Abstract
Single motor unit (SMU) analysis provides a means to examine the motor control of a muscle. SMUs in the genioglossus show considerable complexity, with several different firing patterns. Two of the primary stimuli that contribute to genioglossal activation are carbon dioxide (CO2) and negative pressure, which act through chemoreceptor and mechanoreceptor activation, respectively. We sought to determine how these stimuli affect the behavior of genioglossus SMUs. We quantified genioglossus SMU discharge activity during periods of quiet breathing, elevated CO2 (facilitation), and continuous positive airway pressure (CPAP) administration (inhibition). CPAP was applied in 2-cmH2O increments until 10 cmH2O during hypercapnia. Five hundred ninety-one periods (each ∼3 breaths) of genioglossus SMU data were recorded using wire electrodes(n = 96 units) from 15 awake, supine subjects. Overall hypercapnic stimulation increased the discharge rate of genioglossus units (20.9 ± 1.0 vs. 22.7 ± 0.9 Hz). Inspiratory units were activated ∼13% earlier in the inspiratory cycle, and the units fired for a longer duration (80.6 ± 5.1 vs. 105.3 ± 4.2% inspiratory time; P < 0.05). Compared with baseline, an additional 32% of distinguishable SMUs within the selective electrode recording area were recruited with hypercapnia. CPAP led to progressive SMU inhibition; at ∼6 cmH2O, there were similar numbers of SMUs active compared with baseline, with peak frequencies of inspiratory units close to baseline, despite elevated CO2 levels. At 10 cmH2O, the number of units was 36% less than baseline. Genioglossus inspiratory phasic SMUs respond to hypercapnic stimulation with changes in recruitment and rate coding. The SMUs respond to CPAP with derecruitment as a homogeneous population, and inspiratory phasic units show slower discharge rates. Understanding upper airway muscle recruitment/derecruitment may yield therapeutic targets for maintenance of pharyngeal patency.
Keywords: motoneurons, respiration, tongue, lung, airway, muscle, sleep, apnea
obstructive sleep apnea (OSA) affects at least 2–4% of adults (24, 54) and has major adverse consequences. Together with compromised upper airway anatomy, decreased neural drive to the upper airway muscles during sleep can result in airway closure and obstructive apnea (e.g., Refs. 2, 35). The genioglossus in humans has been widely studied because it is the largest upper airway dilator muscle (1, 2, 12, 49), is readily accessible (3, 21, 44), and its activity is thought to be representative of phasic upper airway dilator muscles. While pharmacological agents that increase genioglossal muscle activity (such as ampakines) may be a viable strategy for OSA therapy (e.g., Refs. 28, 41, 53), the ideal therapeutic targets remain unclear.
Given that genioglossus muscle activation appears necessary and sufficient to stabilize breathing in OSA patients during sleep (23, 25), understanding the responses to both chemoreceptive and mechanoreceptive stimuli is critical to upper airway motor control. Chemoreceptor activation (increased CO2) increases hypoglossal motor output, as indicated by multiunit electromyographic (EMG) activity of respiratory muscles of animals and humans (7, 22, 27, 31). Continuous positive airway pressure (CPAP) decreases the “phasic” multiunit EMG signal (4), and the signal can be completely abolished after anesthetizing the upper airway (13). CPAP raises the transmural airway pressure (i.e., reduces negative airway pressure) and increases lung volume to prevent airway collapse (4). However, the effect of CPAP on genioglossal single motor units (SMUs) has not been investigated.
As motoneurons are the final common pathway of neural output, assessing their responses to stimuli provides insight into motor control of a muscle. The regulation of skeletal muscle force/output is controlled in two interactive ways: 1) changing the number of units that are active at any moment with recruitment/derecruitment, and 2) by varying the discharge frequency and pattern of action potentials of SMUs (rate coding) (for review, see Ref. 10). During eupneic breathing, SMU activity of the human genioglossus exhibits discharge patterns that both correlate to the respiratory cycle (inspiratory phasic, inspiratory tonic, expiratory phasic, and expiratory tonic units), while other units have no distinct respiratory modulation (tonic and tonic other units) (38). However, the relative contribution of recruitment and rate coding over the physiological range is largely unknown for the upper airway. Two recent studies have demonstrated increased recruitment of SMUs with mild hypercapnic stimulation in healthy young populations without increases in rate coding (30, 36). Similarly, at arousal from sleep, the increased EMG activity is also primarily due to recruitment, with a predominant increase in the number of inspiratory phasic units, and not through rapid increases in peak discharge rates (51). However, while these studies have demonstrated recruitment of previously silent units, the units that are already active may be capable of augmenting their discharge rate (possibly in a narrower range), in response to increased total muscle output (8). Consistent with the above studies (30, 36, 51), at the alpha-to-theta transition, there was primarily derecruitment of previously active inspiratory units, and those that continued to discharge reduced their duty cycle (52). However, the tonic and expiratory classes of units seem to be unaffected at sleep onset, showing an absence of derecruitment and no alteration in their discharge pattern (52).
Therefore, we aimed to determine how the different discharge patterns modify their frequency or pattern of discharge to chemoreceptive and mechanoreceptive stimuli. We hypothesized that the different classes of motor units, previously described (38), would respond as a single population through increases in rate coding (increased firing frequency) and earlier activation of active units, together with recruitment of additional inspiratory phasic units under higher conditions of elevated CO2 (10). In addition, because premotor inputs and their various thresholds for activation may alter the activation patterns (6, 22), we hypothesized a marked reduction in the firing rates and derecruitment of inspiratory units with CPAP. Some of the results of these studies have been previously reported in the form of abstracts (37, 42). Given the importance of these muscles in stabilizing breathing, insights into recruitment and rate-coding strategies of genioglossal activity could have therapeutic implications for sleep apnea.
METHODS
Complete data were successfully obtained in 15 healthy human subjects. Demographic details of the group are given in Table 1. Twenty-one subjects were studied in total; however, six subjects were excluded due to trouble with vigilance (n = 2), the observation of erratic breathing patterns (n = 1), end-tidal CO2 (PetCO2) unstable (n = 1), or no decomposable SMU activity (n = 2). The data for the excluded subjects are not included in Table 1. All subjects gave written, informed consent before participation in this study, which was approved by the local Human Research Committee of the Brigham and Women's Hospital and conformed to the Declaration of Helsinki.
Table 1.
Subject demographics
| Group Subject Characteristics | |
|---|---|
| Subjects, no. | 15 |
| Female/male, no. | 5/10 |
| Age, yr | 32.9 ± 3.1 |
| Height, cm | 172.7 ± 1.9 |
| Weight, kg | 68.8 ± 4.0 |
| Body mass index, kg/m2 | 23.8 ± 0.7 |
| Units per subject, no. (range) | 6.3 ± 1.3 (1–19) |
| Inferior margin of geniohyoid muscle, mm | 12.4 ± 0.9 |
| Inferior margin of genioglossus muscle, mm | 20.7 ± 1.0 |
| Genioglossus width, mm | 15.4 ± 0.5 |
Values are means ± SE, unless otherwise specified. Shown are the number of subjects, female-to-male ratio, age, height, weight, body mass index, number of units, and ultrasound measurements of the upper airway. Ultrasound measurements from the skin to the inferior margin of the geniohyoid and genioglossus muscles and the mean width genioglossus are also given.
General procedures.
To determine the depth and location for the recording electrodes in genioglossus, the local anatomy of the upper airway musculature was examined with ultrasonography (12L high-frequency linear array transducer, Vivid i GE Healthcare, Chalfont St. Giles, Bucks, UK) (14, 38, 39). The distance from the skin to the inferior margin of the genioglossus and geniohyoid muscles and the lateral width of genioglossus were recorded using an electronic caliper (14). Before the insertion of wires, a topical anesthetic cream was placed under the chin surface for a minimum of ∼30 min. The chin was thoroughly cleaned using disposable topical antiseptic wipes, and a small reference cross was drawn under the chin 10 mm posterior to genial turbercle, using a sterile skin marker (DEVON tyco/Healthcare Group, Chicopee, MA). Since this area had been examined by ultrasonography, we defined in each individual subject the minimal depth to obtain recordings from the correct muscle and marked this on the needle (see Table 1). The three 27-gauge needles were inserted at 90° to the skin surface and advanced through the mylohyoid and geniohyoid muscles into the genioglossus (>21 mm) with the subjects relaxed, lying comfortably supine. The lumen of each hypodermic needle contained a single Teflon-coated 51-μm hooked stainless steel fine-wire electrode (A & M Systems, Carlsborg, WA) with ∼0.5-mm recording area exposed. The three fine-wire electrodes were inserted under the chin; the most anterior electrode was positioned a minimum of 10 mm posterior to the inner border of the mental protuberance and just lateral to the midline; and the two posterior electrodes were positioned a further ∼10 mm behind the anterior electrode ∼5 mm lateral to each side of the midline. The fine-wire electrodes were referenced to a surface electrode positioned over the bony mandible (MEDI-TRACE 100 series, Kendall Healthcare, Mansfield, MA). A large flexible ground electrode (1180, 3M Health Care, St. Paul, MN) was placed over the right clavicle. Genioglossus EMG signals were filtered (30 Hz to 3 kHz), amplified (×1,000–10,000) (Grass Instruments), sampled at 10 kHz, and stored on computer for offline analysis (Spike2 with 1401 interface, Cambridge Electronic Design, Cambridge, UK). Genioglossus EMG activity was recorded over the entire protocol (see below).
For the entire protocol, the subjects breathed through a nasal mask (Gel Mask, Respironics, Murrysville, PA) attached to a heated pneumotachograph (model 3700A, Hans-Rudolph, Kansas City, MO) and a differential pressure transducer (Validyne, Northbridge, CA). Airway pressure and PetCO2 at the nares were measured through ports in the nasal mask. The flow signal was integrated to give volume. To increase and hold the PetCO2 constant, the pneumotachograph was connected in series to a bleed port for compressed medical air (dry air: 79% N2, 21% O2) titration, and two corrugated respiratory tubes (each 25 mm in diameter × 180 cm length) were connected directly to a CPAP device (Philips Respironics, Murrysville, PA).
Throughout the procedure, subjects lay supine comfortably and relaxed, breathed quietly, and remained awake. Wakefulness was confirmed either with the investigators directly watching the subjects or by monitoring the electroencephalogram (C3-A1, Oz-A2; based on α- or β-EEG activity).
Protocol.
The subjects were asked to lie comfortably supine while remaining awake during the following protocol.
1) A baseline period of 3 min of data collection enabled a stable recording with the PetCO2 levels monitored and recorded.
2) Two corrugated respiratory tubes to increase the dead space were connected to the mask, and, for ∼3 min, compressed medical air was turned on to a level at which the PetCO2 was maintained at similar levels to the baseline flow rate.
3) In rebreathing, the flow rate of the compressed medical air was then reduced to a point at which rebreathing ensued (∼60 s) until peak PetCO2 elevation was 10 Torr. The EMG signal was also used to regulate the peak PetCO2, as when SMU activity approached near saturation for decomposition, the PetCO2 level was maintained.
4) CPAP was then applied at 2-cmH2O increments for 1 min until a level of 10 cmH2O was obtained. Hypercapnia through rebreathing was maintained during CPAP application.
5) CPAP was terminated.
Sorting of SMU activity.
Motor unit potentials were extracted from the raw EMG signal offline using a spike-triggered threshold. The individual SMUs were sorted into “templates” based on their detailed morphology and amplitude (Spike2 analysis system, Cambridge Electronic Design). Manual inspection of all motor unit potentials confirmed the sorting. Instantaneous frequency plots were derived from the time of discharge of the unit (Fig. 1).
Fig. 1.
Typical example of two concurrently recorded single motor units during quiet breathing to show the measurements used in the analysis. From bottom to top: raw electromyographic (EMG; at left) with overlaid motor unit potentials from units (at right of the instantaneous frequency plots), the corresponding volume signal, and the instantaneous frequency plot of the two units (dashed line represents the running average of the unit calculated over 200 ms). The shaded area represents the inspiratory time (Ti) for which the discharge times were all normalized (0% Ti and 100% Ti). The vertical dashed lines represent the Ti (0% Ti and 75% Ti) for which calculations were performed on the percentage of the number of units active or not active. The various measurements are shown for both the top inspiratory tonic unit and the lower inspiratory phasic unit, including the onset time (dti) from the first motor unit potential in each breath (or where the frequency first increased for the tonic unit) and onset frequency (fi) taken from the first interspike interval in each breath, and the end time (dte) and end frequency (fe) taken from the last potential in each breath (or where the frequency decreased for the tonic unit). The time of the peak frequency (dtp and fp) was measured from a running average over 200 ms (dashed line). For each unit, these variables were measured for three breaths and then averaged.
The activity of each motor unit was then classified based on its pattern of discharge during the respiratory cycle, which was determined from the airflow signal (see Fig. 4) (38, 39). Briefly, all units were classified into tonic or phasic categories, depending on whether they discharged throughout both inspiration and expiration (tonic), or only during either inspiration or expiration (phasic). Units were further classified according to the timing of their peak activity: “inspiratory phasic” motor units discharged phasically with their peak frequency during inspiration (see Fig. 2); “expiratory phasic” units discharged phasically with their peak frequency during expiration; “inspiratory tonic” units discharged through inspiration and expiration, but increased their discharge frequency during inspiration; and “expiratory tonic” units discharged through inspiration and expiration, but increased their discharge during expiration (see Fig. 3). “Tonic” units discharged throughout inspiration and expiration with no obvious respiratory modulation of discharge frequency. A small number of units discharged continuously with some modulation, but not in time with the respiratory cycle, and they were classified as “tonic other” units. In addition to the visual categorization of the different classes of motor unit activity, cross correlations between volume (from the integrated flow signal) and instantaneous firing frequency (smoothed over 200 ms) were calculated for all possible phase differences between the two signals on a breath-by-breath basis (see Supplemental Table S1; the online version of this article contains supplemental data). The strength of each correlation was evaluated by calculating the linear coefficient of determination (r2) and the timing of the maximal value of this coefficient. The respiratory phase of the maximal r2 (lag time) indicated whether the unit had a predominant inspiratory or expiratory modulation. Figure 4 displays the confirmation of the computed cross correlations with a plot of the r2 value against the lag time (s) for all units in the eight conditions (A–H).
Fig. 4.
Classification of single motor unit in genioglossus based on their firing frequency-volume coefficient of determination. A–H: plots of the strength (coefficient, r2) and the time of the peak cross correlations for each unit (mean). The correlations were calculated between the volume signal and the discharge frequency (smoothed over 200 ms) of the motor unit by computing all possible x-axis phase differences between the two signals. Time zero represents the end of inspiration. Units were classified into inspiratory phasic (solid circles), inspiratory tonic (half-solid circles), expiratory phasic (solid squares), expiratory tonic (half-solid squares), and tonic and tonic other units (plus signs). Inspiratory units had a peak r2 before the end of inspiration (negative times), and expiratory units had a peak r2 after the end of inspiration during expiration (positive times). Inspiratory phasic r2 values for CO2 condition were greater than 10-cmH2O condition (P < 0.05). The inspiratory tonic units had a trend toward alterations in the lag times of these units between the baseline (−1.2 ± 0.1 s), 8 cmH2O (−0.9 ± 0.1 s), and 10 cmH2O (−0.8 ± 0.1 s; all nonsignificant).
Fig. 2.
Recording of single motor unit activity in the genioglossus. A typical example of a recording from a single subject, in each of the eight conditions [baseline, CO2, 2 cmH2O, 4 cmH2O, 6 cmH2O, 8 cmH2O, 10 cmH2O, and continuous positive airway pressure (CPAP) off]. Traces from bottom to top: mask pressure, end-tidal CO2 (PetCO2), volume, raw EMG from two electrode sites (a and b), with both rectified and integrated EMG (time constant, 100 ms, calibration 0–10 μV), instantaneous frequency plots for three units, and the superimposed motor unit potentials for each unit (inset). The simultaneously recorded units increased their discharge in phase with inspiration. All three units were classed as inspiratory phasic (see Fig. 1 and methods for description of the classification). With CO2, the units tended to increase their activity, and graded suppression was observed with the application of positive airway pressure. Unit 1 was recorded on EMG (a), and units 2 and 3 were recorded on EMG (b). Inset calibrations: 100 μV and 2 ms.
Fig. 3.
Genioglossus single motor unit activity during the eight experimental conditions. A: representative example of a recording from a single subject through the eight conditions. Traces from bottom to top, the mask pressure through (left to right), PetCO2, volume, the raw EMG from one electrode site, rectified and integrated EMG (time constant 100 ms, calibration 0–10 μV), instantaneous frequency plots for a single unit, and the superimposed motor unit potentials for the unit under each condition. Calibrations: 250 μV and 2 ms. B: expanded section of 2 and 6 cmH2O. Note that, in the 2-cmH2O condition, the unit clearly increases its discharge in phase with expiration and was classed as expiratory tonic 0.80 r2. However, at 6 cmH2O, this unit displayed multiple peaks, both inspiratory and expiratory, while it maintained a reduced expiratory peak cross-correlation with the volume signal 0.59 r2 (see methods for description of the classification).
Measurement of respiratory variables and discharge properties of SMUs.
Respiratory variables, including inspiratory time (Ti; s), expiratory time (s), minute ventilation (l/min), respiratory frequency, tidal volume (liter), and PetCO2 (Torr) were analyzed over the breaths, where the motor units were sorted with the aid of custom-designed semi-automated software (Spike2, Cambridge Electronic Design); averaged means were calculated for each of the conditions.
Measurements of SMU discharge behavior were derived from instantaneous frequency plots for each motor unit (Spike2 Cambridge Electronic Design, script courtesy of the Gandevia Laboratory, Randwick, Sydney, Australia). Discharge timing was expressed relative to the integrated flow signal. Motor units were measured over three continuous breaths in each category or until the unit became inactive (baseline, CO2, 2 cmH2O + CO2, 4 cmH2O + CO2, 6 cmH2O + CO2, 8 cmH2O + CO2, 10 cmH2O + CO2, and with CPAP off). However, in rare circumstances, units were accepted if sorted for two consistent breaths. Contamination of the EMG signal due to events such as sighs or swallows was avoided in this analysis by discarding these data.
For phasic units, the onset discharge time was measured at the first discharge for each breath, and the end time was measured at the last discharge in each breath (See Fig. 1). For inspiratory and expiratory tonic units, onset time was taken visually from when the discharge frequency modulation first increased above the tonic levels, and the end time when the discharge frequency first returned to the tonic level (See Fig. 1). Onset discharge frequency for phasic units was calculated from the first interspike interval for each breath. For inspiratory tonic and expiratory tonic units, onset firing frequency was measured at the first increase in the discharge frequency above the tonic level. Peak discharge frequencies were derived from the peak of the instantaneous frequency with a running average for each breath (smoothed over 200 ms).
All variables were averaged across the sorted consecutive breaths. Statistical differences for the discharge parameters were assessed using one-way (ANOVA) with Student-Newman-Keuls post hoc analysis. If data were not normally distributed, then the Kruskal-Wallis test was applied or the two-way ANOVA was performed on ranks (SigmaPlot 11). Statistical significance was defined by P < 0.05. Units that met the criterion for the inspiratory phasic and inspiratory tonic classes under the conditions, CO2, 4 cmH2O, 6 cmH2O, 8 cmH2O, and 10 cmH2O (unit must have been active and not changing class), were selected for a one-way repeated-measures analysis using Student-Newman-Keuls post hoc analysis; one-way repeated-measures ANOVA on ranks was used where units were not normally distributed. The population analysis was calculated using a mixed-model analysis, taking into consideration the subject, number of units, class of the units, condition, and peak firing rates (SAS statistics). Values are given as means ± SE.
RESULTS
Successful unitary recordings, using selective intramuscular fine-wire electrodes, were made from 96 SMUs in the genioglossus muscle during normal eupnic breathing, hypercapnic breathing (through a rebreathing circuit), and under five levels of CPAP. A total of 591 sections (each ∼3 breaths) of genioglossus SMU data were sorted and analyzed in this study. Typical recordings for each of the eight conditions are shown in Figs. 2 and 3. Concurrent recordings of multiple SMUs were obtained in Fig. 2. In this example, units 1 and 2 were both suppressed by the application of CPAP, and, to confirm the unit was not “lost” due to displacement of the electrode, the units were also decomposed in the CPAP off condition. Figure 3 illustrates the activity of an expiratory tonic motor unit that was not suppressed with the application of CPAP through the eight experimental conditions. This unit displays multiple peak discharges that may be explained by convergence of two pacemakers (see Fig. 3B). The correlation and lag time calculated between each unit's firing frequency (smoothed over 200 ms) with the signal of lung volume, for each of the six classes of units in the genioglossus muscle, in each of the eight conditions are shown in Fig. 4, A–H. Figure 4 shows the timing of the peak correlation [time (s)] for each motor unit plotted against strength of the coefficient of determination (r2). Inspiratory units had a peak r2 before the end of inspiration (negative times), and expiratory units had a peak r2 after the end of inspiration during expiration (positive times; see methods and Refs. 38–40). In each of the eight conditions, the activity of 42–94 SMUs was recorded, and the predominant class of motor unit activity exhibited was inspiratory phasic activity (Fig. 5, see Table 3; P < 0.01, mixed-model analysis).
Fig. 5.
The time and frequency plots. A–H: each individual unit in each condition. Each horizontal line represents the firing of a genioglossus single motor unit (591 in total). Vertical dotted lines represent the onset (0%) and end (100%) of inspiration. For each motor unit, the onset time (small colored circle on left) of firing is joined by a thick horizontal line to its end discharge time (small colored circle on right). The color of the circles indicates the onset and end firing frequencies (see inset). The mean peak firing frequency is indicated by the color of the thick horizontal line, and the time of the peak firing frequency is indicated by a black circle. For units that discharged throughout both phases of the respiratory cycle, a thin horizontal line is colored to code the tonic firing frequency. Units are ordered in each category (phasic or tonic), according to their onset discharge time. With hypercapnic stimulation, the peak discharge rate for the population is increased, and more single motor units were activated (63 vs. 93; both P < 0.01). A greater percentage of the inspiratory phasic motor units was activated before the onset of flow 0% Ti (20%, P = 0.02) and continued to discharge after 75% Ti (60%, P < 0.001). The stepwise application of CPAP led to reduced discharge frequencies for the population at 6, 8, and 10 cmH2O (P < 0.01). The firing times were also markedly altered under CPAP with a 22% Ti decrease in the duration of discharge at 8 cmH2O. The onset firing times of these units that were preinspiratory with CO2 (−3.7% Ti) were delayed with the application of 8-cmH2O CPAP to postinspiratory activation (7.7% Ti).
Table 3.
Percent distribution of the different classes of units throughout the protocol
| No. of Units | Inspiratory Phasic Units, % | Inspiratory Tonic Units, % | Expiratory Phasic Units, % | Expiratory Tonic Unit, % | Tonic and Tonic Other Units, % | |
|---|---|---|---|---|---|---|
| Baseline | 63 | 59 | 21 | 3 | 10 | 8 |
| CO2 | 93 | 53 | 31 | 1 | 8 | 8 |
| 2-cmH2O CPAP | 91 | 56 | 25 | 2 | 11 | 5 |
| 4-cmH2O CPAP | 82 | 59 | 23 | 4 | 11 | 4 |
| 6-cmH2O CPAP | 67 | 54 | 24 | 7 | 10 | 4 |
| 8-cmH2O CPAP | 59 | 56 | 27 | 2 | 8 | 7 |
| 10-cmH2O CPAP | 42 | 45 | 31 | 0 | 10 | 14 |
| CPAP off | 94 | 57 | 22 | 3 | 10 | 7 |
| Total | 591 | 55 | 25 | 3 | 10 | 7 |
Ventilatory data.
Respiratory parameters were altered with the application of CPAP and the rebreathing protocol (see Table 2). There were no significant differences for Ti, expiratory time, and respiratory frequency between the eight conditions. Values for minute ventilation tended to be elevated under all conditions compared with baseline and were significantly greater than baseline at the higher levels of CPAP 4–10 cmH2O (P < 0.05). Similarly, tidal volume increased under all levels of CPAP compared with baseline (P < 0.05). PetCO2 was elevated under the CO2 condition by 6 ± 0.3 Torr and remained 12% higher until the CPAP off condition (P < 0.05).
Table 2.
Ventilatory data during electromyographic recordings, one value for each subject/condition
| Baseline | CO2 | 2 cmH2O | 4 cmH2O | 6 cmH2O | 8 cmH2O | 10 cmH2O | CPAP Off | |
|---|---|---|---|---|---|---|---|---|
| Inspiratory time, s | 1.9 ± 0.2 | 2.0 ± 0.1 | 1.9 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.2 | 2.0 ± 0.2 |
| Expiratory time, s | 2.7 ± 0.2 | 2.3 ± 0.1 | 2.5 ± 0.2 | 2.7 ± 0.3 | 2.8 ± 0.3 | 2.8 ± 0.3 | 2.8 ± 0.3 | 2.4 ± 0.3 |
| Minute ventilation, l/min | 7.7 ± 0.7 | 13.8 ± 1.0 | 15.9 ± 1.1* | 15.9 ± 1.2* | 17.0 ± 1.3* | 17.5 ± 1.2* | 16.4 ± 1.4* | 14.0 ± 1.3 |
| Respiratory frequency | 14.4 ± 0.9 | 14.8 ± 0.8 | 14.6 ± 0.8 | 14.7 ± 0.9 | 15.2 ± 1.0 | 14.9 ± 1.0 | 14.9 ± 1.5 | 15.5 ± 1.0 |
| Tidal volume, liter | 0.6 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1* | 1.1 ± 0.1* | 1.2 ± 0.1* | 1.2 ± 0.1* | 1.2 ± 0.1* | 1.0 ± 0.1 |
| End-tidal CO2, Torr | 39.7 ± 0.8 | 46.1 ± 0.6*† | 46.2 ± 0.6*† | 45.9 ± 0.6*† | 45.8 ± 0.5*† | 45.2 ± 0.7*† | 44.5 ± 0.8*† | 41.4 ± 0.7 |
Values are means ± SE. For each variable, a mean value was derived from the mean for each subject under each condition (N = 15). There were no significant differences for inspiratory time, expiratory time, and respiratory frequency between the eight groups.
Significant difference compared with the baseline condition for a given variable;
significant difference compared with the continuous positive airway pressure (CPAP) off condition for a given variable: P < 0.05.
Changes in the number of units and discharge characteristics with rebreathing.
Compared with the baseline condition, significantly more (32%) SMUs were recorded under the condition of elevated CO2 (63 vs. 93; P < 0.01, mixed-model analysis). During the hypercapnic condition, the inspiratory phasic units increased their peak discharge by ∼2 Hz (17.9 ± 0.8 vs. 20.1 ± 0.7 Hz; P = 0.037; see Fig. 6). In addition, the inspiratory phasic motor units were recruited earlier in inspiration under the CO2 condition by an average of ∼16% Ti (%Ti; see Fig. 1 for how the timing variables are defined, P = 0.02; χ2). There were no such differences for the time to peak firing and end-firing time for inspiratory phasic units, which occurred at the same time under baseline and CO2 conditions (peak times 39.8 ± 3.8 vs. 44.2 ± 3.5% Ti; end times 81.8 ± 6.2 vs. 95.9 ± 3.7% Ti, P > 0.05). Despite no significant change in end-firing times, the number of units that continued to fire for longer than 75% Ti was significantly increased from baseline to the CO2 condition (20 vs. 80%, P < 0.001; χ2). The increase in the number of units firing over 75% Ti was also seen in the inspiratory phasic units as 43% stopped discharging in the baseline condition; only 20% of units ceased discharge in the CO2 condition (P < 0.001, χ2).
Fig. 6.
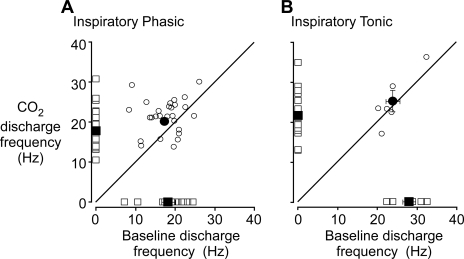
Peak discharge frequency of inspiratory phasic and inspiratory tonic single motor units active in the condition of elevated CO2 compared with baseline (x-axis). The peak discharge rate of inspiratory phasic units (A) and inspiratory tonic units (B) active in CO2 breaths is plotted against their peak discharge rate in the baseline condition. Units active in both CO2 and baseline condition are represented by open circles. Units that were recruited and derecruited between conditions are represented by open squares. Solid circles and squares represent mean ± SE values. The subtle changes in the levels of rate coding can be observed between the conditions: a slight increase in rate coding can be observed with CO2 compared with baseline in the inspiratory phasic units. Only two of the inspiratory tonic units did not respond with an increase in rate coding to hypercapnic stimulation (the lower number of units most likely contributed to the lack of significance). This figure further reveals that the newly recruited units' [indicated by the squares on the y-axis] mean peak discharge frequency (tonic 22 Hz and phasic 18 Hz) was lower than the mean discharge frequency of the units that were already increasing their rate coding in response to the CO2 condition (tonic 25 Hz and phasic 22 Hz).
In contrast, the population of inspiratory tonic SMUs decreased their peak discharge frequency from 25.6 ± 1.3 to 22.2 ± 1.0 Hz from the baseline to CO2 condition, but Fig. 6 illustrates this was largely due to the newly recruited units discharging at lower peak frequencies (Kruskal-Wallis Dunns post hoc; P < 0.05). While it appeared that an additional 10% of inspiratory tonic motor units commenced before the onset of inspiratory flow, this was not significant (P = 0.15; χ2). All of the inspiratory tonic units discharged at over 75% Ti at an elevated rate (above the expiratory tonic frequency) during the baseline condition. During the CO2 condition, there was a little reduction (−10%) in the number of units that maintained this firing at an elevated rate of over 75% Ti.
Changes in the number of units and discharge characteristics with positive airway pressure.
While there was no change in the discharge rates of individual motor units with CPAP, there was an abrupt and marked change in the numbers of units recorded. Once CPAP reached 6 cmH2O, there were fewer units active than in the increased CO2 condition (67 units, CPAP + CO2 vs. 93 units CO2 alone; P < 0.01, mixed-model analysis; see Table 3, Fig. 5, and Fig. 7). This suppression effect was more marked as the pressure increased to 10 cmH2O, when there were fewer units active than under the baseline condition (42 CPAP + CO2 vs. 63 baseline units; P = 0.01 mixed-model analysis).
Fig. 7.
Peak discharge frequency of all single motor units active in the condition of elevated CO2 compared with seven conditions (x-axis). Data are from recordings in the genioglossus muscle from 15 subjects. A–G: the peak discharge rate of units active in CO2 breaths is plotted against their peak discharge rate in seven conditions. Units active in both CO2 and conditions (baseline, 2 cmH2O, 4 cmH2O, 6 cmH2O, 8 cmH2O, 10 cmH2O, and CPAP off; A–G, respectively) are represented by open circles. Units that were recruited and derecruited between conditions are represented by open squares. Solid circles and squares represent mean ± SE values. During baseline, the discharge rate of 63 active motor units is plotted against their peak rate in CO2. Under the hypercapnic condition, an additional 33 new motor units discharged. In contrast, during the higher CPAP levels (E and F), the number of active units was reduced to below baseline conditions. At 10 cmH2O, the number of active units was 36% fewer than baseline. The subtle changes in the levels of rate coding can be observed between the conditions as a slight facilitation with CO2 compared with baseline and progressive inhibition with the increased levels of CPAP pressure. In all panels, the mean peak discharge frequency of motor units active with CO2, but not the condition compared, was lower than the mean frequency of those units that remained active.
There was a significant change in the timing of motor unit activity over the conditions of increased CPAP levels. The number of inspiratory phasic units with recruitment times before inspiratory flow commenced were initially 31% with CO2, and the number declined to 21% during the period of 10 cmH2O (P < 0.001, χ2 test). The inspiratory phasic units with end-firing times before 75% Ti progressively increased from 20% under the CO2 condition to a maximum of 47% at 10 cmH2O (P < 0.001). The graded reduction reached significance at 6 cmH2O with 36% of units ending their firing (P = 0.02). The number of units that were active after 75% Ti returned to control values with CPAP off.
The background “tonic” discharge frequency of the inspiratory tonic units was similar between conditions (baseline: 14.0 ± 0.9 Hz; CO2: 12.0 ± 0.6 Hz; 2 cmH2O: 11.8 ± 0.9 Hz; 4 cmH2O: 16.0 ± 4.0 Hz; 6 cmH2O: 10.8 ± 0.8 Hz; 8 cmH2O: 12.0 ± 0.7 Hz; 10 cmH2O: 9.7 ± 0.8 Hz; CPAP off: 13.1 ± 0.8 Hz, P = 0.057). Due to the low number of units in the expiratory (tonic and phasic) and tonic classes (see Table 3), detailed subanalyses were not powered to detect changes.
Repeated-measures analysis for inspiratory phasic motor units during CO2 and CPAP conditions.
In these experiments, we attempted to follow motor units throughout the entire protocol. However, with the recruitment and derecruitment of motor units under the eight conditions, a subanalysis with repeated measures could be performed on only 20 inspiratory phasic motor units. These units were chosen as they remained active through the conditions CO2, 4 cmH2O, 6 cmH2O, and 8 cmH2O. Thus the analysis was performed to examine the progressive effects of increased CPAP on the mechanoreceptor activation.
There were a reduced number of motor unit action potentials within each breath under CPAP compared with the CO2 condition. Under the CO2 condition, the mean number of motor unit action potentials was 34.4 ± 3.9, and this declined to 24.1 ± 3.0 at 4 cmH2O, 20.4 ± 3.5 at 6 cmH2O, and 21.0 ± 4.6 at 8 cmH2O (see Fig. 2 for raw data; P < 0.05). Despite no change in the onset discharge rates, the peak discharge frequency for the 20 inspiratory phasic SMUs was found to have significant reductions at 8-cmH2O CPAP (18.4 ± 1.2 Hz) compared with CO2 (21.7 ± 1.1 Hz; P = 0.03).
The timing of the motor units relative to the onset of flow was also altered. The preinspiratory firing during CO2 (−3.7 ± 6.3% Ti) was delayed at 4 cmH2O (6.9 ± 4.3% Ti), 6 cmH2O (12.9 ± 5.7% Ti), and 8 cmH2O (7.7 ± 5.0% Ti; all P < 0.05). Consistent with fewer motor unit potentials activated, the end-firing times also showed a consistent and progressive reduction from the CO2 to the 8-cmH2O condition, with the end time reduced from 94.8 ± 5.0 to 77.8 ± 6.4% Ti (see Fig. 5 for the motor unit responses; P < 0.05).
DISCUSSION
This study provides new data on the adjustments in human genioglossus motor unit discharge behavior in response to chemoreceptor and mechanoreceptor stimulus manipulation. Using stimulation of chemoreceptors to increase ventilation and test motoneuron responses, we have observed that genioglossus inspiratory phasic SMUs significantly increase their firing rates by ∼2 Hz. However, the discharge rate does not increase equally for each class of motor unit, as the inspiratory tonic motor unit class did not increase. Facilitation of the motoneuron pool by increased chemical drive to breathe led to a substantial increase in the number of motor units active. Accordingly, these data suggest that recruitment of new units is an important mechanism by which work output of the genioglossus muscle is achieved. Furthermore, with the application of CPAP, there was a reduction in the firing rates of inspiratory phasic units and total number of active motor units, despite the elevated levels of CO2. These data support the influence for a strong inhibitory role that reduced input from mechanoreceptors has on the output of the hypoglossal motor nucleus (13, 48).
Contrary to our initial hypothesis, our results indicate that the different classes of motor units did not modify their behavior as a single population in response to elevated CO2. Rather, the peak firing frequencies of the inspiratory phasic and inspiratory tonic classes of units were altered in a disparate manner. Interestingly, the five different classes of units did respond in a homogeneous manner in terms of their percentile distribution of active units under the eight conditions. Furthermore, for the inspiratory units that were already active, there was earlier activation of units with CO2, together with delayed activation of inspiratory modulated units during CPAP.
It is well established that the output of motoneurons depends on their properties and responses to synaptic inputs (8, 34, 45). Changing the number of motor units that are active (motor unit recruitment) and the rates at which motoneurons discharge action potentials (rate coding) will alter the force that a muscle exerts (8). However, recruitment and rate coding strategies between muscles are known to vary throughout the range of the contraction (8, 10). In the genioglossus muscle during low-level contractions, the discharge rates of the motor units are already high compared with limb and respiratory pump muscles (40, 43). Therefore, it may not seem surprising that the relative increase in rate coding seems minimal (at ∼2 Hz) with moderately high levels of CO2 in the waking state. Therefore, the genioglossal motor units were consistent with the phenomenon that the newly recruited units (or higher threshold) tended to discharge at lower peak rates than the earlier recruited low-threshold units (15). The mechanical impact of a 2.0-Hz increase in firing is uncertain, particularly because the maximum firing rate (and tetanic threshold) for respiratory stimulation of genioglossus remains unclear.
Baseline vs. CO2.
Multiple studies have shown chemoreceptor activation (increased Pco2) to increase the “global” upper airway muscle activity during hypercapnia in animal models (e.g., Refs. 5, 9, 46) and human studies (e.g., Refs. 31, 32, 47). The excitation of hypoglossal motoneurons achieved with increased ventilation is consistent with the effect produced at the motoneuron pools of the parasternal intercostals and scalenes that show more prominent recruitment than frequency modulation (16). This finding is in contrast to phrenic output in humans that shows a preferential increase in rate coding with CO2 stimulation (16). Inspiratory phasic hypoglossal motoneurons discharge for longer proportions of inspiration, whereas the phasic component of inspiratory tonic motor units does not exhibit this effect. This difference in behavior between inspiratory classes of motor units suggests that there may be different drives to these motoneurons, and they may have different premotor sources (36). Alternatively, intrinsic properties of the motoneurons themselves, such as size-related properties, or active neuromodulators, may control or limit increases in discharge (8, 34). Our data suggest that the increased excitation of the hypoglossal nucleus produces increases in rate coding in the phasic class of motor units, while the tonic motor units do not receive increases in their already fast discharge rates (see Fig. 7). There is convincing evidence that mild hypercapnia is associated with increases in the underlying electrical activity within the respiratory muscles (16, 36), although how this is reflected in neuronal discharge patterns is a matter of contention across different motoneuron pools. Recent investigations concluded that recruitment (or derecruitment) of units is the predominant manner in which the human genioglossus EMG signal is increased (or decreased) (30, 51, 52). However, on the basis of the present study, we argue that a higher stimulus intensity, in contrast to earlier studies (30, 36), results in a small increase in rate coding of “phasic” units, together with the recruitment of previously silent units. This result has implications for the interpretation of previous work in OSA vs. control subjects, which showed that OSA patients had an ∼3-Hz higher rate in the inspiratory phasic motor units, together with an ∼3-Hz lower rate in the inspiratory tonic motor units (39). We interpreted this as indicating a lack of evidence for increased global central drive. However, increases in central drive, based on our present data, may only show trends for such increases in discharge frequency for inspiratory tonic units (18), but rather small increases in the frequency of inspiratory phasic units. This finding suggests that OSA patients may have increased central drive. Based on our new data, we believe the observed decrease in discharge rates of inspiratory tonic units during the rebreathing condition is due to the recruitment of more inspiratory tonic units at lower discharge frequencies (possibly driven by persistent inward currents) (18). Motor units with persistent inward currents have the ability for self-sustained, rate-limiting discharge that is induced after a brief input with mediation through monoaminergic drive (18). Thus, once inspiratory tonic units are active, additional synaptic input may minimally affect their discharge rate (18).
Pressure changes on genioglossus muscle respiratory activity.
Both animal and human studies have demonstrated a mechanoreceptor pressure-driven activation of upper airway muscles (13, 19, 20, 29, 48, 50). Given that negative pressure activates genioglossus through stimulation of mechanoreceptors, we sought to determine the pattern of deactivation of genioglossus SMUs, which would occur with the application of positive airway pressure (13). The application of positive airway pressure in 2-cmH2O increments had a graded response in inhibiting the activity of units that were active across all unit classes. This pattern of derecruitment is different from other situations, such as in sleep onset, where a predominant decrease in only the inspiratory (phasic and tonic) classes of units has been observed (52). Our findings have implications for patients using chronic CPAP, since the observed histochemical changes in the genioglossus (26, 33) with treatment in prior studies (11) is possibly a result of reduced genioglossal motor unit activity for extended periods i.e., overnight (e.g., Ref. 17).
Methodological considerations.
First, we used eight conditions to record genioglossus activity and assess the responsiveness of the same motor units to increased ventilation and monitor the graded effects of positive airway pressure over successive 1-min periods. A 10-Torr increase in PetCO2 was used as a cutoff for each trial in each subject. However, our ability to follow the discharge of the same units through the protocol with increased respiratory drive led us to accept an increase of 6 Torr in PetCO2 that was associated with a clear increase in the multiunit signal, where the single-unit activity could still be monitored. Second, it has been previously observed that genioglossus units can switch between classes in transitions from wakefulness to sleep onset (52); however, we note that this “class switching” can also happen in wakefulness, and the strength of the central pattern generators to the motoneurons may be influenced over time (unpublished observations; see Fig. 3). The main analysis was performed on the population responses of motor units due to the intermittent recruitment/derecruitment of the SMUs. The limitation of this approach was that it did not give us detailed information as to how the same units were responding across each condition. Consequently, we performed a subset analysis of the same genioglossus SMUs across multiple conditions. In this subset of inspiratory phasic units, we elected not to use the 10-cmH2O condition, as the small amount of washout of CO2 from the CPAP may have confounded the analysis. Despite this issue, we found clear evidence for graded responses with the application of CPAP at 8 cmH2O with a 3.3-Hz decrease in discharge rates from CO2. The firing times were also markedly altered with a 22% Ti decrease in the duration of discharge at 8 cmH2O compared with hypercapnic stimulation. This observation suggests that, despite a minimal washout occurring at the highest level of CPAP, meaningful conclusions could still be drawn. Finally, we recognize that, although we used standard respiratory stimuli (i.e., CO2 rebreathing and CPAP), these manipulations are complex (e.g., CO2 could increase negative airway pressure during hyperventilation and CPAP can influence end-expiratory lung volume), making definitive conclusions complex.
Summary and conclusions.
In summary, these data provide new information on how chemoreceptor and mechanoreceptors affect the activity of genioglossus motor units in humans. The various classes of genioglossus SMUs do not increase their firing rates and preinspiratory timing in a uniform manner across the motoneuron pool. Chemoreceptor activation substantially increased ventilation and the number of motor units active, suggesting that recruitment remains an important mechanism by which the genioglossus muscle is controlled. The increase in the discharge rates of inspiratory phasic motor units is in contrast to the population of inspiratory tonic units, which show no such increase. Of note, we show that the application of CPAP reduced the firing rates of inspiratory phasic units and the total number of active motor units. This finding supports the influence of mechanoreceptors on hypoglossal motor output, which is capable of outweighing the chemoreceptor excitatory response. These data may have clinical implications for novel therapeutic targets in OSA.
GRANTS
This work was supported by the American Heart Association Grant 0840159N and National Heart, Lung, and Blood Institute Grants R01 HL085188, R01 HL090897, K24 HL093218, and P01 HL095491. J. P. Saboisky is supported by the American Heart Association Founders Affiliate Postdoctoral Fellowship Award (0826061D). D. J. Eckert is supported by Overseas Biomedical (CJ Martin) Fellowship from the National Health and Medical Research Council of Australia (510392).
DISCLOSURES
D. P. White is the Chief Medical Officer for Philips Respironics. D. J. Eckert consults for Apnex Medical. A. Malhotra has received consulting and/or research income from Philips, Apnex Medical, Medtronic, Ethicon, Pfizer, Merck, SGS, SHC, Itamar, Cephalon, and Sepracor. A. S. Jordan consults for Apnex Medical. A number of the authors have industry affiliations related to the treatment of sleep apnea. This study examining physiological mechanisms was funded via peer review grant agencies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Karen Stevenson for the recruitment of subjects and all staff at the Sleep Disorders Program of the Brigham & Women's Hospital for assistance.
REFERENCES
- 1. Abd-El- Malek S. A contribution to the study of the movements of the tongue in animals, with special reference to the cat. J Anat 73: 15–31, 1938 [PMC free article] [PubMed] [Google Scholar]
- 2. Adachi S, Lowe AA, Tsuchiya M, Ryan CF, Fleetham JA. Genioglossus muscle activity and inspiratory timing in obstructive sleep apnea. Am J Orthod Dentofacial Orthop 104: 138–145, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Akahoshi T, White DP, Edwards JK, Beauregard J, Shea SA. Phasic mechanoreceptor stimuli can induce phasic activation of upper airway muscles in humans. J Physiol 531: 677–691, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alex CG, Aronson RM, Onal E, Lopata M. Effects of continuous positive airway pressure on upper airway and respiratory muscle activity. J Appl Physiol 62: 2026–2030, 1987 [DOI] [PubMed] [Google Scholar]
- 5. Bailey EF, Fregosi RF. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J Appl Physiol 96: 440–449, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Bailey EF, Fridel KW, Rice AD. Sleep/wake firing patterns of human genioglossus motor units. J Neurophysiol 98: 3284–3291, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Bailey EF, Jones CL, Reeder JC, Fuller DD, Fregosi RF. Effect of pulmonary stretch receptor feedback and CO(2) on upper airway and respiratory pump muscle activity in the rat. J Physiol 532: 525–534, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Binder MD, Heckman CJ, Powers RK. The physiological control of motoneuron activity. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, chapt. 1, p. 3–53 [Google Scholar]
- 9. Brennick MJ, Parisi RA, England SJ. Genioglossal length and EMG responses to static upper airway pressures during hypercapnia in goats. Respir Physiol 127: 227–239, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Burke RE. Motor units: anatomy, physiology, and functional organization. In: Handbook of Physiology. The Nervous System. Motor Control. Bethesda, MD: Am. Physiol. Soc., 1981, sect. 1, vol. II, part 1, p. 345–422 [Google Scholar]
- 11. Carrera M, Barbe F, Sauleda J, Tomas M, Gomez C, Agusti AG. Patients with obstructive sleep apnea exhibit genioglossus dysfunction that is normalized after treatment with continuous positive airway pressure. Am J Respir Crit Care Med 159: 1960–1966, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Cheng S, Butler JE, Gandevia SC, Bilston LE. Movement of the tongue during normal breathing in awake healthy humans. J Physiol 586: 4283–4294, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deegan PC, Nolan P, Carey M, McNicholas WT. Effects of positive airway pressure on upper airway dilator muscle activity and ventilatory timing. J Appl Physiol 81: 470–479, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Eastwood PR, Allison GT, Shepherd KL, Szollosi I, Hillman DR. Heterogeneous activity of the human genioglossus muscle assessed by multiple bipolar fine-wire electrodes. J Appl Physiol 94: 1849–1858, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Freund HJ. Motor unit and muscle activity in voluntary motor control. Physiol Rev 63: 387–436, 1983 [DOI] [PubMed] [Google Scholar]
- 16. Gandevia SC, Gorman RB, McKenzie DK, De Troyer A. Effects of increased ventilatory drive on motor unit firing rates in human inspiratory muscles. Am J Respir Crit Care Med 160: 1598–1603, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Gordon T, Thomas CK, Munson JB, Stein RB. The resilience of the size principle in the organization of motor unit properties in normal and reinnervated adult skeletal muscles. Can J Physiol Pharmacol 82: 645–661, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist 14: 264–275, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horner RL, Innes JA, Holden HB, Guz A. Afferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesia. J Physiol 436: 31–44, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol 436: 15–29, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hudgel DW, Harasick T. Fluctuation in timing of upper airway and chest wall inspiratory muscle activity in obstructive sleep apnea. J Appl Physiol 69: 443–450, 1990 [DOI] [PubMed] [Google Scholar]
- 22. Hwang JC, Bartlett D, Jr, St. John WM. Characterization of respiratory-modulated activities of hypoglossal motoneurons. J Appl Physiol 55: 793–798, 1983 [DOI] [PubMed] [Google Scholar]
- 23. Jordan AS, White DP, Lo YL, Wellman A, Eckert DJ, Yim-Yeh S, Eikermann M, Smith SA, Stevenson KE, Malhotra A. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep 32: 361–368, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim J, In K, You S, Kang K, Shim J, Lee S, Lee J, Park C, Shin C. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med 170: 1108–1113, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Kobayashi I, Perry A, Rhymer J, Wuyam B, Hughes P, Murphy K, Innes JA, McIvor J, Cheesman AD, Guz A. Inspiratory coactivation of the genioglossus enlarges retroglossal space in laryngectomized humans. J Appl Physiol 80: 1595–1604, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Kuna ST, Bedi DG, Ryckman C. Effect of nasal airway positive pressure on upper airway size and configuration. Am Rev Respir Dis 138: 969–975, 1988 [DOI] [PubMed] [Google Scholar]
- 27. Lo YL, Jordan AS, Malhotra A, Wellman A, Heinzer RC, Schory K, Dover L, Fogel RB, White DP. Genioglossal muscle response to CO2 stimulation during NREM sleep. Sleep 29: 470–477, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lorier AR, Funk GD, Greer JJ. Opiate-induced suppression of rat hypoglossal motoneuron activity and its reversal by ampakine therapy. PLoS ONE 5: e8766, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mathew OP, Abu-Osba YK, Thach BT. Influence of upper airway pressure changes on genioglossus muscle respiratory activity. J Appl Physiol 52: 438–444, 1982 [DOI] [PubMed] [Google Scholar]
- 30. Nicholas C, Bei B, Worsnop C, Malhotra A, Jordan A, Saboisky J, Chan J, Duckworth E, White D, Trinder J. Motor unit recruitment in human genioglossus muscle in response to hypercapnia. Sleep. In press [PMC free article] [PubMed] [Google Scholar]
- 31. Onal E, Lopata M, O'Connor TD. Diaphragmatic and genioglossal electromyogram responses to CO2 rebreathing in humans. J Appl Physiol 50: 1052–1055, 1981 [DOI] [PubMed] [Google Scholar]
- 32. Pillar G, Malhotra A, Fogel RB, Beauregard J, Slamowitz DI, Shea SA, White DP. Upper airway muscle responsiveness to rising Pco2 during NREM sleep. J Appl Physiol 89: 1275–1282, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Rapoport DM, Garay SM, Goldring RM. Nasal CPAP in obstructive sleep apnea: mechanisms of action. Bull Eur Physiopath Respir 19: 616–620, 1983 [PubMed] [Google Scholar]
- 34. Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev 80: 767–852, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 44: 931–938, 1978 [DOI] [PubMed] [Google Scholar]
- 36. Richardson PA, Bailey EF. Tonically discharging genioglossus motor units show no evidence of rate coding with hypercapnia. J Neurophysiol 103: 1315–1321, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saboisky J, Eckert D, Jordan A, Kelly E, Trinder J, Nicholas C, White D, Malhotra A. Human genioglossus single motor units discharge properties in quiet breathing, CO2 and CPAP. Proc Am Thor Soc ?: ?–?, 2010 [Google Scholar]
- 38. Saboisky JP, Butler JE, Fogel RB, Taylor JL, Trinder JA, White DP, Gandevia SC. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol 95: 2213–2221, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Saboisky JP, Butler JE, McKenzie DK, Gorman RB, Trinder JA, White DP, Gandevia SC. Neural drive to human genioglossus in obstructive sleep apnoea. J Physiol 585: 135–146, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saboisky JP, Butler JE, Walsh LD, Gandevia SC. New display of the timing and firing frequency of single motor units. J Neurosci Methods 162: 287–292, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Saboisky JP, Chamberlin NL, Malhotra A. Potential therapeutic targets in obstructive sleep apnoea. Expert Opin Ther Targets 13: 795–809, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saboisky JP, Eckert D, Jordan A, Trinder J, White D, Malhotra A. Behaviour of human genioglossus single motor units (SMU) discharge properties in quiet breathing, CO2 and CPAP. Proc ANS and AuPS, Sydney, NSW Australia 40: 143, 2010 [Google Scholar]
- 43. Saboisky JP, Gorman RB, De Troyer A, Gandevia S, Butler JE. Differential activation among five human inspiratory motoneuron pools during tidal breathing. J Appl Physiol 102: 772–780, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Sauerland EK, Mitchell SP. Electromyographic activity of the human genioglossus muscle in response to respiration and to positional changes of the head. Bull Los Angeles Neurol Soc 35: 69–73, 1970 [PubMed] [Google Scholar]
- 45. Schwindt PC, Crill WE. Factors influencing motoneuron rhythmic firing: results from a voltage-clamp study. J Neurophysiol 48: 875–890, 1982 [DOI] [PubMed] [Google Scholar]
- 46. Sood S, Liu X, Liu H, Nolan P, Horner RL. 5-HT at hypoglossal motor nucleus and respiratory control of genioglossus muscle in anesthetized rats. Respir Physiol Neurobiol 138: 205–221, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Stanchina ML, Malhotra A, Fogel RB, Ayas N, Edwards JK, Schory K, White DP. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med 165: 945–949, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Strohl KP, Redline S. Nasal CPAP therapy, upper airway muscle activation, and obstructive sleep apnea. Am Rev Respir Dis 134: 555–558, 1986 [DOI] [PubMed] [Google Scholar]
- 49. Takemoto H. Morphological analyses of the human tongue musculature for three-dimensional modeling. J Speech Lang Hear Res 44: 95–107, 2001 [DOI] [PubMed] [Google Scholar]
- 50. van Lunteren E, Van de Graaff WB, Parker DM, Mitra J, Haxhiu MA, Strohl KP, Cherniack NS. Nasal and laryngeal reflex responses to negative upper airway pressure. J Appl Physiol 56: 746–752, 1984 [DOI] [PubMed] [Google Scholar]
- 51. Wilkinson V, Malhotra A, Nicholas CL, Worsnop C, Jordan AS, Butler JE, Saboisky JP, Gandevia SC, White DP, Trinder J. Discharge patterns of human genioglossus motor units during arousal from sleep. Sleep 33: 379–387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wilkinson V, Malhotra A, Nicholas CL, Worsnop C, Jordan AS, Butler JE, Saboisky JP, Gandevia SC, White DP, Trinder J. Discharge patterns of human genioglossus motor units during sleep onset. Sleep 31: 525–533, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Younes M, Park E, Horner RL. Pentobarbital sedation increases genioglossus respiratory activity in sleeping rats. Sleep 30: 478–488, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328: 1230–1235, 1993 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.