Abstract
The process by which a single fertilized egg develops into a human being with more than 200 cell types—each with a distinct gene expression pattern controlling its cellular state—is poorly understood. Knowledge of the transcriptional regulatory circuitry that establishes and maintains gene expression programs in mammalian cells is fundamental to understanding development and should provide the foundation for improved diagnosis and treatment of disease. Although it is not yet feasible to map the entirety of this circuitry in vertebrate cells, recent work in embryonic stem (ES) cells has demonstrated that core features of the circuitry can be discovered through studies involving selected regulators. Here, we highlight the fundamental insights that have emerged from studies that examined the role of transcription factors, chromatin regulators, signaling pathways, and noncoding RNAs in the regulatory circuitry of ES cells. Maps of regulatory circuitry and the insights that have emerged from these studies have improved our understanding of global gene expression and are facilitating efforts to reprogram cells for disease therapeutics and regenerative medicine.
More than 200 different cell fates are generated during vertebrate development, each with a gene expression program unique to that cell type (Su et al. 2004; Shyamsundar et al. 2005; Vickaryous and Hall 2006). The expression programs are controlled by transcription factors, chromatin modifiers, and regulatory RNAs that can be influenced by signals from the extracellular environment. The subset of regulators that are expressed in each cell type have a key role in establishing and maintaining cell state. How these regulators control global gene expression programs is almost entirely unmapped in vertebrates because of limitations in our knowledge of the transcriptional, chromatin, and signaling components that are key to the control of each cell type. Nonetheless, multiple groups have begun to tackle the challenge of mapping transcriptional regulatory circuitry, particularly in ES cells, and have demonstrated that important insights into the global control of cell state can be obtained from these efforts (Boyer et al. 2005, 2006; Chew et al. 2005; Bernstein et al. 2006; Lee et al. 2006; Loh et al. 2006; Wu et al. 2006; Pan et al. 2007; Zhao et al. 2007; Chen et al. 2008; Cole et al. 2008; Endoh et al. 2008; Jiang et al. 2008; Kim et al. 2008a; Tam et al. 2008).
Mapping transcriptional regulatory circuitry is important because it provides insights into the regulators and mechanisms that control global gene expression, cell state, and development. Because deficiencies in control of gene expression can contribute to many human diseases such as cancer, immune disease, and diabetes (Villard 2004; Kloosterman and Plasterk 2006; Latchman 2008), knowledge of normal and abnormal transcriptional regulatory circuitries may provide new approaches to disease diagnosis and therapy. Here, we describe initial efforts to dissect core features of the ES cell transcriptional regulatory network and highlight the key insights that have emerged from such studies.
Molecular Mechanisms Controlling Eukaryotic Transcription
Transcription factors, chromatin regulators, signaling pathways, and noncoding RNAs are among the key components that control mRNA gene expression (Fig. 1). The molecular mechanisms by which these regulators control expression of individual genes have been studied extensively and are reviewed elsewhere (Lee and Young 2000; Orphanides and Reinberg 2002; Berger 2007; Li et al. 2007; Core and Lis 2008; Hobert 2008). Our understanding of these mechanisms suggests how to organize models of the transcriptional regulatory circuitry, as described below.
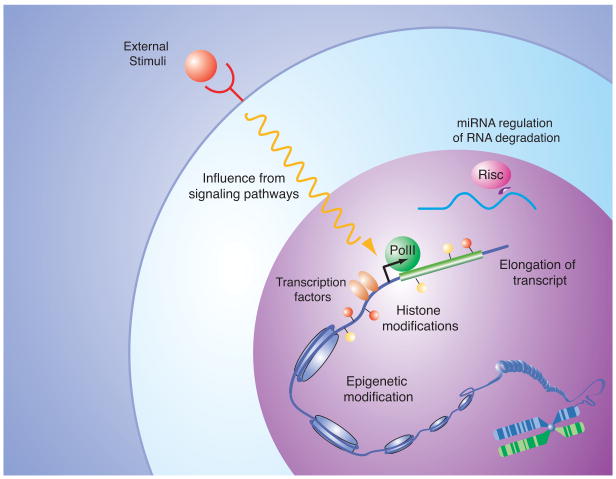
Figure 1.
Mechanisms controlling eukaryotic gene expression: Examples of various types of inputs contributing to the control of mRNA levels within a cell. Site-specific transcription factors, recruitment and control of RNA polymerase initiation and elongation, DNA and histone modifications, input from signal transduction pathways, and the actions of miRNAs on mRNA levels can influence the gene expression program of a cell. Knowledge of the molecular mechanisms involved in control of gene expression can guide efforts to elucidate the transcriptional regulatory circuitry that produces a specific gene expression program. For further details and references, see text.
DNA-binding transcription factors recognize sequence motifs, are key to specific gene regulation, and can thus be used to anchor transcriptional regulatory networks (Harrison 1991; Pabo and Sauer 1992; Kadonaga 2004; Remenyi et al. 2004). Transcription factors are also the single largest protein family encoded in the human genome, where they account for approximately 10% of protein-coding genes (Lander et al. 2001; Levine and Tjian 2003). They bind to both promoter-proximal and -distal regulatory DNA sequences and can aid or inhibit recruitment of the transcription apparatus at target genes (Latchman 1997; Blackwood and Kadonaga 1998; Ogata et al. 2003; West and Fraser 2005). At most well-studied promoters, it is evident that multiple transcription factors are bound, which allows for the combinatorial control of gene expression (Evans et al. 1990; Greene 1990; Harbison et al. 2004; Panne et al. 2004; Remenyi et al. 2004).
Chromatin regulators are often recruited to specific portions of the genome by DNA-binding transcription factors or the transcription apparatus where they act to augment gene expression or repression through their effects on chromatin state (Berger 2007; Kouzarides 2007; Li et al. 2007). Chromatin regulators that methylate DNA and certain nucleosomal histone residues have been implicated in heritable chromatin states and thus have important roles in developmental control. Methylation of CpG islands in the promoter regions of some genes by DNA methyltransferases contributes to their repression and is maintained during development by maintenance methylases (Goll and Bestor 2005; Turek-Plewa and Jagodziński 2005; Klose and Bird 2006). Methylation of nucleosomal histones by Trithorax and Polycomb group protein complexes is also thought to be important for maintaining gene expression programs associated with specific cell states; Trithorax group complexes are associated with actively transcribed genes, whereas Polycomb group regulators are associated with repression of most genes that they occupy (Pirrotta 1998; Orlando 2003; Ringrose and Paro 2004; Schuettengruber et al. 2007; Schwartz and Pirrotta 2007).
Signaling pathways act to maintain or initiate changes in the regulatory circuitry in response to environmental or developmental cues. The terminal components of signaling pathways are often protein kinases that can phosphorylate and activate transcriptional regulators or are themselves transcription factors and chromatin modifiers (Hunter 2000; Brivanlou and Darnell 2002; Yang et al. 2003; Pokholok et al. 2006). Knowledge of the target genes of each of the signaling pathways that contribute to control of cell state is critical to understanding how these pathways control the gene expression program associated with such states.
Noncoding RNAs can influence gene expression and chromatin state (Goodrich and Kugel 2006; Amaral et al. 2008; Hawkins and Morris 2008). For example, a large class of noncoding RNAs, termed microRNAs (miRNAs), modify gene expression by regulating translation and degradation of mRNA transcripts (Ambros 2004; Bartel 2004; Valencia-Sanchez et al. 2006; Meister 2007; Makeyev and Maniatis 2008). Noncoding RNA species have also been implicated in control of chromatin state (Verdel et al. 2004; Moazed et al. 2006; Grewal and Elgin 2007; Rinn et al. 2007; Zaratiegui et al. 2007). We have limited understanding of the regulation of expression of noncoding RNA species, and in most cases, we have yet to identify the specific set of genes that are under the control of these noncoding RNA species.
Concept of Core Transcriptional Regulatory Circuitry
Hundreds of gene expression regulators are present in each cell, making it a challenge to map the regulatory network that they form in even one cell type, much less in 200 cell types (Lander et al. 2001; Brivanlou and Darnell 2002). For this reason, even the most ambitious global studies have examined only a handful of transcriptional regulators and then in only a few cell types (Cawley et al. 2004; Odom et al. 2004, 2006; Boyer et al. 2005; Rada-Iglesias et al. 2005, 2008; Loh et al. 2006; Barski et al. 2007; Mikkelsen et al. 2007; Chen et al. 2008; Cole et al. 2008; Jaenisch and Young 2008; Jiang et al. 2008; Kim et al. 2008a; Komashko et al. 2008; Marson et al. 2008b; Park et al. 2008; Reed et al. 2008; Wang et al. 2008). However, several lines of evidence argue that a small subset of transcription factors and other regulators have a key role in the control of cell state. Cells can be reprogrammed into other cell states through forced expression of a very small number of transcription factors. For example, fibroblasts can be reprogrammed into induced pluripotent stem cells upon forced expression of four transcription factors (Takahashi and Yamanaka 2006; Okita et al. 2007; Takahashi et al. 2007; Wernig et al. 2007; Yu et al. 2007). Similarly, fibroblasts and other cells can take on a skeletal muscle state when the myogenic transcription factor MyoD is expressed (Davis et al. 1987; Weintraub et al. 1989, 1991; Choi et al. 1990). Screens to identify genes that are key to maintaining the ES cell state have identified only a small number of all of the transcription factor genes that are expressed in these cells (Ivanova et al. 2006; Zhang et al. 2006; Fazzio et al. 2008). Furthermore, several studies have shown that many transcription factors can be eliminated without dire consequences for the cell (Winzeler et al. 1999; Giaever et al. 2002; Kemphues 2005). The small set of transcription factors that have been demonstrated to be important for establishment or maintenance of a cell state will henceforth be termed “key regulators.”
A simplified version of the transcriptional regulatory circuitry of a cell can thus be deduced by discovering the population of genes that are occupied and controlled by the key regulators for that cell type. We call this simplified network the “core transcriptional regulatory circuitry.” Given current experimental limitations to elucidating complete vertebrate circuitry, we propose that the mapping of core regulatory circuitry provides a shortcut to discovering key network themes, a concept that we believe has been validated with the study of ES cells.
Key ES Cell Transcription Factors Establish a Core Regulatory Circuitry
Initial studies of transcription factors in the ES cell transcriptional regulatory network focused on the key regulators Oct4, Sox2, and Nanog (Boyer et al. 2005; Loh et al. 2006). Knowledge of genetic phenotypes, expression profiles, and molecular relations was leveraged to identify these factors as key components of the ES cell network. Genetic studies demonstrated functional consequences in ES cells of inappropriate expression of these factors, the expression of Oct4 and Nanog was found to be specific to pluripotent cells, and Sox2 was known to form a heterodimer with Oct4 (Schöler et al. 1990; Ambrosetti et al. 1997; Nichols et al. 1998; Avilion et al. 2003; Chambers et al. 2003; Mitsui et al. 2003; Hart et al. 2004). Because of the overwhelming evidence for key roles for these regulators in the ES cell network, multiple groups have mapped their target genes in human and murine ES cells (Boyer et al. 2005; Loh et al. 2006).
Several important themes emerged from the study of target genes for Oct4, Sox2, and Nanog (Fig. 2). The key regulators clearly prefer to cooccupy their target genes, thus forming a network structure called a multi-input motif (Fig. 2A) (Lee et al. 2002; Boyer et al. 2005; Loh et al. 2006; Alon 2007). Because they form a heterodimer, Oct4 and Sox2 were expected to bind the same target genes, but Nanog was also found to occupy a large percentage of the Oct4-Sox2 bound genes. More recent studies have mapped additional transcription factors in ES cells and have found that they also follow the theme of target gene cooccupancy (Wu et al. 2006; Chen et al. 2008; Jiang et al. 2008; Kim et al. 2008a). These studies, and similar investigations of key regulators in other cell types (Odom et al. 2004, 2006), suggest that the multi-input motif is an important theme in the transcriptional regulatory circuitry of vertebrate cells.
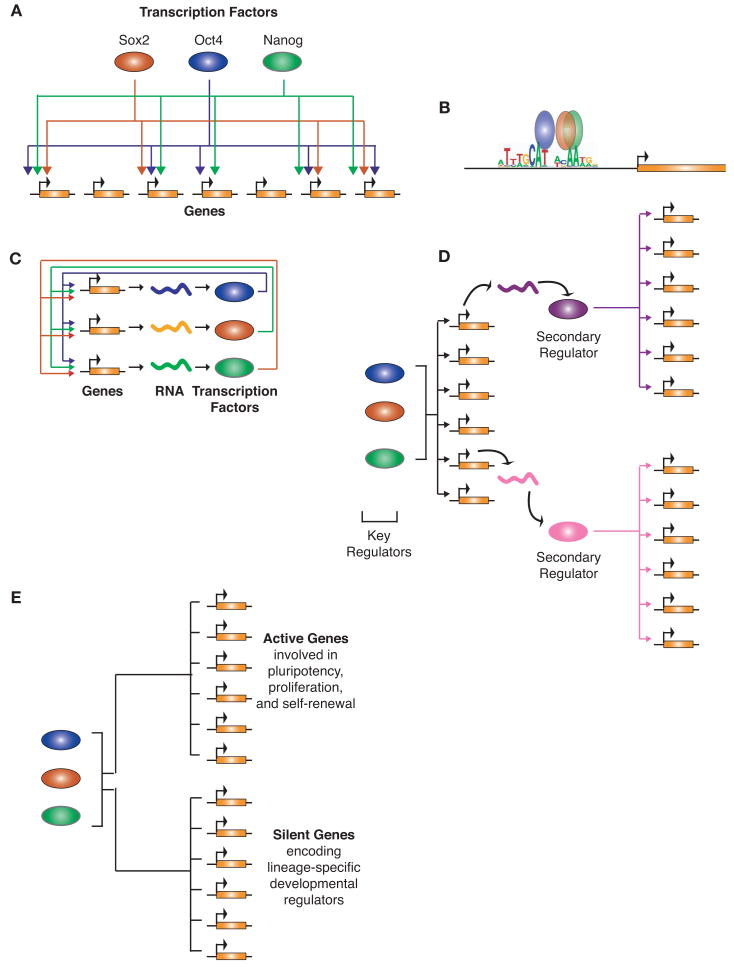
Figure 2.
Major themes emerging from studies of key transcription factors in ES cells. (A) Key regulators largely target the same set of genes and thus form a multi-input motif. Multi-input motifs consist of a set of regulators that bind to a common set of genes. (Colored ovals) Proteins; (orange rectangles) genes. An arrow from a protein to a gene indicates direct regulation of the gene by the protein. (B) Key transcription factors often bind in extremely close proximity to one another on DNA. The motif associated with the key transcription factors in ES cells is depicted in the promoter region for a gene targeted by all three factors. The average location of binding with respect to this motif as determined by large-scale binding profiles is shown and demonstrates the proximity of binding. (Colored ovals) Proteins; (orange rectangle) gene. (C) Key transcription factors often form interconnected autoregulatory loops where the transcription factors together bind to and regulate each of their promoters. (Colored ovals) Proteins; (orange rectangles) genes; (squiggly lines) their resulting transcripts. An arrow from a protein to a gene indicates direct regulation of the gene by the protein. (D) Key transcription factors regulate genes encoding secondary regulators, thus allowing the key regulators ultimate control over many more genes and therefore a larger effect on the complete transcriptional program. (Colored ovals) Proteins; (orange rectangles) genes; (squiggly lines) their resulting transcripts. An arrow from a protein to a gene indicates direct regulation of the gene by the protein. (E) Key transcription factors in ES cells bind to the promoters of both active genes involved in pluripotency and self-renewal and to the promoters of inactive genes encoding lineage-specific developmental regulators that are silent in ES cells. (Colored ovals) Proteins; (orange rectangles) genes. A line from a protein to a gene indicates direct regulation of the gene by the protein.
A related feature or theme that emerged from these global binding studies is that the key regulators tend to occupy DNA sequences in very close proximity to one another (Fig. 2B) (Boyer et al. 2005; Loh et al. 2006; Cole et al. 2008; Marson et al. 2008b). Oct4, Sox, and Nanog were often found to bind within 25 bp of one another at target genes (Marson et al. 2008b). This proximity suggests that these factors are forming tightly associated complexes on DNA to coordinately affect transcription. Some of these transcription factors are competing for binding to overlapping or similar DNA sequences, and because the data come from a population of cells, it is also possible that the complete set of transcription factors is not simultaneously bound at these sites in individual cells. Further studies into the biochemical nature of these binding events are needed to test these possibilities.
One of the more important themes that emerged from the initial studies of the key regulators of ES cells was that Oct4, Sox2, and Nanog together cooccupy their own promoter regions and thus form a network structure called an interconnected autoregulatory loop (Fig. 2C) (Boyer et al. 2005; Loh et al. 2006). This network motif may have two purposes: Feedback gene regulation by these transcription factors may contribute to the stability of the core ES cell transcriptional regulatory network, yet this network structure may also allow for a rapid change in core regulatory circuitry if one regulator is eliminated upon receipt of differentiation signals. Indeed, the circuit formed by Oct4, Sox2, and Nanog could apparently act as a bistable switch controlling ES cell maintenance versus differentiation (Chickarmane et al. 2006).
The early global studies also revealed that the key regulators occupy and control genes encoding many other transcriptional regulators that are expressed in ES cells (Boyer et al. 2005; Loh et al. 2006), forming a hierarchical regulatory network structure (Fig. 2D). The target genes of Oct4, Sox2, and Nanog were significantly enriched for transcription factors and developmental regulators (Boyer et al. 2005). The control of these secondary regulators allows key transcription factors to indirectly control a much larger set of genes. This hierarchical network structure has been described in model organisms and will likely prove to be a common vertebrate network architecture (Martinez-Antonio and Collado-Vides 2003; Ma et al. 2004; Farkas et al. 2006). This network structure may allow for rapid large-scale changes in the transcription program in response to signals that may only directly target a handful of key regulators.
One additional fundamental theme that has emerged from global binding studies is that Oct4, Sox2, and Nanog occupy both actively transcribed genes encoding ES cell transcription factors and repressed genes encoding lineage-specific developmental regulators (Fig. 2E) (Boyer et al. 2005; Loh et al. 2006). This observation suggested that regulation of silent developmental regulators in ES cells might be critical for pluripotency. Inappropriate expression of lineage-specific developmental regulators could initiate gene expression programs for other cell states, and thus it appears to be important to maintain these key regulators of other cell types in a repressed state in ES cells. Precisely how Oct4, Sox2, and Nanog contribute to repression of lineage-specific developmental regulators is not known.
Polycomb and Trithorax Chromatin Regulators in the Core Circuitry
The genomic locations and functions of Polycomb and Trithorax group (PcG and TrxG) proteins, along with the histone modifications catalyzed by these chromatin regulators, have been the subject of much study in ES cells (Bernstein et al. 2006; Boyer et al. 2006; Lee et al. 2006; Pan et al. 2007; Zhao et al. 2007; Endoh et al. 2008). There is considerable genetic evidence that these chromatin regulators have an important role in early development (Faust et al. 1998; O'Carroll et al. 2001; Pasini et al. 2004; Breiling et al. 2007). Studies of their role in the ES cell network have revealed several important insights that are likely to become general themes of vertebrate transcriptional regulatory networks.
One key insight that emerged from studying these chromatin regulators is that the previous assumption that TrxG complexes are associated with actively transcribed genes, whereas PcG regulators are associated with repressed genes, is imperfect (Bernstein et al. 2006; Boyer et al. 2006; Lee et al. 2006; Pan et al. 2007; Zhao et al. 2007; Endoh et al. 2008). The set of silent genes encoding lineage-specific developmental regulators that are occupied by Oct4, Sox2, and Nanog was also occupied by both PcG and TrxG complexes and contained nucleosomes trimethylated at both histone H3 lysine (K)4 and H3K27. These silent genes encoding developmental regulators were therefore described as being bivalently marked by both activating and repressive marks.
Further studies revealed that the transcription apparatus was recruited to the promoters of these bivalently marked genes encoding developmental regulators and that transcription was initiated but full-length transcript was not produced (Guenther et al. 2007; Stock et al. 2007). Studies in Drosophila suggest that transcriptional pausing is a conserved regulatory feature at genes encoding silent developmental regulators in embryonic tissues (Muse et al. 2007; Zeitlinger et al. 2007; Hendrix et al. 2008; Nechaev and Adelman 2008). This regulatory feature may keep genes encoding developmental regulators in a poised expression state, allowing rapid transcription of certain genes upon induction of differentiation. How only a specific subset of these Pc-repressed genes is induced to overcome transcriptional pausing to permit lineage-specific differentiation is not yet understood.
Signaling Pathways Bring Developmental Cues to the Network
Recent studies have revealed how some signal transduction pathways contribute to control of the ES cell transcriptional regulatory network (Pereira et al. 2006; Chen et al. 2008; Cole et al. 2008; Tam et al. 2008; Yi et al. 2008). The Wnt signaling pathway has important roles throughout development and can influence ES cell state (Logan and Nusse 2004; Reya and Clevers 2005). A terminal component of this pathway, the transcription factor Tcf3, was identified as a likely key regulator in ES cells due to its genetic and expression phenotypes (Korinek et al. 1998; Merrill et al. 2004; Pereira et al. 2006).
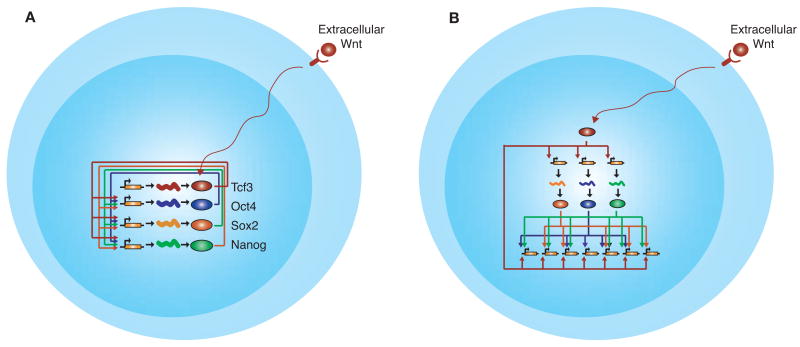
Subsequent genome-wide studies of Tcf3 in ES cells revealed that the Wnt signaling pathway is intimately connected to the core transcriptional circuitry of ES cells (Fig. 3) (Cole et al. 2008; Marson et al. 2008b; Tam et al. 2008; Yi et al. 2008). Tcf3 occupies promoters of the key transcription factors Oct4, Sox2, and Nanog, and these factors, together with Tcf3, occupy the Tcf3 promoter (Fig. 3A). Thus, Tcf3 is a component of the interconnected autoregulatory loop that is at the core of ES cell transcriptional regulatory circuitry. These studies also revealed that Tcf3 cooccupied the genome with the key transcription factors, suggesting that the Wnt signaling pathway can affect cellular state by directly connecting to the core circuitry. In this manner, cells could respond to Wnt signaling through a feedforward loop where the key ES cell regulators as well as their targets are immediately targeted by Tcf3 (Fig. 3B). This network structure would allow for both a rapid and stable response to environmental stimuli.
Figure 3.
Key themes from studies of signaling pathways in ES cells. (A) Signaling pathways can directly connect to key transcription factors in the core interconnected autoregulatory loop through terminal components of their signal transduction pathway. (Colored ovals) Proteins; (orange rectangles) genes; (squiggly lines) their resulting transcripts. An arrow from a protein to a gene indicates direct regulation of the gene by the protein. (Red Y shape and long squiggly arrow) Wnt signal transduction pathway. (B) The terminal component of signaling pathways such as the Wnt pathway can form a feedforward loop by regulating both the key regulators and their target genes. Proteins, genes, and regulation are depicted as in A.
Manipulation of the canonical Wnt pathway through Tcf3 can affect the balance between pluripotency and differentiation in ES cells (Cole et al. 2008). High Wnt pathway activity favors pluripotency, whereas low activity favors differentiation (Sato et al. 2004; Ogawa et al. 2006; Singla et al. 2006; Miyabayashi et al. 2007). Tcf3 and its associated proteins apparently contribute to gene activation when the Wnt pathway is activated and to repression when the pathway is not (Cole et al. 2008; Tam et al. 2008). This suggests that under conditions of high Wnt activity, Tcf3 and the key transcription factors Oct4, Sox2, and Nanog generally function to activate target gene expression (although such activity can be overridden by PcG proteins and other repressors). In contrast, under conditions of low Wnt activity, Tcf3 acts to repress target gene expression and may thus counter the activating functions of Oct4, Sox2, and Nanog. These opposing inputs thus allow ES cells to modulate the level of target gene expression in the core circuitry on the basis of the cell's external environment, which in turn influences the balance between pluripotency and differentiation.
Noncoding RNAs Add Another Layer of Regulation to the Circuitry
miRNAs are critical for normal ES cell self-renewal and differentiation and have demonstrated roles in early development (Bernstein et al. 2003; Kanellopoulou et al. 2005; Murchison et al. 2005; Wang et al. 2007; Sinkkonen et al. 2008; Stefani and Slack 2008). Recent studies have revealed how the miRNA class of noncoding RNAs is controlled in ES cells, and this information has been incorporated into a model of the core regulatory circuitry of ES cells (Marson et al. 2008b). This class of non-coding RNAs adds another layer to the regulation of gene expression because the RNAs act posttranscriptionally to influence mRNA stability and translation. miRNAs can regulate the expression of many protein-coding genes (Farh et al. 2005; Krek et al. 2005; Lewis et al. 2005; Lim et al. 2005) and thus form a number of interesting control circuits in cells where they are expressed.
miRNA gene expression is regulated in a manner similar to that of regulation of protein-coding genes in ES cells (Fig. 4A) (Marson et al. 2008b). The key transcription factors Oct4, Sox2, Nanog, and Tcf3 occupy and positively regulate the promoters of miRNA genes that are actively expressed in ES cells. These key transcription factors also occupy a set of silent miRNA genes that are expressed later during differentiation. This set of silent miRNA genes is occupied by PcG proteins in ES cells, thus poising these miRNA genes for expression during development in a lineage-specific fashion.
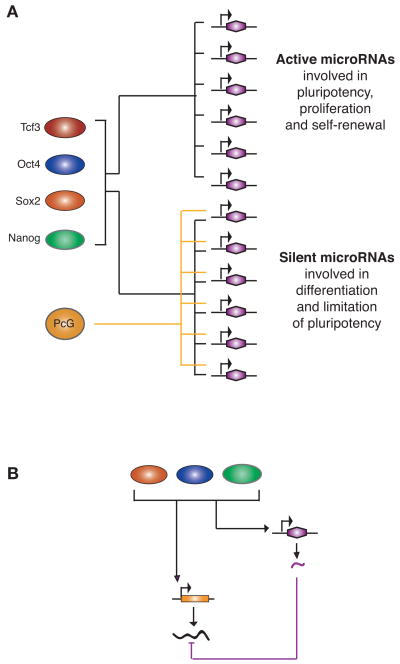
Figure 4.
Key themes from studies of miRNAs in ES cells. (A) miRNA genes are regulated in a manner similar to that of protein-coding genes. Key transcription factors cooccupy the promoters of active miRNAs and, along with Pc proteins, cooccupy the promoters of silent miRNAs. (Colored ovals) Proteins; (purple hexagons) miRNA genes. A line from a protein to a gene indicates direct regulation of the gene by the protein. (B) miRNAs can participate in an incoherent feedforward loop with key transcription factors to modulate the level of target gene expression. Key transcription factors bind and activate an miRNA gene along with another target gene that specifies an mRNA; the miRNA represses by reducing translation or stability of the mRNA. (Colored ovals) Proteins; (orange rectangle) protein-coding gene; (black squiggly line) its mRNA; (purple hexagon) miRNA gene; (squiggly lines) resulting transcripts. Regulation is depicted by arrows and t bars.
Studies of miRNAs and the core circuitry of ES cells also revealed recognizable network motifs that provide insights into how networks can control cell state (Marson et al. 2008b). Certain miRNAs, such as mir-290-295, form a common network motif termed an incoherent feed-forward loop with the key transcription factors in ES cells (Fig. 4B) (Alon 2007). This network architecture may allow ES cells to fine-tune gene expression levels of important target genes and facilitate removal of certain ES-cell-specific mRNAs when cells are stimulated to differentiate.
Model of ES Cell Core Regulatory Circuitry
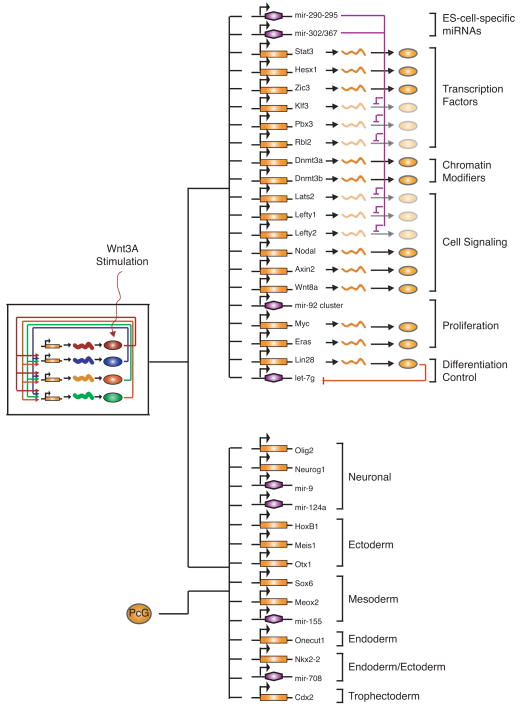
A model for ES cell core regulatory circuitry has recently been described that incorporates key transcription factors, chromatin regulators, the Wnt signaling pathway, and miRNAs (Fig. 5) (Marson et al. 2008b). This model represents only a portion of the available data, but it serves to illustrate several important features of ES cell regulatory circuitry. The transcription factors Oct4, Sox2, Nanog, and Tcf3 form an interconnected autoregulatory loop, to which the Wnt signaling pathway connects. The key transcription factors occupy and regulate a set of actively transcribed protein-coding and noncoding genes whose functions contribute to the ES cell state. The products of some of these genes add another layer of regulation; for example, the miRNAs fine-tune the levels of mRNAs for certain protein-coding genes. The key transcription factors also occupy silent protein-coding and noncoding genes involved in lineage-specific functions, and these genes appear to experience transcription initiation, but transcript completion is prevented by PcG proteins and perhaps additional repressors (Guenther et al. 2007; Stock et al. 2007).
Figure 5.
Model of the ES cell transcriptional regulatory circuitry. The interconnected autoregulatory loop formed by Oct4, Sox2, Nanog, and Tcf3, with input from the Wnt pathway, is shown to the left. (Top right) Active transcripts; (bottom right) inactive transcripts. (Colored ovals) Proteins; (orange rectangles) protein-coding genes; (squiggly lines) their resulting transcripts; (purple hexagons) miRNA genes. Regulation is depicted by lines, arrows, or t bars.
Reprogramming Transcriptional Regulatory Circuitries to the ES Cell State
Somatic cells can be reprogrammed into induced pluripotent stem (iPS) cells by ectopic expression of four or fewer transcriptional regulators (Takahashi and Yamanaka 2006; Meissner et al. 2007; Okita et al. 2007; Takahashi et al. 2007; Wernig et al. 2007, 2008; Yu et al. 2007; Aoi et al. 2008; Jaenisch and Young 2008; Kim et al. 2008b; Nakagawa et al. 2008; Park et al. 2008). The transcription factors that have been used for iPS cell generation have typically included a combination of Oct4, Sox2, Klf4, and cMyc or a mix of Oct4, Sox2, Nanog, and Lin28. Knowledge of the transcriptional regulatory circuitry has already provided insights into the mechanisms by which forced expression of these transcription factors leads to reprogramming of somatic cells (Jaenisch and Young 2008). For example, the interconnected autoregulatory loop of ES cells—composed of genes encoding the transcription factors Oct4, Sox2, Nanog, and Tcf3—can be jump-started by transient expression of these reprogramming factors.
Knowledge of the transcriptional regulatory circuitry has recently been used to improve the reprogramming process (Marson et al. 2008a; Mikkelsen et al. 2008). The discovery that the Wnt pathway is connected directly to ES cell core regulatory circuitry (Cole et al. 2008; Tam et al. 2008; Yi et al. 2008) suggested that manipulation of the Wnt pathway might facilitate reprogramming. Indeed, addition of the Wnt3a ligand allows efficient reprogramming even in the absence of c-Myc (Marson et al. 2008a), which is important because the presence of the exogenous c-Myc oncogene in iPS cells leads to tumors in animals derived from these cells (Okita et al. 2007; Jaenisch and Young 2008). For somatic cells to adopt ES cell transcriptional regulatory circuitry, it is thought that they must silence the expression of key regulators of the somatic cell state. This was confirmed by experiments revealing that repression of key regulators of the somatic cell circuitry using RNA inhibition can substantially improve reprogramming efficiency (Mikkelsen et al. 2008).
Concluding Remarks
Knowledge of the transcriptional regulatory circuitry is important because it provides insights into the mechanisms by which key regulators control global gene expression, cell state, and development. Despite having identified only core elements of the ES cell transcriptional regulatory circuitry, important insights have been gained into the control of pluripotency and self-renewal in these cells. This knowledge has also provided insights into the mechanisms involved in reprogramming of cell state (Jaenisch and Young 2008) and has led to improved methods for reprogramming (Marson et al. 2008a; Mikkelsen et al. 2008). The new understanding of the core transcriptional regulatory circuitry in ES cells is also likely to shed light on key aspects of cancer, because many features of the ES cell gene expression program are recapitulated in cancer cells (Ben-Porath et al. 2008; Wong et al. 2008). These advances highlight the importance of future work to further map the regulatory circuitry of a wide range of cells, particularly those of medical importance.
Acknowledgments
We are grateful for the contributions made by many members of the Young and Jaenisch labs to the insights and concepts described in this paper.
References
- Alon U. Network motifs: Theory and experimental approaches. Nat Rev Genet. 2007;8:450–461. doi: 10.1038/nrg2102. [DOI] [PubMed] [Google Scholar]
- Amaral PP, Dinger ME, Mercer TR, Mattick JS. The eukaryotic genome as an RNA machine. Science. 2008;319:1787–1789. doi: 10.1126/science.1155472. [DOI] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Ambrosetti DC, Basilico C, Dailey L. Synergistic activation of the fibroblast growth factor 4 enhancer by Sox2 and Oct-3 depends on protein-protein interactions facilitated by a specific spatial arrangement of factor binding sites. Mol Cell Biol. 1997;17:6321–6329. doi: 10.1128/mcb.17.11.6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- Blackwood EM, Kadonaga JT. Going the distance: A current view of enhancer action. Science. 1998;281:60–63. doi: 10.1126/science.281.5373.60. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- Breiling A, Sessa L, Orlando V. Biology of Polycomb and Trithorax group proteins. Int Rev Cytol. 2007;258:83–136. doi: 10.1016/S0074-7696(07)58002-2. [DOI] [PubMed] [Google Scholar]
- Brivanlou AH, Darnell JE., Jr Signal transduction and the control of gene expression. Science. 2002;295:813–818. doi: 10.1126/science.1066355. [DOI] [PubMed] [Google Scholar]
- Cawley S, Bekiranov S, Ng HH, Kapranov P, Sekinger EA, Kampa D, Piccolboni A, Sementchenko V, Cheng J, Williams AJ, et al. Unbiased mapping of transcription factor binding sites along human chromosomes 21 and 22 points to widespread regulation of noncoding RNAs. Cell. 2004;116:499–509. doi: 10.1016/s0092-8674(04)00127-8. [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Chew JL, Loh YH, Zhang W, Chen X, Tam WL, Yeap LS, Li P, Ang YS, Lim B, Robson P, Ng HH. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol Cell Biol. 2005;25:6031–6046. doi: 10.1128/MCB.25.14.6031-6046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chickarmane V, Troein C, Nuber UA, Sauro HM, Peterson C. Transcriptional dynamics of the embryonic stem cell switch. PLoS Comput Biol. 2006;2:e123. doi: 10.1371/journal.pcbi.0020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Costa ML, Mermelstein CS, Chagas C, Holtzer S, Holtzer H. MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc Natl Acad Sci. 1990;87:7988–7992. doi: 10.1073/pnas.87.20.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 2008;22:746–755. doi: 10.1101/gad.1642408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core LJ, Lis JT. Transcription regulation through promoter-proximal pausing of RNA polymerase II. Science. 2008;319:1791–1792. doi: 10.1126/science.1150843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Endoh M, Endo TA, Endoh T, Fujimura Y, Ohara O, Toyoda T, Otte AP, Okano M, Brockdorff N, Vidal M, Koseki H. Polycomb group proteins Ring1A/B are functionally linked to the core transcriptional regulatory circuitry to maintain ES cell identity. Development. 2008;135:1513–1524. doi: 10.1242/dev.014340. [DOI] [PubMed] [Google Scholar]
- Evans T, Felsenfeld G, Reitman M. Control of globin gene transcription. Annu Rev Cell Biol. 1990;6:95–124. doi: 10.1146/annurev.cb.06.110190.000523. [DOI] [PubMed] [Google Scholar]
- Farh KK, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, Burge CB, Bartel DP. The widespread impact of mammalian microRNAs on mRNA repression and evolution. Science. 2005;310:1817–1821. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- Farkas IJ, Wu C, Chennubhotla C, Bahar I, Oltvai ZN. Topological basis of signal integration in the transcriptional-regulatory network of the yeast, Saccharomyces cere-visiae. BMC Bioinformatics. 2006;7:478. doi: 10.1186/1471-2105-7-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust C, Lawson KA, Schork NJ, Thiel B, Magnuson T. The Polycomb-group gene eed is required for normal morphogenetic movements during gastrulation in the mouse embryo. Development. 1998;125:4495–4506. doi: 10.1242/dev.125.22.4495. [DOI] [PubMed] [Google Scholar]
- Fazzio TG, Huff JT, Panning B. An RNAi screen of chromatin proteins identifies Tip60-p400 as a regulator of embryonic stem cell identity. Cell. 2008;134:162–174. doi: 10.1016/j.cell.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G, Chu AM, Ni L, Connelly C, Riles L, Veron-neau S, Dow S, Lucau-Danila A, Anderson K, Andre B, et al. Functional profiling of the Saccharomyces cere-visiae genome. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- Goll MG, Bestor TH. Eukaryotic cytosine methyl-transferases. Annu Rev Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- Goodrich JA, Kugel JF. Non-coding-RNA regulators of RNA polymerase II transcription. Nat Rev Mol Cell Biol. 2006;7:612–616. doi: 10.1038/nrm1946. [DOI] [PubMed] [Google Scholar]
- Greene WC. Regulation of HIV-1 gene expression. Annu Rev Immunol. 1990;8:453–475. doi: 10.1146/annurev.iy.08.040190.002321. [DOI] [PubMed] [Google Scholar]
- Grewal SI, Elgin SC. Transcription and RNA interference in the formation of heterochromatin. Nature. 2007;447:399–406. doi: 10.1038/nature05914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;130:77–88. doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison CT, Gordon DB, Lee TI, Rinaldi NJ, Macisaac KD, Danford TW, Hannett NM, Tagne JB, Reynolds DB, Yoo J, et al. Transcriptional regulatory code of a eukaryotic genome. Nature. 2004;431:99–104. doi: 10.1038/nature02800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SC. A structural taxonomy of DNA-binding domains. Nature. 1991;353:715–719. doi: 10.1038/353715a0. [DOI] [PubMed] [Google Scholar]
- Hart AH, Hartley L, Ibrahim M, Robb L. Identification, cloning and expression analysis of the pluripotency promoting Nanog genes in mouse and human. Dev Dyn. 2004;230:187–198. doi: 10.1002/dvdy.20034. [DOI] [PubMed] [Google Scholar]
- Hawkins PG, Morris KV. RNA and transcriptional modulation of gene expression. Cell Cycle. 2008;7:602–607. doi: 10.4161/cc.7.5.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix DA, Hong JW, Zeitlinger J, Rokhsar DS, Levine MS. Promoter elements associated with RNA pol II stalling in the Drosophila embryo. Proc Natl Acad Sci. 2008;105:7762–7767. doi: 10.1073/pnas.0802406105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O. Gene regulation by transcription factors and microRNAs. Science. 2008;319:1785–1786. doi: 10.1126/science.1151651. [DOI] [PubMed] [Google Scholar]
- Hunter T. Signaling—2000 and beyond. Cell. 2000;100:113–127. doi: 10.1016/s0092-8674(00)81688-8. [DOI] [PubMed] [Google Scholar]
- Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, DeCoste C, Schafer X, Lun Y, Lemischka IR. Dissecting self-renewal in stem cells with RNA interference. Nature. 2006;442:533–538. doi: 10.1038/nature04915. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Chan YS, Loh YH, Cai J, Tong GQ, Lim CA, Robson P, Zhong S, Ng HH. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10:353–360. doi: 10.1038/ncb1698. [DOI] [PubMed] [Google Scholar]
- Kadonaga JT. Regulation of RNA polymerase II transcription by sequence-specific DNA binding factors. Cell. 2004;116:247–257. doi: 10.1016/s0092-8674(03)01078-x. [DOI] [PubMed] [Google Scholar]
- Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19:489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues K. Essential genes. WormBook (The C elegans Research Community, WormBook) 2005:1–7. doi: 10.1895/wormbook.1.57.1. http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008a;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, Zaehres H, Wu G, Gentile L, Ko K, Sebastiano V, Araúzo-Bravo MJ, Ruau D, Han DW, Zenke M, Schöler HR. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008b;454:646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Klose RJ, Bird AP. Genomic DNA methylation: The mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Komashko VM, Acevedo LG, Squazzo SL, Iyengar SS, Rabinovich A, O'Geen H, Green R, Farnham PJ. Using ChIP-chip technology to reveal common principles of transcriptional repression in normal and cancer cells. Genome Res. 2008;18:521–532. doi: 10.1101/gr.074609.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V, Barker N, Willert K, Molenaar M, Roose J, Wagenaar G, Markman M, Lamers W, Destree O, Clevers H. Two members of the Tcf family implicated in Wnt/β-catenin signaling during embryogenesis in the mouse. Mol Cell Biol. 1998;18:1248–1256. doi: 10.1128/mcb.18.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Latchman DS. Transcription factors: An overview. Int J Biochem Cell Biol. 1997;29:1305–1312. doi: 10.1016/s1357-2725(97)00085-x. [DOI] [PubMed] [Google Scholar]
- Latchman DS. Eukaryotic transcription factors. 5th. Elsevier/Academic; New York: 2008. Transcription factors and human disease; pp. 373–448. chap. 9. [Google Scholar]
- Lee TI, Young RA. Transcription of eukaryotic protein-coding genes. Annu Rev Genet. 2000;34:77–137. doi: 10.1146/annurev.genet.34.1.77. [DOI] [PubMed] [Google Scholar]
- Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TI, Rinaldi NJ, Robert F, Odom DT, Bar-Joseph Z, Gerber GK, Hannett NM, Harbison CT, Thompson CM, Simon I, et al. Transcriptional regulatory networks in Saccharomyces cerevisiae. Science. 2002;298:799–804. doi: 10.1126/science.1075090. [DOI] [PubMed] [Google Scholar]
- Levine M, Tjian R. Transcription regulation and animal diversity. Nature. 2003;424:147–151. doi: 10.1038/nature01763. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- Ma HW, Kumar B, Ditges U, Gunzer F, Buer J, Zeng AP. An extended transcriptional regulatory network of Escherichia coli and analysis of its hierarchical structure and network motifs. Nucleic Acids Res. 2004;32:6643–6649. doi: 10.1093/nar/gkh1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeyev EV, Maniatis T. Multilevel regulation of gene expression by microRNAs. Science. 2008;319:1789–1790. doi: 10.1126/science.1152326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson A, Foreman R, Chevalier B, Bilodeau S, Kahn M, Young R, Jaenisch R. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008a;3:132–135. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson A, Levine SS, Cole MF, Frampton GM, Brambrink T, Johnstone S, Guenther MG, Johnston WK, Wernig M, Newman J, et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008b;134:521–533. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Antonio A, Collado-Vides J. Identifying global regulators in transcriptional regulatory networks in bacteria. Curr Opin Microbiol. 2003;6:482–489. doi: 10.1016/j.mib.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25:1177–1181. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- Meister G. miRNAs get an early start on translational silencing. Cell. 2007;131:25–28. doi: 10.1016/j.cell.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Merrill BJ, Pasolli HA, Polak L, Rendl M, Garcia-Garcia MJ, Anderson KV, Fuchs E. Tcf3: A transcriptional regulator of axis induction in the early embryo. Development. 2004;131:263–274. doi: 10.1242/dev.00935. [DOI] [PubMed] [Google Scholar]
- Mikkelsen TS, Hanna J, Zhang X, Ku M, Wernig M, Schorderet P, Bernstein BE, Jaenisch R, Lander ES, Meissner A. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Miyabayashi T, Teo JL, Yamamoto M, McMillan M, Nguyen C, Kahn M. Wnt/β-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc Natl Acad Sci. 2007;104:5668–5673. doi: 10.1073/pnas.0701331104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D, Buhler M, Buker SM, Colmenares SU, Gerace EL, Gerber SA, Hong EJ, Motamedi MR, Verdel A, Villen J, Gygi SP. Studies on the mechanism of RNAi-dependent heterochromatin assembly. Cold Spring Harbor Symp Quant Biol. 2006;71:461–471. doi: 10.1101/sqb.2006.71.044. [DOI] [PubMed] [Google Scholar]
- Murchison EP, Partridge JF, Tam OH, Cheloufi S, Hannon GJ. Characterization of Dicer-deficient murine embryonic stem cells. Proc Natl Acad Sci. 2005;102:12135–12140. doi: 10.1073/pnas.0505479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muse GW, Gilchrist DA, Nechaev S, Shah R, Parker JS, Grissom SF, Zeitlinger J, Adelman K. RNA polymerase is poised for activation across the genome. Nat Genet. 2007;39:1507–1511. doi: 10.1038/ng.2007.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Nechaev S, Adelman K. Promoter-proximal pol II: When stalling speeds things up. Cell Cycle. 2008;7:1539–1544. doi: 10.4161/cc.7.11.6006. [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Schöler H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- O'Carroll D, Erhardt S, Pagani M, Barton SC, Surani MA, Jenuwein T. The Polycomb-group gene Ezh2 is required for early mouse development. Mol Cell Biol. 2001;21:4330–4336. doi: 10.1128/MCB.21.13.4330-4336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom DT, Dowell RD, Jacobsen ES, Nekludova L, Rolfe PA, Danford TW, Gifford DK, Fraenkel E, Bell GI, Young RA. Core transcriptional regulatory circuitry in human hepatocytes. Mol Syst Biol. 2006;2:0017. doi: 10.1038/msb4100059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom DT, Zizlsperger N, Gordon DB, Bell GW, Rinaldi NJ, Murray HL, Volkert TL, Schreiber J, Rolfe PA, Gifford DK, Fraenkel E, Bell GI, Young RA. Control of pancreas and liver gene expression by HNF transcription factors. Science. 2004;303:1378–1381. doi: 10.1126/science.1089769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata K, Sato K, Tahirov TH. Eukaryotic transcriptional regulatory complexes: Cooperativity from near and afar. Curr Opin Struct Biol. 2003;13:40–48. doi: 10.1016/s0959-440x(03)00012-5. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Nishinakamura R, Iwamatsu Y, Shimosato D, Niwa H. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem Biophys Res Commun. 2006;343:159–166. doi: 10.1016/j.bbrc.2006.02.127. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Orlando V. Polycomb, epigenomes, and control of cell identity. Cell. 2003;112:599–606. doi: 10.1016/s0092-8674(03)00157-0. [DOI] [PubMed] [Google Scholar]
- Orphanides G, Reinberg D. A unified theory of gene expression. Cell. 2002;108:439–451. doi: 10.1016/s0092-8674(02)00655-4. [DOI] [PubMed] [Google Scholar]
- Pabo CO, Sauer RT. Transcription factors: Structural families and principles of DNA recognition. Annu Rev Biochem. 1992;61:1053–1095. doi: 10.1146/annurev.bi.61.070192.005201. [DOI] [PubMed] [Google Scholar]
- Pan G, Tian S, Nie J, Yang C, Ruotti V, Wei H, Jonsdottir GA, Stewart R, Thomson JA. Whole-genome analysis of histone H3 lysine 4 and lysine 27 methylation in human embryonic stem cells. Cell Stem Cell. 2007;1:299–312. doi: 10.1016/j.stem.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Panne D, Maniatis T, Harrison SC. Crystal structure of ATF-2/c-Jun and IRF-3 bound to the interferon-β enhancer. EMBO J. 2004;23:4384–4393. doi: 10.1038/sj.emboj.7600453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Pasini D, Bracken AP, Jensen MR, Lazzerini Denchi E, Helin K. Suz12 is essential for mouse development and for EZH2 histone methyltransferase activity. EMBO J. 2004;23:4061–4071. doi: 10.1038/sj.emboj.7600402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L, Yi F, Merrill BJ. Repression of Nanog gene transcription by Tcf3 limits embryonic stem cell self-renewal. Mol Cell Biol. 2006;26:7479–7491. doi: 10.1128/MCB.00368-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V. Polycombing the genome: PcG, trxG, and chromatin silencing. Cell. 1998;93:333–336. doi: 10.1016/s0092-8674(00)81162-9. [DOI] [PubMed] [Google Scholar]
- Pokholok DK, Zeitlinger J, Hannett NM, Reynolds DB, Young RA. Activated signal transduction kinases frequently occupy target genes. Science. 2006;313:533–536. doi: 10.1126/science.1127677. [DOI] [PubMed] [Google Scholar]
- Rada-Iglesias A, Ameur A, Kapranov P, Enroth S, Komorowski J, Gingeras TR, Wadelius C. Whole-genome maps of USF1 and USF2 binding and histone H3 acetylation reveal new aspects of promoter structure and candidate genes for common human disorders. Genome Res. 2008;18:380–392. doi: 10.1101/gr.6880908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada-Iglesias A, Wallerman O, Koch C, Ameur A, Enroth S, Clelland G, Wester K, Wilcox S, Dovey OM, Ellis PD, et al. Binding sites for metabolic disease related transcription factors inferred at base pair resolution by chromatin immunoprecipitation and genomic microarrays. Hum Mol Genet. 2005;14:3435–3447. doi: 10.1093/hmg/ddi378. [DOI] [PubMed] [Google Scholar]
- Reed BD, Charos AE, Szekely AM, Weissman SM, Snyder M. Genome-wide occupancy of SREBP1 and its partners NFY and SP1 reveals novel functional roles and combinatorial regulation of distinct classes of genes. PLoS Genet. 2008;4:e1000133. doi: 10.1371/journal.pgen.1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remenyi A, Schöler HR, Wilmanns M. Combinatorial control of gene expression. Nat Struct Mol Biol. 2004;11:812–815. doi: 10.1038/nsmb820. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet. 2004;38:413–443. doi: 10.1146/annurev.genet.38.072902.091907. [DOI] [PubMed] [Google Scholar]
- Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Schöler HR, Dressler GR, Balling R, Rohdewohld H, Gruss P. Oct-4: A germline-specific transcription factor mapping to the mouse t-complex. EMBO J. 1990;9:2185–2195. doi: 10.1002/j.1460-2075.1990.tb07388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–745. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Schwartz YB, Pirrotta V. Polycomb silencing mechanisms and the management of genomic programmes. Nat Rev Genet. 2007;8:9–22. doi: 10.1038/nrg1981. [DOI] [PubMed] [Google Scholar]
- Shyamsundar R, Kim YH, Higgins JP, Montgomery K, Jorden M, Sethuraman A, van de Rijn M, Botstein D, Brown PO, Pollack JR. A DNA microarray survey of gene expression in normal human tissues. Genome Biol. 2005;6:R22. doi: 10.1186/gb-2005-6-3-r22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla DK, Schneider DJ, LeWinter MM, Sobel BE. wnt3a but not wnt11 supports self-renewal of embryonic stem cells. Biochem Biophys Res Commun. 2006;345:789–795. doi: 10.1016/j.bbrc.2006.04.125. [DOI] [PubMed] [Google Scholar]
- Sinkkonen L, Hugenschmidt T, Berninger P, Gaidatzis D, Mohn F, Artus-Revel CG, Zavolan M, Svoboda P, Filipowicz W. MicroRNAs control de novo DNA methylation through regulation of transcriptional repressors in mouse embryonic stem cells. Nat Struct Mol Biol. 2008;15:259–267. doi: 10.1038/nsmb.1391. [DOI] [PubMed] [Google Scholar]
- Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9:219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- Stock JK, Giadrossi S, Casanova M, Brookes E, Vidal M, Koseki H, Brockdorff N, Fisher AG, Pombo A. Ring1-mediated ubiquitination of H2A restrains poised RNA polymerase II at bivalent genes in mouse ES cells. Nat Cell Biol. 2007;9:1428–1435. doi: 10.1038/ncb1663. [DOI] [PubMed] [Google Scholar]
- Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tam WL, Lim CY, Han J, Zhang J, Ang YS, Ng HH, Yang H, Lim B. T-cell factor 3 regulates embryonic stem cell pluripotency and self-renewal by the transcriptional control of multiple lineage pathways. Stem Cells. 2008;26:2019–2031. doi: 10.1634/stemcells.2007-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek-Plewa J, Jagodziński PP. The role of mammalian DNA methyltransferases in the regulation of gene expression. Cell Mol Biol Lett. 2005;10:631–647. [PubMed] [Google Scholar]
- Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- Verdel A, Jia S, Gerber S, Sugiyama T, Gygi S, Grewal SI, Moazed D. RNAi-mediated targeting of heterochromatin by the RITS complex. Science. 2004;303:672–676. doi: 10.1126/science.1093686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickaryous MK, Hall BK. Human cell type diversity, evolution, development, and classification with special reference to cells derived from the neural crest. Biol Rev Camb Philos Soc. 2006;81:425–455. doi: 10.1017/S1464793106007068. [DOI] [PubMed] [Google Scholar]
- Villard J. Transcription regulation and human diseases. Swiss Med Wkly. 2004;134:571–579. doi: 10.4414/smw.2004.10191. [DOI] [PubMed] [Google Scholar]
- Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet. 2007;39:380–385. doi: 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, Cui K, Roh TY, Peng W, Zhang MQ, Zhao K. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40:897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H, Tapscott SJ, Davis RL, Thayer MJ, Adam MA, Lassar AB, Miller AD. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci. 1989;86:5434–5438. doi: 10.1073/pnas.86.14.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell TK, Turner D, Rupp R, Hollenberg S, et al. The myoD gene family: Nodal point during specification of the muscle cell lineage. Science. 1991;251:761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Cassady JP, Jaenisch R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell. 2008;2:10–12. doi: 10.1016/j.stem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- West AG, Fraser P. Remote control of gene transcription. Hum Mol Genet. 2005;14:R101–R111. doi: 10.1093/hmg/ddi104. spec. no. 1. [DOI] [PubMed] [Google Scholar]
- Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke JD, Bussey H, et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2:333–344. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Chen X, Zhang J, Loh YH, Low TY, Zhang W, Sze SK, Lim B, Ng HH. Sall4 interacts with Nanog and co-occupies Nanog genomic sites in embryonic stem cells. J Biol Chem. 2006;281:24090–24094. doi: 10.1074/jbc.C600122200. [DOI] [PubMed] [Google Scholar]
- Yang SH, Sharrocks AD, Whitmarsh AJ. Transcriptional regulation by the MAP kinase signaling cascades. Gene. 2003;320:3–21. doi: 10.1016/s0378-1119(03)00816-3. [DOI] [PubMed] [Google Scholar]
- Yi F, Pereira L, Merrill BJ. Tcf3 functions as a steady-state limiter of transcriptional programs of mouse embryonic stem cell self-renewal. Stem Cells. 2008;26:1951–1960. doi: 10.1634/stemcells.2008-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zaratiegui M, Irvine DV, Martienssen RA. Non-coding RNAs and gene silencing. Cell. 2007;128:763–776. doi: 10.1016/j.cell.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Zeitlinger J, Stark A, Kellis M, Hong JW, Nechaev S, Adelman K, Levine M, Young RA. RNA polymerase stalling at developmental control genes in the Drosophila melanogaster embryo. Nat Genet. 2007;39:1512–1516. doi: 10.1038/ng.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JZ, Gao W, Yang HB, Zhang B, Zhu ZY, Xue YF. Screening for genes essential for mouse embryonic stem cell self-renewal using a subtractive RNA interference library. Stem Cells. 2006;24:2661–2668. doi: 10.1634/stemcells.2006-0017. [DOI] [PubMed] [Google Scholar]
- Zhao XD, Han X, Chew JL, Liu J, Chiu KP, Choo A, Orlov YL, Sung WK, Shahab A, Kuznetsov VA, et al. Whole-genome mapping of histone H3 Lys4 and 27 trimethylations reveals distinct genomic compartments in human embryonic stem cells. Cell Stem Cell. 2007;1:286–298. doi: 10.1016/j.stem.2007.08.004. [DOI] [PubMed] [Google Scholar]