Our discussion regarding the dominant mechanism responsible for hypertension calls to mind the famous poem, “The Blind Men and the Elephant,” by John Godfrey Saxe based on an ancient fable from India. In essence, the blind men described the elephant based on their specific encounter, thus concluding that the elephant was a wall, a spear, a snake, a tree, a fan, or a rope. The lesson is that our interpretations regarding a specific experience are very much dependent on how it presented itself. Because of my predoctoral and postdoctoral training experiences with Arthur Guyton (10) and the fact that my research has been highly focused on the cardinal role of the kidneys in the pathophysiology of hypertension, I support the concept that alterations in kidney function in hypertension are predominantly due to an inappropriately increased activity of the intrarenal renin-angiotensin system (RAS; Refs. 12, 17, 19, 21). While the nervous system is important (4, 5), I contend that chronic activation of the sympathetic nervous system is not the dominant contributor to hypertension, but may also contribute to the activation of the intrarenal RAS that is characteristic of many forms of hypertension (3, 19, 22).
Hypertension is characterized by increased peripheral vascular resistance due to increased vascular smooth muscle contractile activity and endothelial dysfunction (15, 29), but it is more likely that the vasculature is the victim rather than the culprit of the injurious processes that occur in hypertension (27). The nervous system regulates blood pressure by integrating signals coming from all parts of the body and sending neural signals to various organ systems. However, there is limited evidence that chronic increases in sympathetic activity serve as the dominant contributor to most forms of hypertension. Because the neurocentric view is being addressed by Esler, Lambert, and Schlaich (6), I will defer further consideration of this issue to them. Nevertheless, it is important to point out that the successful treatment of resistant hypertension using catheter-based renal sympathetic denervation was restricted to selected patients that were resistant to standard therapy (14).
Through its multiple actions, the kidneys exert a predominant role to regulate arterial pressure (10, 12, 19, 24). Importantly, transplantation studies have demonstrated that the hypertension follows the kidneys (9). There are many models of experimental hypertension but most of them involve procedures or genetic manipulations that activate the RAS (8, 11, 13, 22, 23). While enhanced activity of renal sympathetic nerves contributes to the magnitude of the hypertension, the hypertensive response is only attenuated and the increases in intrarenal ANG II are similar in denervated kidneys as in innervated kidneys (11). In chronic ANG II-infused rabbits, the hypertension was not altered by renal denervation and renal sympathetic nerve activity was not changed (2). Furthermore, there was a general augmentation of vascular reactivity leading to augmented depressor responses to ganglionic blockade, but there was not an increased renal sympathetic activity (18). These studies have not shown differences in renal sympathetic nerve activity among rabbits with various forms of hypertension (2, 18). Prior renal denervation did not blunt the hypertension, suggesting that the sympathetic nervous system does not exert a direct role in the development of ANG II-induced hypertension (18).
Derangements in kidney function that prevent maintenance of balance between salt excretion and salt intake vary from overt renal disease causing reduced excretory capability to transport derangements causing excess sodium reabsorption. Importantly, the studies of monogenetic diseases demonstrate that mutations associated with hypertension are consistently associated with altered tubular reabsorptive function (16). Liddle's syndrome, characterized by overactive amiloride sensitive sodium channels in the principal cells of collecting ducts, is a classic example (26). These single gene mutations demonstrate that transport alterations can cause hypertension independent of neural contributions. Although most forms of hypertension involve multiple gene effects, the final consequences are strikingly similar in that an inappropriate stimulation of tubular reabsorption leads to sodium retention and hypertension (19, 22, 26). Inappropriate activation of the intrarenal RAS is a very powerful hypertensinogenic mechanism because the pleiotropic actions of ANG II lead to increased renal vascular resistance, decreased renal blood flow, and glomerular filtration rate, increased aldosterone release and increased fractional sodium reabsorption at both proximal and distal nephron segments (12, 17, 20, 24, 30).
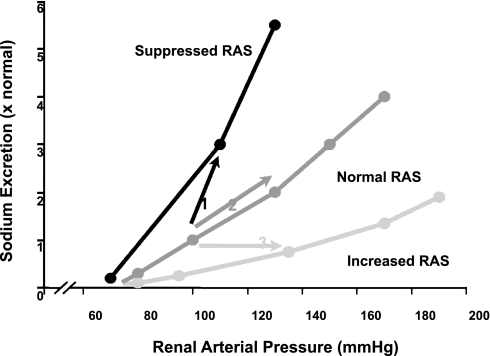
Excess salt retention may also be caused by derangements in neurohormonal communication pathways that maintain normal renal function (19). When inappropriately activated, however, these signals may alter renal hemodynamics and/or tubular transport to prevent appropriate sodium excretion at normal arterial pressure. The excess salt and volume retention leads to increased arterial pressure which then elicits a pressure natriuresis response (Fig. 2), allowing the kidneys to restore sodium homeostasis but at the cost of an elevated arterial pressure (19, 22).
Fig. 2.
Pressure-natriuresis relationships and the responses to increases in salt intake or activation of the intrarenal renin-angiotensin system (RAS). Each pressure natriuresis relationship represents the acute responses in sodium excretion to changes in arterial pressure with all other systems maintained. The suppressed relationship occurs when there is overactivation of sodium retaining mechanisms such as increased RAS. The steeper curve represents the relationship when sodium retaining mechanisms are suppressed or sodium excretory mechanisms are activated such as with RAS inhibition or increased ANP. In normal individuals, a high salt intake leads to a shift in the pressure-natriuresis curve represented as arrow 1 such that sodium excretion increases with only small increases in arterial pressure. When the RAS is not appropriately suppressed, a high salt intake must lead to an increased arterial pressure to maintain sodium balance represented as arrow 2. If there is an augmented activation of the RAS, then even normal salt intake may require an increase in blood pressure to allow maintenance of sodium balance as depicted by arrow 3. While many other systems can modulate the magnitude of the responses, the final outcome always has to be the blood pressure where sodium balance can be maintained.
The RAS has a powerful role because it is both an intrarenal system and a powerful extrarenal system that influences essentially every organ system (20, 24). In addition to the liver, the proximal tubular cells also produce abundant angiotensinogen that help maintain intratubular and renal interstitial ANG II concentrations greater than those in the systemic circulation (12, 25, 28). Furthermore, renin is formed in cells of the juxtaglomerular apparatus and also in principal cells of the collecting ducts, allowing secretion into the tubular fluid to form ANG I from angiotensinogen derived from the proximal nephron (23–25). Thus, the ANG II-mediated increased vascular resistance along with increased tubular reabsorption mediated by an augmented intratubular RAS leads to a sustained decreased sodium excretion and hypertension (10, 19).
An enhanced intrarenal RAS leads to enhanced ANG II throughout the body, thus contributing to increased cardiac contractility, increased peripheral vascular resistance, increased aldosterone release, and hypertensinogenic actions throughout the body (3, 12). Importantly, the pressure natriuresis mechanism, which is responsible for restoring sodium balance when there is inappropriate salt retention (10), does not escape the powerful influence of an augmented RAS (17, 19). As depicted in Fig. 2, augmented intrarenal ANG II levels suppress sodium excretion at any arterial pressure (17, 19). Nevertheless, with sufficient increases in arterial pressure, the increases in sodium excretion restore sodium balance, but again only at the expense of an elevated arterial pressure. The dominant role of the RAS in hypertension is reflected by the fact that blockade of the RAS with ACE inhibitors, ARBs, or the newer renin inhibitor is rapidly being recognized as one of the most effective antihypertensive therapeutic strategies (1, 7).
Conclusion.
In summary, many interacting physiological systems provide homeostatic regulation of arterial pressure, and derangements in any one of them can contribute to hypertension. While the nervous system provides regulatory inputs and stability to the blood pressure mechanisms, the primary responsibility for the long-term regulation of arterial pressure is vested in the kidneys' capability to integrate endocrine, neural, and hemodynamic inputs to maintain sodium balance and arterial blood pressure. Of the many mechanisms contributing to these alterations, the RAS axis plays a most vital role in regulating both sodium balance and blood pressure through its pleotropic actions on multiple vascular, endocrine, and renal mechanisms. Accordingly, it is the intrarenal/intratubular RAS that is the final dominant arbiter responsible for hypertension!
GRANTS
The author's research has been supported by grants from National Heart, Lung, and Blood Institute, National Institute of Diabetes and Digestive and Kidney Diseases, American Heart Association, and NCRR.
ACKNOWLEDGMENTS
I thank Debbie Olavarrieta for assistance in the preparation of the manuscript.
REFERENCES
- 1. Bakris GL, Toto RD, McCullough PA, Rocha R, Purkayastha D, Davis P. Effects of different ACE inhibitor combinations on albuminuria: results of the GUARD study. Kidney Int 73: 1303–1309, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Burke SL, Evans RG, Moretti JL, Head GA. Levels of renal and extrarenal sympathetic drive in angiotensin II-induced hypertension. Hypertension 51: 878–883, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 103: 17985–17990, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davisson RL, Yang G, Beltz TG, Cassell MD, Johnson AK, Sigmund CD. The brain renin-angiotensin system contributes to the hypertension in mice containing both the human renin and human angiotensinogen transgenes. Circ Res 83: 1047–1058, 1998 [DOI] [PubMed] [Google Scholar]
- 5. DiBona GF. The sympathetic nervous system and hypertension: recent developments. Hypertension 43: 147–150, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Esler M, Lampber E, Schlaich M. Point: Chronic activation of the sympathetic nervous system is the dominant contributor to systemic hypertension. J Appl Physiol; doi:10.1152/japplphysiol.00182.2010 [DOI] [PubMed] [Google Scholar]
- 7. Fisher ND, Hollenberg NK. Renin inhibition: what are the therapeutic opportunities? J Am Soc Nephrol 16: 592–599, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Gonzalez-Villalobos RA, Seth DM, Satou R, Horton H, Ohashi N, Miyata K, Katsurada A, Tran DV, Kobori H, Navar LG. Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin II-infused mice. Am J Physiol Renal Physiol 295: F772–F779, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guidi E, Menghetti D, Milani S, Montagnino G, Palazzi P, Bianchi G. Hypertension may be transplanted with the kidney in humans: a long-term historical prospective follow-up of recipients grafted with kidneys coming from donors with or without hypertension in their families. J Am Soc Nephrol 7: 1131–1138, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Guyton AC. Blood pressure control—special role of the kidneys and body fluids. Science 252: 1813–1816, 1991 [DOI] [PubMed] [Google Scholar]
- 11. Ichihara A, Inscho EW, Imig JD, Michel RE, Navar LG. Role of renal nerves in afferent arteriolar reactivity in angiotensin-induced hypertension. Hypertension 29: 442–449, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension 43: 1126–1132, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 373: 1275–1281, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 40: 511–515, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell 104: 545–556, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Mitchell KD, Braam B, Navar LG. Hypertensinogenic mechanisms mediated by renal actions of renin-angiotensin system. Hypertension 19, Suppl I: I-18–I-27, 1992 [DOI] [PubMed] [Google Scholar]
- 18. Moretti JL, Burke SL, Evans RG, Lambert GW, Head GA. Enhanced responses to ganglion blockade do not reflect sympathetic nervous system contribution to angiotensin II-induced hypertension. J Hypertens 27: 1838–1848, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Navar LG, Hamm LL. The kidney in blood pressure regulation. In: Atlas of Diseases of the Kidney. Hypertension and the Kidney, edited by Wilcox CS. Philadelphia: Current Medicine, 1999, p. 1.1–1.22 [Google Scholar]
- 20. Navar LG, Harrison-Bernard LM, Imig JD, Mitchell KD. Renal actions of angiotensin II at AT1 receptor blockers. In: Angiotensin II Receptor Antagonists, edited by Epstein M, Brunner HR. Philadelphia: Hanley & Belfus, 2000, p. 189–214 [Google Scholar]
- 21. Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension 39: 316–322, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Navar LG, Ploth DW. Pathophysiology of renovascular hypertension. In: Hypertension Primer: The Essentials of High Blood Pressure, edited by Izzo JL, Black HR, Sica DA. Philadelphia: Lippincott Williams & Wilkins, 2008, p. 162–165 [Google Scholar]
- 23. Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE, Navar LG. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip Goldblatt hypertensive rats. Hypertension 51: 1590–1596, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prieto-Carrasquero MC, Kobori H, Navar LG. The intrarenal renin-angiotensin system. In: Hypertension and Hormone Mechanisms, edited by Carey RM. Totowa, NJ: Humana, 2007, p. 3–22 [Google Scholar]
- 25. Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension 34: 1265–1274, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Rossier BC, Schild L. Epithelial sodium channel: Mendelian versus essential hypertension. Hypertension 52: 595–600, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Suzuki Y, Mezzano S, Plaza JJ, Egido J. Role of the renin-angiotensin system in vascular diseases: expanding the field. Hypertension 38: 1382–1387, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Schunkert H, Ingelfinger JR, Jacob H, Jackson B, Bouyounes B, Dzau VJ. Reciprocal feedback regulation of kidney angiotensinogen and renin mRNA expressions by angiotensin II. Am J Physiol Endocrinol Metab 263: E863–E869, 1992 [DOI] [PubMed] [Google Scholar]
- 29. Touyz RM. The role of angiotensin II in regulating vascular structural and functional changes in hypertension. Curr Hypertens Rep 5: 155–164, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Zhao D, Seth DM, Navar LG. Enhanced distal nephron sodium reabsorption in chronic angiotensin II-infused mice. Hypertension 54: 120–126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]