Abstract
Itch is relieved by scratching, but the neural mechanisms that are responsible for this are unknown. Spinothalamic tract (STT) neurons respond to itch-producing agents and transmit pruritic information to the brain. We observed that scratching the cutaneous receptive field of primate STT neurons produced inhibition during histamine-evoked activity but not during spontaneous activity or activity evoked by a painful stimulus, suggesting that scratching inhibits the transmission of itch in the spinal cord in a state-dependent manner.
Itch is an unpleasant sensation that is associated with the desire to scratch. For most itches, relief is obtained by scratching in or around the region of itchy skin. However, itch that is coincident with skin disease or systemic disorders can be severe. In these circumstances, the desire to scratch is often overwhelming, but incessant scratching is harmful and leads to itch-scratch cycles that damage the skin and exacerbate the problem1. The mechanism by which scratching sup-presses itch is unknown. However, it has been hypothesized that this mechanism occurs in the CNS because noxious counterstimuli (for example, scratching) reduce itch when delivered many centimeters away from the site of itching and itch does not develop in a zone of cutaneous centrally mediated allodynia2-5. Histamine-sensitive dorsal horn neurons with unidentified projections are variably depressed by counterstimuli in rats6; however, unlike monkeys7, rats do not scratch in response to histamine, complicating the interpretation of these data with regard to itch.
In humans, anterolateral cordotomy eliminates the perception of itch from contralateral body sites below the lesion, implicating the STT in the transmission of pruritic information to the brain8. STT neurons can be activated for many minutes following the cutaneous application of itch-producing agents such as histamine9-11, matching the sensation of itch in humans12. Therefore, we examined whether the responses to histamine in primate STT neurons could be inhibited by scratching in the receptive field. STT neurons were recorded in the lumbar dorsal horn and were identified by antidromic stimulation (Supplementary Methods and Supplementary Fig. 1 online). Neurons were functionally characterized and their responses to initial scratching of the receptive field with a hand-held metal edge were determined. Every neuron tested had a mechanically sensitive receptive field and was excited by scratching (n = 28). Repeated scratches to the receptive field of single STT neurons reliably evoked similar discharges (Fig. 1). Two-thirds of STT neurons showed an after-discharge following scratching (Fig. 1b).
Figure 1.
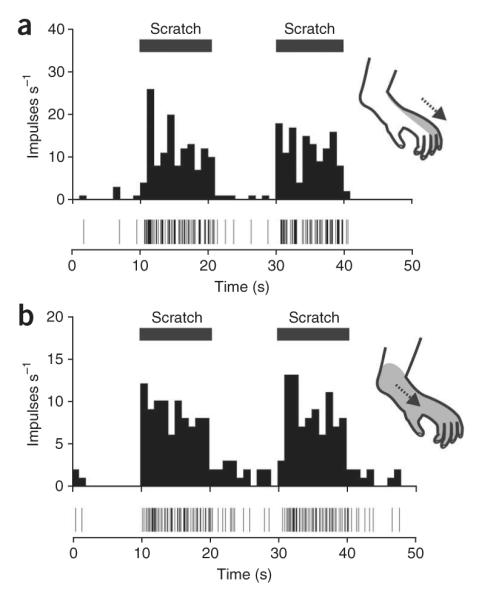
STT neurons are reliably activated by scratching, often with an after-discharge. (a) A histamine-sensitive STT neuron scratched before the histamine application (inset, receptive field and direction of scratching). (b) A histamine-insensitive STT neuron scratched before the histamine application. The time of each action potential is represented by a vertical line located below the histogram. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Minnesota.
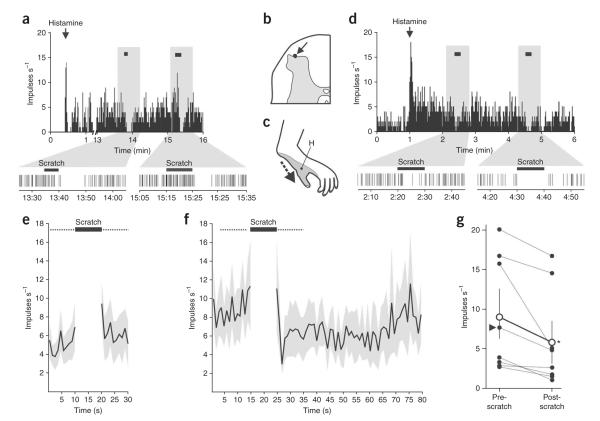
Histamine (20 μg in 10 μL) was then injected intradermally into the receptive field. In eight neurons that responded to histamine (Supplementary Fig. 2 online), the receptive field was scratched for 10 s during the response. Each histamine-responsive neuron showed fewer action potentials during the 10-s period immediately following the scratch than during the 10-s period before the scratch (Fig. 2). Most neurons increased their discharge during scratching, but two cells appeared to reduce their discharge during scratching (Fig. 2d). Scratching the receptive field of histamine-responsive neurons before the application of histamine did not produce inhibition (Figs. 2e and 3). During the response to histamine, however, the mean discharge during the 10 s immediately following scratching was reduced to 62 ± 8% of the pre-scratch level (Fig. 2f,g). Neurons showed lower-frequency discharge rates for about 30 s following scratch and then returned to pre-scratch levels (Fig. 2f). Neurons were recorded in the marginal zone (n = 4; Fig. 2b) and in the deep dorsal horn (n=4) and were classified as being of wide dynamic range (7 out of 8 neurons) or high threshold (1 out of 8 neurons). Histamine-insensitive neurons were not inhibited following scratching (Fig. 3c).
Figure 2.
Scratching inhibits histamine-evoked activation of STT neurons. (a) Discharge rates of an STT neuron activated by histamine before, during and following repeated scratching (black horizontal bars) of the cutaneous receptive field. The time scale is magnified below. (b,c) Recording site (arrow, b) and receptive field (c) of the neuron shown in a. H, histamine injection site. (d) Another histamine-sensitive STT neuron that was inhibited by repeated scratching. (e) Firing rates 10 s before and after (dotted lines) scratching in histamine-responsive STT neurons before the histamine application were not different (mean ± s.e.m., n = 8, Wilcoxon signed rank test, P = 0.2). (f) The time course before and after scratching of the firing rate during a response to histamine (mean ± s.e.m.). (g) Mean discharge rate of each histamine-responsive STT neuron during the 10 s before and the 10 s immediately after scratching during the response to histamine. Arrowhead indicates the high-threshold STT neuron. Open circles represent the group mean ± s.e.m., which was significantly reduced after scratching (n = 8, Wilcoxon signed rank test, P = 0.008).
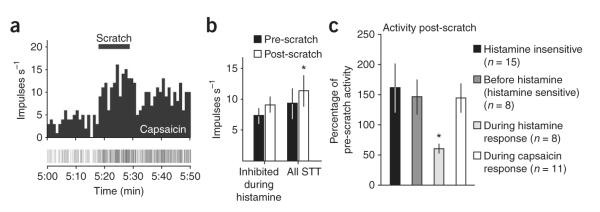
Figure 3.
State-dependency of inhibition. (a) The neuron shown in Figure 2d was not inhibited following scratching during a response to capsaicin. (b) Discharge rate (mean ± s.e.m.) during the response to capsaicin was not inhibited after scratch in histamine-responsive neurons (n = 4, Wilcoxon signed rank test, P = 0.25) and was increased for all STT neurons scratched during capsaicin treatment (n = 11, Wilcoxon signed rank test, P = 0.02). (c) Post-scratch activity given as a percentage of the pre-scratch activity (mean ± s.e.m. were measured). Only histamine-sensitive neurons scratched during the histamine response were inhibited (Kruskal-Wallis ANOVA and Dunn’s post-test, P = 0.009).
We further examined whether the scratch-induced inhibition of the histamine response was a result of ‘fatigue’ of STT neurons from the combined excitatory responses produced by a chemical stimulus and scratching. At least 20 min after the response to histamine, the chemical algogen capsaicin (10 μg in 10 μL) was injected intradermally into the receptive field 1–2 cm from the site of the histamine injection. All histamine responsive STT neurons responded to capsaicin (8 out of 8), as expected because histamine-sensitive peripheral fibers respond to capsaicin and are TRPV1 positive13,14. The receptive fields from four histamine-responsive neurons that were previously inhibited by scratching during histamine were scratched again during their response to capsaicin (Fig. 3a). Unlike during the response to histamine, none of these neurons showed a decrease in discharge rate during the 10-s period following scratching (Fig. 3b). Seven additional STT neurons were exposed to scratching during a response to capsaicin and, together with the four neurons from the histamine-responsive group, showed an overall increase in mean discharge rate after scratching (Fig. 3b). These data indicate that STT neurons responding to a potent chemical stimulus are capable of higher-frequency discharge following a counterstimulus. Scratching inhibited STT neurons only during a response to histamine (Fig. 3c).
Histamine elicits itching in humans12 and scratching is an effective means of partially reducing that itch5. A noxious counter-stimulus was previously shown to reduce activity in brain regions activated by histamine15. Our data suggest that relief of itch by scratching is the result of a reduction in the discharge rate of STT neurons responding to an itch-producing stimulus. Ongoing activity of STT neurons was not reduced when scratching was given before histamine or during a response to capsaicin. The reduced activity following scratching occurred only when delivered during a response to histamine, suggesting that itch produces a state during which scratching engages a central inhibitory mechanism. These data provide a mechanism by which supraspinal activity and sensation regarding itch can be modulated by changes in activity at the level of the spinal cord. Future work should determine whether the state-dependent inhibition of pruriceptive STT neurons is mediated by local inhibitory interneurons and/or a descending mechanism from the brain6,15.
Supplementary Material
ACKNOWLEDGMENTS
We thank H. Truong for his valuable technical assistance. This work was supported by US National Institutes of Health/National Institute of Neurological Disorders and Stroke grants NS-047399 and NS-059199 and by the Graduate School of the University of Minnesota.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
Reprints and permissions information is available online at http://www.nature.com/reprintsandpermissions/
References
- 1.Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. Nat. Rev. Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- 2.Graham DT, Goodell H, Wolff HG. J. Clin. Invest. 1951;30:37–49. doi: 10.1172/JCI102414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward L, Wright E, McMahon SB. Pain. 1996;64:129–138. doi: 10.1016/0304-3959(95)00080-1. [DOI] [PubMed] [Google Scholar]
- 4.Brull SJ, Atanassoff PG, Silverman DG, Zhang J, LaMotte RH. Somatosens. Mot. Res. 1999;16:299–303. doi: 10.1080/08990229970366. [DOI] [PubMed] [Google Scholar]
- 5.Yosipovitch G, Duque MI, Fast K, Dawn AG, Coghill RC. Br. J. Dermatol. 2007;156:629–634. doi: 10.1111/j.1365-2133.2006.07711.x. [DOI] [PubMed] [Google Scholar]
- 6.Carstens E. J. Neurophysiol. 1997;77:2499–2514. doi: 10.1152/jn.1997.77.5.2499. [DOI] [PubMed] [Google Scholar]
- 7.Johanek LM, et al. J. Neurosci. 2008;28:7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White JC, Sweet WH. CC Thomas; Springfield, Illinois: 1969. [Google Scholar]
- 9.Andrew D, Craig AD. Nat. Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- 10.Simone DA, et al. J. Neurophysiol. 2004;91:213–222. doi: 10.1152/jn.00527.2003. [DOI] [PubMed] [Google Scholar]
- 11.Davidson S, et al. J. Neurosci. 2007;27:10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simone DA, et al. Somatosens. Res. 1987;5:81–92. doi: 10.3109/07367228709144620. [DOI] [PubMed] [Google Scholar]
- 13.Schmelz M, et al. J. Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- 14.Shim WS, et al. J. Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mochizuki H, et al. Pain. 2003;105:339–346. doi: 10.1016/s0304-3959(03)00249-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.