Abstract
Tendon disuse, or stress deprivation, frequently accompanies clinical disorders and treatments, yet the metabolism of tendons subject to stress deprivation has rarely been investigated systematically. The effects of stress deprivation on canine flexor tendon were investigated in this study. One adult canine forepaw was suspended for 21 or 42 days. Control forepaws were collected from dogs that had no intervention on their limbs and paws. The expression of collagen I and III was not significantly altered in the tendons disused for 21 days but was significantly decreased at 42 days (P < 0.03). The expression of collagen II, aggrecan, decorin, and fibronectin was significantly decreased in the tendons in the suspended limbs at 21 days (P < 0.002) and further reduced at 42 days. With stress deprivation, the expression of matrix metalloproteinase 2 (MMP2) was significantly increased (P < 0.004) at 21 and 42 days. The expression of MMP3 was significantly decreased at 21 and 42 days (P < 0.03). The expression of MMP13 was not altered with stress deprivation at 21 and 42 days. The expression of MMP14 was significantly increased at 21 days (P = 0.0015) and returned to the control level at 42 days. Tissue inhibitor of metalloproteinase 1 (TIMP1) expression was decreased after the limbs were suspended for 42 days (P = 0.0043), but not 21 days. However, TIMP2 expression was not significantly different from control at 21 or 42 days. Furthermore, the cross-sectional area of the stress-deprived tendons at 42 days was decreased compared with the control group (P < 0.01). The intervention method in this study did not result in any alteration of stiffness of the tendon. Our study demonstrated that stress deprivation decreases the anabolic process and increases the catabolic process of extracellular matrix in flexor tendon.
Keywords: stress deprivation, expression of extracellular matrix-related genes, mechanical property
tendon primarily transmits tensile force between muscle and bone. It is a metabolically active structure. Mechanical stress plays an important role in the development, degeneration, and regeneration of the tendon (25, 40, 53).
The tendon remodels in response to exercise, which impacts tendon composition, structure, and mechanical properties (13, 26). The synthesis of collagen I, a major extracellular matrix (ECM) component in the tendon, increases in human tendon in response to acute exercise (21, 31, 35) and prolonged training (30). The mRNA levels for collagen I and III increase in response to training (6, 22, 37).
With the increase of collagen synthesis following exercise, collagen degradation, which is regulated by matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs), also increases (28), so that collagen turnover is significantly increased after exercise (25), resulting in an increased net synthesis of collagen in the tendon. In accordance with the net collagen synthesis, in human and animal models, exercise results in enlargement of tendon diameter (10, 27, 42, 53) and increase of tensile strength and stiffness (29, 41, 53). In contrast, excessive loading or overuse can result in deterioration in the mechanical properties of the tendon, including decreased modulus of elasticity and decreased maximum stress to failure (46).
At the opposite extreme from normal use, exercise, and overuse of tendons, tendon disuse, or stress deprivation, also occurs. Tendon disuse frequently accompanies a number of disorders, such as infection, tumors, congenital deformities, degenerative diseases, trauma, and treatments for these disorders, which frequently include immobilization, prohibition of weight bearing, and bed rest. For example, the flexor digitorum profundus (FDP) tendon is normally deprived of stress for several months by limiting its movement and loading after it is injured and repaired (47). A better understanding of the effects of stress deprivation could improve our understanding of the recuperation process and, potentially, improve rehabilitation protocols.
Although a number of studies have investigated the effects of tendon disuse and immobilization, the results with regard to protein synthesis, gene expression, and mechanical properties have been limited and inconsistent. A number of studies have reported that disuse results in a decrease in collagen synthesis (18, 24, 43), but an increase in collagen synthesis has also been reported, although it is associated with an increase in collagen degradation, in a rabbit patellar tendon immobilization model (5, 15, 20). In addition, few studies have investigated the effects of disuse on other important ECM components in the tendon, such as decorin and fibronectin, and the components related to catabolism of ECM, such as MMPs and TIMPs. More studies are necessary to further clarify and characterize the effects of stress deprivation on the tendon. In this study, we investigated the effect of stress deprivation on the cross-sectional area and tensile property of canine flexor tendon and metabolism of ECM components, such as collagen I, collagen II, collagen III, aggrecan, decorin, fibronectin, MMPs, and TIMPs, in the tendon in vivo.
MATERIALS AND METHODS
Animals and tendons.
Forty FDP tendons were harvested from the third and fourth digits of the forepaws of 20 adult mongrel dogs (10–15 mo old, 20–25 kg body wt) that had been killed in the course of other studies (49, 55, 56) approved by our Institutional Animal Care and Use Committee. As a part of those studies, which involved surgery on the second and fifth digit tendons, the dogs were treated with a non-weight-bearing protocol, in which the operated forepaw in each dog was splinted in wrist flexion and a sling was used to maintain the paw underneath the chest with a custom-made canine jacket (Fig. 1) for 21 (n = 10) or 42 (n = 10) days. Postoperative care included 10 min of passive motion exercise of the paw and digits twice daily, 7 days/wk, to prevent joint contracture and adhesion formation in the operated digits.
Fig. 1.
Technique for suspension of canine forelimb. A jacket was used to suspend 1 forelimb.
As a control group, we used 20 FDP tendons harvested from forepaws of 10 dogs of similar age, sex, and breed that were involved in unrelated Institutional Animal Care and Use Committee-approved studies of cardiac physiology that did not include interventions involving their legs or paws. In each group, 10 FDP tendons from the third digit were used for analysis of gene expression with real-time RT-PCR, and 10 FDP tendons from the fourth digit were used for evaluations of cross-sectional area and tensile property.
Real-time RT-PCR.
The metabolism of the tendon was investigated by quantification of the expression of ECM-related genes in the tendons with real-time RT-PCR. After the dogs were killed, 10-mm-long segments underneath the distal pulley were harvested from flexor tendons of the third digits. Tendon pieces were stored at −80°C until RNA extraction. Each specimen was homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA) with a MIKRO Dismembrator (B. Braun Biotech). Total RNA was extracted from the tendon segment according to the manufacturer's protocol. Contaminating genomic DNA was digested with DNase (Roche Applied Science, Indianapolis, IN) and further removed using the RNeasy Mini Kit (Qiagen, Valencia, CA). RNA concentration was determined using a RiboGreen RNA quantification kit (Invitrogen), then RNA was reverse transcribed into single-stranded cDNA using the Transcriptor First-Strand cDNA Synthesis Kit (Roche Applied Science). Quantitative RT-PCR was performed with LightCycler 1.5 (Roche Applied Science) to measure the gene expressions of collagen I, collagen II, collagen III, aggrecan, decorin, fibronectin, MMP2 (gelatinase A), MMP3 (stromelysin 1), MMP13 (collagenase 3), MMP14 (membrane-type MMP1), TIMP1, and TIMP2 with an annealing temperature of 60–62°C and magnesium concentration of 3.5 mM. The PCR primers designed from canine-specific cDNA sequences are listed in Table 1.
Table 1.
Primers and amplicon information
| Primer (5′–3′) |
||||
|---|---|---|---|---|
| Gene | GeneBank Accession No. | Sense | Antisense | Size, bp |
| Col1A1 | AF153062 | TGGTTCTCCTGGCAAAGAT | ATCACCGGGTTCACCTTTA | 232 |
| Col2A1 | AF035120 | GTAACCACCAAGGAAATGGCTA | TGGAACCTTGCAGAATGAC | 154 |
| Col3A1 | XM_535997 | ACAGCAGCAAGCTATTGAT | GGACAGTCTAATTCTTGTTCGT | 156 |
| Aggrecan | CFU65989 | GCACAACAGTCACACCT | GCTGACAAAGAAGTTGTCGG | 189 |
| Decorin | NM_001003228 | CAACAGAGAGGCTTATTTGACTTTA | TGGTACTTTGTCCAGACCC | 174 |
| Fibronectin | CFU52106 | GATGACTCGTGCTTCGAC | CTTCTCGCCAATCTTGTAGTT | 183 |
| MMP2 | AF177217 | AGCTACTTCTTCAAGGGTG | GTGTGCAGAAGGCAATG | 150 |
| MMP3 | NM_001002967 | GGAGAGGCTGACATAAAGATT | GATGTATCGCTTGTCCATTG | 161 |
| MMP13 | AF201729 | TACAACTTGTTCCTTGTCGC | CTGGGCCATAGAGAGACT | 166 |
| MMP14 | AY534615 | AAGCACTGGGTGTTTGAT | GTTCCTCGTTGAAACGGT | 169 |
| TIMP1 | AY534616 | CAGCGAGGAGTTTCTGG | GGTAAACACTGTGCACCC | 157 |
| TIMP2 | NM_001003082 | GAGGAAAGAAGGAGTATCTCATTG | CCGGAGACGAGATATAGC | 195 |
| β-Actin | Z70044 | CTGGCACCACACCTTCTA | GCCAGAGGCATACAGGGA | 184 |
| GAPDH | AB038240 | TATGATTCTACCCACGGCAA | CAGTGGACTCCACAACATAC | 154 |
Col1A1, Col2A1, and Col3A1, collagen I, II, and III; MMP2, MMP3, MMP13, and MMP14, matrix metalloproteinase 2, 3, 13, and 14; TIMP1 and TIMP2, tissue inhibitor of metalloprotease 1 and 2.
To find a reference gene for RT-PCR, we tested β-actin and GAPDH. The β-actin-to-RNA ratio significantly changed with the suspension of the limb. However, there was no significant difference in the GAPDH-to-RNA ratio between the normal tendon and the tendon in the limbs suspended for up to 6 wk. Therefore, GAPDH was chosen as reference gene, as in previous studies (7, 8, 48).
Cross-sectional area measurement and tensile test.
Cross-sectional area and tensile strength of the flexor tendon segments from the fourth digits were measured. After the PCR samples were harvested from the third digits, the remaining parts of the undissected forepaws were placed in plastic bags and stored at −80°C. The paws were thawed 1 night before the dissection of flexor tendons in fourth digits for mechanical testing. After the tendon was harvested, its size was measured with a digital caliper for calculation of cross-sectional area. The tendons were then mounted in a servohydraulic testing machine (MTS Systems, Minneapolis, MN) using clamps with interdigitating grooves. The barbed anchor pins at each end of a differential variable-reluctance transducer (MicroStrain, Burlington, VT) were inserted into the tendon to surround the region of interest with an initial distance of 12–14 mm to measure the displacement of the tendon under loading. A preload of 2 N was applied to the tendons before they were stretched. Tendons were distracted at a rate of 20 mm/min. Tensile force and displacement data were collected at a rate of 20 Hz. Throughout the testing, the tendons were kept moist with physiological saline solution. Stiffness was measured from the slope of the load-elongation curve in the linear region.
Statistical methods.
Means and SDs for expression of each gene, cross-sectional area, and stiffness were calculated for each group. The overall comparisons of gene expression, cross-sectional area, and stiffness among the control group and two stress-deprivation groups were analyzed by one-way ANOVA. The Tukey-Kramer honestly significant difference test was used as a post hoc test. P < 0.05 was considered to indicate statistical significance. All statistical analyses were performed with JMP 8 (SAS Institute, Cary, NC).
RESULTS
Stress deprivation resulted in reduction of the expression of collagens in the disuse tendons compared with the tendons in the limbs with free motion (Fig. 2). After stress deprivation for 21 days, the expression of collagen I in the FDP tendons was reduced to 72% of that of the control tendon, but this difference was not statistically significant (P = 0.2933). After stress deprivation for 42 days, the expression of collagen I in the FDP tendon was 26% of that of the control tendon (P = 0.0075). Collagen II demonstrated a more dramatic reduction than collagen I. With limb suspension for 21 and 42 days, the expression of collagen II was significantly reduced to 9% and 2%, respectively, of that of the control tendon. In contrast, the expression of collagen III was reduced less than the expression of collagen I, with no difference at 21 days, and was reduced to 40% of the control tendon at 42 days (P = 0.0222).
Fig. 2.
Gene expression of collagen I, II, and III (Col1A1, Col2A1, and Col3A1) in canine flexor FDP tendons in control and stress-deprived limbs at 21 and 42 days. Significant difference is shown by different letters (a, b).
Stress deprivation also resulted in a significant reduction in the expression of aggrecan, decorin, and fibronectin (Fig. 3), which further decreased with the longer period of stress deprivation. After stress deprivation for 21 days, the expression of aggrecan, decorin, and fibronectin in the flexor tendons was reduced to 23%, 52%, and 52% of that in the control tendons, respectively (all P < 0.002). With stress deprivation for 42 days, the expression of aggrecan, decorin, and fibronectin was further reduced to 4%, 30%, and 24%, respectively (all P < 0.0001).
Fig. 3.
Gene expression of aggrecan, decorin, and fibronectin in canine FDP tendons in control and stress-deprived limbs at 21 and 42 days. Significant difference is shown by different letters.
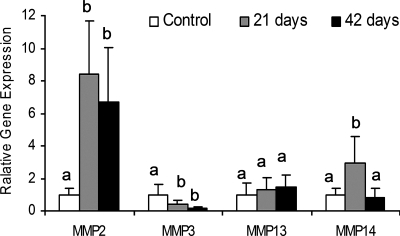
Stress deprivation had different effects on the expression of MMP2, MMP3, MMP13, and MMP14 (Fig. 4). With stress deprivation, the expression of MMP2 was significantly increased (P < 0.004) at 21 and 42 days. The expression of MMP3 was significantly decreased at 21 and 42 days (P < 0.03). The expression of MMP13 was not altered with stress deprivation. The expression of MMP14 was significantly increased at 21 days (P = 0.0015), but not 42 days. Stress deprivation for 21 days did not result in a reduction of the expression of TIMP1 and TIMP2 (Fig. 5). However, a significant decrease of TIMP1 (P = 0.0043), but not TIMP2 (P = 0.1489), was found after stress deprivation for 42 days.
Fig. 4.
Gene expression of matrix metalloproteinases (MMP2, MMP3, and MMP14) in canine FDP tendons in control and stress-deprived limbs at 21 and 42 days. Significant difference is shown by different letters.
Fig. 5.
Gene expression of tissue inhibitors of metalloproteinases (TIMP1 and TIMP2) in canine FDP tendons in control and stress-deprived limbs at 21 and 42 days. Significant difference is shown by different letters.
The cross-sectional area of the tendons decreased with stress deprivation. The cross-sectional area of the control tendons was 7.13 ± 1.16 mm2. With stress deprivation for 21 days, the cross-sectional area of the tendons was reduced to 6.79 ± 0.77 mm2, but this difference was not significant (P = 0.6673). Stress deprivation for 42 days did result in a significant decrease in tendon cross-sectional area (5.93 ± 0.63 mm2) compared with the control tendons (P = 0.0093), but not the tendons subjected to 21 days of stress deprivation (P = 0.1302).
Stress deprivation did not alter the stiffness of the tendons (P > 0.05). The stiffness of the control tendons and the 21- and 42-day stress-deprived tendons was 346 ± 80, 331 ± 68, and 359 ± 63 N/mm, respectively.
DISCUSSION
It has been over four decades since the effects of stress deprivation on tendons were first investigated (1). The studies mainly focused on human Achilles (15, 16, 44) and patellar (17, 18) tendons and animal Achilles (2, 8, 19, 23, 24, 34, 43), patellar (5, 20, 32, 50, 51, 54), and tibialis anterior (24, 43) tendons. Clinically, however, the human finger FDP tendon is also commonly stress deprived because of the frequency of hand injuries and the need for stress deprivation in the course of treatment (47). In this study, we studied stress deprivation of canine FDP tendons by splinting and sling immobilization, both of which are common clinical techniques.
Collagen synthesis.
Collagen is the principal structural component of the tendon. A number of techniques have been used to study collagen synthesis in tendons. Radioactive proline was used to label collagen to study collagen synthesis in human patellar tendon in vivo (18). The tendon collagen synthetic rate fell with disuse of the tendon at 10 and 21 days. The same technique was used to trace the collagen synthesis and degradation in rabbit patellar tendons after 9 wk of immobilization; however, at this longer time point, collagen synthesis and degradation were increased compared with control values (5).
In addition to measurement of proline uptake, the NH2-terminal propeptide of type I collagen has also been used as a biomarker of collagen synthesis. The technique of microdialysis was performed to collect the NH2-terminal propeptide of type I collagen in the peritendinous space of human Achilles tendons. Studies using this method have shown that collagen synthesis did not change after immobilization for 2 wk but increased after immobilization for 7 wk (15, 16).
Prolyl 4-hydroxylase and galactosylhydroxylysyl glucosyltransferase catalyze posttranslational modifications of collagen biosynthesis. Their activities are associated with the rate of collagen synthesis. The activities of these two enzymes in rat Achilles and tibialis anterior tendons were measured after cast immobilization for 1 and 3 wk (24, 43). In these studies, collagen biosynthesis decreased in Achilles and tibialis anterior tendons, which were casted in a position that relieved tension on the tendons, but collagen biosynthesis did not change in the tendons that were casted in a stretched position. The message from this study appears to be that it is not simply a lack of motion that downregulates tendon collagen synthesis: there must be some degree of unloading as well.
Collagen synthesis can also be assessed by quantification of collagen mRNA with real-time RT-PCR. Training and exercise increase the expression levels of collagen I and III (6, 22, 37). Heinemeier et al. (22) normalized the gene expression to tissue weight and found that the expression of collagen I and III in rat Achilles tendons increased in response to concentric, eccentric, or isometric training. However, they found no change in collagen I and III mRNA expression in response to hindlimb suspension for 1 or 2 wk (23). Arruda et al. (8) found that the expression of collagen I and III decreased in rat Achilles tendon after 5 wk of denervation.
In this study, we used real-time RT-PCR to measure the synthesis of collagen I, II, and III in FDP tendon with or without stress deprivation. Our results are in agreement with previous reports that the expression of collagen I and III in the tendon is unaltered or slightly decreased with stress deprivation for a short period of time, in our case 3 wk. Our data are at variance with some previous studies, which have variably shown increases and decreases in collagen synthesis over longer time frames. We believe that our findings may be explained by reference to the above studies, which have tended to show decreases of collagen synthesis when immobilization is combined with a lack of loading over a longer term.
In addition to collagen I and III, there is some collagen II in certain tendons, mainly in compressive regions where tendons go around bony pulleys or bear weight. In these locations, intratendinous nodules of fibrocartilage are present. There is a rapid depletion of collagen II and fibrocartilage in the rabbit hindpaw flexor tendon within 4 wk after elimination of compression by transposition (33). The volar aspect of the tendon segments in our study includes one area of fibrocartilage. We found that the expression of collagen II was significantly reduced with stress deprivation, to the point of nearly complete inhibition. These data suggest that collagen II might be a more sensitive marker of tendon stress deprivation.
Proteoglycan expression.
In addition to collagens, the tendon also consists of a number of proteoglycans. Aggrecan, which holds water and resists compression (52), was identified in the fibrocartilaginous region of the tendon. Decorin, which has been identified at the interface of the collagen fibrils, is believed to facilitate fibrillar slippage (39). Fibronectin serves as a cell attachment molecule and induces cell spreading and adhesion to various substrata; it is also involved in wound healing and tendon lubrication (3, 4, 9, 12).
Little information available regarding changes in individual proteoglycan in stress-deprived tendons is available. A prior study investigated uronic acid in the hydrolysates of rat patellar tendons (51). Hindlimb suspension for 4 wk resulted in a significant decrease of uronic acid in rat patellar tendons. The authors proposed that proteoglycans in the stress-deprived patellar tendon were decreased compared with the normal control tendons.
We studied the expression of aggrecan, decorin, and fibronectin. The expression of all these proteoglycans significantly decreased with stress deprivation. Longer-duration stress deprivation resulted in larger reductions in the expression of these proteoglycans. The expression of aggrecan was most severely affected. As aggrecan is also present in fibrocartilage, these changes, which mirror those of collagen II, seem logical. We conclude that markers of proteoglycan synthesis might also be useful in the assessment of tendon stress deprivation.
MMP and TIMP expression.
Collagen degradation has been measured with a number of experimental techniques, such as radioactive labeling (5) and microdialysis of the collagen COOH-terminal telopeptide (15, 16). However, the details of collagen degradation in the stress-deprived tendon are unknown. The real-time RT-PCR technique is sensitive and is able to detect the individual MMPs, TIMPs, and other proteins related to degradation of the tendon. We believe that RT-PCR assessment of MMPs and TIMPs could be useful in the study of degradation of collagen and other ECM components in response to stress deprivation.
MMPs are a large family of enzymes that degrade ECM components; their activities are inhibited by TIMPs (36). MMP2, MMP3, and MMP14 participate in collagen degradation, as well as collagen remodeling, during flexor tendon healing (38). MMP13 participates only in collagen degradation. TIMP1 and TIMP2 are capable of inhibiting activities of various MMPs (11, 25). However, the roles of MMPs and TIMPs during stress deprivation of the tendon are unknown.
This study found that the expression of MMP2, MMP3, MMP13, and MMP14 in the flexor tendon varied in their response to stress deprivation. MMP2 (gelatinase A), which has the capability to digest a broad range of substrates in addition to gelatin (11, 45), such as collagen I, collagen III, aggrecan, and fibronectin, appeared to play the most important role in remodeling of the stress-deprived tendon, as it was the most significantly upregulated. The expression of MMP14 (membrane-type MMP1), which digests collagen I, II, III, aggrecan, and fibronectin, may also play a role in remodeling of disused tendon initially, as it was elevated at 3 wk, but not 6 wk. MMP3 and MMP13 appeared to be much less involved in remodeling of the stress-deprived tendon. The disuse did not stimulate the increase but, rather, the decrease of the expression of MMP3 (stromelysin 1) in the tendon.
The increase of MMP expression and the decrease of TIMP expression that we found could indicate an increase of the rate of tendon remodeling. Again, measurement of these markers, especially MMP2, might be very useful in the assessment of stress deprivation in the tendon.
Cross-sectional area and tensile property.
Long-term exercise can increase animal and human tendon diameter (10, 27, 42, 53). Our data show that stress deprivation resulted in a decrease of the cross-sectional area of canine flexor tendon. This finding is consistent with the decrease in expression of collagens and proteoglycans that we also observed. It would appear from the published studies that, in more tendinous areas, stress deprivation does not greatly affect the cross-sectional areas of rat and rabbit Achilles tendon (19, 34) or human Achilles and patellar tendons (17, 18, 44). We can only speculate as to the changes that might occur in the fibrocartilaginous area in our model. This is also consistent with previous work showing the rapid depletion of fibrocartilage in the rabbit model, as mentioned above (33).
Stress deprivation results in a decrease in tendon stiffness or modulus in animal and human tendons (2, 17, 18, 32, 34, 40, 44, 50, 54). However, this study did not find that the stiffness of canine flexor tendon was significantly altered after stress deprivation for 3 or 6 wk. This could result from the method of stress deprivation used in this study, which was not absolute: the animals could move their limbs within the sling, and they had passive therapy every day. We believe that our method of stress deprivation may have less effect on stiffness of the tendon than the methods used in previous studies.
There are several limitations to this study. 1) This study took advantage of tissue remaining after the completion of other projects, none of which was designed to assess the effects of stress deprivation. Nonetheless, given the paucity of data, we believe that our results are useful, particularly in identifying some markers of stress deprivation of the tendon that might be helpful in the future. 2) The surgical intervention that was performed in the neighboring digits may have affected the gene expression in the intact tendons. However, mRNA levels of a number of growth factors returned to normal levels 9 days after surgery (14). Therefore, we believe that the results of gene expression obtained in this study mainly result from the stress deprivation. 3) Our study focused only on gene expression, which does not represent the total amount of proteins and the total MMP activity. However, we believe that this study potentially increases our understanding of the molecular mechanisms involved in the tendon disuse process, because the genes that are expressed are a reflection of current, rather than accumulated, activity.
Conclusions.
We observed that stress deprivation downregulates the anabolic process of the ECM in flexor tendon by reducing the expression of collagen I, collagen II, collagen III, aggrecan, decorin, and fibronectin. At the same time, stress deprivation increases the catabolic process of the ECM by increasing the expression of MMPs, especially MMP2 and MMP14, and decreasing the expression of TIMP1 and TIMP2. The expression of collagen I, collagen III, TIMP1, and TIMP2 responded to stress deprivation more slowly than the expression of collagen II, aggrecan, decorin, fibronectin, MMP2, MMP3, and MMP14. MMP2 may play an important role in the remodeling of stress-deprived tendon. The loss of collagens and proteoglycans results in a smaller cross-sectional area in the fibrocartilaginous zone of tendons exposed to stress deprivation.
GRANTS
This study was supported by a grant from the Mayo Foundation. Tissue was obtained from studies funded by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants AR-44391 and AR-049407.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Akeson WH, Amiel D, LaViolette D, Secrist D. The connective tissue response to immobility: an accelerated ageing response? Exp Gerontol 3: 289–301, 1968 [DOI] [PubMed] [Google Scholar]
- 2. Almeida-Silveira MI, Lambertz D, Perot C, Goubel F. Changes in stiffness induced by hindlimb suspension in rat Achilles tendon. Eur J Appl Physiol 81: 252–257, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Amiel D, Foulk RA, Harwood FL, Akeson WH. Quantitative assessment by competitive ELISA of fibronectin (Fn) in tendons and ligaments. Matrix 9: 421–427, 1989 [DOI] [PubMed] [Google Scholar]
- 4. Amiel D, Gelberman R, Harwood F, Siegel D. Fibronectin in healing flexor tendons subjected to immobilization or early controlled passive motion. Matrix 11: 184–189, 1991 [DOI] [PubMed] [Google Scholar]
- 5. Amiel D, Woo SL, Harwood FL, Akeson WH. The effect of immobilization on collagen turnover in connective tissue: a biochemical-biomechanical correlation. Acta Orthop Scand 53: 325–332, 1982 [DOI] [PubMed] [Google Scholar]
- 6. Archambault JM, Hart DA, Herzog W. Response of rabbit Achilles tendon to chronic repetitive loading. Connect Tissue Res 42: 13–23, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Arnoczky SP, Tian T, Lavagnino M, Gardner K. Ex vivo static tensile loading inhibits MMP-1 expression in rat tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J Orthop Res 22: 328–333, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Arruda EM, Mundy K, Calve S, Baar K. Denervation does not change the ratio of collagen I and collagen III mRNA in the extracellular matrix of muscle. Am J Physiol Regul Integr Comp Physiol 292: R983–R987, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Banes AJ, Link GW, Bevin AG, Peterson HD, Gillespie Y, Bynum D, Watts S, Dahners L. Tendon synovial cells secrete fibronectin in vivo and in vitro. J Orthop Res 6: 73–82, 1988 [DOI] [PubMed] [Google Scholar]
- 10. Birch HL, McLaughlin L, Smith RK, Goodship AE. Treadmill exercise-induced tendon hypertrophy: assessment of tendons with different mechanical functions. Equine Vet J Suppl 30: 222–226, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Bramono DS, Richmond JC, Weitzel PP, Kaplan DL, Altman GH. Matrix metalloproteinases and their clinical applications in orthopaedics. Clin Orthop Relat Res 272–285, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Brigman BE, Hu P, Yin H, Tsuzaki M, Lawrence WT, Banes AJ. Fibronectin in the tendon-synovial complex: quantitation in vivo and in vitro by ELISA and relative mRNA levels by polymerase chain reaction and Northern blot. J Orthop Res 12: 253–261, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Buchanan CI, Marsh RL. Effects of exercise on the biomechanical, biochemical and structural properties of tendons. Comp Biochem Physiol A Mol Integr Physiol 133: 1101–1107, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Chen CH, Cao Y, Wu YF, Bais AJ, Gao JS, Tang JB. Tendon healing in vivo: gene expression and production of multiple growth factors in early tendon healing period. J Hand Surg [Am] 33: 1834–1842, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Christensen B, Dyrberg E, Aagaard P, Enehjelm S, Krogsgaard M, Kjaer M, Langberg H. Effects of long-term immobilization and recovery on human triceps surae and collagen turnover in the Achilles tendon in patients with healing ankle fracture. J Appl Physiol 105: 420–426, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Christensen B, Dyrberg E, Aagaard P, Kjaer M, Langberg H. Short-term immobilization and recovery affect skeletal muscle but not collagen tissue turnover in humans. J Appl Physiol 105: 1845–1851, 2008 [DOI] [PubMed] [Google Scholar]
- 17. de Boer D, Ring C, Wood M, Ford C, Jessney N, McIntyre D, Carroll D. Time course and mechanisms of mental stress-induced changes and their recovery: hematocrit, colloid osmotic pressure, whole blood viscosity, coagulation times, and hemodynamic activity. Psychophysiology 44: 639–649, 2007 [DOI] [PubMed] [Google Scholar]
- 18. de Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV, Rennie MJ. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol 585: 241–251, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eliasson P, Fahlgren A, Pasternak B, Aspenberg P. Unloaded rat Achilles tendons continue to grow, but lose viscoelasticity. J Appl Physiol 103: 459–463, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Harwood FL, Amiel D. Differential metabolic responses of periarticular ligaments and tendon to joint immobilization. J Appl Physiol 72: 1687–1691, 1992 [DOI] [PubMed] [Google Scholar]
- 21. Heinemeier K, Langberg H, Olesen JL, Kjaer M. Role of TGF-β1 in relation to exercise-induced type I collagen synthesis in human tendinous tissue. J Appl Physiol 95: 2390–2397, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Heinemeier KM, Olesen JL, Haddad F, Langberg H, Kjaer M, Baldwin KM, Schjerling P. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol 582: 1303–1316, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heinemeier KM, Olesen JL, Haddad F, Schjerling P, Baldwin KM, Kjaer M. Effect of unloading followed by reloading on expression of collagen and related growth factors in rat tendon and muscle. J Appl Physiol 106: 178–186, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Karpakka J, Vaananen K, Virtanen P, Savolainen J, Orava S, Takala TE. The effects of remobilization and exercise on collagen biosynthesis in rat tendon. Acta Physiol Scand 139: 139–145, 1990 [DOI] [PubMed] [Google Scholar]
- 25. Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84: 649–698, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Kjaer M, Magnusson P, Krogsgaard M, Boysen Moller J, Olesen J, Heinemeier K, Hansen M, Haraldsson B, Koskinen S, Esmarck B, Langberg H. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat 208: 445–450, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kongsgaard M, Aagaard P, Kjaer M, Magnusson SP. Structural Achilles tendon properties in athletes subjected to different exercise modes and in Achilles tendon rupture patients. J Appl Physiol 99: 1965–1971, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Koskinen SO, Heinemeier KM, Olesen JL, Langberg H, Kjaer M. Physical exercise can influence local levels of matrix metalloproteinases and their inhibitors in tendon-related connective tissue. J Appl Physiol 96: 861–864, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Kubo K, Kanehisa H, Fukunaga T. Effects of resistance and stretching training programmes on the viscoelastic properties of human tendon structures in vivo. J Physiol 538: 219–226, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Langberg H, Rosendal L, Kjaer M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol 534: 297–302, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Langberg H, Skovgaard D, Petersen LJ, Bulow J, Kjaer M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol 521: 299–306, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Majima T, Yasuda K, Fujii T, Yamamoto N, Hayashi K, Kaneda K. Biomechanical effects of stress shielding of the rabbit patellar tendon depend on the degree of stress reduction. J Orthop Res 14: 377–383, 1996 [DOI] [PubMed] [Google Scholar]
- 33. Malaviya P, Butler DL, Boivin GP, Smith FN, Barry FP, Murphy JM, Vogel KG. An in vivo model for load-modulated remodeling in the rabbit flexor tendon. J Orthop Res 18: 116–125, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Matsumoto F, Trudel G, Uhthoff HK, Backman DS. Mechanical effects of immobilization on the Achilles' tendon. Arch Phys Med Rehabil 84: 662–667, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 567: 1021–1033, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem 274: 21491–21494, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Olesen JL, Heinemeier KM, Haddad F, Langberg H, Flyvbjerg A, Kjaer M, Baldwin KM. Expression of insulin-like growth factor I, insulin-like growth factor binding proteins, and collagen mRNA in mechanically loaded plantaris tendon. J Appl Physiol 101: 183–188, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Oshiro W, Lou J, Xing X, Tu Y, Manske PR. Flexor tendon healing in the rat: a histologic and gene expression study. J Hand Surg [Am] 28: 814–823, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Pins GD, Christiansen DL, Patel R, Silver FH. Self-assembly of collagen fibers. Influence of fibrillar alignment and decorin on mechanical properties. Biophys J 73: 2164–2172, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reeves ND, Maganaris CN, Ferretti G, Narici MV. Influence of 90-day simulated microgravity on human tendon mechanical properties and the effect of resistive countermeasures. J Appl Physiol 98: 2278–2286, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol 548: 971–981, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosager S, Aagaard P, Dyhre-Poulsen P, Neergaard K, Kjaer M, Magnusson SP. Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand J Med Sci Sports 12: 90–98, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Savolainen J, Myllyla V, Myllyla R, Vihko V, Vaananen K, Takala TE. Effects of denervation and immobilization on collagen synthesis in rat skeletal muscle and tendon. Am J Physiol Regul Integr Comp Physiol 254: R897–R902, 1988 [DOI] [PubMed] [Google Scholar]
- 44. Shin D, Finni T, Ahn S, Hodgson JA, Lee HD, Edgerton VR, Sinha S. Effect of chronic unloading and rehabilitation on human Achilles tendon properties: a velocity-encoded phase-contrast MRI study. J Appl Physiol 105: 1179–1186, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Somerville RP, Oblander SA, Apte SS. Matrix metalloproteinases: old dogs with new tricks. Genome Biol 4: 216, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, Carpenter JE. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg 9: 79–84, 2000 [PubMed] [Google Scholar]
- 47. Strickland J. Biologic rationale, clinical application and results of early motion following flexor tendon repair. J Hand Ther 2: 71–83, 1989 [Google Scholar]
- 48. Sullivan BE, Carroll CC, Jemiolo B, Trappe SW, Magnusson SP, Dossing S, Kjaer M, Trappe TA. Effect of acute resistance exercise and sex on human patellar tendon structural and regulatory mRNA expression. J Appl Physiol 106: 468–475, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tanaka T, Zhao C, Sun YL, Zobitz ME, An KN, Amadio PC. The effect of carbodiimide-derivatized hyaluronic acid and gelatin surface modification on peroneus longus tendon graft in a short-term canine model in vivo. J Hand Surg [Am] 32: 876–881, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Uchida H, Tohyama H, Nagashima K, Ohba Y, Matsumoto H, Toyama Y, Yasuda K. Stress deprivation simultaneously induces over-expression of interleukin-1β, tumor necrosis factor-α, and transforming growth factor-β in fibroblasts and mechanical deterioration of the tissue in the patellar tendon. J Biomech 38: 791–798, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Vailas AC, Deluna DM, Lewis LL, Curwin SL, Roy RR, Alford EK. Adaptation of bone and tendon to prolonged hindlimb suspension in rats. J Appl Physiol 65: 373–376, 1988 [DOI] [PubMed] [Google Scholar]
- 52. Vogel KG, Koob TJ. Structural specialization in tendons under compression. Int Rev Cytol 115: 267–293, 1989 [DOI] [PubMed] [Google Scholar]
- 53. Woo SL, Ritter MA, Amiel D, Sanders TM, Gomez MA, Kuei SC, Garfin SR, Akeson WH. The biomechanical and biochemical properties of swine tendons–long term effects of exercise on the digital extensors. Connect Tissue Res 7: 177–183, 1980 [DOI] [PubMed] [Google Scholar]
- 54. Yamamoto N, Ohno K, Hayashi K, Kuriyama H, Yasuda K, Kaneda K. Effects of stress shielding on the mechanical properties of rabbit patellar tendon. J Biomech Eng 115: 23–28, 1993 [DOI] [PubMed] [Google Scholar]
- 55. Zhao C, Sun YL, Kirk RL, Thoreson AR, Jay GD, Moran SL, An KN, Amadio PC. Effects of a lubricin-containing compound on the results of flexor tendon repair in a canine model in vivo. J Bone Joint Surg Am 92: 1453–1461, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhao C, Zobitz ME, Sun YL, Predmore KS, Amadio PC, An KN, Moran SL. Surface treatment with 5-fluorouracil after flexor tendon repair in a canine in vivo model. J Bone Joint Surg Am 91: 2673–2682, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]