Abstract
NADH-localized fluorometry was used as a noninvasive technique to monitor changes in the energy state of intact tissue (muscle and connective tissue), without anesthesia, as a function of blood plasma O2-carrying capacity in the hamster window chamber model. Acute moderate isovolemic hemodilution was induced by two isovolemic hemodilution steps: in the first step, 6% 70-kDa dextran (Dex70) was used to induce an acute anemic state (18% Hct); in the second step, exchange transfusion of polyethylene glycol (PEG) maleimide-conjugated Hb (4 g/dl, PEG-Hb) or Dex70 (6 g/dl) was used to reduce erythrocytes to 75% of baseline (11% Hct). PEG-Hb had six copies of PEG (5 kDa) conjugated to each human Hb (0.48 g PEG/g Hb) through extension arm-facilitated chemistry. Systemic parameters, microvascular perfusion, functional capillary density, intravascular and interstitial Po2, and intracellular NADH fluorescence were monitored. Mean arterial blood pressure after extreme hemodilution was statistically significantly reduced for Dex70 compared with PEG-Hb. The presence of PEG-Hb in the circulation maintained positive acid-base balance. While microvascular blood flows were not different, functional capillary density was significantly higher for PEG-Hb than Dex70. Arteriolar Po2 was higher in the presence of PEG-Hb than Dex70, but tissue and venular Po2 were not different. Cellular energy metabolism (intracellular O2) in the tissues was improved with PEG-Hb. Moderate hemodilution to 18% Hct (6.4 g Hb/dl) brings tissue O2 delivery to the verge of inadequacy. Extreme hemodilution to 11% Hct (3.7 g Hb/dl) produces tissue anoxia, and high-O2-affinity PEG-Hb (Po2 at which blood is 50% saturated with O2 = 4 Torr, 1.1 g Hb/dl) only partially decreases anaerobic metabolism without increasing tissue Po2.
Keywords: microcirculation, functional capillary density, oxygen release, polyethylene glycol maleimide-conjugated hemoglobin, critical oxygen supply, NADH fluorescence
hemoglobin (Hb)-based O2 carriers (HbOCs) are being developed, in part, to supplement or substitute autologous blood transfusion use during surgical interventions, to provide additional O2-carrying capacity (23, 29). The most extensively studied and financed blood substitute was diaspirin cross-linked Hb (40). Intramolecular cross-linking of Hb overcame the nephrotoxicity but remained vasoconstrictive (40). The vasoactivity was credited to nitric oxide (NO) scavenging, Hb extravasation, and vascular hyperoxygenation (40). Polyethylene glycol (PEG)-conjugated bovine Hb was introduced by Enzon (17). This product carried 10 copies of 5-kDa PEG chains conjugated to the protein using succinimidyl carbonate chemistry and was not vasoconstrictive (17). Neutralization of vasoactivity was attributed to its enhanced molecular volume, viscosity, and high colloid osmotic pressure (COP), leading to a new approach for producing nonhypertensive Hb materials (33).
The application of PEGylated Hb (PEG-Hb) as O2-carrying plasma expanders is counterintuitive to the conventional wisdom of O2-carrying blood substitutes, because PEG-Hb exhibits high O2 affinity and is effective at low Hb concentrations. During experimental studies and clinical trials, PEG-Hb has been shown to be more effective than earlier formulations of blood substitute based on mimicking blood O2 transport properties (high Hb concentration and normal O2 affinity) (12, 38). PEG-Hb is not a blood substitute in the conventional sense, since it is designed to deliver O2 at a lower Po2 than red blood cells (RBCs) (38).
Experimental procedures that test the capacity of PEG-Hb to deliver O2 to the tissue are limited by its high O2 affinity, which precludes PEG-Hb taking part in the normal tissue O2 supply when tissue Po2 is ∼20 Torr. Reducing O2 availability lowers tissue Po2 and allows PEG-Hb to release O2. However, O2 release to the cellular O2 metabolism cannot be confirmed by tissue Po2 measurements, as they do not represent the oxidative state of the mitochondria. Since tissue Po2 after extreme hemodilution with PEG-Hb does not increase compared with identical interventions using non-O2-carrying plasma expanders, techniques to study intracellular O2 levels are needed to determine whether the circulation benefits from the presence of PEG-Hb (9).
The NADH/NAD+ redox state is an indicator of the decrease of intracellular O2 concentration (10, 11), which causes NAD+ and other members of the mitochondrial respiratory chain to be reduced (10, 11). Glycolysis yields pyruvate and ATP, in addition to NADH. When O2 is present, the NADH generated by glycolysis is oxidized by the mitochondria via the electron transport chain, resulting in ATP and NAD+. In the absence of enough intracellular O2 or other causes of inhibition of the electron transport chain, NADH accumulates, and the supply of NAD+ is depleted. NADH concentration can be measured based on the difference in the absorption spectrum of its reduced form; however, this approach is not suitable to the study of mitochondria NADH levels. More specific than absorption spectroscopy, fluorescence spectrophotometry in the near-ultraviolet range can be applied for NADH measurements in vivo. NADH absorbs light at 320–380 nm and emits fluorescent light at 420–480 nm; therefore, it is possible to evaluate the redox state of the mitochondria by monitoring the fluorescence of NADH (31).
We developed an experimental model in which NADH fluorescence can be recorded from the tissue of the hamster window model under different oxygenation conditions to assess changes in the NADH/NAD+ redox state of intact tissue to understand intracellular Po2 changes (31). It should be noted that the hamster skinfold chamber model includes muscle and connective tissue. This model, in combination with systemic and microvascular parameters and microvascular O2 levels after extreme hemodilution with PEG-Hb, was used to study how PEG-Hb induced changes in mitochondrial redox state. To achieve conditions of O2 supply limitation, the experimental hamster window chamber model was subjected to moderate hemodilution (MH) via two isovolemic exchanges with 6% 70-kDa dextran (Dex70) to induce an acute anemic state (18% Hct). After this moderate anemia, the animals were randomly exchange-transfused with PEG-Hb [P5K6-Hb, 6 copies of 5-kDa PEG conjugated through extension arm chemistry (EAC) to each human Hb] or 6% Dex70 to a condition of extreme hemodilution (11% Hct). Changes in microvascular function were characterized by determining effects on capillary flow, microhemodynamic changes, and oxygenation, and the tissue O2 state was assessed by NADH fluorescence.
METHODS
Animal preparation.
Male golden Syrian hamsters (55–65 g body wt; Charles River Laboratories, Boston, MA) were fitted with a dorsal window chamber. Animal handling and care followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the local animal care committee. The hamster window chamber model is widely used for microvascular studies in the unanesthetized state, and the complete surgical technique is described in detail elsewhere (13, 14). Catheters were tunneled under the skin, exteriorized at the dorsal side of the neck, and securely attached to the window frame.
Inclusion criteria.
Animals were suitable for the experiments if 1) systemic parameters were within normal range, namely, heart rate (HR) >340 beats/min, mean arterial blood pressure (MAP) >80 mmHg, systemic Hct >45%, and arterial Po2 >50 Torr, and 2) microscopic examination of the tissue in the chamber observed under a ×650 magnification did not reveal signs of edema or bleeding.
Test materials.
PEG-Hb (P5K6-Hb), succinimidophenyl PEG-Hb conjugate, was obtained through EAC of thiolated human Hb, with six copies of 5-kDa PEG, two of which are conjugated to the thiol of β-Cys93 and four to the ε-amino groups of lysine residues. The latter is accomplished through EAC introduced by reaction of iminothiolane with Hb (to introduce sulfhydryl groups to lysine residues) (1, 3, 5, 26). Hb (0.5 mM) was incubated with 5 mM iminothiolane in the presence of 10 mM 5-kDa maleimidophenyl PEG in PBS at 4°C overnight; then the PEGylated protein was isolated by tangential flow filtration (Minim, Pall, Port Washington, NY) with a 50-kDa membrane. The molecular mass of PEG-Hb is ∼95,000. Concentrations of Hb refer to grams of Hb tetramer. O2 equilibrium curves were measured using the method described previously (4), and other physical characterizations were P50 (i.e., Po2 at which Hb is 50% saturated with O2) of 5.6 Torr and Hill coefficient of 1.26.
O2 saturation.
O2 saturation curves for hamster blood and PEG-Hb were obtained by deoxygenation of O2-equilibrated samples in Hemox buffer at 37°C using a Hemox analyzer (TCS Scientific, New Hope, PA).
Experimental groups.
Animals were randomly divided into two experimental groups before the experiment: 1) Dex70 animals were treated with 6% 70-kDa dextran (Pharmacia, Uppsala, Sweden) and 2) PEG-Hb animals were treated with 4.0 g Hb/dl and 1.88 g PEG/dl.
Acute isovolemic exchange-transfusion (hemodilution) protocol.
Acute anemia was induced by two isovolemic hemodilution steps, as described in detail in our previous reports (4, 6, 36). Briefly, the volume of each exchange-transfusion step was calculated as a percentage of the blood volume, estimated as 7% of body weight. The acute anemic state was induced by lowering systemic Hct to 18% by progressive isovolemic hemodilution using 6 g Dex70/dl.
MH was randomly assigned to three experimental groups using a sorting scheme based on a list of random numbers (2). Group 1 was used to study microvascular O2 distribution at MH. In groups 2 and 3, 35% of blood volume was exchanged with Dex70 and PEG-Hb, respectively.
Blood was simultaneously withdrawn at the same rate from the carotid artery catheter according to a previously established protocol (4). Blood samples were withdrawn at the end of the experiment for subsequent analysis of viscosity and COP. The duration of the experiments was 4 h. Each exchange and the respective observation time point after exchange were completed in 1 h. Systemic and microcirculation data were taken after a stabilization period of 10 min.
Systemic parameters.
MAP and HR were recorded continuously (MP 150, Biopac System, Santa Barbara, CA). Hct was measured from centrifuged arterial blood samples collected in heparinized capillary tubes. Hb content was determined spectrophotometrically (B-Hemoglobin, Hemocue, Stockholm, Sweden).
Blood chemistry and biophysical properties.
Arterial blood was collected in heparinized glass capillaries (50 μl) and immediately analyzed for Po2, Pco2, base excess, and pH with use of a blood chemistry analyzer (model 248, Bayer, Norwood, MA). Blood samples for viscosity and COP measurements were quickly withdrawn into heparinized 5-ml syringes at the end of the experiment. Viscosity was measured in a DV-II Plus (Brookfield, Middleboro, MA). COP was measured using a colloid osmometer (model 4420, Wescor, Logan, UT).
Microvascular experimental setup.
The unanesthetized animal was placed in a restraining tube with a longitudinal slit from which the window chamber protruded and then fixed to the microscopic stage for transillumination with the intravital microscope (model BX51WI, Olympus, New Hyde Park, NY). Animals were given 20 min to adjust to the tube environment before any measurement. Tissue image was projected onto a charge-coupled device camera (COHU 4815) connected to a videocassette recorder and viewed on a monitor. Measurements were carried out using a ×40 (LUMPFL-WIR, 0.8 numerical aperture, Olympus) water-immersion objective. The same sites of study were followed throughout the experiment, so that comparisons could be made directly with baseline levels.
Functional capillary density.
Functional capillaries, defined as those capillary segments that have RBC transit of at least a single RBC in a 45-s period in 10 successive microscopic fields, were assessed, totaling a region of 0.46 mm2. Each field had between two and five capillary segments with RBC flow. Functional capillary (FCD, cm−1), i.e., total length of RBC-perfused capillaries divided by area of microscopic field of view, was evaluated by measuring and adding the length of capillaries that had RBC transit in the field of view. The relative change in FCD from baseline levels after each intervention is indicative of the extent of capillary perfusion (7).
Microhemodynamics.
A video image-shearing method was used to measure vessel diameter (D) (20). Changes in arteriolar and venular diameter from baseline were used as indicators of a change in vascular tone. Arteriolar and venular centerline velocities were measured online by the photodiode cross-correlation method (photodiode/velocity tracker model 102B, Vista Electronics, San Diego, CA). The measured centerline velocity (V) was corrected according to vessel size to obtain the mean RBC velocity (24). Blood flow (Q̇) was calculated from the measured values as follows: Q̇ = π × V(D/2)2. This calculation assumes a parabolic velocity profile and has been found to be applicable to 15- to 80-μm-ID tubes and 6–60% Hct (24).
Measurement of NADH.
Tissue fluorescence is a widely used and accepted method to measure NADH in vivo (15, 30–32). Areas of interest were located using the microscope, and the edge of the vessels was used to focus the image with bright-field transillumination and fluorescence epi-illumination. Fluorescence emission signals (420 and 460 nm filter; Thorslabs, Newton, NJ) were recorded using a photomultiplier (model R928, Hamamatsu) during fluorescent excitation (360 nm filter; Thorslabs) using a dichroic mirror (model XF2003 390DRLP, Omega Optical, Brattleboro, VT). The photomultiplier photometric head was mounted on the camera port of the trinocular head of the microscope. Fluorescence intensities were recorded as the tissue was excited (model MP 150, Biopac System; 200-Hz sampling rate). Filter wheels were rotated to switch from 420 to 460 nm. Fluorescence was excited with a mercury arc lamp (200 W); the lamp shutter was opened only for 2-s intervals to prevent photobleaching. To circumvent optical problems associated with thickness of the tissue, a partial confocal effect was created by placing a 150-μm pinhole in the excitation light path (19). The fluorescence ratio gives concentration-independent, NADH-sensitive fluorescence measurements (15, 30, 32). Fluorescence ratio was formed as follows: RNADH = (I460 − Iback460)/(I420 − Iback420). Background fluorescence intensities for each wavelength (Iback420 and Iback460) were recorded before fluorescent excitation.
Photometric assembly was combined with an adjustable rectangular diaphragm, mounted in front of the photomultiplier unit. The rectangular area was backilluminated and visualized through the binocular observation port with exact size and superimposed on the view field. This design allows precise location of the photometric window relative to the anatomic details of the study tissue. Photomultiplier signal output was calibrated in terms of the number of detected photons per unit area of emission. The photomultiplier current output was measured as voltage across a 10-kΩ resistor. NADH fluorescence was detected at 0.5–1.0 V with a window size of 15 × 15 μm.
Microvascular Po2 distribution.
High-resolution noninvasive microvascular Po2 measurements were made using phosphorescence quenching microscopy (PQM) (21, 36). PQM is based on the O2-dependent quenching of phosphorescence emitted by albumin-metalloporphyrin complex after pulsed light excitation. PQM is independent of the dye concentration within the tissue and is well suited for detecting hypoxia, because its decay time is inversely proportional to the Po2 level, causing the method to be more precise at low Po2. This technique is used to measure intravascular and extravascular Po2, since the albumin-dye complex continuously extravasates the circulation into the interstitial fluid (21, 36). Extravascular fluid Po2 (interstitial fluid) was measured in tissue regions between functional capillaries. PQM allows for precise localization of the Po2 measurements without subjecting the tissue to injury. These measurements provide a detailed understanding of microvascular O2 distribution and indicate whether O2 is delivered to the interstitial areas.
O2 delivery and extraction.
The microvascular methodology used in our studies allows a detailed analysis of O2 supply in the tissue. Calculations are made using Eqs. 1 and 2 (7)
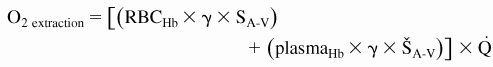 |
where RBCHb is Hb from RBCs [i.e., total Hb − plasma Hb (g Hb/dl blood)], plasmaHb is acellular Hb (g Hb/dl blood), γ is O2-carrying capacity of saturated Hb (1.34 ml O2/g Hb), SA is arteriolar blood O2 saturation, ŠA is arteriolar acellular Hb O2 saturation, A-V indicates arteriolar-venular differences, and Q̇ is microvascular flow. O2 saturations were measured as described above.
Data analysis.
Results are presented as means ± SD. The box-whisker plot separates the data into quartiles, with the top of the box defining the 75th percentile, the line within the box the median, and the bottom of the box the 25th percentile. The upper “whisker” defines the 95th percentile and the lower whisker the 5th percentile. Grubbs' method was used to assess closeness for all measured parameter values at baseline. This method quantifies how far each parameter value is from the other values obtained, computing a P value supposing that all the values were really sampled from a Gaussian population. Data within each group were analyzed using analysis of variance for repeated measurements (ANOVA, Kruskal-Wallis test). When appropriate, post hoc analyses were performed with Dunn's multiple comparison test. Microhemodynamic data are presented as absolute values and ratios relative to baseline values. A ratio of 1.0 signifies no change from baseline; lower and higher ratios are indicative of changes proportionally lower and higher than baseline (i.e., 1.5 would mean a 50% increase from the baseline level). The same vessels and capillary fields were followed, so that direct comparisons with their baseline levels could be performed, allowing for more robust statistics for small sample populations. All statistics were calculated using Prism 4.01 (GraphPad Software, San Diego, CA). Changes were considered statistically significant if P < 0.05.
RESULTS
Eighteen animals entered the study; all animals tolerated the entire hemodilution protocol without visible signs of discomfort. The animals were randomly assigned to the following experimental groups: MH (n = 6, 62 ± 4 g), Dex70 (n = 6, 63 ± 3 g), and PEG-Hb (n = 6, 60 ± 3 g). All animals included in the study passed the Grubbs' test, ensuring that all the measured parameter values at baseline were within a similar population (P < 0.05). Similarities between groups at baseline and prior randomization were statistically verified among groups (P > 0.30).
Systemic parameters.
The first and second hemodilution exchanges with Dex70 significantly reduced Hct and Hb. Blood gas parameters are presented in Table 1. Exchange with PEG-Hb and Dex70 decreased Hct to a similar degree. PEG-Hb presented higher total Hb compared with Dex10 due to the acellular Hb. MAP did not decrease from baseline after the first exchange with Dex70 (101 ± 6 mmHg), and upon the second hemodilution step, MAP significantly decreased from baseline. MAP and HR are presented in Table 1. Arterial blood gas analysis showed a significant increase in arterial Po2 and Pco2 from baseline for all groups. Arterial pH was not different from baseline, although PEG-Hb was different from Dex70. Base excess was significantly reduced for Dex70 compared with baseline, MH, and PEG-Hb. Comparison of rheological properties and COP of the blood for all study groups is presented in Table 2.
Table 1.
Systemic parameters before and after exchange protocol
| Extreme Hemodilution |
||||
|---|---|---|---|---|
| Baseline (n = 18) | Moderate Hemodilution (n = 18) | Dex70 (n = 6) | PEG-Hb (n = 6) | |
| Hct, % | 49.4 ± 1.0 | 18.9 ± 0.6† | 11.7 ± 0.5†‡ | 11.4 ± 0.4†‡ |
| Hb, g/dl | 14.7 ± 0.4 | 6.4 ± 0.4† | 3.7 ± 0.3†‡ | 4.9 ± 0.3*†‡ |
| Plasma Hb, g/dl | 1.1 ± 0.1 | |||
| MAP, mmHg | 112 ± 8 | 97 ± 7† | 67 ± 6†‡ | 86 ± 5*† |
| HR, beats/min | 430 ± 20 | 438 ± 26 | 403 ± 31 | 428 ± 29 |
| PO2, Torr | 57.3 ± 6.0 | 76.4 ± 7.0† | 101.0 ± 10.6†‡ | 88.4 ± 7.3*† |
| PCO2, Torr | 53.4 ± 5.1 | 49.0 ± 4.9† | 39.7 ± 6.4†‡ | 45.0 ± 5.4*† |
| pH | 7.345 ± 0.021 | 7.357 ± 0.036 | 7.326 ± 0.029 | 7.372 ± 0.024* |
| BE, mmol | 3.1 ± 1.4 | 1.3 ± 1.2† | −4.2 ± 2.0†‡ | 0.5 ± 1.1*† |
Values are means ± SD. Baseline includes all animals in the study. Dex70, 70-kDa dextran; PEG-Hb, polyethylene glycol-conjugated Hb; Hct, systemic hematocrit; MAP, mean arterial blood pressure; PO2 and PCO2, arterial PO2 and PCO2; BE, arterial base excess.
P < 0.05 vs. Dex70.
P < 0.05 vs. baseline.
P < 0.05 vs. moderate hemodilution.
Table 2.
Blood physical properties
| Moderate Hemodilution | Dex70 | PEG-Hb | |
|---|---|---|---|
| Hct, % | 18 | 11 | 11 |
| Viscosity,* cP | |||
| Plasma | 1.3 | 1.4 | 1.3 |
| Blood | 2.9 | 2.1 | 1.8 |
| COP, mmHg | 18 | 17 | 21 |
COP, colloid osmotic pressure at 27°C. Hct values are presented in Table 1.
Shear rate = 160 s−1 at 37°C.
Microhemodynamics.
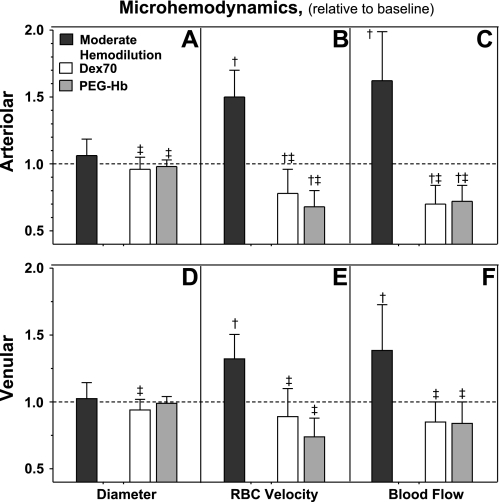
Microvascular changes, i.e., diameter, RBC velocity, and blood flow in arterioles (46–78 and 44–80 μm), were measured after each hemodilution step. Microvascular diameter and blood flow are presented in Fig. 1, A and D. Arteriolar diameter was unchanged after the first exchange. MH produced arteriolar dilation from baseline. Extreme hemodilution with Dex70 and PEG-Hb restored arteriolar vessel diameter to baseline. Venule diameters were not different from baseline after MH. Extreme hemodilution with Dex70 constricted venules compared with baseline and MH. Venular diameters with PEG-Hb were not different from baseline.
Fig. 1.
Changes in arteriolar and venular hemodynamics after moderate and extreme hemodilution with 70-kDa dextran (Dex70) and polyethylene glycol-conjugated Hb (PEG-Hb). Dashed line, baseline. †P < 0.05 vs. baseline. ‡P < 0.05 vs. moderate hemodilution. Diameters (μm, mean ± SD) in each animal group were as follows: 67.2 ± 8.0 (n = 52) for arterioles and 69.0 ± 8.8 (n = 60) for venules at baseline. RBC velocities (mm/s, mean ± SD) in each animal group were as follows: 4.7 ± 0.7 for arterioles and 1.9 ± 0.5 for venules at baseline. Flow (nl/s, mean ± SD) in each animal group was as follows: 14.7 ± 4.4 for arterioles and 6.2 ± 2.0 for venules at baseline.
RBC velocity in arterioles and venules is presented in Fig. 1, B and E. Arteriolar and venular RBC velocities increased from baseline after MH. Extreme hemodilution with Dex70 and PEG-Hb reduced arteriolar and venular RBC velocities from baseline and MH. The arteriolar and venular blood flows after hemodilution are presented in Fig. 1, C and F. Arteriolar and venular blood flow were significantly increased from baseline after MH. Further hemodilution with Dex70 and PEG-Hb decreased arteriolar and venular blood flow from baseline and MH. Arteriolar and venular flow for Dex70 and PEG-Hb were not different.
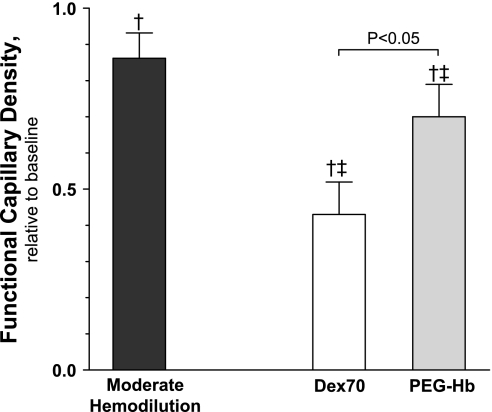
FCD.
FCD is shown in Fig. 2. MH decreased FCD from baseline. After extreme hemodilution with Dex70 and PEG-Hb, FCD was further reduced from baseline and MH.
Fig. 2.
Functional capillary density (FCD) after moderate and extreme hemodilution with Dex70 and PEG-Hb. †P < 0.05 vs. baseline. ‡P < 0.05 vs. moderate hemodilution.
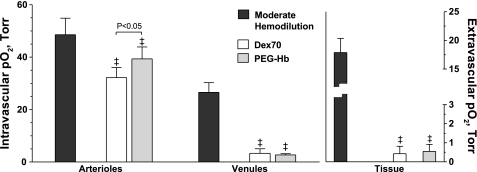
Microvascular O2 distribution.
Intravascular and extravascular Po2 is presented in Fig. 3. Increasing plasma O2-carrying capacity with PEG-Hb significantly increased arteriolar Po2 compared with Dex70, although venular and extravascular Po2 were not different among extreme hemodiluted groups. According to previous studies, normal extravascular Po2 for the hamster window model is 21.7 ± 3.5 Torr (22).
Fig. 3.
Intravascular and extravascular Po2 after moderate and extreme hemodilution with Dex70 and PEG-Hb. ‡P < 0.05 vs. moderate hemodilution.
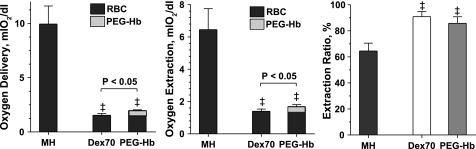
Calculated O2 delivery and extraction.
Figure 4 shows the analysis of O2 delivery and extraction in the microcirculation. PEG-Hb increased microvascular O2 delivery and extraction compared with Dex70. The contribution of native RBCs was similar among extreme hemodiluted groups.
Fig. 4.
Arterial O2 delivery and extraction after moderate hemodilution (MH) and extreme hemodilution with Dex70 and PEG-Hb. ‡P < 0.05 vs. MH. O2 transport is not directly measurable; however, it can be calculated using measured parameters. Delivery and extraction were calculated from averaging arterioles and venules of each animal. Difference in O2 delivery and extraction between PEG-Hb and Dex70 was statistically significant, P < 0.05.
NADH levels.
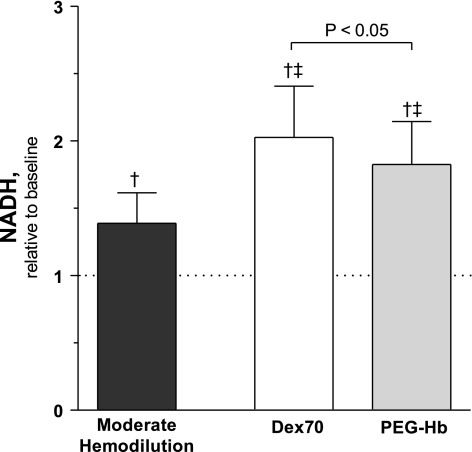
Figure 5 shows NADH fluorescence from baseline. MH increased NADH fluorescence from baseline. Extreme hemodilution with Dex70 and PEG-Hb increased NADH fluorescence compared with baseline and MH. NADH fluorescence was higher for Dex70 than PEG-Hb.
Fig. 5.
NADH extravascular levels after moderate and extreme hemodilution with Dex70 and PEG-Hb. Dashed line, baseline. †P < 0.05 vs. baseline. ‡P < 0.05 vs. moderate hemodilution. Difference in tissue NADH levels between PEG-Hb and Dex70 was statistically significant, P < 0.05.
DISCUSSION
The principal finding of this study is that extreme hemodilution (11% Hct) with PEG-Hb and Dex70 produced similar interstitial fluid O2 levels in the hamster window model. However, tissue anoxia characterized by local NADH fluorescence was significantly reduced with PEG-Hb compared with Dex70. Accordingly, the intravascular presence of high-affinity acellular Hb increases calculated microvascular O2 delivery and extraction, without increasing extravascular Po2 (9, 37). Determination of NADH fluorescence and calculated O2 delivery and extraction coincided to show that the hamster window chamber tissue is O2-depleted after extreme hemodilution and that PEG-Hb reduced NADH accumulation by partially sustaining O2 metabolic needs. After extreme hemodilution, PEG-Hb (1.1 g Hb/dl blood) increased total blood O2-carrying capacity by 32% compared with animals hemodiluted with only dextran. In addition to the increase in blood O2-carrying capacity, when the extravascular Po2 drops to the levels attained after extreme hemodilution, the importance of PEG-Hb-facilitated O2 diffusion increases significantly. PEG-Hb increased plasma O2 concentration by at least one order of magnitude compared with dissolved O2, although the PEG-Hb-mediated increase in plasma O2 is partially counterbalanced by its slower diffusion coefficient compared with physically dissolved O2 (25).
Our results show that PEG-Hb increases O2 delivery in conditions where native O2-carrying capacity is severely decreased. In the extreme anemic conditions of this study (11% Hct), RBCs are <50% saturated with O2 (hamster P50 is 32 Torr) as they arrive at the microcirculation, while at the microcirculation the high O2 affinity of PEG-Hb was >95% saturated with O2, and, therefore, higher concentrations of O2 were available to the tissue. The PEG-Hb used in this study was produced by a one-step method of OxyHb thiolation and PEG maleimidation; it offers a simple, reliable method to produce and modify Hb to produce an O2 carrier. The six succinimidophenyl PEG chains were conjugated through EAC onto thiolated human Hb. Two PEG chains were conjugated to the thiols of β-Cys93 units of the Hb, and four were conjugated to the ε-amino groups of lysine residues distributed on the individual α- and β-globin subunits of the Hb. The PEG mass distribution (5 kDa) is narrow; thus the sites of modification under the standardized reaction conditions had limited variability. The thiolation/PEGylation reaction conditions with OxyHb narrow the number of sites from 20 potential sites of modification identified (3, 26).
In these experiments of extreme hemodilution with PEG-Hb, ∼22% of the total Hb was acellular Hb. This fraction of plasma Hb provided 27% of the microvascular O2 delivered and 20% of the total microvascular O2 extracted. Conversely, microvascular O2 extraction when extreme hemodilution was induced with a non-O2 carrier (Dex70) was only 78% of that when PEG-Hb was present in the circulation. PEG-Hb also led to higher FCD after extreme hemodilution, an additional factor that promotes O2 release. Thus, O2 release, by high-O2-affinity Hb solutions, such as PEG-Hb, partially prevented tissue anoxic states, as supported by the measured NADH fluorescence.
The principal microhemodynamic difference between extreme hemodilution with PEG-Hb and Dex70 was capillary perfusion (FCD). In studies of resuscitation from hemorrhagic shock, FCD was a more reliable indicator of survival than blood flow or tissue Po2 (22). Microvascular perfusion is governed by FCD, which is regulated by arteriolar tone (7, 8). Vascular tone is controlled, to a large degree, by NO, primarily generated by endothelial NO (28). When acellular Hb solutions are present in the circulation, NO reacts rapidly with acellular OxyHb to produce methemoglobin and nitrate (16). This rapid and irreversible reaction at high concentrations of intravascular acellular OxyHb implies that the endothelium-derived NO is insufficient for normal autocrine diffusion to smooth muscle (16, 33). Our results suggest that a plasma PEG-Hb concentration of ∼1 g Hb/dl is insufficient for causing a significant inactivation of endothelial NO.
The introduction of acellular Hb solutions in conditions of maximally reduced native O2-carrying capacity highlights vasoactive effects. Previous studies show that vasoactive Hb solutions, such as Oxyglobin Biopure (Boston, MA) and αα-Hb, decrease microvessel diameter, blood flow, and FCD compared with PEG-Hb (8, 37). Thus, vasoconstriction cannot solely be explained by NO scavenging. Two complementary hypotheses for acellular Hb vasoactivity are as follows: 1) large Hb molecules have slow diffusion properties and, therefore, do not overload arterioles with O2 (a cause of vasoconstriction due to metabolic autoregulation); and 2) Hb molecules with large hydrodynamic diameter are not effective NO scavengers, because they do not extravasate and intercalate between the endothelium and smooth muscle. Given the proposed sequence of events that follows infusion of acellular Hb, a critical parameter for successful acellular HbOCs is to reduce molecular diffusivity by increasing size and viscosity, according to the Stokes-Einstein law. The diffusion coefficient for acellular Hb increases proportionally to the absolute temperature and inversely to the solution viscosity and molecular radius. PEG-Hb has increased molecular radius and viscosity compared with early-generation HbOCs (26, 41). Comparative analysis of the results obtained in the hamster window model tends to support the O2 autoregulation mechanism, since vasoconstriction has been observed with mostly low-O2-affinity Hb solutions. Low-affinity Hb solutions release O2 prior to arriving to the microcirculation, which activates the autoregulatory mechanism to limit O2 delivery by increasing peripheral vascular resistance.
MAP was higher in PEG-Hb than Dex70 animals, however, a simple mathematical approximation of vascular resistance using the Hagen-Poiseuille equation indicated that local resistances were not different at the microvascular level, since diameter, velocity, and blood flow were not different between groups. This supports the concept that low precapillary (arteriolar) O2 release prevents arteriolar vasoconstriction, allowing systemic pressure to arrive at the capillary network (8). Our microvascular results with PEG-Hb are similar to previous findings showing maintenance of microvascular function and FCD within normal physiological limits after hemodilution (9, 37). During extreme hemodilution, only high-viscosity plasma expanders maintain FCD by redistributing microvascular pressure (8). However, PEG-Hb does not increase plasma viscosity; therefore, PEG-Hb efficacy should be attributable to its O2-carrying capacity properties (high O2 affinity and moderate concentration) (18, 37).
NADH is a naturally occurring fluorophore that transfers electrons by means of an electron transport chain located in the inner membrane of mitochondria (10, 34, 35). Under anoxic conditions, intracellular NADH accumulates. Unlike the oxidized form NAD+, NADH is highly fluorescent (39). Therefore, changes in NADH fluorescence are due to changes in O2 supply or consumption. The hamster window model includes muscle and connective tissue with stable O2 consumption; therefore, predominant changes of O2 supply determine the tissue O2 state. The paradoxical reduction of NADH fluorescence after extreme hemodilution with PEG-Hb without considerable effects on extravascular Po2 (1.7 ± 1.1 and 1.3 ± 1.2 Torr for PEG-Hb and Dex70, respectively) suggests that the similar extravascular Po2 measured are due to higher tissue O2 consumption that released in both groups. The differences in intracellular redox state measured by the fluorescence of the accumulated NADH are the consequence of higher O2 released by the high-O2-affinity PEG-Hb transiting through open capillaries. Although microvascular O2 delivery cannot be directly measured, it can be calculated as presented in Fig. 4. Accordingly, the intravascular presence of high-O2-affinity PEG-Hb increases calculated microvascular O2 delivery and extraction, results that are partially verified with the measurement of NADH fluorescence. O2 transport from the blood to intracellular mitochondria is a process governed by the law of diffusion. Therefore, Po2 should be substantially lower at the mitochondria than in the extracellular fluid and the blood vessel lumen. Although accurate measurements of cellular and intracellular O2 gradients remained elusive because of technical difficulties, it is highly likely that the mitochondria have lower local Po2 than other cell compartments, such as the cytoplasm (27).
NADH fluorescence was used in this study as a marker for tissue O2 state. Results are presented as changes relative to baseline. In vivo fluorescence from reduced pyridine nucleotides, NADH, can be difficult to distinguish from reduced NADPH because of spectral and temporal emission similarities (39). Thus the fluorescence measured could be the sum of both, although their relative contributions cannot be determined. Therefore, some caution must be used when interpreting these data. NADPH is used extensively in biosynthetic pathways in major organs (15, 30, 35), but these pathways are not present in the muscle and skin tissue. Furthermore, previous studies in this type of tissue report small cellular concentrations of NADPH compared with NADH (15, 30, 39). Additionally, interpretation of fluorescence measurements from tissue can be confounded by tissue heterogeneity. However, in the hamster window model, the tissue is a thin preparation, precluding this problem.
After MH, extravascular Po2 decreased slightly from baseline (21.7 ± 3.5 Torr at baseline and 18.2 ± 2.4 Torr with MH), and, surprisingly, NADH fluorescence increased 40% from baseline. The increase in NADH fluorescence is not due to changes in optical properties of the tissue, since our technique normalizes the NADH fluorescence signal (460 nm) relative to the tissue optical properties at 420 nm. Use of two tissue isobestic wavelengths accounts for possible alterations in myoglobin light absorption, water content, and other fluorescent elements (32). Additionally, because NADH fluorescence was measured at locations without blood vessels, change in blood properties (blood light absorption) should not affect our results. Therefore, the increase in NADH fluorescence after MH is likely to be an indication of tissue O2 deficit or disruption of cellular O2 balance.
In conclusion, a general problem in supplying O2 to the tissues is transport of O2 from the lung with minimal losses, so that the bulk of O2 can be released where needed, rather than in compartments with an adequate O2 supply. Molecular Hb solutions have the capacity to enhance O2 transport from blood to tissue because of facilitated diffusion and the direct molecular contact between the O2 carrier and the blood vessel wall. Conversely, the introduction of a high-O2-affinity acellular Hb allows for O2 to remain in the circulating blood, while allowing its release in O2-deficient localities, such as the capillaries, without triggering autoregulatory protective mechanisms. Arterial O2 content and extravascular Po2 are basic indicators of appropriate tissue O2 supply; however, in conditions close to extreme anoxia, NADH levels should be a more reliable indicator of tissue metabolic conditions. In this context, the presence of moderate concentrations of a high-affinity acellular Hb in the circulation appears to significantly improve oxidative metabolism in the tissue during extreme anemic conditions.
GRANTS
This work was partially supported by National Heart, Lung, and Blood Institute Bioengineering Research Partnership Grant R24-HL-64395, Program Project Grant P01-HL-071064, and Grants R01-HL-62354 and R01-HL-62318.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Froilan P. Barra and Cynthia Walser for the surgical preparation of the animals.
REFERENCES
- 1. Acharya AS, Manjula BN, Smith PK. Hemoglobin Crosslinkers. US Patent 5,585,484, 1996
- 2. Altman DG, Bland JM. Statistics notes: how to randomise. BMJ 319: 703–704, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ananda K, Acharya SA. Role of extension arm in PEG-Hb conjugates on the stability of the tetramer: non-conservative EAF maleimide thio-PEG mediated PEGylation. Artif Cells Blood Substit Immobil Biotechnol 36: 499–512, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Cabrales P, Kanika ND, Manjula BN, Tsai AG, Acharya SA, Intaglietta M. Microvascular Po2 during extreme hemodilution with hemoglobin site specifically PEGylated at Cys-93(β) in hamster window chamber. Am J Physiol Heart Circ Physiol 287: H1609–H1617, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Cabrales P, Nacharaju P, Manjula BN, Tsai AG, Acharya SA, Intaglietta M. Early difference in tissue pH and microvascular hemodynamics in hemorrhagic shock resuscitation using polyethylene glycol-albumin- and hydroxyethyl starch-based plasma expanders. Shock 24: 66–73, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Cabrales P, Tsai AG, Frangos JA, Briceno JC, Intaglietta M. Oxygen delivery and consumption in the microcirculation after extreme hemodilution with perfluorocarbons. Am J Physiol Heart Circ Physiol 287: H320–H330, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Cabrales P, Tsai AG, Intaglietta M. Microvascular pressure and functional capillary density in extreme hemodilution with low and high plasma viscosity expanders. Am J Physiol Heart Circ Physiol 287: H363–H373, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Cabrales P, Tsai AG, Winslow RM, Intaglietta M. Effects of extreme hemodilution with hemoglobin-based O2 carriers on microvascular pressure. Am J Physiol Heart Circ Physiol 288: H2146–H2153, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Cabrales P, Tsai AG, Winslow RM, Intaglietta M. Extreme hemodilution with PEG-hemoglobin vs. PEG-albumin. Am J Physiol Heart Circ Physiol 289: H2392–H2400, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Chance B, Oshino N, Sugano T, Mayevsky A. Basic principles of tissue oxygen determination from mitochondrial signals. Adv Exp Med Biol 37A: 277–292, 1973 [DOI] [PubMed] [Google Scholar]
- 11. Chance B, Williams GR. A method for the localization of sites for oxidative phosphorylation. Nature 176: 250–254, 1955 [DOI] [PubMed] [Google Scholar]
- 12. Chang TM. Therapeutic applications of polymeric artificial cells. Nat Rev Drug Discov 4: 221–235, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Colantuoni A, Bertuglia S, Intaglietta M. Quantitation of rhythmic diameter changes in arterial microcirculation. Am J Physiol Heart Circ Physiol 246: H508–H517, 1984 [DOI] [PubMed] [Google Scholar]
- 14. Endrich B, Asaishi K, Götz A, Messmer K. A new chamber technique for microvascular studies in unanaesthetized hamsters. Res Exp Med (Berl) 177: 125–134, 1980 [DOI] [PubMed] [Google Scholar]
- 15. Eng J, Lynch RM, Balaban RS. Nicotinamide adenine dinucleotide fluorescence spectroscopy and imaging of isolated cardiac myocytes. Biophys J 55: 621–630, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gladwin MT, Crawford JH, Patel RP. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation. Free Radic Biol Med 36: 707–717, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Gulati A, Barve A, Sen AP. Pharmacology of hemoglobin therapeutics. J Lab Clin Med 133: 112–119, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Hellums JD, Nair PK, Huang NS, Oshima N. Simulation of intraluminal gas transport processes in the microcirculation. Ann Biomed Eng 24: 1–24, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and Po2 gradients in solid tumors in vivo: high resolution measurements reveal a lack of correlation. Nat Med 3: 177–182, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Intaglietta M, Tompkins WR. Microvascular measurements by video image shearing and splitting. Microvasc Res 5: 309–312, 1973 [DOI] [PubMed] [Google Scholar]
- 21. Kerger H, Groth G, Kalenka A, Vajkoczy P, Tsai AG, Intaglietta M. Po2 measurements by phosphorescence quenching: characteristics and applications of an automated system. Microvasc Res 65: 32–38, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Kerger H, Saltzman DJ, Menger MD, Messmer K, Intaglietta M. Systemic and subcutaneous microvascular Po2 dissociation during 4-h hemorrhagic shock in conscious hamsters. Am J Physiol Heart Circ Physiol 270: H827–H836, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Kreimeier U, Messmer K. Hemodilution in clinical surgery: state of the art 1996. World J Surg 20: 1208–1217, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Lipowsky HH, Zweifach BW. Application of the “two-slit” photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res 15: 93–101, 1978 [DOI] [PubMed] [Google Scholar]
- 25. McCarthy MR, Vandegriff KD, Winslow RM. The role of facilitated diffusion in oxygen transport by cell-free hemoglobins: implications for the design of hemoglobin-based oxygen carriers. Biophys Chem 92: 103–117, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Meng F, Manjula BN, Tsai AG, Cabrales P, Intaglietta M, Smith PK, Prabhakaran M, Acharya SA. Hexa-thiocarbamoyl phenyl PEG5K Hb: vasoactivity and structure: influence of rigidity of the conjugation linkage on the PEGylation induced plasma expander-like solution properties of PEG-Hb adducts. Protein J 28: 199–212, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Mik EG, Stap J, Sinaasappel M, Beek JF, Aten JA, van Leeuwen TG, Ince C. Mitochondrial Po2 measured by delayed fluorescence of endogenous protoporphyrin IX. Nat Methods 3: 939–945, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Moncada S, Palmer MJ, Higgs EA. Nitric oxide: physiology, pathophysiology, pharmacology. Pharmacol Rev 43: 109–134, 1991 [PubMed] [Google Scholar]
- 29. Monk TG, Goodnough LT, Birkmeyer JD, Brecher ME, Catalona WJ. Acute normovolemic hemodilution is a cost-effective alternative to preoperative autologous blood donation by patients undergoing radical retropubic prostatectomy. Transfusion 35: 559–565, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Nuutinen EM. Subcellular origin of the surface fluorescence of reduced nicotinamide nucleotides in the isolated perfused rat heart. Basic Res Cardiol 79: 49–58, 1984 [DOI] [PubMed] [Google Scholar]
- 31. Richmond KN, Shonat RD, Lynch RM, Johnson PC. Critical Po2 of skeletal muscle in vivo. Am J Physiol Heart Circ Physiol 277: H1831–H1840, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Riess ML, Camara AK, Chen Q, Novalija E, Rhodes SS, Stowe DF. Altered NADH and improved function by anesthetic and ischemic preconditioning in guinea pig intact hearts. Am J Physiol Heart Circ Physiol 283: H53–H60, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Rohlfs RJ, Brunner E, Chiu A, Gonzales A, Gonzales ML, Magde D, Magde MD, Jr, Vandegriff KD, Winslow RM. Arterial blood pressure responses to cell-free hemoglobin solutions and the reaction with nitric oxide. J Biol Chem 273: 12128–12134, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Rosenthal M, Martel D, LaManna JC, Jobsis FF. In situ studies of oxidative energy metabolism during transient cortical ischemia in cats. Exp Neurol 50: 477–494, 1976 [DOI] [PubMed] [Google Scholar]
- 35. Tischler ME, Friedrichs D, Coll K, Williamson JR. Pyridine nucleotide distributions and enzyme mass action ratios in hepatocytes from fed and starved rats. Arch Biochem Biophys 184: 222–236, 1977 [DOI] [PubMed] [Google Scholar]
- 36. Tsai AG, Friesenecker B, McCarthy M, Sakai H, Intaglietta M. Plasma viscosity regulates capillary perfusion during extreme hemodilution in hamster skin fold model. Am J Physiol Heart Circ Physiol 275: H2170–H2180, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Tsai AG, Vandegriff KD, Intaglietta M, Winslow RM. Targeted O2 delivery by low-P50 hemoglobin: a new basis for O2 therapeutics. Am J Physiol Heart Circ Physiol 285: H1411–H1419, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Vandegriff KD, Malavalli A, Woodridge J, Lohman J, Winslow RM. MP4, a new nonvasoactive PEG-Hb conjugate. Transfusion 43: 509–516, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Wakita M, Nishimura G, Tamura M. Some characteristics of the fluorescence lifetime of reduced pyridine nucleotides in isolated mitochondria, isolated hepatocytes, and perfused rat liver in situ. J Biochem (Tokyo) 118: 1151–1160, 1995 [DOI] [PubMed] [Google Scholar]
- 40. Winslow RM. Alternative oxygen therapeutics: products, status of clinical trials, and future prospects. Curr Hemat Rep 2: 503–510, 2003 [PubMed] [Google Scholar]
- 41. Winslow RM. MP4, a new nonvasoactive polyethylene glycol-hemoglobin conjugate. Artif Organs 28: 800–806, 2004 [DOI] [PubMed] [Google Scholar]