Abstract
Specific ventilation (SV) is the ratio of fresh gas entering a lung region divided by its end-expiratory volume. To quantify the vertical (gravitationally dependent) gradient of SV in eight healthy supine subjects, we implemented a novel proton magnetic resonance imaging (MRI) method. Oxygen is used as a contrast agent, which in solution changes the longitudinal relaxation time (T1) in lung tissue. Thus alterations in the MR signal resulting from the regional rise in O2 concentration following a sudden change in inspired O2 reflect SV—lung units with higher SV reach a new equilibrium faster than those with lower SV. We acquired T1-weighted inversion recovery images of a sagittal slice of the supine right lung with a 1.5-T MRI system. Images were voluntarily respiratory gated at functional residual capacity; 20 images were acquired with the subject breathing air and 20 breathing 100% O2, and this cycle was repeated five times. Expired tidal volume was measured simultaneously. The SV maps presented an average spatial fractal dimension of 1.13 ± 0.03. There was a vertical gradient in SV of 0.029 ± 0.012 cm−1, with SV being highest in the dependent lung. Dividing the lung vertically into thirds showed a statistically significant difference in SV, with SV of 0.42 ± 0.14 (mean ± SD), 0.29 ± 0.10, and 0.24 ± 0.08 in the dependent, intermediate, and nondependent regions, respectively (all differences, P < 0.05). This vertical gradient in SV is consistent with the known gravitationally induced deformation of the lung resulting in greater lung expansion in the dependent lung with inspiration. This SV imaging technique can be used to quantify regional SV in the lung with proton MRI.
Keywords: functional magnetic resonance imaging, respiration, gravity, oxygen-enhanced magnetic resonance imaging
specific ventilation (SV) is the ratio of the volume of fresh gas (ΔV) moving into a region of the lung to the end-expiratory volume (V0) of that region, SV = ΔV/V0. SV is thus a dimensionless quantity that provides a measure of how efficiently a given lung region is ventilated. Regional SV is an important metric from a physiological standpoint (14, 16, 23).
Three approaches have been used in the past to quantify the distribution of SV in humans. 1) Inhalation of radioactive 133Xe gas (or other tracers) yields information on the spatial distribution of ventilation; however, the radiation dose limits the applicability of this method in repeated-measurement studies (14, 22). 2) Using multiple-breath nitrogen washouts, Lewis et al. (16) described a method to estimate the distribution of SV; however, this method does not yield spatial information. 3) More recently, magnetic resonance imaging (MRI) using hyperpolarized gases (129Xe and, more commonly, 3He) has been used to quantify ventilation (1, 17, 21). MRI measurements of hyperpolarized gas provide good spatial resolution and do not require ionizing radiation, but they have several disadvantages: 3He is a rare gas; 129Xe, although more readily available, is soluble in blood and has anesthetic properties; and hyperpolarization requires dedicated hardware and a specific 3He (or 129Xe)-dedicated MR imaging coil, different from those used for standard proton (1H) MRI. All of these factors limit its generalization to routine use.
With proton MRI, it has been shown that inhaled oxygen (O2) can be used as a contrast agent to verify the presence or absence of ventilation (5). Oxygen is weakly paramagnetic, and when in solution in lung tissues it produces a measurable decrease in the longitudinal relaxation time (T1) of tissues, increasing the signal intensity of an appropriately timed inversion recovery proton MR image. A small body of work has been published on oxygen-enhanced ventilation, mainly focusing on optimizing MRI acquisition and postprocessing of image data (3, 18–20, 27) and on detecting ventilatory defects (25, 28, 29) (reviewed recently in Ref. 26). These publications demonstrate the use of O2 as a contrast agent in the lung and have shown the ability to detect ventilation defects. However, O2-enhanced MRI signal contains more information than just the presence or absence of ventilation. The change in O2 concentration in lung tissues (determining the local change in T1) depends on the rate of change of the alveolar O2 concentration, which is a function of the regional SV (16). Thus measurement of the regional rate of change of the MRI signal allows the quantification of SV: after a change in inspired fractional O2 content (FiO2), units that have higher SV reach the new equilibrium faster than units that have a lower SV. Thus the rapidity of the change in the MRI signal in a particular voxel or region following a change in FiO2 is a quantitative measure of the SV of that portion of the lung.
Previous studies (14, 23) have shown a vertical gradient in SV, i.e., SV is gravity dependent, with the dependent lung being better ventilated (higher SV) than the nondependent lung. We hypothesized that we could measure SV with proton MRI by using O2 as a contrast agent. With this approach we have mapped the distribution of SV in the lung and, as a test case, its gravitationally dependent gradient.
METHODS
Quantification of Specific Ventilation: Basis of Measurement
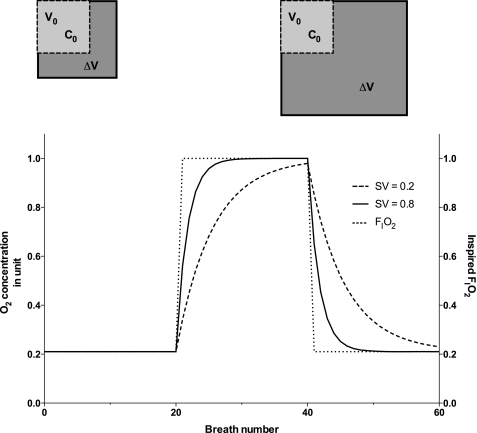
After a sudden change in inspired fraction of O2 (FiO2), the rate of change of the alveolar O2 concentration is a function of the local SV: lung units with higher SV reach a new equilibrium faster than units with a lower SV. Therefore, the time it takes to reach a new equilibrium is a measure of the local SV. This time is reflected in the MR images. For a series of inversion recovery images acquired with appropriate scanning parameters, the signal intensity change observed after a change in inspired gas is determined by the change in T1, a change driven by the local amount of O2 in solution, which is a function of the local O2 partial pressure. Therefore, the time course of the regional MR signal intensity resulting from a change in FiO2 reflects local SV. More specifically, the rise time of the MRI signal intensity following the onset of O2 inhalation—the time required for the signal to change to a new equilibrium level—is directly related to SV (Fig. 1).
Fig. 1.
Specific ventilation (SV) of a simple unit: V0 is the end-expiratory volume of the unit and ΔV the volume increase that occurs during inspiration. C0 is the initial concentration at end expiration. SV is defined as the ratio ΔV/V0. Top left: schematic view depicts a low-SV unit. Top right: a unit with higher SV. Bottom: simulated response of both units to a sudden change in inspired O2 fraction (FiO2; dotted line). The high-SV unit equilibrates faster (continuous line, SV = 0.8) than the lower-SV unit (dashed line, SV = 0.2).
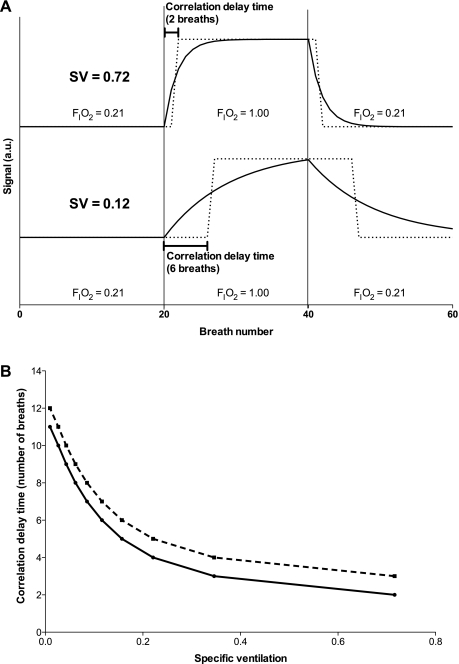
As an empirical measure of the rise time, we used the time delay that maximizes the cross-correlation of the measured signal time course from an image voxel with a square wave representing the onset of increased inspired O2 (dashed lines, Fig. 2A), and we refer to this as the correlation delay time. This approach is illustrated in Fig. 2A. Mathematically, the cross-correlation is maximized when the response is shifted by about half the rise time, so that different rise times effectively translate to different delays in the cross-correlation analysis, allowing us to use the correlation delay time as an empirical index of the rise time. This calculation is done for the entire time series corresponding to each image voxel. (Note that dead space in the plumbing leading from the gas containers to the subject introduces a true global delay, but this delay is calculated from the geometry and flow rate of the delivery system and eliminated before the cross-correlation analysis.) To relate the correlation delay time to SV, we modeled the experiment with a simple lung unit and simulated the signal changes during the series of consecutive breaths following a sudden change in FiO2. The simulated data for units with different SVs were then analyzed with the identical cross-correlation method used for the experimental data to derive a calibration curve of SV versus correlation delay time. This calibration curve was used to convert the measured correlation delay times into a quantitative estimate of SV for each image voxel (Fig. 2B). The details of these steps are described below.
Fig. 2.
A: correlation delay time for 2 simulated units with different SV (SV = 0.72 and SV = 0.12, continuous lines). Vertical lines (breaths 20 and 40) represent changes in inspired gas. The shifted driving function that maximizes the correlation with each unit is represented (square wave, dashed line). The integer amount of breath by which the driving function is shifted so as to maximize correlation is the correlation delay time (2 breaths for SV = 0.72 and 6 breaths for SV = 0.12). Only the first 60 breaths are represented here, yet the entire series (220 breaths) was used in the computation of the correlation delay time. B: translation function from measured correlation delay time (y-axis) to SV for 2 different values of delay due to plumbing—zero delay (continuous line) and 1-breath delay (dashed line). The addition of a plumbing delay resulted, as expected, in a parallel vertical displacement of the curve.
Model and Simulated Data
To translate the MRI signal rise time into a quantification of SV, we constructed a model of a lung unit corresponding to the voxel size measured during the MRI imaging (see below). The only variable of interest in the model is the rate of equilibration—the rise time—and not the equilibrium values. Therefore, dead space, water vapor, and end-tidal Pco2, factors that change the steady-state equilibrium alveolar O2 partial pressure (PaO2) but remain largely constant during the experiment, are ignored in the following description. The model describes solely the temporal behavior of the O2 concentration from the air-breathing equilibrium value to the new 100% O2 steady state.
Different ventilation-perfusion ratios (V̇/Q̇) result in different steady-state O2 concentrations, for both inspired air (∼range 50–140 mmHg, for V̇/Q̇ between 0.001 and 10) and 100% inspired O2 (∼550–600 mmHg, for the same range of V̇/Q̇) (37). These steady-state equilibrium conditions provide adequate (and essentially similar) contrast for all reasonable V̇/Q̇. Furthermore, our model is independent of the equilibrium or steady-state conditions—it depends solely on the rate of equilibration. Two lung units presenting identical V̇/Q̇ and different SVs are still distinguishable from each other by the different rates of equilibration, with the unit presenting a higher SV having a faster turnover, and thus equilibrating faster.
The two assumptions in this model are that 1) voxel dimension (1.6 × 1.6 mm in the imaging plane, for a 15-mm-thick slice, volume ∼40 mm3) is small enough such that concentrations inside the unit at end expiration can be considered uniform, and therefore each voxel can be treated as a single ventilatory unit and 2) the time interval between two consecutive acquired images (>5 s) is long enough so that equilibrium between oxygen in the gas phase and in solution in blood and tissues is attained by end expiration. The first of these assumptions is supported by simulation work by Paiva (30), Davidson (4), and Engel (7). Using either continuous (trumpet model) or discrete (asymmetric branch point) models of the acinus, these approaches have shown that at the scale of our resolution O2 concentration would be expected to be constant within a single respiratory pathway. Simulations in asymmetric branch point models suggest that differences in O2 concentration can persist among parallel respiratory units, yet within each unit the O2 concentration gradient is essentially abolished, matching our assumption.
Subjects self-gate their breathing to the 5-s interval between consecutive image acquisitions. Oxygen dissolves in tissues very rapidly compared with this 5-s interval between images. For instance, for a normal, room air inspired breath, it takes ∼0.25 s for oxygen to diffuse through a 0.5-μm-thick capillary wall and reach its equilibrium with hemoglobin in the pulmonary capillary (36), a process that includes dissolving in tissue as well as other processes. The process of simple dissolution in tissue thus is assumed to be complete within this same time frame, as in our second assumption.
Each voxel is simulated as a single ventilatory unit, with end-expiratory volume V0, to which inspiration transiently adds ΔV during each breath (Fig. 1). The initial concentration in the unit at end expiration is denoted C0, and Cn (n = 1, 2, 3, … ) denotes the concentration in the unit at end expiration after breath n.
Let Cinspn denote the concentration of oxygen in the inspired gas for breath n; the subjects are breathing air (FiO2 = 0.21) during the first 20 breaths and are then changed to a gas mixture enriched in oxygen (in our experiment, FiO2 = 1.0).
At the end of the first breath following the switch in FiO2 the concentration in the unit is:
| (1) |
The same reasoning can be used to establish a recursive formula for concentration after n breaths:
| (2) |
which in turn can be rewritten as a function of the unit's SV, with SV = ΔV/V0, as:
| (3) |
This equation is used to model oxygen concentration. A simulated response for an individual voxel is obtained, for a given set of FiO2 breaths (dotted line in Fig. 1). Figure 1 presents the time series of two units, one with low SV (SV = 0.2) and a second with high SV (SV = 0.8).
A linear relationship between R1 = 1/T1 and FiO2 has been consistently reported (13, 33). The slope and zero-crossing values reported for the R1-FiO2 function (13) result in a almost linear relationship between T1 and FiO2 in the range used in the present experiment (FiO2 = 0.21 or 1.0).
On the basis of numerical simulations, for the inversion time of our experiment (TI = 1,000 ms) and the variation of T1 with inspired oxygen concentration reported by Jakob et al. (13), the error involved in assuming that the MR signal varies linearly with oxygen concentration is <3% for these studies. Our calibration curve is based on this assumption.
Time-Shifted Cross-Correlation Between Driving Function and Simulated Units
To determine the correlation delay time we computed the cross-correlation between each simulated ventilatory unit response (continuous and dashed lines, Fig. 1, bottom) and FiO2 (dotted line, Fig. 1, bottom). The FiO2 curve was shifted breath by breath, and the time shift that maximizes the time-shifted cross-correlation is a measure of how fast the unit equilibrates—correlation delay time (computed similarly to Ref. 32). This is illustrated, for two simulated units (SV = 0.12 and SV = 0.72), in Fig. 2, top: the continuous line depicts the unit response, and the dashed line depicts the time-shifted FiO2 curve that maximizes cross-correlation.
We simulated units with SV ranging from 0.05 to 1 in steps of 0.05, and the simulated signal was analyzed by using the time-shifted cross-correlation. The outcome of these simulations is shown in Fig. 2B. This figure is a conversion tool, allowing the translation of correlation delay time measured by using the proton-MRI acquired time series into quantitative values of ventilation, on a voxel-per-voxel basis. Figure 2B also shows that when SV is above 0.5 this analysis is incapable of determining differences in SV and lumps all ventilations above that threshold. This is because equilibration for these high-SV units happens sufficiently fast that the correlation delay time is less than 1 breath, and thus not resolvable. However, on the basis of previous studies, relatively few lung units have SV higher than 0.5 (16), and the presence of lung disease is typically associated with the development of regions of reduced SV (11), as opposed to increased SV, making this limitation of relatively minor significance.
Figure 2B also shows that, as expected, for a given measured correlation delay time (y-axis) the corresponding SV depends not only on the SV but also on an extrinsic delay resulting from plumbing volume in the inspiratory line (see Specific ventilation imaging protocol below). In essence, any volume that exists within the experimental configuration between the subject's mouth and the valve used to change between gases of different FiO2 introduces a delay that will appear to artificially reduce SV unless properly accounted for. This emphasizes the need to determine this delay by measuring respiratory flow and inspiratory plumbing volume.
Magnetic Resonance Imaging Data Collection
Subjects.
We studied eight healthy subjects (3 female, 5 male subjects) in the supine posture. Table 1 presents subject characteristics (sex, age, height, and weight) and pulmonary function data [forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and FEV1/FVC]. The Human Subjects Research Protection Program of the University of California, San Diego, approved this study, and subjects participated after giving written informed consent.
Table 1.
Subject characteristics, pulmonary function data, and slope of lung height-specific ventilation relationship for the 8 subjects
| Subject | Sex | Age, yr | Height, m | Weight, kg | FEV1, liters (% predicted) | FVC, liters (% predicted) | FEV1/FVC (% predicted) | −1/slope, cm−1 | Spatial Fractal Dimension |
|---|---|---|---|---|---|---|---|---|---|
| S1 | M | 33 | 1.81 | 81 | 3.99 (88) | 5.17 (93) | 0.77 (95) | 0.020 | 1.15 |
| S2 | M | 26 | 1.85 | 93 | 4.57 (92) | 5.28 (87) | 0.87 (105) | 0.015 | 1.15 |
| S3 | F | 26 | 1.73 | 68 | 4.13 (115) | 5.04 (118) | 0.82 (96) | 0.031 | 1.15 |
| S4 | F | 39 | 1.62 | 66 | 3.03 (101) | 3.65 (99) | 0.83 (101) | 0.038 | 1.09 |
| S5 | F | 24 | 1.76 | 92 | 3.08 (102) | 3.65 (99) | 0.84 (102) | 0.043 | 1.11 |
| S6 | M | 52 | 1.86 | 113 | 4.50 (105) | 5.36 (96) | 0.84 (109) | 0.046 | 1.10 |
| S7 | M | 34 | 1.78 | 83 | 3.84 (89) | 4.98 (93) | 0.77 (95) | 0.021 | 1.16 |
| S8 | M | 28 | 1.70 | 60 | 4.43 (107) | 5.16 (103) | 0.86 (104) | 0.019 | 1.13 |
| Mean ± SD | 33 ± 9 | 1.76 ± 0.08 | 82 ± 17 | 3.85 ± 0.61 (100 ± 10) | 4.79 ± 0.71 (99 ± 9) | 0.82 ± 0.04 (101 ± 5) | 0.029 ± 0.012* | 1.13 ± 0.03 | |
FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Group average significantly different from zero, P = 0.0002.
MRI.
Data were collected with a 1.5-T Signa HDx TwinSpeed MRI system (General Electric Medical Systems, Milwaukee, WI). A single sagittal slice was selected in the right lung, in order to avoid physiological noise arising from cardiac movements. Slice selection was aimed at selecting the slice within the lung presenting the largest anterior-posterior dimension, while avoiding major hilar vessels.
Specific ventilation imaging protocol.
Two-dimensional T1-weighted images were acquired with an inversion recovery (TI = 1,000 ms) single-shot fast spin echo (SSFSE) sequence, with images being acquired with a half-Fourier acquisition [half-Fourier acquisition single-shot turbo spin echo (HASTE)], with a 40 × 40-cm field of view, echo time of ∼30 ms, and a 15-mm image slice thickness. A MRI homodyne reconstruction algorithm rescaled the data to a 256 × 256 matrix, with each voxel thus corresponding to ∼1.6 × 1.6 × 15 mm (∼40 mm3). A TI of 1,000 ms, approximating T1 of lung tissue, ensured maximal sensitivity to changes in O2 concentration (3). The long repetition time used in this SSFSE sequence (5 s, compared with the T1 of blood, 1.4 s) renders the O2-induced contrast independent of lung density; however, lung density does alter the local signal-to-noise ratio. HASTE acquisition was utilized to keep the echo time short to minimize signal loss due to the very short transverse relaxation time (T2*) observed in the lung (9, 34). Images were voluntarily respiratory gated. Subjects were instructed to take a normal breath in following the noise made by the MR image acquisition, and relax back to functional residual capacity (FRC), at a comfortable expiratory flow rate. All our subjects were comfortable with the default 5-s interbreath intervals (12 breaths/min). Images were acquired during a short (approximately hundreds of milliseconds), postexpiratory breath hold at FRC, resulting in a relatively natural respiratory maneuver only constrained by a constant breathing rate.
The addition of O2 as a contrast agent followed a block-design functional MRI (fMRI) approach, typically utilized for neuroimaging studies. A lung image was acquired every 5 s, with 20 images acquired with the subject inspiring air (21% oxygen), and 20 images with the subject inspiring 100% O2. This cycle was repeated five times, and an additional 20 breaths of 100% oxygen were added at the end of the last cycle, making a total of 220 images (total imaging time was 18 min 20 s). Blocks of 20 breaths of air and 100% oxygen were chosen to ensure an approach to full equilibration, for the ranges of SV and V̇/Q̇ observed in normal healthy subjects (16, 35), in accordance with what has been observed in prior multiple-breath washout studies (31). Five cycles of air-oxygen provided an acceptable signal-to-noise ratio and were implemented as a compromise between keeping the total acquisition time below 20 min and an improved signal-to-noise ratio that would result from a longer sequence.
A face mask (Hans Rudolph; dead space 73–113 ml, depending on mask size) equipped with a nonrebreathing T valve (dead space 27.9 ml) was fitted to the subject. One end of the T valve was connected to the inlet, where a remote-controlled three-way pneumatic sliding valve (Hans Rudolph model 8500) allowed rapid switching between room air and O2 contained in a 170-liter Douglas bag (Hans Rudolph type 6170). The inspiratory path resistances on the room air and O2 circuits were matched to eliminate changes in FRC following changes in inspiratory path. The outlet of the T valve was connected to a ∼6-m-long large-bore low-resistance expiratory line, leading out of the scanner room, where expired tidal volume was simultaneously measured with a ParvoMedics Metabolic Measurement System (ParvoMedics, Sandy, UT).
The experimental configuration for controlling the inspired gas (room air, 100% oxygen) introduced an extrinsic plumbing delay in the inspired signal that is determined by the volume of tubing that connects the remote control valve to the subject. This volume was made as small as possible (0.6 l) under constraints imposed by working in an MRI environment. This delay can be computed from each subject's tidal volume and the tubing volume, by dividing the tubing volume by the tidal volume. In practice, this extrinsic delay was taken into account by computing individual inspired fractional oxygen concentration (FiO2) time series on a breath-by-breath basis (Cinspn in Eq. 3), taking the tubing volume as a delay chamber of fixed volume through which the inspired gas must pass. Once this extrinsic plumbing delay is corrected for, what is left corresponds to the signal intensity change over time, allowing the computation of SV as described in Specific ventilation imaging data analysis.
Specific ventilation imaging data analysis.
A time series of the 220 images corresponding to the specific ventilation imaging (SVI) sequence was constructed. Quality control of the acquired image was performed at this stage, and images where the subject was not at FRC based on diaphragm position compared with adjacent images were removed from the series and replaced by an interpolated image constructed from the preceding and following images. A region of interest was manually drawn, and the subsequent analyses were restricted to the voxels inside the lung. The region of interest encompassed the entire lung, yet avoided partial volume effects from regions close to the chest wall and the diaphragm. Data analysis was performed on a voxel-by-voxel basis; the time course of each voxel—MRI signal intensity versus time—was used to compute the regional correlation delay time. All data analysis was performed with Matlab (Mathworks, Natick, MA).
We computed the correlation delay time, using a shifted cross-correlation between the FiO2 and the acquired signal intensity time series. The time-shifted cross-correlation is a fast and simple approach to implement, fitting only one parameter, the correlation delay time, and is independent of the asymptotic signal intensity. By removing this extra degree of freedom, the model can remain simple while capturing only the time course of the transition, leaving out the physiological dead space, water vapor, CO2 concentration, and any effect of varying V̇/Q̇, factors that alter the steady-state concentration but not the time course of the transition. Moreover, the shifted correlation delay approach acts as a smoothing low-pass filter, eliminating some of the high-frequency noise present in our data that did not allow a direct fit of the model (Eq. 3). In practice, the FiO2 (driving function) is correlated with the time course of each voxel, and this process is repeated for delayed versions of the driving function (delayed by an integer number of breaths). The integer delay that maximizes the cross-correlation between the time-shifted driving function and the actual voxel response is the correlation delay time. The correlation delay time computed in this way will only retain voxels whose correlation with the optimal shifted FiO2 was significant (P < 0.05) and the null hypothesis of no correlation rejected in these cases. In voxels in which the null hypothesis was accepted, the corresponding lung voxel was attributed no specific ventilation value and treated hereafter as missing data.
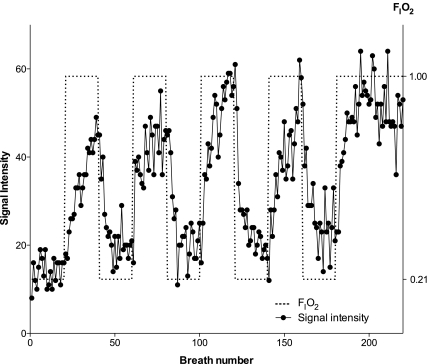
Figure 3 (filled circles, continuous lines) shows the time course of signal intensity for one such voxel. The arbitrarily chosen voxel plotted was located in the dependent portion of the lung. The corresponding driving function representing FiO2 is also shown (Fig. 3, dotted line). The delay between the driving function and the measured signal intensity is a measure of the correlation delay time for that voxel.
Fig. 3.
Time series of signal intensity for a single voxel. When the subject changed from breathing air to oxygen, signal intensity increased. FiO2 is represented by the dashed line. Once the extrinsic plumbing delay has been accounted for, the correlation delay time (expressed in number of breaths) between the FiO2 driving function and each voxel's time series is a quantitative measure of ventilation. Units with higher SV equilibrate faster; thus signal intensity increases faster, and correlation delay time is shorter, than for units with lower SV. With application of the modeling and signal-processing algorithm described in methods, different correlation delay times were converted into a physiologically meaningful measure of SV. The correlation delay time for this voxel was 2 breaths, corresponding to a SV of 0.35 (P < 0.0001).
Statistics
Each series of 220 breaths was considered as a single measure of SV, computed as described above, creating for each subject one map of SV. All the voxels presenting a statistically significant (P < 0.05) correlation with the optimally shifted drive function were included in the analysis. The remaining voxels were treated as missing data. Two different analyses were implemented: a comparison of SV by lung thirds and a finer analysis, on a centimeter-per-centimeter basis moving up the supine lung.
For the lung thirds analysis, SV was partitioned into three gravitational regions, corresponding to thirds of the lung based on equal vertical extent: the dependent portion, the intermediate region, and the nondependent region. The data were reduced to a subject-by-subject average SV per lung gravitational region. One-way repeated-measures ANOVA was used to compare the gravitational gradient in SV across the lung regions (3 levels: dependent, intermediate, nondependent). Where overall significance was present, post hoc testing was conducted with Student's t-test, to determine where this significance occurred.
In a second analysis, linear regression was used to evaluate the linear relationship between the vertical height of the lung and SV, by dividing the data into isogravitational slices of 1-cm thickness (∼6 vertical voxels). As subjects were studied in the supine position, the vertical height of the lung (isogravitational level) was measured along the anterior-posterior axis, with zero height corresponding to the most dependent isogravitational voxels. The linear relationships were evaluated individually for each subject. The intersubject averaged SV versus lung height values correspond to averages over vertical 1-cm regions for the eight subjects. Different subjects have different anterior-posterior lung dimensions; therefore, results are reported only for lung heights that included data from all eight subjects. The slope of the individual relationships between height and SV were compared with a zero slope by a one-group t-test.
All data are presented as means ± SD. When data for the eight subjects were averaged, SD corresponds to the intersubject variability. When relative dispersion is calculated, spatial SD refers to the intervoxel variability within a SV map. Spatial fractal dimension, a scale-independent index of spatial heterogeneity, was also computed for each SV map (8). The null hypothesis (no effect) was rejected when P < 0.05, two tailed, except where otherwise indicated. All statistical analyses were performed with Prism (GraphPad, San Diego, CA).
RESULTS
General Data
Subject descriptive data and pulmonary function measurements are presented in Table 1. The subjects studied had normal spirometry, as indicated by an average FEV1 = 100 ± 10% predicted, FVC = 99 ± 9% predicted, and FEV1/FVC = 101 ± 5% predicted (Table 1). Subjects maintained a relatively constant expired tidal volume throughout the experiment, averaging 0.87 ± 0.18 liters. Heart rate during SVI data acquisition averaged 65 ± 9 beats/min (no difference between inspiring air and 100% oxygen), and arterial oxygen saturation (SaO2) measured by pulse oximetry was 97.4 ± 1.7% during breathing of room air and increased to 97.8 ± 1.5% during inspiration of 100% oxygen. The selected breathing rate was followed by all subjects, and tidal volume was relatively stable throughout the 220 breaths [the average coefficient of variation (CV) for the 8 subjects was 16%]. Tidal volume did not change when comparing breaths taken while inspiring air and oxygen (average over 8 subjects: 0.86 ± 0.15 vs. 0.87 ± 0.17 l; 2-tailed t-test paired comparison, P = 0.82).
Specific Ventilation Imaging
Figure 3 presents the time series of a voxel (continuous line) together with the inspired FiO2 (dotted line) for a voxel in the dependent lung region. Five repetitions of a block of 20 air breaths and 20 100% oxygen breaths were performed. Signal intensity increased when the inspired line was changed from air to 100% oxygen and decreased for the opposite change, from oxygen back to room air. After the five cycles of air-100% oxygen, 20 additional breaths on 100% oxygen were added. Noise, expressed as the CV of the steady-state signal (first 20 breaths on air and last 20 breaths inspiring O2) averaged over the entire lung of the eight subjects studied, was 22.8 ± 1.5% for air and 18.4 ± 1.8% for oxygen. To test the robustness of the algorithm we have run the model with similar levels of random noise. The average SV estimation error introduced by 20% noise levels is extremely small (average error in estimating SV ∼0.003); the maximal SV estimation error was ∼0.013 and was observed for very low-SV units (SV < 0.02), below the normal healthy range (16).
The convergence of the data processing algorithm to a solution was tested by using simulated data with 25% added noise (consistent with the experimental data). The data processing algorithm estimated SV by using an increasing number of air-oxygen cycles (first 40, 80, 120, 160 breaths, corresponding to 1, 2, 3, and 4 cycles, respectively). These estimations were compared with the best estimation of SV, obtained by using the entire 220-breath simulated time series. When the analysis was restricted to the first 40 breaths 51.6% of simulated units had converged to their best estimation. As expected, as the number of cycles increased, so did the number of units having converged: 90.9% for 2 cycles, 97.7% for 3 cycles, and 98.8% when the first 160 breaths were used for the analysis (4 cycles). The number of units presenting a significant correlation with the driving function increased as the analysis was extended to an increasing number of cycles.
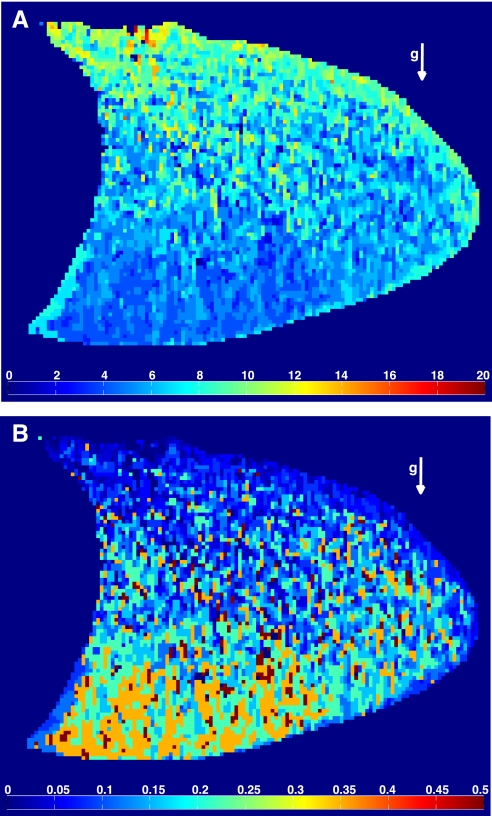
From each individual voxel response, a map of the correlation delay time for all voxels within the lung was computed and is presented for a representative subject in Fig. 4A. The arrow indicates the direction of gravity; the head is located to the right of the image, and the diaphragm to the left. In Fig. 4A, warmer colors represent portions of the lung with a longer correlation delay time. The extrinsic delay resulting from the inspiratory plumbing was accounted for before the analysis. With the results presented in Fig. 2B, this correlation delay time was then translated into a quantitative measure of SV (Fig. 4B). In Fig. 4B, warmer colors represent regions of the lung with higher SV. Average SV in a slice of the right lung, averaged over all subjects, was 0.33 ± 0.11. Overall SV heterogeneity, as measured by the relative dispersion, averaged 0.63 ± 0.11 (individual range 0.50–0.79), comparable to that previously reported for perfusion (12). The average spatial fractal dimension of the SV maps was 1.13 ± 0.03 (individual range 1.09–1.16; Table 1), similar to that derived for perfusion maps (15).
Fig. 4.
A: correlation delay time map (in number of breaths) in a sagittal slice of the right lung of a typical subject. B: corresponding SV map, using the “translation” shown in Fig. 2B. In this plane, the head is located on right and the diaphragm on left. The vector g indicates the direction of gravity. The subject was supine. Note the shorter correlation delay time in the dependent regions (A), which indicates a greater SV in these regions than in the nondependent lung (B).
On average (over all subjects), 3.7% of the lung failed to pass the null hypothesis test in the shifted correlation (individual range 0.1–17.8%; only a single subject had >5% of voxels), showing no statistically significant changes with changes in FiO2. These voxels were excluded from the subsequent analysis.
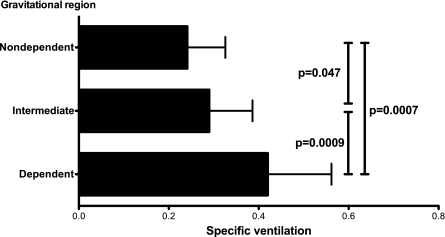
In Fig. 4B a clear vertical (gravitational) gradient of SV is present. Units in the dependent portions of the lung have higher SV than those in the nondependent portions of the lung. This relationship is quantified in Fig. 5, where results for the regional dependence of SV over the eight subjects bring out such a relationship: a statistically significant difference in SV was observed, with the most dependent third of the lung having a SV of 0.42 ± 0.14, the intermediate third 0.29 ± 0.10, and the nondependent third 0.24 ± 0.08 (all differences, P < 0.05).
Fig. 5.
SV per third of the lung, grouped by the vertical distance from the most dependent portion. ANOVA for repeated measures comparing all 3 regions showed a highly significant difference (F = 26.8, P < 0.0001). Post hoc testing (paired t-test) P values are shown (7 degrees of freedom, t values of 5.5, 2.4, and 5.8 for the dependent-intermediate, intermediate-nondependent, and dependent-nondependent region paired comparisons, respectively).
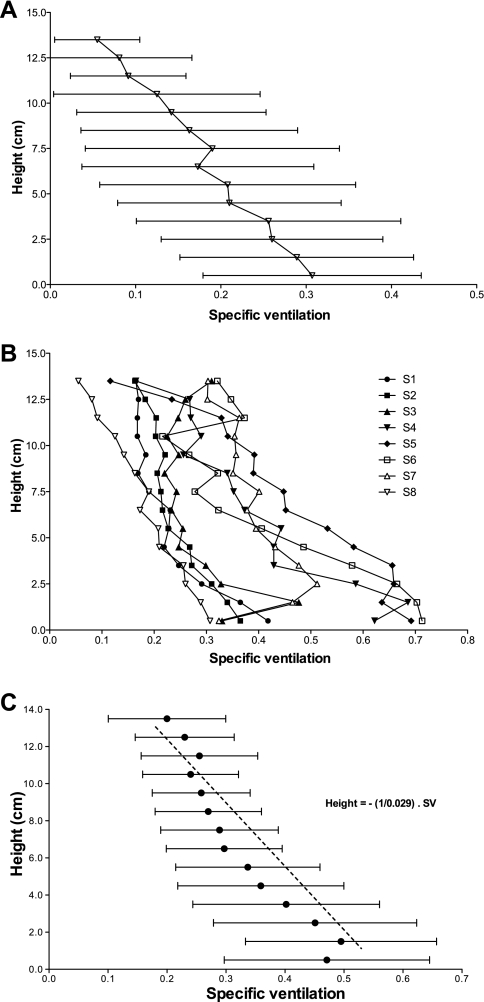
An approximately linear decrease in SV with height was observed, as evidenced in Fig. 6, where SV is plotted against height for one subject (Fig. 6A) and for the eight individual subjects (Fig. 6B). The average over all subjects is presented in Fig. 6C. The inverse of the slope of this line is a measure of the decrease in SV per centimeter. SV was found to decrease 0.029 ± 0.012 cm−1 (average slope over the 8 individual subjects). Subject-by-subject fitted slopes, reported in Table 1, showed a decrease in SV with increasing height ranging from 0.015 to 0.046 cm−1. The slope of the height-SV relationship was significantly different from a zero slope (P = 0.0002). An exception to the linearity is present in the most dependent portions of the lung (height < 1.0 cm). This most dependent portion of the lung had, on average, lower SV than the layers immediately above them; however, this difference failed to reach statistical significance.
Fig. 6.
SV averaged along the isogravitational lines, in 1-cm bins, plotted vs. the vertical distance from the most dependent portion of the lung for the right lung slice. Height increases up along the y-axis. As different subjects have different lung heights, data were only plotted when all the subjects contributed to the average. A: SV plotted against height of the lung for a single subject (S8), with horizontal error bars corresponding to the variability (SD) present in each isogravitational bin. B: individual height profiles of mean SV for the 8 subjects studied, each determined as in A. C: average height profiles of SV, with error bars corresponding to intersubject variability. The dashed line corresponds to the average slope determined by averaging the individual slopes of the 8 individual height-SV profiles (B).
Two independent SV maps of subject S1 were obtained, 20 days apart. A Bland-Altman analysis on the centimeter-per-centimeter SV data was performed; the average difference between the two runs was found to be 3.0%, with a 95% confidence interval of ±9.5%.
DISCUSSION
This study shows that with O2-enhanced proton MRI regional quantification of SV is possible, extracting more information from the method than the mere absence or presence of ventilation. From a physiological standpoint, SVI shows a clear gravitational gradient, with higher values seen in dependent lung as expected. The springlike self-deformation of the lung (23), the Slinky effect (12, 23), is likely the main determinant of the gravitational gradient observed in SV. However, in the most dependent third of the supine lung there are deviations from this overall behavior, suggesting important influences of other factors such as the nonlinearity of the pressure-volume curve of the lung, the large vessels in the lung, and dependent airways closure.
Vertical Gradient in Specific Ventilation
The measurements show a clear, and for the most part, linear vertical gradient in SV (Figs. 4 and 6). As expected, the dependent portions of the lung have a higher SV than the nondependent portions (Fig. 5). Because the dependent portion of the lung partially supports the weight of the upper portions, it is more “compressed,” and therefore its V0 is smaller. Furthermore, it is subject to a higher (less negative) pleural pressure than an equivalent nondependent region, therefore placing it on a steeper portion of the pressure-volume curve. Therefore, a given change in pleural pressure resulting from an inspiratory effort will result in a greater increase in volume. Both the lower initial (end-expiratory) volume and the increased change in volume would be expected to contribute to the higher SV observed in the dependent regions of the lung compared with the nondependent region (36).
Comparison with Published Specific Ventilation Distributions
Kaneko et al. (14), using a 133Xe technique, reported a vertical gradient of SV for three supine subjects (normal healthy subjects of similar age to the group studied here) of 0.020, 0.022, and 0.026 cm−1, in close accordance with the 0.029 cm−1 measured with our SVI technique.
Using two different krypton isotopes, Amis et al. (2) reported a vertical gradient of SV in three supine subjects and found a ∼45% decrease from the most dependent part of the lung to the most nondependent portion, in close accordance with the ∼53% decrease found in our data. Using PET, Musch et al. (24) reported a SV gradient relative to the mean, in the supine posture, of −4.5 ± 2.5%, slightly lower than the −8.4 ± 1.9% (range 5.3–10.2%) we observed in our group. The breathing pattern used for image acquisition in this particular study is not directly comparable to that presented here, for it starts with a 40-s breath hold, followed by ∼160 s of free breathing.
Prisk et al. (31) reported an average SV of 0.364 ± 0.028, in a population of four supine subjects somewhat older than those studied here (average age 43 yr compared with 33 yr in this study) and with tidal volume constrained to ∼650 ml. This compares with an average of 0.33 ± 0.11 in our subject group (individual range 0.19–0.47) and suggests a close agreement between the techniques. Comparison with upright subjects is inappropriate since there are large changes in FRC with posture (6), which serves to alter SV.
Possible Effects of Airways Closure
The approximately linear increase in SV with decreasing lung height is not always seen in the most dependent portion of the lung (Fig. 6C). Airway closure occurs when small airways, thought to be respiratory bronchioles, collapse, resulting in trapped gas in the alveoli distal to that point. Trapped gas means higher initial volumes and also lower volumes of inspired air, both effects contributing to a decrease in SV (36). We observed SV patterns consistent with airway closure in four of the eight subjects imaged (subjects S3, S4, S7, and, to a lesser extent, S5). For these subjects, SV in the most dependent part of the lung (height < 1 cm) was lower than in the subsequent 1- to 2-cm-height slice.
Assumptions and Limitations
The technique is currently limited to a single lung imaging slice. For each subject, we selected a midlung sagittal slice in the right lung that presented a low percentage of large vessels. Large pulmonary veins transport to the imaging plane changes in O2 concentration that occur in distant regions of the lung. In different lung slices or imaging planes, this can be a confounding effect, addressable by masking of large vessels based on voxel intensity (10).
The present voxel size corresponds to rodlike shaped structures, with 1.6 × 1.6 mm in the plane surface but 15 mm in the perpendicular direction. An individual voxel may contain multiple adjacent acini, and as such the method measures the average response. The true resolution obtained in SVI is somewhat lower than the nominal resolution, because of small registration errors that inevitably occur from image to image, resulting in some averaging of responses of neighboring in-plane voxels.
In our present implementation, we have assumed that the subject reaches a constant and reproducible FRC for each acquired image and that each breath is identical (a tidal breath). Based on the measured tidal volume data this was true in this subject population; however, this might prove not to be the case for studies in some patient populations. Patients might not be able to comply with the 220-breath sequence and might be less proficient in attaining a reproducible FRC. In such circumstances, subject training, respiratory MR image acquisition triggering, image registration, or an individualized approach to translating correlation delay time to SV in the face of changing total ventilation might be required. Misregistration resulting from patient movement or failure to attain FRC at the time of imaging introduces an additional error in the measurement of SV. Image registration of deformable structures such as the lung is not an easy task, yet it will likely be required for the extension of the proposed method to patients suffering from severe pulmonary disorders. We anticipate that the signal-to-noise ratio associated with our measurement will be worse in patients with lung disease such as chronic obstructive pulmonary disease (COPD). Low signal-to-noise ratio might only impact specific regions of the lung, namely regions with lower SV than that observed in normal healthy subjects; low-SV regions are known to be present in patient populations (16). To capture regions of lower SV, longer blocks of air-oxygen would be required; longer blocks improve contrast, especially in low-SV units, as they allow more time for equilibration. Depending on the specific signal-to-noise conditions of the population and their ability to cope with longer periods in the scanner, this can be achieved either by reducing the number of cycles or by extending the total acquisition time.
Breathing 100% O2 will result in a small increase of hemoglobin-bound O2 (by ∼1% in this study). The main effect of hemoglobin-bound O2 is a T2* change and a much smaller effect on the T1 (5). Adapting the data reported by Silvennoinen et al. for a 1.5-T field (33), a 1% SaO2 increase would be expected to result in a <0.5% change in the T1 of blood, a negligible effect compared with the observed 12.5% change in T1 between breathing 21% O2 and 100% O2 (3, 13). Thus the measurement is dominated by the increase in the amount of oxygen in solution in blood and tissues and not by the increase in hemoglobin-bound oxygen.
Conclusion
Quantification of SV in the human lung is practical with proton MRI SVI, which uses O2 as a contrast agent. As expected, there was a clearly defined vertical gradient in SV in the supine human lung, and this was most likely a result of the self-deformation of the lung (the Slinky effect). However, in the most dependent third of the lung, other factors such as dependent airways closure, the nonlinear nature of the lung pressure-volume curve, and the large pulmonary vessels likely play important roles in determining regional SV over and above Slinky behavior.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants HL-080203 and HL-081171. R. C. Sá's work was supported by the National Space Biomedical Research Institute through National Aeronautics and Space Administration NCC 9-58. A. C. Henderson was supported by NIH Grant 1K99-HL-093064 and D. J. Dubowitz by NIH Grant NS-053934.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Albert MS, Cates GD, Driehuys B, Happer W, Saam B, Springer CSJ, Wishnia A. Biological magnetic resonance imaging using laser-polarized 129Xe. Nature 370: 199–201, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Amis TC, Jones HA, Hughes JM. Effect of posture on inter-regional distribution of pulmonary ventilation in man. Respir Physiol 56: 145–167, 1984 [DOI] [PubMed] [Google Scholar]
- 3. Chen Q, Jakob PM, Griswold MA, Levin DL, Hatabu H, Edelman RR. Oxygen enhanced MR ventilation imaging of the lung. MAGMA 7: 153–161, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Davidson MR. Further considerations in a theoretical description of gas-transport in lung airways. Bull Math Biol 43: 517–548, 1981 [DOI] [PubMed] [Google Scholar]
- 5. Edelman RR, Hatabu H, Tadamura E, Li W, Prasad PV. Noninvasive assessment of regional ventilation in the human lung using oxygen-enhanced magnetic resonance imaging. Nat Med 2: 1236–1239, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Elliott AR, Prisk GK, Guy HJ, West JB. Lung volumes during sustained microgravity on Spacelab SLS-1. J Appl Physiol 77: 2005–2014, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Engel LA. Gas mixing within the acinus of the lung. J Appl Physiol 54: 609–618, 1983 [DOI] [PubMed] [Google Scholar]
- 8. Glenny RW, Robertson HT. Fractal properties of pulmonary blood flow: characterization of spatial heterogeneity. J Appl Physiol 69: 532–545, 1990 [DOI] [PubMed] [Google Scholar]
- 9. Hatabu H, Alsop DC, Listerud J, Bonnet M, Gefter WB. T2* and proton density measurement of normal human lung parenchyma using submillisecond echo time gradient echo magnetic resonance imaging. Eur J Radiol 29: 245–252, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Henderson AC, Prisk GK, Levin DL, Hopkins SR, Buxton RB. Characterizing pulmonary blood flow distribution measured using arterial spin labeling. NMR Biomed 22: 1025–1035, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hickam JB, Blair E, Frayser R. An open-circuit helium method for measuring functional residual capacity and defective intrapulmonary gas mixing. J Clin Invest 33: 1277–1286, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hopkins SR, Henderson AC, Levin DL, Yamada K, Arai TJ, Buxton RB, Prisk GK. Vertical gradients in regional lung density and perfusion in the supine human lung: the Slinky effect. J Appl Physiol 103: 240–248, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jakob PM, Wang T, Schultz G, Hebestreit H, Hebestreit A, Hahn D. Assessment of human pulmonary function using oxygen-enhanced T1 imaging in patients with cystic fibrosis. Magn Reson Med 51: 1009–1016, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Kaneko K, Milic-Emili J, Dolovich MB, Dawson A, Bates DV. Regional distribution of ventilation and perfusion as a function of body position. J Appl Physiol 21: 767–777, 1966 [DOI] [PubMed] [Google Scholar]
- 15. Levin DL, Buxton RB, Spiess JP, Arai T, Balouch J, Hopkins SR. Effects of age on pulmonary perfusion heterogeneity measured by magnetic resonance imaging. J Appl Physiol 102: 2064–2070, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Lewis SM, Evans JW, Jalowayski AA. Continuous distributions of specific ventilation recovered from inert-gas washout. J Appl Physiol 44: 416–423, 1978 [DOI] [PubMed] [Google Scholar]
- 17. MacFall JR, Charles HC, Black RD, Middleton H, Swartz JC, Saam B, Driehuys B, Erickson C, Happer W, Cates GD, Johnson GA, Ravin CE. Human lung air spaces: potential for MR imaging with hyperpolarized He-3. Radiology 200: 553–558, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Mai VM, Bankier AA, Prasad PV, Li W, Storey P, Edelman RR, Chen Q. MR ventilation-perfusion imaging of human lung using oxygen-enhanced and arterial spin labeling techniques. J Magn Reson Imaging 14: 574–579, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Mai VM, Chen Q, Bankier AA, Edelman RR. Multiple inversion recovery MR subtraction imaging of human ventilation from inhalation of room air and pure oxygen. Magn Reson Med 43: 913–916, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Mai VM, Tutton S, Prasad PV, Chen Q, Li W, Chen C, Liu B, Polzin J, Kurucay S, Edelman RR. Computing oxygen-enhanced ventilation maps using correlation analysis. Magn Reson Med 49: 591–594, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Middleton H, Black RD, Saam B, Cates GD, Cofer GP, Guenther R, Happer W, Hedlund LW, Johnson GA, Juvan K. MR imaging with hyperpolarized 3He gas. Magn Reson Med 33: 271–275, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Milic-Emili J. Radioactive xenon in the evaluation of regional lung function. Semin Nucl Med 1: 246–262, 1971 [DOI] [PubMed] [Google Scholar]
- 23. Milic-Emili J, Henderson JA, Dolovich MB, Trop D, Kaneko K. Regional distribution of inspired gas in the lung. J Appl Physiol 21: 749–759, 1966 [DOI] [PubMed] [Google Scholar]
- 24. Musch G, Layfield JD, Harris RS, Vidal Melo MF, Winkler T, Callahan RJ, Fischman AJ, Venegas JG. Topographical distribution of pulmonary perfusion and ventilation, assessed by PET in supine and prone humans. J Appl Physiol 93: 1841–1851, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Naish JH, Parker GJ, Beatty PC, Jackson A, Young SS, Waterton JC, Taylor CJ. Improved quantitative dynamic regional oxygen-enhanced pulmonary imaging using image registration. Magn Reson Med 54: 464–469, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Ohno Y, Hatabu H. Basics concepts and clinical applications of oxygen-enhanced MR imaging. Eur J Radiol 64: 320–328, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Ohno Y, Hatabu H, Higashino T, Kawamitsu H, Watanabe H, Takenaka D, van Cauteren M, Sugimura K. Centrically reordered inversion recovery half-Fourier single-shot turbo spin-echo sequence: improvement of the image quality of oxygen-enhanced MRI. Eur J Radiol 52: 200–205, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Ohno Y, Hatabu H, Takenaka D, Adachi S, Van Cauteren M, Sugimura K. Oxygen-enhanced MR ventilation imaging of the lung: preliminary clinical experience in 25 subjects. AJR Am J Roentgenol 177: 185–194, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Ohno Y, Hatabu H, Takenaka D, Uematsu H, Ohbayashi C, Higashino T, Nogami M, Yoshimura M, Fujii M, Sugimura K. Dynamic MR imaging: value of differentiating subtypes of peripheral small adenocarcinoma of the lung. Eur J Radiol 52: 144–150, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Paiva M. Gas transport in the human lung. J Appl Physiol 35: 401–410, 1973 [DOI] [PubMed] [Google Scholar]
- 31. Prisk GK, Guy HJ, Elliott AR, Paiva M, West JB. Ventilatory inhomogeneity determined from multiple-breath washouts during sustained microgravity on Spacelab SLS-1. J Appl Physiol 78: 597–607, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Prisk GK, Hammer J, Newth CJ. Techniques for measurement of thoracoabdominal asynchrony. Pediatr Pulmonol 34: 462–472, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Silvennoinen MJ, Kettunen MI, Kauppinen RA. Effects of hematocrit and oxygen saturation level on blood spin-lattice relaxation. Magn Reson Med 49: 568–571, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Theilmann RJ, Arai TJ, Samiee A, Dubowitz DJ, Hopkins SR, Buxton RB, Prisk GK. Quantitative MRI measurement of lung density must account for the change in T2* with lung inflation. J Magn Reson Imaging 30: 527–534, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wagner PD, Laravuso RB, Uhl RR, West JB. Continuous distributions of ventilation-perfusion ratios in normal subjects breathing air and 100 per cent O2. J Clin Invest 54: 54–68, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. West JB. Respiratory Physiology—The Essentials. Philadelphia, PA: Lippincott Williams & Wilkins, 2008 [Google Scholar]
- 37. West JB. State of the art: ventilation-perfusion relationships. Am Rev Respir Dis 116: 919–943, 1977 [DOI] [PubMed] [Google Scholar]