Abstract
Wang et al. in a recent study in Nature shed new and contrasting insights into the role of GSK-3 as a negative regulator of proliferation and self-renewal.
GSK-3 was originally identified and later embodied as a member of the insulin-signalling pathway responsible for phosphorylation and inactivation of glycogen synthase, a major regulatory enzyme of glycogen metabolism. But it was soon recognized that this kinase paints with far broader strokes and influences processes that govern cell metabolism, polarity, transcription, cell cycle division, apoptosis, development and cell fate. In mammals, GSK-3 exists as two isoforms, encoded by separate genes. GSK-3α (51 kDa) and GSK3-β (47 kDa) are highly conserved and widely expressed kinases that share 98% sequence identity within their catalytic domains [1]. While structurally related, these isoforms are not functionally equivalent.
Unlike most protein kinases, GSK-3 is active under resting conditions and is rapidly inhibited by diverse stimuli. For example, insulin, via PI3/AKT/PKB, induces the inactivation of GSK-3. Many of its cellular targets are held in an inactive state through inhibitory phosphorylation. Phosphorylation by GSK-3 can also promote the ubiquitination and degradation of target proteins. Dysregulation of GSK-3, or the pathways that control it, have been implicated in various human diseases, such as muscle hypertrophy, diabetes, cancer, bipolar mood disorder, schizophrenia, and neurodegenerative diseases [2].
The therapeutic potential of GSK-3 inhibitors in these disease states has been actively pursued and several potent, chemical inhibitors of GSK-3 have been developed, most of which are ATP-competitive and do not discriminate between GSK-3α and GSK-3β. Administration of these inhibitors improves glucose homeostasis and insulin action in rodent models of diabetes and obesity and there is evidence that the inhibitors may be useful for conditions associated with inflammation such as ischemia, sepsis and colitis as well as neurodegenerative accumulation of hyperphosphorylated Tau [2]. Cumulatively, these data suggest a promising future for GSK-3 antagonists. However, their progress into clinical trials has been clouded by the concern that inhibition of GSK-3 will promote oncogenesis. GSK-3 is a key suppressor of the Wnt, Hedgehog and Notch pathways that control cellular fate determination and stem cell maintenance. Within these pathways, GSK-3 serves to phosphorylate the pro-oncogenic molecules β-catenin, c-Myc and c-Jun targeting them for degradation or inactivation, thereby inhibiting proliferation and self-renewal. However, these pathways are commonly deregulated in human cancers and, furthermore, gain-of-function mutations in these three proteins that interfere with GSK-3 inhibition have been found in cancers of the skin, colon, prostate and liver [3]. Thus, GSK-3 inhibition could mimic ectopic signalling of these pathways and promote tumorigenesis. However, no direct in vivo evidence has indicated that such a phenomenon occurs upon administration of GSK-3 inhibitors. Rather, recent studies in prostate, pancreatic and colorectal cancer cell lines indicate GSK-3 inhibitors leads to significant reduction in cell growth and proliferation [2,4], suggesting that inhibiting GSK-3 may actually be beneficial for the treatment of specific cancers.
Here, Wang et al. [5] have provided strong evidence demonstrating that GSK-3 activity is essential for maintenance of a subset of leukemias driven by the translocation of the MLL (mixed lineage leukemia) proto-oncogene. Leukemias that harbor MLL rearrangements are found in approximately 10% of human cases, with > 70 % frequency of occurrence in infant leukemia. Over 50 different translocation fusion partners have been identified. Patients with MLL fusions often have a poor clinical outcome and thus much effort has focused on seeking new treatments [6].
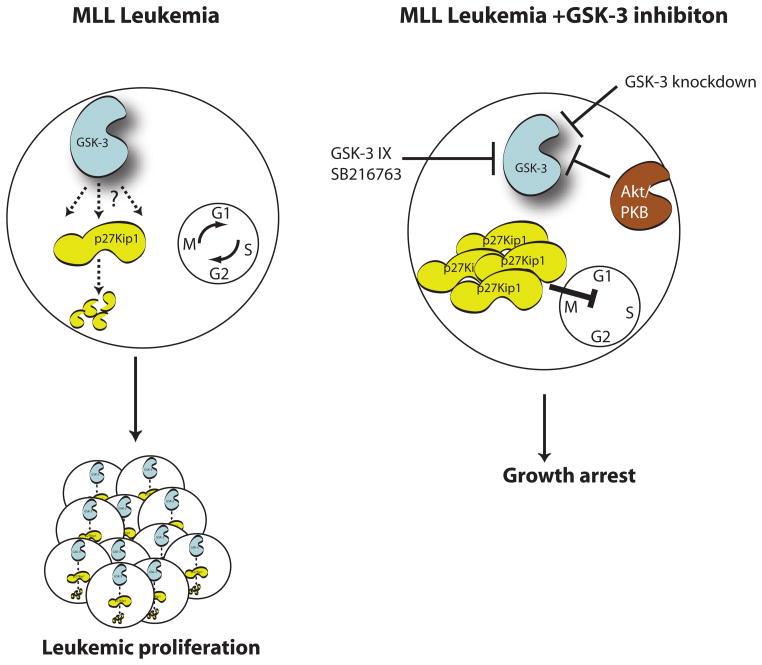
Using a pharmacological screen, Wang et al. [5] found that selective inhibitors of GSK-3 (GSK-3 IX, SB216763 and Alsterpaullone) specifically inhibited the growth of human MLL but not non-MLL leukaemia cells. In addition, GSK-3 inhibitors reduced clonogenic potential and proliferation of MLL-transduced murine myeloid or B cell progenitors. Importantly, normal primary myeloid progenitors and progenitors transduced by non-MLL fusions, displayed little sensitivity to drug treatment. These authors demonstrated that this inhibitor effect is indeed mediated by GSK-3, as genetic ablation of GSK-3β and shRNA-mediated knockdown of GSK-3α phenocopied the reduced self-renewing capacity of MLL-transformed progenitors in vitro and leukemogenesis in transplanted mice. The Wang et al. study also demonstrated that both GSK-3β and GSK-3α play redundant roles in maintenance of MLL transformed leukemias and that greater than 75% reduction in kinase activity is required to invoke impairment of leukemogenicity. This resembles the functionally redundant role of GSK-3 within the Wnt pathway in maintaining low cytoplasmic levels of β-catenin in resting cells, such that removal of at least 3 of the 4 GSK-3 alleles is required for any significant Wnt signaling [7]. Mechanistically, GSK-3 inhibitors expectedly increased β-catenin levels. While the extent of β-catenin stabilization was similar in both non- and MLL-human leukaemia cell lines, they observed that inhibition of GSK-3 induced expression of the p27Kip1 CDK inhibitor in only MLL-transformed cells. Furthermore, shRNA-p27Kip1 prevented GSK-3 inhibitor-induced growth arrest of MLL-leukemic cells (figure 1).
Figure 1.
The model of Wang et al demonstrates that GSK-3 supports the maintenance of MLL leukemia cells by continuous degradation of the cyclin dependent kinase inhibitor, p27Kip1. Suppression of GSK-3 leads to the stabilisation of p27Kip1 and subsequent growth arrest in MLL but not non-MLL leukemic or normal cells.
These findings shed new and contrasting insights into the role of GSK-3 as a negative regulator of proliferation and self-renewal. GSK-3 has been shown to modulate human stem cells (HSC) activity in vivo, suggesting that administration of GSK-3 inhibitors may directly enhance the repopulating capacity of transplanted HSCs [8]. Recent data indicate that certain leukemias are maintained by a population of self-renewal leukemia stem cells (LSC), and that they originate from transformed HSC and committed myeloid progenitors. A major concern is whether LSCs can be specifically targeted without affecting normal HSC. Wang et al. provide compelling evidence that inhibitors of GSK-3 could indeed be useful in targeting specifically the LSC population with MLL rearrangements without perturbing the normal HSC compartment [5]. There are some interesting questions that arise from their studies. Firstly, why are MLL-rearranged- but not non-MLL leukemia cells vulnerable to GSK-3 inhibitors? Wang et al. show that the abundance of the MLL-fusion oncogenes is not altered by GSK-3 inhibition, but the mechanism by which these cells are sensitized remains unclear, as does the molecular means by which GSK-3 influences p27Kip1. Recently, it was shown that a set of non-MLL leukemia cells exhibited over-expression of c-Myc and that the capacity of GSK-3 to associate and target Myc for degradation was reduced. This ‘GSK-3 resistance’ may offer an explanation as to why non-MLL cells are insensitive to GSK-3 inhibitors [9].
Secondly, what is the molecular mechanism by which GSK-3 supports MLL-induced oncogenesis? The authors allude to the existence of an Akt/GSK-3/p27Kip1 pathway within these cells. They show that a constitutively active Akt mutant suppresses MLL-positive cells and this is abolished by co-expression of a GSK-3 mutant that can no longer be phosphorylated/inhibited by Akt (figure 1). It would be of interest to determine whether MLL-induced leukemogenesis can occur in GSK-3α/β knockin mice expressing non-Akt-inhibitable mutants of GSK-3 [10] and if so, the status of p27Kip1 expression in the tumor cells. Thirdly, and most importantly, what are the long-term effects of high level GSK-3 inhibition as a therapy for MLL leukemias? This new study raises the possibility that activation of p27Kip1 and its growth inhibitory action will trump pro-oncogenic effects on proteins such as β-catenin. Wang et al. also demonstrated that exposure of animals with MLL leukaemia-like leukemia to lithium, which inhibits GSK-3, prolonged the survival of the treated mice by 40–50%. For decades, lithium has been widely used for the treatment of bipolar disorder, and there has been no evidence that these patients have increased incidences of cancers. Indeed, mice that have enhanced Wnt signaling and multiple intestinal polyps due to mutation of one copy of APC do not show further increased numbers of polyps when treated with lithium [reviewed in 1]. Thus, it appears that, despite initial concerns that long-term use of GSK3 inhibitors may promote oncogenesis, there is evidence accumulating to suggest that these drugs may, in fact, be effective in the treatment of certain cancers.
Acknowledgments
S.P. and J.W. are supported by funding from the Canadian Institutes of Health Research and Canadian Diabetes Association.
References
- 1.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez A. Preclinical efficacy on GSK-3 inhibitors: towards a future generation of powerful drugs. Medicinal Research Reviews. 2008;28:773–796. doi: 10.1002/med.20119. [DOI] [PubMed] [Google Scholar]
- 3.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Ougolkov AV, Fernandez-Zapico ME, Bilim VN, Smyrk TC, Chari ST, Billadeau DD. Aberrant nuclear accumulation of glycogen synthase kinase-3beta in human pancreatic cancer: association with kinase activity and tumor dedifferentiation. Clinical Cancer Research. 2006;12:5074–5081. doi: 10.1158/1078-0432.CCR-06-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Smith KS, Murphy M, Piloto O, Somervaille TC, Cleary ML. Glycogen synthase kinase 3 in MLL leukaemia maintenance and targeted therapy. Nature. 2008 doi: 10.1038/nature07284. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nature Reviews Cancer. 2007;7:823–833. doi: 10.1038/nrc2253. [DOI] [PubMed] [Google Scholar]
- 7.Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell. 2007;12:957–971. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmes T, O’Brien TA, Knight R, Lindeman R, Symonds G, Dolnikov A. The role of glycogen synthase kinase-3beta in normal haematopoiesis, angiogenesis and leukaemia. Curr Med Chem. 2008;15:1493–1499. doi: 10.2174/092986708784638834. [DOI] [PubMed] [Google Scholar]
- 9.Malempati S, Tibbitts D, Cunningham M, Akkari Y, Olson S, Fan G, Sears RC. Aberrant stabilization of c-Myc protein in some lymphoblastic leukemias. Leukemia. 2006;20:1572–1581. doi: 10.1038/sj.leu.2404317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]