Abstract
The aim of this study was to explore heat acclimation effects on cutaneous vascular responses and sweating to local ACh infusions and local heating. We also sought to examine whether heat acclimation altered maximal skin blood flow. ACh (1, 10, and 100 mM) was infused in 20 highly trained cyclists via microdialysis before and after a 10-day heat acclimation program [two 45-min exercise bouts at 50% maximal O2 uptake (V̇o2max) in 40°C (n = 12)] or control conditions [two 45-min exercise bouts at 50% V̇o2max in 13°C (n = 8)]. Skin blood flow was monitored via laser-Doppler flowmetry (LDF), and cutaneous vascular conductance (CVC) was calculated as LDF ÷ mean arterial pressure. Sweat rate was measured by resistance hygrometry. Maximal brachial artery blood flow (forearm blood flow) was obtained by heating the contralateral forearm in a water spray device and measured by Doppler ultrasound. Heat acclimation increased %CVCmax responses to 1, 10, and 100 mM ACh (43.5 ± 3.4 vs. 52.6 ± 2.6% CVCmax, 67.7 ± 3.4 vs. 78.0 ± 3.0% CVCmax, and 81.0 ± 3.8 vs. 88.5 ± 1.1% CVCmax, respectively, all P < 0.05). Maximal forearm blood flow remained unchanged after heat acclimation (290.9 ± 12.7 vs. 269.9 ± 23.6 ml/min). The experimental group showed significant increases in sweating responses to 10 and 100 mM ACh (0.21 ± 0.03 vs. 0.31 ± 0.03 mg·cm−2·min−1 and 0.45 ± 0.05 vs. 0.67 ± 0.06 mg·cm−2·min−1, respectively, all P < 0.05), but not to 1 mM ACh (0.13 ± 0.02 vs. 0.18 ± 0.02 mg·cm−2·min−1, P = 0.147). No differences in any of the variables were found in the control group. Heat acclimation in highly trained subjects induced local adaptations within the skin microcirculation and sweat gland apparatus. Furthermore, maximal skin blood flow was not altered by heat acclimation, demonstrating that the observed changes were attributable to improvement in cutaneous vascular function and not to structural changes that limit maximal vasodilator capacity.
Keywords: local heating, maximal skin blood flow
it is well established that individuals who undergo a period of chronic heat exposure (heat acclimation) have improved ability to thermoregulate, especially while exercising in a hot environment (9, 33, 36, 37). An increased ability of the cardiovascular system to perfuse the skin microcirculation, together with enhanced evaporative cooling due to higher sweat rates (SRs) at a given core temperature, widens the core-to-skin thermal gradient (9, 37) and allows heat dissipation from the body core to the environment. Although there has been some research on the effects of heat acclimation on skin blood flow (SkBF), the findings on the specific mechanism by which SkBF is increased after heat acclimation remain inconclusive (36, 48, 55). Roberts et al. (36) reported that a 10-day period of heat acclimation lowered the internal temperature threshold for cutaneous vasodilation, without a significant change in the slope of the relationship relative to internal temperature. They postulated that these changes were caused by a central mechanism. Conversely, other studies showed that heat acclimation increases the slope of the relationship of forearm vascular conductance or SR to internal temperature during exercise in the heat (41, 48). Differences in the heat acclimation protocols (ambient temperature and intensity and duration of exercise) and the type of heat test (at rest or during exercise) may account for such discordant findings. However, in all these studies the changes in cutaneous blood flow were induced by an increased internal temperature. Therefore, it remains uncertain whether these changes are centrally mediated or whether there are functional or structural changes occurring within the cutaneous vasculature. Furthermore, the effects on heat acclimation on maximal SkBF remain unknown. This is important, as it remains unknown whether SkBF following heat acclimation may be augmented by an increased overall ability of the skin vessels to vasodilate (i.e., increased maximal SkBF secondary to structural changes within the vasculature) or by an improved vasodilatory responsiveness to a given stimulus (i.e., increased sensitivity).
Analysis of the vascular responsiveness to local acetylcholine (Ach) administration and analysis of the vascular responsiveness to locally applied heat (thermal hyperemia) are two of the most commonly used approaches for examining cutaneous vascular function (7, 29). ACh is an endothelium-dependent vasodilator that works predominantly through a prostanoid-dependent pathway, with a smaller role for nitric oxide (NO) and a likely role for an endothelium-derived hyperpolarizing factor (EDHF) (17, 22). The cutaneous response to local hyperthermia involves a bimodal response, in which the initial vasodilatory peak is predominantly mediated by local sensory nerves, and a secondary plateau phase that is predominantly mediated by NO. Thus these different approaches would allow us to interrogate some of the underlying mechanisms of observed improvements in cutaneous vascular function following heat acclimation.
Because of the improvements in cardiovascular and thermoregulatory function that follow a period of heat acclimation, SR is higher at a given exercise intensity or core temperature (11, 33, 44, 54). This is in part a centrally mediated response (5, 24, 31, 36, 46, 54). However, other studies have shown evidence that the increased sweat output following heat acclimation is due primarily to local adaptations of the sweat gland apparatus (2, 3, 10, 18, 31, 38). In other words, there might be some underlying adaptations that can modify sweating independent of a central drive. It is also known that exercise training confers some degree of heat acclimation (12). It has not been demonstrated whether highly trained, competitive endurance athletes can further increase their SR response to controlled doses of ACh following a period of heat acclimation.
Therefore, our primary purpose was to investigate whether a period of heat acclimation could modify local SkBF responses. We accomplished this by locally stimulating the skin in resting subjects in a thermoneutral room with specific concentrations of the endothelium-dependent vasodilator ACh infused via microdialysis and by performing a standardized local heating protocol (17, 20, 27) while measuring the SkBF responses. We also aimed to determine whether heat acclimation impacts maximal SkBF. Finally, we tested the heat acclimation effects on sweating responses in trained athletes to local stimulation with ACh. We hypothesized that, in response to a given ACh concentration or during thermal hyperemia, SkBF would be higher after heat acclimation but that absolute maximal SkBF would remain unchanged. In addition, we hypothesized that SRs would be higher at a given ACh concentration after heat acclimation.
METHODS
Study design.
This study is part of a larger project that investigated effects of heat acclimation on performance variables in endurance-trained cyclists. Briefly, participants were subjected to a battery of physiological and performance tests under two environmental conditions followed by a heat acclimation or an exercising control program, and then the tests were repeated. The heat acclimation protocol consisted of 10 bouts of cycling exercise at 40°C and 30% relative humidity (wet bulb globe temperature = 35°C). Subjects performed two 45-min bouts at 50% of their maximal O2 uptake (V̇o2max), with 10 min of rest between bouts. A control group exercised at the same intensity, but with the chamber set at 13°C and 30% relative humidity (wet bulb globe temperature = 12°C). The heat acclimation or control exposures were completed in 10–14 days. The 50% V̇o2max exercise intensity was selected, as it would represent compensable heat stress sufficient to induce heat acclimation but would not be sufficient to induce training adaptations for our highly trained athletes. Subjects were instructed to maintain their normal training routines during the 10-day intervention period to maintain their fitness level. Preacclimation studies were performed the week (i.e., ≤7 days) prior to the beginning of the intervention, and postacclimation studies were completed within 1 wk of the conclusion of the heat acclimation period. Because of the possibility of losing some of the heat acclimation effects, we did not control for menstrual cycle in the three female participants. However, the two women in the experimental group entered the study at different phases of their menstrual cycle (1 in the early follicular phase and 1 during the midluteal phase).
Subjects.
Prior to participation, each subject gave written and verbal informed consent. All protocols were approved by the Institutional Review Board of the University of Oregon. Twelve highly trained endurance cyclists [10 men and 2 women, 24 ± 6 (SD) yr old] completed the heat acclimation protocol; height, body weight, and body mass index of the subjects was as follows: 175 ± 6 cm, 67.7 ± 8.1 kg, and 22.1 ± 3.9 kg/m2, respectively. To rule out the possibility that any potential changes in the experimental group could be due to a training effect, eight matched subjects (7 men and 1 woman, 26 ± 4 yr old) completed the control protocol; their stature, body weight, and body mass index were as follows: 174 ± 6 cm, 70.2 ± 4.1 kg, and 23.1 ± 3.1 kg/m2, respectively. Of these, four men (28 ± 5 yr old) performed the control protocol followed by the heat acclimation exposures and experimental tests; their stature, body weight, and body mass index were as follows: 176 ± 4 cm, 73.1 ± 1.5 kg, and 23.5 ± 2.8 kg/m2, respectively. Having some subjects complete the exercise control protocol followed by heat acclimation allowed us to assess intrasubject effects of the two interventions. In other words, we wanted to ensure that no changes in our measured variables would result from the control protocol and that a subsequent heat acclimation period would cause the expected changes in that same individual. The heat acclimation group and control group were matched for maximal aerobic power (66.9 and 66.8 ml·kg−1·min−1, respectively; Table 1) and training experience. A complete description of the subject groups is presented in Table 1. No attempt was made to control for training or heat acclimation status during the lead-in phase of the study, although subjects were recruited from the same cycling team and, thus, had the same competition schedule and essentially identical training routines. Therefore, we are confident that both groups had the same level of training or heat acclimation status, so no heat tolerance test was conducted prior to the experimental trials.
Table 1.
Physiological characteristics of heat acclimation and control groups
| Heat Acclimation Group (n = 12) | Control Group (n = 8) | |
|---|---|---|
| Maximal O2 uptake | ||
| l/min | 4.47 ± 0.21 (3.00–5.51) | 4.70 ± 0.14 (4.25–5.51) |
| ml·kg −1·min−1 | 66.9 ± 2.1 (57.0–76.1) | 66.8 ± 1.7 (59.1–76.6) |
| Maximal power output | ||
| W | 370 ± 15 (260–430) | 381 ± 11 (340–420) |
| W/kg | 5.5 ± 0.2 (4.7–6.0) | 5.4 ± 0.2 (5.0–5.9) |
Values are means ± SE, with range in parentheses. Maximal O2 consumption and maximal power output values were from maximal O2 uptake test done in cool (13°C) environment.
Subject monitoring.
On each study visit, subjects reported to the laboratory after a 2-h fast and were well hydrated. Studies were performed in an air-conditioned (22–24°C) laboratory with the subjects in a supine position and the experimental arm extended at the right side at heart level. Subjects were instructed to avoid consumption of alcohol or caffeine for ≥12 h prior to the study. In addition, they were not allowed to exercise on the same day prior to the study and were told to avoid ingestion of nonprescription drugs for the entire duration of the multiple study visits. Blood pressure (Cardiocap, Datex Ohmeda) was measured via auscultation in the left arm every 5–7 min throughout the entire experiment to rule out that changes in red blood cell (RBC) flux were due to pressure changes. Initial resting plasma volume (pretreatment, or day 1) was calculated from body mass by the equation of Sawka et al. (43), and posttreatment (day 10) plasma volume was calculated by correction of that initial value for the percent change in plasma volume (8). To ensure that the subjects were properly hydrated, nude body weight was measured every day the subjects came to the laboratory and plasma osmolality was measured on days 1 and 10 of the heat acclimation protocol. Euhydration was demonstrated by nude body mass within 1% of the 5-day average and plasma osmolality <290 mosmol/kgH2O (40).
SkBF and SR measurements.
As an index of SkBF, RBC flux was measured by noninvasive laser-Doppler flowmetry (moorLab, Moor Instruments, Devon, UK). Two probes were used in conjunction with two SR capsules to continuously monitor RBC flux at each site. In addition, two probes were combined with local skin heating devices and placed on the forearm to investigate SkBF responses to a local skin heating protocol.
SRs were quantitatively measured by the resistance hygrometry technique (1). Briefly, dry nitrogen was supplied to the sweat capsules (0.5 cm2 area) at a fixed rate of 0.2 l/min. The humidity of the air flowing out of the capsules was measured with capacitance hygrometers that were successfully calibrated by the manufacturer (model HMP230, Vaisala, Helsinki, Finland) and did not require daily recalibration. The same units were used on the experimental and control groups, and all the timing of the studies was counterbalanced. SR was calculated based on measurements of relative humidity (Δrh) in the air as it passed through the capsule, the airflow rate (AF), the density of saturated steam at the given temperature (D), and the capsule's surface area (SA) using the following equation: SR = [AF*(Δrh/100)*D]/SA.
Specific protocol.
Two microdialysis fibers (MD 2000, Bioanalytical Systems) with a membrane length of 10 mm and a 20-kDa membrane cutoff were placed ≥5 cm apart in the forearm skin of the right arm of the subject. Placement of the microdialysis fibers was achieved by insertion of a 25-gauge needle through the skin, with entry and exit points ∼2.5 cm apart. No anesthesia was used for microdialysis probe placement. The microdialysis fiber was then threaded though the lumen of the needle. The needle was withdrawn from the skin, leaving the microdialysis membrane in place.
After the needle insertion, the trauma response was allowed to resolve for 90–120 min. During this time, the microdialysis fibers were continuously perfused with Ringer solution at a rate of 2.0 μl/min. Then integrated laser-Doppler probes and SR capsules were placed directly over the microdialysis membranes to continuously measure RBC flux and SR. Both sites were monitored continuously until a stable 10-min baseline was recorded before the first ACh infusion. Subjects then received perfusate containing 1, 10, and 100 mM ACh dissolved in Ringer solution. ACh concentrations were determined on the basis of previous research done in human skin utilizing microdialysis delivery of this agonist (28, 47). Each infusion lasted for ≥20 min, or until there was a clear plateau in the SkBF and SR recordings. Finally, maximal RBC flux was achieved by infusion of 28 mM sodium nitroprusside (SNP; Nitropress, Ciba Pharmaceuticals), which is known to result in maximal dilation of skin sites (19).
The local skin heating devices were turned on and held constant at 33°C for 10 min during baseline data collection. After the baseline period, the temperature of the local heaters was increased at a rate of 0.5°C every 5 s to 42°C. This rate of local heating does not result in any pain sensation (30). The local heaters were held constant at 42°C until SkBF reached a stable 10-min plateau. The temperature of the local heaters was then raised to 44°C to elicit maximal cutaneous vasodilation (27).
During part of the study, the left forearm was locally heated in a cylindrical water spray device that sprayed heated water from jets encircling the suspended forearm (25, 49). At the same time, brachial artery diameters and blood velocity were measured using a Doppler ultrasound machine (Terason, Burlington, MA) with a 10-MHz linear array transducer. The transducer was placed 3–10 cm proximal to the antecubital fossa, and the entire width of the artery was insonated with an angle of 60°. The forearm was heated for 45 min, and measurements were obtained for 2 min before forearm heating (baseline) and at 13, 28, and 43 min. During each measurement, blood flow to the hand was occluded with a blood pressure cuff placed around the wrist distal to the spray device to prevent inclusion of the hand circulation in the calculations of brachial blood flow. Forearm skin temperatures remained between 42 and 43°C.
Data analyses.
Data were digitized and saved on a computer at 40 Hz using Windaq data acquisition software (Dataq Instruments, Akron, OH). Data were analyzed offline using signal-processing software. RBC flux values from the laser-Doppler units were divided by mean arterial pressure (MAP) to yield an index of cutaneous vascular conductance (RBC flux ÷ MAP = CVC). In addition, RBC flux values were calibrated to 100% CVC during maximal blood flow (i.e., 28 mM SNP infusion successfully elicits maximal skin vasodilation). Expression of data in this manner takes into account any changes in blood flow due to changes in blood pressure and, thus, better reflects changes in SkBF. Therefore, data are presented as a percentage of maximal CVC (%CVCmax). Because of the transient nature of the initial peak during a local skin heating protocol, a 5- to 10-s period of SkBF was used for analysis. For the plateau during local heating and drug infusions, a stable 5- to 7-min period of SkBF was used for subsequent analyses. SR was calculated on the basis of relative humidity, air temperature, skin surface area, and airflow and is expressed as mg·cm−2·min−1 (1). Brachial artery diameter and blood velocity were recorded to a computer interfaced with custom-designed edge-detection and wall-tracking analysis software (DICOM, Perth, Australia). The custom analysis software allows real-time video images of the brachial artery to be captured from the ultrasound machine, encoded, and stored at 30 frames/s for later analysis of vessel diameter in synchrony with end diastole (53).
SkBF (%CVCmax) and SR outcome data were compared between groups and across acclimation status (i.e., before and after heat acclimation) using two-way repeated-measures ANOVA, with planned comparisons performed on each ACh concentration (baseline, 1 mM, 10 mM, and 100 mM), local heating phase (peak and plateau), and brachial artery blood flow with Student-Newman-Keuls method for post hoc comparisons where significance was detected at the 0.05 level. To confirm that no differences existed between experimental and control groups prior to the intervention (heat acclimation or exercise control), unpaired t-tests were performed for each measurement across ACh doses, local heating phase, and brachial artery blood flow.
RESULTS
Table 1 shows the initial aerobic performance results of the heat acclimation and control groups. Note that the heat acclimation and control groups have essentially identical maximal aerobic power (66.9 and 66.8 ml·kg−1·min−1, respectively) and maximal power output (5.5 and 5.4 W/kg, respectively). All subjects completed the 10-day heat acclimation and control group exercise program.
Table 2 provides resting plasma volume, hematocrit, hemoglobin, and heart rate and core temperature responses at the end of the second exercise bout on day 1 and day 10 of the heat acclimation program or control group exercise program. The heat acclimation group demonstrated a reduction (P < 0.001) in heart rate (15 beats/min) and a reduction (P < 0.002) in core temperature (0.5°C), while no differences were observed in the control group. The heat acclimation group demonstrated an increase (6.5%, P < 0.05), while the control group demonstrated a reduction (4.6%, P > 0.05), in plasma volume (at rest). Body weight did not change in either group between the pre- and posttreatment protocols. The average power output during the intervention period was 165 and 170 W for the heat acclimation and exercise control groups, respectively, calculated on the basis of 50% of each individual's V̇o2max.
Table 2.
Mean differences between day 1 and day 10 of heat acclimation or exercise control period
| Heat Acclimation Group (n = 12) |
Control Group (n = 8) |
|||
|---|---|---|---|---|
| Day 1 | Day 10 | Day 1 | Day 10 | |
| Final heart rate, beats/min | 165 ± 2 | 150 ± 3* | 130 ± 3 | 127 ± 5 |
| Final Tc, °C | 39.3 ± 0.1 | 38.8 ± 0.1* | 38.1 ± 0.1 | 38.1 ± 0.1 |
| Hemoglobin, g/dl | 13.8 ± 0.3 | 13.3 ± 0.3 | 13.7 ± 0.2 | 14.0 ± 0.5 |
| Hematocrit | 0.43 ± 0.01 | 0.42 ± 0.01 | 0.42 ± 0.01 | 0.43 ± 0.01 |
| Plasma volume, liters | 3.0 ± 0.1 | 3.2 ± 0.1* | 3.1 ± 0.1 | 2.9 ± 0.1 |
| Plasma volume change, % | 6.5 ± 1.2† | −4.6 ± 2.7 | ||
Values are means ± SE. Resting plasma volume, hematocrit, hemoglobin, and final heart rate and core temperature (Tc) were measured at end of the 2nd exercise bout.
P < 0.05 vs. day 1.
P < 0.05 vs. control group.
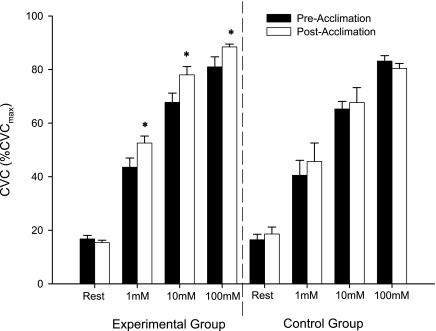
Figure 1 shows heat acclimation effects on cutaneous vascular responses to specific concentrations of ACh. The experimental group showed significant increases in the cutaneous vascular responses to 1, 10, and 100 mM ACh (43.5 ± 3.4 vs. 52.6 ± 2.6% CVCmax, 67.7 ± 3.4 vs. 78.1 ± 3.1% CVCmax, and 81.0 ± 3.8 vs. 88.5 ± 1.1% CVCmax, respectively, all P < 0.05). No significant changes were found in the control group in cutaneous vascular responses to all concentrations of ACh (40.5 ± 5.6 vs. 45.7 ± 6.9% CVCmax, 65.3 ± 2.8 vs. 67.7 ± 5.6% CVCmax, and 83.2 ± 2.0 vs. 80.4 ± 1.9% CVCmax). In addition, there were no differences between the experimental group and control group in the preintervention ACh doses.
Fig. 1.
Effect of heat acclimation on cutaneous vascular conductance (CVC) in response to 1, 10, and 100 mM ACh. Values are means ± SE for 12 experimental and 8 control subjects. *P < 005 vs. preacclimation within concentration.
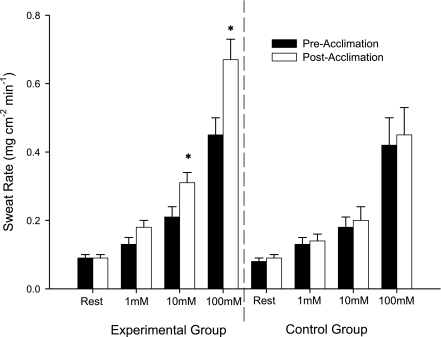
Figure 2 shows heat acclimation effects on local SR responses to specific concentrations of ACh. The experimental group showed significant increases in sweating responses to 10 and 100 mM ACh (0.21 ± 0.03 vs. 0.31 ± 0.03 and 0.45 ± 0.05 vs. 0.67 ± 0.06 mg·cm−2·min−1, respectively, all P < 0.05), but not to 1 mM ACh (0.13 ± 0.02 vs. 0.18 ± 0.02 mg·cm−2·min−1, P = 0.147). No significant changes were found in the control group in SR responses to the same concentrations of ACh (0.13 ± 0.02 vs. 0.14 ± 0.02, 0.18 ± 0.03 vs. 0.20 ± 0.04, and 0.42 ± 0.08 vs. 0.45 ± 0.08 mg·cm−2·min−1, respectively). In addition, there were no differences between the experimental group and control group in the preintervention ACh doses.
Fig. 2.
Effect of heat acclimation on sweat rate responses to 1, 10, and 100 mM ACh. Values are means ± SE for 12 experimental and 8 control subjects. *P < 005 vs. preacclimation within concentration.
Table 3 shows heat acclimation effects on vascular responses during skin local heating and forearm heating protocols. There were no significant changes in any of the variables in the control group or experimental group. In addition, there were no differences between the experimental group and control group in the preintervention tests.
Table 3.
Vascular responses from skin local heating and forearm heating protocols
| Experimental Group (n = 12) |
Control Group (n = 8) |
|||
|---|---|---|---|---|
| Preacclimation | Postacclimation | Preacclimation | Postacclimation | |
| Skin local heating protocol | ||||
| Local heating initial peak, %CVCmax | 66.4 ± 2.1 (49.4–75.9) | 68.2 ± 2.5 (54.3–81.1) | 68.7 ± 2.0 (61.8–74.3) | 66.6 ± 2.7 (51.3–73.5) |
| Local heating plateau, %CVCmax | 79.6 ± 2.0 (64.3–90.4) | 80.9 ± 1.7 (72.4–96.5) | 77.9 ± 2.3 (68.2–89.1) | 77.8 ± 1.7 (70.9–87.4) |
| Maximal skin blood flow, RBC flux | 286 ± 24 (131–464) | 302 ± 15 (222–393) | 310 ± 24 (198–397) | 308 ± 16 (246–383) |
| Forearm heating protocol | ||||
| Brachial artery blood flow, ml/min | 291 ± 13 (227–329) | 270 ± 24 (211–407) | 302 ± 31 (195–475) | 292 ± 14 (239–362) |
Values are means ± SE, with range in parentheses. CVCmax, maximal cutaneous vascular conductance. There were no significant changes in any of the variables in the control or experimental group.
DISCUSSION
To our knowledge, this is the first study to investigate potential peripheral adaptations in the cutaneous vasculature following a period of heat acclimation. Furthermore, this is the first study to examine whether maximal SkBF (estimated from maximal brachial artery blood flow) is altered by heat acclimation. This latter issue may seem relatively minor; however, findings of greater SkBF following heat acclimation in previous studies may have been explained by changes in maximal vasodilator capacity of the skin or a change in vascular responsiveness to a given level of stimulation (or a combination of both). The major findings of the present study are as follows. 1) Heat acclimation improved the cutaneous vascular responses to locally applied ACh in the skin microcirculation, but not to local thermal hyperemia. 2) Absolute maximal forearm SkBF does not change following a period of heat acclimation. 3) Our data confirm previous reports that the increased SR observed after heat acclimation is caused, at least in part, by local sweat gland adaptations (2, 3, 18, 31, 38) and extend these findings to demonstrate the adaptation in highly trained endurance athletes. Importantly, we can exclude a possible training effect during heat acclimation on our findings, an issue that has not consistently been adequately controlled in previous studies. In support of this latter point, the athletes were already highly trained, the workload during acclimation was too low to confer training adaptations, and no changes were observed in a matched control group (which exercised at the same intensity as the experimental group but in a cool environment).
Our observations of augmented cutaneous vascular regulation following heat acclimation are consistent with other reports (11, 36, 55), and we have extended these findings by demonstrating increased sensitivity in the CVC to a local stimulus. Specifically, heat acclimation may cause an increase in the number of muscarinic receptors, enhance their sensitivity, cause a decrease in the cholinesterase activity that leads to an improved vascular response to a given ACh level, or alter the pathway of vasodilation within the smooth muscle or the endothelial cells. Interestingly, we found that heat acclimation increased SkBF responses to specific ACh concentrations, but the response to skin local heating remained unchanged. It is generally agreed that ACh mediates increases in SkBF via multiple potential pathways, with a profound role for prostanoids (17, 22, 28) and lesser roles for NO, especially at the lower doses of ACh (17, 22, 28), and, possibly, an EDHF mechanism (34). The SkBF response to rapid local heating has been shown to be mediated mostly by local sensory nerves (initial peak) and NO (15, 20, 30). On the other hand, there is evidence against roles for prostanoids (13, 26) or histamine (via H1 receptors) (13, 52) in the skin vasodilation in response to local heating. Taken together, it seems that the augmented SkBF response to ACh after heat acclimation may be due to upregulation of the cyclooxygenase (COX) pathway leading to the production of prostanoids or to an enhanced EDHF mechanism. However, further studies using specific NO synthase (NOS) inhibitors are needed to completely rule out any NO component on the enhanced skin vasodilatory response to ACh infusions after heat acclimation.
Much of the research performed on heat acclimation effects on SkBF during whole body heat stress suggests that the increased SkBF after a period of heat acclimation is caused by a central mechanism (11, 36, 55). A reduction of the internal temperature threshold for forearm vasodilation, without changes in the slope of the forearm blood flow-internal temperature relationship, has been reported (36, 55). Therefore, it has been speculated that heat acclimation modifies thermoregulatory responses in the skin by central mechanisms. These previous studies, however, employed increases in internal temperature to elicit skin vasodilation; therefore, any potential peripheral adaptations in cutaneous vascular function per se cannot be excluded. In addition, SkBF was estimated in these studies from forearm blood flow measurements via venous occlusion plethysmography. Using the microdialysis technique, we were able to administer fixed concentrations of an endothelium-dependent agonist in a small area of the skin, thereby tightly controlling the stimulus for vasodilation. In addition, with laser-Doppler flowmetry, we were able to estimate changes in blood flow (from direct measurements of RBC flux) that occur only within the skin microcirculation and compare these changes with a maximal vasodilation. Therefore, the utilization of these combined methodologies and expression of values as percent CVCmax allowed a more consistent comparison between subjects and drug concentrations. On this basis, our results agree with our hypothesis that there are local adaptations within the skin microcirculation following heat acclimation, although a centrally mediated adaptation during hyperthermia or exercise was not investigated and cannot be ruled out.
Although the specific pathways leading to cutaneous vasodilation in response to increases in body temperature remain enigmatic, this mechanism is believed to be mediated by a cholinergic cotransmitter system, with ACh contributing up to 20% of the vasodilation and some other substance(s) coreleased from cholinergic terminals responsible for the rest (21). The SkBF changes observed after heat acclimation in this study are remarkable, given that ACh contributes only a small percentage during active cutaneous vasodilation (21). Larger changes may be discovered if other possible cotransmitters (e.g., VIP or substance P) were used to cause skin vasodilation. Nevertheless, the fact that this study used local stimulation and, therefore, no central mechanism was activated supports the theory that peripheral adaptations to the cutaneous circulation play a role in the enhanced SkBF observed after heat acclimation.
Numerous studies have shown that NO may contribute up to 40–50% of active vasodilation (19, 45, 50). Recently, Kellogg et al. (23) demonstrated that much of active cutaneous vasodilation may be due to the neuronal NOS isoform, which may suggest that this pathway is upregulated following heat acclimation, but that the endothelial NOS pathway is not. In addition, one study provides evidence for a role of prostanoids in active cutaneous vasodilation in young adults (26), although another study suggests a limited role for COX-derived vasodilator prostanoids in middle-aged individuals (16). Differences in the average age of the subject population (22 vs. 53 yr old) may explain the mixed findings between these two studies. In fact, age has been shown to alter the prostacyclin-mediated vasodilation in the human forearm (32). If the increased SkBF response to ACh observed in the present study is due to an increase in the COX pathway enzymes, then this common pathway with active vasodilation may partially explain the higher SkBFs observed during hyperthermia after acclimation. In any case, the stimulus for the improvements in SkBF during heat acclimation also remains an issue in need of clarification. In a recent study by Green et al. (14), it was determined that a greater SkBF following chronic local heating was dependent on an elevated shear stress during the heating. When shear stress was minimized with partial blood flow occlusion, local adaptations in SkBF were not observed. Although there are differences between the two studies (chronic local heating vs. whole body hyperthermia and exercise), it is possible that a greater shear stress attending an elevated SkBF results in improvements in the pathways of vasodilation in the skin. Clearly, more studies investigating specific mechanisms of the post-heat-acclimation changes in SkBF are needed.
To our knowledge, there has not been published research on the effect of heat acclimation on maximal SkBF. At issue is whether previous reports of elevated SkBF after heat acclimation are attributable to functional changes in SkBF regulation or whether maximal vasodilator capacity was increased due to structural changes within the skin or cutaneous vasculature. Previous studies showed that locally heating the forearm to 42°C with a warm water spray device for 35–45 min successfully achieves maximal skin vasodilation (25, 49); thus we employed this approach in our study. Our data suggest that the maximal ability of the skin vessels to vasodilate is not altered with heat acclimation. In addition, the maximal RBC flux values for laser-Doppler measurements also remained unchanged after heat acclimation (Table 3). This study is the first to provide evidence that heat acclimation does not alter maximal SkBF. Instead, an increased sensitivity of the skin microvasculature to vasodilation likely plays a role in the augmented SkBF observed after heat acclimation.
Previous research focused on the effects of heat acclimation on sweating responses has received much more attention than regulation of SkBF. Studies have demonstrated that heat acclimation lowers the internal temperature threshold for sweating (31, 36, 41, 55), suggesting a role for central mechanisms. Our observations support several studies that propose that heat acclimation induces changes at the level of the sweat gland as well (2–4, 6, 10, 18, 38), suggesting increased cholinergic sensitivity of the eccrine sweat gland or increased glandular hypertrophy (39). It is also particularly interesting that we observed such pronounced improvements in sweating response after heat acclimation (∼50% increase at 10 and 100 mM ACh) in our subjects. This is a remarkable adaptation, given that there was no central stimulus to induce sweating and that the subject population was highly trained and, thus, already partially heat acclimated (12). Our observation of increases in sweating and SkBF, despite no activation of the nerves that regulate their function, is interesting and may suggest that the higher SRs were observed because of a higher SkBF, or vice versa. A very recent study showed that decreased SkBF and decreased local temperature each independently attenuate the sweating response during passive heat stress in humans (51). The independent and integrated regulation of sweat glands and the cutaneous vasculature is not fully understood, and further studies are warranted to evaluate these interactions.
In most of the heat acclimation protocols that involve exercise, there is a chance that the changes observed after heat acclimation could be due to a training effect (42, 56). We believe that this is not the case in our study for several reasons. The combination of low exercise intensity during the heat acclimation process (50% of their V̇o2max) and the subjects' high fitness level (mean V̇o2max of 66 ml·kg−1·min−1) make the training effect unlikely (35). In addition, we did not observe significant changes in the control group, which exercised at the same intensity as the experimental group. Although no attempt was made to control for training or pre-heat-acclimation status during the lead-in phase of the study, all the subjects who participated in this study were recruited from the same club team and, thus, had the same competition schedule and essentially identical training routines. In addition, we do not believe that the differences observed after heat acclimation were related to the subjects' training background. There were no differences in the effect of the intervention between the subjects who completed the study in early winter (and, thus, were probably riding mostly greater distances) and those who completed the study during the spring/summer (and, presumably, were working out at a higher intensity). Finally, the experimental and control groups were studied concurrently to ensure that both groups were closely matched for training status and pretest heat exposure.
In summary, our results provide evidence demonstrating improved cutaneous vascular responses to ACh stimulation following heat acclimation. Furthermore, our findings demonstrate that the maximal vasodilator capacity of the skin is not increased following a period of heat acclimation. Finally, SR responses to a given ACh concentration are augmented by heat acclimation in highly trained cyclists.
Significance.
The overarching finding from this project is that heat acclimation can enhance SkBF and sweating in highly trained cyclists by peripheral adaptations. These thermoregulatory adaptations may confer possible ergogenic benefits by multiple potential mechanisms: 1) an increased SR will decrease skin temperature (assuming adequate sweat evaporation), which may limit the rise in core temperature, decrease SkBF requirements, and allow a greater fraction of the available cardiac output to be directed to active muscles; and 2) an increased SkBF per given local stimulus will enhance convective cooling and deliver more blood to the cooled skin. An expanded plasma volume attending the heat acclimation would allow a greater SkBF without compromising blood pressure regulation. The enhanced thermoregulation may allow highly trained cyclists to better regulate their core temperature and improve their exercise capacity, especially in hot environments. Although maximal SkBF was not modified by heat acclimation, this is less important during exercise, as SkBF does not approach maximal levels. It is evident that a period of heat acclimation can alter SkBF and sweating, even in highly trained cyclists. These benefits and changes in the thermoregulatory effector sites may even be more pronounced in the general population.
GRANTS
Funding of this research project was provided by the Clarissa and Evonuk Memorial Fellowship and National Heart, Lung, and Blood Institute Grant HL-081671 (C. T. Minson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors appreciate the considerable time and effort of the subjects who participated in this project. The authors thank Sarah Huelskamp, Krista Jensen, and Tom Stocklin-Enright for help with data collection.
REFERENCES
- 1. Bullard RW. Continuous recording of sweating rate by resistance hygrometry. J Appl Physiol 17: 735–737, 1962 [DOI] [PubMed] [Google Scholar]
- 2. Buono MJ, Martha SL, Heaney JH. Peripheral sweat gland function is improved with humid heat acclimation. J Therm Biol 34: 127–130, 2009 [Google Scholar]
- 3. Buono MJ, Numan TR, Claros RM, Brodine SK, Kolkhorst FW. Is active sweating during heat acclimation required for improvements in peripheral sweat gland function? Am J Physiol Regul Integr Comp Physiol 297: R1082–R1085, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen WY, Elizondo RS. Peripheral modification of thermoregulatory function during heat acclimation. J Appl Physiol 37: 367–373, 1974 [DOI] [PubMed] [Google Scholar]
- 5. Colin J, Houdas Y. Initiation of sweating in man after abrupt rise in environmental temperature. J Appl Physiol 20: 984–990, 1965 [DOI] [PubMed] [Google Scholar]
- 6. Collins KJ, Crockford GW, Weiner JS. The local training effect of secretory activity on the response of eccrine sweat glands. J Physiol 184: 203–214, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci 27: 503–508, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37: 247–248, 1974 [DOI] [PubMed] [Google Scholar]
- 9. Eichna LW, Park CR, Nelson N, Horvath SM, Palmes ED. Thermal regulation during acclimatization in a hot, dry (desert type) environment. Am J Physiol 163: 585–597, 1950 [DOI] [PubMed] [Google Scholar]
- 10. Fox RH, Goldsmith R, Hampton IF, Lewis HE. The nature of the increase in sweating capacity produced by heat acclimatization. J Physiol 171: 368–376, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fox RH, Goldsmith R, Kidd DJ, Lewis HE. Blood flow and other thermoregulatory changes with acclimatization to heat. J Physiol 166: 548–562, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gisolfi C, Robinson S. Relations between physical training, acclimatization, and heat tolerance. J Appl Physiol 26: 530–534, 1969 [DOI] [PubMed] [Google Scholar]
- 13. Gooding KM, Hannemann MM, Tooke JE, Clough GF, Shore AC. Maximum skin hyperaemia induced by local heating: possible mechanisms. J Vasc Res 43: 270–277, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Green DJ, Carter HH, Fitzsimons MG, Cable NT, Thijssen DH, Naylor LH. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J Physiol 588: 1571–1577, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hodges GJ, Johnson JM. Adrenergic control of the human cutaneous circulation. Appl Physiol Nutr Metab 34: 829–839, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Holowatz LA, Jennings JD, Lang JA, Kenney WL. Ketorolac alters blood flow during normothermia but not during hyperthermia in middle-aged human skin. J Appl Physiol 107: 1121–1127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol 563: 965–973, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inoue Y, Havenith G, Kenney WL, Loomis JL, Buskirk ER. Exercise- and methylcholine-induced sweating responses in older and younger men: effect of heat acclimation and aerobic fitness. Int J Biometeorol 42: 210–216, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Kellogg DLJ, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol 85: 824–829, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Kellogg DLJ, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol 86: 1185–1190, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Kellogg DLJ, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res 77: 1222–1228, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Kellogg DLJ, Zhao JL, Coey U, Green JV. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol 98: 629–632, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Kellogg DLJ, Zhao JL, Wu Y. Neuronal nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. J Physiol 586: 847–857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuno Y. Human Perspiration. Springfield, IL: Thomas, 1956 [Google Scholar]
- 25. Martin HL, Loomis JL, Kenney WL. Maximal skin vascular conductance in subjects aged 5–85 yr. J Appl Physiol 79: 297–301, 1995 [DOI] [PubMed] [Google Scholar]
- 26. McCord GR, Cracowski JL, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Integr Comp Physiol 291: R596–R602, 2006 [DOI] [PubMed] [Google Scholar]
- 27. McCord GR, Minson CT. Cutaneous vascular responses to isometric handgrip exercise during local heating and hyperthermia. J Appl Physiol 98: 2011–2018, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Medow MS, Glover JL, Stewart JM. Nitric oxide and prostaglandin inhibition during acetylcholine-mediated cutaneous vasodilation in humans. Microcirculation 15: 569–579, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Minson CT. Thermal provocation to evaluate microvascular reactivity in human skin. J Appl Physiol. 27, 2010; doi:10:1152/japplphysiol.00414.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Nadel ER, Pandolf KB, Roberts MF, Stolwijk JA. Mechanisms of thermal acclimation to exercise and heat. J Appl Physiol 37: 515–520, 1974 [DOI] [PubMed] [Google Scholar]
- 32. Nicholson WT, Vaa B, Hesse C, Eisenach JH, Joyner MJ. Aging is associated with reduced prostacyclin-mediated dilation in the human forearm. Hypertension 53: 973–978, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nielsen B, Hales JR, Strange S, Christensen NJ, Warberg J, Saltin B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol 460: 467–485, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526, 1987 [DOI] [PubMed] [Google Scholar]
- 35. Pollock ML. The quantification of endurance training programs. Exerc Sport Sci Rev 1: 155–188, 1973 [PubMed] [Google Scholar]
- 36. Roberts MF, Wenger CB, Stolwijk JA, Nadel ER. Skin blood flow and sweating changes following exercise training and heat acclimation. J Appl Physiol 43: 133–137, 1977 [DOI] [PubMed] [Google Scholar]
- 37. Rowell LB, Kraning KK, Kennedy JW, Evans TO. Central circulatory responses to work in dry heat before and after acclimatization. J Appl Physiol 22: 509–518, 1967 [DOI] [PubMed] [Google Scholar]
- 38. Sato F, Owen M, Matthes R, Sato K, Gisolfi CV. Functional and morphological changes in the eccrine sweat gland with heat acclimation. J Appl Physiol 69: 232–236, 1990 [DOI] [PubMed] [Google Scholar]
- 39. Sato K, Sato F. Individual variations in structure and function of human eccrine sweat gland. Am J Physiol Regul Integr Comp Physiol 245: R203–R208, 1983 [DOI] [PubMed] [Google Scholar]
- 40. Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS. American College of Sports Medicine position stand: Exercise and fluid replacement. Med Sci Sports Exerc 39: 377–390, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Sawka MN, Gonzalez RR, Young AJ, Dennis RC, Valeri CR, Pandolf KB. Control of thermoregulatory sweating during exercise in the heat. Am J Physiol Regul Integr Comp Physiol 257: R311–R316, 1989 [DOI] [PubMed] [Google Scholar]
- 42. Sawka MN, Young AJ, Cadarette BS, Levine L, Pandolf KB. Influence of heat stress and acclimation on maximal aerobic power. Eur J Appl Physiol Occup Physiol 53: 294–298, 1985 [DOI] [PubMed] [Google Scholar]
- 43. Sawka MN, Young AJ, Pandolf KB, Dennis RC, Valeri CR. Erythrocyte, plasma, and blood volume of healthy young men. Med Sci Sports Exerc 24: 447–453, 1992 [PubMed] [Google Scholar]
- 44. Senay LC, Mitchell D, Wyndham CH. Acclimatization in a hot, humid environment: body fluid adjustments. J Appl Physiol 40: 786–796, 1976 [DOI] [PubMed] [Google Scholar]
- 45. Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol 85: 830–834, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Shvartz E, Bhattacharya A, Sperinde SJ, Brock PJ, Sciaraffa D, Van Beaumont W. Sweating responses during heat acclimation and moderate conditioning. J Appl Physiol 46: 675–680, 1979 [DOI] [PubMed] [Google Scholar]
- 47. Stewart JM, Medow MS, Minson CT, Taneja I. Cutaneous neuronal nitric oxide is specifically decreased in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 293: H2161–H2167, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Takeno Y, Kamijo YI, Nose H. Thermoregulatory and aerobic changes after endurance training in a hypobaric hypoxic and warm environment. J Appl Physiol 91: 1520–1528, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Taylor WF, Johnson JM, O'Leary D, Park MK. Effect of high local temperature on reflex cutaneous vasodilation. J Appl Physiol 57: 191–196, 1984 [DOI] [PubMed] [Google Scholar]
- 50. Wilkins BW, Holowatz LA, Wong BJ, Minson CT. Nitric oxide is not permissive for cutaneous active vasodilatation in humans. J Physiol 548: 963–969, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wingo JE, Low DA, Keller DM, Brothers RM, Shibasaki M, Crandall CG. Skin blood flow and local temperature independently modify sweat rate during passive heat stress in humans. J Appl Physiol 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wong BJ, Williams SJ, Minson CT. Minimal role for H1 and H2 histamine receptors in cutaneous thermal hyperemia to local heating in humans. J Appl Physiol 100: 535–540, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol 91: 929–937, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Wyndham CH, Rogers GG, Senay LC, Mitchell D. Acclimization in a hot, humid environment: cardiovascular adjustments. J Appl Physiol 40: 779–785, 1976 [DOI] [PubMed] [Google Scholar]
- 55. Yamazaki F, Hamasaki K. Heat acclimation increases skin vasodilation and sweating but not cardiac baroreflex responses in heat-stressed humans. J Appl Physiol 95: 1567–1574, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Young AJ, Sawka MN, Levine L, Cadarette BS, Pandolf KB. Skeletal muscle metabolism during exercise is influenced by heat acclimation. J Appl Physiol 59: 1929–1935, 1985 [DOI] [PubMed] [Google Scholar]