Abstract
Ambient temperature and physical activity modulate bone elongation in mammals, but mechanisms underlying this plasticity are a century-old enigma. Longitudinal bone growth occurs in cartilaginous plates, which receive nutritional support via delivery of solutes from the vasculature. We tested the hypothesis that chronic exercise and warm temperature promote bone lengthening by increasing solute delivery to the growth plate, measured in real time using in vivo multiphoton microscopy. We housed 68 weanling female mice at cold (16°C) or warm (25°C) temperatures and allowed some groups voluntary access to a running wheel. We show that exercise mitigates the stunting effect of cold temperature on limb elongation after 11 days of wheel running. All runners had significantly lengthened limbs, regardless of temperature, while nonrunning mice had shorter limbs that correlated with housing temperature. Tail length was impacted only by temperature, indicating that the exercise effect was localized to limb bones and was not a systemic endocrine reaction. In vivo multiphoton imaging of fluoresceinated tracers revealed enhanced solute delivery to tibial growth plates in wheel-running mice, measured under anesthesia at rest. There was a minimal effect of rearing temperature on solute delivery when measured at an intermediate room temperature (20°C), suggesting that a lasting increase in solute delivery is an important factor in exercise-mediated limb lengthening but may not play a role in temperature-mediated limb lengthening. These results are relevant to the study of skeletal evolution in mammals from varying environments and have the potential to fundamentally advance our understanding of bone elongation processes.
Keywords: bone, cartilage, nutrient supply, phenotypic plasticity, multiphoton microscopy
assessment of limb length variation among mammals, including humans, requires an understanding of the extent to which a growing bone can plastically respond to its external environment. Although bones have an intrinsic growth potential (37, 66), extrinsic variables, such as ambient temperature and physical activity, can modulate the skeleton's genetic blueprint and elicit permanent changes in limb length. Warm temperature and exercise promote longer limb growth, whereas relatively cooler temperatures and less active behavior produce shorter limbs (6, 11, 80, 83, 90, 106); the mechanisms by which they do so have remained elusive for over a century.
Longitudinal growth occurs at the ends of long bones in cartilaginous growth plates (35). Unlike many other organs, growth plate cartilage does not have a penetrating blood supply (20). Growth plates receive nutritional support via delivery of solutes from the vasculature located in and around the adjacent bone (17, 105). Exercise and temperature can alter the volume and velocity of blood flow to bone (43, 77, 80, 83, 91), but it is unclear how these changes translate to growth plates where nutrients are transferred from the bloodstream. Developments in imaging technology offer a new opportunity to investigate this problem with the advent of methods for measuring solute delivery to growth plate cartilage in vivo (36, 82, 102). These tools are a key advancement in skeletal biology research because they allow us to revisit many long-standing research questions about how environmental variables, such as ambient temperature and voluntary exercise, influence bone elongation in mammals.
Exercise and Temperature Effects on Limb Elongation
As early as 1896, Beyer (12) noted enhanced linear growth in young Navy cadets who underwent regimented gymnasium exercise compared with cadets under normal conditions. Adams (2) later found that adult women who performed manual labor during adolescence were taller than their nonlaboring peers. Many others have since reported a positive relationship between increased activity and increased bone length in humans (21, 62) and animals in natural and controlled experimental contexts (53, 59, 90). These findings are not limited to mammals, as Losos and colleagues (60) were able to experimentally induce longer limb growth in lizards by varying their perch size during growth. They found that this within-generation increase in limb length parallels the morphology of natural-dwelling lizards from different habitats (60, 61). At the other extreme, lower activity levels result in decreased limb length (11, 13, 93, 106), similar to an effect of low rearing temperature.
Sumner (85) first documented the impact of ambient temperature on extremity size in laboratory mice over a century ago. Subsequent experimental work has clearly demonstrated that warm rearing temperature increases, and cold decreases, limb elongation (3, 6, 48, 49, 80, 99). This interesting pattern closely resembles the ecogeographical trend, described in “Allen's rule,” that extremities are shorter in animals naturally occupying colder climates (4).
Role of the Vasculature in Exercise- and Temperature-Modulated Growth
Evidence suggests that environmental temperature and exercise may modify extremity growth by inducing changes in limb vasculature (17, 94). Processes of bone elongation depend on the delivery of nutrients and hormones from the bloodstream (17, 63, 94). High temperature, exercise, and stimulated muscle contractions increase blood supply to bone (38, 43, 56, 59, 63, 64, 77, 91), while cold temperature and muscle disuse decrease bone perfusion (14, 20, 25, 42, 43, 45, 77, 80, 87). Increased or decreased blood supply can enhance (17–19, 68, 95) or restrict (16, 46, 89) bone elongation, respectively, suggesting that some of the growth effects common to activity and temperature could stem from changes in the vasculature. The purpose of this study is to test the hypothesis that chronic wheel-running exercise and warm rearing temperature promote bone lengthening by increasing solute delivery to cartilage growth plates.
Cold Rearing Temperature as a Natural Experimental Model
Cold rearing temperature presents a unique model to evaluate the interaction between exercise, solute delivery, and limb elongation. Previous work has shown that low rearing temperature causes a marked reduction in limb length and vascular supply in the absence of pharmacological or surgical intervention and with no impact on body mass (78, 80). This creates a well-suited model to assess the growth-promoting effects of exercise beyond those that might occur within the normal constraints of physiology and genetics (57). By simply lowering ambient temperature, the potential of a direct impact of exercise on bone length and vascular supply can be tested in a model where these factors are naturally reduced. The hypothesis that solute delivery mediates the exercise-induced increase in limb elongation predicts that growth plate solute delivery and limb length should be increased by exercise in the cold. The cold phenotype should be at least partially reversed by exercise.
Growth Plate Imaging Using Multiphoton Microscopy
Measurement of solute delivery to skeletal growth plates of a living animal is a nontrivial technological challenge. Classic in vivo bone and cartilage imaging is conducted on a macroscopic level and lacks the resolution required for visualization of the growth plate in detail (22, 34, 107). High-resolution methods preserve excellent cellular detail and vascular structure in postmortem samples, but with a loss of physiological integrity (30, 41, 51, 104). To bridge these dynamic and static approaches, our laboratory developed a novel platform for imaging tibial growth plates of live young mice using multiphoton microscopy (MPM) (36, 102). This three-dimensional resolved form of microscopy renders optical sections of tissues from a live intact animal in unprecedented detail (31, 98, 110). We are the first group to use these pioneering methods to obtain high-resolution images up to 150 μm deep into the growth plate of a living animal with all surrounding vasculature intact (36, 82, 102).
We employ the distinct powers of MPM to measure solute delivery to cartilage in vivo by imaging fluorescent tracers as they enter the tibial growth plate from the vasculature in real time. We follow a rigorous protocol to quantify tracer amount based on fluorescence intensity at a defined focal depth, and we standardize growth plate intensities to those in the vasculature to enable comparisons between animals (82). Among the key advantages of this system is second-harmonic generation from collagen, a unique feature of nonlinear microscopy that enables identification of the perichondrium as a surrounding landmark of the growth plate (103, 109). Localization of the perichondrium is critical, because it allows consistent imaging in a defined growth plate region at a specified depth. The relatively long-wavelength excitation of the multiphoton laser alleviates tissue scattering and damage, allowing visualization of cellularly resolved detail 150 μm into the growth plate (36, 102). The integrity of the intact animal is maintained by examination of these skeletal tissues in vivo, enabling the assessment of responses to experimental conditions in real time (82).
We previously described a protocol that used an acute temperature model of warming a single limb to examine the effect of short-term (≤4 h) temperature exposure on solute delivery to tibial growth plates of live young mice (82). Here we apply this established methodology to study the impact of chronic temperature exposure and voluntary wheel-running exercise in mice over an 11-day period. We define chronic as continuous treatment over multiple days, as opposed to acute exposure, which lasts only several minutes or hours. We tested the hypothesis that exercise and warm rearing temperature promote bone lengthening by increasing solute delivery to the growth plate. Solute delivery is quantitatively defined here by the total amount of intravenous tracer entry in mouse tibial growth plates, analyzed using in vivo multiphoton imaging (82). Relative availability of this low-molecular-weight tracer is expected to be proportional to that of soluble nutrients and essential growth factors from the bloodstream. We housed weanling mice at warm (25°C) or cold (16°C) temperatures, with and without access to a running wheel, during an 11-day period that is characterized by exceptionally rapid growth in mice (8, 54, 78, 81). We tested three a priori predictions: 1) cold rearing temperature decreases solute delivery to the growth plate; 2) exercise increases solute delivery to the growth plate; and 3) exercise rescues the cold limb phenotype.
MATERIALS AND METHODS
Animals and Treatment
All procedures were submitted to and approved by the Institutional Animal Care and Use Committee at Cornell University (protocol 2007-0179). Female C57BL/6J mice (n = 68) were received from Jackson Laboratories at 21 days of age. After a 72-h shipping acclimation period, 24-day-old mice were individually caged and randomly assigned to four experimental groups (Table 1): warm (25°C) wheel (WW), warm no wheel (WN), cold (16°C) wheel (CW), and cold no wheel (CN). We previously showed that temperature has a gradient-like effect on limb lengthening (78, 80); therefore, rather than designating one specific temperature as “control,” we emphasize the cold-warm differential. We do, however, suggest that warm temperature without exercise can generally be considered the standard with which the other groups are compared, since this reflects normal animal housing conditions.
Table 1.
Body mass, quadriceps mass, and long bone lengths after 11 days of treatment
| Warm (25°C) |
Cold (16°C) |
||||
|---|---|---|---|---|---|
| Wheel (n = 15) | No wheel (n = 16) | Wheel (n = 16) | No wheel (n = 15) | Pairwise Differences* | |
| Body mass, g | 15.90 (0.92) | 15.15 (0.81) | 16.31 (0.68) | 15.36 (0.85) | WW > WN |
| CW > WN | |||||
| CW > CN | |||||
| Quadriceps mass, g | 0.103 (0.01) | 0.085 (0.01) | 0.096 (0.01) | 0.084 (0.01) | WW > WN |
| WW > CN | |||||
| CW > WN | |||||
| CW > CN | |||||
| Femur length, mm | 12.88 (0.20) | 12.71 (0.18) | 12.82 (0.21) | 12.58 (0.18) | WW > WN |
| WW > CN | |||||
| CW > CN | |||||
| Tibia length, mm | 15.45 (0.34) | 15.30 (0.26) | 15.46 (0.32) | 15.19 (0.21) | WW > CN |
| CW > CN | |||||
| Humerus length, mm | 10.07 (0.14) | 9.97 (0.12) | 10.14 (0.21) | 10.02 (0.15) | CW > WN |
| Radius length, mm | 9.90 (0.11) | 9.83 (0.15) | 9.85 (0.24) | 9.62 (0.63) | WW > CN |
| WN > CN | |||||
| CW > CN | |||||
Values are means (SD). WW, warm wheel; WN, warm no wheel; CW, cold wheel; CN, cold no wheel.
P < 0.05 by 1-way ANOVA and Tukey's honestly significant difference test.
Female mice were selected, because they are known for reliable exercise wheel usage, running farther and longer than their male counterparts (5). Treatments lasted for 11 days, and mice were euthanized by anesthesia overdose after multiphoton imaging at 35 days of age. This was the end point, because 5 wk is the optimal age for in vivo imaging (36, 102). All mice were housed in standard plastic cages with corn cob bedding, 12:12-h light-dark cycle, and ad libitum access to food and water.
Mice in the cold temperature groups were housed in a 26-ft3 commercial refrigerator (model MK-26GD, Koolaire) modified at Cornell University to provide an operating temperature of 16°C and approximately four air changes per minute via connection to in-house ventilation. Temperature was regulated using a refrigeration temperature controller (model T4031, Honeywell) and monitored using a wireless thermometer/hygrometer (model RMS300, Oregon Scientific). The refrigeration unit had a glass panel front door and was equipped with a fluorescent lamp to provide the same light quantity as the warm-temperature mice housed in the same room.
The exercise groups were provided continuous free access to running wheels (Fast-Trac, Bio-Serv) equipped with digital bicycle counters (model BC-906, Sigma Sport) to assess wheel usage. Wheels were fitted with high-power magnets (Power Magnet, Sigma Sport) that could be positioned up to 20 mm away from the counter, enabling placement of the counting device outside the cage. Since the mouse wheels (471 mm) were smaller than the minimum wheel size entry accepted in the cycle computer, wheel circumference was set to 1,413 mm, and output data were corrected by 1/3 to obtain actual distance run in kilometers.
In Vivo Multiphoton Imaging
We followed our previously described methods for measuring solute delivery in growth plates using MPM (82). We quantitatively assessed rates of entry and movement of systemically delivered fluorescent tracers from the vasculature into the proximal tibial growth plate of 35-day-old mice. All animals were subjected to identical conditions (39) of anesthesia (1.5% isoflurane) and room temperature (20°C), regardless of prior wheel or temperature treatment (see Caveats and Technical Considerations).
Tibial growth plates were exposed through a minor surgical incision in the superficial fascia of the biceps femoris and gastrocnemius muscles between the medial collateral ligament and saphenous vessels, as previously described (36, 82). For water-immersion imaging using a ×20/0.95 numerical aperture objective (Olympus XLUMPlanFl), limbs were bathed in lactated Ringer solution that was warmed to 34°C to maintain physiological limb temperature (10), while body core and respiration were held constant at 36°C and 120 breaths/min. After identification of a standard imaging depth in the growth plate (50 μm below the deepest edge of the perichondrium), mice were given an intracardiac injection of fluorescein tracer (50 μl, 0.5%), and time-lapse multiphoton images (880 nm wavelength) were captured every ∼6 s spanning 10 min. Image collection did not continue beyond 10 min, because our prior work (36, 82) showed that the fluorescein accumulation curves plateau by ∼8 min after injection. We collected data beyond this point until 10 min to verify that the growth plate and surrounding vasculature were fully saturated.
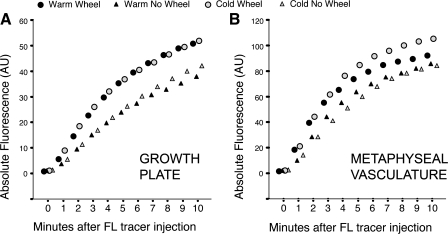
After in vivo imaging was completed, all image processing and subsequent analyses were done blind to group to prevent experimenter bias. Fluorescence was quantified in sample regions within the growth plate and vasculature at 1-min intervals using ImageJ (version 1.37, National Institutes of Health) and was evaluated at time points midway (5 min) and at the end (10 min) of the series following our published methods (82). To facilitate interanimal comparisons, fluorescence in the growth plate was standardized to peak fluorescence in the metaphyseal vasculature by calculation of a ratio of average growth plate fluorescence to average vascular fluorescence measured 8 min after tracer injection (82). This time point marked a plateau in the accumulation curves among the groups (see Fig. 3B), ensuring that peak levels in the vascular compartment had been attained across groups.
Fig. 3.
Timed accumulation curves of fluorescein (FL) tracer in the growth plate and metaphyseal vasculature. A: exercise significantly increased FL arrival and entry into the growth plate. Within 5 min after tracer injection, absolute FL concentrations were ∼1.5-fold greater in the growth plates of the wheel-running than nonrunning groups, independent of temperature. B: there was also more tracer in the adjacent metaphyseal vasculature of the wheel-running groups: >1.3 times more FL had accumulated in the metaphyseal region of the runners by 5 min (temperatures pooled), indicating enhanced tracer delivery into and around the growth plates of exercised mice. These differences in absolute values, however, were not statistically significant because of within-group variation. Group means are shown without error bars to facilitate viewing.
Morphometry
Body mass and tail length were recorded for each mouse at the start (24 days) and end (35 days) of the treatment period following methods from a prior study (78). Quadriceps muscles were dissected from the right femur after death and weighed to the nearest 0.001 g following published methods (47). Long bones were dissected and measured from digital images acquired using a flatbed scanner (Perfection V30, Epson) following published methods (108) (Fig. 1). As with image processing, all postmortem measurements were done blind to group to prevent experimenter bias. Total lengths of the humerus, radius, femur, and tibia were recorded. Humerus length was defined by the distance between the most proximal and distal articular surfaces; radius length was the distance between the head and distal point on the styloid process; femur length was measured from the most proximal point on the greater trochanter to the most distal point on the medial condyle in a line running parallel to the shaft; and tibia length was measured as the distance between the proximal articular surface and the most distal point on the medial malleolus. Some mice (n = 6) were euthanized at 24 days without wheel or temperature treatment to obtain average representative starting limb length. All measurements were made in ImageJ (version 1.37).
Fig. 1.
Temperature effects on femur length in wheel-running and non-wheel-running mice housed at warm (25°C) and cold (16°C) temperatures. Cold housing without an exercise wheel decreased bone length, but wheel exercise increased limb length at both temperatures. All long bone lengths measured from digital images were, in general, as follows: WW ≈ CW > WN > CN, where WW is warm wheel, CW is cold wheel, WN is warm no wheel, and CN is cold no wheel. Scale bar, 1 mm.
Statistical Analysis
Statistical analyses were performed using SPSS 11.0 software, with α = 0.05 for all procedures. Data were evaluated using one- and two-way ANOVA designs. A two-way factorial model with temperature and exercise as fixed factors was employed to identify main effects of these variables and their potential interaction. One-way ANOVA with group (WW, WN, CW, and CN) as the independent variable was also used to more clearly distinguish differences among the four separate treatments. Tukey's honestly significant difference multiple comparison test was used to identify significant pairwise differences. Independent comparisons between groups and treatments were performed as appropriate using Student's t-test. Relationships among variables were assessed using Pearson's product-moment correlation.
RESULTS
Warm Temperature and Exercise Increase Limb Length
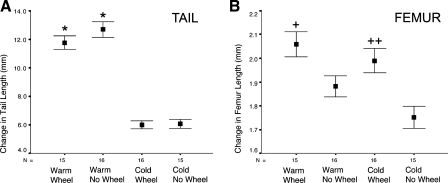
Compared with their cold and nonexercised counterparts, warm temperature and wheel exercise increased limb length in mice after the 11-day treatment period (Fig. 1). Average long bone lengths measured from digital images (Table 1) were, in general, as follows: WW ≈ CW > WN > CN. Two-way ANOVA revealed no effect of exercise on tail elongation (F = 1.4, P = 0.25) but showed a significant and sizable temperature effect (F = 216.3, P < 0.001). Change in tail length in the cold mice measured only half of that in the warm groups, regardless of exercise (Fig. 2A). Femur length, by contrast, varied with temperature and exercise (Figs. 1 and 2B). With the exception of radius length, there was no temperature-by-exercise interaction in any of the variables (all F ≤ 0.6, P ≥ 0.45). The temperature-by-exercise interaction approached significance in the radius (F = 3.1, P = 0.09), with more pronounced shortening in CN mice (Table 1). There were significant (P < 0.05, 1-tailed) effects of temperature and exercise on femur length (temperature: F = 4.2, P = 0.02; exercise: F = 18.3, P < 0.001) and radius length (temperature: F = 8.5, P = 0.002; exercise: F = 10.4, P = 0.001). There was a large main effect of exercise on body mass (F = 16.4, P < 0.001), quadriceps mass (F = 31.4, P < 0.001), and lengths of the tibia (F = 8.7, P = 0.003) and humerus (F = 7.4, P = 0.005), but the temperature effect on these variables was not significant when tested with a two-by-two factorial ANOVA.
Fig. 2.
Temperature effects on tail and femur length in wheel-running and non-wheel-running mice. A: tail lengths confirmed previous findings that extremities are shorter at cold temperatures. There was a significant and sizable temperature effect on tail elongation (2-way ANOVA: F = 216.3, P < 0.001) but no effect of wheel-running exercise. Change in tail length in the cold mice measured only half of that in the warm groups, regardless of exercise. B: femur length varied with temperature and exercise. Conclusion is that the exercise effect was localized to limb bones and was not a systemic or endocrine reaction. Femur is plotted as change in length from a common baseline starting point, determined by average femur length of 8 mice at 24 days age. Tail is plotted as change in length from the start, measured for each individual mouse. Values are group means ± SE. Statistically significant (P < 0.05) by 1-way ANOVA and Tukey's test for multiple independent comparisons: *WW and WN vs. CW and CN in pairwise comparisons; +WW > WN and WW > CN; ++CW > CN.
To more clearly distinguish among the four treatment groups, we next applied one-way ANOVA and Tukey's post hoc test (P < 0.05). Table 1 lists significant pairwise differences for each variable. Both exercise groups had increased body mass and quadriceps muscle mass. On average, mice in the WW group had the longest limb bones, with the exception of the humerus, which was slightly longer in the CW group.
In summary, mice with exercise wheels were larger, more muscular, and had significantly longer limbs than those without wheels, regardless of temperature. CN mice had the shortest limbs, confirming the predicted temperature effect (Table 1). Tail length was increased by temperature but was unaffected by wheel running, indicating that the exercise effect occurred directly on the weight-bearing limbs.
Wheel Exercise Increases Fluorescein Tracer Intensities in and Around the Growth Plate
MPM was used to image solute delivery to the growth plate using fluorescein, a low-molecular-weight tracer, as a proxy for soluble factors in the bloodstream. Timed accumulation curves showed that exercise, but not temperature, increased fluorescein arrival and entry into the growth plate (Fig. 3A). Within 5 min after tracer injection, absolute fluorescein intensities were ∼1.5-fold greater in the growth plates of the wheel-running than non-wheel-running mice, regardless of temperature. This difference, however, only approached significance due to large within-group variation (wheel vs. nonwheel, temperatures pooled: Student's t = 1.4, P = 0.08). The wheel-no wheel difference declined slightly over time, but at 10 min there was still, on average, ∼1.3-fold more tracer in the growth plates of the wheel groups.
Also, more tracer was measured in the metaphyseal vasculature adjacent to the growth plate in the wheel-running mice (Fig. 3B): >1.3 times more fluorescein had accumulated in the metaphyseal vasculature of the runners by 5 min (temperatures pooled), indicating enhanced tracer delivery into and around the growth plates of exercised mice. Fluorescein intensities in the growth plate were nearly the same between the two exercised groups (Fig. 3A); however, there was more fluorescein in the metaphyseal vasculature of the CW mice than the other groups (Fig. 3B). These differences, however, were not statistically significant because of large variation in the measurement (CW vs. CN at 5 min: Student's t = 1.1, P = 0.14).
In summary, exercise markedly increased fluorescein tracer intensities in the growth plate and metaphyseal vasculature of wheel-running mice. There was no appreciable effect of temperature on fluorescein levels in the growth plate or vasculature. Because of marked variation in absolute fluorescence values among individuals, a standardization factor was applied to enable more rigorous among-group comparisons.
Wheel Exercise Increases Relative Fluorescein Accumulation in the Growth Plate
Measured fluorescence intensities can vary among individuals as a function of imaging conditions (82), rendering absolute values difficult to interpret. Fluorescein intensities in the growth plate were therefore standardized to peak local vascular intensities in each mouse for additional comparisons (Fig. 4). This standardization ensured that the intergroup differences in absolute intensities were not an artifact of imaging quality, since growth plate and vascular intensities were always highly correlated in each individual (Pearson's r = 0.85, P < 0.001). In other words, when imaging conditions rendered lower intensities in the growth plate, a similar lower intensity was found in the adjacent metaphyseal vasculature of the same animal.
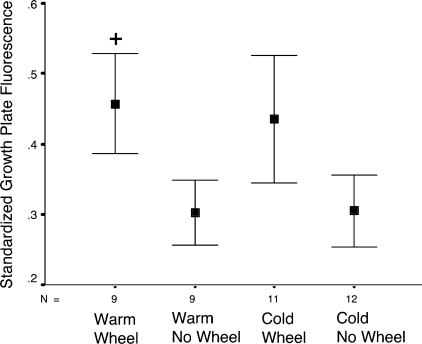
Fig. 4.
Plot of relative growth plate fluorescence measured 5-min after fluorescein tracer injection. Fluorescein levels in the growth plate were standardized to peak concentrations in the metaphyseal bone region to account for variation in imaging conditions between mice (36, 82). Two-way ANOVA applied to these standardized data revealed a significant exercise effect (F = 4.3, P = 0.02) and confirmed the apparent absence of a temperature effect on relative fluorescein accumulation in the growth plate. Values are group means ± SE. +Statistical significance (P < 0.05) by 1-way ANOVA and Tukey's test for multiple independent comparisons: WW > WN and WW > CN.
Two-way ANOVA applied to standardized data at the 5-min time point (Fig. 4) revealed a significant exercise effect (F = 4.3, P = 0.02) and confirmed the apparent absence of a temperature effect on relative fluorescein accumulation in the growth plate (F = 0.02, P > 0.80). Multiple independent comparisons using Student's t-test showed significantly more fluorescein in growth plates of the WW group than the WN (t = 1.8, P < 0.05) and CN (t = 1.8, P < 0.05) groups. Although visibly greater, standardized values in the CW mice were not statistically different from those in the CN (t = 1.3, P = 0.10) and WN (t = 1.2, P = 0.12) groups, likely due to increased vascular levels in the CW group (Fig. 3B), which slightly skewed the ratios. Relative growth plate fluorescence did not differ by temperature between WW and CW (t = 0.2, P > 0.86) or between WN and CN (t = 0.03, P > 0.98) groups, as indicated by ANOVA.
In summary, standardized fluorescein values in the growth plate were elevated in the wheel-running mice compared with their nonrunning counterparts, but there was no difference due to temperature. Exercise was the main effect.
Variation in Wheel Usage Relates to Differences in Limb Length and Solute Delivery
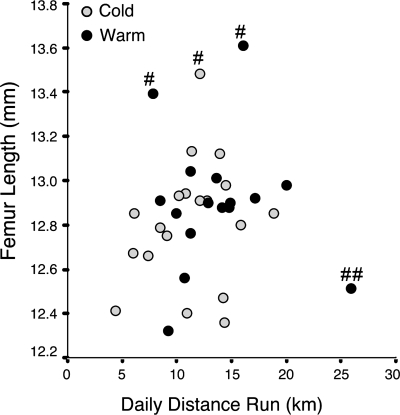
There was considerable variation in voluntary wheel usage among mice. Daily distances ranged from 5 to 25 km/day (Fig. 5), but there was no significant difference between cold and warm groups (Student's t = 1.5, P = 0.15). Given that exercise impacts limb elongation, this fivefold range in average daily distance could account for the increased variation in other data collected (Table 1, Fig. 4). Standard errors were consistently elevated in the wheel groups for nearly every variable measured, but tests for homogeneity of variances indicated that these were nonsignificant.
Fig. 5.
Relationship between femur length and wheel-running distance. A: voluntary wheel usage varied from 5 to 25 km/day, but there was no significant difference in average distance between cold and warm groups. There was a slight positive correlation between distance run and femur length (Pearson's r = 0.3, P = 0.03, outliers excluded as denoted by # and ##), suggesting that the amount of wheel running could be related to degree of limb lengthening. This 5-fold range in average daily distance may also account for increased variation in other data collected (see Figs. 2 and 4). #Mice with unusually long femurs; ##mouse with extremely high running activity.
There appeared to be a slight positive relationship between distance run and femur length (Fig. 5), but this was not a statistically significant correlation (Pearson's r = 0.1, P = 0.28). However, three outliers had unusually long femurs compared with any other mouse in the sample (>13.2 mm), and one mouse exhibited extremely high wheel-running activity (>25 km/day). The three mice with the unusually long femurs were also among the largest animals at the start of the experiments, suggesting a potential unintended sampling bias. We therefore omitted these outliers on scientific grounds for an additional analysis to better assess the relationship between the variables. Excluding these outliers yielded a stronger positive correlation between femur length and distance run (Pearson's r = 0.3, P = 0.03), suggesting that there may be a trend between amount of wheel running and limb lengthening.
Since we were interested in the relationship between exercise-mediated limb lengthening and solute delivery to growth plate cartilage, we applied partial correlation analysis to account for variation in amount of wheel running. When we controlled for distance run, partial correlation analysis of relative growth plate fluorescence and femur length yielded a moderate, although nonsignificant, positive correlation (r = 0.3, P = 0.09).
In summary, wheel usage varied among mice and showed a positive, yet nonsignificant, relationship with femur length and fluorescein tracer intensity in the growth plate.
DISCUSSION
Warm Temperature and Exercise Increase Limb Length
We used an experimental system that integrated warm and cold rearing temperatures with wheel-running exercise in young mice to examine their individual and combined effects on bone elongation. We used warm temperature without a wheel as a comparison standard, because this is the normal housing condition for mice in our facility. We avoid the term “control,” however, because the temperature effect on limb elongation follows a gradient pattern, and the presence of a temperature differential is the point of importance (78, 80).
After an 11-day treatment period of naturally rapid growth in mice (8, 54, 78, 81), we found that cold housing temperature decreased and exercise increased limb elongation as predicted. When combined, wheel exercise compensated for the shortening effect of cold temperature. All wheel-running mice had similarly lengthened limbs, regardless of temperature, while nonrunning mice had shorter limbs that correlated with housing temperature. Femurs were, on average, 0.3 mm longer in the WW than CN group (Fig. 2). While this may seem minimal, consider that total femur length in the WW mice only increased by ∼2 mm from the 24-day starting point. The 0.3-mm intergroup difference, therefore, represents a substantial 15% of the total length obtained during the study.
The bone most affected by temperature was the radius, which was distinctly shorter in CN mice. WW mice had the longest radii, illustrating a significant temperature-by-exercise interaction. We hypothesize this is because >70% of radius lengthening occurs at the wrist (29, 70, 71), and so this growth plate had greatest exposure to ambient temperature by virtue of its anatomic location in the distal forelimb. Indeed, others have shown a similar proximal-to-distal gradient in appendage lengthening, temperature, and blood flow (3, 27, 65, 67, 78, 92). The differential impact of exercise on the radius also is not surprising, since its active growth plate is at a primary weight-bearing site of the mouse forelimb. We did not image the distal radius growth plate because of methodological constraints; however, we focused multiphoton imaging on the proximal (knee) growth plate of the tibia, where ∼60% of its lengthening occurs at a high-impact site (29).
The individual effects of temperature and exercise are consistent with prior work (3, 80, 83, 90); however, their combined effects on limb length are a novel result of this study. Interestingly, we found no impact of exercise on tail elongation, yet tail length was significantly reduced at cold temperature, as shown previously (Fig. 2) (80). Others have also reported that exercise does not impact tail elongation (33), and we suspect that this is because the tail does not experience an impact load. For instance, moderate compression of articular cartilage has an anabolic effect on chondrocytes by stimulating changes in cell matrix, fluid flow, and biochemical activity (72). Since growth plate cartilage is composed of the same resident cell type, it is possible that increased compression of the long bones stimulates the same changes in growth plate cartilage to enhance its growth potential, whereas temperature acts through alternate mechanisms (80). The tail, therefore, absent loading on its articular disks, served as an exquisite control variable for the temperature effect.
Although exercise and warm temperature can increase levels of systemic factors, such as growth hormone (40, 97), our observations using the tail as a temperature control support the concept that exercise affects the limbs directly, rather than generally promoting growth through systemic mechanisms. This direct, local effect is especially likely, since exercise can also increase circulating levels of stress hormones (84), which are known to inhibit bone elongation (24). We found that body mass was greater in mice in the exercise groups than in their counterparts without wheels, which we attribute in part to increased muscle mass. Mice with wheels had significantly larger quadriceps muscles (Table 1), and we qualitatively noted that these animals were leaner and more muscular overall. The increased muscle mass, therefore, could be expected to exert larger loads on the skeleton (47), especially the weight-bearing sites. Since the tail does not experience load bearing and its length was not affected by wheel running, the conclusion is that the exercise effect was localized to the weight-bearing skeleton and was not a general systemic response to heightened activity. The ability to discriminate a local vs. systemic reaction to exercise has key relevance to studies on skeletal loading and mechanotransduction in bone (55, 74, 96).
Wheel Exercise Increases Fluorescein Tracer Intensity in the Growth Plate
Since our results indicated that the exercise response was localized to limb bones, we used in vivo multiphoton imaging to test the hypothesis that exercise promotes bone elongation by enhancing solute delivery to the growth plate. We used fluorescein as a proxy for solutes in the bloodstream, because it is a nontoxic, low-molecular-weight tracer that enters the growth plate with relative ease (36, 82, 102). We measured fluorescein in the proximal tibial growth plate after an intracardiac injection to determine whether solute delivery differed in groups exposed to chronic temperature and/or exercise treatment. Fluorescein levels in the growth plates and adjacent metaphyseal vasculature of wheel-running mice were increased as predicted. However, there were no temperature differences as hypothesized. This suggests that exercise has the primary effect on solute delivery.
The absence of a temperature effect on solute delivery in nonrunning mice was surprising, because their limb lengths were affected by housing temperature (Figs. 1 and 2). In a prior study using microspheres to measure total bone blood flow in warm- and cold-housed mice, Serrat et al. (80) described a threshold response to temperature. They found that bone perfusion was only reduced in mice housed at their coldest study temperature (7°C) and did not differ between two warmer-housed groups (21°C and 27°C), even though limb lengths differed. It is possible that the temperatures used in the present study (16°C and 25°C) were above a similar cold threshold that maintains normal solute delivery to cartilage. In fact, the 16°C cold temperature in our experiments was mild compared with 0–10°C in other studies (3, 6, 9, 85). Importantly, 16°C was sufficient to induce a differential growth response relative to the 25°C warm-housed mice (note tails in Fig. 2). If the threshold idea is correct, then it suggests that exercise must induce physiological changes to increase solute delivery above this normal threshold.
Mechanistically, one way to increase solute delivery to the growth plate would be to increase the total volume of blood arriving at a bone by increasing flow rate, enlarging vessel diameter (vasodilation), and/or increasing vessel numbers (angiogenesis). Our results complement published data that show that total bone blood flow is increased with exercise (43, 56, 59, 63, 64, 91) and are especially relevant in light of the known correlation between increased blood flow and bone elongation (17–19, 68, 95). Our data are a key advancement, because we illustrate for the first time that these exercise effects can occur locally in growth plate cartilage. These data could not be obtained using other methods.
Fluorescein Tracer Intensities Differ in Growth Plate and Vasculature
It is interesting and unexpected that the WW mice had slightly lower levels of fluorescein in the vasculature, yet their relative growth plate levels did not differ from their CW counterparts. Vascular fluorescein levels were measured as the fluorescence intensity in the region of the metaphyseal bone adjacent to the growth plate, which contains a rich network of blood vessels (20). One potential explanation is that cold-reared mice have bones that are less dense, causing the fluorescein concentrations to appear higher in that region because of decreased bone density. Delahunty and colleagues (28) found a seasonal reduction in femur mineral content in female mice in the winter, suggesting that bone density could indeed be lower in the cold-reared mice. Radial bone expansion peaks during this stage of rapid postnatal growth (3–5 wk of age in mice) (23), and so bone density changes might be expected in our experiments. We are currently investigating bone mineral density from micro-CT scans to address this possibility, and our preliminary results show a temperature effect despite the relatively short treatment period (unpublished data). Steinberg and Trueta (83) detected bone loss in comparable-aged rats as early as 1 wk following hindlimb immobilization, supporting the idea that the growing skeleton responds rapidly to changes in mechanical stimuli.
Variation in Wheel Usage Relates to Differences in Limb Length and Solute Delivery
Running distances in both groups ranged from 5 to 25 km/day, with an average of ∼12 km/day. This is consistent with ranges published by others (5, 52), with the variation expected since the activity was voluntary. We hypothesize that variation in wheel-running activity may, in part, account for the increased variation we detected in other variables (see error bars in Figs. 2 and 4). Indeed, using the same strain of mice, Isaksson et al. (52) reported increased within-group variation in bone mechanical properties and collagen networks of the animals that voluntarily ran on wheels. We found a slight positive correlation between femur length and running distance, suggesting that variation in wheel usage could account for some of the variation in limb length. However, the correlation was only marginally significant when several outliers were removed on scientific grounds (see above), and so these results need further confirmation. Future studies using defined exercise regimens could clarify this issue by eliminating the variation introduced by voluntary exercise. However, defined exercise must be employed cautiously because of the potential for a negative physiological impact of overstimulation (32, 58, 73).
Caveats and Technical Considerations
While our study provides fundamental data regarding the role of solute delivery in exercise-mediated limb lengthening, our results must be considered in light of several technical constraints. Most important for discussion is the length of treatment and the difference between rearing and imaging temperatures.
Length of treatment.
While a longer treatment period would have been favorable to reveal greater intergroup differences, the 35-day end point was specific to the requirements of this study. We started with 24-day weanlings purchased from a commercial vendor to ensure that age- and sex-matched cohorts were available for regular weekly imaging. We previously found that 35 days of age is optimal for visualization of the proximal tibial growth plate in vivo (36, 82, 102), since growth plate size and growth rate decline substantially thereafter in mice (8).
It is essential to note that the 11-day treatment occurred during a period of naturally rapid growth in mice, in terms of body size (54) and growth plate activity (8, 81). Animals in this study showed a 55% increase in body mass (10 g at start to 15.5 g at end point). It is therefore impressive, but not surprising, that the bone elongation differences emerged within this relatively short time period. These data support previous ideas that the postnatal skeleton is especially responsive to external stimuli during a critical postparturition window of heightened sensitivity (79).
Rearing and imaging temperatures.
Temperature change was one of the most difficult aspects of our experiments to control. Mice were reared chronically at cold and warm temperatures of 16°C and 25°C, respectively. Multiphoton imaging was done at a standard intermediate temperature of 20°C. Mice reared in the cold experienced short-term (up to several hours) acute warming when brought to the imaging facility, while the warm-reared mice experienced acute cooling. It was logistically not possible to avoid these temperature changes because of the sensitive nature of the microscopy equipment. However, maintaining the mice at the same neutral imaging temperature was the best way to ensure that we were investigating a permanent chronic change in solute delivery, rather than a transient effect of room temperature.
To minimize experimentally induced variation, we maintained rigorous control over core and limb temperature during imaging and otherwise subjected all mice to the same conditions (82). Published data suggest that any impact of imaging conditions may actually only be minimal. Serrat (78) used microspheres to measure bone blood supply in cold- and warm-reared mice and found that surgical room temperature did not significantly affect bone perfusion results. Others have shown that anesthesia minimally impacts bone blood flow if normal core body temperature is maintained (1). Furthermore, acute exercise effects on bone blood supply are known to persist for 1 h after rest (91), and chronic changes have even been shown in the resting state (56, 87). We were interested in detecting a chronic change that could account for differences in bone elongation rate, and so we believe these methods are appropriate to the research question.
Summary and Implications
Using an experimental model that combines effects of temperature and wheel-running exercise on skeletal growth in mice, this study is the first to provide evidence for a role of solute delivery in exercise-augmented limb elongation. We found that limb length varied with temperature and exercise but tail length varied with temperature alone. This demonstrates that exercise effects were limited to the weight-bearing skeleton. Such effects were manifested in a long-term increase in solute delivery to the growth plate, which was clearly evident in the resting state. These results indicate that exercise may modulate limb growth by permanently enhancing availability of essential nutrients and soluble factors in the growth plate. It is significant, then, that temperature impacted extremity growth without altering solute delivery to the growth plate, suggesting that temperature effects on limb elongation occur through other physiological mechanisms (80).
Our data demonstrate that in vivo multiphoton imaging is a reliable tool for measuring chronic changes in solute delivery to tibial growth plates of mice. These analytic methods will be useful for assessing the role of nutrient transport in natural and experimental models of growth plate dysfunction, particularly chondrodysplasias, where defects in the extracellular matrix could impede molecular transport in cartilage, leading to impaired bone growth (see review in Ref. 82).
These findings are relevant for assessing skeletal variation among mammals in different environmental contexts, particularly those where temperature and exercise could have a dual effect on morphology. For example, in an analysis of body shape in humans from two archaeological sites in Alaska with marked differences in winter temperatures (Point Hope vs. Kodiak Island), Holliday and Hilton (50) found that the groups had similar limb proportions, despite a 14°C difference in January high temperatures. They had expected to find relatively shorter limbs in the colder-dwelling Point Hope sample, on the basis of the prediction that their more extreme climate would have driven selection for a more cold-adapted body shape (50). While genetic and environmental components must be considered, one speculation for their similarity in body form could be that heightened physical activity characteristic of both circumpolar groups mitigated some of the impact of cold temperature on limb length. Although this developmental plasticity hypothesis cannot be directly tested in fossils, the work presented here sheds important light on what remains a perplexing issue in human and mammalian evolution.
By illuminating exercise and temperature effects on bone elongation in a controlled setting, these laboratory experiments make evolutionary scenarios regarding the heritability of limb length problematic when viewed as simple adaptations for thermoregulation (69, 76, 86, 88) and/or performance (15, 75). On the other hand, if these environmentally induced morphologies do confer an evolutionary advantage, they could potentially become fixed in a population through natural selection and/or genetic assimilation (7, 26, 44, 80).
Building on the classic genetic assimilation work of Waddington and Baldwin, West-Eberhard (100, 101) elegantly described how morphologically plastic traits could evolve through the process of natural selection. Indeed, if exercise- or temperature-induced developmental lability could produce advantageous traits in the context of a challenging environment (7, 26, 44, 60, 61, 86), it is likely that the underlying mechanisms could be under intense natural selection and, ultimately, become fixed in a population after many generations (80). Indeed, West-Eberhard (100) suggested that environmentally driven phenotypic plasticity could actually have greater evolutionary potential than random mutation in terms of natural selection. Since skeletal material is often the sole reference for reconstructing an animal's biology and behavior, it is critical that we continue forging efforts to understand the causes and extent of this intriguing bone elongation plasticity. These efforts will be key to making better-informed assessments of skeletal adaptations among animals in our remote and distant past.
GRANTS
This work was made possible by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant RO1 AR-052003-05 to C. E. Farnum. M. A. Serrat is an American Association of Anatomists Scholar, and this research was in part funded by a postdoctoral fellowship from the American Association of Anatomists (www.anatomy.org).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
DISCLAIMER
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ACKNOWLEDGMENTS
The authors thank W. Zipfel and J. McMullen for assistance with the multiphoton microscope; S. Allen, R. Barrier, R. Combs, and Cornell Laboratory Animal Services for animal housing and husbandry; B. Linnehan for research aid; and E. Donnelly and S. Chang for technical advice and critical suggestions.
REFERENCES
- 1. Aardal NP, Svanes K, Egenberg KE. Effect of hypothermia and pentobarbital anaesthesia on the distribution of cardiac output in rabbits. Eur Surg Res 5: 372, 1973 [DOI] [PubMed] [Google Scholar]
- 2. Adams EH. A comparative anthropometric study of hard labor during youth as a stimulator of physical growth of young colored women. Res Q 9: 102–108, 1938 [Google Scholar]
- 3. Al-Hilli F, Wright EA. The effects of changes in the environmental temperature on the growth of bones in the mouse: radiological and morphological study. Br J Exp Pathol 64: 43–52, 1983 [PMC free article] [PubMed] [Google Scholar]
- 4. Allen JA. The influence of physical conditions in the genesis of species. Radical Rev 1: 108–140, 1877 [Google Scholar]
- 5. American Physiological Society Resource Book for the Design of Animal Exercise Protocols. Bethesda, MD: Am. Physiol. Soc., 2006 [Google Scholar]
- 6. Ashoub MA. Effect of two extreme temperatures on growth and tail-length of mice. Nature 181: 284, 1958 [DOI] [PubMed] [Google Scholar]
- 7. Badyaev AV. Evolutionary significance of phenotypic accommodation in novel environments: an empirical test of the Baldwin effect. Phil Trans R Soc Lond B Biol Sci 364: 1125–1141, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bailón-Plaza A, Lee AO, Veson EC, Farnum CE, van der Meulen MCH. BMP-5 deficiency alters chondrocytic activity in the mouse proximal tibial growth plate. Bone 24: 211–216, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Barnett SA, Scott SG. Some effects of cold and of hybridity on the growth of mice. J Embryol Exp Morphol 11: 35–51, 1963 [PubMed] [Google Scholar]
- 10. Becher C, Springer J, Feil S, Cerulli G, Paessler HH. Intra-articular temperatures of the knee in sports—an in-vivo study of jogging and alpine skiing. BMC Musculoskelet Disord 9: 46, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bertram JE, Greenberg LS, Miyake T, Hall BK. Paralysis and long bone growth in the chick: growth shape trajectories of the pelvic limb. Growth Dev Aging 61: 51–60, 1997 [PubMed] [Google Scholar]
- 12. Beyer HG. The influence of exercise on growth. J Exp Med 1: 546–558, 1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Biewener AA, Bertram JEA. Structural response of growing bone to exercise and disuse. J Appl Physiol 76: 946–955, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Bloomfield SA. Does altered blood flow to bone in microgravity impact on mechanotransduction? J Musculoskelet Neuronal Interact 6: 324–326, 2006 [PubMed] [Google Scholar]
- 15. Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature 432: 345–352, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Brashear HR. Epiphyseal avascular necrosis and its relation to longitudinal bone growth. J Bone Joint Surg Am 45: 1423–1438, 1963 [PubMed] [Google Scholar]
- 17. Brodin H. Longitudinal bone growth and nutrition of the epiphyseal cartilages and the local blood supply: an experimental study in the rabbit. Acta Orthop Scand Suppl 20: 9–92, 1955 [PubMed] [Google Scholar]
- 18. Brookes M. Blood supply of developing bone and its possible bearing on malformation of the limbs and face in congenital haemangiomatous disorders. Proc R Soc Med 65: 597–599, 1972 [PMC free article] [PubMed] [Google Scholar]
- 19. Brookes M, May KU. The influence of temperature on bone growth in the chick. J Anat 111: 351–363, 1972 [PMC free article] [PubMed] [Google Scholar]
- 20. Brookes M, Revell WJ. Blood Supply of Bone: Scientific Aspects. New York: Springer, 1998 [Google Scholar]
- 21. Buskirk ER, Andersen KL, Brozek J. Unilateral activity and bone and muscle development in the forearm. Res Q 27: 127–131, 1956 [Google Scholar]
- 22. Cairns R. Magnetic resonance imaging of the growth plate: pictorial essay. Can Assoc Radiol J 54: 234–242, 2003 [PubMed] [Google Scholar]
- 23. Callewaert F, Venken K, Kopchick JJ, Torcasio A, van Lenthe GH, Boonen S, Vanderschueren D. Sexual dimorphism in cortical bone size and strength but not density is determined by independent and time-specific actions of sex steroids and IGF-1: evidence from pubertal mouse models. J Bone Miner Res 25: 617–626, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Chagin AS, Karimian E, Sundström K, Eriksson E, Sävendahl L. Catch-up growth after dexamethasone withdrawal occurs in cultured postnatal rat metatarsal bones. J Endocrinol 204: 21–29, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Colleran PN, Wilkerson MK, Bloomfield SA, Suva LJ, Turner RT, Delp MD. Alterations in skeletal perfusion with simulated microgravity: a possible mechanism for bone remodeling. J Appl Physiol 89: 1046–1054, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Crispo E. The Baldwin effect and genetic assimilation: revisiting two mechanisms of evolutionary change mediated by phenotypic plasticity. Evolution 61: 2469–2479, 2007 [DOI] [PubMed] [Google Scholar]
- 27. De Neve W, Van Der Elst J, Geerts F, Van Loon R, De Clerck V, Storme G. The influence of ambient temperature on tumor growth, metastasis and survival in mice. Clin Exp Metastasis 6: 213–219, 1988 [DOI] [PubMed] [Google Scholar]
- 28. Delahunty K, Horton L, Coombs H, Shultz K, Svenson K, Marion M, Holick M, Beamer W, Rosen CJ. Gender- and compartment-specific bone loss in C57BL/6J mice: correlation to season? J Clin Densitom 12: 89–94, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Digby KH. The measurement of diaphyseal growth in proximal and distal directions. J Anat Physiol 50: 187–188, 1916 [PMC free article] [PubMed] [Google Scholar]
- 30. Draenert K, Draenert Y. The role of the vessels in the growth plate: morphological examination. Scann Electron Microsc 1: 339–344, 1985 [PubMed] [Google Scholar]
- 31. Dunn KW, Sutton TA. Functional studies in living animals using multiphoton microscopy. ILAR J 49: 66–77, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Duyar I. Growth patterns and physical plasticity in adolescent laborers. Coll Antropol 32: 403–412, 2008 [PubMed] [Google Scholar]
- 33. Erich WB, Peltenberg AL, Minkhorst J, VanDessel B, Biersteker-Hubben MW, Zonderland ML, Huisveld IA. The influence of physical exercise on growth and sexual maturation in young female rats. Growth 49: 131–140, 1985 [PubMed] [Google Scholar]
- 34. Etchebehere E, Caron M, Pereira JA, Lima M, Santos AO, Ramos CD, Barros FB, Sanches A, Santos-Jesus R, Belangero W, Camargo EE. Activation of the growth plates on three-phase bone scintigraphy: the explanation for the overgrowth of fractured femurs. Eur J Nucl Med 28: 72–80, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Farnum CE. Postnatal growth of fins and limbs through endochondral ossification. In: Fins Into Limbs: Evolution, Development, and Transformation. Chicago: University of Chicago Press, 2007, p. 118–151 [Google Scholar]
- 36. Farnum CE, Lenox M, Zipfel W, Horton W, Williams R. In vivo delivery of fluoresceinated dextrans to the murine growth plate: imaging of three vascular routes by multiphoton microscopy. Anat Rec A Discov Mol Cell Evol Biol 288: 91–103, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Felts WJ. Transplantation studies of factors in skeletal organogenesis. I. The subcutaneously implanted immature long-bone of the rat and mouse. Am J Phys Anthropol 17: 201–215, 1959 [DOI] [PubMed] [Google Scholar]
- 38. Feucht BL, Richardson AW, Hines HM. Effect of hot foments on volume of blood flow in extremities of dogs. Arch Phys Med Rehabil 30: 687–690, 1949 [PubMed] [Google Scholar]
- 39. Flesken-Nikitin A, Williams RM, Zipfel W, Webb WW, Nikitin AY. Use of multiphoton imaging for studying cell migration in the mouse. Methods Mol Biol 294: 335–345, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Ftaiti F, Jemni M, Kacem A, Zaouali M, Tabka Z, Zbidi A, Grélot L. Effect of hyperthermia and physical activity on circulating growth hormone. Appl Physiol Nutr Metab 35: 350–357, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Garofalo S, Vuorio E, Metsaranta M, Rosati R, Toman D, Vaughan J, Guillermina L, Mayne R, Ellard J, Horton W, De Crombrugghe B. Reduced amounts of cartilage collagen fibrils and growth plate anomalies in transgenic mice harboring a glycine-to-cysteine mutation in the mouse type II procollagen α1-chain gene. Proc Natl Acad Sci USA 88: 9648–9652, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Geiser M, Trueta J. Muscle action, bone rarefaction and bone formation: an experimental study. J Bone Joint Surg Br 40: 282–311, 1958 [DOI] [PubMed] [Google Scholar]
- 43. Genant HK, Bautovich GJ, Singh M, Lathrop KA, Harper PV. Bone-seeking radionucleotides: an in vivo study of factors affecting skeletal uptake. Radiology 113: 373–382, 1974 [DOI] [PubMed] [Google Scholar]
- 44. Ghalambor CK, McKay JK, Carroll SP, Reznick DN. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21: 394–407, 2007 [Google Scholar]
- 45. Greyson ND, Tepperman PS. Three-phase bone studies in hemiplegia with reflex sympathetic dystrophy and the effect of disuse. J Nucl Med 25: 423–429, 1984 [PubMed] [Google Scholar]
- 46. Haas SL. The relation of the blood supply to the longitudinal growth of bone. Am J Orthop Surg 15: 157–171, 1917 [Google Scholar]
- 47. Hamrick MW, McPherron AC, Lovejoy CO, Hudson J. Femoral morphology and cross-sectional geometry of adult myostatin-deficient mice. Bone 27: 343–349, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Harland SC. Effect of temperature on growth in weight and tail-length of inbred and hybrid mice. Nature 186: 446, 1960 [Google Scholar]
- 49. Harrison GA. Environmental modification of mammalian morphology. Man 60: 3–6, 1960 [Google Scholar]
- 50. Holliday TW, Hilton CE. Body proportions of circumpolar peoples as evidenced from skeletal data: Ipiutak and Tiagra (Point Hope) versus Kodiak Island Inuit. Am J Phys Anthropol 142: 287–302, 2010 [DOI] [PubMed] [Google Scholar]
- 51. Hunter WL, Arsenault AL. Vascular invasion of the epiphyseal growth plate: analysis of metaphyseal capillary ultrastructure and growth dynamics. Anat Rec 227: 223–231, 1990 [DOI] [PubMed] [Google Scholar]
- 52. Isaksson H, Tolvanen V, Finnilä MAJ, Iivarinen J, Tuukkanen J, Seppänen K, Arokoski JPA, Brama PA, Jurvelin JS, Helminen HJ. Physical exercise improves properties of bone and its collagen network in growing and maturing mice. Calcif Tissue Int 85: 247–256, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Iwamoto J, Shimamura C, Takeda T, Abe H, Ichimura S, Sato Y, Toyama Y. Effects of treadmill exercise on bone mass, bone metabolism, and calciotropic hormones in young growing rats. J Bone Miner Metab 22: 26–31, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Jackson Laboratory Body Weight Information for Jax Mice Strain C57BL/6J. Bar Harbor, ME: Jackson Laboratory, 2010 [Google Scholar]
- 55. Jee WSS. The past, present, and future of bone morphometry: its contribution to an improved understanding of bone biology. J Bone Miner Metab 23 Suppl: 1–10, 2005 [DOI] [PubMed] [Google Scholar]
- 56. Jurvelin J, Lahtinen T, Kiviranta I, Arnala I, Lappalainen R, Tammi M, Helminen HJ. Blood flow, histomorphology and elemental composition of the canine femur after physical training or immobilization. Acta Physiol Scand 132: 385–389, 1988 [DOI] [PubMed] [Google Scholar]
- 57. Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol 25: 629–648, 2009 [DOI] [PubMed] [Google Scholar]
- 58. Kiiskinen A. Physical training and connective tissues in young mice: physical properties of Achilles tendons and long bones. Growth 41: 123–137, 1977 [PubMed] [Google Scholar]
- 59. Kiiskinen A, Suominen H. Blood circulation of long bones in trained growing rats and mice. Eur J Appl Physiol 34: 303–309, 1975 [DOI] [PubMed] [Google Scholar]
- 60. Losos JB, Creer DA, Glossip D, Goellner R, Hampton A, Roberts G, Haskell N, Taylor P, Ettling J. Evolutionary implications of phenotypic plasticity in the hindlimb of the lizard Anolis sagrei. Evolution 54: 301–305, 2000 [DOI] [PubMed] [Google Scholar]
- 61. Losos JB, Schoener TW, Warheit KI, Creer D. Experimental studies of adaptive differentiation in Bahamian Anolis lizards. Genetica 112–113: 399–415, 2001 [PubMed] [Google Scholar]
- 62. Malina RM. Exercise as an influence upon growth. Review and critique of current concepts. Clin Pediatr 8: 16–26, 1969 [DOI] [PubMed] [Google Scholar]
- 63. McDonald F, Pitt Ford TR. Blood flow changes in the tibia during external loading. J Orthop Res 11: 36–48, 1994 [DOI] [PubMed] [Google Scholar]
- 64. Mohr T, Akers TK, Wessman HC. Effect of high voltage stimulation on blood flow in the rat. Phys Ther 67: 526–533, 1987 [DOI] [PubMed] [Google Scholar]
- 65. Mostafa A, Attar SE, Mili F, Wright EA. The effect of environmental temperature on the rate of healing of fractures in tail vertebrae of mice. J Bone Joint Surg Br 62: 102–103, 1980 [DOI] [PubMed] [Google Scholar]
- 66. Murray PDF, Selby D. Intrinsic and extrinsic factors in the primary development of the skeleton. Dev Genes Evol 122: 629–662, 1930 [DOI] [PubMed] [Google Scholar]
- 67. Nakano T, Thompson JR, Christopherson RJ, Aherne FX. Blood flow distribution in hind limb bones and joint cartilage from young growing pigs. Can J Vet Res 50: 96–100, 1986 [PMC free article] [PubMed] [Google Scholar]
- 68. Noel JF, Wright EA. The growth of transplanted mouse vertebrae: effects of transplantation under the renal capsule, and the relationship between the rate of growth of the transplant and the age of the host. J Embryol Exp Morphol 28: 633–645, 1972 [PubMed] [Google Scholar]
- 69. Nudds RL, Oswald SA. An interspecific test of Allen's rule: evolutionary implications for endothermic species. Evolution 61: 2839–2848, 2007 [DOI] [PubMed] [Google Scholar]
- 70. Payton CG. The growth in length of the long bones in the madder-fed pig. J Anat 66: 414–425, 1932 [PMC free article] [PubMed] [Google Scholar]
- 71. Pritchett JW. Growth plate activity in the upper extremity. Clin Orthop Relat Res 268: 235–242, 1991 [PubMed] [Google Scholar]
- 72. Ramage L, Nuki G, Salter DM. Signalling cascades in mechanotransduction: cell-matrix interactions and mechanical loading. Scand J Med Sci Sports 19: 457–469, 2009 [DOI] [PubMed] [Google Scholar]
- 73. Reich A, Jaffe N, Tong A, Lavelin I, Genina O, Pines M, Sklan D, Nussinovitch A, Monsonego-Ornan A. Weight loading young chicks inhibits bone elongation and promotes growth plate ossification and vascularization. J Appl Physiol 98: 2381–2389, 2005 [DOI] [PubMed] [Google Scholar]
- 74. Robling AG, Turner CH. Mechanical signaling for bone modeling and remodeling. Crit Rev Eukaryotic Gene Expr 19: 319–338, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ruff C. Variation in human body size and shape. Annu Rev Anthropol 31: 211–232, 2002 [Google Scholar]
- 76. Ruff CB. Morphological adaptation to climate in modern and fossil hominids. Yearbook Phys Anthropol 37: 65–107, 1994 [Google Scholar]
- 77. Schoutens A, Bergmann P, Verhas M. Bone flood flow measured by 85Sr microspheres and bone seeker clearances in the rat. Am J Physiol Heart Circ Physiol 236: H1–H6, 1979 [DOI] [PubMed] [Google Scholar]
- 78. Serrat MA. Environmentally-Determined Tissue Temperature Modulates Extremity Growth in Mammals: A Potential Comprehensive Explanation of Allen's Rule (Ph.D. thesis) Kent, OH: Kent State University, 2007 [Google Scholar]
- 79. Serrat MA, Farnum CE, Williams RM, Lovejoy CO. Environment influences bone elongation during a critical period of postnatal growth (Abstract). Am J Phys Anthropol Suppl 44: 364, 2009 [Google Scholar]
- 80. Serrat MA, King D, Lovejoy CO. Temperature regulates limb length in homeotherms by directly modulating cartilage growth. Proc Natl Acad Sci USA 105: 19347–19352, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Serrat MA, Lovejoy CO, King D. Age- and site-specific decline in insulin-like growth factor-I receptor expression is correlated with differential growth plate activity in the mouse hindlimb. Anat Rec 290: 375–381, 2007 [DOI] [PubMed] [Google Scholar]
- 82. Serrat MA, Williams RM, Farnum CE. Temperature alters solute transport in growth plate cartilage measured by in vivo multiphoton microscopy. J Appl Physiol 106: 2016–2025, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Steinberg ME, Trueta J. Effects of activity on bone growth and development in the rat. Clin Orthop Relat Res 156: 52–60, 1981 [PubMed] [Google Scholar]
- 84. Stuempfle KJ, Nindl BC, Kamimori GH. Stress hormone responses to ultraendurance race in the cold. Wilderness Environ Med 21: 22–27, 2010 [DOI] [PubMed] [Google Scholar]
- 85. Sumner FB. Some effects of external conditions upon the white mouse. J Exp Zool 7: 97–155, 1909 [Google Scholar]
- 86. Symonds MR, Tattersall GJ. Geographical variation in bill size across bird species provides evidence for Allen's rule. Am Nat 176: 188–197, 2010 [DOI] [PubMed] [Google Scholar]
- 87. Thorp BH, Duff SRI. Effect of exercise on the vascular pattern in the bone extremities of broiler fowl. Res Vet Sci 45: 72–77, 1988 [PubMed] [Google Scholar]
- 88. Tilkens MJ, Wall-Scheffler C, Weaver TD, Steudel-Numbers K. The effects of body proportions on thermoregulation: an experimental assessment of Allen's rule. J Hum Evol 53: 286–291, 2007 [DOI] [PubMed] [Google Scholar]
- 89. Tomita Y, Tsai TM, Steyers C, Ogden L, Jupiter JB, Kutz JE. The role of the epiphyseal and metaphyseal circulations on longitudinal growth in the dog: an experimental study. J Hand Surg [Am] 11: 375–382, 1986 [DOI] [PubMed] [Google Scholar]
- 90. Tomljenovic Borer K, Kuhns LR. Radiographic evidence for acceleration of skeletal growth in adult hamsters by exercise. Growth 41: 1–13, 1977 [PubMed] [Google Scholar]
- 91. Tøndevold E, Bülow J. Bone blood flow in conscious dogs at rest and during exercise. Acta Orthop Scand 54: 53–57, 1983 [DOI] [PubMed] [Google Scholar]
- 92. Tothill P, MacPherson JN. The distribution of blood flow to the whole skeleton in dogs, rabbits and rats measured with microspheres. Clin Phys Physiol Meas 7: 117–123, 1986 [DOI] [PubMed] [Google Scholar]
- 93. Trueta J. Skeletal shape and muscle power. Bull Hosp Joint Dis 21: 332–345, 1960 [PubMed] [Google Scholar]
- 94. Trueta J. Studies of the Development and Decay of the Human Frame. Philadelphia: Saunders, 1968 [Google Scholar]
- 95. Trueta J. The influence of the blood supply in controlling bone growth. Bull Hosp Joint Dis 14: 147–157, 1953 [PubMed] [Google Scholar]
- 96. Turner CH, Warden SJ, Bellido T, Plotkin LI, Kumar N, Jasiuk I, Danzig J, Robling AG. Mechanobiology of the skeleton. Sci Signal 28: pt 3, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Vendelbo MH, Jørgensen J, Pedersen S, Gormsen L, Lund S, Schmitz O, Jessen N, Møller N. Exercise and fasting activate growth hormone-dependent myocellular signal transducer and activator of transcription-5b phosphorylation and insulin-like growth factor-I messenger ribonucleic acid expression in humans. J Clin Endocrinol Metab 95: E64–E68, 2010 [DOI] [PubMed] [Google Scholar]
- 98. Wang BG, Konig K, Halbhuber KJ. Two-photon microscopy of deep intravital tissues and its merits in clinical research. J Microsc 238: 1–20, 2010 [DOI] [PubMed] [Google Scholar]
- 99. Weaver ME, Ingram DL. Morphological changes in swine associated with environmental temperature. Ecology 50: 710–713, 1969 [Google Scholar]
- 100. West-Eberhard MJ. Developmental plasticity and the origin of species differences. Proc Natl Acad Sci USA 102: 6543–6549, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. West-Eberhard MJ. Phenotypic plasticity and the origins of diversity. Annu Rev Ecol Syst 20: 249–278, 1989 [Google Scholar]
- 102. Williams RM, Zipfel WR, Tinsley ML, Farnum CE. Solute transport in growth plate cartilage: in vitro and in vivo. Biophys J 93: 1039–1050, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Williams RM, Zipfel WR, Webb WW. Interpreting second-harmonic generation images of collagen I fibrils. Biophys J 88: 1377–1386, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wilsman NJ, Farnum CE, Leiferman EM, Fry M, Barreto C. Differential growth by growth plates as a function of multiple parameters of chondrocyte kinetics. J Orthop Res 14: 927–936, 1996 [DOI] [PubMed] [Google Scholar]
- 105. Wirth T, Syed Ali MM, Rauer C, Süss D, Griss P, Syed Ali S. The blood supply of the growth plate and the epiphysis: a comparative scanning electron microscopy and histological experimental study in growing sheep. Calcif Tissue Int 70: 312–319, 2002 [DOI] [PubMed] [Google Scholar]
- 106. Wisely SM, Santymire RM, Livieri TM, Marinari PE, Kreeger JS, Wildt DE, Howard J. Environment influences morphology and development for in situ and ex situ populations of the black-footed ferret (Mustela nigripes). Anim Conserv 8: 321–328, 2005 [Google Scholar]
- 107. Yang KTA, Yang AD. Evaluation of activity of epiphyseal plates in growing males and females. Calcif Tissue Int 78: 348–356, 2006 [DOI] [PubMed] [Google Scholar]
- 108. Young NM, Hallgrímsson B. Serial homology and the evolution of mammalian limb covariation structure. Evolution 59: 2691–2704, 2002 [PubMed] [Google Scholar]
- 109. Zipfel WR, Williams RM, Christie R, Nikitin AY, Hyman BT, Webb WW. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc Natl Acad Sci USA 100: 7075–7080, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol 21: 1369–1377, 2003 [DOI] [PubMed] [Google Scholar]