Abstract
We quantified energy production in 7 prepubescent boys (11.7 ± 0.6 yr) and 10 men (35.6 ± 7.8 yr) using 31P-magnetic resonance spectroscopy to investigate whether development affects muscle energetics, given that resistance to fatigue has been reported to be larger before puberty. Each subject performed a finger flexions exercise at 0.7 Hz against a weight adjusted to 15% of their maximal voluntary strength for 3 min, followed by a 15-min recovery period. The total energy cost was similar in both groups throughout the exercise bout, whereas the interplay of the different metabolic pathways was different. At the onset of exercise, children exhibited a higher oxidative contribution (50 ± 15% in boys and 25 ± 8% in men, P < 0.05) to ATP production, whereas the phosphocreatine breakdown contribution was reduced (40 ± 10% in boys and 53 ± 12% in men, P < 0.05), likely as a compensatory mechanism. The anaerobic glycolysis activity was unaffected by maturation. The recovery phase also disclosed differences regarding the rates of proton efflux (6.2 ± 2.5 vs. 3.8 ± 1.9 mM·pH unit−1·min−1, in boys and men, respectively, P < 0.05), and phosphocreatine recovery, which was significantly faster in boys than in men (rate constant of phosphocreatine recovery: 1.3 ± 0.5 vs. 0.7 ± 0.4 min−1; Vmax: 37.5 ± 14.5 vs. 21.1 ± 12.2 mM/min, in boys and men, respectively, P < 0.05). Our results obtained in vivo clearly showed that maturation affects muscle energetics. Children relied more on oxidative metabolism and less on creatine kinase reaction to meet energy demand during exercise. This phenomenon can be explained by a greater oxidative capacity, probably linked to a higher relative content in slow-twitch fibers before puberty.
Keywords: children, skeletal muscle, 31P-magnetic resonance spectroscopy
it is well recognized that numerous functional changes occur throughout growth and maturation. More particularly, at the skeletal muscle level, while muscle mass and the associated maximal strength significantly increase, muscle sensitivity to fatigue would be paradoxically enhanced (23, 31, 55, 57, 74). Indeed, during repeated high-intensity exercise bouts, i.e., maximal running or cycling sprints (23, 50, 52) and maximal isokinetic leg extension exercise (29, 66), the decline in short-term muscle power or strength has been reported to be less important in children than in young men. Although this higher resistance to fatigue reported in children has still to be clarified, several hypotheses have been put forth. Among them and considering that muscle fatigue can be associated with accumulation of metabolic by-products within exercising muscle (16), changes in energy metabolism during puberty have been advocated. However, the corresponding studies addressing the issue of a modified muscle energetics in children are scarce, likely as a result of the invasiveness of muscle biopsy measurements. A few comparative analyses between children and adults have been reported on the basis of 31P-magnetic resonance spectroscopy (31P-MRS) measurements, allowing noninvasive documenting of metabolic changes occurring in exercising muscle (2, 39, 51, 56, 65, 75).
On the one hand, on the basis of pH changes recorded in exercising muscle, some authors concluded to a lower anaerobic glycolytic activity before puberty (39, 65, 75), whereas others reported opposite results (51, 56). However, one should keep in mind that pH changes recorded either in bloodstream or in exercising muscle cannot be considered as a reliable index of anaerobic glycolytic activity. Regarding blood measurements, kinetics of lactate and protons transport into the bloodstream, together with buffering capacity, have to be taken into account. As previously illustrated, anaerobic ATP production in exercising muscle when coupled to ATP hydrolysis is related to proton accumulation, whereas proton efflux, buffering capacity, and phosphocreatine (PCr) consumption have the opposite effects, i.e., muscle alkalinization (24). On that basis, anaerobic ATP production cannot be investigated without an appropriate analysis of proton handling (36).
On the other hand, on the basis of 31P-MRS measurements performed during the postexercise recovery period, it has been suggested that the higher resistance to fatigue in children would be related to a larger muscle oxidative capacity, allowing a faster energy replenishment following repeated exercise bouts (31, 56, 65). However, similar measurements did not confirm this enhanced aerobic capacity in prepubescent boys compared with men (2, 39). Moreover, similar contradictions have been shown regarding the oxygen uptake kinetics (V̇o2) response to exercise. Some studies reported that the time constant of V̇o2 at the onset of moderate to heavy exercise was faster before puberty (1, 72), whereas Hebestreit and coworkers (21) found a similar rate of V̇o2 transients in children and adults. Nevertheless, in line with an enhanced oxidative capacity before puberty, it has been reported several times that children displayed a higher oxygen cost compared with men during the transition from rest to moderate or heavy cycle and running exercises (1, 21, 72). However, information related to aerobic ATP production in activated muscle during exercise is still missing.
Overall, while a reduced glycolytic contribution to ATP production and an enhanced oxidative contribution might be plausible to explain the higher resistance to fatigue in children, a detailed quantitative analysis of ATP production, which would be crucial to clearly determine the exact effects of maturation on muscle energetics, has never been reported so far.
The relative contributions of oxidative and anaerobic processes are tightly linked to exercise modality, i.e., intensity and duration, and individual capacity, e.g., fiber-type composition and training status (43). While differences among protocols might explain the various results reported so far, these issues also underline the importance of exercise standardization to properly compare subjects with obvious different muscle capacities.
In the present study, we aimed at determining, through a quantitative analysis, whether muscle energy production is influenced by growth and maturation under standardized exercise conditions.
METHODS
Subjects.
The study was performed on the dominant forearm of 7 prepubescent boys (11.7 ± 0.6 yr old, range 11.3–12.8 yr old) and 10 men (35.6 ± 7.8 yr old, range 24.6–46.5 yr old). Written, informed consent was obtained from all the subjects and from the parents of each under 18-yr-old volunteer. The protocol was approved by the Ethics Committee of Timone Hospital (Marseille, France).
Pubertal stage was determined according to pubic hair and gonadal development described by Tanner and Whitehouse using a self-reporting method (64). Subjects were then classified as prepubertal when the combined assessment of development sites was not greater than stage 2. None of the subjects was involved in any intensive specific training program, but all of them were physically active.
Experimental design.
All subjects were tested on the laboratory on two occasions, separated by at least 48 h. During the first session, each volunteer performed a maximal handgrip test to standardize the exercise conditions, and we gathered their physical characteristics. A magnetic resonance imaging (MRI) investigation was performed before the handgrip test. During the second session, each subject performed a standardized rest-exercise-recovery protocol during which 31P-MRS measurements were performed on the finger flexor muscles.
Maximum voluntary isometric force measurement.
Maximal isometric strength of the dominant forearm (Fmax) was measured using a dedicated ergometer designed and built in our laboratory. Each subject sat on a chair and held a handle bar at the distal phalange joint level, while the dominant forearm was positioned horizontally to reproduce the position adopted throughout the 31P-MRS measurements.
After a familiarization period, each subject was verbally encouraged to perform a maximal handgrip contraction for a 3-s period. This measurement was repeated three times, with at least a 60-s resting period between each measurement. Force output was measured using a calibrated force transducer (0 to 2,000 N force range, ZF, Scaime, France) connected to the handle bar and transmitted to a personal computer using an analog-to-digital converter, thereby providing a visual feedback to the subject. The maximum force was determined as the mean of three reproducible measurements. The variability of each trial was similar whatever the age (2.9 ± 2.3% on average).
MRI.
MRI investigations were performed at 1.5 T on a whole body Siemens-Vision Plus Imaging system (Siemens). The dominant arm was placed in the middle of an “extremity coil” (central diameter = 25 cm). Subjects were scanned in a prone position with the arm fully extended and relaxed. For each subject, mid-forearm was ink-marked, and the corresponding mark was aligned with the crosshairs of the MRI system so that identical positioning was ensured in the magnet bore for each subject. Transverse T1-weighted images (9–13 slices, depending on the forearm length) were recorded from the wrist to the elbow with the following parameters (repetition time = 490 ms, echo time = 12 ms, 200-mm field of view, 512 × 512 acquisition matrix, slice thickness 5 mm, 10-mm gap, and a total duration of 122 s).
31P-MRS.
31P-MRS investigations were performed at 4.7 T using a Bruker 47/30 Biospec spectrometer (Bruker) interfaced to a 30-cm-wide bore magnet. Magnetic field homogeneity was optimized by monitoring the signal from water and lipid protons at 200.14 MHz. Pulsing conditions (1.8-s interpulse delay, 120-μs pulse length) were chosen to optimize the 31P signal obtained with a 50-mm-diameter surface coil (double tuned for 31P and 1H) positioned over the belly of the flexor digitorum muscles at the maximum diameter of the forearm.
During the standardized rest-exercise-protocol, spectra were time averaged as blocks of 32 scans (time resolution = 57.6 s), except during the exercise and the early part (4 min) of the transition from exercise to rest, during which spectra were recorded as 16 scan-blocks to improve the temporal resolution (28.8 s).
At rest, a fully relaxed spectrum (20-s interpulse delay, 8 scans) was recorded, and the comparative analysis between fully relaxed and partially relaxed spectra provided the basis for the partial saturation correction, as previously described (3).
31P-MRS experimental protocol.
The subjects sat on a chair close to the magnet and inserted their dominant forearm horizontally into the magnet bore. The forearm was placed approximately at the same height as the shoulder to ensure a good venous return. After a 5-min resting period, the subjects performed a dynamic exercise for 3 min, followed by a 15-min recovery period. Exercise consisted of a series of finger flexions against a weight adjusted to 15% of the Fmax at 0.7 Hz. Contraction-relaxation cycles were gated to the magnetic resonance acquisition on the basis of an audible signal, so that spectra were always recorded in a relaxed state.
The sliding amplitude of the weight was recorded using a home-built displacement transducer connected to a personal computer running ATS software (SYSMA-France). Calculated power output and exercise frequency were averaged over each 15-s exercise period.
Spectra analysis.
Spectra were fitted in the time domain using a nonlinear least squares algorithm (AMARES) (69). After correction for partial saturation, the area of each signal was translated as absolute concentration on the basis of a 8.2 mM ATP concentration ([ATP]) at rest (66). According to ATP biochemical assays previously reported, we assumed a similar [ATP] in children and adults (14). Intracellular pH (pHi) was calculated from the chemical shift of Pi relative to PCr in parts per million (δ) (49), according the following equation:
The free cytosolic ADP concentration ([ADP]) was calculated according to the method previously described by Kemp and coworkers (33), assuming that PCr represents 85% of the total creatine content (Cr + PCr) (7):
where Kobs is the apparent equilibrium constant of creatine kinase (CK) reaction calculated as a function of the binding of H+, K+ and Mg2+, which is, in turn, a function of pHi, as follows:
where KCK = 1.75 × 10−8 (20), and f terms are the fractions of the total reactants in these specific forms, which can be derived for any assumed set of ionic dissociations for each reactant (ADP, PCr, and ATP), according to the method of Harkema and Meyer (20), modified to include K+ binding, as previously described by Kemp et al. (33).
ATP synthesis rates.
Rates of ATP synthesis from the net PCr breakdown, anaerobic glycolysis, and oxidative phosphorylation were calculated throughout the 3-min exercise session.
The rate of ATP synthesized from the net breakdown of PCr (ATPCK, mM ATP/min) was determined from the rate of PCr decrease at any time point during the exercise (32):
ATP production from anaerobic glycolysis (ATPgly, mM ATP/min) was calculated from exercise-induced changes in pHi, taking into account proton consumption within the CK reaction (HCK+), proton efflux (Hefflux+), and buffering capacity (Hβ+) (32).
HCK+ (in mM ATP/min) is the amount of H+ consumed by CK reaction, calculated from the time-dependent changes in [PCr] as follows: HCK+ = γdPCr/dt, where γ is the stoichiometric coefficient of the coupled Lohman reaction, as described originally by Kushmerick [γ = 27.239 − 13.593 (pH) + 2.144 (pH2) − 0.10887 (pH3)] (40).
Hβ+ was determined from the rate of pHi changes corrected by the apparent buffer capacity, βtotal (in slykes, mmole acid added per unit change in pHi) as follows: Hβ+ = −βtotal dpHi/dt, where βtotal = βt + βPi + βPME + βCO2, in which βt, the inherent buffering capacity of the muscle, was 20 slykes, according to results obtained in previous studies (38, 68) and under ischemic exercise conditions in McArdle's patients (35).
The buffering capacities of Pi (βPi) and phosphomonoester (PME; βPME) was calculated as a function of the corresponding concentrations and pHi, according to the formula:
where x is either Pi or PME, and pK = 6.75 for Pi and 6.20 for PME (9, 32).
The bicarbonate contribution (βCO2) was calculated using the equation:
where S is the solubility of CO2 in muscle water (0.3 mM/kPa) and Pco2 is the partial pressure of CO2 (5 slykes) (32).
Hefflux+ (in mM/min) was calculated for each time point of exercise using the rate constant (λ in mM·min−1·pH unit−1) calculating during the early recovery period as follows:
During the recovery period, proton efflux (Veff, mM·pH unit−1·min−1) was calculated, as previously described by Kemp et al. (35) from the changes in PCr and pH during the first 24 s of recovery (32), according to the equation:
where γ is the amount of protons consumed per mole of PCr consumed (40), m is the number of protons produced per mole of ATP oxidatively produced [m = 0.16/(1 + 10(6.1 − pH))] (35).
The rate of oxidative ATP production (ATPox, mM ATP/min) was calculated throughout the 3 min of exercise using the maximal rate of oxidative ATP production (Vmax) calculated during the recovery period and [ADP] (32):
Energy cost.
The total energy turnover (ET; mM ATP/min) was determined throughout the exercise as the sum of ATP rates:
As a standardization procedure, the energy cost [EC; mM ATP/min/(W/dm3)] was calculated as ET scaled to the ratio between power output and forearm muscle volume. The relative contribution of each pathway was expressed in percentage of the total EC at each time point of exercise.
PCr recovery kinetics.
During the recovery period, PCr time changes were fitted to a monoexponential function expressed by:
where [PCrcons] is the amount of PCr consumed during exercise calculated from the difference between the PCr concentration measured at rest ([PCrrest]) and the PCr concentration ([PCr]) at the end of the exercise, which were expressed in mM. The time t is expressed in minutes, and kPCr is the rate constant of PCr recovery (min−1).
The initial rate of PCr recovery (ViPCr) was calculated as follows:
The maximal rate of oxidative phosphorylation (Vmax, mM/min) was calculated according to the model of Michaëlis Menten, as previously described (34), taking into account [ADP] measured at the end of exercise ([ADPend]), the initial rate of PCr resynthesis (ViPCr), and the Michaëlis-Menten constant (Km), which was assumed to be 30 μM (32)
Statistical analyses.
The statistical analyses have been performed using the general linear model procedure of SAS software (SAS Institute, Cary, NC), which takes into account the present unbalanced population.
Between groups (boys and men), comparative analyses of variables changing with respect to time were performed using a two-way analyses of variance with repeated measures over time. Univariate tests were performed to analyze the effects of time and the interaction between time and group. In case of violation of the sphericity assumption (investigated using Mauchly's sphericity test), we adjusted the univariate test degrees of freedom using the Huynh Feldt epsilon correction factor; the P values indicated in the paper were those corrected (for the time and the interaction time × group). When the time × group interaction was significant, post hoc repeated comparisons between groups were performed (Bonferroni contrast) for mean values at corresponding time point. Other variables were compared using appropriate (Student t or Mann-Whitney nonparametric) statistical tests. Relationships between variables were analyzed using linear regressions, and the corresponding strength was assessed using Pearson's correlation coefficient. Probability values for testing hypotheses were significant at 0.05. In Tables 1–4, text, and Figs. 1–3, results are reported as means ± SD.
Table 1.
Physical characteristics
| Children | Adults | P | |
|---|---|---|---|
| Age, yr | 11.7 ± 0.6 | 35.6 ± 7.8 | <0.05 |
| Height, cm | 149 ± 3 | 177 ± 5 | <0.05 |
| Weight, kg | 35.9 ± 2.0 | 74.2 ± 9.3 | <0.05 |
| BMI, kg/m2 | 16.4 ± 1.0 | 22.7 ± 2.5 | <0.05 |
| Fmax, N | 181 ± 36 | 452 ± 53 | <0.05 |
| VM, cm3 | 227 ± 50 | 498 ± 84 | <0.05 |
| Fmax/VM, N/cm3 | 0.82 ± 0.17 | 0.84 ± 0.09 | NS |
Values are means ± SD. BMI, body mass index; Fmax, finger flexor muscle's maximal voluntary isometric force; VM, forearm muscle volume measured by MRI; Fmax/VM, ratio of Fmax to VM; NS, nonsignificant.
Table 2.
Resting metabolic variables
| Children | Adults | P | |
|---|---|---|---|
| pHi | 7.05 ± 0.05 | 7.05 ± 0.04 | NS |
| [PCr], mM | 29.7 ± 1.9 | 32.5 ± 4.5 | NS |
| [Pi], mM | 4.9 ± 1.0 | 4.0 ± 1.2 | NS |
| [PCr]/[Pi] | 6.6 ± 1.4 | 8.8 ± 1.8 | <0.05 |
| [ADP], μM | 9.1 ± 3.3 | 9.2 ± 1.7 | NS |
Values are means ± SD. pHi, intracellular pH; [PCr], phosphocreatine concentration; [Pi], inorganic phosphate concentration; [PCr]/[Pi], PCr-to-Pi concentration ratio; [ADP], adenosine diphosphate concentration.
Table 3.
Exercise-induced metabolic changes
| Children | Adults | P | |
|---|---|---|---|
| End-exercise pHi | 6.57 ± 0.15 | 6.47 ± 0.16 | NS |
| ΔpH | 0.5 ± 0.2 | 0.6 ± 0.2 | NS |
| End-exercise [PCr], mM | 15.6 ± 3.5 | 11.6 ± 4.0 | <0.05 |
| [PCrcons], % | 46.9 ± 8.4 | 62.2 ± 13.2 | <0.01 |
| End-exercise [Pi], mM | 11.2 ± 3.8 | 18.5 ± 6.6 | <0.01 |
| End-exercise PCr/Pi | 1.5 ± 0.5 | 0.8 ± 0.5 | <0.01 |
| End-exercise [ADP], μM | 30.2 ± 13.1 | 46.8 ± 19.5 | <0.01 |
| ATPPCr max, mM ATP/min/(W/dm3) | 6.0 ± 3.3 | 8.4 ± 5.7 | NS |
| ATPgly max, mM ATP/min/(W/dm3) | 14.4 ± 7.1 | 12.4 ± 4.9 | NS |
| ATPox max, mM ATP/min/(W/dm3) | 10.2 ± 3.8 | 5.1 ± 2.6 | <0.05 |
| ECmean, mM ATP/min/(W/dm3) | 19.2 ± 8.4 | 13.1 ± 4.8 | NS |
Values are means ± SD. ΔpHi, difference between pHi measured at rest and at end of exercise; [PCrcons], the amount of PCr concentration consumed throughout the exercise expressed as %resting value; ATPPCr max, ATPgly max, and ATPox max: maximal rates of ATP derived from the net breakdown of PCr, anaerobic glycolysis, and oxidative phosphorylation, respectively, throughout the exercise; ECmean, total energy cost averaged over the 3 min of exercise; dm, dry muscle. ATP flux and EC are expressed in millimoles of ATP per minute related to the power output to forearm muscle volume ratio (W/dm3).
Table 4.
Metabolic variables measured throughout the postexercise recovery period
| Children | Adults | P | |
|---|---|---|---|
| λ, mM/pH unit | 14.5 ± 6.8 | 6.8 ± 3.2 | <0.01 |
| Veff max, mM/min | 6.2 ± 2.5 | 3.8 ± 1.9 | <0.05 |
| βtot, slykes | 30.7 ± 5.7 | 34.1 ± 5.8 | NS |
| kPCr, min−1 | 1.3 ± 0.5 | 0.7 ± 0.4 | <0.05 |
| ViPCr, mM/min | 18.1 ± 8.1 | 11.7 ± 4.1 | <0.05 |
| Vmax, mM/min | 37.5 ± 14.5 | 21.1 ± 12.2 | <0.05 |
Values are means ± SD. λ, Apparent efflux rate, i.e., the rate of pHi change scaled to the pHi difference recorded at end of exercise; Veff max, maximum rate of proton efflux; βtot, total buffering capacity; kPCr, rate constant of PCr recovery; ViPCr, initial rate of PCr resynthesis; Vmax, theoretical maximum rate of oxidative phosphorylation.
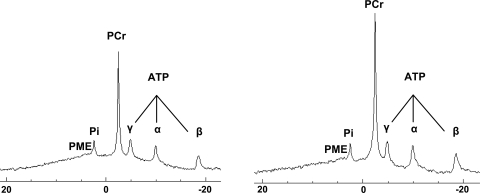
Fig. 1.
Typical spectra recorded at rest in a prepubescent boys (left) and a man (right). PCr, phosphocreatine; PME, phosphomonoester. Pi, inorganic phospate.
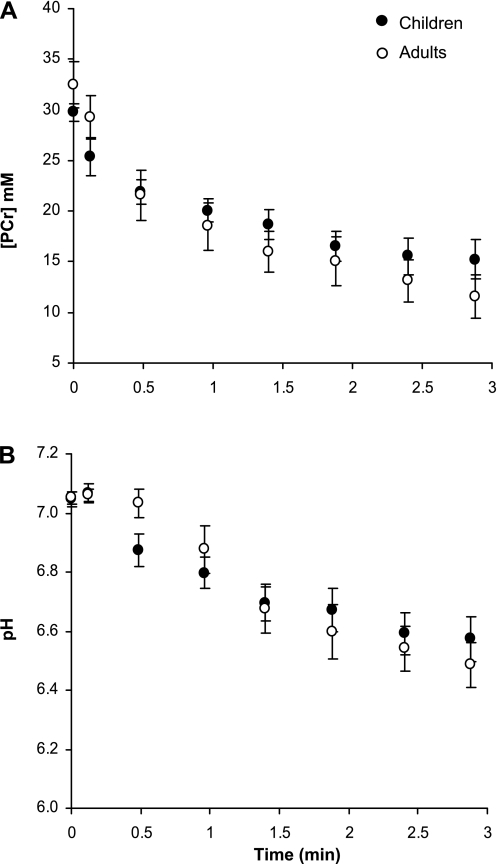
Fig. 2.
Exercise-induced changes in PCr concentration ([PCr]; A) and intracellular pH (B). Values are means ± SD in both groups.
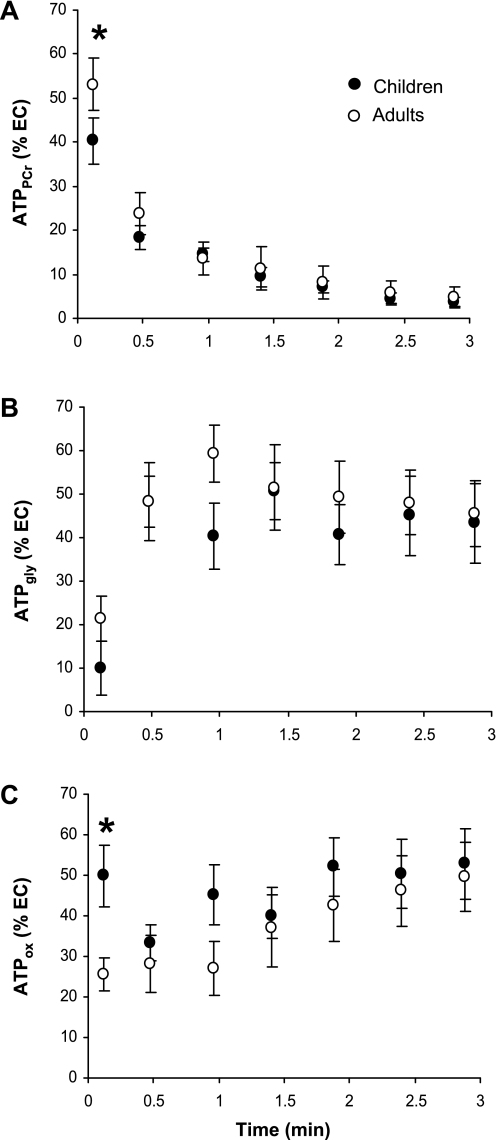
Fig. 3.
Relative contributions of PCr (ATPPCr; A), anerobic glycolysis (ATPgly; B), and oxidative phosphorylation to the total ATP turnover (ATPox; C). Values are expressed as percentage of the standardized energy cost (EC) calculated as the total ATP synthesis rate related to the power output-to-muscle volume ratio at each time point. *Significant difference between children and men (P < 0.05).
RESULTS
Part of the exercise and recovery data analyzed in the present study has been previously reported (56). The physical characteristics of each group are reported in Table 1. As expected, muscle volume (Vm) and Fmax were both significantly lower in boys, whereas the corresponding ratio, i.e., Fmax scaled to Vm, was similar in both groups (Table 1). Accordingly, considering the whole set of force values, Fmax was positively related to muscle volume as measured using MRI (y = 0.81x, R2 = 0.93, and P < 0.001).
Typical spectra recorded at rest in a child and an adult are displayed in Fig. 1. The PCr-to-ATP ratio was similar in boys (3.7 ± 0.6) and men (3.9 ± 0.5). Similarly Pi/ATP did not differ with respect to age (0.6 ± 0.1 and 0.5 ± 0.2 in children and adults, respectively).
Similarly, at rest, pHi and [PCr], [Pi], and [ADP] were similar in both groups (Table 2). On the contrary, children exhibited a significantly lower PCr-to-Pi ratio (Table 2).
The workload during exercise was adjusted to a given percentage of the maximal voluntary contraction, ensuring that each subject performed the finger flexions exercise at the same relative intensity. As a result, the absolute mechanical power output was significantly lower in boys (0.5 ± 0.2 W) compared with men (1.5 ± 0.3 W, P < 0.001). However, related to muscle volume, similar mechanical performance (Watt per unit of muscle volume) was found whatever the age (2.2 ± 0.6 and 2.7 ± 0.5 W/dm3 in boys and men, respectively). Additionally, a significant positive relationship between power output and muscle volume was found (R2 = 0.84, P < 0.01).
Exercise-induced changes in pHi and PCr are illustrated in Fig. 2. The total amount of PCr consumed throughout the exercise, defined as the difference between the [PCr] measured at rest and the end of exercise content, was significantly larger in men (62.2 ± 13.2% of the resting value) than in boys (46.9 ± 8.4% of the resting value) and was associated with a significantly higher Pi and ADP accumulation at the end of exercise (Table 3). Accordingly, the PCr-to-Pi ratio was significantly lower in men than in boys (Table 3). On the contrary, we observed no difference in the pHi time-dependent changes between the two groups (Fig. 2B).
The total rate of proton (H+) production calculated from pHi and PCr changes and taking into account the buffering capacity (βtotal) and efflux (Hefflux+) at each time point of exercise was similar in both groups. However, the detailed analysis of each mechanism involved in H+ balance illustrated a few differences. We found a significant time × group interaction effect for the amount of proton consumed through the CK reaction (P < 0.05). More particularly, given that the initial rate of PCr consumption (ViPCr ex) was significantly lower in children (12.4 ± 3.7 mM/min; the values were ranging from 7.2 to 18.2 mM/min, and the median value was 12.6 mM/min) compared with men (24.1 ± 15.5 mM/min; the values were ranging from 6.6 to 62.3 mM/min, and the median value was 21.6 mM/min), the corresponding rate of H+ consumed by this process was reduced at the onset of exercise in children (2.1 ± 0.6 mM/min) compared with adults (4.2 ± 2.8 mM/min; P < 0.05). After the first 30 s of exercise, the time-dependent changes in H+ consumption due to PCr breakdown were similar in both groups, illustrating the reduction of the PCr contribution to the total energy production (Fig. 3A). The Pi accumulation associated with the PCr breakdown was reduced in boys compared with men, and the corresponding buffering capacity (significant time × group interaction effect, P < 0.001), calculated at end of exercise was significantly higher in men (9.3 ± 3.2 slykes) than in boys (6.0 ± 2.0 slykes; P < 0.05). No significant difference in the contribution of PME and bicarbonate to the total buffering capacity was found. However, the total buffering capacity taking into account Pi, bicarbonate, and PME similarly increased in both groups from 29.0. ± 1.9 and 28.6 ± 1.6 to 33.0 ± 6.6 and 37.6 ± 5.3 slykes in children and adults, respectively.
During exercise, the interaction effect showed a significant increase of Hefflux+ with respect to intracellular acidosis in both groups (P < 0.01), which was significantly higher (time × group interaction, P < 0.05) after the first 30 s of exercise in boys compared with men. At the onset of exercise, it was similar in boys (0.1 ± 0.1 mM/min) and in men (0.2 ± 0.3 mM/min). At the end of exercise, this rate was 6.2 ± 2.5 mM/min in boys and 3.8 ± 1.9 mM/min in men (P < 0.01).
The standardized EC was similar in both groups throughout the whole exercise duration (Table 3). The relative contributions of the CK reaction, anaerobic glycolysis, and aerobic metabolism to ATP synthesis are displayed in Fig. 3. We found a significant time × group interaction effect for both PCr (P < 0.05) and aerobic (P < 0.05) contributions to the total energy demand. Post hoc analyses illustrated a significant difference for the first time point of exercise for these two parameters (P < 0.05 and P < 0.001 for CK reaction and aerobic metabolism, respectively). The maximal rate of ATP produced from the PCr breakdown (ATPPCr) was observed in both groups at the onset of exercise. While the absolute value of ATPPCr did not differ with respect to age, the corresponding relative contribution, i.e., scaled to the total EC, was significantly higher in men (53 ± 12%) than in boys (40 ± 10%) (Fig. 3A). During the remaining time in exercise, both the absolute and relative PCr contributions to ATP consumption decreased similarly in both groups (Fig. 3A) from 4.0 ± 1.9 and 3.7 ± 1.1 to 0.8 ± 0.5 and 0.4 ± 0.4 mM ATP/min/(W/dm3) in children and adults, respectively. The corresponding relative contributions to the total energy demand declined from 18 ± 5 to 4 ± 2% in children and from 24 ± 9 to 5 ± 4% in men.
On the basis of PCr and pHi changes during exercise, we calculated ATPgly. Both absolute and relative glycolytic contributions to ATP production were identical in children and adults throughout the entire exercise period (Fig. 3B). Similarly, the maximal anaerobic glycolytic flux did not differ with respect to age (Table 3).
Throughout the exercise, we observed a continuous increase in the rate of aerobic ATP production expressed in absolute terms in both adults (from 3.9 ± 2.4 to 4.5 ± 1.9 mM ATP·min−1·W−1·dm−3) and children (from 3.9 ± 2.3 to 9.6 ± 4.1 mM ATP/min/(W/dm3) groups. Although no significant difference was found when ATP flux was expressed in absolute terms, the relative aerobic contribution to the total ATP production at the onset of exercise was significantly larger in boys (50 ± 15%) compared with men (25 ± 8%) (Fig. 3C). During the remaining exercise period, the absolute and relative oxidative ATP contributions were similar in both groups (Fig. 3C).
Overall, a significant interaction time by group effect has been found regarding the total anaerobic contribution to the total energy demand (P < 0.05). The post hoc analysis revealed that anaerobic ATP provision amounted to 75 ± 8% of the total EC at the onset of exercise in men was significantly reduced in boys, i.e., 50 ± 15% of the total EC at the onset of exercise (P < 0.001). At end of exercise, the anaerobic contribution to ATP production was similar in both groups (50 ± 17 and 47 ± 17% of the total EC in men and boys, respectively).
The kinetics of postexercise pHi recovery was significantly different between the two groups. The initial rate of pHi recovery was significantly faster in children (0.03 ± 0.09 pH units/min) compared with adults (−0.04 ± 0.04 pH units/min) for whom an initial acidosis was measured. The PCr recovery kinetics was also faster in boys than in men, indicating a larger proton load in children (Table 4). The faster kinetics of pHi and PCr postexercise recovery in children indicated a faster rate of proton efflux in children (6.2 ± 2.4 mM/min) compared with adults (3.9 ± 1.9 mM/min) (Table 4). The corresponding apparent proton efflux rate constant (λ) was also significantly larger in children (14.5 ± 6.8 pH unit−1) compared with men (6.8 ± 3.2 pH unit−1).
DISCUSSION
We mainly showed in the present study that the EC of contraction was unaffected throughout the maturation process, whereas the relative contribution of each metabolic pathway to ATP production during a standardized exercise changed with respect to age. The increased aerobic contribution to energy production measured in prepubescent boys was compensated by a reduced PCr consumption. On the contrary, the anaerobic glycolysis activity was unaffected by the maturational degree. To our knowledge, this study is the first ever comparing quantitatively changes in ATP production rates and proton handling during growth and maturation.
The larger aerobic contribution to the total energy demand, inferred at the onset of exercise in boys compared with men, is in line with previous studies reporting a greater oxygen cost at the onset of cycling or running exercise (including the O2 gain of the cardiodynamic and primary phases of the V̇o2) in children compared with adults (1, 21, 72). More specifically, previous studies have reported a greater average oxygen cost of cycling exercise in prepubescent boys compared with men (1, 21). Similarly, taking into account the running speed and the body mass, Williams et al. (72) reported that the initial O2 cost (calculated from the amplitude of V̇o2 at the end of the primary component) was significantly higher in boys than in men during moderate and heavy running tasks. Before puberty, children would be able to adapt their oxidative metabolism faster than adults to meet the energy demand at the onset of exercise. In this line, some authors reported a faster initial V̇o2 adjustment in children compared with adults (1, 72). For instance, Williams et al. (72) showed that the primary phase time constant of V̇o2 was significantly faster in boys than in men during the heavy exercise. Interestingly, a greater oxygen cost and a faster primary phase time constant (54) have been reported in athletes with a high type I fiber content. More specifically, Pringle et al. (54) reported a significant negative correlation between the time constant of the V̇o2 primary component and the fiber-type distribution. Subjects with a high type I fiber relative content displayed a significantly faster V̇o2 adjustment at the onset of a heavy exercise. On that basis, one can hypothesize that the present results obtained in children could be in line with a greater proportion of type I fibers, as previously showed by Lexell et al. (44). These authors reported a significant reduction in the proportion of type I fibers from ∼65% at the age of 5 yr to 50% at the age of 20 yr (44). Hebestreit et al. (21) reported opposite results with similar time constants for the phase II in boys (age ranging from 9 to 12 yr old) and young men during cycle exercise performed at 50, 100, or 130% of peak V̇o2 and maintained during 210, 120, and 75 s, respectively. However, it has been argued that the exercise duration was too short so that V̇o2 kinetics could not be accurately investigated (18, 107). In addition, the significant decrease of the phase II time constant between exercises performed at 100 and 130% of peak V̇o2 further confirm the inaccuracy of the method. Indeed, it has been clearly shown that this time constant remained unchanged or increased with exercise intensity (71).
In addition, a faster pulmonary V̇o2 adjustment should diminish the oxygen-deficit extent in children, thereby reducing the anaerobic contribution to ATP production at the onset of exercise, i.e., PCr breakdown and/or anaerobic glycolysis. We reported a similar anaerobic glycolysis activity in both groups, whereas the significantly reduced PCr breakdown, measured at the onset of exercise in children, is in line with a reduced oxygen deficit before puberty. In addition, the reduced contribution from PCr breakdown to energy demand may reflect a lower CK activity before puberty, as previously reported by Kaczor et al. (30). These authors showed that the specific CK activity, i.e., related to muscle wet weight was 28% lower in children (age ranging from 3 to 11 yr old) compared with adults (30). However, this result should be considered with caution, given that, once related to the total protein content, they found, in agreement with previous results (5, 19), that CK activity was not affected by maturation (30). In addition, one has to keep in mind that normal CK activity is far too high for a 30% decrease to make any difference to rates of PCr depletion. It is noteworthy that, although 95% of the total CK activity is lost in MM CK knockout mice, the time course of PCr depletion during stimulation and ischemia was unaltered (26, 59). Moreover, given that the total EC measured in the present study was similar in both groups, the cause must indeed lie in altered regulation of oxidative ATP synthesis. The spared PCr store during exercise before puberty could explain the greater muscle fatigue resistance commonly reported in prepubescent children compared with young adults during high-intensity intermittent exercises (55, 57, 74).
On the basis of a complete analysis of proton handling in exercising muscle, we found no maturation-related changes in anaerobic glycolytic metabolism. During the exercise bout, in both groups, the anaerobic glycolytic flux quickly increased during the first half of the exercise and then decreased slightly until the end of exercise. To our knowledge, no corresponding data have been reported so far in children. This rapid increase and modest decline in glycolytic ATP production is consistent with previous data from other investigators observed in finger flexor muscles and calf muscles during nonischemic, dynamic (33, 36, 45, 70), and isometric exercises (41, 50). However, the anaerobic glycolytic activity mainly depends on exercise modalities (i.e., intensity and duration), so that direct comparisons are difficult.
Our results contrast with those commonly reported in the pediatric literature regarding a limited glycolytic activity before puberty and clearly indicate that glycolysis is fully efficient before puberty. At least regarding previous 31P-MRS studies (39, 65, 75), the discrepancy might be related to methodological differences, related to the quantification of glycolytic activity. These studies reported a lower acidosis in boys at the end of an incremental exercise performed until exhaustion and concluded, on that basis, that the glycolytic activity was immature before puberty. However, one should clearly have in mind that pH changes in exercising muscle illustrate the balance between mechanisms of protons production and consumption. More exactly, while the exercise-induced acidosis is partly related to an increased glycolytic activity, other mechanisms contribute to these changes either positively (oxidative proton production) or negatively (PCr consumption, buffering capacity, and proton efflux) (37). In other words, a reduced end-of-exercise acidosis could be explained, either by a lower glycolytic activity, or by a higher rate of proton removal. As a result, no conclusion regarding the glycolytic activity can be withdrawn from the sole pH values, and an accurate account of mechanisms of protons consumption and production must be performed.
In addition, apart from these methodological considerations, other concerns could be addressed regarding the conclusions of an immature glycolytic activity in children (39, 65, 75). Exercise was conducted until exhaustion in the corresponding studies, and one could argue that children could have stopped exercise prematurely due to a lack of motivation. In the present study, each subject was verbally encouraged to perform their maximum force, which was averaged over three reproducible measurements. The within-subject variation for Fmax was low for the entire population (2.7 ± 1.3%) and not significantly different between boys and men, indicating that all of the volunteers gave their maximum during the maximal handgrip test.
Moreover, it is of interest that the results obtained during the postexercise recovery period, i.e., a faster PCr and pH recovery kinetics, which has been shown to be independent of the muscle mass (48), also support a larger aerobic capacity in children compared with men, as previously described (65). At the end of exercise, the proton efflux calculated, taking into account both pH changes (corrected by buffering and oxidative proton production) and proton load linked to PCr resynthesis, was significantly faster in prepubescent boys than in men. Although no corresponding results are available in children for the purpose of comparison, the results we obtained in adults are consistent with previous studies with similar end-of-exercise acidosis (61) and similar exercise conditions (45). Given that similar acceleration of pH recovery kinetics has been reported in highly endurance-trained athletes compared with less-trained subjects (25, 43), this result provides additional support for an enhanced oxidative capacity before puberty.
In exercising muscle, proton efflux is controlled by cell membrane transporters, especially the monocarboxylate transporters (MCT), and blood flow (29). It has been shown that, during the first phase of recovery from intense exercise, the lactate-H+ cotransport accounted for ∼50% of the total H+ release (52), thereby illustrating the significant involvement of the MCT. On that basis, our results suggest that the faster proton efflux found in children compared with adults could be related to an increased MCT expression or activity before puberty. This hypothesis is consistent with the faster blood lactate appearance after exercise reported in prepubescent children compared with men after intense exercise (4, 6, 12, 22). Furthermore, a larger membrane transport capacity for lactate and proton has been linked to a higher slow-twitch fiber content (53). In that respect, some studies reported a significantly higher MCT expression, especially the type I isoform (MCT1), in endurance-trained athletes compared with less-trained subjects (13, 67). Overall, the faster rate of proton efflux measured in children would also be consistent with a higher percentage of slow-twitch fibers, which would account for the larger aerobic capacity. In that respect, MCT1 content has been shown to be highly correlated to citrate synthase (specific enzyme of Krebs cycle) activity in both human (13) and rat muscle (46), regardless of the training status.
On the other hand, age-related changes in muscle blood flow could play a prominent role in determining proton efflux rates. It has been shown that an increased blood flow accounted for an enhanced pH recovery capacity in both adults (73) and prepubescent children (11) throughout an active recovery process. An improved blood flow in children could be mediated by a higher capillary density or a smaller intramuscular perfusion distance, which could facilitate metabolites diffusion from muscle to blood, as previously suggested (15). Interestingly, capillary density has been shown in animals to decrease during maturation, i.e., as the fiber cross-sectional area increases with respect to growth (58, 62, 63). A higher functional capillarization would also explain the faster lactate clearance previously reported after exercise (4, 6, 12, 22).
As reported in a previous study from our laboratory (56), PCr recovery kinetics were faster in children compared with men, thereby further illustrating the larger aerobic capacity measured during the exercise bout. These findings are in agreement with the results of Taylor et al. (65) obtained after a graded plantar flexion exercise performed until exhaustion. In contrast, Kuno et al. (39) reported similar PCr recovery rate constants (kPCr) in children and adults after an incremental calf test to exhaustion. However, given that end-of-exercise metabolic conditions were different in children and adults, the corresponding conclusions might be erroneous. Indeed, it has been shown that kPCr values are modulated by the end-of-exercise acidosis so that comparisons are only valid if end-of-exercise conditions are similar (60).
More recently, Barker et al. (2) reported no age-related difference in PCr recovery kinetics after a moderate leg extension exercise on the basis of kPCr and Vmax measurements. However, in their study, the Vmax calculation was oversimplified as the product of kPCr and the resting [PCr], as previously described (10, 32, 47). This oversimplification is actually derived from the calculation of the ViPCr from an exponential fitting. In that case, ViPCr is represented by the product of kPCr and the amount of PCr consumed at the end of exercise. On that basis, a few authors have calculated Vmax similarly to ViPCr, with the amount of PCr consumed being replaced by the resting [PCr]. Although mathematically plausible, this calculation is not supported by any control model, and the corresponding Vmax is devoid of any enzymatic meaning, as is the case for the calculation of Vmax using ADP within a Michaelis-Menten framework. This method is then largely questionable because it ignores the commonly accepted theory considering ADP as a key regulator of oxidative ATP synthesis (34). In addition, with such an approximate calculation, Vmax becomes strongly dependent on [PCr] measured at rest. In other words, with the use of this method, a higher resting PCr value will be directly linked to a higher maximal oxidative capacity. In their study, Barker et al. (2) reported a significantly higher [PCr] measured at rest in men (46.1 ± 8.1 mM) compared with the boys (35.9 ± 3.7 mM). Given that kPCr values were similar, the corresponding calculated Vmax values were higher in men than in boys, even if no significant difference had been reported (1.4 ± 0.2 and 2.4 ± 1.2 mM/s, respectively, in children and adults). However, compared with other published data of [PCr] resting values estimated by 31P-MRS in quadriceps muscle in normally active men, which range from 27 to 34 mM (10, 25, 28, 43), the level reported by Barker and coworkers (2) seems largely overestimated in adults. Consequently, this result should be interpreted with caution. However, the differences with Barker et al. cannot only be explained by the analytic model choice. Indeed, in the present study, the higher Vmax value in children compared with men was associated with a larger kPCr value, contrary to what was reported by Barker et al. Given the similar end-of-exercise pH values in both groups, we were able to reliably compare the recovery variables between the two groups (60). This result strongly supported an enhanced oxidative capacity in children compared with men. Other factors, such as the muscles investigated or the exercise intensity, could account for these discrepancies. However, it has been reported that Vmax was independent of the exercise intensity (60). Further investigations would be warranted to determine whether maturation is muscle specific.
In comparative analysis between boys and men, care must be taken to properly consider morphological differences, i.e., muscle mass for exercise standardization and localization of activated muscles with respect to the magnetic resonance signal sampling.
One could argue that muscle mass difference could have affected the phosphorylated metabolite quantification and, more specifically, the relative PCr depletion, as recorded using unlocalized spectroscopy, i.e., using surface coils. Actually, the real issue is to determine whether the surface coil used for these experiments was sampling a different mixture of active and inactive muscle fibers in children compared with adults. In a previous work from our laboratory (56), we clearly showed that, with the 5-cm-diameter surface coil and the pulse sequence we used in the present study, we did not sample the extensor muscles in either group and sampled the same relative amount of active and inactive muscles in both groups, despite the clear muscle mass difference. Consequently, the metabolic differences we reported are really due to physiological difference and not to any methodological bias.
As a standardization procedure, the exercise intensity we used in the present work was calculated on the basis of maximum voluntary contraction measurements, and the corresponding mechanical results were scaled to muscle volume. This was done according to the results of Fowler et al. (17), who previously illustrated that normalizing power to muscle volume results in a similar metabolic stress between individuals of different sizes. They reported a highly linear relationship between power output normalized to muscle volume and the corresponding metabolic changes, i.e., PCr consumption. In that respect, metabolic changes occurring under those standardized exercise conditions can be reliably compared and do not suffer from bias, such as those related to different workloads, relative activated muscle mass, maximal capacity etc. Interestingly, although the absolute power output developed by men was significantly larger compared with that of boys during the exercise bout, the corresponding value scaled to muscle volume was independent of age. The reliability of the standardization procedure was further illustrated by the significant positive relationship between power output (P) and muscle volume (VM) (P = 0.003VM, R2 = 0.84, P < 0.01).
Another issue related to the in vivo assessment of mitochondrial activity is the accurate calculation of [ADP], which is tightly linked to the accurate determination of the Kobs, taking into account H+, K+, and Mg2+ influence. In the present study, we assumed free [Mg2+] to be 1 mM, as previously described (20, 42). More recently, Iotti et al. (27) developed a method allowing [ADP] calculations on the basis of pH and pMg changes. Using this method, we found, in agreement with Iotti et al., that, in exercising muscle, [ADP] rose from 0.24 ± 0.02 to 0.44 ± 0.18 mM in boys and from 0.24 ± 0.05 to 0.38 ± 0.23 mM in men. The differences between our two groups were similar, regardless of the way ADP and pH were calculated, although the [ADP] were twofold higher than the values reported so far (33, 41, 68, 70).
In conclusion, we clearly showed that maturation significantly affects skeletal muscle energetics. Our results illustrated for the first time that, for a similar total EC, the aerobic contribution to ATP production was significantly higher in prepubescent boys and compensated for by a reduced PCr breakdown, while anaerobic glycolytic activity was similar whatever the age, thereby illustrating a larger oxidative capacity and a fully efficient glycolytic activity in children.
GRANTS
This work was supported by the Association Française Contre les Myopathies.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank all of the participants for effort and patience during data collection.
REFERENCES
- 1. Armon Y, Cooper DM, Flores R, Zanconato S, Barstow TJ. Oxygen uptake dynamics during high-intensity exercise in children and adults. J Appl Physiol 70: 841–848, 1991 [DOI] [PubMed] [Google Scholar]
- 2. Barker AR, Welsman JR, Fulford J, Welford D, Armstrong N. Muscle phosphocreatine kinetics in children and adults at the onset and offset of moderate-intensity exercise. J Appl Physiol 105: 446–456, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Bendahan D, Confort-Gouny S, Kozak-Reiss G, Cozzone PJ. Heterogeneity of metabolic response to muscular exercise in humans. New criteria of invariance defined by in vivo phosphorus-31 NMR spectroscopy. FEBS Lett 272: 155–158, 1990 [DOI] [PubMed] [Google Scholar]
- 4. Beneke R, Hutler M, Jung M, Leithauser RM. Modeling the blood lactate kinetics at maximal short-term exercise conditions in children, adolescents, and adults. J Appl Physiol 99: 499–504, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Berg A, Kim SS, Keul J. Skeletal muscle enzyme activities in healthy young subjects. Int J Sports Med 7: 236–239, 1986 [DOI] [PubMed] [Google Scholar]
- 6. Berthoin S, Allender H, Baquet G, Dupont G, Matran R, Pelayo P, Robin H. Plasma lactate and plasma volume recovery in adults and children following high-intensity exercises. Acta Paediatr 92: 283–290, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Boska M. ATP production rates as a function of force level in the human gastrocnemius/soleus using 31P MRS. Magn Reson Med 32: 1–10, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Chance B, Leigh JS, Jr, Clark BJ, Maris J, Kent J, Nioka S, Smith D. Control of oxidative metabolism and oxygen delivery in human skeletal muscle: a steady-state analysis of the work/energy cost transfer function. Proc Natl Acad Sci U S A 82: 8384–8388, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Conley KE, Blei ML, Richards TL, Kushmerick MJ, Jubrias SA. Activation of glycolysis in human muscle in vivo. Am J Physiol Cell Physiol 273: C306–C315, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol 526: 203–210, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dotan R, Falk B, Raz A. Intensity effect of active recovery from glycolytic exercise on decreasing blood lactate concentration in prepubertal children. Med Sci Sports Exerc 32: 564–570, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Dotan R, Ohana S, Bediz C, Falk B. Blood lactate disappearance dynamics in boys and men following exercise of similar and dissimilar peak-lactate concentrations. J Pediatr Endocrinol Metab 16: 419–429, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Dubouchaud H, Butterfield GE, Wolfel EE, Bergman BC, Brooks GA. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am J Physiol Endocrinol Metab 278: E571–E579, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Eriksson O, Saltin B. Muscle metabolism during exercise in boys aged 11 to 16 years compared to adults. Acta Paediatr Belg 28, Suppl: 257–265, 1974 [PubMed] [Google Scholar]
- 15. Falk B, Dotan R. Child-adult differences in the recovery from high-intensity exercise. Exerc Sport Sci Rev 34: 107–112, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev 74: 49–94, 1994 [DOI] [PubMed] [Google Scholar]
- 17. Fowler MD, Ryschon TW, Wysong RE, Combs CA, Balaban RS. Normalized metabolic stress for 31P-MR spectroscopy studies of human skeletal muscle: MVC vs. muscle volume. J Appl Physiol 83: 875–883, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Gaesser GA, Poole DC. The slow component of oxygen uptake kinetics in humans. Exerc Sport Sci Rev 24: 35–71, 1996 [PubMed] [Google Scholar]
- 19. Haralambie G. Enzyme activities in skeletal muscle of 13–15 years old adolescents. Bull Eur Physiopath Respir 18: 65–74, 1982 [PubMed] [Google Scholar]
- 20. Harkema SJ, Meyer RA. Effect of acidosis on control of respiration in skeletal muscle. Am J Physiol Cell Physiol 272: C491–C500, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Hebestreit H, Kriemler S, Hughson RL, Bar-Or O. Kinetics of oxygen uptake at the onset of exercise in boys and men. J Appl Physiol 85: 1833–1841, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Hebestreit H, Meyer F, Htay H, Heigenhauser GJ, Bar-Or O. Plasma metabolites, volume and electrolytes following 30-s high-intensity exercise in boys and men. Eur J Appl Physiol Occup Physiol 72: 563–569, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Hebestreit H, Mimura K, Bar-Or O. Recovery of muscle power after high-intensity short-term exercise: comparing boys and men. J Appl Physiol 74: 2875–2880, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Hochachka PW, Mommsen TP. Protons and anaerobiosis. Science 219: 1391–1397, 1983 [DOI] [PubMed] [Google Scholar]
- 25. Hug F, Bendahan D, Le Fur Y, Cozzone PJ, Grelot L. Metabolic recovery in professional road cyclists: a 31P-MRS study. Med Sci Sports Exerc 37: 846–852, 2005 [DOI] [PubMed] [Google Scholar]
- 26. in't Zandt HJ, Wieringa B, Heerschap A. Creatine kinase knockout mice–what is the phenotype: skeletal muscle. MAGMA 6: 122–123, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Iotti S, Frassineti C, Alderighi L, Sabatini A, Vacca A, Barbiroli B. In vivo (31)P-MRS assessment of cytosolic [Mg(2+)] in the human skeletal muscle in different metabolic conditions. Magn Reson Imaging 18: 607–614, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Jubrias SA, Esselman PC, Price LB, Cress ME, Conley KE. Large energetic adaptations of elderly muscle to resistance and endurance training. J Appl Physiol 90: 1663–1670, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Juel C. Regulation of cellular pH in skeletal muscle fiber types, studied with sarcolemmal giant vesicles obtained from rat muscles. Biochim Biophys Acta 1265: 127–132, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Kaczor JJ, Ziolkowski W, Popinigis J, Tarnopolsky MA. Anaerobic and aerobic enzyme activities in human skeletal muscle from children and adults. Pediatr Res 57: 331–335, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Kanehisa H, Okuyama H, Ikegawa S, Fukunaga T. Fatigability during repetitive maximal knee extensions in 14-year-old boys. Eur J Appl Physiol Occup Physiol 72: 170–174, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Kemp GJ, Radda GK. Quantitative interpretation of bioenergetic data from 31P and 1H magnetic resonance spectroscopic studies of skeletal muscle: an analytical review. Magnes Res 10: 43–63, 1994 [PubMed] [Google Scholar]
- 33. Kemp GJ, Roussel M, Bendahan D, Le Fur Y, Cozzone PJ. Interrelations of ATP synthesis and proton handling in ischaemically exercising human forearm muscle studied by 31P magnetic resonance spectroscopy. J Physiol 535: 901–928, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kemp GJ, Taylor DJ, Radda GK. Control of phosphocreatine resynthesis during recovery from exercise in human skeletal muscle. NMR Biomed 6: 66–72, 1993 [DOI] [PubMed] [Google Scholar]
- 35. Kemp GJ, Taylor DJ, Styles P, Radda GK. The production, buffering and efflux of protons in human skeletal muscle during exercise and recovery. NMR Biomed 6: 73–83, 1993 [DOI] [PubMed] [Google Scholar]
- 36. Kemp GJ, Thompson CH, Barnes PR, Radda GK. Comparisons of ATP turnover in human muscle during ischemic and aerobic exercise using 31P magnetic resonance spectroscopy. Magn Reson Med 31: 248–258, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Kemp GJ, Thompson CH, Sanderson AL, Radda GK. pH control in rat skeletal muscle during exercise, recovery from exercise, and acute respiratory acidosis. Magn Reson Med 31: 103–109, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Kemp GJ, Thompson CH, Taylor DJ, Radda GK. Proton efflux in human skeletal muscle during recovery from exercise. Eur J Appl Physiol Occup Physiol 76: 462–471, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Kuno S, Takahashi H, Fujimoto K, Akima H, Miyamaru M, Nemoto I, Itai Y, Katsuta S. Muscle metabolism during exercise using phosphorus-31 nuclear magnetic resonance spectroscopy in adolescents. Eur J Appl Physiol Occup Physiol 70: 301–304, 1995 [DOI] [PubMed] [Google Scholar]
- 40. Kushmerick MJ. Multiple equilibria of cations with metabolites in muscle bioenergetics. Am J Physiol Cell Physiol 272: C1739–C1747, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Lanza IR, Befroy DE, Kent-Braun JA. Age-related changes in ATP-producing pathways in human skeletal muscle in vivo. J Appl Physiol 99: 1736–1744, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Lawson JW, Veech RL. Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem 254: 6528–6537, 1979 [PubMed] [Google Scholar]
- 43. Layec G, Bringard A, Vilmen C, Micallef JP, Le Fur Y, Perrey S, Cozzone PJ, Bendahan D. Does oxidative capacity affect energy cost? An in vivo MR investigation of skeletal muscle energetic. Eur J Appl Physiol 106: 229–242, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Lexell J, Sjostrom M, Nordlund AS, Taylor CC. Growth and development of human muscle: a quantitative morphological study of whole vastus lateralis from childhood to adult age. Muscle Nerve 15: 404–409, 1992 [DOI] [PubMed] [Google Scholar]
- 45. Mattei JP, Bendahan D, Roussel M, Lefur Y, Cozzone PJ. Gender modulates the energy cost of muscle contraction in untrained healthy subjects. A 31P magnetic resonance spectroscopy analysis. FEBS Lett 450: 173–177, 1999 [DOI] [PubMed] [Google Scholar]
- 46. McCullagh KJ, Poole RC, Halestrap AP, O'Brien M, Bonen A. Role of the lactate transporter (MCT1) in skeletal muscles. Am J Physiol Endocrinol Metab 271: E143–E150, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Meyer RA. A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol Cell Physiol 254: C548–C553, 1988 [DOI] [PubMed] [Google Scholar]
- 48. Minotti JR, Johnson EC, Hudson TL, Zuroske G, Fukushima E, Murata G, Wise LE, Chick TW, Icenogle MV. Training-induced skeletal muscle adaptations are independent of systemic adaptations. J Appl Physiol 68: 289–294, 1990 [DOI] [PubMed] [Google Scholar]
- 49. Moon RB, Richards JH. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem 248: 7276–7278, 1973 [PubMed] [Google Scholar]
- 50. Newcomer BR, Boska MD. Adenosine triphosphate production rates, metabolic economy calculations, pH, phosphomonoesters, phosphodiesters, and force output during short-duration maximal isometric plantar flexion exercises and repeated maximal isometric plantar flexion exercises. Muscle Nerve 20: 336–346, 1997 [DOI] [PubMed] [Google Scholar]
- 51. Petersen SR, Gaul CA, Stanton MM, Hanstock CC. Skeletal muscle metabolism during short-term, high-intensity exercise in prepubertal and pubertal girls. J Appl Physiol 87: 2151–2156, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Pilegaard H, Domino K, Noland T, Juel C, Hellsten Y, Halestrap AP, Bangsbo J. Effect of high-intensity exercise training on lactate/H+ transport capacity in human skeletal muscle. Am J Physiol Endocrinol Metab 276: E255–E261, 1999 [DOI] [PubMed] [Google Scholar]
- 53. Pilegaard H, Terzis G, Halestrap A, Juel C. Distribution of the lactate/H+ transporter isoforms MCT1 and MCT4 in human skeletal muscle. Am J Physiol Endocrinol Metab 276: E843–E848, 1999 [DOI] [PubMed] [Google Scholar]
- 54. Pringle JS, Doust JH, Carter H, Tolfrey K, Campbell IT, Sakkas GK, Jones AM. Oxygen uptake kinetics during moderate, heavy and severe intensity “submaximal” exercise in humans: the influence of muscle fibre type and capillarisation. Eur J Appl Physiol 89: 289–300, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Ratel S, Bedu M, Hennegrave A, Dore E, Duche P. Effects of age and recovery duration on peak power output during repeated cycling sprints. Int J Sports Med 23: 397–402, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Ratel S, Tonson A, Le Fur Y, Cozzone P, Bendahan D. Comparative analysis of skeletal muscle oxidative capacity in children and adults: a 31P-MRS study. Appl Physiol Nutr Metab 33: 720–727, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Ratel S, Williams CA, Oliver J, Armstrong N. Effects of age and mode of exercise on power output profiles during repeated sprints. Eur J Appl Physiol 92: 204–210, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Ripoll E, Sillau AH, Banchero N. Changes in the capillarity of skeletal muscle in the growing rat. Pflügers Arch 380: 153–158, 1979 [DOI] [PubMed] [Google Scholar]
- 59. Roman BB, Meyer RA, Wiseman RW. Phosphocreatine kinetics at the onset of contractions in skeletal muscle of MM creatine kinase knockout mice. Am J Physiol Cell Physiol 283: C1776–C1783, 2002 [DOI] [PubMed] [Google Scholar]
- 60. Roussel M, Bendahan D, Mattei JP, Le Fur Y, Cozzone PJ. 31P magnetic resonance spectroscopy study of phosphocreatine recovery kinetics in skeletal muscle: the issue of intersubject variability. Biochim Biophys Acta 1457: 18–26, 2000 [DOI] [PubMed] [Google Scholar]
- 61. Roussel M, Mattei JP, Le Fur Y, Ghattas B, Cozzone PJ, Bendahan D. Metabolic determinants of the onset of acidosis in exercising human muscle: a 31P-MRS study. J Appl Physiol 94: 1145–1152, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Smith D, Green H, Thomson J, Sharratt M. Capillary and size interrelationships in developing rat diaphragm, EDL, and soleus muscle fiber types. Am J Physiol Cell Physiol 256: C50–C58, 1989 [DOI] [PubMed] [Google Scholar]
- 63. Tamaki N. Effect of growth on muscle capillarity and fiber type composition in rat diaphragm. Eur J Appl Physiol Occup Physiol 54: 24–29, 1985 [DOI] [PubMed] [Google Scholar]
- 64. Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child 51: 170–179, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Taylor DJ, Kemp GJ, Thompson CH, Radda GK. Ageing: effects on oxidative function of skeletal muscle in vivo. Mol Cell Biochem 174: 321–324, 1997 [PubMed] [Google Scholar]
- 66. Taylor DJ, Styles P, Matthews PM, Arnold DA, Gadian DG, Bore P, Radda GK. Energetics of human muscle: exercise-induced ATP depletion. Magn Reson Med 3: 44–54, 1986 [DOI] [PubMed] [Google Scholar]
- 67. Thomas C, Perrey S, Lambert K, Hugon G, Mornet D, Mercier J. Monocarboxylate transporters, blood lactate removal after supramaximal exercise, and fatigue indexes in humans. J Appl Physiol 98: 804–809, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Trenell MI, Sue CM, Kemp GJ, Sachinwalla T, Thompson CH. Aerobic exercise and muscle metabolism in patients with mitochondrial myopathy. Muscle Nerve 33: 524–531, 2006 [DOI] [PubMed] [Google Scholar]
- 69. Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129: 35–43, 1997 [DOI] [PubMed] [Google Scholar]
- 70. Walter G, Vandenborne K, Elliott M, Leigh JS. In vivo ATP synthesis rates in single human muscles during high intensity exercise. J Physiol 519: 901–910, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wilkerson DP, Jones AM. Influence of initial metabolic rate on pulmonary O2 uptake on-kinetics during severe intensity exercise. Respir Physiol Neurobiol 152: 204–219, 2006 [DOI] [PubMed] [Google Scholar]
- 72. Williams CA, Carter H, Jones AM, Doust JH. Oxygen uptake kinetics during treadmill running in boys and men. J Appl Physiol 90: 1700–1706, 2001 [DOI] [PubMed] [Google Scholar]
- 73. Yoshida T, Watari H, Tagawa K. Effects of active and passive recoveries on splitting of the inorganic phosphate peak determined by 31P-nuclear magnetic resonance spectroscopy. NMR Biomed 9: 13–19, 1996 [DOI] [PubMed] [Google Scholar]
- 74. Zafeiridis A, Dalamitros A, Dipla K, Manou V, Galanis N, Kellis S. Recovery during high-intensity intermittent anaerobic exercise in boys, teens, and men. Med Sci Sports Exerc 37: 505–512, 2005 [DOI] [PubMed] [Google Scholar]
- 75. Zanconato S, Buchthal S, Barstow TJ, Cooper DM. 31P-magnetic resonance spectroscopy of leg muscle metabolism during exercise in children and adults. J Appl Physiol 74: 2214–2218, 1993 [DOI] [PubMed] [Google Scholar]