Abstract
We examined the effects of exercise intensity and training on rates of lipolysis, plasma free fatty acid (FFA) appearance (Ra), disappearance (Rd), reesterification (Rs), and oxidation (RoxP) in postmenopausal (PM) women. Ten sedentary but healthy women (55 ± 0.6 yr) completed 12 wk of supervised endurance exercise training on a cycle ergometer [5 days/wk, 1 h/day, 65% peak oxygen consumption (V̇o2peak)]. Flux rates were determined by continuous infusion of [1-13C]palmitate and [1,1,2,3,3-2H5]glycerol during 90 min of rest and 60 min of cycle ergometer exercise during one pretraining exercise trial [65% V̇o2peak (PRE)] and two posttraining exercise trials [at power outputs that elicited 65% pretraining V̇o2peak (absolute training; ABT) and 65% posttraining V̇o2peak (relative training; RLT)]. Initial body weights (68.2 ± 4.5 kg) were maintained over the course of study. Training increased V̇o2peak by 16.3 ± 3.9% (P < 0.05) (Zarins ZA, Wallis GA, Faghihnia N, Johnson ML, Fattor JA, Horning MA and Brooks GA. Metabolism 58: 9: 1338–1346, 2009). Glycerol Ra and Rd were elevated in the RLT trial (P < 0.05), but not the ABT trial after training. Rates of plasma FFA Ra, Rd, and RoxP were elevated during the ABT compared with PRE trial (P < 0.05). FFA Rs accounted for most (50–70%) of Rd during exercise; training reduced FFA Rs during ABT, but not RLT compared with PRE. We conclude that, despite the large age-related decrease in metabolic scope in PM women, endurance training increases the capacities for FFA mobilization and oxidation during exercises of a given power output. However, after menopause, total lipid oxidation capacity remains low, with reesterification accounting for most of FFA Rd.
Keywords: stable isotopes, substrate utilization, fat metabolism, glycerol, lipolysis, gender, female, exercise training, lipid metabolism
menopause is accompanied by declining levels of the female sex hormones estrogen (17β-estradiol, E2) and progesterone (P4), and reductions in these ovarian steroids may affect lipid metabolism (3, 4). Also, an age-related decline in metabolic scope [maximum O2 uptake (V̇o2max)/resting metabolic rate] may cause any exercise task to be performed at a higher percentage of V̇o2max, thus shifting substrate use to carbohydrate, as opposed to lipid-derived, energy sources (2).
Despite far-ranging interest in the metabolic consequences of aging, the effects of menopause on free fatty acid (FFA) mobilization [FFA rate of appearance (Ra)], reesterification (Rs), and rate of oxidation of plasma FFAs (RoxP) have been little studied in humans. To date, studies on the effects of endurance training on FFA mobilization and oxidation during exercise in older individuals have grouped men and women together in the analysis. For example, Sial et al. (24) reported a significantly higher lipid oxidation and a lower whole body respiratory exchange ratio (RER) in elderly men and women after training during exercise at a given absolute power output (PO), without any training effect on rates of lipolysis and plasma FFA mobilization. In comparing untrained young to older subjects at the same absolute PO, Sial et al. (23) also reported similar rates of lipolysis in young compared to elderly subjects, while higher FFA mobilization, but lower fat oxidation, was found in the elderly persons. Moreover, at a similar relative exercise intensity (and higher PO), young subjects had greater rates of lipolysis, FFA mobilization, and lipid oxidation (23).
Age-related changes in fatty acid Rs may contribute to the observed differences in fat oxidation. If fatty acids were mobilized but reesterified, less lipid would be oxidized during an exercise bout. Consistent with a role of altered reesterification, Lange et al. (17) observed less than expected FFA release from abdominal adipose tissue in exercising older (75 ± 2 yr) women. However, in a recent report on older women (>60 yr), Yeckel et al. (29) utilized the glycerol Ra to FFA concentration (expressed as a ratio) as an index of whole body fatty acid Rs during hyperinsulinemic, euglycemic clamp tests in women with abdominal obesity (circumference > 97.5 cm). Their results suggested markedly blunted reesterification, and the investigators concluded that dysregulation of Rs contributed to insulin resistance in older women with abdominal obesity. Fatty acid Rs is difficult to study, as it requires independent estimates of FFA and glycerol Ra.
We examined the effects of exercise intensity and training on lipolysis, FFA Ra, rate of disappearance (Rd), Rs, and RoxP in healthy, middle-aged, postmenopausal (PM) women during a moderate endurance exercise task. Because metabolic scope declines with age, we measured these parameters in women exercising at a given absolute PO and at the same exercise intensity relative to the pretraining V̇o2max. Based on previous research on young women and the effects of E2 and P4 on lipid flux and whole body substrate partitioning, we predicted that, after menopause, women would experience decreased FFA mobilization and oxidation at rest and during exercise. However, based on experience with young men and women (9, 11), we posited that endurance training would act to compensate for the effects of age and menopause on the ability to utilize lipid energy sources. Our results show that weight-stable PM women respond in a similar manner as younger women to endurance training. However, while endurance training results in significant improvements in FFA mobilization and oxidation, the total lipid oxidation capacity remains low in PM women. A large redirection of FFA flux to Rs caused the decreased capacity for lipid oxidation in PM women; this condition was only partially corrected by endurance training.
MATERIALS AND METHODS
This paper is part of a larger investigation of substrate metabolism during endurance training in PM women. Some of the data have been reported elsewhere (30, 31), but, for convenience of readers, are repeated here.
Subjects.
Ten healthy, nonsmoking, weight-stable PM women (55 ± 0.6 yr) were recruited from the University of California campus and the surrounding community by posted notices and internet advertisements. Women were considered to be PM if they had not menstruated for at least 1 yr, and their plasma follicle-stimulating hormone (FSH) levels were >30 mIU/ml. Subjects were considered sedentary if they participated in <2 h of regular strenuous activity per week for the previous year and if they had peak oxygen consumptions (V̇o2peak) between 15 and 35 ml·kg−1·min−1, as determined by a leg cycle ergometer stress test. The women were admitted into the study if they met the following criteria: 1) were diet and weight stable for >6 mo; 2) had not taken estrogen for >6 mo or blood thinners such as aspirin for >3 mo before the study; 3) had not had a hysterectomy; 4) had normal lung function (forced expiratory volume in 1 s of 70% or more); and 5) were disease and injury free, as determined by a health history questionnaire and physical examination. Subjects were excluded if they had metabolic syndrome, as defined by the National Institutes of Health Adult Treatment Panel III (15). The University of California Committee for the Protection of Human Subjects approved the study (protocol no. 2005–10-29), and subjects provided informed consent.
Screening tests.
Tests to determine V̇o2peak were conducted on a cycle ergometer (Monark Ergometric 839E, Vansbro, Sweden) under medical supervision as per American College of Sports Medicine guidelines (16). The test started at 50 W and was increased by 25 W every 3 min until volitional exhaustion. Respiratory gases were continuously monitored throughout the test using an open-circuit online automated gas analysis system (ParvoMedics TrueMax 2400, Salt Lake City, UT) that was calibrated using room air and a certified calibration gas. Heart rate was monitored continuously using a Quinton 759 electrocardiogram (Seattle, WA), and blood pressure was measured by auscultation. Subjects were considered to have reached their V̇o2peak when the following criteria were met: 1) leveling off of oxygen consumption with increasing workload; 2) RER values >1.1; and 3) a heart rate within 10% of their age-predicted maximum. All women had an RER value >1.15 when tested for V̇o2peak, and all but two subjects had heart rates within 10% of their age-predicted maximum. None of the study subjects had a leveling off of oxygen consumption with increasing workload.
Body composition was measured by dual-energy X-ray absorptiometry; waist circumference was determined as the smallest circumference between the xiphoid process and the anterior iliac crest; hip circumference was measured as the largest circumference around the buttocks; and a 12-h fasting blood sample was taken from each of the prospective subjects to obtain baseline measurements of plasma glucose, FSH, triglyceride (TG), HDL, and total cholesterol. FSH levels were used to confirm PM status, while TG and HDL levels were used in conjunction with other parameters to assess whether the subjects had metabolic syndrome. Three-day diet records (2 weekdays and 1 weekend day) were recorded and analyzed before and after the 12-wk endurance-training program to monitor subjects' caloric intake and macronutrient composition and to ensure that the subjects had maintained the same macronutrient distribution throughout the course of the study (DietAnalysis Plus, version 6.1 ESHA Research, Salem, OR). Furthermore, participants were weighed before every training session and trial and asked to increase their energy intake to maintain weight stability.
Experimental design.
After the preliminary screening, subjects underwent the first isotope infusion trial. The subjects then underwent a 12-wk endurance-training program. Two posttraining isotope infusion trials were performed in a randomized order at weeks 10 and 12 of the training period, and training continued between the two trials. Every trial consisted of a 90-min rest period followed by 60 min of continuous pedaling on a cycle ergometer. The task was selected as it is consistent in duration with Institute of Medicine physical activity recommendations, and because, from practical experience, it was the most strenuous 1-h exercise task that sedentary participants were capable of completing (27a). Subjects drank tap water ad libitum during each trial to maintain hydration status. The pretraining trial was performed at 65% of V̇o2peak (PRE). One of the posttraining trials was performed at the same absolute workload (65% of pretraining V̇o2peak, ABT), while the other posttraining trial was performed at the same relative workload (65% of new, posttraining V̇o2peak, RLT).
Study participants were studied in each of the three trials with a three-tracer combination of [1-13C]palmitate, [1,1,2,3,3-2H5]glycerol (D5-glycerol), and [6,6,-2H2]glucose (D2-glucose). Data on glucose are reported elsewhere (30). Participants rested the day before the day in which exercise was performed, but continued typical activities of daily living. Subjects were fed standardized diets to cover energy needs (2,051 ± 58 total calories: 24% fat, 58% carbohydrate, and 18% protein) the day before trials, based on Institute of Medicine predictive equations for total energy expenditure, assuming a physical activity level of 1.5, low active (physical activity level = total energy expenditure/basal energy expenditure). Subjects were instructed not to drink caffeine-containing beverages 24 h before the trials.
The training protocol was performed on a leg cycle ergometer and has been reported elsewhere (30), but is abstracted here for convenience to the reader. Training sessions were gradually increased from 30 to 60 min over the first 4 wk. Training sessions started at 3 days/wk for the first 3 wk and then were increased to 5 days/wk during weeks 5–12. By week 5, subjects were exercising for 60 min at 65% of V̇o2peak. Interval training was added to the training during the last 4 wk, during which subjects performed four 1-min sprints at a PO that elicited 100% V̇o2peak. The four 1-min sprints are included in the training protocol to increase the exercise-training stimulus and force a larger adaptation to endurance training. Undergraduate University of California-Berkeley student trainers who had completed course work in exercise physiology and were Red Cross cardiopulmonary resuscitation certified supervised participant training. Each training session, trainers were responsible for no more than two subjects and monitored heart rate every 10 min via Polar heart rate monitors and data from the intermediate (week 5) V̇o2peak test. All subjects complied with the exercise training protocol and remained in the study for the entire duration.
Tracer protocol.
Subjects reported to the laboratory 9 h fasted on the morning of the trial, and catheters were then placed into a hand vein to obtain an “arterialized” blood sample using the heated hand vein technique for measurements of fasting blood glucose and background blood palmitate and glycerol enrichments. A previous study in our laboratory (15) showed that measurement of blood glucose isotopic enrichment (IE) was the same whether it was taken from the radial artery or used the heated hand vein technique.
After collection of the background blood and breath measurements, subjects were given a standardized pretrial meal (breakfast in 9 of 10 cases comprising 560 kcal; 60% carbohydrate, 26% fat, and 14% protein) to consume in the laboratory. Due to a work conflict, one subject completed her isotope trials in the afternoon, arriving at the laboratory at noon to begin each trial. However, the subject consumed the same standardized pretrial meal as the other subjects. A different breakfast (565 kcal; 61% carbohydrate, 27% fat, 12% protein) was provided to this subject to eat on the morning of the trials. We choose to study subjects under postabsorptive conditions to mimic the normal free-living conditions and report data on PM women with stable exercise blood glucose levels and normal preexercise liver glycogen stores.
Isotope tracers were obtained from Cambridge Isotope Laboratories (Woburn, MA), diluted in 0.9% sterile saline (D2-glucose and D5-glycerol), or bound to 5% human albumin ([1-13C]palmitate) (11, 16), tested for sterility and pyrogenicity (University of California, San Francisco, School of Pharmacy), and passed through a 0.2-μm Millipore filter (Nalgene, Rochester, NY) on the day of each experimental trial.
Ninety minutes after the subjects finished consuming the standardized pretrial meal, a venous catheter was placed in the contralateral arm and a 15-ml priming bolus of D5-glycerol was administered at a rate that was 125 times the resting infusion rate. D5-glycerol and [1-13C]palmitate were continuously infused (Baxter Travenol 6300 infusion pump) for the 90-min rest period while subjects rested supine or semisupine. The D5-glycerol resting infusion rate was 0.32 mg/min. Infusion rates were doubled during the PRE and ABT trials, and tripled for the RLT trial. The resting infusion rate of [1-13C]palmitate was 0.57 mg/min and was doubled for exercise from rest. These infusion rates were chosen based on experience to maintain stable IE values for the different exercise workloads to perform the kinetic calculations.
Respiratory gas exchange measurements and indirect calorimetry samples were collected at 0, 60, 75, and 90 min of rest and at 15, 30, 45, and 60 min of exercise using the ParvoMedics indirect calorimetry system. Heart rate and blood pressure measurements were recorded throughout rest and exercise at the same frequency as the blood and breath sampling.
Blood sampling.
Blood was drawn before tracer infusion to obtain background IEs. After background sampling and subsequent to the start of the tracer infusion, blood samples were taken at 60, 75, and 90 min of rest and at 15, 30, 45, and 60 min of exercise from the heated hand vein catheter. Hematocrit was measured at each of the time points using a circular microcapillary tube reader (no. 2201, International) and verified to be stable so as not to compromise the metabolite concentration measurements. Samples for the analysis of blood glycerol concentration and IE were immediately deproteinized with 8% perchloric acid. Samples for the analysis of plasma FFA concentration and palmitate IE were collected in EDTA tubes. One milliliter of plasma for FFA analysis was extracted with 4 ml of 30:70 heptane-isopropanol with pentadecanoic acid as internal standard and subsequently mixed with 2 ml 0.003 M sulfuric acid before storage at −20°C.
Glycerol analyses.
Glycerol concentrations and IEs were determined by gas chromatography/mass spectrometry (GC/MS; GC model 6890 series and MS model 5973N, Agilent Technologies) of the triacetate derivative using an Agilent DB-225 GC column. Details of the glycerol analysis by GC/MS have been described in detail previously (13). Briefly, in preparation for GC/MS, each sample was spiked with a known amount of [U-13C]glycerol as internal standard for concentration measurement, neutralized with 2 N potassium hydroxide, transferred to an ion exchange column with a mixture of cation and anion exchange resin (AG 50W-X8, 50–100 mesh H+ resin; AG 1-X8, 100–200 mesh formate resin), and eluted with deionized water. The effluent was lyophilized and subsequently derivatized with 100 ml of a 2:1 mixture of acetic anhydride-pyridine at 65°C for 10 min. The reagent was evaporated under N2, and the samples were reconstituted in ethyl acetate for GC/MS analysis. The GC injector temperature was set at 250°C; the initial oven temperature was 110°C and was gradually increased by 35°C until it reached a final temperature of 240°C. The transfer line was set at 250°C, the source temperature at 200°C, and the quadrapole temperature at 116°C. The carrier gas was helium, and the split less injection was used with a 35:1 ml/min ratio. Methane was used to monitor mass-to-charge ratios of 159, 162, and 164 for endogenous glycerol, [U-13C3]glycerol internal standard, and [1,1,2,3,3-2H5]glycerol tracer, respectively. Selected ion abundances were compared against external standard curves for calculation of concentration and IE.
FFA analyses.
Individual fatty acid concentrations and palmitate IEs were determined by split injection GC/MS/flame ionization (GC model 6890 series and MS model 5973N, Agilent Technologies) of the fatty acid methyl ester derivative using an Agilent DB-225 GC column and are described in detail here (13). FFA were isolated from plasma extracts by thin layer chromatography, subsequently derivatized with 12% boron trichloride in methanol to fatty acid methyl ester derivatives, dried under N2 gas, and reconstituted in heptane. The GC injector temperature was set at 250°C. The initial oven temperature was held at 55°C for 30 s and was gradually increased by 25°C/min until it reached 195°C and was then ramped at 3°C/min to 205°C and 8°C/min to 230°C. The carrier gas was helium in a constant flow mode of 2.7 ml/min and an average velocity of 60 cm/s. Electron impact ionization and selective ion monitoring were performed for mass-to-charge ratios of 270:271 for endogenous palmitate and [1-13C]palmitate tracer, respectively. Selected ion abundances were compared against external standard curves for calculation of concentration and IE. Flame ionization detection for concentration of individual fatty acids allowed for calculation of individual, total fatty acids, and %palmitate of total for modeling FFA kinetics. Individual FFA concentrations at rest include the background sample (after 9-h overnight fast) labeled “postabsorptive” and the 3-h post-meal rest sample labeled “postprandial.”
Calculations.
FFA and glycerol Ra, Rd, and metabolic clearance rate (MCR) were calculated using the last measurement period during rest (spanning 15 min, thus including time points −15 and 0) and two last measurement periods during exercise (spanning 30 min, thus including time points 30, 45, and 60 of exercise) using the equations of Steele (27), as modified for use with stable isotopes (28).
where F is the tracer infusion rate; V is the estimated volume of distribution (40 ml/kg for palmitate and 270 ml/kg for glycerol); C1 and C2 are concentrations at sampling times t1 and t2, respectively; IE1 and IE2 are IEs at sampling times t1 and t2, respectively; V̇co2 is CO2 production; and IECO2 is IE of CO2. IEs were corrected for background enrichments in blood samples collected before tracer infusion. Rate of energy expenditure and total fat oxidation (RoxT) and carbohydrate oxidation were calculated from pulmonary gas exchange using the equations of Frayn (8) for which was assumed that the rate of amino acid oxidation over 24 h was equal to the amino acid content of the standardized daily diet for each individual study participant. Percentage of Rd oxidized (Rdox) was from FFA Rd and corrected for with a bicarbonate correction factor (k) (14). Other FFA oxidation (RoxO) was calculated as the total FFA oxidation minus the RoxP. The rate of whole body Rs was estimated as the difference between the lipolytic rate (3 times glycerol Ra) and rate of total FFA oxidation (RoxT).
Statistical analyses.
Values are mean ± SE. For evaluation of significance of responses to exercise (from rest) and training on parameters of metabolism, values for the last 15 min of rest (75 and 90 min) and the last 30 min of exercise (30, 45, and 60 min) were averaged to give representative values. Because metabolite concentrations did not always stabilize during the exercise tests (see Fig. 1), we used separate two-way repeated-measures analyses of variance (RANOVAs) to evaluate significance of time on variations in blood metabolite concentrations during the three trials studied. Because training did not significantly affect resting blood metabolite concentrations, or any other variable, data from three trials were pooled to obtain representative values on resting subjects. To assess the effects of training on blood metabolite kinetics during exercise, separate RANOVAs were run on variables of interest obtained during rest, PRE, ABT, and RLT trials. If a significant F value (P < 0.05) was obtained from RANOVA, a Dunnett's test was performed to make multiple comparisons using PRE condition values as controls, while maintaining α = 0.05 (12). Statistical analyses were performed using SPSS Graduate Pack 11.0 software.
Fig. 1.
A: plasma glycerol concentration (μM) at rest and during exercise before and after 12 wk of endurance training. The pretraining (PRE) trial was at 65% peak O2 uptake (V̇o2peak). The absolute workload posttraining (ABT) trial was at the same absolute exercise power output that elicited 65% V̇o2peak before training, and the relative (RLT) exercise task elicited 65% of the posttraining V̇o2peak. B: free fatty acid (FFA) concentrations (μM) over time in middle-aged (55 ± 0.6 yr) postmenopausal women at rest and during exercise before and after 12 wk of endurance training. C: molar percent excess (MPE) of D5-glycerol over time for the 3 isotope trials. D: MPE of [1-13C]palmitate over time for the 3 isotope trials. Values are means ± SE. *Significantly different from pretraining 65% (PRE) at P < 0.05.
RESULTS
Subject characteristics.
Physical characteristics and work capacities of the subjects before and after training were reported previously (31) and are reported in Table 1 for the readers' convenience. Exercise trial work capacities and glucose concentration are detailed in Table 2 and were reported previously (31). Briefly, subjects were weight stable throughout the intervention, and measurement by dual-energy X-ray absorptiometry showed no change in body composition due to training. Maximal workload increased by 25.3 ± 3.4%, from 55.5 ± 5.98 W in PRE to 74.93 ± 4.07 W in RLT, with the ABT performed at 55.63 ± 6.02 W. V̇o2peak increased by 16.3 ± 3.9% as a result of training (P < 0.05) (31). Due to the training increase in aerobic capacity, the ABT posttraining trial was conducted at an exercise PO eliciting 55% of the posttraining V̇o2peak. Training also lowered resting heart rate, pulmonary minute ventilation, blood pressure, and mean arterial pressure during ABT (P < 0.05), but not RLT (31).
Table 1.
Subject characteristics before and after 12 wk of endurance training
| Variable | Pretraining | Posttraining | Difference, % |
|---|---|---|---|
| Age, yr | 55 ± 0.61 | ||
| Years postmenopausal | 5 ± 0.95 | ||
| Height, cm | 162.4 ± 1.44 | ||
| Weight, kg | 68.2 ± 4.50 | 67.7 ± 4.56 | −0.78 ± 0.35 |
| BMI, kg/m2 | 25.9 ± 1.71 | 25.7 ± 1.71 | −0.75 ± 0.36 |
| Body fat, % | 38.0 ± 2.90 | 38.0 ± 2.92 | −3.28 ± 2.58 |
| Fat mass, kg | 25.5 ± 3.55 | 25.7 ± 3.78 | −3.87 ± 3.12 |
| Fat-free mass, kg | 39.0 ± 1.30 | 39.1 ± 1.32 | 1.57 ± 1.03 |
| Peak power output, W | 117 ± 7.08 | 145 ± 6.21* | 25.3 ± 3.38 |
| V̇o2peak | |||
| ml · kg−1 · min−1 | 25.5 ± 1.80 | 29.4 ± 1.85* | 16.3 ± 3.93 |
| l/min | 1.69 ± 0.08 | 1.95 ± 0.08* | 16.3 ± 4.26 |
Values are means ± SE; n = 10 subjects. BMI, body mass index; V̇o2peak, peak oxygen consumption.
Significantly different from pretraining by Student's t-test, P < 0.05. Data were previously reported (31).
Table 2.
Physiological parameters of subjects during rest and exercise, before and after training
| Rest |
Exercise |
||||
|---|---|---|---|---|---|
| Variable | Pretraining | Posttraining | PRE | ABT | RLT |
| Workload, W | 55.50 ± 5.98 | 55.63 ± 6.02 | 74.93 ± 4.07†‡ | ||
| V̇o2, ml · kg−1 · min−1 | 3.46 ± 0.14 | 3.55 ± 0.16 | 16.8 ± 1.18* | 16.3 ± 1.29 | 18.69 ± 1.38†‡ |
| Plasma glucose, mmol/l | 6.85 ± 0.39 | 6.48 ± 0.32 | 5.36 ± 0.27 | 5.42 ± 0.26* | 4.51 ± 0.16*†‡ |
| Glucose Ra, mg · kg−1 · min−1 | 3.7 ± 1.4* | 3.9 ± 0.63* | 5.21 ± 0.48 | 4.58 ± 0.39* | 5.85 ± 0.36 |
Values are means ± SE; n = 10 subjects. V̇o2, oxygen consumption; Ra, rate of appearance; PRE, 65% of V̇o2peak pretraining; ABT, same absolute workload (65% of pretraining V̇o2peak); RLT, same relative workload (65% of new V̇o2peak).
Significantly different from resting conditions at P < 0.05.
Significantly different from pretraining 65% (PRE) at P < 0.05.
Metabolite concentrations and IEs.
Blood glycerol concentration was affected by preliminary procedures (e.g., ECG electrode placement, catheterization, background blood sampling, the presence of multiple investigators, etc.), but stabilized during the hour before exercise. During exercise, blood glycerol concentrations increased during all three trials. However, during exercise, there were no significant differences in glycerol concentrations between trials (Fig. 1A).
As with glycerol, plasma FFA concentrations were elevated by preliminary procedures, but stabilized in the hour before exercise (Fig. 1B). Plasma FFA concentrations increased in all three trials throughout exercise, and a two-way RANOVA revealed a training effect on FFA concentration during exercise (P < 0.05) (Table 3; see also Table 5). Multiple comparisons using Dunnett's showed a significant difference between FFA concentration in PRE and ABT trials at 30 min into exercise (Fig. 1B) (P < 0.05). Individual FFA concentrations for plasma myristate, palmitate, palmitoleate, stearate, oleate, linoleate, and total plasma FFA concentration averaged by trial are listed in Table 4. The percentage contribution of palmitate to total plasma FFA concentration did not change between the trials before or after training. IEs for glycerol (Fig. 1C), palmitate (Fig. 1D), and 13CO2 (data not shown) were used to calculate metabolite kinetics.
Table 3.
FFA kinetics in postmenopausal women during rest and exercise
| Rest |
Exercise |
||||
|---|---|---|---|---|---|
| Variable | Pretraining | Posttraining | PRE | ABT | RLT |
| Glycerol and FFA kinetics | |||||
| Glycerol Rd | |||||
| mmol · kg−1 · min−1 | 1.3 ± 0.3* | 1.6 ± 0.3* | 3.4 ± 0.7 | 3.4 ± 0.5 | 4.2 ± 0.8 |
| mmol · kg FFM−1 · min−1 | 2.3 ± 0.4* | 2.7 ± 0.4* | 6.0 ± 0.9 | 6.1 ± 0.7 | 7.7 ± 1.0* |
| Glycerol MCR | |||||
| ml · kg−1 · min−1 | 25.9 ± 6.9 | 31.5 ± 6.7 | 20.1 ± 4.7 | 18.9 ± 2.3 | 27.1 ± 4.7 |
| ml · kg · FFM−1 · min−1 | 41.6 ± 8.6* | 50.9 ± 9.4* | 32.9 ± 5.8 | 33.1 ± 2.9 | 45.2 ± 6.2* |
| FFA Ra | |||||
| mmol · kg−1 · min−1 | 1.7 ± 0.4* | 2.2 ± 0.2* | 5.7 ± 0.8 | 8.2 ± 1.2* | 7.2 ± 0.6 |
| mmol · kg FFM−1 · min−1 | 3.3 ± 0.6* | 3.7 ± 0.3* | 9.5 ± 1.7 | 13.3 ± 1.7* | 11.5 ± 1.1 |
| FFA MCR | |||||
| ml · kg−1 · min−1 | 11.5 ± 0.9 | 10.8 ± 1.3 | 11.8 ± 1.2 | 14.0 ± 1.7 | 12.5 ± 0.9 |
| ml · kg FFM−1 · min−1 | 19.6 ± 1.4 | 18.2 ± 1.9 | 19.1 ± 1.8 | 22.9 ± 2.2 | 20.1 ± 1.0 |
| FFA oxidation | |||||
| Total, μmol · kg−1 · min−1 | 0.7 ± 0.1* | 0.7 ± 0.1* | 4.3 ± 0.8 | 6.3 ± 1.2 | 5.5 ± 1.0 |
| Plasma, μmol · kg−1 · min−1 | 0.2 ± 0.0* | 0.2 ± 0.0* | 3.1 ± 0.4 | 4.9 ± 0.6* | 4.4 ± 0.4 |
| Other FFA, μmol · kg−1 · min−1 | 0.6 ± 0.1 | 0.5 ± 0.1 | 1.1 ± 0.6 | 1.8 ± 1.2 | 1.2 ± 0.8 |
| FFA from other†, % | 81.1* | 70.0* | 26.3 | 29.4 | 22.0 |
| FFA Rd oxidized, % | 7.6 ± 0.6* | 7.7 ± 0.6* | 59.8 ± 3.9 | 64.9 ± 2.9 | 67.5 ± 4.3* |
| Reesterification rate, μmol · kg−1 · min−1 | 3.8 ± 0.6* | 3.3 ± 0.5* | 8.1 ± 0.8 | 6.2 ± 1.2 | 11.3 ± 2.0* |
Values are means ± SE. FFA, free fatty acid; Rd, rate of disappearance; MCR, metabolic clearance rate; FFM, fat-free mass.
Significantly different from pretraining 65% (PRE) at P < 0.05.
An estimate of the %total FFA oxidation coming from nonplasma sources, calculated as the %other FFA oxidation from total FFA oxidation.
Table 5.
Summary of statistical comparisons
| RANOVA |
Dunnett's Test, P value |
||||||
|---|---|---|---|---|---|---|---|
| Parameter | DF between | DF within conditions | DF within residual | F | PRE vs. Rest | PRE vs. ABT | PRE vs. RLT |
| FFA Ra, mmol · kg−1 · min−1 | 9 | 3 | 27 | 17.96 | <0.01 | <0.05 | |
| FFA Ra, mmol · kg FFM−1 · min−1 | 9 | 3 | 27 | 14.40 | <0.01 | <0.05 | |
| FFA Rd, mmol · kg−1 · min−1 | 9 | 3 | 27 | 15.17 | <0.01 | <0.05 | |
| Glycerol Ra, mmol · kg−1 · min−1 | 8 | 3 | 24 | 20.63 | <0.01 | <0.05 | |
| Glycerol Rd, mmol · kg−1 · min−1 | 7 | 3 | 21 | 10.01 | <0.01 | ||
| Glycerol Rd, mmol · kg FFM−1 · min−1 | 8 | 2 | 24 | 16.29 | <0.01 | <0.01 | |
| Glycerol MCR, mmol · kg FFM−1 · min−1 | 8 | 3 | 24 | 5.51 | <0.01 | <0.01 | |
| Individual FFA concentrations | 9 | 3 | 27 | 25.36 | <0.01 | <0.01 | <0.01 |
| Total lipid metabolism | 8 | 3 | 24 | 20.04 | <0.01 | ||
| FFA oxidation | |||||||
| Total, μmol · kg−1 · min−1 | 8 | 3 | 24 | 18.40 | <0.01 | ||
| Plasma, μmol · kg−1 · min−1 | 9 | 3 | 27 | 18.60 | <0.01 | <0.05 | |
| FFA from other, % | 8 | 3 | 24 | 5.46 | <0.05 | ||
| FFA Rd oxidized, % | 9 | 3 | 27 | 124.68 | <0.01 | <0.01 | |
| Reesterification rate, μmol · kg−1 · min−1 | 7 | 3 | 21 | 12.84 | <0.01 | <0.05 | |
F-value from one-way repeated-measures ANOVA (RANOVA) testing for effect of training. Dunnett's test was used for multiple comparisons against a single (PRE) control group to reveal significance between trials. Degrees of freedom (DF) are reported between and within (conditions + residual) subjects.
Table 4.
Individual FFA concentrations in postmenopausal women postabsorptive, postprandial, and during exercise
| Rest |
Exercise |
||||
|---|---|---|---|---|---|
| FFA | Postabsorptive | Postprandial | PRE | ABT | RLT |
| Myristic, μmol/l | 9.3 ± 0.7 | 2.7 ± 0.4 | 7.9 ± 1.0 | 8.0 ± 0.8 | 8.5 ± 0.7 |
| Palmitate, μmol/l | 183.4 ± 10.4 | 67.1 ± 5.0 | 154.1 ± 16.3 | 189.0 ± 14.9 | 180.7 ± 9.5 |
| Palmitoleic, μmol/l | 15.7 ± 1.5 | 3.0 ± 0.4 | 13.0 ± 2.2 | 13.5 ± 1.9 | 14.6 ± 1.3 |
| Stearic, μmol/l | 86.9 ± 8.2 | 30.1 ± 2.2 | 46.4 ± 4.3 | 76.1 ± 9.6 | 66.2 ± 4.2 |
| Oleic, μmol/l | 284.7 ± 18.4 | 74.8 ± 7.6 | 209.0 ± 23.3 | 269.8 ± 26.8 | 260.0 ± 20.2 |
| Linoleic, μmol/l | 111.6 ± 6.5 | 39.2 ± 4.5 | 104.3 ± 12.8 | 108.2 ± 11.1 | 111.7 ± 10.7 |
| Linolenic, μmol/l | 10.2 ± 1.3 | 10.1 ± 1.2 | 8.3 ± 1.3 | 11.7 ± 2.1 | 8.7 ± 1.2 |
| Total, μmol/l | 701.0 ± 40* | 226.3 ± 18* | 542.9 ± 58 | 675.5 ± 55* | 649.3 ± 41* |
| Palmitate, % | 26 | 30 | 28 | 28 | 28 |
Values are means ± SE.
Significantly different from pretraining 65% (PRE), P < 0.05. Symbols are same as in Fig. 1.
Metabolite kinetics.
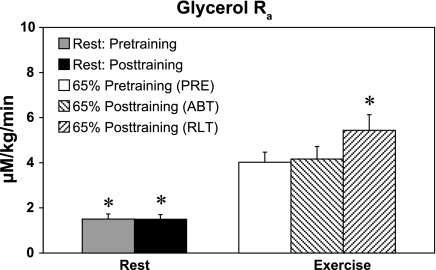
A one-way RANOVA revealed a training effect on glycerol Ra (P < 0.05). Multiple comparisons using a Dunnett's test showed a significant increase between PRE and rest, as well as PRE and RLT, but not between PRE and ABT (P < 0.05) (Fig. 2). The patterns for glycerol Rd and MCR during rest and exercise before and after training were similar to those of glycerol Ra (Table 3). There were no significant changes in glycerol MCR when expressed per unit body weight after training at rest or during exercise (Table 3). However, RANOVA revealed a training effect on glycerol MCR when expressed in terms of fat free mass (P < 0.05). Multiple comparisons using Dunnett's test isolated a significant difference between PRE, and rest as well as between PRE and RLT, but not between PRE and ABT (P < 0.05).
Fig. 2.
Effect of 12 wk of endurance training on plasma glycerol rate of appearance (Ra) in postmenopausal (PM) women. Values are means ± SE of the last 15 min of rest and the last 30 min of exercise. Symbols are same as in Fig. 1. *Significantly different from pretraining 65% (PRE) at P < 0.05.
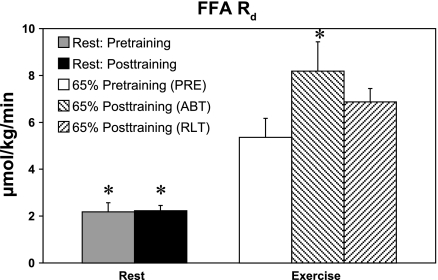
Resting FFA Ra and Rd were unaffected by training, and during exercise the pattern of response for FFA Ra (Table 3) was similar to that of FFA Rd (Fig. 3). RANOVA revealed significant training effects on FFA Ra and Rd (P < 0.05). Multiple comparisons using a Dunnett's test isolated a significant decrease between rest and PRE and a significant increase between PRE and ABT, whether expressed in terms of total body weight or fat-free mass (Table 3) (P < 0.05).
Fig. 3.
Effect of 12 wk of endurance training on plasma FFA rate of disappearance (Rd) in PM women. Values are means ± SE of the last 15 min of rest and the last 30 min of exercise. Symbols are same as in Fig. 1. *Significantly different from pretraining 65% (PRE) at P < 0.05.
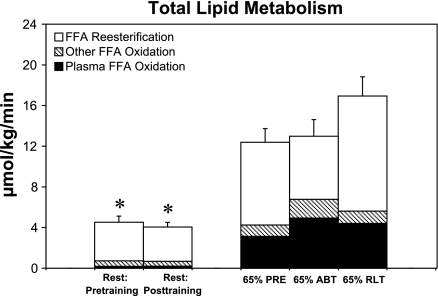
Rs dominated FFA Rd (Rs, open bars, Fig. 4). Rs during the PRE, ABT, and RLT trials comprised 66, 48, and 64% of total lipid metabolism, respectively.
Fig. 4.
Effect of 12 wk of endurance training on fatty acid reesterification and other components of total lipid metabolism in PM women. Values are means ± SE of the last 15 min of rest and the last 30 min of exercise. Symbols are same as in Fig. 1 and are for total lipid metabolism. *Significantly different from pretraining 65% (PRE) at P < 0.05.
Comparing the PRE and ABT trial, total lipid metabolism did not change (Fig. 4), but RoxP and RoxO increased, whereas Rs decreased. In part, the increase in RoxP during ABT compared with PRE can be attributed to the increase in percent Rd oxidized (Table 3). RANOVA revealed a significant training effect on total lipid metabolism (P < 0.05). Multiple comparisons using a Dunnett's test showed a significant decrease between PRE and rest, but no significant differences between PRE and ABT or PRE and RLT (P < 0.05). Total oxidative energy sources based on energy expenditure of trials is reported elsewhere (31). The rate of total body Rs did not differ at rest before and after training, but RANOVA revealed a training effect (P < 0.05). Multiple comparisons using a Dunnett's test showed PRE was significantly higher than rest and that RLT was significantly higher than PRE (P < 0.05). During exercise, there was a reduction in Rs during the ABT trial compared with the PRE trial. but this was not statistically significant.
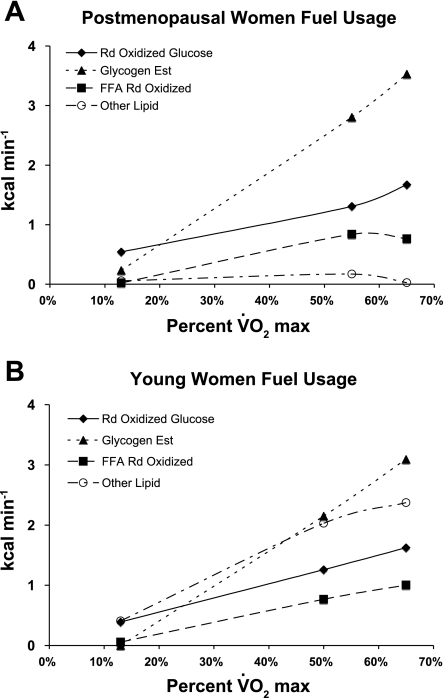
Using data taken from a study on younger women (9), Fig. 5 presents a comparison of the endogenous substrates oxidized in PM and younger women during exercise. While the usages of glycogen, blood glucose, and blood-borne FFAs are similar between age groups, the “other lipid depot,” purported to represent intramyocellular TGs and other depots, is not accessed in PM women.
Fig. 5.
A: contribution of different fuels used in PM women exercising at intensities ranging from rest to 65% maximum O2 uptake (V̇o2max). Rd oxidized glucose was calculated as 50% of Rd; FFA Rd oxidized was calculated as percent Rd FFA oxidized at given power output posttraining (see Table 3). Glycogen estimation is from total carbohydrate minus Rd oxidized glucose. Other lipid is calculated as total lipid oxidized minus Rd oxidized FFA Rd. B: data taken from Friedlander et al. (9, 10).
DISCUSSION
In weight-stable PM women, parameters of FFA mobilization and oxidation respond to exercise and endurance training much like they do in younger individuals. However, while endurance training results in significant improvements in FFA mobilization and oxidation, even after training total lipid oxidation capacity remains low in PM women due to their reduced metabolic scope and a large redirection of FFA flux to Rs compared with younger women.
Endurance training increased FFA Rd by an average of 52% during continuous exercise at a given moderate submaximal PO in PM women. In concordance with results of earlier training studies on older individuals (24), the increases in FFA Rd by oxidation we observed in PM women exercising at a given exercise PO after training demonstrates significant metabolic plasticity in response to an endurance training program. In this context, we note that the posttraining increase in FFA Rd by oxidation at a given absolute PO (i.e., ABT) shows that the Crossover Concept of energy-substrate partitioning in fuel selection during exercise applies to aging, as well as younger, human populations (2). Because relative exercise intensity is the largest determinant of energy-substrate partitioning during exercise, an increase in total aerobic capacity allows for a shift in substrate utilization in endurance-trained individuals exercising at a given absolute PO. Now we report that PM women are capable of many of the same endurance training-induced adaptations as their younger counterparts. We also report with confidence that these effects are due to endurance training and not caloric restriction, changes in dietary composition, or body weight alterations, because we successfully controlled for those independent modulators of metabolism over the course of the training study. However, PM women are unable to achieve increased rates of FFA oxidation at a relatively high (65% V̇o2peak) workload even after 12 wk of training.
Total lipid metabolism was less in middle-aged PM women compared with those seen in previous studies on young women under similar experimental conditions (1, 9, 10). As noted in our laboratory's companion papers (30, 31), and reproduced in the literature in older populations (6, 25), a nascent insulin-resistant state most likely accounts for the decrease in total lipid metabolism determined in PM women 3 h after a standardized breakfast. The lower total lipid metabolism during exercise is in part due to the decreased ability to consume oxygen in PM women compared with the younger population. When expressed in liters per minute, V̇o2max was 47% less in PM women than in their younger counterparts (9). While an age-related decline in V̇o2max is well documented, the reason for the decline is not known (18, 26). Importantly, however, given the metabolic plasticity of PM women in response to endurance training, we interpret the results to mean that the PM decrease in total work capacity is not caused by an older body becoming less metabolically adaptable; instead, the metabolic responses we observed are attributable to age-related decreases in metabolic scope. As well, the age-related decline in metabolic scope may have been exacerbated by decades of a sedentary lifestyle that could not be reversed completely by 12 wk of endurance training. In support of this interpretation, we note that Short et al. (22) observed similar degrees of mitochondrial adaptation in young and elderly subjects in response to 16 wk of endurance training. However, even after training, mitochondrial capacity was less in trained older compared with that in young women.
The significant increase in FFA Rd in the ABT trial has not previously been seen in PM women, but the increase mimics the response seen in younger women (9, 20) and men (1, 5) and the trend seen by Sial et al. (24) in older individuals. The data we now report are also consistent with training and comparison studies in older subjects utilizing indirect calorimetry to assess whole body substrate selection that have primarily been performed on men or pooled cohorts of men and women in the analysis (24). In an earlier report, Sial et al. suggested that the increase in lipid oxidation is due to metabolic changes at the muscle level. Our data support this conclusion, in part, because of a higher FFA MCR during the ABT trial, lower Rs, and increased RoxP.
Never measured before in PM women was the finding of a lower FFA Rd in RLT trial. The lack of E2 in PM women is a likely explanation for the decrease in FFA Rd at the same RLT workload. E2 administration to men and amenorrhic women has demonstrated increase in fat oxidation and a decrease in glucose Ra (4, 21). Mechanistically, Campbell and Febbraio (3) reported E2 administration in rats increased the activity of carnitine palmitoyltransferase I and β-3-hydroxyacyl-CoA dehydrogenase, and withdrawal of E2 following menopause may alter muscle biochemistry in favor of carbohydrate metabolism during exercise.
Whole body lipolysis, as represented by glycerol Ra, scaled to exercise intensity such that the greatest glycerol Ra was achieved during the RLT trial (Fig. 2). Because the same pattern of response was observed in young women studied in the early follicular menstrual cycle phase (9), there is no evidence for an effect of chronic withdrawal of ovarian hormones on lipolysis in the women studied here during exercise. Our values for glycerol flux rates observed in PM women are lower than those observed in younger women and men at the same relative workloads (5, 11), but higher than those reported in elderly men and women at a lower work intensity (26). Hence, the present results on lipolysis we observed in PM women show both aging (i.e., suppressed lipolysis in older individuals), as well as sex effects (i.e., greater lipolytic rates in women compared with men).
As a percentage of lipolysis, fatty acid Rs rates were three times higher in PM women compared with rates in their younger counterparts (Table 3, Fig. 4). These data are interpreted to mean that PM women are able to mobilize TGs at a similar rate as younger women, but after menopause women are unable to oxidize available FFAs, a finding that supports the notion that older individuals have a muscle-specific defect in the ability to oxidize lipid (9, 23). A comparison of PRE and ABT trials shows that 12 wk of endurance training partially compensates for the PM imbalance between FFA disposal via Rs and oxidation as Rs decreases and RoxP increases after training (Fig. 4). The limitation in ability to oxidize FFA in PM compared with younger women is further illustrated in Fig. 5. While the absolute energy (kcal) derived from blood glucose, glycogen, and plasma FFA does not differ vastly from the PM and young women; the “other lipid” (RoxO = RoxT − RoxP) is much less in PM than in younger women. An inability of PM women to access intramuscular TG use is a possibility, although methodological considerations limit the current knowledge as to their participation to exercising muscle substrate oxidation during exercise (1, 19). Another possibility is that, in PM women, inactive muscle and other tissues are unable to use lipid during exercise and instead use carbohydrate-derived fuels. This alternative possibility is supported by the fact that the women in the present study displayed such high glucose disposal rates that blood glucose concentration was suppressed during the RLT trial (Table 4). In contrast, glycemia was well maintained in younger women during similar exercise bouts (10, 30). Additionally, a comparison of glycerol Ra and FFA Rs values determined during ABT and RLT trials allowed assessment of the effects of exercise intensity on fatty acid mobilization and Rs. By those measures, exercise intensity affects both FFA mobilization and Rs (Table 3 and Fig. 2).
While there is a paucity of data on fatty acid Rs in older women, our present results in elevated Rs are consistent with data of Lange et al. (17). They observed low net FFA compared with glycerol release from abdominal adipose tissue in exercising older (75 ± 2 yr) women. Additionally, Lange et al. also described an inability of working muscles to oxidize FFAs in the older women. Now, our results (Table 3) provide the first direct measurement of whole body Rs during exercise in older women. Recently also Yeckel et al. (29) reported on fatty acid Rs in older women, and like us those authors also concluded that Rs was dysregulated. Using an indirect measurement, Yeckel et al. concluded that Rs was blunted in abdominally obese PM women compared with nonabdominally obese PM women. Seemingly, the two research groups initiated very different studies on different populations. Perhaps most importantly, whereas we studied women 3–4 h following a small, standardized meal, they studied fasted women using a hyperinsulinemic, euglycemic clamp using high- and low-insulin infusion rates. Hence, the treatments (exercise-low insulin vs. clamp-high insulin) were very different. Unfortunately, for the purposes of comparison, Yeckel et al. did not report basal glycerol Ra values, and their methodology did not involve an assessment of FFA Ra. However, an earlier training study by DiePietro et al. (7) in PM women reported glycerol Ra data during a hyperinsulinemic/euglycemic clamp and reported increased insulin sensitivity of glycerol Ra after higher intensity training. Hence, for the present, it can only be said that dysregulated FFA Rs is suspected in PM women.
In evaluating significance of our results, it must be acknowledged that, because of the presence of multiple comparisons, the P values and dependent conclusions must be interpreted cautiously. In our statistical analysis, we corrected for the effects of repeated trials on the same set of subjects by means of RANOVAs followed by Dunnett's tests. Use of those statistical tools assumed that each parameter measured was independent of every other. However, in some cases, the parameters measured were, in fact, biologically related (e.g., FFA Ra and glycerol Ra). In such cases, the reader needs to assess physiological significance of differences. In the example mentioned, because glycerol Ra and FFA Ra were related parameters, but responded differently to treatment, the differences observed in PM women are judged to be physiologically, as well as significantly, different.
Because of problems associated with adjusting α when calculating P values for making multiple comparisons on a relatively small number of subjects, we reevaluated the data using a mixed-effects regression model and STATA 11.0 software (Table 5). That approach gave similar results as shown in Table 3 obtained using RANOVAs and Dunnett's tests. Hence, we feel justified in our data analyses and interpretations.
In summary, despite a large age-related decrease in metabolic scope and corresponding decrements in total lipid metabolism, PM women retain the capacity for endurance training-induced increases in FFA mobilization and disposal by oxidation during continuous exercise at a given moderate-intensity submaximal exercise PO. Along with increases in the abilities to mobilize and utilize lipid as a fuel source, improved V̇o2peak, lower heart rate, ventilation, and mean arterial pressure at rest and during the same absolute PO, we report that weight-stable PM women are capable of many of the same endurance training-induced adaptations as seen in younger counterparts. In this context, it is remarkable that, despite the age-related decline in metabolic scope, energy substrate utilization patterns in PM women are similar to those predicted under the crossover concept (2), as observed on younger individuals (1, 9, 13). In other words, there do not appear to be age-related changes in energy substrate utilization that cause changes in metabolic scope; instead, energy substrate utilization patterns in PM women are predictable based on metabolic scope, which is reduced in aging. However, while after menopause women are capable of mobilizing lipid energy sources, 12 wk of endurance training was inadequate to prevent loss in the ability to maintain substantial rates of lipid oxidation at high relative workloads.
GRANTS
This study was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R01 AR042906 to G. A. Brooks.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENT
We thank the subjects for participation and compliance with training and experimental procedures and good cheer. They are truly amazing and accomplished people. We also thank T. Mau, N, Faghihnia, P. Nguyen, T. Nguyen, M. Patella, E. Mayeda, B. Martinelli, and N. Wortham for technical support and assistance, and C. Chang, S. Dixit, A. Luke, and H. Masket for providing medical coverage during exercise stress testing.
REFERENCES
- 1. Bergman BC, Butterfield GE, Wolfel EE, Casazza GA, Lopaschuk GD, Brooks GA. Evaluation of exercise and training on muscle lipid metabolism. Am J Physiol Endocrinol Metab 276: E106–E117, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the “crossover” concept. J Appl Physiol 76: 2253–2261, 1994 [DOI] [PubMed] [Google Scholar]
- 3. Campbell SE, Febbraio MA. Effect of ovarian hormones on mitochondrial enzyme activity in the fat oxidation pathway of skeletal muscle. Am J Physiol Endocrinol Metab 281: E803–E808, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Carter S, McKenzie S, Mourtzakis M, Mahoney DJ, Tarnopolsky MA. Short-term 17β-estradiol decreases glucose Ra but not whole body metabolism during endurance exercise. J Appl Physiol 90: 139–146, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Carter SL, Rennie C, Tarnopolsky MA. Substrate utilization during endurance exercise in men and women after endurance training. Am J Physiol Endocrinol Metab 280: E898–E907, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Defronzo RA. Glucose intolerance and aging: evidence for tissue insensitivity to insulin. Diabetes 28: 1095–1101, 1979 [DOI] [PubMed] [Google Scholar]
- 7. DePietro L, Dziura J, Yeckel CW, Neufer PD. Exercise and improved insulin sensitivity in older women: evidence of the enduring benefits of higher intensity training. J Appl Physiol 100: 142–149, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55: 628–634, 1983 [DOI] [PubMed] [Google Scholar]
- 9. Friedlander AL, Casazza GA, Horning MA, Budinger TF, Brooks GA. Effects of exercise intensity and training on lipid metabolism in young women. Am J Physiol Endocrinol Metab 275: E853–E863, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Friedlander AL, Casazza GA, Horning MA, Huie MJ, Piacentini MF, Trimmer JK, Brooks GA. Training-induced alterations of carbohydrate metabolism in women: women respond differently from men. J Appl Physiol 85: 1175–1186, 1998 [DOI] [PubMed] [Google Scholar]
- 11. Friedlander AL, Casazza GA, Horning MA, Usaj A, Brooks GA. Endurance training increases fatty acid turnover, but not fat oxidation, in young men. J Appl Physiol 86: 2097–2105, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Glantz SA. Primer of Biostatistics (6th Ed.). New York: McGraw-Hill Medical, 2005 [Google Scholar]
- 13. Henderson GC, Fattor JA, Horning MA, Faghihnia N, Johnson ML, Mau TL, Luke-Zeitoun M, Brooks GA. Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J Physiol 584: 963–981, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henderson GC, Fattor JA, Horning MA, Faghihnia N, Luke-Zeitoun M, Brooks GA. Retention of intravenously infused [13C]bicarbonate is transiently increased during recovery from hard exercise. J Appl Physiol 103: 1604–1612, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Horning MA, Friedlander AL, Casazza GA, Huie MJ, Brooks GA. Arterial and “arterialized” sampling sites do not change isotopic enrichment using [6,6-d-glucose] and [1,1,2,3,3,-d-glycerol] (Abstract). FASEB J 12: A854, 1998 [Google Scholar]
- 16. Institute of Medicine Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington. DC: National Academies Press, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Lange KH, Lorentsen J, Isaksson F, Simonsen L, Juul A, Christensen NJ, Kjaer M, Bulow J. Subcutaneous abdominal adipose tissue lipolysis during exercise determined by arteriovenous measurements in older women. J Am Geriatr Soc 50: 275–281, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Lanza IR, Nair KS. Muscle mitochondrial changes with aging and exercise. Am J Clin Nutr 89: 467S–71S, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roepstorff C, Vistisen B, Kiens B. Intramuscular triacylglycerol in energy metabolism during exercise in humans. Exerc Sport Sci Rev 33: 182–188, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Romijn JA, Coyle EF, Sidossis LS, Rosenblatt J, Wolfe RR. Substrate metabolism during different exercise intensities in endurance-trained women. J Appl Physiol 88: 1707–1714, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Ruby BC, Robergs RA, Waters DL, Burge M, Mermier C, Stolarczyk L. Effects of estradiol on substrate turnover during exercise in amenorrheic females. Med Sci Sports Exerc 29: 1160–1169, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 52: 1888–1896, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Sial S, Coggan AR, Carroll R, Goodwin J, Klein S. Fat and carbohydrate metabolism during exercise in elderly and young subjects. Am J Physiol Endocrinol Metab 271: E983–E989, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Sial S, Coggan AR, Hickner RC, Klein S. Training-induced alterations in fat and carbohydrate metabolism during exercise in elderly subjects. Am J Physiol Endocrinol Metab 274: E785–E790, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Soler JT, Folsom AR, Kaye SA, Prineas RJ. Associations of abdominal adiposity, fasting insulin, sex hormone binding globulin, and estrone with lipids and lipoproteins in post-menopausal women. Atherosclerosis 79: 21–27, 1989 [DOI] [PubMed] [Google Scholar]
- 26. Spina RJ, Ogawa T, Kohrt WM, Martin WH, 3rd, Holloszy JO, Ehsani AA. Differences in cardiovascular adaptations to endurance exercise training between older men and women. J Appl Physiol 75: 849–855, 1993 [DOI] [PubMed] [Google Scholar]
- 27. Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci 82: 420–430, 1959 [DOI] [PubMed] [Google Scholar]
- 28. Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss, 1992 [Google Scholar]
- 29. Yeckel CW, Dziura J, DiPietro L. Abdominal obesity in older women: potential role for disrupted fatty acid reesterification in insulin resistance. J Clin Endocrinol Metab 93: 1285–1291, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zarins ZA, Johnson ML, Faghihnia N, Horning MA, Wallis GA, Fattor JA, Brooks GA. Training improves the response in glucose flux to exercise in postmenopausal women. J Appl Physiol 107: 90–97, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zarins ZA, Wallis GA, Faghihnia N, Johnson ML, Fattor JA, Horning MA, Brooks GA. Effects of endurance training on cardiorespiratory fitness and substrate partitioning in postmenopausal women. Metabolism 58: 1338–1346, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]