Abstract
Recent advances have identified an important role of bone morphogenetic protein 4 (BMP4) in pulmonary vascular remodeling, yet the underlying mechanisms remain largely unexplored. We have previously found that Ca2+ influx through store-operated calcium channels (SOCC), which are mainly thought to be composed of canonical transient receptor potential (TRPC) proteins, likely contribute to the pathogenic development of chronic hypoxic pulmonary hypertension. In this study, we investigated the effect of BMP4 on expression of TRPC and store-operated Ca2+ entry (SOCE) in pulmonary arterial smooth muscle cells (PASMCs). Real-time quantitative PCR and Western blotting revealed that treatment with BMP4 (50 ng/ml, 60 h) increased TRPC1, TRPC4, and TRPC6 mRNA and protein expression in growth-arrested rat distal PASMCs. Moreover, in comparison to vehicle control, cells treated with BMP4 also exhibited enhanced SOCE, and elevated basal intracellular calcium concentration ([Ca2+]i) as determined by fluorescent microscopy using the Ca2+ indicator Fura-2 AM. Perfusing cells with Ca2+-free Krebs-Ringer bicarbonate solution (KRBS) or KRBS containing SOCC antagonists SKF-96365 or NiCl2 attenuated the increases in basal [Ca2+]i caused by BMP4. Specific knockdown of BMP4 by small interference RNA significantly decreased the mRNA and protein expression of TRPC1, TRPC4, and TRPC6 and reduced SOCE and basal [Ca2+]i in serum-stimulated PASMCs. We conclude that BMP4 regulates calcium signaling in PASMCs likely via upregulation of TRPC expression, leading to enhanced SOCE and basal [Ca2+]i in PASMCs, and by this mechanism contributes to pulmonary vascular remodeling during pulmonary arterial hypertension.
Keywords: calcium signaling, intracellular calcium concentration
bone morphogenetic proteins (BMPs) are multifunctional growth factors of the transforming growth factor-β superfamily that regulate growth and differentiation in many cell types and play remarkable roles in embryonic development, organogenesis, and adult tissue remodeling (6, 25, 27). Members of the BMP family signal through the complex of type I (i.e., Alk2, Alk3, and Alk-6) and type II (i.e., BMPRII, ActRIIa, and ActRIIb) serine/threonine kinase receptors, leading to activation of Smad-dependent and Smad-independent (e.g., ERK, JNK, and p38 MAP kinase pathways) regulation of gene expression (6, 25). Recent evidence has identified multiple abnormalities in BMP signaling associated with pulmonary arterial hypertension (PAH), a lethal complication featured by profound remodeling and sustained vasoconstriction in pulmonary vasculature (9). For example, loss-of-function germline mutations of BMPRII were found in many cases of familial and sporadic PAH (5, 20, 24, 40). Marked reduction of BMPRII expression in pulmonary vasculature was found to be associated with primary pulmonary hypertension and in animal models of pulmonary hypertension induced by monocrotaline or chronic hypoxia (1, 28, 39, 10). Increased expression of BMP ligands (e.g., BMP2, BMP4, and BMP7) or downstream SMAD and p38MAPK/ERK signaling was observed in hypoxic mouse lung (10). In the past decade, more and more effort has been devoted to understanding the role and mechanism of aberrant BMP signaling in the development of PAH.
It is generally accepted that elevation of intracellular Ca2+ concentration ([Ca2+]i) facilitates the contraction and proliferation of PASMCs and is therefore considered to be a key contributor to the pathogenesis of pulmonary hypertension (3, 26, 14). The increase in [Ca2+]i could result from the following (16, 36): 1) release of Ca2+ from internal storage sites, such as sarcoplasmic reticulum (SR); 2) influx of Ca2+ from extracellular fluid through L-type voltage-dependent Ca2+ channels (VDCC), receptor-operated Ca2+ channels, or SOCC; and/or 3) reduced efflux of Ca2+ through plasmalemmal Ca2+-ATPases and Na+/Ca2+ exchange. Ca2+ entry via SOCC, so-called store-operated Ca2+ entry (SOCE), is triggered by depletion of SR Ca2+ stores and is essential to refill Ca2+ in SR, to maintain intracellular Ca2+ homeostasis, and to elicit pulmonary vasoconstriction (30, 37, 34, 45). In pulmonary arterial smooth muscle cells (PASMCs), we and others have previously found that Ca2+ entry via SOCC other than VDCC provides an important pathway for hypoxic increases of [Ca2+]i (21, 23, 42–44). In response to growth factors, such as PDGF, proliferation of PASMCs occurs with increased SOCE and [Ca2+]i (11, 12, 38, 50). The molecular identity of SOCC is not completely understood. It is believed that SOCC are composed of a subgroup members of the transient receptor potential (TRP) superfamily, termed canonical transient receptor potential (TRPC) proteins (31). Of the seven TRPC (TRPC1–7) isoforms identified so far, we recently demonstrated that only TRPC1, TRPC4, and TRPC6 are substantially expressed in rat distal pulmonary artery smooth muscle and PASMCs (22, 41, 43).
BMP4, a member of the BMP family, was recently recognized as an important factor promoting PASMC proliferation and migration, and ultimately mediating pulmonary vascular remodeling in chronically hypoxic pulmonary hypertension (CHPH) (10); however, the detailed mechanisms remain unknown. In this study we examined the regulation of the calcium signaling by BMP4 in PASMCs. Our results indicate that BMP4 augments Ca2+ influx through SOCC and elevates basal [Ca2+]i in PASMCs likely via induction of TRPC expression, thus providing a mechanism of its contribution to the pathogenesis of PAH.
MATERIALS AND METHODS
Isolation and culture of rat distal pulmonary arterial smooth muscle cells.
Animal protocols were approved by the Animal Care and Use Committee of the Johns Hopkins Medical Institutions. Distal pulmonary arteries (PA, >4th generation) were dissected from lungs of male Wistar rats (300–500 g body wt) and anesthetized with pentobarbital sodium (65 mg/kg ip) as previously described (41). Adventitia were removed from the isolated PA, and endothelium was denuded by opening the vessel longitudinally and rubbing the luminal surface with a cotton swab. PASMCs were harvested from these vessels enzymatically and plated onto 25-mm coverslips in six-well dishes and cultured for 3–5 days in smooth muscle growth media (SMGM-2, Clonetics, Walkersville, MD) in a humidified atmosphere of 5% CO2-95% air at 37°C. Cellular purity was >95%, as assessed by morphological appearance under phase-contrast microscopy and immunofluorescence staining for α-actin.
BMP4 treatment.
PASMCs at 50–60% confluence were growth-arrested in smooth muscle basal media (SMBM, Clonetics, Walkersville, MD) containing 0.3% FBS for 24 h and then treated with 5–250 ng/ml recombinant human BMP4 (rhBMP4, R&D systems, Minneapolis, MN) for 24, 48, or 60 h, before they were subjected to Ca2+ assays or gene expression measurements.
Small interference RNA transfection.
BMP4 small interference RNA (siRNA) was designed and synthesized by Dharmacon (Lafayette, CO). It is a siGENOME SMARTpool containing four individual duplexes targeting to different areas of BMP4 mRNA (accession no. NM_012827). Nontargeting siRNA Pool (Dharmacon) served as a control. Rat distal PASMCs grown with SMGM-2 were transfected with 25 nM siRNA for 6 h in serum-free SMBM using GeneSilencer (Genlantis, San Diego, CA) according to the manufacturer's instructions, then cultured for 42 h in SMBM containing 5% FBS before being subjected to gene expression measurements, [Ca2+]i and Ca2+ influx assays. Cell viability after siRNA treatment was assessed under fluorescence confocal microscopy using a LIVE/DEAD viability/cytotoxicity reagent (Invitrogen, Carlsbad, CA).
Measurement of [Ca2+]i.
As we described previously (41), coverslips with PASMCs were loaded with 7.5 μM Fura-2 AM (Invitrogen) for 60 min at 37°C under 5% CO2-95% air, and each was mounted in a closed polycarbonate chamber clamped in a heated aluminum platform (PH-2, Warner Instruments, Hamden, CT) on the stage of a Nikon TSE 100 Ellipse inverted microscope (Nikon, Melville, NY). Cells were perfused at 0.5–1 ml/min with Krebs-Ringer bicarbonate solution (KRBS), which was composed of (in mM) 118 NaCl, 4.7 KCl, 2.5 CaCl2, 0.57 MgSO2, 1.18 KH2PO4, 25 NaHCO3, and 10 glucose, equilibrated in heated reservoirs with 5% CO2 and 16% O2 and led to the chamber through stainless steel tubing. Chamber temperature was maintained at 37°C with an in-line heat exchanger and dual-channel heater controller (models SF-28 and TC-344B, Warner Instruments). After 10 min of initial perfusion to remove extracellular dye, [Ca2+]i was measured using a xenon arc lamp, interference filters, electronic shutter, ×20 fluorescence objective, and a cooled charge-coupled device imaging camera, and determined at 30- to 60-s intervals from the ratio of Fura-2 fluorescence emitted at 510 nm with excitation at 340 nm to that with excitation at 380 nm (F340/F380). Data were collected online with InCyte software (Intracellular Imaging, Cincinnati, OH). Background images were acquired by unfocusing the imaging field. The background values were subtracted from the mean fluorescence intensities at each wavelength before calculation of the ratio values. [Ca2+]i was estimated from standard curves established with calibration solutions containing 0–1,350 nM Ca2+ (Invitrogen) and is presented as an average from 20–30 cells.
Measurement of SOCE.
PASMCs were perfused for at least 10 min with Ca2+-free KRBS containing 1 mM EGTA to chelate residual Ca2+, 5 μM nifedipine (Sigma-Aldrich, St. Louis, MO) to prevent calcium entry through L-type VDCC and 10 μM cyclopiazonic acid (CPA, Sigma-Aldrich) to deplete SR Ca2+ stores. SOCE was assessed in two ways, as described previously (22, 41). First, [Ca2+]i was measured before and after restoration of extracellular [Ca2+] to 2.5 mM. SOCE was evaluated from the peak increase in [Ca2+]i caused by restoration of extracellular Ca2+ in the continued presence of nifedipine and CPA. Second, we monitored Fura-2 fluorescence excited at 360 nm at 30-s intervals before and after addition of MnCl2 (200 μM) to the cell perfusate; SOCE was evaluated from the rate at which Fura-2 fluorescence was quenched by Mn2+, which entered the cell as a Ca2+ surrogate and reduced Fura-2 fluorescence upon binding to the dye. Fluorescence excited at 360 nm was the same for Ca2+-bound and Ca2+-free Fura-2; therefore, changes in fluorescence were caused by Mn2+ alone.
RNA extraction and real-time quantitative PCR.
Total RNA in PASMCs was extracted using RNeasy kit (Qiagen, Valencia, CA). DNA contamination in RNA preparations was removed by on-column DNase digestion using RNeasy column and RNase-free DNaseI (Qiagen). Transcript mRNA was reverse transcribed in a reaction mixture of 20 μl containing 250 ng total RNA using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA), followed by quantification with real-time PCR using QuantiTect SYBR Green PCR Master Mix (Qiagen) in a iCycler IQ (Bio-Rad) machine. The PCR reaction mixture of 25 μl was composed of 400 nM forward and reverse primers and cDNA template from 6.25 ng RNA. Primer sequences specific for rat TRPC1, TRPC4, TRPC6, BMP2, BMP4, or cyclophilin B are listed in Table 1. The program of real-time quantitative PCR consisted of a hot start at 95°C for 15 min, 45 cycles with each containing 94°C for 15 s, 57.5°C for 20 s and 72°C for 20 s, and melting curves performed at 95°C for 1 min, 55°C for 1 min, and an increment of 0.5°C for 80 repeats. Specificity of the PCR products was sequentially verified by melting curves, agarose gel electrophoresis, and DNA sequencing. Detection threshold cycle (CT) values were generated by iCyclerIQ software. PCR efficiency of each pair of primers was obtained from measurements of five-point serial dilutions of an unknown cDNA sample. Relative concentration of each transcript was calculated using the Pfaffl method (32). TRPC or BMP mRNA copies were normalized to cyclophilin B and are expressed as percent change from control values.
Table 1.
Real-time qPCR primers for rat TRPC, BMP, and cyclophilin B
| Gene | Accession No. | Primer Sequence (Left/Right) | Product Size, bp | Location in Sequence |
|---|---|---|---|---|
| TRPC1 | NM_053558 | 5′-AGCCTCTTGACAAACGAGGA-3′ | 146 | 797–942 |
| 5′-ACCTGACATCTGTCCGAACC-3′ | ||||
| TRPC4 | NM_053434 | 5′-GACACGGAGTTCCAGAGAGC-3′ | 142 | 771–912 |
| 5′-GTTGGGCTGAGCAACAAACT-3′ | ||||
| TRPC6 | NM_053559 | 5′-TACTGGTGTGCTCCTTGCAG-3′ | 141 | 1267–1407 |
| 5′-GAGCTTGGTGCCTTCAAATC-3′ | ||||
| BMP2 | NM_017178 | 5′-AAGCGTCAAGCCAAACACAAAC-3′ | 107 | 896–1002 |
| 5′-GCCACGATCCAGTCATTCCAC-3′ | ||||
| BMP4 | BC078901 | 5′-CAGAGCCAACACTGTGAGGA-3′ | 108 | 477–584 |
| 5′-GGGATGCTGCTGAGGTTAAA-3′ | ||||
| Cyclophilin B | NM_022536 | 5′-ACGGTCAGGTCATCACTATC-3′ | 118 | 170-287 |
| 5′-TGCCACAGGATTCCATACC-3′ |
qPCR, quantitative PCR; BMP, bone morphogenetic protein; TRPC, canonical transient receptor potential.
Western blot analysis.
PASMCs following various treatments were scraped and lysed in ice-cold Laemmli sample buffer containing 62.5 mM Tris·HCl (pH 6.8), 2% sodium dodecyl sulfate (SDS), 10% glycerol, 5% protease inhibitor cocktail, 1 mM EDTA, and 200 μM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride. Cell lysate proteins were quantified using the bicinchoninic acid protein assay (Pierce, Rockford, IL). BMP2 and BMP4 proteins in cell-conditioned media were pulled down using heparin-conjugated Sepharose bead and were suspended in sample buffer. Cell lysate or conditioned media proteins were denatured by adding dithiothreitol to 150 mM and heating at 95°C for 3 min and resolved by 10% SDS-PAGE. Separated proteins were transferred onto polyvinylidene difluoride membranes (pore size 0.45 μM, Bio-Rad). Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.2% Tween 20 and blotted with affinity-purified rabbit polyclonal antibodies specific for TRPC proteins, goat polyclonal antibody to BMP2, or mouse monoclonal antibodies to BMP4 or α-actin. Bound antibodies were probed with horseradish peroxidase-conjugated anti-rabbit, anti-goat, or anti-mouse IgG (Kirkegaard and Perry Laboratories, Gaithersburg, MD) and detected using an enhanced chemiluminescence system (ECL, GE healthcare, Piscataway, NJ).
Drugs and materials.
Unless otherwise specified, all reagents were obtained from Sigma-Aldrich. TRPC antibodies other than TRPC1 were obtained from Alomone Laboratories (Jerusalem, Israel). BMP2 and BMP4 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). rhBMP4 stock solutions at 50 μg/ml were made in 4 mM HCl containing 0.1% BSA. Stock solutions (30 mM) of CPA and nifedipine were made in dimethyl sulfoxide (DMSO). Fura-2 AM was prepared on the day of the experiment as a 2.5 mM stock solution in DMSO containing 20% pluronic F-127 (Invitrogen).
Statistical analysis.
Data are expressed as means ± SE, and n is the number of experiments performed, which equals the number of animals providing cells. Statistical analyses were performed using Student's t-test. Differences were considered significant when P < 0.05.
RESULTS
Treatment with BMP4 increased TRPC expression in PASMCs.
Treatments of BMP4 at 5 ng/ml, 50 ng/ml, or 250 ng/ml for 60 h dose dependently increased the mRNA expression of TRPC1, TRPC4, and TRPC6 in PASMCs (Fig. 1A). The respective inductions of these three transcripts by 50 ng/ml and 250 ng/ml BMP4 treatments were not significantly different. Therefore, the optimal dosage of BMP4 for maximal induction was considered to be 50 ng/ml, which caused increases of mRNA expression of TRPC1, TRPC4, and TRPC6 by 166.2%, 230.3%, and 180.9%, respectively (Fig. 1A). Time course study at 24 h, 48 h, and 60 h indicated that the most drastic increases of TRPC mRNA by 50 ng/ml BMP4 treatment were at 60 h (Fig. 1B). Under the optimal treatment condition (50 ng/ml, 60 h), BMP4 also increased the protein expression of TRPC1, TRPC4, and TRPC6 in PASMCs, as confirmed by Western blotting (Fig. 2).
Fig. 1.
A: expression of canonical transient receptor potential (TRPC) mRNA relative to cyclophilin B in pulmonary arterial smooth muscle cells (PASMCs) treated with bone morphogenetic protein 4 (BMP4) at 5 ng/ml, 50 ng/ml, 250 ng/ml, or vehicle (control) for 60 h as determined by real-time quantitative PCR (n = 4 in each group). B: expression of TRPC mRNA relative to cyclophilin B in PASMCs treated with 50 ng/ml BMP4 or vehicle control for 24 h, 48 h, or 60 h as determined by real-time quantitative PCR (n = 4 in each group). Data are presented as percent change from control. *P < 0.05 vs. vehicle control cells.
Fig. 2.
Representative Western blots for TRPC1, TRPC4, TRPC6, and α-actin in PASMCs treated with 50 ng/ml BMP4 or vehicle (control) for 60 h. Bar graph shows mean protein expression for TRPC1, TRPC4, and TRPC6 relative to α-actin (n = 4 on samples from 4 animals; *P < 0.05 vs. vehicle control cells). Bar values are means ± SE.
Treatment with BMP4 increased SOCE in PASMCs.
SOCE was assessed in two ways, Ca2+ restoration and Mn2+ quenching. In the Ca2+ restoration assessment, CPA given in the absence of extracellular Ca2+ and presence of nifedipine caused an initial transient increase in [Ca2+]i, indicating Ca2+ release which was not different between control PASMCs and cells treated with BMP4 (Fig. 3A). Subsequent restoration of extracellular Ca2+ induced a second increase in [Ca2+]i, which quickly achieved a peak Δ[Ca2+]i of 373.8 ± 53.7 nM (n = 4) in PASMCs with BMP4 treatment; this peak change in [Ca2+]i was greater compared with that measured in vehicle control cells (Δ[Ca2+]i 210.4 ± 36.7 nM, n = 4) (P < 0.05; Fig. 3A).
Fig. 3.
A: representative traces of intracellular Ca2+ concentration ([Ca2+]i) responses to restoration of extracellular [Ca2+] to 2.5 mM after perfusion with Ca2+-free Krebs-Ringer bicarbonate solution (KRBS; 0 Ca2+) containing cyclopiazonic acid (CPA; 10 μM), nifedipine (Nif, 5 μM), and EGTA (1 mM) in rat distal PASMCs treated with BMP4 (n = 4 experiments in 109 cells), or vehicle for 60 h (n = 4 experiments in 105 cells). Bar graph indicates that maximum increase in [Ca2+]i after restoration of extracellular [Ca2+] was greater in BMP4-treated PASMCs than that in control cells (*P < 0.001). B: time course of quenching of Fura-2 AM fluorescence at 360 nm by 200 μM Mn2+ after perfusion with Ca2+-free KRBS (0 Ca2+) containing nifedipine (5 μM) and CPA (10 μM) in rat distal PASMCs treated with 50 ng/ml BMP4 (n = 3 experiments in 88 cells), or vehicle for 60 h, normalized to fluorescence at time 0 (F/F0). Bar graph shows that Mn2+ quenching, expressed as percent decrease in fluorescence at time 10 min from time 0, was greater in BMP4-treated PASMCs compared with that in control cells (*P < 0.001). Bar values are means ± SE.
Mn2+ quenching, which is measured as the rate at which Mn2+ quenched Fura-2 fluorescence, is thought to be a more specific index of Ca2+ influx. SOCE evaluated by Mn2+ quenching and expressed as the percent decrease in fluorescence at time 10 min from time 0 of perfusion was greater in BMP4-treated PASMCs (55.0 ± 1.7%, n = 3) compared with that in vehicle control cells (30.3 ± 1.2%, n = 3; P <0.001; Fig. 3B).
Treatment with BMP4 increased basal [Ca2+]i in PASMCs.
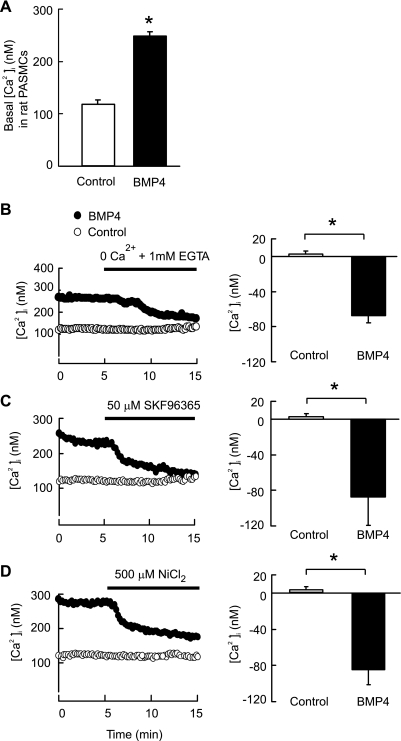
Basal [Ca2+]i was increased from 112.4 ± 7.4 nM (n = 16) in control cells exposed to vehicle (4 mM HCl with 0.1% BSA) to 255.6 ± 5.2 nM (n = 16) in PASMCs treated with 50 ng/ml BMP4 for 60 h (P < 0.001; Fig. 4A). Removal of extracellular Ca2+ by perfusion with Ca2+-free KRBS containing 1 mM EGTA for 10 min did not affect basal [Ca2+]i in control cells (n = 4); however, it caused a 75.6 ± 10.5 nM reduction of [Ca2+]i in cells treated with BMP4 (n = 4; P < 0.05 vs. control) (Fig. 4B). Moreover, perfusion with KRBS containing SOCC antagonist SKF-96365 (50 μM) or NiCl2 (500 μM) for 10 min in PASMCs treated with BMP4 decreased basal [Ca2+]i about 87.3 ± 31.7 nM (n = 4; P < 0.05 vs. control) or 84.5 ± 16.5 nM (n = 4; P < 0.05 vs. control), respectively; however, neither SKF-96365 nor NiCl2 altered basal [Ca2+]i in control cells (Fig. 4, C and D).
Fig. 4.
A: changes in basal [Ca2+]i in rat distal PASMCs treated with 50 ng/ml BMP4 (n = 16 from 365 cells) for 60 h. Bar values are means ± SE. *P < 0.001 vs. vehicle control cells (n = 16 from 358 cells). B: removal of extracellular Ca2+ reduced basal [Ca2+]i in rat distal PASMCs treated with BMP4 (50 ng/ml, 60 h) (n = 4 in 115 cells), but not in control cells treated with vehicle (n = 4 in 136 cells). C: store-operated calcium channel (SOCC) antagonist SKF-96365 and NiCl2 attenuated basal [Ca2+]i in rat distal PASMCs treated with BMP4 (50 ng/ml, 60 h) (n = 4 in 109 cells), but not in control cells treated with vehicle (n = 4 in 121 cells). D: SOCC antagonist attenuated basal [Ca2+]i in PASMCs treated with BMP4 (50 ng/m, 60 h) (n = 4 in 112 cells), but not in control cells treated with vehicle (n = 4 in 119 cells). Values are means ± SE. *Significantly different from vehicle control: P < 0.05.
Knockdown of BMP4 reduced TRPC expression, SOCE, and basal [Ca2+]i in serum-stimulated PASMCs.
In this study, we found that BMP4 is endogenously produced by serum-stimulated proliferating PASMCs; therefore it could act on cells via an autocrine mechanism (Fig. 5, A and B). To verify the above effect of BMP4 on Ca2+ signaling in PASMCs, we used siRNA targeted to BMP4 (BMP4 siRNA) to knock down BMP4 expression in the culture. The specificity of BMP4 siRNA was verified by determining the expression of BMP2, which shares high sequence homology and hence belongs to the same subgroup with BMP4 in the BMP family. As seen in Fig. 5, A and B, exposure of PASMCs to BMP4 siRNA resulted in 94.3% reduction of BMP4 mRNA and in significant decreases of BMP4 protein in conditioned media compared with that in nontargeting siRNA (NT siRNA)-treated cells. Treatment with BMP4 siRNA did not alter BMP2 expression in PASMCs, as demonstrated by real-time PCR for its mRNA (Fig. 5A), and Western blotting for its protein level in the cell conditioned media (Fig. 5B), suggesting a specific knockdown of BMP4 in PASMCs. Next, we examined the effect of decrement in BMP4 on TRPC expression, and the subsequent functional influences on SOCE and basal [Ca2+]i in rat distal PASMCs. As indicated in Fig. 5, C and D, knockdown of BMP4 by BMP4 siRNA caused significant decreases of mRNA (Fig. 5C) and protein (Fig. 5D) expression of TRPC1, TRPC4, and TRPC6 compared with that in cells treated with NT siRNA. Consequently, SOCE was decreased by 52.3% (P < 0.001, Fig. 6A) and 46.4% (P < 0.05; Fig. 6B) as measured by [Ca2+]i responses to extracellular Ca2+ restoration and Mn2+ quenching, respectively, in PASMCs treated with BMP4 siRNA. In addition, basal [Ca2+]i was reduced from 166.0 ± 7.5 nM (n = 4) in cells treated with NT siRNA to 106.9 ± 8.1 nM (n = 4) in cells treated with BMP4 siRNA (P < 0.01; Fig. 7).
Fig. 5.
A: BMP4 and BMP2 mRNA relative to cyclophilin B as determined by real-time quantitative (q)PCR in PASMCs treated with BMP4 small interference (si)RNA or nontargeting (NT) control siRNA (n = 3 for each group). *P < 0.05 vs. NT siRNA-treated cells. B: representative Western blots of the secreted BMP4 and BMP2 protein in conditioned media from PASMC cultures treated with BMP4 siRNA or NT control siRNA. C: TRPC1, TRPC4, and TRPC6 mRNA relative to cyclophilin B as determined by real-time qPCR in PASMCs treated with BMP4 siRNA or NT control siRNA (n = 3 for each group; *P < 0.05 vs. NT siRNA-treated cells). D: representative Western blots of TRPC1, TRPC4, and TRPC6 protein in PASMCs treated with BMP4 siRNA or NT control siRNA. Bar graph shows mean protein expression for TRPC1, TRPC4, and TRPC6 relative to α-actin (n = 3 for each group; *P < 0.05 vs. NT siRNA treated cells). Bar values are means ± SE.
Fig. 6.
A: representative traces of [Ca2+]i responses to restoration of extracellular [Ca2+] to 2.5 mM after perfusion with Ca2+-free KRBS (0 Ca2+) containing CPA (10 μM), nifedipine (Nifed; 5 μM), and EGTA (1 mM) in rat distal PASMCs treated with BMP4 siRNA (n = 3 experiments in 86 cells) or NT siRNA (n = 3 experiments in 87 cells). Bar graph indicates that maximum increase in [Ca2+]i after restoration of extracellular [Ca2+] was reduced in BMP4 siRNA-treated PASMCs compared with that in NT siRNA-treated cells (*P < 0.001). B: time course of quenching of Fura-2 AM fluorescence at 360 nm by 200 μM Mn2+ after perfusion with Ca2+-free KRBS (0 Ca2+) containing nifedipine (5 μM) and CPA (10 μM) in rat distal PASMCs treated with BMP4 siRNA (n = 3 experiments in 83 cells), or NT siRNA, normalized to fluorescence at time 0 (F/F0). Bar graph shows that Mn2+ quenching, expressed as percent decrease in fluorescence from time 0, was reduced in BMP4 siRNA-treated PASMCs compared with that in NT siRNA-treated cells (*P < 0.05). Bar values are means ± SE.
Fig. 7.
Changes in basal [Ca2+]i in rat distal PASMCs treated with BMP4 siRNA (n = 3 from 88 cells) or NT siRNA. Bar values are means ± SE. *P < 0.01 vs. NT siRNA-treated cells (n = 3 from 89 cells).
DISCUSSION
This study presents important evidence indicating that BMP4 acts as a regulator of Ca2+ signaling in PASMCs.
First of all, we found that treatment with BMP4 increased TRPC1, TRPC4, and TRPC6 expression in growth-arrested PASMCs. The function of TRPC1, TRPC4, and TRPC6 proteins has been mainly interpreted as components of SOCC, mediating SOCE, thus regulating diverse cellular activity, such as proliferation, migration, and contraction in response to various stimuli. Upregulated TRPC1, TRPC4, and TRPC6 expression was found to correlate with increased SOCE in proliferate PASMCs (12, 38). Overexpression of human TRPC1 enhanced contractile responses to CPA in rat pulmonary arterial rings (18). PASMCs treated with PDGF or ATP exhibited an increase in TRPC6 and TRPC4 expression, correlating with enhanced SOCE (50, 53). In contrast, reduction of TRPC1, TRPC4, or TRPC6 with antisense oligonucleotide, siRNA, bosentan, or sildenafil resulted in attenuated amplitude of SOCE induced by mitogen or hypoxia in human or rat PASMCs (17, 23, 38, 50, 53).
If TRPC1, TRPC4, and TRPC6 are indeed components of SOCC in PASMCs, the increased expression of these Ca2+ channel proteins could increase the number of SOCC and lead to enhanced SOCE in cells treated with BMP4. Therefore, we next tested whether treatment with BMP4 indeed influenced SOCE in PASMCs. Measurement of SOCE via [Ca2+]i responses to extracellular Ca2+ restoration following passive store depletion of intracellular Ca2+ stores with CPA revealed a greater response in BMP4-treated PASMCs. Because the increase in [Ca2+]i following Ca2+ restoration can be influenced by both Ca2+ influx through SOCC and Ca2+ efflux, we also used Mn2+ quenching of fluorescence excited at 360 nm (F360) as a more direct evaluation of Ca2+ entry. Following store depletion with CPA, Mn2+ caused significantly greater quenching of F360 in PASMCs from BMP4 treatment compared with that in cells from vehicle control treatment. The increased amplitude of [Ca2+]i following restoration of extracellular Ca2+ and faster rate of Mn2+ quenching of F360 in cells from BMP4 treatment indicate enhanced Ca2+ influx through SOCC. One possible explanation for this increased SOCE is that it results from an increased number of SOCC due to increased expression of TRPC. Another possibility is a greater degree of store depletion; however, the increase of [Ca2+]i in response to CPA was not significantly different in vehicle and BMP4-treated PASMCs, suggesting equal depletion of stored Ca2+. Hence, differences in store depletion do not account for enhanced SOCE caused by BMP4. Our data suggest that the enhanced SOCE in cells treated with BMP4 could indeed be due to greater number of SOCC. Finally, we found that BMP4 treatment enhanced basal [Ca2+]i in PASMCs. This increase in basal [Ca2+]i was abolished by removal of extracellular Ca2+ or perfusing cells with SOCC inhibitor SKF-96365 (50 μmol/l) or NiCl2 (500 μmol/l) at concentrations that inhibited SOCE by >80% (41), demonstrating the requirement of Ca2+ influx through SOCC.
Contrary to the upregulatory effect of BMP4, knockdown of endogenous BMP4 expression in these cells by specific siRNA resulted in reduced expression of TRPC1, TRPC4, and TRPC6 and consequently reduced SOCE and basal [Ca2+]i in proliferating rat PASMCs stimulated with serum, confirming the regulatory role of BMP4 in expression of TRPC and Ca2+ signaling in PASMCs.
Substantial evidence indicates that the rise of [Ca2+]i is an important trigger for pulmonary vascular medial hypertrophy by stimulating PASMC proliferation and migration (2, 33). Mitogenic stimulation of PASMCs leads to elevated [Ca2+]i by triggering Ca2+ release from the SR and Ca2+ influx through SOCC (2, 4, 7, 14, 15, 30, 34, 54). Removal of extracellular Ca2+ and chelation of intracellular Ca2+ markedly inhibited PASMC proliferation induced by serum or growth factors, e.g., PDGF (50, 38). While other BMPs, such as BMP2 and BMP7, seem to be mainly apoptotic or inhibitory to PASMC proliferation, BMP4 was found to stimulate proliferation and migration of mouse PASMCs, and human cells from peripheral arteries (8, 10, 13, 19, 29, 47, 51). Heterozygous BMP4-null mice were absent of hypoxic increases of BMP4 expression and protected from hypoxia-induced vascular smooth muscle cell proliferation and vascular remodeling during development of CHPH (10). Our findings of the enhancement of expression of TRPC1, TRPC4, and TRPC6 by BMP4, and, consequently, basal [Ca2+]i elevation in PASMCs, suggest that the BMP4 regulation of PASMC proliferation and pulmonary vascular remodeling is likely mediated through increases in basal [Ca2+]i. In contrast to the enhancive effects of BMP4, there were also other observations indicating that BMP4 is anti-proliferative or apoptotic to PASMCs (19, 48). The contradictory effects of BMP4 in PASMCs from different studies likely reflect that the specific effect of BMP4 varies depending on the functional receptors it primarily binds to when tested in cells from different anatomic location, or the system the culture takes place.
In common with other BMPs, BMP4 transduces its signals though type I and type II receptors, leading to the activation of Smad and MAPK pathways (47, 48). Normally, BMP receptor 2 (BMPR2) is the major type II receptor that responds to BMP4. Studies have suggested that ablation of BMPR2 may facilitate the binding of BMP4 to other type II receptors such as ActRII through the utilization of coreceptor repulsive guidance molecule RGMa and lead to attenuated activation of Smad1/5, without disrupting the activation of p38MAPK and ERK1/2 in PASMCs (35, 46, 47). Activation of Smad signaling has been confirmed to be associated with growth inhibition and apoptosis, while activation of p38MAPK/ERK leads to enhanced proliferation of PASMCs (19, 47, 48). Further study is needed to validate whether our data obtained in cell cultures are relevant to reality in vivo with respect to the development of CHPH, and to further elucidate the signaling pathways involved in the upregulation of TRPC by BMP4, particularly in cells anchored with loss-of-function BMPR2 mutant.
In summary, this is the first report, to our knowledge, to show that BMP4 regulates TRPC expression, SOCE, and basal [Ca2+]i in PASMCs. These effects could provide a mechanism of increased contraction and proliferation of PASMCs in the pathogenesis of PAH, such as CHPH, in which the expression of BMP4 ligand was found to be upregulated.
GRANTS
This work was supported by National Institutes of Health Research Grants R01-HL-093020 and K02-HL-079981 (to J. Wang), American Lung Association of Maryland (to J. Wang), National Natural Science Foundation of China (30770953, 81070043, 81071917), Chinese Central Government Key Research Projects of the 973 grants (2009CB522107), Changjiang Scholars and Innovative Research Team in University grant (to J. Wang), Guangdong Department of Science and Technology of China (2009B050700041), Guangdong Natural Science Foundation Team Grant (to W. Lu), and Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2008, to J. Wang), China.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Drs. J. T. Sylvester and L. Shimoda for constructive discussion during the study of this manuscript.
REFERENCES
- 1.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 105: 1672–1678, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ. Calcium signalling and cell proliferation. Bioessays 17: 491–500, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Boulay G, Zhu X, Peyton M, Jiang M, Hurst R, Stefani E, Birnbaumer L. Cloning and expression of a novel mammalian homolog of Drosophila transient receptor potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. J Biol Chem 272: 29672–29680, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, Hodge SE, Knowles JA. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 67: 737–744, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425: 577–584, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Doi S, Damron DS, Horibe M, Murray PA. Capacitative Ca2+ entry and tyrosine kinase activation in canine pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 278: L118–L130, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Dorai H, Vukicevic S, Sampath TK. Bone morphogenetic protein-7 (osteogenic protein-1) inhibits smooth muscle cell proliferation and stimulates the expression of markers that are characteristic of SMC phenotype in vitro. J Cell Physiol 184: 37–45, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Eickelberg O, Morty RE. Transforming growth factor beta/bone morphogenic protein signaling in pulmonary arterial hypertension: remodeling revisited. Trends Cardiovasc Med 17: 263–269, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Frank DB, Abtahi A, Yamaguchi DJ, Manning S, Shyr Y, Pozzi A, Baldwin HS, Johnson JE, de Caestecker MP. Bone morphogenetic protein 4 promotes pulmonary vascular remodeling in hypoxic pulmonary hypertension. Circ Res 97: 496–504, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Golovina VA. Cell proliferation is associated with enhanced capacitative Ca2+ entry in human arterial myocytes. Am J Physiol Cell Physiol 277: C343–C349, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol 280: H746–H755, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, Rabinovitch M. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest 118: 1846–1857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyvelin JM, Guibert C, Marthan R, Savineau JP. Cellular mechanisms and role of endothelin-1-induced calcium oscillations in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 275: L269–L282, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha1-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res 88: 325–332, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, Miyamoto S, Nakazawa H, Won KJ, Sato K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev 49: 157–230, 1997 [PubMed] [Google Scholar]
- 17.Kunichika N, Landsberg JW, Yu Y, Kunichika H, Thistlethwaite PA, Rubin LJ, Yuan JX. Bosentan inhibits transient receptor potential channel expression in pulmonary vascular myocytes. Am J Respir Crit Care Med 170: 1101–1107, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Kunichika N, Yu Y, Remillard CV, Platoshyn O, Zhang S, Yuan JX. Overexpression of TRPC1 enhances pulmonary vasoconstriction induced by capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 287: L962–L969, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Lagna G, Nguyen PH, Ni W, Hata A. BMP-dependent activation of caspase-9 and caspase-8 mediates apoptosis in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 291: L1059–L1067, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, 3rd, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat Genet 26: 81–84, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Lu W, Wang J, Shimoda LA, Sylvester JT. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 295: L104–L113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu W, Ran P, Zhang D, Peng G, Li B, Zhong N, Wang J. Sildenafil inhibits chronically hypoxic upregulation of canonical transient receptor potential expression in rat pulmonary arterial smooth muscle. Am J Physiol Cell Physiol 298: C114–C123, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Machado RD, Aldred MA, James V, Harrison RE, Patel B, Schwalbe EC, Gruenig E, Janssen B, Koehler R, Seeger W, Eickelberg O, Olschewski H, Elliott CG, Glissmeyer E, Carlquist J, Kim M, Torbicki A, Fijalkowska A, Szewczyk G, Parma J, Abramowicz MJ, Galie N, Morisaki H, Kyotani S, Nakanishi N, Morisaki T, Humbert M, Simonneau G, Sitbon O, Soubrier F, Coulet F, Morrell NW, Trembath RC. Mutations of the TGF-beta type II receptor BMPR2 in pulmonary arterial hypertension. Hum Mutat 27: 121–132, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev 14: 627–644, 2000 [PubMed] [Google Scholar]
- 26.McDaniel SS, Platoshyn O, Wang J, Yu Y, Sweeney M, Krick S, Rubin LJ, Yuan JX. Capacitative Ca2+ entry in agonist-induced pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol 280: L870–L880, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Morrell NW. Pulmonary hypertension due to BMPR2 mutation: a new paradigm for tissue remodeling? Proc Am Thorac Soc 3: 680–686, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Morty RE, Nejman B, Kwapiszewska G, Hecker M, Zakrzewicz A, Kouri FM, Peters DM, Dumitrascu R, Seeger W, Knaus P, Schermuly RT, Eickelberg O. Dysregulated bone morphogenetic protein signaling in monocrotaline-induced pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 27: 1072–1078, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Nakaoka T, Gonda K, Ogita T, Otawara-Hamamoto Y, Okabe F, Kira Y, Harii K, Miyazono K, Takuwa Y, Fujita T. Inhibition of rat vascular smooth muscle proliferation in vitro and in vivo by bone morphogenetic protein-2. J Clin Invest 100: 2824–2832, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng LC, Gurney AM. Store-operated channels mediate Ca2+ influx and contraction in rat pulmonary artery. Circ Res 89: 923–929, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev 85: 757–810, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Remillard CV, Yuan JX. TRP channels, CCE, and the pulmonary vascular smooth muscle. Microcirculation 13: 671–692, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Robertson TP, Hague D, Aaronson PI, Ward JP. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol 525: 669–680, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudarakanchana N, Flanagan JA, Chen H, Upton PD, Machado R, Patel D, Trembath RC, Morrell NW. Functional analysis of bone morphogenetic protein type II receptor mutations underlying primary pulmonary hypertension. Hum Mol Genet 11: 1517–1525, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Shimoda LA, Wang J, Sylvester JT. Ca2+ channels and chronic hypoxia. Microcirculation 13: 657–670, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Snetkov VA, Aaronson PI, Ward JP, Knock GA, Robertson TP. Capacitative calcium entry as a pulmonary specific vasoconstrictor mechanism in small muscular arteries of the rat. Br J Pharmacol 140: 97–106, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 283: L144–L155, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Takahashi H, Goto N, Kojima Y, Tsuda Y, Morio Y, Muramatsu M, Fukuchi Y. Downregulation of type II bone morphogenetic protein receptor in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 290: L450–L458, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliott GC, Ward K, Yacoub M, Mikhail G, Rogers P, Newman J, Wheeler L, Higenbottam T, Gibbs JS, Egan J, Crozier A, Peacock A, Allcock R, Corris P, Loyd JE, Trembath RC, Nichols WC. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J Med Genet 37: 741–745, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L848–L858, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Shimoda LA, Weigand L, Wang W, Sun D, Sylvester JT. Acute hypoxia increases intracellular [Ca2+] in pulmonary arterial smooth muscle by enhancing capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 288: L1059–L1069, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res 98: 1528–1537, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Weigand L, Sylvester J, Shimoda L. Enhanced capacitative Ca2+ entry contributes to elevated resting Ca2+ and tension in pulmonary arterial smooth muscle from rats exposed to chronic hypoxia (Abstract). Am J Respir Crit Care Med 169: A400, 2004 [Google Scholar]
- 45.Weigand L, Foxson J, Wang J, Shimoda LA, Sylvester JT. Inhibition of hypoxic pulmonary vasoconstriction by antagonists of store-operated Ca2+ and nonselective cation channels. Am J Physiol Lung Cell Mol Physiol 289: L5–L13, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Xia Y, Yu PB, Sidis Y, Beppu H, Bloch KD, Schneyer AL, Lin HY. Repulsive guidance molecule RGMa alters utilization of bone morphogenetic protein (BMP) type II receptors by BMP2 and BMP4. J Biol Chem 282: 18129–18140, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Yang X, Long L, Southwood M, Rudarakanchana N, Upton PD, Jeffery TK, Atkinson C, Chen H, Trembath RC, Morrell NW. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res 96: 1053–1063, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Yu PB, Deng DY, Beppu H, Hong CC, Lai C, Hoyng SA, Kawai N, Bloch KD. Bone morphogenetic protein (BMP) type II receptor is required for BMP-mediated growth arrest and differentiation in pulmonary artery smooth muscle cells. J Biol Chem 283: 3877–3888, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA 101: 13861–13866, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol 284: C316–C330, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Zhang S, Fantozzi I, Tigno DD, Yi ES, Platoshyn O, Thistlethwaite PA, Kriett JM, Yung G, Rubin LJ, Yuan JX. Bone morphogenetic proteins induce apoptosis in human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 285: L740–L754, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Zhang S, Patel HH, Murray F, Remillard CV, Schach C, Thistlethwaite PA, Insel PA, Yuan JX. Pulmonary artery smooth muscle cells from normal subjects and IPAH patients show divergent cAMP-mediated effects on TRPC expression and capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 292: L1202–L1210, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Zhang S, Remillard CV, Fantozzi I, Yuan JX. ATP-induced mitogenesis is mediated by cyclic AMP response element-binding protein-enhanced TRPC4 expression and activity in human pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 287: C1192–C1201, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Zhang WM, Yip KP, Lin MJ, Shimoda LA, Li WH, Sham JS. ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors. Am J Physiol Lung Cell Mol Physiol 285: L680–L690, 2003 [DOI] [PubMed] [Google Scholar]