisk. Thr, 3 amino acids before transmembrane segment IVS6 (Thr1354 in human and rabbit), is indicated by a light-gray double arrowhead. As indicated by a black double arrowhead, a conserved Ser is found at the same position in CaVα1C and CaVα1D, similar to the MH mutation T1354S. Sequences for cat and horse CaVα1C and CaVα1D, for chicken CaVα1S, and for frog CaVα1S and CaVα1C were extracted from genomic assemblies at http://www.ensembl.org using a BLAST search with rabbit CaVα1S, CaVα1C, or CaVα1D cDNA sequences. GeneBank Accession Nos. for CaVα1S, CaVα1C, or CaVα1D, respectively, of the different species are as follows: human (Homo sapiens) NM_000069 (α1S), NM_199460 (α1C), NM_000720 (α1D); rabbit (Oryctolagus cuniculus) NM_001101720 (α1S), NM_001136522 (α1C); rat (Rattus norvegicus) NM_017298 (α1D), mouse (Mus musculus) NM_014193 (α1S), NM_009781 (α1C), NM_028981 (α1D); dog (Canis lupus familiaris) XM_843592 (α1S), XM_534932 (α1C), XM_844338 (α1D); cat (Felis catus) NM_001038605 (α1S); horse (Equus caballus) XM_001916128 (α1S); chicken (Gallus gallus) XM_416388 (α1C), NM_205034 (α1D); and frog (Xenopus tropicalis) NM_001079461 (α1D).
Mutation T1354S Accelerates Skeletal Muscle CaV Current Kinetics
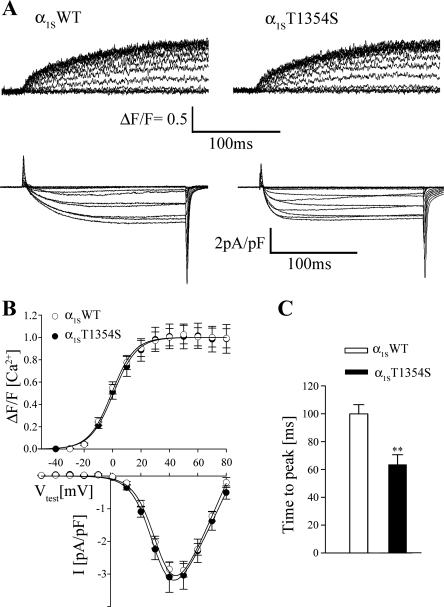
To test the functional role of this newly discovered MH mutation, we introduced the T1354S mutation into the well-characterized GFP-tagged CaVα1S of rabbit and transfected this construct (α1ST1354S) into dysgenic (CaVα1S-null) myotubes (32, 12). Double fluorescence immunocytochemistry showed that α1ST1354S correctly incorporates into the plasma membrane and colocalizes with RyR1 (Supplemental Fig. 1) in a punctuate expression pattern identical to that of the wild-type α1S (α1SWT). After immunocytochemistry demonstrated that mutation T1354S does not interfere with proper CaVα1S membrane targeting, we addressed the question whether mutation T1354S affects voltage sensing and/or Ca2+-conductivity of the skeletal muscle CaV. Whole cell patch-clamp current recordings combined with intracellular Ca2+-release measurements were performed in transfected dysgenic myotubes loaded with the fluorescent Ca2+ indicator Fluo-4. Recordings from myotubes (Fig. 3A), transfected with α1SWT (left) and α1ST1354S (right), showed that both α1S constructs restored robust L-type Ca2+ currents (bottom traces) as well as Ca2+ transients (top traces). The averaged amplitudes and voltage dependences of both Ca2+ inward currents and intracellular Ca2+ transients of myotubes expressing α1ST1354S were comparable (P > 0.05) to those of myotubes expressing α1SWT (Fig. 3B and Table 2).
Fig. 3.
L-type Ca2+ currents are accelerated in myotubes expressing α1ST1354S. A: representative traces of intracellular Ca2+ release (top) and L-type Ca2+ currents (bottom) recorded in response to 200-ms test pulses from dysgenic myotubes expressing wild-type α1S (α1SWT) and α1ST1354S. B: mean voltage dependences and amplitudes of peak Ca2+ transients (ΔF/F; α1SWT, n = 40, and α1ST1354S, n = 27) and of L-type Ca2+ currents (α1SWT, n = 31, and α1ST1354S, n = 26) are indistinguishable in α1SWT- and α1ST1354S-expressing myotubes. I-V, current-voltage relationship. C: time to peak of Ca2+ currents is significantly (**P < 0.01) reduced in myotubes expressing α1ST1354S (α1SWT, n = 31, and α1ST1354S, n = 26).
Table 2.
Properties of intracellular Ca2+ transients and L-type Ca2+ currents
| α1SWT | α1ST1354S | |
|---|---|---|
| Ca2+ transient | ||
| (ΔF/F0)max | 1.81 ± 0.14 | 1.74 ± 0.19 |
| n | 40 | 27 |
| Ca2+ currents | ||
| Imax, pA/pF | 2.97 ± 0.20 | 3.26 ± 0.44 |
| Vact1/2, mV | 34.68 ± 0.95 | 33.85 ± 0.82 |
| Vrev, mV | 80 ± 1.25 | 83 ± 1.76 |
| ttp, ms | 99.8 ± 6.6† | 67.1 ± 7.3 |
| n | 31 | 26 |
| Double exponential fit | ||
| τslow, ms | 30.4 ± 3.38* | 19.8 ± 3.7 |
| τfast, ms | 5.05 ± 0.36* | 3.69 ± 0.5 |
| n | 18 | 13 |
| Monoexponential fit | ||
| τmono, ms | 35 ± 3.6 | 24.3 ± 5.0 |
| n | 13 | 13 |
Data are means ± SE for n myotubes, analyzed by fitting each recording with appropriate equations and their definitions as described in materials and methods. Properties of depolarization-induced Ca2+ transients and L-type Ca2+ currents were determined by combined whole cell patch-clamp and intracellular Fluo-4 Ca2+ recordings from dysgenic myotubes expressing wild-type α1S (α1SWT) and α1ST1354S.
P < 0.05;
P < 0.01.
Nevertheless, α1ST1354S-expressing myotubes showed significantly distinct Ca2+ current kinetics compared with α1SWT. In Fig. 3A, a representative recording from an α1SWT-transfected myotube shows slow-activating Ca2+ currents as typical for CaVα1S (bottom traces, left), which is in contrast to the more accelerated inward currents, recorded from an α1ST1354S-transfected myotube (bottom traces, right). On average, time to peak values of maximal current traces (40 or 50 mV) were significantly (P < 0.01) reduced from 99.87 ± 6.6 ms in α1SWT traces (n = 31) to 67.1 ± 7 ms in α1ST1354S (n = 26; Fig. 3C).
Fast Activation Kinetics is an Intrinsic Property of α1ST1354S
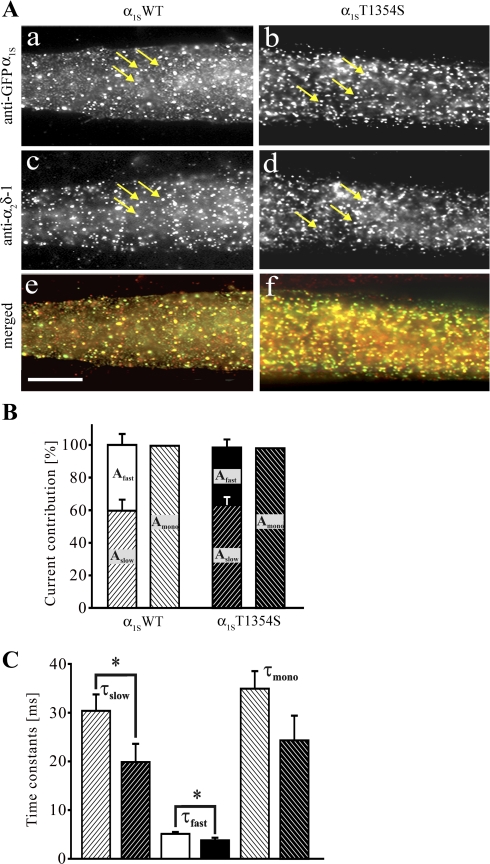
We previously demonstrated that the siRNA knockdown of the auxiliary α2δ-1-subunit of the skeletal muscle CaV in myotubes leads to accelerated activation kinetics of the skeletal muscle L-type Ca2+ current (30). As the T1354S mutation accelerated the current activation kinetics, and considering its localization in an extracellular loop, it appears possible that this mutation might disturb the interaction between CaVα1S and α2δ-1. To evaluate the localization and interaction of CaVα1S and α2δ-1, double immunofluorescence labeling of α1S and α2δ-1 was performed in dysgenic myotubes transfected with α1SWT and α1ST1354S. Figure 4A demonstrates that the T1354S mutation does not notably alter the distribution pattern of α2δ-1. The staining patterns of α1ST1354S-expressing myotubes were indistinguishable from those of myotubes expressing α1SWT. To elucidate whether mutation T1354S could affect the functional interaction of α1S and α2δ-1, the activation of the Ca2+ currents was further analyzed. As previously described the activation phase of the skeletal muscle Ca2+ current can be best described by monoexponential or double exponential functions depending on the individual recording (2, 4). In double-exponentially fitted recordings, the fast component represents a population of channels not associated with α2δ-1, whereas the slow component represents a population of channels regulated by α2δ-1 (30). Kinetic analysis showed that in both channels (α1SWT and α1ST1354S) the slow and the fast components (Aslow and Afast) contribute with comparable fractions (60 and 40% for WT and 64 and 36% for T1354S) to the total current amplitude (Fig. 4B), indicating similar interactions with α2δ-1. In contrast, both time constants (τslow and τfast) were significantly (P < 0.05) reduced (19.8 ± 3.7 ms and 3.7 ± 0.5 ms; n = 13) in α1ST1354S compared with those of α1SWT (30.4 ± 3.38 ms and 5.05 ± 0.36 ms; n = 18; Fig. 4C and Table 2). A similar trend was found with the recordings adequately fitted monoexponentially. Although not statistically significant, the time constant (τmono) showed a tendency to be shorter (P = 0.1) in α1ST1354S (24.3 ± 3.6 ms; n = 13) compared with α1SWT (35 ± 3.6 ms; n = 13; Fig. 4C and Table 2).
Fig. 4.
Interaction between CaVα1S and α2δ-1 is not affected by the T1354S mutation. A: double immunofluorescence labeling of GFP-tagged α1S (a and b, clusters indicated by arrows) and of α2δ-1 (c and d, clusters indicated by arrows) in myotubes expressing α1SWT (left) and α1ST1354S (right). Merged images (e and f) show proper colocalization of both α1S-constructs with the endogenous α2δ-1. B and C: activating phases of the Ca2+ currents from Fig. 3A were fitted either by double or monoexponential functions and corresponding contributions to the total current amplitudes (B) and time constants of activation (C) were analyzed. B: relative contribution of fast and slow fractions to total current amplitude was similar for α1SWT (white background, black hatched bars) and α1ST1354S (black background, white hatched bars). C: time constants for all slow and fast components (τslow, τfast, α1SWT, n = 18, and α1ST1354S, n = 13) were significantly reduced (*P < 0.05) in α1ST1354S-expressing (black background, white hatched bars) compared with α1SWT-expressing (white background, black hatched bars) myotubes. A similar tendency was observed for τmono (α1SWT, n = 13, and α1ST1354S, n = 13) (C).
Caffeine Sensitivity of SR Ca2+ Release in α1ST1354S-Expressing Myotubes
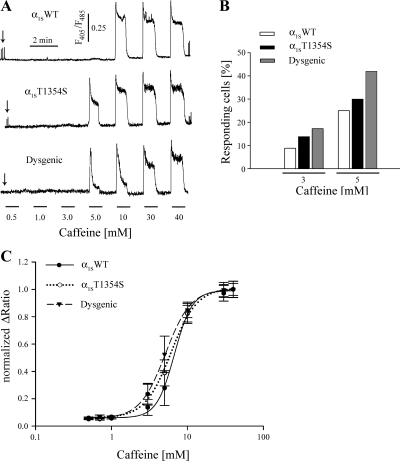
Clinical manifestations of MH episodes are associated with a nonphysiological increase of myoplasmic Ca2+ concentrations. Skeletal muscles from MHS individuals show a higher response to RyR1 activators like caffeine, halothane. and 4-chloro-m-cresol (42, 44). It has been shown that Ca2+ release induced by caffeine is downregulated by an intact interaction of RyR1 with the α1S-subunit (41). Accordingly, we considered the possibility that the T1354S mutation accounts for an increased caffeine sensitivity of RyR1. To this aim, we generated caffeine dose-response curves of α1SWT- and α1ST1354S-expressing myotubes as well as of α1S-null (dysgenic) myotubes (Fig. 5A). Before the application of varying concentrations of caffeine, three extracellular electrical pulses were applied to each cell to check for intact CaV-RyR1 coupling. As expected, no electrically evoked Ca2+ transients were observed in dysgenic myotubes. The response of myotube to 60-s sequential exposures to caffeine concentrations of 0.5, 1, 3, 5, 10, 30, and 40 mM was determined. As shown in Fig. 5B, myotubes started to respond at a 3 mM caffeine concentration. More responding cells at 3 and 5 mM caffeine were observed in cultures expressing α1ST1354S compared with cultures expressing α1SWT, however, not as many as in untransfected dysgenic myotubes (Fig. 5B).
Fig. 5.
Caffeine sensitivity of RyR1 in α1ST1354S-expressing myotubes. A: representative caffeine concentration-response curves recorded from Indo-1-loaded myotubes transfected with α1SWT (top), α1ST1354S (middle), and untransfected dysgenic myotubes (bottom). At the beginning of each trace, a train of 3 extracellular electrical stimuli was applied to elicit voltage-gated Ca2+ release (black arrows). No electrically induced Ca2+ release was observed in untransfected dysgenic myotubes. Subsequently, increasing concentrations of caffeine were applied for 60 s to each myotube separated by 60-s washing steps. B: number of cells (%) responding to caffeine concentrations of 3 and 5 mM. C: concentration dependence of caffeine-induced Ca2+ release for α1SWT (●, n = 19), α1ST1354S (○, n = 22), and dysgenic myotubes (▾, n = 21).
Accordingly, also the dose-response curve of Ca2+ release in untransfected dysgenic myotubes tended to shift towards lower caffeine concentrations compared with α1SWT-expressing myotubes. In α1ST1354S-expressing myotubes, the sensitivity of caffeine-induced Ca2+ release was closer to that of dysgenic myotubes than to α1SWT-expressing (Fig. 5C). The EC25 values that best describe the differences between the dose-response curves were 4.78 ± 0.47 mM for dysgenic, 4.6 ± 0.50 mM for α1ST1354S-expressing, and 5.34 ± 0.50 mM for α1SWT-expressing myotubes.
The averaged values of the maximal caffeine-induced Ca2+ release were similar in untransfected myotubes (0.416 ± 0.018; n = 21) and in α1ST1354S (0.426 ± 0.025; n = 22)- and α1SWT (0.424 ± 0.025; n = 19)-expressing myotubes.
Accelerated Ca2+ Current Activation Through α1ST1354S Leads to an Increase of SR Ca2+ Release in the Presence of Caffeine
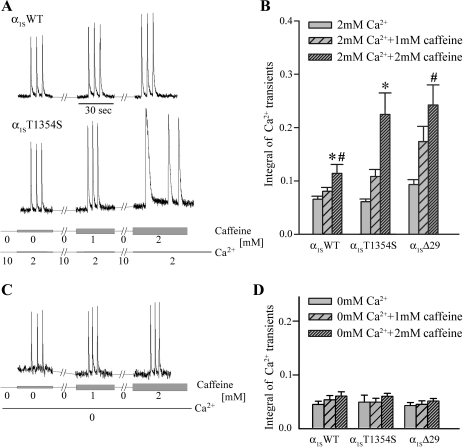
Above we showed that L-type Ca2+ currents through α1ST1354S have accelerated activation kinetics. It is to be expected that faster activation leads to higher Ca2+ influx during the time frame of a skeletal muscle action potential (3–5 ms). This correlation is reflected as a trend by integrating the early phase of L-type Ca2+ current traces through α1SWT- and α1ST1354S-expressing myotubes (Supplemental Fig. 2). Consequently, we were interested whether this higher initial Ca2+ influx leads to an increased activation of SR Ca2+ release under the influence of caffeine. To test this hypothesis, we analyzed action-potential-induced Ca2+ release induced by extracellular electrical stimuli in α1SWT- and α1ST1354S-expressing myotubes in presence of subthreshold (1 and 2 mM) concentrations of caffeine (Fig. 6). Three single electrical pulses were applied to the myotubes during sequential 30-s application of 0, 1, and 2 mM caffeine under exposure to 2 mM extracellular Ca2+ (Fig. 6A). Between each electrical stimulation phase, myotubes were incubated for 2 min with 10 mM extracellular Ca2+ to allow optimal loading of the SR. To test the potential effect of higher CaVα1S Ca2+ influx on SR Ca2+ release, we also included control experiments on myotubes expressing construct α1SΔ29, a CaVα1S splice variant with an eightfold increased current density and accelerated current activation (38). Figure 6B shows that Ca2+ influx through the distinct CaV constructs indeed correlates with intracellular Ca2+ release under caffeine trigger. In the presence of 2 mM caffeine, RyR1 Ca2+ release was significantly (P < 0.05) increased in myotubes expressing α1ST1354S (0.22 ± 0.04; n = 23) and α1SΔ29 (0.24 ± 0.04; n = 29) compared with those expressing α1SWT (0.11 ± 0.02; n = 19). Additionally, we measured action potential-induced Ca2+ release during sequential 30-s application of 0, 1, and 2 mM caffeine in Ca2+ free extracellular solution (Fig. 6C). Ca2+ release was undistinguishable in α1SWT (n = 13)-, α1ST1354S (n = 6)-, and α1SΔ29 (n = 14)-expressing myotubes also in the presence of 1 and 2 mM caffeine (Fig. 6D).
Fig. 6.
Ca2+ release induced by extracellular electrical stimuli in α1SWT- and α1ST1354S-expressing myotubes. A and C: Ca2+ transients from myotubes were elicited by 3 extracellular electrical pulses during sequential 30-s application of 0, 1, and 2 mM caffeine (indicated by gray bars of different width) in extracellular solutions containing 2 mM Ca2+ (A) and 0 mM Ca2+ (C, bottom lines). Between the pulse trains myotubes were incubated for 2 min with extracellular caffeine-free solution containing 10 mM Ca2+ for SR reloading (A). B: integral of Ca2+ transients in 2 mM extracellular Ca2+ as a measure for total Ca2+ release in response to one action potential. A trend towards higher Ca2+ release with constructs α1ST1354S (n = 23) and α1SΔ29 (n = 29) compared with α1SWT (n = 19) is observable under 1 mM caffeine and is significant under 2 mM caffeine (*P, #P < 0.05, respectively). D: integral of Ca2+ transients in 0 mM extracellular Ca2+ shows no difference between α1SWT (n = 13), α1ST1354S (n = 6), and α1SΔ29 (n = 14) in relation to different caffeine exposures.
DISCUSSION
While >178 MH-causing mutations are described for the skeletal muscle RyR1, with 29 of them functionally characterized (33), so far only two MH mutations for CaVα1S, R1086H, and R174W were identified (5, 26) with only one (R1086H) physiologically characterized (41). Here, we identified and functionally characterized a T1354S exchange in CaVα1S found in an Italian MHS family as the third MHS-related mutation in the CACNA1S gene. While the previously described mutation R174W leads to a loss of a positive charge in the IS4 voltage sensing segment and the R1086H mutation involves the intracellular linker between repeat III and IV, the newly identified T1354S mutation is located in the extracellular portion of the linker between the pore loop and the S6 transmembrane helix in homologous repeat IV. Intuitively, it might appear unlikely that a mutation in an extracellular domain could influence RyR1 Ca2+ release. However, in our experiments this mutation indeed influences the activation properties of the skeletal muscle CaV and in consequence leads to increased RyR1 Ca2+ release under caffeine exposure. Extracellular regions of voltage-dependent ion channels were already identified to be important for the regulation of inward currents. Episodic ataxia in humans has been associated with the G293R missense mutation in the N-terminal extracellular part of the S5-S6 linker of homologous repeat I of the neuronal Ca2+ channel CaVα1A (43). When heterologously expressed, mutant CaVα1AG293R leads to reduced current density without changing single channel conductance, to a shift in the current-voltage relationship to more positive potentials, and to faster inactivation (40) of Ca2+ currents. Similarly, the P601L mutation in the extracellular S5-S6 linker region in repeat II of mouse CaVα1A (11, 13) induces the tottering phenotype, a neurological disorder, characterized by seizures and ataxia. If mutant CaVα1AP601L was heterologously expressed, considerably reduced Ca2+ currents were recorded despite intact gating charges (39). This current reduction was shown to be a consequence of reduced channel open probability (11). Recently, an embryonic splice variant of CaVα1S has been identified in which the lack of exon 29 shortens the extracellular linker connecting transmembrane segments IVS3 and IVS4 (38). This splice variant is characterized by an eightfold increase in current density and a 30-mV left shift in the voltage dependence of the current. Consequently, the observed acceleration of Ca2+ inward currents induced by the T1354S mutation is not entirely unexpected.
Ca2+ currents recorded from skeletal myotubes, in which the α2δ-1-subunit was depleted display current kinetics that are very similar to the recordings obtained with α1ST1354S (30). Like in α1ST1354S-expressing myotubes, Ca2+ inward currents in α2δ-1-depleted myotubes do not show a change in maximal current density compared with wild-type myotubes but display significantly accelerated current activation. Because of these similarities and due to the fact that mutation T1354S is located in an extracellular region, potentially in the interaction domain with the extracellular portion of the α2δ-1 (17), we tested whether T1354S exchange might influence the interaction with α2δ-1. However, immunocytochemical staining revealed perfect colocalization of α1ST1354S with α2δ-1. Further, it was shown that the acceleration of Ca2+ currents in α2δ-1-depleted myotubes results from a strong reduction or even elimination of the slow component contribution to the total current amplitude, whereas no change was observed in the time constants of the individual component (30). In contrast, the recordings with α1ST1354S showed reduced time constants for both kinetic components without a change in the relative contribution of fast and slowly activating channel populations. Furthermore, the time constant of the currents, best fitted by a monoexponential function, was also reduced. Thus the number of channels with and without association to α2δ-1 does not appear to change upon expression of α1ST1354S. Instead, these data support a model that understands the acceleration of activation as an intrinsic property of CaVα1S induced by the T1354S mutation.
We propose that the acceleration of CaVα1S Ca2+ inward currents leads to an increased influx of Ca2+ during the skeletal muscle action potential. Although peak current sizes of α1ST1354S were identical to α1SWT after the current has reached its maximal amplitude in 200-ms test pulses, only the initial phase of these 200-ms is relevant for the in vivo situation, as action potentials in skeletal muscle last only a few milliseconds. In this initial phase, an accelerated current activation can greatly contribute to the total Ca2+ influx into the myotube. Indeed, integration of the first milliseconds (5, 10, or 15 ms) of the traces recorded from α1ST1354S suggested increased net Ca2+ influxes compared with α1SWT (Supplemental Fig. 2). According to our model, this elevated Ca2+ influx leads to an additional Ca2+-dependent activation of RyR1 especially after sensitization of the RyR1 under caffeine (Figs. 5 and 6) and thus induces enhanced SR Ca2+ release. Thus, while in cardiac muscle-type EC coupling the fast Ca2+ influx through CaVα1C essentially determines RyR2 Ca2+ release (3), we show here that also in skeletal muscle the Ca2+ influx through a mutated CaVα1S can amplify EC coupling, at least under the influence of RyR1 activators, to a significant extent.
We further show that RyR1 exhibits a tendency to a higher sensitivity to caffeine when it is under the control of α1ST1354S similar to the previously described CaVα1S mutation R1086H. The combination of the higher Ca2+ influx due to accelerated Ca2+ currents together with the amplification of RyR1 sensitivity for caffeine (and supposedly also for other RyR1 activators) might appropriately explain the MHS phenotype in subjects carrying the CaVα1S T1354S mutation.
The lack of an enhancing effect of the T1354S mutation on Ca2+ release in the absence of extracellular Ca2+ (Fig. 6, C and D) and the unchanged resting Ca2+ levels between α1SWT- and α1ST1354S-expressing myotubes (Supplemental Fig. 3) strengthen the hypothesis that only the combined effect of both, caffeine and enhanced Ca2+ influx affect the SR Ca2+ release in the MH phenotype and do not support the idea of a pure activating effect of the T1354S mutation on RyR1.
GRANTS
This work was supported by the Austrian Science Fund (FWF-16098-B04 to M. G. and FWF-P20059-B05 to B. E. F.), by the Medizinische Forschungsförderung Innsbruck (MFI-6180 to J. S. and MFI-2007-417 to P. T.), by the grant of MIUR-Prin 2007, Italy (20074T4MLS_002 to A. C.), by the Ministero della Salute (Roma), Convenzione CEINGE-MIUR (2000) art 5.2 (to F. S.) and Convenzione CEINGE-Regione Campania (to F. S.), and by the progetto S.co.Pe, Centro di eccellenza riconosciuto dal MIUR ex DM 11/2000.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Sandra Schleret for technical help and Dr. Paul D. Allen for the MDG cell line.
REFERENCES
- 1.Adams BA, Tanabe T, Mikami A, Numa S, Beam KG. Intramembrane charge movement restored in dysgenic skeletal muscle by injection of dihydropyridine receptor cDNAs. Nature 346: 569–572, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Avila G, Dirksen RT. Functional impact of the ryanodine receptor on the skeletal muscle L-type Ca2+ channel. J Gen Physiol 115: 467–480, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Caffrey JM. Kinetic properties of skeletal-muscle-like high-threshold calcium currents in a non-fusing muscle cell line. Pflügers Arch 427: 277–288, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Carpenter D, Ringrose C, Leo V, Morris A, Robinson RL, Halsall PJ, Hopkins PM, Shaw MA. The role of CACNA1S in predisposition to malignant hyperthermia. BMC Med Genet 10:104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carsana A, Fortunato G, De Sarno C, Brancadoro V, Salvatore F. Identification of new polymorphisms in the CACNA1S gene. Clin Chem Lab Med 41: 20–22, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Cottingham RWJ, Idury RM, Schäffer AA. Faster sequential genetic linkage computations. Am J Hum Genet 53: 252–263, 1993 [PMC free article] [PubMed] [Google Scholar]
- 8.Denborough MA, Lovell RRH. Anaesthetic deaths in a family. Lancet 276: 45, 1960 [DOI] [PubMed] [Google Scholar]
- 9.Deufel T, Golla A, Iles D, Meindl A, Meitinger T, Schindelhauer D, DeVries A, Pongratz D, MacLennan DH, Johnson KJ, Lehmann-Horn F. Evidence for genetic heterogeneity of malignant hyperthermia susceptibility. Am J Hum Genet 50: 1151–1161, 1992 [PMC free article] [PubMed] [Google Scholar]
- 10.Dirksen RT. Bi-directional coupling between dihydropyridine receptors and ryanodine receptors. Front Biosci 7: d659–670, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Doyle J, Ren X, Lennon G, Stubbs L. Mutations in the CACNL1A4 calcium channel gene are associated with seizures, cerebellar degeneration, and ataxia in tottering and leaner mutant mice. Mamm Genome 8: 113–120, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Felder E, Protasi F, Hirsch R, Franzini-Armstrong C, Allen PD. Morphology and molecular composition of sarcoplasmic reticulum surface junctions in the absence of DHPR and RYR in mouse skeletal muscle. Biophys J 82: 3144–3149, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fletcher CF, Lutz CM, O'Sullivan TN, Shaughnessy JDJ, Hawkes R, Frankel WN, Copeland NG, Jenkins NA. Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell 87: 607–617, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Flucher BE, Andrews SB, Daniels MP. Molecular organization of transverse tubule/sarcoplasmic reticulum junctions during development of excitation-contraction coupling in skeletal muscle. Mol Biol Cell 5: 1105–1118, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V. The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J Cell Biol 128: 893–904, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grabner M, Dirksen RT, Beam KG. Tagging with green fluorescent protein reveals a distinct subcellular distribution of L-type and non-L-type Ca2+ channels expressed in dysgenic myotubes. Proc Natl Acad Sci USA 95: 1903–1908, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurnett CA, De Waard M, Campbell KP. Dual function of the voltage-dependent Ca2+ channel α2/δ subunit in current stimulation and subunit interaction. Neuron 16: 431–440, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Iles DE, Lehmann-Horn F, Scherer SW, Tsui LC, Olde Weghuis D, Suijkerbuijk RF, Heytens L, Mikala G, Schwartz A, Ellis FR, Stewart AD, Deufel T, Wieringa B. Localization of the gene encoding the α2/δ-subunits of the L-type voltage-dependent calcium channel to chromosome 7q and analysis of the segregation of flanking markers in malignant hyperthermia susceptible families. Hum Mol Genet 3: 969–975, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Jurkat-Rott K, McCarthy T, Lehmann-Horn F. Genetics and pathogenesis of malignant hyperthermia. Muscle Nerve 23: 4–17, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Kugler G, Grabner M, Platzer J, Striessnig J, Flucher BE. The monoclonal antibody mAB 1A binds to the excitation-contraction coupling domain in the II-III loop of the skeletal muscle calcium cannel α1S subunit. Arch Biochem Biophys 427: 91–100, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Lathrop GM, Lalouel JM, Julier C, Ott J. Multilocus linkage analysis in humans: detection of linkage and estimation of recombination. Am J Hum Genet 37: 482–498, 1985 [PMC free article] [PubMed] [Google Scholar]
- 22.Levitt RC, Olckers A, Meyers S, Fletcher JE, Rosenberg H, Isaacs H, Meyers DA. Evidence for the localization of a malignant hyperthermia susceptibility locus (MHS2) to human chromosome 17q. Genomics 14: 562–566, 1992 [DOI] [PubMed] [Google Scholar]
- 23.MacLennan DH, Duff C, Zorzato F, Fujii J, Phillips M, Korneluk RG, Frodis W, Britt BA, Worton RG. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature 343: 559–561, 1990 [DOI] [PubMed] [Google Scholar]
- 24.McCarthy TV, Healy JM, Heffron JJ, Lehane M, Deufel T, Lehmann-Horn F, Farrall M, Johnson K. Localization of the malignant hyperthermia susceptibility locus to human chromosome 19q12–13.2. Nature 343: 562–564, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Mickelson JR, Louis CF. Malignant hyperthermia: excitation-contraction coupling, Ca2+ release channel, and cell Ca2+ regulation defects. Physiol Rev 76: 537–592, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Monnier N, Procaccio V, Stieglitz P, Lunardi J. Malignant-hyperthermia susceptibility is associated with a mutation of the α1-subunit of the human dihydropyridine-sensitive L-type voltage-dependent calcium-channel receptor in skeletal muscle. Am J Hum Genet 60: 1316–1325, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morton ME, Froehner SC. Monoclonal antibody identifies a 200-kDa subunit of the dihydropyridine-sensitive calcium channel. J Biol Chem 262: 11904–11907, 1987 [PubMed] [Google Scholar]
- 28.Morton ME, Froehner SC. The α1 and α2 polypeptides of the dihydropyridine-sensitive calcium channel differ in developmental expression and tissue distribution. Neuron 2: 1499–1506, 1989 [DOI] [PubMed] [Google Scholar]
- 29.Neuhuber B, Gerster U, Döring F, Glossmann H, Tanabe T, Flucher BE. Association of calcium channel α1S and β1a subunits is required for the targeting of β1a but not of α1S into skeletal muscle triads. Proc Natl Acad Sci USA 95: 5015–5020, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obermair GJ, Kugler G, Baumgartner S, Tuluc P, Grabner M, Flucher BE. The Ca2+ channel α2δ-1 subunit determines Ca2+ current kinetics in skeletal muscle but not targeting of α1S or excitation-contraction coupling. J Biol Chem 280: 2229–2237, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Ording H, Brancadoro V, Cozzolino S, Ellis FR, Glauber V, Gonano EF, Halsall PJ, Hartung E, Heffron JJ, Heytens L, Kozak-Ribbens G, Kress H, Krivosic-Horber R, Lehmann-Horn F, Mortier W, Nivoche Y, Ranklev-Twetman E, Sigurdsson S, Snoeck M, Stieglitz P, Tegazzin V, Urwyler A, Wappler F. In vitro contracture test for diagnosis of malignant hyperthermia following the protocol of the European MH Group: results of testing patients surviving fulminant MH and unrelated low-risk subjects. The European Malignant Hyperthermia Group. Acta Anaesthesiol Scand 41: 955–966, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Powell JA, Petherbridge L, Flucher BE. Formation of triads without the dihydropyridine receptor alpha subunits in cell lines from dysgenic skeletal muscle. J Cell Biol 134: 375–387, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson RL, Carpenter D, Shaw MA, Halsall PJ, Hopkins PM. Mutations in RYR1 in malignant hyperthermia and central core disease. Hum Mutat 27: 977–989, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Robinson RL, Monnier N, Wolz W, Jung M, Reis A, Nuernberg G, Curran JL, Monsieurs K, Stieglitz P, Heytens L, Fricker R, van Broeckhoven C, Deufel T, Hopkins PM, Lunardi J, Mueller CR. A genome wide search for susceptibility loci in three European malignant hyperthermia pedigrees. Hum Mol Genet 6: 953–961, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Sudbrak R, Golla A, Hogan K, Powers P, Gregg R, Du Chesne I, Lehmann-Horn F, Deufel T. Exclusion of malignant hyperthermia susceptibility (MHS) from a putative MHS2 locus on chromosome 17q and of the α1, β1, and γ subunits of the dihydropyridine receptor calcium channel as candidates for the molecular defect. Hum Mol Genet 2: 857–862, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Sudbrak R, Procaccio V, Klausnitzer M, Curran JL, Monsieurs K, van Broeckhoven C, Ellis R, Heyetens L, Hartung EJ, Kozak-Ribbens G, Heilinger D, Weissenbach J, Lehman-Horn F, Mueller CR, Deufel T, Stewart AD, Lunardi J. Mapping of a further malignant hyperthermia susceptibility locus to chromosome 3q13.1. Am J Hum Genet 56: 684–691, 1995 [PMC free article] [PubMed] [Google Scholar]
- 37.The European Malignant Hyperpyrexia Group A protocol for the investigation of malignant hyperpyrexia (MH) susceptibility. Br J Anaesth 56: 1267–1269, 1984 [DOI] [PubMed] [Google Scholar]
- 38.Tuluc P, Molenda N, Schlick B, Obermair GJ, Flucher BE, Jurkat-Rott K. A CaV1.1 Ca2+ channel splice variant with high conductance and voltage-sensitivity alters EC coupling in developing skeletal muscle. Biophys J 96: 35–44, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wakamori M, Yamazaki K, Matsunodaira H, Teramoto T, Tanaka I, Niidome T, Sawada K, Nishizawa Y, Sekiguchi N, Mori E, Mori Y, Imoto K. Single tottering mutations responsible for the neuropathic phenotype of the P-type calcium channel. J Biol Chem 273: 34857–34867, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Wappl E, Koschak A, Poteser M, Sinnegger MJ, Walter D, Eberhart A, Groschner K, Glossmann H, Kraus RL, Grabner M, Striessnig J. Functional consequences of P/Q-type Ca2+ channel CaV2.1 missense mutations associated with episodic ataxia type 2 and progressive ataxia. J Biol Chem 277: 6960–6966, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Weiss RG, O'Connell KMS, Flucher BE, Allen PD, Grabner M, Dirksen RT. Functional analysis of the R1086H malignant hyperthermia mutation in the DHPR reveals an unexpected influence of the III-IV loop on skeletal muscle EC coupling. Am J Physiol Cell Physiol 287: C1094–C1102, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Westerblad H, Andrade FH, Islam MS. Effects of ryanodine receptor agonist 4-chloro-m-cresol on myoplasmic free Ca2+ concentration and force of contraction in mouse skeletal muscle. Cell Calcium 24: 105–115, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Yue Q, Jen JC, Nelson SF, Baloh RW. Progressive ataxia due to a missense mutation in a calcium-channel gene. Am J Hum Genet 61: 1078–1087, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zorzato F, Scutari E, Tegazzin V, Clementi E, Treves S. Chlorocresol: an activator of ryanodine receptor-mediated Ca2+ release. Mol Pharmacol 44: 1192–1201, 1993 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.