Summary
Glycogen synthase kinase–3 (GSK-3) is a multifunctional serine/threonine kinase found in all eukaryotes. The enzyme is a key regulator of numerous signalling pathways, including cellular responses to Wnt, receptor tyrosine kinases and G protein-coupled receptors and is involved in a wide swathe of cellular processes ranging from glycogen metabolism to cell cycle regulation and proliferation. GSK-3 is unusual in that it is usually constitutively active in cells and is regulated through inhibition of its activity. Another peculiarity compared with other protein kinases is GSK-3’s preference for primed substrates, i.e. substrates previously phosphorylated by another kinase. This article presents an overview of the many ways in which GSK-3 is regulated and focuses on recent advances in our understanding of GSK-3 regulation in multiple pathways. Specifically addressed are: implications of the recent solution of the crystal structure of GSK-3 with respect to GSK-3’s penchant for primed substrates and the regulation of GSK-3 by serine phosphorylation, new findings related to GSK-3 regulation of the Wnt/β-catenin pathway, the newly described involvement of GSK-3 in the Hedgehog pathway, and a discussion of new GSK-3 inhibitors and what they are revealing about this unusual enzyme.
Introduction
Glycogen synthase kinase – 3 (GSK-3) is a serine/threonine kinase that was first isolated and purified as an activity capable of phosphorylating and inactivating the enzyme glycogen synthase (Embi et al., 1980; Woodgett and Cohen, 1984). We now know that beyond its role in glycogen metabolism, GSK-3 acts as a downstream regulatory switch that determines the output of numerous signaling pathways initiated by diverse stimuli (recently reviewed in: (Frame and Cohen, 2001; Grimes and Jope, 2001; Woodgett, 2001). The pathways in which GSK-3 acts as a key regulator, when dysregulated, have been implicated in the development of human diseases such as diabetes, Alzheimer’s disease, bipolar disorder and cancer.
Given its involvement in many pathophysiological processes and diseases, GSK-3 is a tempting therapeutic target. However, its proclivity for involvement in multiple pathways also raises the issue of selectivity – how might one process be impacted while leaving others untouched. It is also imperative to assess the full spectrum of GSK-3 cell functions to avoid later surprises. For example, while inhibition of GSK-3 may be desirable in one context, say, in preventing neuronal apoptosis, it could have serious implications in accelerating hyperplasia should, another function of GSK-3 be impacted - such as the regulation of cell proliferation by deregulation of β-catenin. That said, the recent development of small molecule inhibitors of GSK-3 has provided new tools for visualizing the cell from GSK-3’s perspective. Since GSK-3 is constitutively active in most cell types, regulation of substrate phosphorylation occurs either by inactivation of GSK-3 or by changing substrate accessibility or recognition. In this review we present an overview of the various processes in which GSK-3 plays a key role and describe the current status of knowledge of GSK-3 regulation.
GSK-3 variants
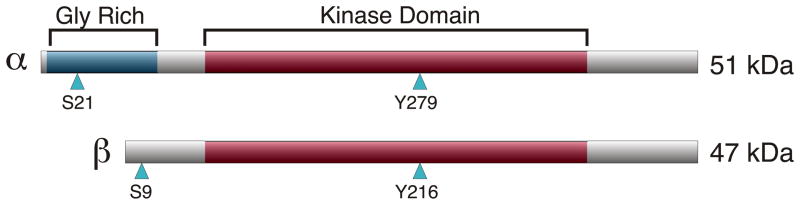
There are two mammalian GSK-3 isoforms that are encoded by distinct genes: GSK-3α and GSK-3β (Woodgett, 1990) (fig. 1). GSK-3α has a mass of 51 kDa, while GSK-3β encodes a protein of 47 kDa. The difference in size between the two isoforms is due to a glycine rich extension found only at the amino terminus of GSK-3α. Although highly homologous within their kinase domains (98% identity), the two gene products share only 36% identity in the last 76 C-terminal amino acid residues of the proteins and the mass difference is a result of a glycine-rich amino terminal extension in the α isoform (Woodgett, 1990). Homologues of GSK-3 have been found in all eukaryotes examined to date, and display a high degree of homology, with isoforms from species as distant as flies and humans displaying over 90% homology within the kinase domain (reviewed in Ali et al., 2001).
Fig. 1.
Schematic representation of mammalian GSK-3α and GSK-3β. Sites of serine and tyrosine phosphorylation are indicated with blue arrowheads. The glycine-rich amino-terminal domain unique to GSK-3α and the conserved kinase domain shared by both isoforms are highlighted.
GSK-3α and GSK-3β, although structurally similar, are not functionally identical. This became obvious upon ablation of the GSK-3β isoform in mice, resulting in an embryonic lethal phenotype (Hoeflich et al., 2000). Embryo’s carrying homozygous deletions of exon 2 of GSK-3β die around embryonic day 16 due to massive liver degeneration caused by extensive hepatocyte apoptosis. This phenotype is remarkably similar to that observed in animalslacking RelA or I-κB kinase 2 (Beg et al., 1995; Li et al., 1999), components of the NF-κB signaling pathway (Ghosh and Karin, 2002; Rothwarf and Karin, 1999). Regulation of NF-κB nuclear import was not disrupted in embryonic fibroblasts isolated from the GSK-3β null mice, indicating that GSK-3 regulates a component(s) affecting some other level of NF-κB regulation. The specific target(s) of GSK-3β in the regulation of NF-κB signaling has not been identified. Other protein kinases have been shown to phosphorylate p65, with consequent increases in transcriptional transactivation (Jang et al., 2001; Wang et al., 2000). Indeed, GSK-3β is capable of phosphorylating the C-terminal domain (residues 354–551) of the p65 subunit of NF-κB in vitro (Schwabe and Brenner, 2002), but further studies are required to verify if p65 is a physiological GSK-3β substrate in vivo and to assess the effect of this modification.
A minor (~15% of total) splice variant of GSK-3β, termed GSK-3β2, has recently been identified which contains a 13 amino acid insert within the kinase domain (Mukai et al., 2002). Analysis of the in vitro kinase activity of GSK-3β2 revealed reduced activity towards the microtubule-associated protein, tau, compared to “unspliced” GSK-3β. An antibody selective for the novel splice insertion polypeptide revealed that GSK-3β2 is localized primarily to neuronal cell bodies, unlike unspliced GSK-3β that is also found in neuronal processes. It is unclear whether these substrate and subcellular localization differences can be generalized to other proteins or cells. Given the location of the insert within a highly conserved sequence, the splice-encoded peptide likely forms a loop/hook which may allow differential binding of the splice variant to scaffolding proteins that then exposes the isoform to a distinct subset of target proteins (Mukai et al., 2002).
GSK-3 regulation by insulin and growth factors: inhibition by serine phosphorylation
Stimulation of cells with insulin causes inactivation of GSK-3 via a phosphatidylinositol 3-kinase (PI3K)-dependent mechanism. PI3K-induced activation of PKB (also termed Akt) results in PKB phosphorylation of both GSK-3 isoforms (serine 9 of GSK-3β; serine 21 of GSK-3α) (Cross et al., 1995). Phosphorylation of these residues inhibits GSK-3 activity (see below). PI3K-dependent inhibition of GSK-3 leads to the dephosphorylation of GSK-3 substrates including glycogen synthase and eukaryotic protein synthesis initiation factor-2B (eIF-2B), resulting in their functional activation and leading to increased glycogen and protein synthesis (reviewed in Cohen et al., 1997). GSK-3 has an unusual preference for target proteins that are pre-phosphorylated at a “priming” residue located C-terminal to the site of GSK-3 phosphorylation (Fiol et al., 1987). The consensus sequence for GSK-3 substrates is Ser/Thr–X–X–X-Ser/Thr-P, where the first Ser or Thr is the target residue, X is any amino acid (but often proline), and the last Ser-P/Thr-P is the site of priming phosphorylation. Although not strictly required, priming phosphorylation greatly increases the efficiency of substrate phosphorylation of most GSK-3 substrates by a factor of 100-1000X (Thomas et al., 1999). For example, glycogen synthase, the prototypical primed substrate, requires a priming phosphorylation event by casein kinase II (CK2) and then undergoes sequential multi-site phosphorylation by GSK-3 (Fiol et al., 1988; Fiol et al., 1990).
The crystal structure of GSK-3 was recently determined by three groups (Bax et al., 2001; Dajani et al., 2001; ter Haar et al., 2001). The structure has provided insight into the preference of GSK-3 for primed, pre-phosphorylated substrates. Protein kinases related to GSK-3, such as CDK2, p38γ and ERK2, require phosphorylated residues on their activation loops as a prerequisite for activity (Bellon et al., 1999; Brown et al., 1999; Canagarajah et al., 1997). A phospho-threonine is used by all three related kinases to align intramolecularly key β-strand and α-helical domains. In addition, a phosphotyrosine residue is employed by p38γ and ERK2 to open up the catalytic site for substrate access. The activation loop of GSK-3 is also phosphorylated. This so-called “T-loop” habours a phosphorylated tyrosine at Y216/Y279 (GSK-3β/GSK-3α, respectively). Although Y216/Y279 phosphorylation could play a role in forcing open the substrate binding site, there appear to be no constraints preventing the open conformation in the unphosphorylated state (Dajani et al., 2001). Thus, it is suggested that T-loop phosphorylation of GSK-3 facilitates substrate phosphorylation, but is not strictly required for kinase activity (Hughes et al., 1993; Dajani et al., 2001). The function expected for the “missing” phospho-threonine in GSK-3’s activation loop is believed to be replaced by the phosphorylated residue of a primed substrate, which binds to a positively charged pocket comprised of R96, R180, and K205 (residue numbers for GSK-3β). This not only optimizes orientation of kinase domains, but also places the substrate at the correct position within the catalytic groove for phosphorylation to occur. There are some substrates that lack a priming site. These proteins often display negatively charged residues at or near the priming position that may mimic a phospho-residue.
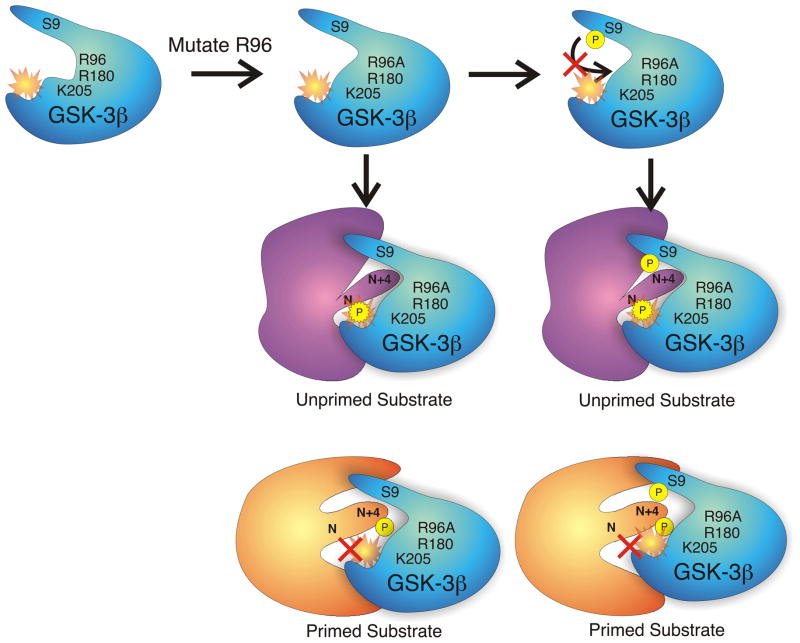
The crystal structure of GSK-3 also helps to explain the inhibitory role of serine phosphorylation near the amino terminus (see fig. 2A). Phosphorylation of S9 of GSK-3β or S21 of GSK-3α creates a primed pseudo-substrate that is capable of intramolecularly binding to the positively charged pocket mentioned above. This folding precludes phosphorylation of any substrates because the catalytic groove is occupied. Note that the mechanism of inhibition is competitive. A consequence of this mode of inhibition is that primed substrates, in high enough concentrations, can compete off the pseudosubstrate, leading to their phosphorylation (Frame et al., 2001). Thus, although less efficient, un-primed substrates may provide a more accurate measure of GSK-3 activity in kinase assays performed in vitro. Mutation of R96 to alanine disrupts the pocket of positive charge (fig. 2B) (Frame et al., 2001). Interestingly, although a peptide 11 amino acids long, matching the phosphorylated N-terminus of GSK-3β (NTptide-11), acts as a competitive inhibitor for both primed and unprimed substrates, a truncated version of this peptide (NTptide-8) only inhibits GSK-3 phosphorylation of primed substrates (Frame et al., 2001). Thus, small molecule inhibitors modeled to fit in the positively charged pocket of GSK-3’s kinase domain could potentially be very effective for selective inhibition of primed substrates.
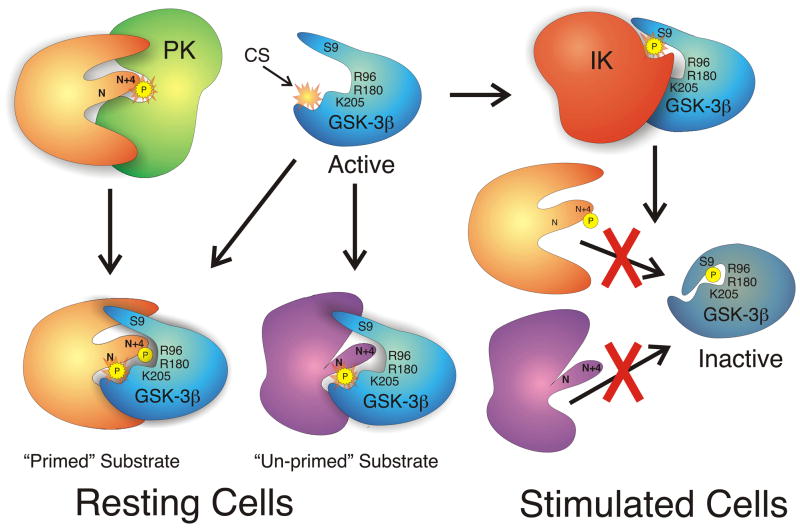
Fig. 2.
Fig. 2A. Regulation of GSK-3β activity by serine phosphorylation. In the resting cell, GSK-3β is constitutively active. Both un-primed substrates and substrates phosphorylated by a priming kinase (PK) are capable of being phosphorylated by the active GSK-3β. The priming phospho-residue at position N + 4, binds a pocket of positive charge arising from the arginine (R) and lysine (K) residues indicated. This directs a serine or threonine at position N to the active catalytic site (c.s.). When an inactivating kinase (IK) such as PKB/Akt phosphorylates GSK-3β on serine 9 (S9), the phosphorylated amino-terminus becomes a primed pseudo-substrate that occupies the positive binding pocket and active site of the enzyme, acting as a competitive inhibitor for true substrates. This prevents phosphorylation of any substrates. 2B. Effect of mutating arginine 96 to alanine (R96A) on GSK-3β activity. Since R96 is a crucial component of the positive pocket that binds primed substrates, its mutation to an uncharged ala residue disrupts the pocket so that primed substrates can no longer bind. The enzyme retains activity. Also, the S9-phosphorylated pseudosubstrate is no longer capable of inactivating the enzyme. As a consequence, GSK-3β whether S9-phosphorylated or not, can phosphorylate unprimed substrates, but not primed substrates.
Numerous other stimuli other than insulin have been shown to lead to inactivation of GSK-3 through serine phosphorylation. Alternate signaling pathways reported to converge on GSK-3 inactivation through serine phosphorylation include: 1) Activation of the mitogen-activated protein kinase (ERK/MAPK) pathway by growth factors such as EGF, and PDGF (Brady et al., 1998; Saito et al., 1994), via stimulation of the GSK-3-inactivating kinase p90RSK (also known as MAPKAP-K1); 2) Activation of p70 ribosomal S6 kinase (p70S6K) by addition of amino acids to cultured myocytes (Armstrong et al., 2001), perfused rat hearts (Terruzzi et al., 2002), or isolated rat hepatocytes (Krause et al., 2002); 3) Activation of cAMP activated protein kinase (PKA) with cell permeable cAMP analogues or stimulation with forskolin (Fang et al., 2000; Li et al., 2000; Tanji et al., 2002); 4) Activation of PKC by lysophosphatidic acid (LPA) or phenylephrine (Ballou et al., 2001; Fang et al., 2002). “Cross-talk” between the different signaling pathways described above is commonly observed, with many of the stimuli working through more than one pathway. For instance, EGF and PDGF also activate PKB which leads to Serine 9/21 phosphorylation of GSK-3 (Brady et al., 1998; Cross et al., 1997).
With so many pathways converging at a single node of GSK-3 inactivation, how are specific physiological responses generated to the different stimuli? Cells appear to use several different mechanisms to impart signalling specificity mediated through GSK-3 inactivation. An obvious means is to employ additional and agonist-specific signaling events in parallel to GSK-3 inactivation. For instance, in adipocytes, insulin not only leads to GSK-3 inactivation, but also stimulates glucose uptake and activation of glycogen-associated type 1 protein phosphatase (PP-1C), all of which are required to promote glycogen synthesis (Dent et al., 1990; Robinson et al., 1993). EGF causes inactivation of GSK-3 primarily through activation of the MAPK/p90 ribosomal protein S6 kinase (p90RSK) signaling pathway (Eldar-Finkelman et al., 1995). EGF-treated adipocytes exhibit reduced GSK-3 activity, but do not show enhanced glycogen synthesis since neither PP-1C is activated nor is glucose uptake increased, possibly due to short-lived PKB activation (Cross et al., 1997). Similar results were obtained when adipocytes were stimulated with PDGF (Brady et al., 1998). Other mechanisms regulating the specificity of the outcome of GSK-3 inactivation are described below.
GSK-3 regulation by tyrosine phosphorylation: a putative role in neuronal apoptosis
As described above, GSK-3β is fully activated through phosphorylation of Y216 in its activation loop. The physiological significance of Y216 phosphorylation in mammalian cells is unclear since this phosphorylation is constitutive in resting cells (Hughes et al., 1993). Indeed, the kinase(s) responsible for GSK-3 tyrosine phosphorylation in unstimulated cells has not been identified, unlike the tyrosine kinase Zak1 that tyrosine phosphorylates the Y216 equivalent of the slime mould homologue of GSK-3 (Kim et al., 1999; see below). GSK-3β expressed in bacteria shows evidence of autophosphorylation on tyrosine, serine and threonine residues, raising the possibility that tyrosine phosphorylation of GSK-3 in mammalian cells is an autocatalytic event (Wang et al., 1994). GSK-3 immunoprecipitated from mammalian cells does not show this behaviour, however (Hughes et al., 1993)
Although GSK-3 is tyrosine phosphorylated in resting cells, the level may not be stoichiometric. Apoptotic stimuli, such as staurosporine treatment or neurotrophic factor withdrawal, resulted in increased GSK-3 activity and tyrosine phosphorylation, implicating GSK-3 in the development of apoptosis in the neuronal cell lines tested (Bhat et al., 2000; Bijur and Jope, 2001). Also in neuronal cells, LPA, through a pathway involving the G-proteins Gα12 and Gα13, has been shown to increase GSK-3 activity at least in part through enhanced tyrosine phosphorylation of Y216/279 (Sayas et al., 2002). Treatment of primary neurons with LPA resulted in neurite retraction, while treatment of a neuroblastoma cell line, Neuro2A, resulted in cell rounding (Sayas et al., 2002). LPA has also been shown to cause apoptosis of adult neurons (Steiner et al., 2000). No GSK-3 tyrosine kinases were identified in the above studies, but separate studies presented below suggest two possible candidates.
Transient increases in intracellular calcium have been reported to increase GSK-3- mediated phosphorylation of the microtubule associated protein tau, with the increased GSK-3 activity attributed to elevated GSK-3 tyrosine phosphorylation (Hartigan and Johnson, 1999). A tyrosine kinase that has been implicated in mediating this effect: proline-rich tyrosine kinase 2 (PYK)1 since this kinase is sensitive to calcium (Hartigan et al., 2001). The Fyn tyrosine kinase is another potential player implicated in GSK-3 tyrosine Insulin treatment of SH-SY5Y cells for 1 minute caused an increase in Fyn association with GSK-3β, a transient increase in GSK-3β activity with a concomitant transient increase in GSK-3β phosphorylation on Y216, and a transient increase in Tau phosphorylation. The short duration of insulin treatment is crucial for the observed increase in GSK-3 activity (Lesort et al., 1999). Prolonged treatment with insulin, besides the well-characterized inhibition of GSK-3 through serine phosphorylation discussed in the previous section, has also been reported to inhibit GSK-3 by tyrosine-dephosphorylation, in Chinese hamster ovary cells (Murai et al., 1996). Further characterization of the proposed GSK-3 tyrosine kinases is required to substantiate a role for them in physiological processes.
To date, the only organism in which a clear role has been identified for GSK-3 tyrosine phosphorylation is the slime mould, Dictyostelium discoideum. In Dictyostelium, the GSK-3 tyrosine kinase Zak1 has been shown to be essential for activation of GSK-3 during developmental patterning and is regulated downstream of certain cyclic AMP receptors involved in chemotaxis (Kim et al., 1999; Plyte et al., 1999). Unfortunately, identification of a mammalian orthologue of Zak1 has so far proven elusive. An opposing role for a protein tyrosine phosphatase, which dephosphorylates GSK-3 on tyrosine residues and results in a prestalk fate, has been described recently (Kim et al., 2002). Interestingly, phosphorylation of two tyrosine residues, both residing in the activation loop of GSK-3, appear to be modulated by cAMP in Dictyostelium (Kim et al., 2002). Both of these tyrosine residues, Y214 and Y220, are conserved in mammals (Y216 and Y219, respectively). Thus, Y219 may also play a role in GSK-3 regulation in mammals.
The role of GSK-3 in the Wnt/β-catenin pathway
The Wnts are a family of secreted, cysteine-rich, glycosylated, protein ligands that influence cell growth, differentiation, migration and fate (Miller, 2002; Polakis, 2000; Smalley and Dale, 1999). Several members of the Wnt family have been identified in organisms ranging in complexity from the worm Caenorhabditis elegans to humans, with at least 19 different Wnts identified in mammals (Miller, 2002). Severe developmental defects usually occur in mice engineered to be defective in Wnt signaling. One of the pathways regulated by Wnt molecules is termed the canonical Wnt pathway, or the Wnt/β-catenin pathway (reviewed in: Polakis, 2000; Seidensticker and Behrens, 2000; Sharpe et al., 2001) (fig. 3). For the purposes of this review, discussion of Wnt signaling is limited to the Wnt/β-catenin pathway. Wnt signal transduction ultimately results in the activation of genes utilizing the T-cell factor (TCF)/lymphoid enhancer factor (LEF) family of architectural transcription factors (reviewed in Barker et al., 2000; Brantjes et al., 2002; Novak and Dedhar, 1999). The effector molecule responsible for activating TCF/LEF responsive genes is β-catenin, which in this pathway, serves as a transcriptional transactivator than binds to the DNA-bound TCF/LEFs. As in the insulin pathway, GSK-3 plays a key inhibitory role in the Wnt pathway. In unstimulated cells, GSK-3 phosphorylates the N-terminal domain of β-catenin thereby targeting it for ubiquitination and proteasomal degradation. Exposure of cells to Wnts leads to inactivation of GSK-3 through an as yet unclear mechanism. The phospho-protein Dishevelled is required, after receptor-ligand interaction, to transduce the signal that results in the inactivation of GSK-3. As a result, β-catenin is dephosphorylated (van Noort et al., 2002) and escapes the ubiquitination-dependent destruction machinery. Unphosphorylated β-catenin accumulates in the cytoplasm and translocates to the nucleus where it can associates with the TCF/LEFs and becomes a transcriptional transactivator. Mutations in β-catenin that prevent its phosphorylation by GSK-3 (gain-of-function mutations) have been found in cancers of the skin, colon, prostate, liver, endometrium and ovary (reviewed in Polakis, 2000). Many of the components of the Wnt pathway, and their functional roles, were first identified by studying the orthologous Wnt pathway in Drosophila melanogaster, driven by the Wnt homologue, Wingless (reviewed in Dierick and Bejsovec, 1999; Manoukian and Woodgett, 2002; Siegfried and Perrimon, 1994). In Drosophila, the GSK-3 homologue is termed shaggy (Bourouis et al., 1990) or zeste-white3 (Siegfried et al., 1990).
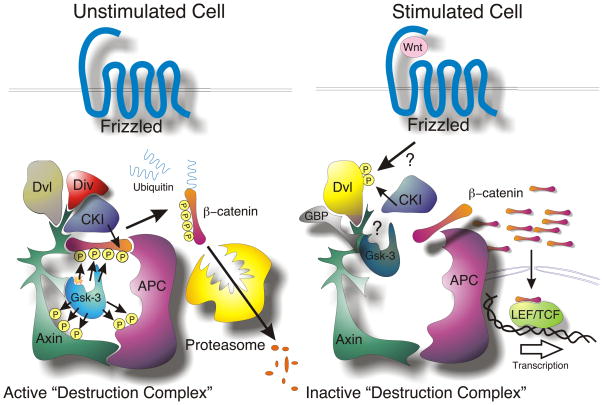
Fig. 3.
Central Role of GSK-3 in the Wnt/β-catenin Pathway. In unstimulated cells, phosphorylation by CKI (S45) primes β-catenin for subsequent phosphorylation by GSK-3 (S41,S37,S33) which targets β-catenin for ubiquitination and proteasomal degradation. The ankyrin repeat protein, Diversin (Div) may help recruit CKI to the destruction complex. Wnt stimulation activates the receptor Frizzled, which then signals through Dishevelled (Dvl), using an unclear mechanism, to inactivate β-catenin phosphorylation. Unphosphorylated β-catenin accumulates and then translocates to the nucleus where it transactivates genes regulated by TCF/LEF transcription factors. The GSK-3 binding protein (GBP/FRAT) may be involved in transmission of a Wnt signal by regulating GSK-3’s binding to the scaffold protein, Axin.
Phosphorylation of β-catenin by GSK-3 occurs in a complex sometimes referred to as the destruction complex, that consists minimally of the proteins GSK-3, β-catenin, Axin/Conductin and adenomatous polyposis coli (APC)(Hinoi et al., 2000). APC is a tumour suppressor protein commonly deleted in familial adenomatous polyposis and sporadic colorectal cancer (reviewed in Polakis, 1997). Axin (and a related protein known as Conductin or Axil) harbours several protein-protein interaction domains and serves as a scaffolding protein that holds together the elements of the β-catenin destruction complex. Both Axin and APC are phosphorylated by GSK-3. Phosphorylation of Axin by GSK-3 is reported to increase its stability and increase its binding to β-catenin (Ikeda et al., 1998; Jho et al., 1999; Yamamoto et al., 1999). Phosphorylation of APC increases its binding to β-catenin (Rubinfeld et al., 1996).
Four groups have recently determined that β-catenin is also a primed substrate for GSK-3, with casein kinase I (CKI) acting as the priming kinase (Amit et al., 2002; Hagen et al., 2002; Hagen and Vidal-Puig, 2002; Liu et al., 2002; Yanagawa et al., 2002). CKI targets serine 45, four residues C-terminal to three GSK-3 targets at serines 33, 37 and 41. In the context of a priming kinase, CKI functions as negative regulator of Wnt signaling. This is in contrast to several previous reports that previously identified CKI as a positive transducer of Wnt signaling (Gao et al., 2002; Kishida et al., 2001; Lee et al., 2001; McKay et al., 2001). CKI has been shown to bind Axin and Dishevelled and to phosphorylate not only β-catenin, but also Axin, Dishevelled, and APC. A model in which CKI can act as both a positive and negative regulator of Wnt signaling has been proposed (Polakis, 2002). In this model, CKI plays a role in the destruction complex as the priming kinase for GSK-3 and is required for transmission of the Wnt signal by assisting in the activation of Dishevelled, perhaps by increasing its affinity for signaling intermediates (see below). A novel ankyrin repeat containing protein, Diversin, has been reported to recruit CKI to the destruction complex (Schwarz-Romond et al., 2002).
These data raise the possibility that GSK-3 plays only a latent role in regulation of β-catenin. For example, if CKI activity is directly regulated by Wnt signalling, then phosphorylation of serine 45 would act as the trigger for subsequent phosphorylation by GSK-3. In this scenario, the activity of GSK-3 could be totally independent of Wnt regulation. However, phosphorylation of serine 45 appears to be constitutive, at least in some cell types. Although it has been reported that serine 45 phosphorylation is decreased upon Wnt stimulation, the phospho-specific antibodies used also detect serine 41, one of the GSK-3 targets (Amit et al., 2002). Antibodies selective for phospho-serine 45 do not report changes in stoichiometry in response to Wnt (Xi He, personal communication). Clearly, cells have evolved complex mechanisms to titrate β-catenin levels, presumably to allow multiple layers of control.
Another interesting player in the regulation of the Wnt pathway, at least in vertebrates, is a GSK-3 binding protein termed GBP (also known as FRAT2) (Farr et al., 2000; Ferkey and Kimelman, 2002; Fraser et al., 2002; Sumoy et al., 1999; Yost et al., 1998). Binding of GBP to GSK-3 precludes GSK-3 from binding Axin and thus interferes with β-catenin phosphorylation. A small peptide derived from FRAT called FRAT-tide is sufficient to prevent Axin-GSK-3 interaction and prevents both Axin and β-catenin phosphorylation (Thomas et al., 1999). Comparison of mutations that affect GSK-3 binding to Axin and GBP as well as analysis of the crystal structure of GSK-3 complexed to FRAT-tide, indicates overlap of the binding sites on GSK-3 for GBP/FRAT and Axin (Bax et al., 2001; Ferkey and Kimelman, 2002; Fraser et al., 2002). Treatment of Xenopus embryo extracts with CKIε has been shown to increase binding of GBP to Dishevelled (Lee et al., 2001). GBP also plays a role in the nuclear export of GSK-3 (Franca-Koh et al., 2002). A mutant of GSK-3 that is incapable of binding GBP accumulates in the nucleus. Moreover, a peptide that interferes with GBP binding to GSK-3 causes endogenous GSK-3 to accumulate in the nucleus. These findings suggest that GBP may regulate GSK-3 access to substrates partitioned between nuclear and cytoplasmic compartments. Since there are two mammalian GBP homologues identified to date (Freemantle et al., 2002), each with dynamically regulated expression patterns during development, GBP could play an important role in modulating GSK-3 function, especially during development. Rather surprisingly, GBP homologues have not been identified in Drosophila or C. elegans implying that it is not a core component of canonical Wnt signaling.
A critical aspect of GSK-3’s role in the Wnt pathway is that GSK-3 appears to be insulated from regulators of GSK-3 that lie outside of the Wnt pathway. For example, insulin signaling leads to inhibition of GSK-3 via serine 9/21 phosphorylation but does not cause accumulation of β-catenin. Conversely, Wnt signaling does not affect insulin signaling (Ding et al., 2000; Yuan et al., 1999). How this insulation occurs is unclear, but likely stems from the effective sequestration of a fraction of GSK-3 with Axin in the destruction complex. Of note, tissues from mice lacking GSK-3β do not show evidence of accumulated β-catenin even though total GSK-3 levels are reduced by 50% and there is zero cellular GSK-3β. Immunoprecipitation of Axin from these tissues reveals that GSK-3β is simply replaced by GSK-3α (in wild type cells, both GSK-3α and GSK-3β are found bound to Axin) (E. Rubie and J. Woodgett, unpublished). Since cellular levels of GSK-3 exceed Axin, the destruction machinery compensates for the loss of GSK-3β by substituting GSK-3α.
GSK-3 and the Hedgehog pathway
Yet another novel function for GSK-3 was recently discovered by groups examining Hedgehog (Hh) signaling in Drosophila (Jia et al., 2002; Price and Kalderon, 2002). The Hh pathway is somewhat similar to the Wnt pathway inasfar as both Wnt and Hh are secreted signaling proteins involved in embryonic patterning that often work in concert (reviewed in: Ingham and McMahon, 2001). While the Wnt pathway utilizes β-catenin to transduce its signals to the nucleus, the Hh pathway employs a protein called Cubitus interruptus (Ci) in flies or Gli in mammals (Ingham and McMahon, 2001). In the absence of a Hh signal, Ci is targeted for proteolysis. Unlike in Wnt signaling, proteolysis does not result in total degradation of Ci, but rather processes it from a 155 amino acid form (Ci155) to a truncated 75 amino acid form (Ci75) that functions as a transcriptional repressor (Aza-Blanc et al., 1997). The role of GSK-3, in combination with CKI and protein kinase A (PKA), acting as a priming kinase, is to phosphorylate Ci155 to target it for proteolytic processing in the absence of a Hh signal (Jia et al., 2002; Price and Kalderon, 2002). Activation of Hh signaling results in translocation of full length Ci155 to the nucleus where it functions to activate Hh target genes. As in the Wnt pathway, several of the molecules involved in Hh signal transduction have been implicated in cancer (reviewed in Taipale and Beachy, 2001). Notably, familial germline mutations in one allele of the Hh receptor, called Patched, are associated with an almost complete penetrance of basal cell carcinoma (Johnson et al., 1996, Taipale, 2001). Although GSK-3 phosphorylation of the mammalian homologues of Ci, a family of three Gli proteins has yet to be reported, all contain multiple GSK-3 consensus sites next to PKA sites (Ruis I Altaba, 1999; Jia et al., 2002). It seems likely, based on the conservation of the Wnt pathway, that the Gli proteins will soon be confirmed as GSK-3 substrates.
Other GSK-3 substrates
There are numerous putative GSK-3 substrates with roles in a wide spectrum of cellular processes including: glycogen metabolism, transcription, translation, cytoskeletal regulation, intracellular vesicular transport, cell cycle progression, circadian rhythm regulation and apoptosis. Phosphorylation of these substrates by GSK-3 is most often inhibitory, as seen with glycogen synthase, β-catenin and Ci. Although a detailed description of the function of all of the substrates is beyond the scope of this review, a listing of many of the best characterized substrates and well as some newly identified substrates, with a brief description of their function and the role of GSK-3 phosphorylation, is shown in Table 1.
Table 1.
Putative GSK-3 substrates. Substrates involved in Alzheimer’s disease are shaded blue, components of the Wnt pathway are shaded yellow, transcription factors are shaded green and proteins involved in protein, insulin and lipid metabolism are shaded red.
| Putative Substrate | Function | Effect of Phosphorylation by GSK-3 | References |
|---|---|---|---|
| Kinesin light chain | Regulatory component of motor protein involved in vesicular transport | Inhibits anterograde vesicular movement | (Morfini et al., 2002) |
| Presenilin 1 (PS1) | Transmembrane protein linked to Alzheimer’s disease; also binds β-catenin | Increases degradation of C-terminal PS 1 fragment | (Kirschenbaum et al., 2001) |
| Tau | Microtubule-associated protein; stabilizes microtubules | Reduced microtubule binding; decreased microtubule stability | (Hanger et al., 1992) |
| β-catenin | Transcription transactivator | Targets for degradation | (Yost et al., 1996) |
| Axin | Scaffold protein in Wnt Pathway | Increased affinity for β-catenin and increased axin stability | (Ikeda et al., 1998; Jho et al., 1999; Yamamoto et al., 1999) |
| Adenomatou s Polyposis Coli | Wnt pathway component | Increased β-catenin binding; decreased microtubule binding | (Rubinfeld et al., 1996) |
| Timeless | Transcription factor regulating Drosophila Circadian Rhythm | Increased heterodimerization with clockgene called period or increased nuclear transport suggested | (Martinek et al., 2001) |
| Nuclear Factor of Activated T-cells c | Transcription factor; early immune response genes | Decreased DNA binding; increased nuclear export | (Beals et al., 1997) |
| Heat Shock Factor-1 | Transcription factor; regulates genes in response to potentially lethal stressors | Inactivates transcription factor activity | (Chu et al., 1996) |
| c-Jun | Transcription factor; component of activator protein-1 (AP-1) that regulates many diverse genes | Decreased DNA binding and transactivation | (Boyle et al., 1991) |
| c-Myc | Transcription factor; regulates genes involved in cell growth, differentiation and apoptosis | Targets for degradation | (Pulverer et al., 1994; Sears et al., 2000) |
| cAMP respo nse element binding protein | Transcription factor; regulates cAMP-responsive genes | Increased transcription factor activity | (Fiol et al., 1994) |
| Microphthalmia-associated transcription factor | Transcription factor; regulates tyrosinase expression | Increased binding to tyrosinase promoter | (Khaled et al., 2002; Takeda et al., 2000) |
| Cylin D1 | Transcription factor; cell cycle regulation | Increased nuclear export; targets for degradation | (Diehl et al., 1998) |
| eIF-2B translation factor | Critical for translation initiation | Inhibits activity | (Singh et al., 1996; Welsh and Proud, 1993) |
| Inhibitor-2 | Regulatory subunit of phosphatase | Activates phosphatase | (Park et al., 1994) |
| Glycogen Synthase | Glycogen metabolism | Inhibits enzyme activity | (Dent et al., 1989; Dent et al., 1990; Fiol et al., 1988) |
| Insulin receptor substrate 1 | Insulin signaling | Inhibits insulin receptor signaling | (Eldar-Finkelman and Krebs, 1997) |
| Acetyl CoA Carboxylase | Key lipogenic enzyme | Inactivates enzyme | (Hughes et al., 1992) |
| ATP-citrate L yase | Fatty acid synthesis | Inactivates enzyme | (Benjamin et al., 1994; Hughes et al., 1992) |
| Mucin 1 / DF 3 Antigen | Transmembrane glycoprotein that binds β-catenin; overexpressed in human carcinomas | Decreases interaction with b-catenin | (Li et al., 1998) |
Other GSK-3 binding proteins
Besides GBP, axin and APC, new GSK-3 binding partners located outside of the Wnt pathway have recently been identified. The tumour-suppressor protein p53 has recently been shown to bind and activate GSK-3 in the nucleus of neuroblastoma cells treated with camptothecin, a topoisomerase I inhibitor that causes DNA damage and subsequently elicits a p53 response (Watcharasit et al., 2002). The activation of GSK-3 occurs without changes in its phosphorylation and is restricted to nuclear GSK-3 via an unknown mechanism. A yeast 2-hybrid screen identified the A-kinase anchoring protein AKAP220 as a GSK-3 binding partner (Tanji et al., 2002). AKAPs are scaffolding proteins and the interaction between AKAP220 and GSK-3 occurred in a complex that also contained PKA and type 1 protein phosphatase. The interaction between AKAP220 and GSK-3 enhances PKA-mediated inhibition of GSK-3.
Presenilin 1 (PS1) is one of the two mammalian presenilins identified via association of mutations occurring in these proteins and early-onset familial Alzheimer’s disease. Besides having a role in proteolytic processing of proteins at the cell membrane such as amyloid precursor protein, presenilin has been shown to be able to bind β-catenin and has been implicated in regulating its cellular levels. PS1 may also function as a scaffolding protein that binds the proteins PKA, GSK-3 and β-catenin (Palacino et al., 2001). This novel quaternary complex functions in a similar manner to the Axin complex, using PS1 to tether the priming phosphorylation by PKA, on S45 of β-catenin, to subsequent phosphorylation by GSK-3, with the same end result; targeting β-catenin for proteasomal degradation.
GSK-3 and human disease
Many of the pathways that use GSK-3 as a regulator have links to human diseases. As described above, GSK-3 is a core component of two pathways involved in cellular fate determination and morphology, the Wnt and Hedgehog pathways, which are both involved in several forms of human cancer. There are also numerous studies linking GSK-3 to Alzheimer’s disease, based on GSK-3-mediated hyperphosphorylation of tau, which is associated with neurofibrillary tangles, one of the hallmark features of Alzheimer’s disease (reviewed in De Ferrari and Inestrosa, 2000; Maccioni et al., 2001; Mattson, 2001). The GSK-3 inhibitor lithium (Stambolic et al., 1996), used for decades as a therapeutic intervention for bipolar disorder, also implicates GSK-3 activity in the underlying causes of this disease (Detera-Wadleigh, 2001; Gould and Manji, 2002; Manji et al., 1999). In support of GSK-3’s role in mood regulation is the fact that another mood-stablilizing drug, valproate, also inhibits GSK-3 (Chen et al., 1999; Li et al., 2002). It should be mentioned that lithium and valproate also affect neuronal inositol metabolism and it is possible that their mood-stabilizing properties are related to this effect (Williams et al., 2002). Apart from a role in neurological pathologies, GSK-3 may also play a role in the development of non-insulin dependent diabetes mellitus (NIDDM), a disease often associated with chronic inhibition of muscle glycogen synthase (reviewed in Kaidanovich and Eldar-Finkelman, 2002).
Small molecule inhibitors of GSK-3
In view of the association of abnormal GSK-3 activity and various human pathological conditions, GSK-3 is emerging as a potential therapeutic target. This is particularly for therapeutic intervention in diseases where its over-expression may be linked to pathology such as NIDDM and neurodegenerative diseases (Eldar-Finkelman, 2002) but may also be relevant for some of the more recently identified functions, such as its role in activating NF-kB, a well characterized target for anti-inflammatory agents (Hoeflich et al., 2000). The best-characterized inhibitor of GSK-3 is lithium (Klein and Melton, 1996; Stambolic et al., 1996). Although fairly specific for GSK-3 compared with other protein kinases, lithium also affects other enzymes and requires a relatively high dose in vitro (Ki is mM), to inhibit GSK-3 activity in cell culture (Stambolic et al., 1996). The mode of lithium inhibition of GSK-3 is through competition for Mg2+ (Ryves and Harwood, 2001). Recently, the bivalent form of zinc, which is insulin-mimetic, has also been shown to be an inhibitor of GSK-3 when used at a concentration of 15 μM in a cell culture system (Ilouz et al., 2002).
Several new GSK-3 inhibitors have recently been developed, most of which are ATP competitive (Martinez et al., 2002a; Martinez et al., 2002b). A list of these drugs, their mode of action and some of their reported effects in cell culture and patients is shown in Table 2.
Table 2.
GSK-3 inhibitors. Abbreviations: N/A, non-applicable.
| Drug Type | Specific Drug Examples | Mode of Action | Clinical Effects / Effects in Cell Culture | Reference |
|---|---|---|---|---|
| Lithium | N/A | Competes for Mg2+ | Mood stabilization; prevents polyglutamine toxicity in Huntington’s Disease | (Carmichael et al., 2002; Klein and Melton, 1996; Stambolic et al., 1996) |
| Bivalent Zinc | N/A | Undetermined; does not compete for substrate | Insulin-mimetic | (Ilouz et al., 2002) |
| Valproate | N/A | Unclear | Mood stabilization | (Chen et al., 1999) |
| Hymenialdisine | Dibromo- hymenialdisine | ATP-competitive | Suppresses inflammation; inhibits tau phosphorylation | (Breton and Chabot-Fletcher, 1997; Meijer et al., 2000) |
| Paullones | Alsterpaullone | ATP-competitive | Inhibits tau phosphorylation; anti-tumoural | (Knockaert et al., 2002; Leost et al., 2000) |
| Indirubins | 5,5′-dibromo- indirubin, | ATP-competitive | Anti-mitotic; anti-tumoural; inhibits tau phosphorylation | (Damiens et al., 2001; Leclerc et al., 2001) |
| Maleimides | Ro 31–8220, SB- 216763, SB-415286 | ATP-competitive | Insulin-mimetic; prevention of neuronal death in culture | (Coghlan et al., 2000; Cross et al., 2001; Hers et al., 1999; Lochhead et al., 2001; Smith et al., 2001) |
| Muscarinic agonists | AF102B, AF150 | Unclear | Inhibits tau phosphorylation | (Forlenza et al., 2000) |
| Thiadiazolidinones | N/A | ATP-non competitive; probably disrupts substrate binding groove | No Data | (Martinez et al., 2002a) |
There may be a skeleton in the closet, however, for anti-GSK-3 therapeutics. In addition to the problem of its broad range of functions, inhibition of the enzyme could presumably lead to enhanced accumulation of β-catenin, a known oncogene. To mitigate this complication, drugs that selectively target non-Axin associated GSK-3 would be desirable, especially for chronic indications, such as diabetes. On the positive side, long term lithium treatment is not associated with enhanced incidence of cancer, although the lithium-treated patient cohort is not typical of the general population.
Perspectives
An enzyme with as many proposed substrates as GSK-3 requires numerous levels of regulation to confer signal-dependent specificity. Several different mechanisms regulating the phosphorylation of GSK-3 substrates have been described: 1) inactivation of GSK-3 through serine phosphorylation; 2) activation of GSK-3 through tyrosine phosphorylation; 3) inactivation of GSK-3 through tyrosine dephosphorylation; 4) covalent modification of substrates through priming phosphorylation; 5) inhibition or facilitation of GSK-3-mediated substrate phosphorylation due to interaction with binding or scaffolding proteins; 6) targeting of GSK-3 to different subcellular localizations; 7) differential usage of isoforms or splice variants to alter subcellular localization or substrate specificity; 8) integration of parallel signals conveyed by a single stimulus.
Although our understanding of GSK-3 regulation is increasing, there are still several outstanding issues: 1) putative GSK-3 substrates need to be verified and classified as to whether they require priming phosphorylation; 2) proteins that have been identified as GSK-3 interacting proteins require characterization to identify sites of interaction and mechanisms that regulating binding; 3) the mechanism of Wnt-mediated inactivation of GSK-3; 4) the basis for the specific requirement for GSK-3β in regulating NFκB function remains (GSK-3α does not substitute); 5) the specificity of newly emerging GSK-3 inhibitors must be validated.
GSK-3 has reliably and regularly provided surprises ever since its discovery and several of these have proven to be new signalling paradigms. There is little reason to doubt that there are more revelations in store but with enhanced tools such as selective inhibitors, knockout mice and modified binding proteins, there’s hope that the true cellular roles for this multi-talented kinase will be appreciated, including its utility as a therapeutic target. But there are certainly easier molecules to study…..
Footnotes
(also known as: cell adhesion kinase, beta; focal adhesion kinase 2; protein tyrosine kinase-2, beta; cell adhesion kinase, beta; calcium dependent protein kinase; related adhesion focal tyrosine kinase)
Frequently Rearranged in Advanced T-Cell Lymphomas
References
- Ali A, Hoeflich KP, Woodgett JR. Glycogen synthase kinase-3: properties, functions, and regulation. Chem Rev. 2001;101:2527–2540. doi: 10.1021/cr000110o. [DOI] [PubMed] [Google Scholar]
- Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–1076. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JL, Bonavaud SM, Toole BJ, Yeaman SJ. Regulation of glycogen synthesis by amino acids in cultured human muscle cells. J Biol Chem. 2001;276:952–956. doi: 10.1074/jbc.M004812200. [DOI] [PubMed] [Google Scholar]
- Aza-Blanc P, Ramirez-Weber FA, Laget MP, Schwartz C, Kornberg TB. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell. 1997;89:1043–1053. doi: 10.1016/s0092-8674(00)80292-5. [DOI] [PubMed] [Google Scholar]
- Ballou LM, Tian PY, Lin HY, Jiang YP, Lin RZ. Dual regulation of glycogen synthase kinase-3beta by the alpha1A-adrenergic receptor. J Biol Chem. 2001;276:40910–40916. doi: 10.1074/jbc.M103480200. [DOI] [PubMed] [Google Scholar]
- Barker N, Morin PJ, Clevers H. The Yin-Yang of TCF/beta-catenin signaling. Adv Cancer Res. 2000;77:1–24. doi: 10.1016/s0065-230x(08)60783-6. [DOI] [PubMed] [Google Scholar]
- Bax B, Carter PS, Lewis C, Guy AR, Bridges A, Tanner R, Pettman G, Mannix C, Culbert AA, Brown MJ, et al. The structure of phosphorylated GSK-3beta complexed with a peptide, FRATtide, that inhibits beta-catenin phosphorylation. Structure (Camb) 2001;9:1143–1152. doi: 10.1016/s0969-2126(01)00679-7. [DOI] [PubMed] [Google Scholar]
- Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–1934. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- Bellon S, Fitzgibbon MJ, Fox T, Hsiao HM, Wilson KP. The structure of phosphorylated p38gamma is monomeric and reveals a conserved activation-loop conformation. Structure Fold Des. 1999;7:1057–1065. doi: 10.1016/s0969-2126(99)80173-7. [DOI] [PubMed] [Google Scholar]
- Benjamin WB, Pentyala SN, Woodgett JR, Hod Y, Marshak D. ATP citrate-lyase and glycogen synthase kinase-3 beta in 3T3-L1 cells during differentiation into adipocytes. Biochem J. 1994;300:477–482. doi: 10.1042/bj3000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat RV, Shanley J, Correll MP, Fieles WE, Keith RA, Scott CW, Lee CM. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc Natl Acad Sci U S A. 2000;97:11074–11079. doi: 10.1073/pnas.190297597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijur GN, Jope RS. Proapoptotic stimuli induce nuclear accumulation of glycogen synthase kinase-3 beta. J Biol Chem. 2001;276:37436–37442. doi: 10.1074/jbc.M105725200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourouis M, Moore P, Ruel L, Grau Y, Heitzler P, Simpson P. An early embryonic product of the gene shaggy encodes a serine/threonine protein kinase related to the CDC28/cdc2+ subfamily. EMBO J. 1990;9:2877–2884. doi: 10.1002/j.1460-2075.1990.tb07477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WJ, Smeal T, Defize LH, Angel P, Woodgett JR, Karin M, Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991;64:573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Brady MJ, Bourbonais FJ, Saltiel AR. The activation of glycogen synthase by insulin switches from kinase inhibition to phosphatase activation during adipogenesis in 3T3-L1 cells. J Biol Chem. 1998;273:14063–14066. doi: 10.1074/jbc.273.23.14063. [DOI] [PubMed] [Google Scholar]
- Brantjes H, Barker N, van EJ, Clevers H. TCF: Lady Justice casting the final verdict on the outcome of Wnt signalling. Biol Chem. 2002;383:255–261. doi: 10.1515/BC.2002.027. [DOI] [PubMed] [Google Scholar]
- Breton JJ, Chabot-Fletcher MC. The natural product hymenialdisine inhibits interleukin-8 production in U937 cells by inhibition of nuclear factor-kappaB. J Pharmacol Exp Ther. 1997;282:459–466. [PubMed] [Google Scholar]
- Brown NR, Noble ME, Endicott JA, Johnson LN. The structural basis for specificity of substrate and recruitment peptides for cyclin-dependent kinases. Nat Cell Biol. 1999;1:438–443. doi: 10.1038/15674. [DOI] [PubMed] [Google Scholar]
- Canagarajah BJ, Khokhlatchev A, Cobb MH, Goldsmith EJ. Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell. 1997;90:859–869. doi: 10.1016/s0092-8674(00)80351-7. [DOI] [PubMed] [Google Scholar]
- Carmichael J, Sugars KL, Bao Y, Rubinsztein DC. GSK-3beta inhibitors prevent cellular polyglutamine toxicity caused by the Huntington’s disease mutation. J Biol Chem. 2002 doi: 10.1074/jbc.M204861200. in press. [DOI] [PubMed] [Google Scholar]
- Chen G, Huang LD, Jiang YM, Manji HK. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J Neurochem. 1999;72:1327–1330. doi: 10.1046/j.1471-4159.2000.0721327.x. [DOI] [PubMed] [Google Scholar]
- Chu B, Soncin F, Price BD, Stevenson MA, Calderwood SK. Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor-1. J Biol Chem. 1996;271:30847–30857. doi: 10.1074/jbc.271.48.30847. [DOI] [PubMed] [Google Scholar]
- Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- Cohen P, Alessi DR, Cross DA. PDK1, one of the missing links in insulin signal transduction? FEBS Lett. 1997;410:3–10. doi: 10.1016/s0014-5793(97)00490-0. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Cross DA, Culbert AA, Chalmers KA, Facci L, Skaper SD, Reith AD. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J Neurochem. 2001;77:94–102. doi: 10.1046/j.1471-4159.2001.t01-1-00251.x. [DOI] [PubMed] [Google Scholar]
- Cross DA, Watt PW, Shaw M, van der Kaay J, Downes CP, Holder JC, Cohen P. Insulin activates protein kinase B, inhibits glycogen synthase kinase-3 and activates glycogen synthase by rapamycin-insensitive pathways in skeletal muscle and adipose tissue. FEBS Lett. 1997;406:211–215. doi: 10.1016/s0014-5793(97)00240-8. [DOI] [PubMed] [Google Scholar]
- Dajani R, Fraser E, Roe SM, Young N, Good V, Dale TC, Pearl LH. Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell. 2001;105:721–732. doi: 10.1016/s0092-8674(01)00374-9. [DOI] [PubMed] [Google Scholar]
- Damiens E, Baratte B, Marie D, Eisenbrand G, Meijer L. Anti-mitotic properties of indirubin-3′-monoxime, a CDK/GSK-3 inhibitor: induction of endoreplication following prophase arrest. Oncogene. 2001;20:3786–3797. doi: 10.1038/sj.onc.1204503. [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Inestrosa NC. Wnt signaling function in Alzheimer’s disease. Brain Res Rev. 2000;33:1–12. doi: 10.1016/s0165-0173(00)00021-7. [DOI] [PubMed] [Google Scholar]
- Dent P, Campbell DG, Hubbard MJ, Cohen P. Multisite phosphorylation of the glycogen-binding subunit of protein phosphatase-1G by cyclic AMP-dependent protein kinase and glycogen synthase kinase-3. FEBS Lett. 1989;248:67–72. doi: 10.1016/0014-5793(89)80433-8. [DOI] [PubMed] [Google Scholar]
- Dent P, Lavoinne A, Nakielny S, Caudwell FB, Watt P, Cohen P. The molecular mechanism by which insulin stimulates glycogen synthesis in mammalian skeletal muscle. Nature. 1990;348:302–308. doi: 10.1038/348302a0. [DOI] [PubMed] [Google Scholar]
- Detera-Wadleigh SD. Lithium-related genetics of bipolar disorder. Ann Med. 2001;33:272–285. doi: 10.3109/07853890108998756. [DOI] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierick H, Bejsovec A. Cellular mechanisms of wingless/Wnt signal transduction. Curr Top Dev Biol. 1999;43:153–190. doi: 10.1016/s0070-2153(08)60381-6. [DOI] [PubMed] [Google Scholar]
- Ding VW, Chen RH, McCormick F. Differential regulation of glycogen synthase kinase 3beta by insulin and Wnt signaling. J Biol Chem. 2000;275:32475–32481. doi: 10.1074/jbc.M005342200. [DOI] [PubMed] [Google Scholar]
- Eldar-Finkelman H. Glycogen synthase kinase 3: an emerging therapeutic target. Trends Mol Med. 2002;8:126–132. doi: 10.1016/s1471-4914(01)02266-3. [DOI] [PubMed] [Google Scholar]
- Eldar-Finkelman H, Krebs EG. Phosphorylation of insulin receptor substrate 1 by glycogen synthase kinase 3 impairs insulin action. Proc Natl Acad Sci USA. 1997;94:9660–9664. doi: 10.1073/pnas.94.18.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar-Finkelman H, Seger R, Vandenheede JR, Krebs EG. Inactivation of glycogen synthase kinase-3 by epidermal growth factor is mediated by mitogen-activated protein kinase/p90 ribosomal protein S6 kinase signaling pathway in NIH/3T3 cells. J Biol Chem. 1995;270:987–990. doi: 10.1074/jbc.270.3.987. [DOI] [PubMed] [Google Scholar]
- Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107:519–527. [PubMed] [Google Scholar]
- Fang X, Yu S, Tanyi JL, Lu Y, Woodgett JR, Mills GB. Convergence of multiple signaling cascades at glycogen synthase kinase 3: Edg receptor-mediated phosphorylation and inactivation by lysophosphatidic acid through a protein kinase C-dependent intracellular pathway. Mol Cell Biol. 2002;22:2099–2110. doi: 10.1128/MCB.22.7.2099-2110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Yu SX, Lu Y, Bast RC, Jr, Woodgett JR, Mills GB. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci U S A. 2000;97:11960–11965. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr GH, 3rd, Ferkey DM, Yost C, Pierce SB, Weaver C, Kimelman D. Interaction among GSK-3, GBP, axin, and APC in Xenopus axis specification. J Cell Biol. 2000;148:691–702. doi: 10.1083/jcb.148.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferkey DM, Kimelman D. Glycogen synthase kinase-3 beta mutagenesis identifies a common binding domain for GBP and Axin. J Biol Chem. 2002;277:16147–16152. doi: 10.1074/jbc.M112363200. [DOI] [PubMed] [Google Scholar]
- Fiol CJ, Haseman JH, Wang YH, Roach PJ, Roeske RW, Kowalczuk M, DePaoli-Roach AA. Phosphoserine as a recognition determinant for glycogen synthase kinase-3: phosphorylation of a synthetic peptide based on the G-component of protein phosphatase-1. Arch Biochem Biophys. 1988;267:797–802. doi: 10.1016/0003-9861(88)90089-6. [DOI] [PubMed] [Google Scholar]
- Fiol CJ, Mahrenholz AM, Wang Y, Roeske RW, Roach PJ. Formation of protein kinase recognition sites by covalent modification of the substrate. Molecular mechanism for the synergistic action of casein kinase II and glycogen synthase kinase 3. J Biol Chem. 1987;262:14042–14048. [PubMed] [Google Scholar]
- Fiol CJ, Wang A, Roeske RW, Roach PJ. Ordered multisite protein phosphorylation. Analysis of glycogen synthase kinase 3 action using model peptide substrates. J Biol Chem. 1990;265:6061–6065. [PubMed] [Google Scholar]
- Fiol CJ, Williams JS, Chou CH, Wang QM, Roach PJ, Andrisani OM. A secondary phosphorylation of CREB341 at Ser129 is required for the cAMP-mediated control of gene expression. A role for glycogen synthase kinase-3 in the control of gene expression. J Biol Chem. 1994;269:32187–32193. [PubMed] [Google Scholar]
- Forlenza OV, Spink JM, Dayanandan R, Anderton BH, Olesen OF, Lovestone S. Muscarinic agonists reduce tau phosphorylation in non-neuronal cells via GSK-3beta inhibition and in neurons. J Neural Transm. 2000;107:1201–1212. doi: 10.1007/s007020070034. [DOI] [PubMed] [Google Scholar]
- Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001;7:1321–1327. doi: 10.1016/s1097-2765(01)00253-2. [DOI] [PubMed] [Google Scholar]
- Franca-Koh J, Yeo M, Fraser E, Young N, Dale TC. The regulation of glycogen synthase Kinse-3 nuclear export by Frat/GBP. J Biol Chem. 2002 doi: 10.1074/jbc.M207265200. in press. [DOI] [PubMed] [Google Scholar]
- Fraser E, Young N, Dajani R, Franca-Koh J, Ryves J, Williams RS, Yeo M, Webster MT, Richardson C, Smalley MJ, et al. Identification of the Axin and Frat binding region of glycogen synthase kinase-3. J Biol Chem. 2002;277:2176–2185. doi: 10.1074/jbc.M109462200. [DOI] [PubMed] [Google Scholar]
- Freemantle SJ, Portland HB, Ewings K, Dmitrovsky F, DiPetrillo K, Spinella MJ, Dmitrovsky E. Characterization and tissue-specific expression of human GSK-3-binding proteins FRAT1 and FRAT2. Gene. 2002;291:17–27. doi: 10.1016/s0378-1119(02)00594-2. [DOI] [PubMed] [Google Scholar]
- Gao ZH, Seeling JM, Hill V, Yochum A, Virshup DM. Casein kinase I phosphorylates and destabilizes the beta-catenin degradation complex. Proc Natl Acad Sci USA. 2002;99:1182–1187. doi: 10.1073/pnas.032468199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Gould TD, Manji HK. The Wnt signaling pathway in bipolar disorder. Neuroscientist. 2002;8:497–511. doi: 10.1177/107385802237176. [DOI] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Hagen T, Di Daniel E, Culbert AA, Reith AD. Expression and characterization of GSK-3 mutants and their effect on beta-catenin phosphorylation in intact cells. J Biol Chem. 2002;277:23330–23335. doi: 10.1074/jbc.M201364200. [DOI] [PubMed] [Google Scholar]
- Hagen T, Vidal-Puig A. Characterisation of the phosphorylation of beta-catenin at the GSK-3 priming site Ser45. Biochem Biophys Res Commun. 2002;294:324–328. doi: 10.1016/S0006-291X(02)00485-0. [DOI] [PubMed] [Google Scholar]
- Hanger DP, Hughes K, Woodgett JR, Brion JP, Anderton BH. Glycogen synthase kinase-3 induces Alzheimer’s disease-like phosphorylation of tau: generation of paired helical filament epitopes and neuronal localisation of the kinase. Neurosci Lett. 1992;147:58–62. doi: 10.1016/0304-3940(92)90774-2. [DOI] [PubMed] [Google Scholar]
- Hartigan JA, Johnson GV. Transient increases in intracellular calcium result in prolonged site-selective increases in Tau phosphorylation through a glycogen synthase kinase 3beta-dependent pathway. J Biol Chem. 1999;274:21395–21401. doi: 10.1074/jbc.274.30.21395. [DOI] [PubMed] [Google Scholar]
- Hartigan JA, Xiong WC, Johnson GV. Glycogen synthase kinase 3beta is tyrosine phosphorylated by PYK2. Biochem Biophys Res Commun. 2001;284:485–489. doi: 10.1006/bbrc.2001.4986. [DOI] [PubMed] [Google Scholar]
- Hers I, Tavare JM, Denton RM. The protein kinase C inhibitors bisindolylmaleimide I (GF 109203x) and IX (Ro 31–8220) are potent inhibitors of glycogen synthase kinase-3 activity. FEBS Lett. 1999;460:433–436. doi: 10.1016/s0014-5793(99)01389-7. [DOI] [PubMed] [Google Scholar]
- Hinoi T, Yamamoto H, Kishida M, Takada S, Kishida S, Kikuchi A. Complex formation of adenomatous polyposis coli gene product and axin facilitates glycogen synthase kinase-3 beta-dependent phosphorylation of beta-catenin and down-regulates beta-catenin. J Biol Chem. 2000;275:34399–34406. doi: 10.1074/jbc.M003997200. [DOI] [PubMed] [Google Scholar]
- Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- Hughes K, Nikolakaki E, Plyte SE, Totty NF, Woodgett JR. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 1993;12:803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K, Ramakrishna S, Benjamin WB, Woodgett JR. Identification of multifunctional ATP-citrate lyase kinase as the alpha-isoform of glycogen synthase kinase-3. Biochem J. 1992;288:309–314. doi: 10.1042/bj2880309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilouz R, Kaidanovich O, Gurwitz D, Eldar-Finkelman H. Inhibition of glycogen synthase kinase-3beta by bivalent zinc ions: insight into the insulin-mimetic action of zinc. Biochem Biophys Res Commun. 2002;295:102–106. doi: 10.1016/s0006-291x(02)00636-8. [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Jang MK, Goo YH, Sohn YC, Kim YS, Lee SK, Kang H, Cheong J, Lee JW. Ca2+/calmodulin-dependent protein kinase IV stimulates nuclear factor-kappa B transactivation via phosphorylation of the p65 subunit. J Biol Chem. 2001;276:20005–20010. doi: 10.1074/jbc.M010211200. [DOI] [PubMed] [Google Scholar]
- Jho E, Lomvardas S, Costantini F. A GSK3beta phosphorylation site in axin modulates interaction with beta-catenin and Tcf-mediated gene expression. Biochem Biophys Res Commun. 1999;266:28–35. doi: 10.1006/bbrc.1999.1760. [DOI] [PubMed] [Google Scholar]
- Jia J, Amanai K, Wang G, Tang J, Wang B, Jiang J. Shaggy/GSK3 antagonizes Hedgehog signalling by regulating Cubitus interruptus. Nature. 2002;416:548–552. doi: 10.1038/nature733. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, Quinn AG, Myers RM, Cox DR, Epstein EH, Jr, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- Kaidanovich O, Eldar-Finkelman H. The role of glycogen synthase kinase-3 in insulin resistance and Type 2 diabetes. Expert Opin Ther Targets. 2002;6:555–61. doi: 10.1517/14728222.6.5.555. [DOI] [PubMed] [Google Scholar]
- Khaled M, Larribere L, Bille K, Aberdam E, Ortonne JP, Ballotti R, Bertolotto C. Glycogen synthase kinase 3b is activated by cAMP and plays an active role in the regulation of melanogenesis. J Biol Chem. 2002 doi: 10.1074/jbc.M202939200. in press. [DOI] [PubMed] [Google Scholar]
- Kim L, Harwood A, Kimmel AR. Receptor-Dependent and Tyrosine Phosphatase-Mediated Inhibition of GSK3 Regulates Cell Fate Choice. Dev Cell. 2002;3:523–532. doi: 10.1016/s1534-5807(02)00269-1. [DOI] [PubMed] [Google Scholar]
- Kim L, Liu J, Kimmel AR. The novel tyrosine kinase ZAK1 activates GSK3 to direct cell fate specification. Cell. 1999;99:399–408. doi: 10.1016/s0092-8674(00)81526-3. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum F, Hsu SC, Cordell B, McCarthy JV. Glycogen synthase kinase-3beta regulates presenilin 1 C-terminal fragment levels. J Biol Chem. 2001;276:30701–30707. doi: 10.1074/jbc.M102849200. [DOI] [PubMed] [Google Scholar]
- Kishida M, Hino S, Michiue T, Yamamoto H, Kishida S, Fukui A, Asashima M, Kikuchi A. Synergistic activation of the Wnt signaling pathway by Dvl and casein kinase Iepsilon. J Biol Chem. 2001;276:33147–33155. doi: 10.1074/jbc.M103555200. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knockaert M, Wieking K, Schmitt S, Leost M, Grant KM, Mottram JC, Kunick C, Meijer L. Intracellular Targets of Paullones. Identification following affinity purification on immobilized inhibitor. J Biol Chem. 2002;277:25493–25501. doi: 10.1074/jbc.M202651200. [DOI] [PubMed] [Google Scholar]
- Krause U, Bertrand L, Maisin L, Rosa M, Hue L. Signalling pathways and combinatory effects of insulin and amino acids in isolated rat hepatocytes. Eur J Biochem. 2002;269:3742–3750. doi: 10.1046/j.1432-1033.2002.03069.x. [DOI] [PubMed] [Google Scholar]
- Leclerc S, Garnier M, Hoessel R, Marko D, Bibb JA, Snyder GL, Greengard P, Biernat J, Wu YZ, Mandelkow EM, et al. Indirubins inhibit glycogen synthase kinase-3 beta and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most cyclin-dependent kinase inhibitors? J Biol Chem. 2001;276:251–260. doi: 10.1074/jbc.M002466200. [DOI] [PubMed] [Google Scholar]
- Lee E, Salic A, Kirschner MW. Physiological regulation of [beta]-catenin stability by Tcf3 and CK1epsilon. J Cell Biol. 2001;154:983–993. doi: 10.1083/jcb.200102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leost M, Schultz C, Link A, Wu YZ, Biernat J, Mandelkow EM, Bibb JA, Snyder GL, Greengard P, Zaharevitz DW, et al. Paullones are potent inhibitors of glycogen synthase kinase-3beta and cyclin-dependent kinase 5/p25. Eur J Biochem. 2000;267:5983–5994. doi: 10.1046/j.1432-1327.2000.01673.x. [DOI] [PubMed] [Google Scholar]
- Lesort M, Jope RS, Johnson GV. Insulin transiently increases tau phosphorylation: involvement of glycogen synthase kinase-3beta and Fyn tyrosine kinase. J Neurochem. 1999;72:576–584. doi: 10.1046/j.1471-4159.1999.0720576.x. [DOI] [PubMed] [Google Scholar]
- Li M, Wang X, Meintzer MK, Laessig T, Birnbaum MJ, Heidenreich KA. Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3beta. Mol Cell Biol. 2000;20:9356–9363. doi: 10.1128/mcb.20.24.9356-9363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- Li X, Bijur GN, Jope RS. Glycogen synthase kinase-3beta, mood stabilizers, and neuroprotection. Bipolar Disord. 2002;4:137–44. doi: 10.1034/j.1399-5618.2002.40201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bharti A, Chen D, Gong J, Kufe D. Interaction of glycogen synthase kinase 3beta with the DF3/MUC1 carcinoma-associated antigen and beta-catenin. Mol Cell Biol. 1998;18:7216–7224. doi: 10.1128/mcb.18.12.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Lochhead PA, Coghlan M, Rice SQ, Sutherland C. Inhibition of GSK-3 selectively reduces glucose-6-phosphatase and phosphatase and phosphoenolypyruvate carboxykinase gene expression. Diabetes. 2001;50:937–946. doi: 10.2337/diabetes.50.5.937. [DOI] [PubMed] [Google Scholar]
- Maccioni RB, Munoz JP, Barbeito L. The molecular bases of Alzheimer’s disease and other neurodegenerative disorders. Arch Med Res. 2001;32:367–381. doi: 10.1016/s0188-4409(01)00316-2. [DOI] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Chen G. Lithium at 50: have the neuroprotective effects of this unique cation been overlooked? Biol Psychiatry. 1999;46:929–940. doi: 10.1016/s0006-3223(99)00165-1. [DOI] [PubMed] [Google Scholar]
- Manoukian AS, Woodgett JR. Role of glycogen synthase kinase-3 in cancer: regulation by Wnts and other signaling pathways. Adv Cancer Res. 2002;84:203–229. doi: 10.1016/s0065-230x(02)84007-6. [DOI] [PubMed] [Google Scholar]
- Martinek S, Inonog S, Manoukian AS, Young MW. A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell. 2001;105:769–79. doi: 10.1016/s0092-8674(01)00383-x. [DOI] [PubMed] [Google Scholar]
- Martinez A, Alonso M, Castro A, Perez C, Moreno FJ. First non-ATP competitive glycogen synthase kinase 3 beta (GSK-3beta) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer’s disease. J Med Chem. 2002a;45:1292–9. doi: 10.1021/jm011020u. [DOI] [PubMed] [Google Scholar]
- Martinez A, Castro A, Dorronsoro I, Alonso M. Glycogen synthase kinase 3 (GSK-3) inhibitors as new promising drugs for diabetes, neurodegeneration, cancer, and inflammation. Med Res Rev. 2002b;22:373–384. doi: 10.1002/med.10011. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Neuronal death and GSK-3beta: a tau fetish? Trends Neurosci. 2001;24:255–256. doi: 10.1016/s0166-2236(00)01838-5. [DOI] [PubMed] [Google Scholar]
- McKay RM, Peters JM, Graff JM. The casein kinase I family in Wnt signaling. Dev Biol. 2001;235:388–396. doi: 10.1006/dbio.2001.0308. [DOI] [PubMed] [Google Scholar]
- Meijer L, Thunnissen AM, White AW, Garnier M, Nikolic M, Tsai LH, Walter J, Cleverley KE, Salinas PC, Wu YZ, et al. Inhibition of cyclin-dependent kinases, GSK-3beta and CK1 by hymenialdisine, a marine sponge constituent. Chem Biol. 2000;7:51–63. doi: 10.1016/s1074-5521(00)00063-6. [DOI] [PubMed] [Google Scholar]
- Miller JR. The Wnts. Genome Biol. 2002;3:3001. doi: 10.1186/gb-2001-3-1-reviews3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Elluru R, Ratner N, Brady ST. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J. 2002;21:281–293. doi: 10.1093/emboj/21.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai F, Ishiguro K, Sano Y, Fujita SC. Alternative splicing isoform of tau protein kinase I/glycogen synthase kinase 3beta. J Neurochem. 2002;81:1073–1083. doi: 10.1046/j.1471-4159.2002.00918.x. [DOI] [PubMed] [Google Scholar]
- Murai H, Okazaki M, Kikuchi A. Tyrosine dephosphorylation of glycogen synthase kinase-3 is involved in its extracellular signal-dependent inactivation. FEBS Lett. 1996;392:153–160. doi: 10.1016/0014-5793(96)00806-x. [DOI] [PubMed] [Google Scholar]
- Novak A, Dedhar S. Signaling through beta-catenin and Lef/Tcf. Cell Mol Life Sci. 1999;56:523–537. doi: 10.1007/s000180050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacino JJ, Murphy MP, Murayama O, Iwasaki K, Fujiwara M, Takashima A, Golde TE, Wolozin B. Presenilin 1 regulates beta-catenin-mediated transcription in a glycogen synthase kinase-3-independent fashion. J Biol Chem. 2001;276:38563–38569. doi: 10.1074/jbc.M105376200. [DOI] [PubMed] [Google Scholar]
- Plyte SE, O’Donovan E, Woodgett JR, Harwood AJ. Glycogen synthase kinase-3 (GSK-3) is regulated during Dictyostelium development via the serpentine receptor cAR3. Development. 1999;126:325–333. doi: 10.1242/dev.126.2.325. [DOI] [PubMed] [Google Scholar]
- Polakis P. The adenomatous polyposis coli (APC) tumor suppressor. Biochim Biophys Acta. 1997;1332:F127–147. doi: 10.1016/s0304-419x(97)00008-5. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Polakis P. Casein Kinase 1: A Wnt’er of Disconnect. Curr Biol. 2002;12:499. doi: 10.1016/s0960-9822(02)00969-7. [DOI] [PubMed] [Google Scholar]
- Price MA, Kalderon D. Proteolysis of the hedgehog signaling effector cubitus interruptus requires phosphorylation by glycogen synthase kinase 3 and casein kinase 1. Cell. 2002;108:823–835. doi: 10.1016/s0092-8674(02)00664-5. [DOI] [PubMed] [Google Scholar]
- Pulverer BJ, Fisher C, Vousden K, Littlewood T, Evan G, Woodgett JR. Site-specific modulation of c-Myc cotransformation by residues phosphorylated in vivo. Oncogene. 1994;9:59–70. [PubMed] [Google Scholar]
- Robinson LJ, Razzack ZF, Lawrence JC, Jr, James DE. Mitogen-activated protein kinase activation is not sufficient for stimulation of glucose transport or glycogen synthase in 3T3-L1 adipocytes. J Biol Chem. 1993;268:26422–26427. [PubMed] [Google Scholar]
- Rothwarf DM, Karin M. The NF-kappa B activation pathway: a paradigm in information transfer from membrane to nucleus. Sci STKE. 1999;1999:RE1. doi: 10.1126/stke.1999.5.re1. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A. Gli proteins and Hedgehog signaling: development and cancer. Trends Genet. 1999;15:418–425. doi: 10.1016/s0168-9525(99)01840-5. [DOI] [PubMed] [Google Scholar]
- Ryves WJ, Harwood AJ. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem Biophys Res Commun. 2001;280:720–725. doi: 10.1006/bbrc.2000.4169. [DOI] [PubMed] [Google Scholar]
- Saito Y, Vandenheede JR, Cohen P. The mechanism by which epidermal growth factor inhibits glycogen synthase kinase 3 in A431 cells. Biochem J. 1994;303:27–31. doi: 10.1042/bj3030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayas CL, Avila J, Wandosell F. Glycogen synthase kinase-3 is activated in neuronal cells by galpha 12 and galpha 13 by rho-independent and rho-dependent mechanisms. J Neurosci. 2002;22:6863–6875. doi: 10.1523/JNEUROSCI.22-16-06863.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe RF, Brenner DA. Role of glycogen synthase kinase-3 in TNF-alpha-induced NF-kappaB activation and apoptosis in hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2002;283:G204–211. doi: 10.1152/ajpgi.00016.2002. [DOI] [PubMed] [Google Scholar]
- Seidensticker MJ, Behrens J. Biochemical interactions in the wnt pathway. Biochim Biophys Acta. 2000;1495:168–182. doi: 10.1016/s0167-4889(99)00158-5. [DOI] [PubMed] [Google Scholar]
- Sharpe C, Lawrence N, Martinez Arias A. Wnt signalling: a theme with nuclear variations. Bioessays. 2001;23:311–318. doi: 10.1002/bies.1045. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Asbrand C, Bakkers J, Kuhl M, Schaeffer HJ, Huelsken J, Behrens J, Hammerschmidt M, Birchmeier W. The ankyrin repeat protein Diversin recruits Casein kinase Iepsilon to the beta-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev. 2002;16:2073–2084. doi: 10.1101/gad.230402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000;14:2501–2514. doi: 10.1101/gad.836800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidensticker MJ, Behrens J. Biochemical interactions in the wnt pathway. Biochim Biophys Acta. 2000;1495:168–182. doi: 10.1016/s0167-4889(99)00158-5. [DOI] [PubMed] [Google Scholar]
- Sharpe C, Lawrence N, Martinez Arias A. Wnt signalling: a theme with nuclear variations. Bioessays. 2001;23:311–318. doi: 10.1002/bies.1045. [DOI] [PubMed] [Google Scholar]
- Siegfried E, Perkins LA, Capaci TM, Perrimon N. Putative protein kinase product of the Drosophila segment-polarity gene zeste-white3. Nature. 1990;345:825–829. doi: 10.1038/345825a0. [DOI] [PubMed] [Google Scholar]
- Siegfried E, Perrimon N. Drosophila wingless: a paradigm for the function and mechanism of Wnt signaling. Bioessays. 1994;16:395–404. doi: 10.1002/bies.950160607. [DOI] [PubMed] [Google Scholar]
- Singh LP, Denslow ND, Wahba AJ. Modulation of rabbit reticulocyte guanine nucleotide exchange factor activity by casein kinases 1 and 2 and glycogen synthase kinase 3. Biochemistry. 1996;35:3206–3212. doi: 10.1021/bi9522099. [DOI] [PubMed] [Google Scholar]
- Smalley MJ, Dale TC. Wnt signalling in mammalian development and cancer. Cancer Metastasis Rev. 1999;18:215–230. doi: 10.1023/a:1006369223282. [DOI] [PubMed] [Google Scholar]
- Smith DG, Buffet M, Fenwick AE, Haigh D, Ife RJ, Saunders M, Slingsby BP, Stacey R, Ward RW. 3-Anilino-4-arylmaleimides: potent and selective inhibitors of glycogen synthase kinase-3 (GSK-3) Bioorg Med Chem Lett. 2001;11:635–639. doi: 10.1016/s0960-894x(00)00721-6. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- Steiner MR, Holtsberg FW, Keller JN, Mattson MP, Steiner SM. Lysophosphatidic acid induction of neuronal apoptosis and necrosis. Ann N Y Acad Sci. 2000;905:132–141. doi: 10.1111/j.1749-6632.2000.tb06545.x. [DOI] [PubMed] [Google Scholar]
- Sumoy L, Kiefer J, Kimelman D. Conservation of intracellular Wnt signaling components in dorsal-ventral axis formation in zebrafish. Dev Genes Evol. 1999;209:48–58. doi: 10.1007/s004270050226. [DOI] [PubMed] [Google Scholar]
- Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- Takeda K, Takemoto C, Kobayashi I, Watanabe A, Nobukuni Y, Fisher DE, Tachibana M. Ser298 of MITF, a mutation site in Waardenburg syndrome type 2, is a phosphorylation site with functional significance. Hum Mol Genet. 2000;9:125–132. doi: 10.1093/hmg/9.1.125. [DOI] [PubMed] [Google Scholar]
- Tanji C, Yamamoto H, Yorioka N, Kohno N, Kikuchi K, Kikuchi A. A-kinase anchoring protein AKAP220 binds to glycogen synthase kinase-3beta (GSK-3beta ) and mediates protein kinase A-dependent inhibition of GSK-3beta. J Biol Chem. 2002 doi: 10.1074/jbc.M206210200. [DOI] [PubMed] [Google Scholar]
- ter Haar E, Coll JT, Austen DA, Hsiao HM, Swenson L, Jain J. Structure of GSK3beta reveals a primed phosphorylation mechanism. Nat Struct Biol. 2001;8:593–596. doi: 10.1038/89624. [DOI] [PubMed] [Google Scholar]
- Terruzzi I, Allibardi S, Bendinelli P, Maroni P, Piccoletti R, Vesco F, Samaja M, Luzi L. Amino acid- and lipid-induced insulin resistance in rat heart: molecular mechanisms. Mol Cell Endocrinol. 2002;190:135–145. doi: 10.1016/s0303-7207(02)00005-9. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Frame S, Goedert M, Nathke I, Polakis P, Cohen P. A GSK3-binding peptide from FRAT1 selectively inhibits the GSK3-catalysed phosphorylation of axin and beta-catenin. FEBS Lett. 1999;458:247–251. doi: 10.1016/s0014-5793(99)01161-8. [DOI] [PubMed] [Google Scholar]
- van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- Wang D, Westerheide SD, Hanson JL, Baldwin AS., Jr Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J Biol Chem. 2000;275:32592–32597. doi: 10.1074/jbc.M001358200. [DOI] [PubMed] [Google Scholar]
- Wang QM, Fiol CJ, DePaoli-Roach AA, Roach PJ. Glycogen synthase kinase-3 beta is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. J Biol Chem. 1994;269:944–954. [PubMed] [Google Scholar]
- Watcharasit P, Bijur GN, Zmijewski JW, Song L, Zmijewska A, Chen X, Johnson GV, Jope RS. Direct, activating interaction between glycogen synthase kinase-3beta and p53 after DNA damage. Proc Natl Acad Sci U S A. 2002;99:7951–7955. doi: 10.1073/pnas.122062299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh GI, Proud CG. Glycogen synthase kinase-3 is rapidly inactivated in response to insulin and phosphorylates eukaryotic initiation factor eIF-2B. Biochem J. 1993;294:625–629. doi: 10.1042/bj2940625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RS, Cheng L, Mudge AW, Harwood AJ. A common mechanism of action for three mood-stabilizing drugs. Nature. 2002;417:292–295. doi: 10.1038/417292a. [DOI] [PubMed] [Google Scholar]
- Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett JR. Judging a protein by more than its name: GSK-3. Sci STKE. 2001;2001:RE12. doi: 10.1126/stke.2001.100.re12. [DOI] [PubMed] [Google Scholar]
- Woodgett JR, Cohen P. Multisite phosphorylation of glycogen synthase. Molecular basis for the substrate specificity of glycogen synthase kinase-3 and casein kinase-II (glycogen synthase kinase-5) Biochim Biophys Acta. 1984;788:339–47. doi: 10.1016/0167-4838(84)90047-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem. 1999;274:10681–10684. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- Yanagawa S, Matsuda Y, Lee JS, Matsubayashi H, Sese S, Kadowaki T, Ishimoto A. Casein kinase I phosphorylates the Armadillo protein and induces its degradation in Drosophila. EMBO J. 2002;21:1733–1742. doi: 10.1093/emboj/21.7.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost C, Farr GH, 3rd, Pierce SB, Ferkey DM, Chen MM, Kimelman D. GBP, an inhibitor of GSK-3, is implicated in Xenopus development and oncogenesis. Cell. 1998;93:1031–1041. doi: 10.1016/s0092-8674(00)81208-8. [DOI] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Yuan H, Mao J, Li L, Wu D. Suppression of glycogen synthase kinase activity is not sufficient for leukemia enhancer factor-1 activation. J Biol Chem. 1999;274:30419–30423. doi: 10.1074/jbc.274.43.30419. [DOI] [PubMed] [Google Scholar]